Abstract
Mammalian sperm must undergo a functionally defined process called capacitation to be able to fertilize oocytes. They become capacitated in vivo by interacting with the female reproductive tract or in vitro in a defined capacitation media that contains bovine serum albumin (BSA), calcium (Ca2+) and bicarbonate (HCO3−). In this work, sperm were double stained with propidium iodide and the Ca2+ dye Fluo-4 AM and analyzed by flow cytometry to determine changes in intracellular Ca2+ concentration ([Ca2+]i). An increase in [Ca2+]i was observed in a subpopulation of capacitated live sperm when compared with non-capacitated ones. Sperm exposed to capacitating medium displayed a rapid increase in [Ca2+]i within 1 min of incubation, which remained sustained for 90 min. These rise in [Ca2+]i after 90 min of incubation in capacitating medium was evidenced by an increase in the normalized median fluorescence intensity (MFI). This increase was dependent on the presence of extracellular Ca2+ and at least in part reflected the contribution of a new subpopulation of sperm with higher [Ca2+]i. In addition, it was determined that the capacitation-associated [Ca2+]i increase was dependent of CatSper channels, as sperm derived from CatSper knockout (CatSper KO) or incubated in the presence of CatSper inhibitors failed to increase [Ca2+]i. Surprisingly, a minimum increase in [Ca2+]i was also observed in CatSper KO sperm suggesting the existence of other Ca2+ transport system. Altogether, these results indicate that a subpopulation of sperm increases [Ca2+]i very rapidly during capacitation mainly due to a CatSper-mediated influx of extracellular Ca2+.
Keywords: Sperm, Calcium, Capacitation, CatSper
Introduction
Freshly ejaculated mammalian sperm do not have the ability to fertilize oocytes. They must undergo a functionally defined process called capacitation (Chang, 1951; Austin, 1952), which allows them to develop hyperactivated motility and the ability to undergo acrosomal exocytosis (Suarez, 2008; Buffone, Hirohashi and Gerton, 2014). Sperm become capacitated in vivo by interacting with the female reproductive tract or in vitro in a defined capacitation media that contains bovine serum albumin (BSA), calcium (Ca2+) and bicarbonate (HCO3−) (Yanagimachi, 1994; Visconti, Bailey, et al., 1995). From a molecular point of view, capacitation leads to an increase in cAMP, Protein Kinase A (PKA, aka cAMP-dependent protein kinase) activity and tyrosine phosphorylation of sperm proteins (Visconti, Moore, et al., 1995; Alvau et al., 2016), membrane hyperpolarization (De La Vega-Beltran et al., 2012) as well as a rise in intracellular pH (pHi) (Stival et al., 2016) and in intracellular Ca2+ concentration ([Ca2+]i) (Ruknudin and Silver, 1990). The increase in [Ca2+]i was shown to be essential for hyperactivation and acrosomal exocytosis in several mammalian species (Marín-Briggiler et al., 2003; Darszon et al., 2011; Correia, Michelangeli and Publicover, 2015). The predominant source of Ca2+ during hyperactivation in the female genital tract is extracellular Ca2+ that enters through CatSper channels (Kirichok, Navarro and Clapham, 2006). CatSper proteins are only expressed in male germ cells and localize specifically to the plasma membrane of the principal piece of the flagellum in mature sperm (Carlson et al., 2005; Jin et al., 2007). Male mice lacking any of the CatSper1–4 genes are infertile and their sperm are unable to undergo hyperactivation (Ren et al., 2001; Quill et al., 2003; Qi et al., 2007; Chung et al., 2011), as are human males with loss-of-function mutations (Avidan et al., 2003; Avenarius et al., 2009; Smith et al., 2013).
Most of our knowledge about capacitation comes from in vitro experiments using whole sperm populations, which derive from average responses of sperm with different degrees of capacitation, that consider not only normal cells but also dead as well as abnormal gametes. Previous observations by multiple laboratories indicate that sperm population is not homogeneous and only a subset of sperm undergoes capacitation (Buffone et al., 2004; Escoffier et al., 2015). Sperm [Ca2+]i is usually assessed using Ca2+ sensitive probes such as Fluo and Fura by fluorometry (Mata-Martínez et al., 2013), flow cytometry (López-González et al., 2014) and single-cell analysis (Romarowski et al., 2016). Single cell analysis has the advantage of studying molecular events in individual sperm and detecting local changes in different sperm compartments. However, this method is not suitable for high-throughput studies. On the other hand, flow cytometry can analyze thousands of cells very rapidly compared to the time required for single-cell studies, and results in a valuable tool for the analysis of different populations of capacitated sperm (Tao et al., 1993; Piehler et al., 2006; Robles and Martínez-Pastor, 2013). Using this approach, it was reported how changes in sodium (Escoffier et al., 2012), membrane potential (Escoffier et al., 2015), membrane fluidity (Gadella et al., 1999), pH (López-González et al., 2014; Puga Molina et al., 2017) and acrosomal exocytosis (Zoppino et al., 2012; Hirohashi et al., 2015; Romarowski et al., 2015) occur in individual sperm during capacitation. However, despite the importance of Ca2+ fluxes in events leading to capacitation, analysis of [Ca2+]i in mouse sperm is still scarce.
In this study, we analyzed [Ca2+]i changes during capacitation from epididymal mouse sperm using flow cytometry. We observed that there is a subpopulation of sperm that increases [Ca2+]i very rapidly during capacitation due to an influx from extracellular Ca2+. The rise in [Ca2+]i appears to be controlled by HCO3− and BSA present in the capacitation media and is mediated by CatSper channels. However, our experiments also suggest the presence of a CatSper-independent Ca2+ influx that could be critical for sperm capacitation.
Materials and Methods
Reagents
Chemicals were obtained from the following sources: bovine serum albumin (BSA) A7906, Ca2+ ionophore A23187, Mibefradil, NNC55-0396 and Ethylene glycol-bis (2-aminoethylether)-N,N,N,N′tetraacetic acid (EGTA) were purchased from Sigma–Aldrich Chemical Co. (St.Louis, MO); Fluo-4 AM and Fluo-3 AM from Molecular Probes, Thermo Fisher Scientific; Pluronic acid from Life Technologies Corporation (Invitrogen); PI from Santa Cruz (Santa Cruz, USA) and Ionomycin from Alomone Labs (Jerusalem, Israel). All other chemicals were of reagent grade. Fluo-4 AM, Fluo-3 AM, Pluronic acid, Ca2+ ionophore A23187 and Ionomycin were dissolved in DMSO; EGTA was dissolved in non-capacitating modified TYH medium without Ca2+ (-HCO3−, -BSA, -Ca2+); while PI, Mibefradil and NNC55-0396 were dissolved in hexa-distilled water.
Animals
Hybrid F1 (C57BL/6 x Balb/C) mature (10–12 weeks-old) male mice were used. CatSper KO (Ren et al., 2001) mice and their corresponding wild-type siblings were on a mixed background (C57BL/6 and CD1), and were provided by Dr. Pablo Visconti from University of Massachusetts (Navarrete et al., 2016). CatSper-null mice were euthanized in accordance with the Animal Care and Use Committee (IACUC) guidelines of UMass-Amherst. In all cases, mice were housed in groups of 4 or 5 in a temperature-controlled room (23°C) with lights on at 07:00 h and off at 19:00 h, and had free access to tap water and laboratory chow. All experimental procedures were carried according to guidelines of the institutional animal care and were reviewed and approved by the Ethical Committee of the Instituto de Biología y Medicina Experimental, Buenos Aires. Experiments were performed in strict accordance with the Guide for Care and Use of Laboratory Animals approved by the National Institutes of Health (NIH).
Sperm medium
The non-capacitating medium used in this study was a modified Toyoda–Yokoyama–Hosi (modified TYH) containing 119.3 mM NaCl, 4.7 mM KCl, 1.71 mM CaCl2.2H2O, 1.2 mM KH2PO4, 1.2 mM MgSO4.7H2O, 0.51 mM sodium pyruvate, 5.56 mM glucose, 20 mM HEPES, 10 μg/ml gentamicin and phenol red 0.0006% (NC medium). For capacitating conditions 15 mM NaHCO3 and 5 mg/ml BSA were added (CAP medium). In the cases where medium without added Ca2+ salts was required, 1.71 mM CaCl2.2H2O was omitted (nominal zero Ca2+ medium, indicated by Ca2+=0). When chelation of the extracellular Ca2+ was needed, we added 1 mM EGTA at nominal zero Ca2+ medium. In all cases, pH was adjusted to 7.4 with NaOH.
Sperm capacitation
Animals were euthanized and cauda epididymal mouse sperm were collected. Both cauda epididymis were placed in 1 ml of non-capacitating modified TYH medium (without BSA and NaHCO3) in the presence or absence of Ca2+ as described for each experiment. After 15 min of incubation at 37 °C (swim-out), epididymis were removed, and sperm were resuspended to a final maximum concentration of 1x107 cells/ml on 100 μl of the appropriate medium depending on the experiment performed. A 10 min pre-incubation in 100 μl of non-capacitating media containing CatSper inhibitors (30 μM Mibefradil or 10 μM NNC55-0396) was done when required. An equal volume (100 μl) of non-capacitating medium or two-fold concentrated capacitating media (30 mM NaHCO3 and 10 mg/ml BSA) was added, and sperm were incubated for different time periods at 37°C.
Determination of [Ca2+]i by flow cytometry
Sperm [Ca2+]i changes were assessed using Fluo-4 AM. After incubation in the appropriate medium, samples were centrifuged at 400 x g for 4 min at room temperature and resuspended in 200 μl of non-capacitating media containing 1 μM Fluo-4 AM and 0.02% Pluronic acid for 20 min at 37°C. Samples were washed again and resuspended in 50 μl of non-capacitating TYH media. Before collecting data 2 ng/μl of PI was added to monitor viability. In some experiments 10 μM of Ca2+ ionophore A23187 was also added to the samples. Data were recorded as individual cellular events using a BD LSRFortessa TM cytometer (Biosciences; Becton, Dickinson and Company) for the experiments involving CatSper KO and their wild-type mice siblings and BD FACSCanto II TM cytometer (Biosciences; Becton, Dickinson and Company) for all other experiments. Side-scatter area (SSC-A) and forward-scatter area (FSC-A) data were collected from 20,000 events per sample in order to define sperm population as previously described (Escoffier et al., 2012). In all cases, doublet exclusion was performed analyzing two-dimensional dot plot FSC-A vs. forward-scatter height (FSC-H). Doublets exhibit a higher signal width or area to height ratio compared to single cells (singlets). Events deviating from the diagonal are doublets. Positive cells for Fluo-4 AM were collected using the filter for Fluorescein isothiocyanate (FITC; 530/30), and for PI the filter for Peridinin chlorophyll protein complex PerCP (670LP). The two indicators had minimal emission overlap, but still compensation was done. Data were analyzed using FlowJo software (V10.0.7).
Live imaging of [Ca2+]i in individual mouse sperm
Sperm were incubated with 2 μM Fluo-3 AM and 0.05% Pluronic acid in non-capacitating TYH modified medium with BSA (5 mg/ml) during 20 min. Once loaded, the cells were centrifuged at 500 x g for 4 min at room temperature and resuspended in 200 μl of non-capacitating media with BSA. Sperm were immobilized on mouse laminin (0.1 mg/ml) coated-cover slips to allow recordings. The chamber was filled with recording medium (non-capacitating TYH). Ca2+ imaging was performed before, during, and after TYH + 30 mM HCO3− or non-capacitating TYH (vehicle) addition. At the end of each recording, 10 μM Ionomicyn was added to assess the viability of the cells. Those cells that did not display a robust Ca2+ response after addition of Ionomicyn were not included in our analysis. The images were taken in a Zeiss LSM880 scan head on an axio observer Z1 inverted microscope with a 60x1.4 AN oil immersion objective. A laser line 488 nm of an argon ion laser was used for the excitation, while the detection was done in a GaAsP spectral detector with a bandwidth between 508 and 588 nm. Bi-directional scanning was used with a Dwell time of 1.03 μsec at 512x512 pixels resulting in an acquisition of 3 frames per second for periods of 8–10 minutes. Movies were processed and analyzed with Image J (v1.38, NIH). Regions of interest (ROIs) were drawn on each sperm for fluorescence quantification. Intracellular [Ca2+] is presented as (F-F0)/F0 ratios after background subtraction, where F-F0 was the change in fluorescence signal intensity and F0 was the baseline as calculated by averaging 180 frames before stimulus application.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) of at least four independent experiments for all determinations. Statistical analyses were performed using the GraphPad Prism 6 software (La Jolla, CA USA). The differences between means of only 2 groups were analyzed by paired t-test (single-cell analysis). Two-way analysis of variance (ANOVA) with repeated measures was used to analyze the effect of incubation medium in normalized median fluorescence intensity (MFI) of Fluo-4 AM and percentage of sperm with high Fluo-4 AM fluorescence, over time. One-way ANOVA for matched data was performed to analyze percentage of sperm with high Fluo-4 AM fluorescence comparing different incubation conditions, while non-parametric Friedman test was performed in combination with Dunn’s multiple comparisons test to analyze normalized MFI of Fluo-4 AM. Two-way ANOVA for independent measures was performed to analyze normalized MFI of Fluo-4 AM and percentage of sperm with high Fluo-4 AM fluorescence, for the effects of: experimental condition x genotype. Post hoc Sidak’s test was employed when necessary. A probability (p) value p<0.05 was considered statistically significant. Parametric or non-parametric comparisons were used as dictated by data distribution.
Results
Only a subpopulation of capacitated mouse sperm displays an increase in [Ca2+]i
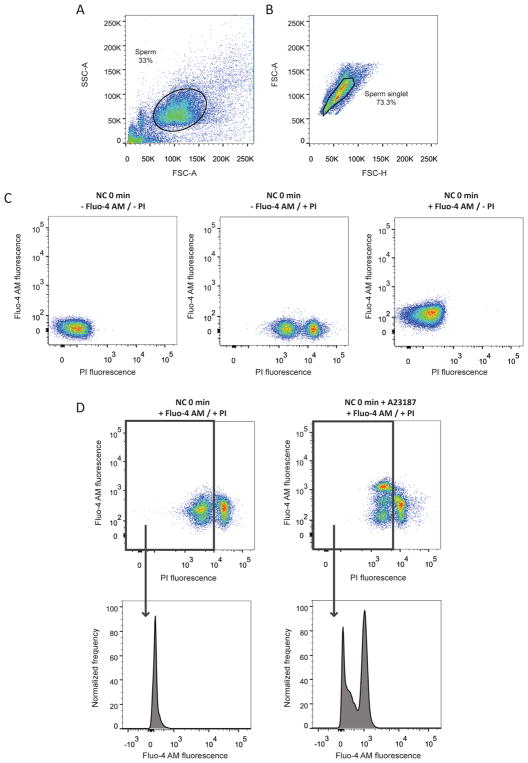
Intracellular [Ca2+] was measured in individual cells using flow cytometry. Sperm were loaded with the Ca2+ probe Fluo-4 AM and PI to differentiate between live and dead sperm. Briefly, the sperm population was defined as previously described (Escoffier et al., 2012), where 0.1% Triton X-100 was used to discriminate non-sperm particles passing through the flow cytometer detector as it solubilizes non-sperm particles. Then those sperm associated in doublets were excluded. Once non-sperm events and sperm doublets were gated out (Figure 1A–B), two-dimensional fluorescence dot plots of Fluo-4 AM (positively correlated with [Ca2+]i) versus PI (to label DNA of dying cells) were created. In order to make compensation, three controls were performed for each experiment: without any staining (-Fluo-4 AM / -PI), with only PI staining (-Fluo-4 AM / +PI), or with only Fluo-4 AM staining (+Fluo-4 AM / -PI) (Figure 1C). In experimental samples, by using the two-dimensional fluorescence dot plots, the live sperm population was gated (negative for PI staining). This population was used for the analysis of [Ca2+]i by performing histograms that display fluorescence of Fluo-4 AM on the x-axis and normalized frequency of sperm on the y-axis (Figure 1D). Both, two-dimensional dot plots and histograms, were used to observe changes in [Ca2+]i of live sperm. An increase in Fluo-4 AM fluorescence was observed in live sperm incubated with Ca2+ ionophore A23187 (Figure 1D right) when compared with non-treated ones (Figure 1D left). This ionophore-induced increase did not occur in dead sperm (PI positive, Figure 1D).
Figure 1. Parameters settings for [Ca2+]i analysis by flow cytometry.
Ca2+ was measured in mouse sperm in TYH medium using 1 μM Fluo-4 AM. A) Representative two-dimensional dot plot side-scatter area (SSC-A) vs. forward-scatter area (FSC-A) analysis of sperm collected after swim-out (NC 0 min) where the population of sperm is defined for each experiment (n=25). B) Representative two-dimensional dot plot FSC-A vs. forward-scatter height (FSC-H) analysis for doublet exclusion. Events deviating from the diagonal are doublets (n=25). C) Flow cytometry compensation controls performed in all experiments (n=25): Representative two-dimensional fluorescence dot plot Fluo-4 AM vs. PI analysis of sperm collected after swim-out (NC 0 min) without any staining (-Fluo-4 AM / -PI), with only PI staining (-Fluo-4 AM / +PI), or only Fluo-4 AM staining (+Fluo-4 AM / -PI). D) Fluo-4 AM vs. PI two-dimensional fluorescence dot plot analysis of sperm collected after swim-out (NC 0 min) incubated with or without Ca2+ ionophore A23187. These two-dimensional dot plots were used to distinguish between sperm with low (live) and high (dead) PI staining. Histogram analysis depicting normalized frequency of sperm and Fluo-4 AM fluorescence were performed in live sperm populations. Representative images of 4 independent experiments are shown.
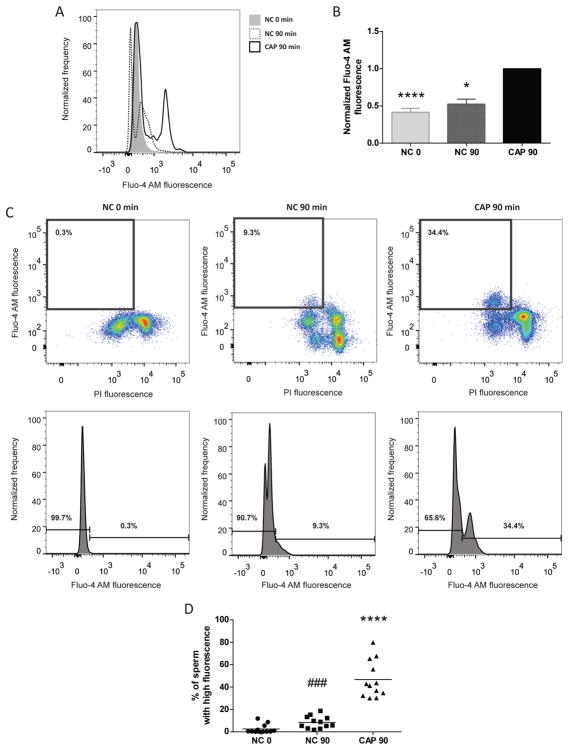
To investigate the effect of capacitation in the regulation of [Ca2+]i, Fluo-4 AM fluorescence was analyzed in sperm treated with three different conditions. First, [Ca2+]i was determined immediately after recovering sperm from the swim-out (grey shadow in Figure 2A); second, the analysis was conducted in sperm incubated for 90 min in conditions that do not support capacitation; and finally, [Ca2+]i was assessed in sperm incubated for 90 min in capacitating medium (Figure 2A). Incubation for 90 min in capacitating medium led to an increase in normalized MFI of Fluo-4 AM in live sperm, which was not observed in non-capacitating conditions (Figure 2B). Interestingly, incubation for 90 min in both, non-capacitating and capacitating conditions led to an increase in Fluo-4 AM fluorescence in a live sperm subpopulation, which can be observed in the dot plots, when compared with those recovered from the swim-out (Figure 2C, upper panels). Once again, this [Ca2+]i increase did not occur in dead sperm (PI positive, Figure 2C, upper panels). However, the percentage of sperm with high [Ca2+]i was significantly higher in the capacitated population. The increase can be also visualized using normalized frequency histograms (Figure 2C, bottom panels). The percentage of sperm undergoing [Ca2+]i increase was plotted for sperm from 12 different mice (Figure 2D). In every case the population of sperm with high fluorescence was determined from the capacitating control condition (CAP 90 min) and extrapolated to the others conditions of each experiment (Figure 2C).
Figure 2. Intracellular Ca2+ levels increased during capacitation.
A) Sperm collected after swim-out (NC 0 min) or incubated for 90 min either under non-capacitating conditions (NC) or capacitating conditions (CAP) were analyzed. Representative histograms of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm are shown. B) Normalized MFI of Fluo-4 AM compared to the control condition (CAP 90 min). Values represent the mean ± SEM of 12 independent experiments. **** p<0.0001 represents statistical significance between control (CAP 90 min) vs. NC 0 min; * p<0.05 represents statistical significance between CAP 90 min vs. NC 90 min. Non-parametric Friedman test was performed in combination with Dunn’s multiple comparisons test. C) Representative two-dimensional Fluo-4 AM vs. PI fluorescence dot plot and their corresponding histograms of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm are shown. We identified the responsive population with high Fluo-4 AM fluorescence levels from the capacitating condition (CAP 90 min) and we established the percentage of sperm that responds increasing the [Ca2+]i. D) Percentage of sperm that increased Fluo-4 AM fluorescence after being incubated for 90 min under non-capacitating (NC 90 min) and capacitating conditions (CAP 90 min) in comparison with sperm collected after swim-out (NC 0 min). Values represent the percentage of sperm of 12 independent experiments. **** p<0.0001 represents statistical significance between control (CAP 90 min) vs. NC 0 and NC 90 min conditions; ### p<0.001 represents statistical significance between NC 90 min vs. NC 0 min condition. One-way ANOVA for matched data was performed, with Tukey’s multiple comparisons test.
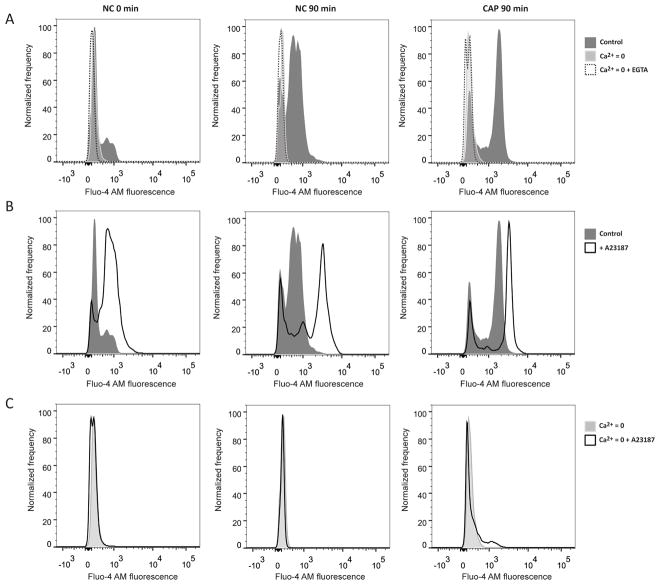
The capacitation-induced increase in [Ca2+]i can be due to an influx from extracellular Ca2+ and/or to the release of this ion from sperm intracellular Ca2+ storages (e.g. acrosome, redundant nuclear envelope). To elucidate the source of Ca2+, Fluo-4 AM fluorescence was analyzed in sperm incubated in Ca2+-free medium (nominal zero Ca2+ concentration, Ca2+=0) or in Ca2+-free medium with the addition of 1 mM EGTA (Ca2+=0 + EGTA), given that Ca2+=0 media still contain contaminant Ca2+ at micromolar concentrations (Marin-Briggiler, 2005). In neither of these conditions the increase in [Ca2+]i in non-capacitating and capacitating media was observed (Figure 3A). These results support the hypothesis that the presence of extracellular Ca2+ is required for the capacitation-associated increase in [Ca2+]i. On the other hand, as a positive control, and to determine the higher sensitivity limit of our assay, sperm were exposed to A23187. In the presence of Ca2+ in the incubation media, addition of Ca2+ ionophore resulted in an increase in [Ca2+]i in every case (Figure 3B) which was not observed when A23187 was added to sperm incubated in Ca2+=0 media (Figure 3C). Previous reports indicate that Ca2+ ionophore A23187 may be used to release Ca2+ from intracellular stores in the absence of extracellular Ca2+ in other cell types (Pelassy, Breittmayer and Aussel, 1992; Rzigalinski, Blackmore and Rosenthal, 1996; Cobbold, Brookes and Wileman, 2000). However, the absence of [Ca2+]i increase with A23187 in Ca2+=0 media indicated that no mobilization of Ca2+ from intracellular stores was induced in sperm (Figure 3C).
Figure 3. Capacitation-associated [Ca2+]i increase depends on the presence of Ca2+ in the incubation medium.
Cauda epididymal sperm were recovered by swim-out (NC 0 min) in media with or without Ca2+ (nominal zero Ca2+, indicated by Ca2+=0). Sperm were incubated for 90 min under non-capacitating (NC) and capacitating conditions (CAP) in media with or without Ca2+ (in the presence of EGTA 1 mM or not). Just before recording, in some cases, 10 μM of A23187 was added. Histograms of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm are shown, which are representative of 4 independent experiments. A) Sperm collected after swim-out (NC 0 min). B) Sperm incubated for 90 min under non-capacitating conditions (NC 90 min). C) Sperm incubated for 90 min under capacitating conditions (CAP 90 min).
The increase in [Ca2+]i is an early capacitation event and is independently regulated by HCO3− and BSA
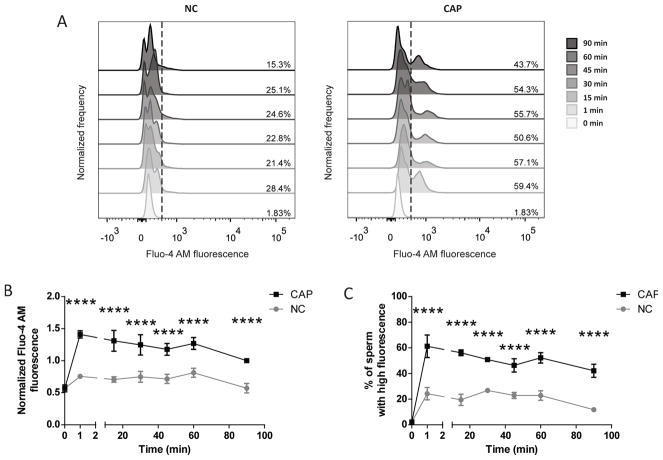
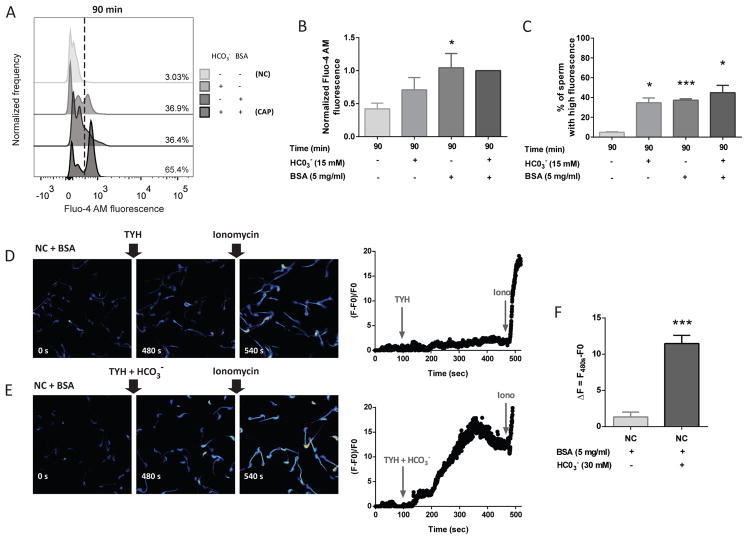
Sperm capacitation is a time-dependent process; while some signaling pathways are activated as soon as the sperm encounters a capacitation-supporting medium, others only occur after several minutes to hours of incubation. To test the kinetics of Ca2+ changes, [Ca2+]i was analyzed in sperm incubated in medium that supports capacitation or not, at different time points. In capacitating conditions, a very rapid rise in [Ca2+]i starting at 1 min of incubation was observed in a sperm subpopulation (Figure 4A). The percentage of sperm with high Ca2+ remains stable for at least 90 min. On the other hand, in non-capacitating conditions, although a percentage of the sperm population also presented higher [Ca2+]i, the relative amount of [Ca2+]i was much lower. The changes in the normalized MFI of Fluo-4 AM and the percentage of sperm that belongs to the responsive population at different time points during capacitation are plotted for 4 independent experiments (Figure 4B and 4C, respectively).
Figure 4. A rise in [Ca2+]i occurs after 1 min of incubation under capacitating conditions.
A) Intracellular [Ca2+] was measured by flow cytometry in sperm collected after swim-out (0 min) and incubated under non-capacitating and capacitating conditions for different time periods (1–90 min). Histograms of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm, with the corresponding percentage of sperm that increased Fluo-4 AM fluorescence, are shown. Histograms are representative of at least 4 independent experiments. B) Normalized MFI, compared to the control condition (CAP 90 min), of Fluo-4 AM. Values represent the mean ± SEM of at least 4 independent experiments. **** p<0.0001 represents statistical significance between control (CAP 90 min) vs. time-matched NC condition. Two-way ANOVA with repeated measures and Sidak’s multiple comparisons test was performed. C) Percentage of sperm that increased Fluo-4 AM fluorescence after being incubated (1–90 min) under non-capacitating (NC) and capacitating conditions (CAP). Values represent the mean ± SEM of at least 4 independent experiments. **** p<0.0001 represents statistical significance between control (CAP 90 min) vs. time-matched NC condition. Two-way ANOVA with repeated measures and Sidak’s multiple comparisons test was performed.
In our experimental conditions, the difference between the medium that supports capacitation and the one that does not is the presence of BSA and HCO3−. To test the participation of these molecules in the capacitation-associated [Ca2+]i increase independently, sperm were incubated for 90 min in the presence or absence of one or both compounds. Incubation with HCO3− alone stimulated an increase in the percentage of sperm with high Ca2+ in comparison with sperm incubated in medium that did not support capacitation (-HCO3−, -BSA, +Ca2+). On the other hand, BSA had an even greater effect stimulating a rise in [Ca2+]i to similar levels as those obtained in the presence of both, HCO3− and BSA (Figure 5A–C). The effect of BSA in the regulation of [Ca2+]i has been analyzed previously in single cells (Xia and Ren, 2009). The flow cytometry experiments suggest that HCO3− alone is also able to induce elevation of [Ca2+]i. To further test this hypothesis, changes in [Ca2+]i were analyzed using single cell imaging of Fluo-3 AM-loaded sperm. Addition of HCO3− resulted in a rapid [Ca2+]i increase (Figure 5E and F) which did not occur when non-capacitating TYH medium (vehicle) was added (Figure 5D and F, and Supplementary Movies S1–S2). Overall, flow cytometry and single cell analyses suggest that both BSA and HCO3− contribute to the increase in [Ca2+]i observed under capacitating conditions.
Figure 5. Either HCO3− or BSA caused independently an increase in Ca2+ response, being the effect of BSA significantly greater.
Assessment of the effects of HCO3− and BSA on the capacitation induced [Ca2+]i increase. A) Sperm were swum-out in non-capacitating medium (NC) and then incubated for 90 min in TYH medium in the presence or absence of different compounds: HCO3− (15 mM) and BSA (5 mg/ml). Histograms, representative of 4 independent experiments, of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm are shown, with the corresponding percentage of sperm that increased Fluo-4 AM fluorescence. B) Normalized MFI, compared to the control condition (CAP), of Fluo-4 AM. Values represent the mean ± SEM of 4 independent experiments. * p<0.05 represents statistical significance between BSA (-HCO3−, +BSA, +Ca2+) vs. NC condition (-HCO3−, -BSA, +Ca2+). Non-parametric Friedman test was performed in combination with Dunn’s multiple comparisons test. C) Percentage of sperm that increased Fluo-4 AM fluorescence after being incubated for 90 min in the different conditions. Values represent the mean ± SEM of 4 independent experiments. *** p<0.001, * p<0.05 represents statistical significance vs. NC condition (-HCO3−, -BSA, +Ca2+). One-way ANOVA for matched data was performed, with Tukey’s multiple comparisons test. HCO3− addition promoted a rapid [Ca2+]i increase in sperm. D–F) Representative fluorescence images of 4 independent experiments, corresponding to [Ca2+]i responses obtained before and after TYH medium or TYH+HCO3− addition, are shown. At the end 10 μM Ionomycin (Iono) was added as a viability control. Right traces show representative single cell [Ca2+]i recordings obtained during each experiment. Arrows indicate additions of TYH or TYH+HCO3− and Ionomycin. D) Sperm exposed to vehicle (TYH medium). E) Addition of TYH with 30 mM HCO3−. F) Intracellular [Ca2+] increase under the indicated condition. ΔF = Fluorescence just before Ionomycin addition (480 s) – F0. Values represent the mean ± SEM of 4 independent experiments. *** p<0.001 represents statistical significance between TYH+HCO3− vs. control condition (TYH addition). Paired t-test was performed.
CatSper activity is required for the capacitation-associated [Ca2+]i increase
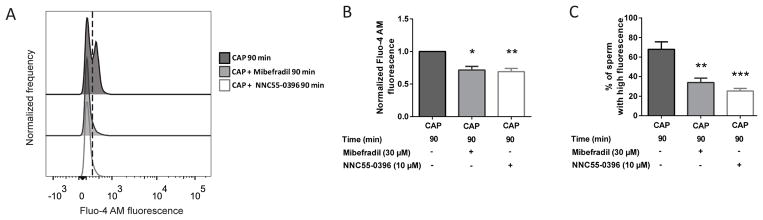
To assess the participation of CatSper, [Ca2+]i was evaluated in the presence or absence of voltage-gated Ca2+ channel (Cav) blockers: Mibefradil and NNC55-0396 (Lishko, Botchkina and Kirichok, 2011; Strünker et al., 2011). Sperm were incubated for 90 min in capacitating conditions in the presence of 30 μM Mibefradil or 10 μM NNC55-0396, concentrations previously shown to block CatSper currents using electrophysiological measurements (Strünker et al., 2011). These concentrations did not affect sperm viability (data not shown). Addition of these compounds suppressed the capacitation-associated [Ca2+]i increase (Figure 6). The inhibition can be visualized by a decrease in the normalized MFI of Fluo-4 AM (Figure 6B) as well as in the percentage of sperm that displayed a rise in [Ca2+]i (Figure 6C).
Figure 6. CatSper inhibition blocked the rise in [Ca2+]i that occurs during capacitation.
A) Sperm incubated for 90 min under capacitating (CAP) conditions in the absence or presence of CatSper inhibitors (Mibefradil and NNC55-0396), were analyzed. Representative histograms of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm, with the corresponding percentage of sperm that increased Fluo-4 AM fluorescence, are shown. B) Normalized MFI, compared to the control condition (CAP without CatSper inhibitor), of Fluo-4 AM. Values represent the mean ± SEM of 8 independent experiments. ** p<0.01, * p<0.05 represents statistical significance vs. CAP condition without CatSper inhibitor. Non-parametric Friedman test was performed in combination with Dunn’s multiple comparisons test. C) Percentage of sperm that increased Fluo-4 AM fluorescence after being incubated for 90 min in the different conditions. Values represent the mean ± SEM of 8 independent experiments. *** p<0.001, ** p<0.01 represents statistical significance vs. (CAP without CatSper inhibitor). One-way ANOVA for matched data with Dunnett’s multiple comparisons test was performed.
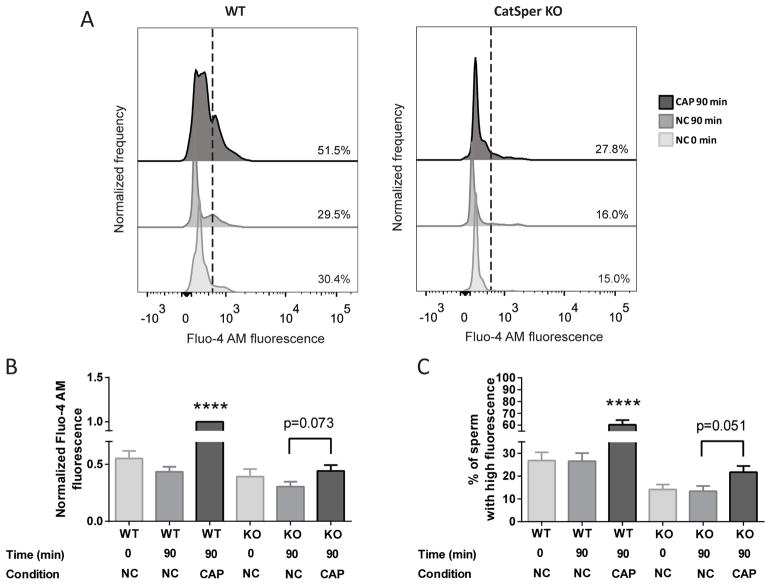
To further test the role of CatSper channels we used CatSper-null sperm. Intracellular [Ca2+] analysis was performed in CatSper KO sperm and compared with sperm from wild-type siblings. The absence of functional CatSper channel displayed a significant decrease in [Ca2+]i in comparison with the rise observed in wild-type mice (Figure 7A), which can also be observed by a decrease in the normalized MFI of Fluo-4 AM (Figure 7B) and in the percentage of sperm that displayed a rise in [Ca2+]i (Figure 7C). Interestingly, in CatSper KO mice, incubation for 90 min in capacitating media led to a slight increase of [Ca2+]i as determined by normalized MFI of Fluo-4 AM (p=0.073; Figure 7B) and by the percentage of sperm with high Fluo-4 AM fluorescence (p=0.051; Figure 7C) in comparison with non-capacitating cells (Figure 7). This result suggests that, even in the absence of CatSper, there is a minimum increase in [Ca2+]i during capacitation.
Figure 7. Functional CatSper channels are required for the capacitation associated [Ca2+]i increase to occur.
A) CatSper KO and wild-type mice sperm collected after swim-out (NC 0 min) or incubated for 90 min either under non-capacitating conditions (NC) or capacitating conditions (CAP) were analyzed. Representative histograms of normalized frequency vs. Fluo-4 AM fluorescence of non-PI stained sperm, with the corresponding percentage of sperm that increased Fluo-4 AM fluorescence, are shown. B) Normalized MFI, compared to the control condition (wild-type CAP), of Fluo-4 AM. Values represent the mean ± SEM of 5 independent experiments. Two-way ANOVA showed a significant interaction (genotype x condition), Pinteraction<0.001. **** p<0.0001 represents statistical significance between control (wild-type CAP condition) vs. all other conditions. Sidak’s multiple comparisons test was performed. To analyzed the differences between CatSper KO NC and CAP conditions, paired t-test was performed (p=0.073). C) Percentage of sperm that increased Fluo-4 AM fluorescence after being incubated in the different conditions. Values represent the mean ± SEM of 5 independent experiments. Two-way ANOVA showed a significant interaction (genotype x condition), Pinteraction<0.001. **** p<0.0001 represents statistical significance between control (wild-type CAP condition) vs. all other conditions. Sidak’s multiple comparisons test was performed. In order to determine the differences between CatSper KO NC and CAP conditions, paired t-test was performed (p=0.051).
Discussion
After ejaculation, sperm are able to move actively but lack fertilizing competence. They acquire the ability to fertilize in the female genital tract in a time-dependent process called capacitation (Austin, 1951; Chang, 1951). Initially, capacitation was defined using fertilization as an end-point. However, a variety of evidence suggests that the functional changes occurring in the sperm during capacitation are not one event, but a combination of sequential and concomitant signaling processes (Visconti et al., 2011) that include complex signaling cascades where intracellular Ca2+ plays a central role. The participation of Ca2+ in the regulation of capacitation process is well documented (Darszon et al., 2011; Visconti et al., 2011). It was clearly observed when sperm were incubated in Ca2+-free media, either with or without chelating agents such as EGTA (Ahmad et al., 1995; Marquez, Ignotz and Suarez, 2007; Torres-Flores et al., 2011; Battistone et al., 2014) or by elevating its levels using Ca2+ ionophores like A23187 (Tateno et al., 2013). In addition, pharmacological and genetic loss-of-function experiments have shown a central role of Ca2+ in the regulation of sperm motility, hyperactivation and acrosomal exocytosis (Suarez and Dai, 1995; Ho and Suarez, 2001; Darszon et al., 2011).
One of the first events that triggers sperm capacitation is the activation of a cAMP pathway (Buffone et al., 2014) after sperm are released from the epididymis and interact with higher HCO3− and Ca2+ concentrations present in the seminal fluid and the female reproductive tract (Okamura et al., 1985). Genetic loss-of-function experiments demonstrated the essential role of proteins involved in the cAMP pathway (Hess et al., 2005) in sperm capacitation and fertilization. In agreement with the role of Ca2+ in these events, mouse sperm exposed to the Ca2+ ionophore A23187 are able to develop hyperactivation, undergo acrosomal exocytosis and acquire fertilizing ability even when the cAMP pathway is completely inhibited (Tateno et al., 2013). In addition, it is considered that Ca2+ has a biphasic role in sperm capacitation signaling pathways (Navarrete et al., 2015). Incubation of mouse sperm in the absence of added extracellular Ca2+ blocked the capacitation-associated increase in tyrosine phosphorylation (Marin-Briggiler, 2005). However, addition of EGTA to further lower the extracellular Ca2+ (traces at micromolar concentrations) promotes a strong increase in tyrosine phosphorylation. A similar effect was also observed when adding calmodulin antagonists or calcineurin inhibitor (Navarrete et al., 2015). These results suggest that Ca2+ ions regulate sperm cAMP and tyrosine phosphorylation pathways in a biphasic manner and that some of its effects are mediated by calmodulin (Navarrete et al., 2015).
Despite all this evidence, there are few reports that directly measure Ca2+ levels during capacitation in mammalian sperm (Jai et al., 1978; Coronel and Lardy, 1987; White and Aitken, 1989; Ruknudin and Silver, 1990; Zhou et al., 1990; Baldi et al., 1991). Ruknudin and Silver reported an increase in [Ca2+]i during mouse sperm capacitation (Ruknudin and Silver, 1990). However, their approach relied on the use of Ca2+-selective microelectrodes to measure changes in [Ca2+]i (Ca2+ uptake). Although it was a reasonable method at that time, these experiments have two major disadvantages: first, the low sensitivity of the method itself; and second, the fact that it only registers changes in the [Ca2+] present in the incubation media. Another attempt to measure Ca2+ was using single cell approaches. However, the time frame of these experiments preclude to evaluate a large number of cells and require sophisticated equipment. We decided to use flow cytometry because it allows the analysis of thousands of cells within seconds resulting in a very useful method to evaluate single cell behavior in a much larger population. Furthermore, a distinction between live and dead sperm can be performed by the addition of viability dyes. Most studies on sperm capacitation have been done with complete sperm suspensions containing sperm in different stages of capacitation, as well as deteriorated sperm cells (Huszar et al., 1998; Buffone et al., 2004; García-Álvarez et al., 2014). On the other hand, different studies have revealed that capacitation generates heterogeneous sperm populations, highlighting the importance of evaluating single cell behavior (Escoffier et al., 2012, 2015; Romarowski et al., 2016). The reason for this heterogeneity is unknown but may be originated from different epididymal maturation states. It is also hypothesized that having sperm at different stages of capacitation could extend the time period in which they are capable to fertilize an ovulated egg.
In this work, flow cytometry was used to analyze changes in [Ca2+]i during capacitation. For this purpose, sperm suspensions were double stained with the Ca2+ dye Fluo-4 AM in combination with PI to analyze these changes in individual live sperm. We observed that only a subpopulation of the sperm incubated under capacitating conditions effectively responds displaying a rapid increase in [Ca2+]i within 1 minute. Our results indicate that sperm incubated in media that do not support capacitation are constituted by mainly two subpopulations. In contrast, sperm incubated under capacitating conditions distributed in three defined subpopulations, where two display similar Fluo-4 AM average fluorescence than the non-capacitated sperm population, while the other one depicted a higher fluorescence.
Two Ca2+ transport systems were clearly identified in mammalian sperm. The first one involves Ca2+ efflux through the Plasma Membrane Ca2+ ATPase (PMCA), and the Na+/Ca2+-exchanger (NCX). PMCA, localized in the principal piece of the flagellum, is crucial for sperm function since its elimination affects sperm motility (Schuh et al., 2004) and sperm from PMCA4b KO mice are deficient in hyperactivated motility and therefore sterile (Okunade et al., 2004). Mitochondrial abnormalities found in PMCA4-deficient spermatozoa (Okunade et al., 2004) suggest Ca2+ overload due to defective Ca2+ extrusion. NCX is present in the plasma membrane of mammalian spermatozoa (Babcock and Pfeiffer, 1987) and is thought to play a crucial role in the regulation of Ca2+ homeostasis (Reddy et al., 2001; Su and Vacquier, 2002). Pharmacological inhibition of NCX leads to a rise in [Ca2+]i and significant inhibition of human sperm motility (Krasznai et al., 2006). The second Ca2+ transport system is related to Ca2+ influx and involves mainly the sperm-specific Ca2+ channel called CatSper (Ren et al., 2001; Quill et al., 2003; Jin et al., 2007; Qi et al., 2007). CatSper is composed by at least 7 subunits, and KO of any of them results in degradation of all other subunits (Qi et al., 2007). Studies using these mice revealed that CatSper is essential for hyperactivation and fertilization. In addition, in contrast to what occurs in wild-type sperm, CatSper1 KO undergoes PKA activation and an increase in tyrosine phosphorylation even in nominal zero Ca2+ media suggesting that CatSper transports the Ca2+ involved in the regulation of PKA/cAMP-dependent pathway required for sperm capacitation (Navarrete et al., 2015). Despite other Ca2+ plasma membrane channels being described, such as voltage-gated Ca2+ channels (Wennemuth et al., 2000; Treviño et al., 2004), cyclic-nucleotide gated Ca2+ channels (Wiesner et al., 1998) and canonical transient receptor potential (TRPC) channels (Treviño et al., 2001), only CatSper (Ren et al., 2001; Quill et al., 2003) was shown to be required for male fertility. In the present study, the increase in [Ca2+]i that occurs under capacitating conditions is absent either in the presence of CatSper inhibitors or in CatSper KO sperm. Surprisingly, a small increase in [Ca2+]i was observed in CatSper KO sperm incubated under capacitating conditions, suggesting that other transporters/channels may be contributing to increase Ca2+ levels during capacitation. This is in agreement with the fact that we have observed a small increase in intracellular Ca2+ in sperm incubated under non-capacitating conditions. Currently, no other Ca2+ currents have been recorded in mature mouse sperm and this possibility requires further experimentation.
Recent groundbreaking work from Chung and coworkers using super-resolution microscopy (STORM) showed that CatSper distributes longitudinally along four backbone lines localized in the plasma membrane close to the fibrous sheath (Chung et al., 2014). Together with CatSper, other signaling molecules display a similar spatial distribution along the principal piece, which reveals a complex organization of signaling pathways in the sperm flagellum. In addition, a variation in subflagellar localization of CatSper domains in capacitated sperm has been described by 3D STORM (Chung et al., 2014). Only 30% of sperm, approximately, presented a quadrilateral CatSper1 domain organization and were the ones able to display hyperactivated motility and tyrosine phosphorylation (Chung et al., 2014). This is consistent with the observation made by different groups that only a subpopulation of sperm achieved hyperactivation upon capacitation (Kulanand and Shivaji, 2001; Buffone et al., 2009; Goodson et al., 2011). In agreement with this evidence, we could determine that approximately 40% of sperm increases [Ca2+]i as a result of capacitation.
Despite the advantage previously described in using flow cytometry, this approach does not provide information about subcellular localization of [Ca2+]i changes, as it does live single cell imaging. Both methods can be combined in a novel and powerful technique to study intracellular events such as the image-based flow cytometry, which allows the analysis of a large number of cells and provides subcellular localization of the labeling. This approach has recently been used with human sperm (Matamoros-Volante et al., 2017) and may contribute to solve the controversy in the field regarding the origin of the [Ca2+]i increase in mammalian sperm, and therefore the nature of the channels involved.
In humans but not mice, progesterone (Lishko, Botchkina and Kirichok, 2011; Strünker et al., 2011) and other steroids activate CatSper via binding to the serine hydrolase ABHD2 (α/β hydrolase domain–containing protein 2) (Miller et al., 2016; Mannowetz, Miller and Lishko, 2017). In both species, CatSper is weakly voltage dependent but its currents are strongly augmented by intracellular alkalinization perhaps related to the remarkable abundance of histidine residues in its amino terminus (Kirichok, Navarro and Clapham, 2006). In addition, previous reports have shown that BSA induces [Ca2+]i influx through CatSper channel activation (Xia and Ren, 2009), because this response is absent in CatSper1 KO sperm. We could determine that the capacitation-associated [Ca2+]i increase is related to both principal components of capacitating media, HCO3− and BSA. HCO3− stimulates a rise in [Ca2+]i very rapidly as observed in live single cell analysis. High concentrations of HCO3− that sperm encounters in the female genital tract could trigger an initial change in the pHi and activation of SLO3 channels (Santi et al., 2010); the resulting membrane hyperpolarization raises pHi, probably through a voltage-sensitive NHE mechanism (Chávez et al., 2014). This intracellular alkalization activates CatSper channel, which results in a very rapid [Ca2+]i increase.
Our work showed that only a subpopulation of sperm increases [Ca2+]i during capacitation. This rise might be associated with the fact that only a fraction of the population undergoes capacitation. Nowadays cell sorting technology is available and may be used to separate capacitated sperm and therefore, improve in vitro fertilization success rates. Moreover, by using the appropriate fluorescent probes, flow cytometry can be also used to simultaneously monitor different capacitation-related events (e.g. Na+, pH, Ca2+, membrane potential, acrosomal exocytosis, etc) in the same sperm population. In this regard, it is well established that Ca2+ is essential for acrosomal exocytosis and evidence provided by different groups suggest that a combination of both, cell surface channels and intracellular stores contribute to initiation of acrosome reaction (De Blas et al., 2002; Herrick et al., 2005; Cohen et al., 2014). Our results will help to understand how Ca2+ is modulated during capacitation in preparation for acrosomal exocytosis. Ideally, an image-based flow cytometry (Matamoros-Volante et al., 2017) experiment where Ca2+ and acrosomal exocytosis is simultaneously monitored would help to understand this complex regulation.
Given the fact that most studies in mammalian sperm capacitation have been conducted in vitro, it is still unclear which are the in vivo mechanisms involved in this process. In this regard, we and others have started analyzing in vivo capacitation using mtDsRed2/Acr-EGFP mouse to monitor the status of the acrosome in live, motile sperm during their transit through the oviduct (Hino et al., 2016; La Spina et al., 2016). We envision that these approaches using new transgenic technologies developed to monitor intracellular events will reveal how capacitation occurs in vivo.
Supplementary Material
Representative movie showing the [Ca2+]i response obtained before and after non-capacitating TYH medium addition. At the end, 10 μM Ionomycin (Iono) was added as a viability control.
Representative movie showing the [Ca2+]i response before and after TYH+HCO3− addition. At the end, 10 μM Ionomycin (Iono) was added as a viability control.
Acknowledgments
Funding: This work was supported by the National Institute of Health (R01TW008662 to MGB), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015-2294 to MGB and PICT 2014-2702 to DK) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development NIH (RO1 HD38082 to PEV).
We would like to thank Dr. Gabriel Rabinovich and Dr. Mariana Salatino for their assistance in the flow cytometry experiments. We also thank Dr. Maria Gracia Gervasi and Dr. Ana Maria Salicioni for their technical assistance, as well as Professor Ricardo Bello for his assistance in statistical analysis. We thank as well Rodrigo Vena for his technical assistance in the use of confocal microscopy. We also would like to thank Rene Baron, Fortabat and Williams foundations. We thank the Government of Extremadura (Spain) and the Fondo Social Europeo (PO14005) for the fellowship to D.M.H. and the Consejo Nacional de Investigaciones Científicas Y Técnicas for fellowships to G.M.L, T.D.M, C.R, L.C.P.M and A.R. The authors do not have conflict of interest to declare.
References
- Ahmad K, Bracho GE, Wolf DP, Tash JS. Regulation of human sperm motility and hyperactivation components by calcium, calmodulin, and protein phosphatases. Archives of andrology. 1995;35(3):187–208. doi: 10.3109/01485019508987871. [DOI] [PubMed] [Google Scholar]
- Alvau A, Battistone MA, Gervasi MG, Navarrete FA, Xu X, Sánchez-Cárdenas C, De la Vega-Beltran JL, Da Ros VG, Greer PA, Darszon A, Krapf D, Salicioni AM, Cuasnicu PS, Visconti PE. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development (Cambrigde) 2016;143(13):2325–33. doi: 10.1242/dev.136499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Australian journal of scientific research. 1951;4(4):581–596. doi: 10.1071/BI9510581. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LLH, Kahrizi K, Najmabadi H, Smith RJH. American Journal of Human Genetics. 4. Vol. 84. The American Society of Human Genetics; 2009. Human Male Infertility Caused by Mutations in the CATSPER1 Channel Protein; pp. 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L, Krasnov T, Ben-Asher E, Olender T, Khen M, Yaniv I, Zaizov R, Shalev H, Delaunay J, Fellous M, Lancet D, Beckmann JS. CATSPER2, a human autosomal nonsyndromic male infertility gene. European journal of human genetics: EJHG. 2003;11(7):497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- Babcock DF, Pfeiffer DR. Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage-dependent and pH-sensitive mechanisms. The Journal of biological chemistry. 1987;262(31):15041–7. [PubMed] [Google Scholar]
- Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. Journal of andrology. 1991;12(5):323–30. [PubMed] [Google Scholar]
- Battistone MA, Alvau A, Salicioni AM, Visconti PE, Da Ros VG, Cuasnicú PS. Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Molecular human reproduction. 2014;20(11):1054–66. doi: 10.1093/molehr/gau073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas G, Michaut M, Treviño CL, Tomes CN, Yunes R, Darszon A, Mayorga LS. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. The Journal of Biological Chemistry. 2002;277(51):49326–49331. doi: 10.1074/jbc.M208587200. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Doncel GF, Calamera JC, Verstraeten SV. Capacitation-associated changes in membrane fluidity in asthenozoospermic human spermatozoa. International Journal of Andrology. 2009;32(4):360–375. doi: 10.1111/j.1365-2605.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Doncel GF, Marín Briggiler CI, Vazquez-Levin MH, Calamera JC. Human sperm subpopulations: relationship between functional quality and protein tyrosine phosphorylation. Human reproduction (Oxford, England) 2004;19(1):139–46. doi: 10.1093/humrep/deh040. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Hirohashi N, Gerton G. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biology of reproduction. 2014;90(5):112. doi: 10.1095/biolreprod.114.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Wertheimer EV, Visconti PE, Krapf D. Biochim Biophys Acta. 164. Vol. 4. Elsevier B.V: 2014. Central role of soluble adenylyl cyclase and cAMP in sperm physiology; pp. 2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF. Identical phenotypes of CatSper1 and CatSper2 null sperm. Journal of Biological Chemistry. 2005;280(37):32238–32244. doi: 10.1074/jbc.M501430200. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168(4277):697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chávez JC, Ferreira JJ, Butler A, De La Vega Beltran JL, Trevino CL, Darszon A, Salkoff L, Santi CM. SLO3 K+ Channels Control Calcium Entry through CATSPER Channels in Sperm. Journal of Biological Chemistry. 2014;289(46):32266–32275. doi: 10.1074/jbc.M114.607556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J-JJ, Shim S-HH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Cell. 4. Vol. 157. Elsevier Inc: 2014. Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility; pp. 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J-J, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nature communications. 2011;2:153. doi: 10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold C, Brookes SM, Wileman T. Biochemical Requirements of Virus Wrapping by the Endoplasmic Reticulum: Involvement of ATP and Endoplasmic Reticulum Calcium Store during Envelopment of African Swine Fever Virus. Journal of Virology. 2000;74(5):2151–2160. doi: 10.1128/JVI.74.5.2151-2160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Buttke DE, Asano A, Mukai C, Nelson JL, Ren D, Miller RJ, Cohen-Kutner M, Atlas D, Travis AJ. Developmental Cell. 3. Vol. 28. Elsevier Inc; 2014. Lipid Modulation of Calcium Flux through CaV2.3 Regulates Acrosome Exocytosis and Fertilization; pp. 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronel CE, Lardy HA. Characterization of Ca2+ uptake by guinea pig epididymal spermatozoa. Biol Reprod. 1987;37(5):1097–1107. doi: 10.1095/biolreprod37.5.1097. [DOI] [PubMed] [Google Scholar]
- Correia J, Michelangeli F, Publicover S. Regulation and roles of Ca2+ stores in human sperm. Reproduction. 2015;150(2):R56–R76. doi: 10.1530/REP-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium Channels in the Development, Maturation, and Function of Spermatozoa. Physiological reviews. 2011;91(4):1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- Escoffier J, Krapf D, Navarrete F, Darszon A, Visconti PE. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. Journal of cell science. 2012;125(Pt 2):473–485. doi: 10.1242/jcs.093344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffier J, Navarrete F, Haddad D, Santi CM, Darszon A, Visconti PE. Flow Cytometry Analysis Reveals That Only a Subpopulation of Mouse Sperm Undergoes Hyperpolarization During Capacitation. Biology of Reproduction. 2015;92(5):2–3. doi: 10.1095/biolreprod.114.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella BM, Miller NGA, Colenbrander B, Van Golde LMG, Harrison RAP. Flow cytometric detection of transbilayer movement of fluorescent phospholipid analogues across the boar sperm plasma membrane: Elimination of labeling artifacts. Molecular Reproduction and Development. 1999;53(1):108–125. doi: 10.1002/(SICI)1098-2795(199905)53:1<108::AID-MRD13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- García-Álvarez O, Maroto-Morales A, Ramón M, del Olmo E, Jiménez-Rabadán P, Fernández-Santos MR, Anel-López L, Garde JJ, Soler AJ. Dynamics of sperm subpopulations based on motility and plasma membrane status in thawed ram spermatozoa incubated under conditions that support in vitro capacitation and fertilisation. Reproduction, fertility, and development. 2014;26(5):725–32. doi: 10.1071/RD13034. [DOI] [PubMed] [Google Scholar]
- Goodson SG, Zhang Z, Tsuruta JK, Wang W, O’Brien DA. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biology of reproduction. 2011;84(6):1207–15. doi: 10.1095/biolreprod.110.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick SB, Schweissinger DL, Kim S-W, Bayan KR, Mann S, Cardullo RA. The acrosomal vesicle of mouse sperm is a calcium store. Journal of Cellular Physiology. 2005;202(3):663–671. doi: 10.1002/jcp.20172. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Stuart B, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental Cell. 2005;9(2):249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino T, Muro Y, Tamura-Nakano M, Okabe M, Tateno H, Yanagimachi R. The Behavior and Acrosomal Status of Mouse Spermatozoa In Vitro, and Within the Oviduct During Fertilization after Natural Mating. Biology of Reproduction. 2016;95(3):50–50. doi: 10.1095/biolreprod.116.140400. [DOI] [PubMed] [Google Scholar]
- Hirohashi N, La Spina FA, Romarowski A, Buffone MG. Redistribution of the intra-acrosomal EGFP before acrosomal exocytosis in mouse spermatozoa. Reproduction. 2015;149(6):657–663. doi: 10.1530/REP-15-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 2001;122(4):519–526. doi: 10.1530/rep.0.1220519. [DOI] [PubMed] [Google Scholar]
- Huszar G, Patrizio P, Vigue L, Willets M, Wilker C, Adhoot D, Johnson L. Cytoplasmic extrusion and the switch from creatine kinase B to M isoform are completed by the commencement of epididymal transport in human and stallion spermatozoa. Journal of andrology. 1998;19(1):11–20. [PubMed] [Google Scholar]
- Jai B, Singh PAL, Babcock DF, Lardy HA. Increased Calcium-Ion Influx is a Component of Capacitation of Spermatozoa. Biochem J. 1978:549–556. doi: 10.1042/bj1720549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, Yan W. Biology of Reproduction. 1. Vol. 77. Society for the Study of Reproduction; 2007. Catsper3 and Catsper4 Are Essential for Sperm Hyperactivated Motility and Male Fertility in the Mouse; pp. 37–44. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006 Feb;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- Krasznai Z, Krasznai ZT, Morisawa M, Bazsáné ZK, Hernádi Z, Fazekas Z, Trón L, Goda K, Márián T. Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation. Cell Motility and the Cytoskeleton. 2006;63(2):66–76. doi: 10.1002/cm.20108. [DOI] [PubMed] [Google Scholar]
- Kulanand J, Shivaji S. Capacitation-associated changes in protein tyrosine phosphorylation, hyperactivation and acrosome reaction in hamster spermatozoa. Andrologia. 2001;33(2):95–104. doi: 10.1046/j.1439-0272.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- De La Vega-Beltran JL, Sánchez-Cárdenas C, Krapf D, Hernandez-González EO, Wertheimer E, Treviño CL, Visconti PE, Darszon A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. Journal of Biological Chemistry. 2012;287(53):44384–44393. doi: 10.1074/jbc.M112.393488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Nature. 7338. Vol. 471. Nature Publishing Group; 2011. Progesterone activates the principal Ca2+ channel of human sperm; pp. 387–91. [DOI] [PubMed] [Google Scholar]
- López-González I, Torres-Rodríguez P, Sánchez-Carranza O, Solís-López A, Santi CM, Darszon AI, Treviño CL. Membrane hyperpolarization during human sperm capacitation. Molecular human reproduction. 2014;20(7):619–29. doi: 10.1093/molehr/gau029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannowetz N, Miller MR, Lishko PV. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proceedings of the National Academy of Sciences. 2017;114(22):5743–5748. doi: 10.1073/pnas.1700367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Briggiler CI. Evidence of the presence of calcium/calmodulin-dependent protein kinase IV in human sperm and its involvement in motility regulation. Journal of Cell Science. 2005;118(9):2013–2022. doi: 10.1242/jcs.02326. [DOI] [PubMed] [Google Scholar]
- Marín-Briggiler CI, Gonzalez-Echeverría F, Buffone MG, Calamera JC, Tezón JG, Vazquez-Levin MH. Calcium requirements for human sperm function in vitro. Fertility and Sterility. 2003;79(6):1396–1403. doi: 10.1016/S0015-0282(03)00267-X. [DOI] [PubMed] [Google Scholar]
- Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Developmental Biology. 2007;303(1):214–221. doi: 10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Martínez E, José O, Torres-Rodríguez P, Solís-López A, Sánchez-Tusie AA, Sánchez-Guevara Y, Treviño MB, Treviño CL. Measuring intracellular Ca2+ changes in human sperm using four techniques: conventional fluorometry, stopped flow fluorometry, flow cytometry and single cell imaging. Journal of visualized experiments: JoVE. 2013;(75):e50344–e50344. doi: 10.3791/50344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros-Volante A, Moreno-Irusta A, Torres-Rodriguez P, Giojalas L, Gervasi MG, Visconti PE, Treviño CL. Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: the case of capacitation-induced tyrosine phosphorylation. MHR: Basic science of reproductive medicine. 2017;24(2):gax062–gax062. doi: 10.1093/molehr/gax062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF, Hill RZ, Bautista DM, Kirichok Y, Lishko PV. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science (New York, NY) 2016;352(6285):555–9. doi: 10.1126/science.aad6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Alvau A, Lee HC, Levin LR, Buck J, Leon PM-D, Santi CM, Krapf D, Mager J, Fissore RA, Salicioni AM, Darszon A, Visconti PE. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Scientific reports. 2016;6:33589. doi: 10.1038/srep33589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, García-Vázquez FA, Alvau A, Escoffier J, Krapf D, Sánchez-Cárdenas C, Salicioni AM, Darszon A, Visconti PE. Biphasic role of calcium in mouse sperm capacitation signaling pathways. Journal of Cellular Physiology. 2015;230(8):1758–1769. doi: 10.1002/jcp.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. Journal of Biological Chemistry. 1985;260(17):9699–9705. [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted Ablation of Plasma Membrane Ca2+-ATPase (PMCA) 1 and 4 Indicates a Major Housekeeping Function for PMCA1 and a Critical Role in Hyperactivated Sperm Motility and Male Fertility for PMCA4. Journal of Biological Chemistry. 2004;279(32):33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- Pelassy C, Breittmayer JP, Aussel C. Agonist-induced inhibition of phosphatidylserine synthesis is secondary to the emptying of intracellular Ca2+ stores in Jurkat T-cells. The Biochemical journal. 1992;288(Pt 3):785–9. doi: 10.1042/bj2880785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehler E, Petrunkina AM, Ekhlasi-Hundrieser M, Töpfer-Petersen E. Dynamic quantification of the tyrosine phosphorylation of the sperm surface proteins during capacitation. Cytometry Part A. 2006;69(10):1062–1070. doi: 10.1002/cyto.a.20338. [DOI] [PubMed] [Google Scholar]
- Puga Molina LC, Pinto NA, Torres Rodríguez P, Romarowski A, Vicens Sanchez A, Visconti PE, Darszon A, Treviño CL, Buffone MG. Essential Role of CFTR in PKA-Dependent Phosphorylation, Alkalinization, and Hyperpolarization During Human Sperm Capacitation. Journal of Cellular Physiology. 2017;232(6):1404–1414. doi: 10.1002/jcp.25634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill Ta Sugden Sa Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14869–74. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PR, Patni A, Sharma A, Gupta S, Tiwary AK. Effect of 2′,4′-dichlorobenzamil hydrochloride, a Na+-Ca2+ exchange inhibitor, on human spermatozoa. European Journal of Pharmacology. 2001;418(1–2):153–155. doi: 10.1016/S0014-2999(01)00892-5. [DOI] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413(6856):603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles V, Martínez-Pastor F. Flow Cytometric Methods for Sperm Assessment’, in. Methods in molecular biology (Clifton, NJ) 2013:175–186. doi: 10.1007/978-1-62703-038-0_16. [DOI] [PubMed] [Google Scholar]
- Romarowski A, Battistone MA, La Spina FA, del Puga Molina LC, Luque GM, Vitale AM, Cuasnicu PS, Visconti PE, Krapf D, Buffone MG. PKA-dependent phosphorylation of LIMK1 and Cofilin is essential for mouse sperm acrosomal exocytosis. Developmental biology. 2015;405(2):237–49. doi: 10.1016/j.ydbio.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romarowski A, Sanchez-Cardenas C, Ramirez-Gomez HV, Puga Molina LDC, Trevino CL, Hernandez-Cruz A, Darszon AI, Buffone MG. A Specific Transitory Increase in Intracellular Calcium Induced by Progesterone Promotes Acrosomal Exocytosis in Mouse Sperm. Biology of Reproduction. 2016 Jan;94:1–12. doi: 10.1095/biolreprod.115.136085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruknudin A, Silver IA. Ca2+ uptake during capacitation of mouse spermatozoa and the effect of an anion transport inhibitor on Ca2+ uptake. Molecular Reproduction and Development. 1990;26(1):63–68. doi: 10.1002/mrd.1080260110. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Blackmore PF, Rosenthal MD. Arachidonate mobilization is coupled to depletion of intracellular calcium stores and influx of extracellular calcium in differentiated U937 cells. Biochimica et biophysica acta. 1996;1299(3):342–52. doi: 10.1016/0005-2760(95)00224-3. [DOI] [PubMed] [Google Scholar]
- Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L, Vega-beltrán JL, De Butler A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS letters. 2010;584(5):1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch K-PP, Neyses L. Journal of Biological Chemistry. 27. Vol. 279. American Society for Biochemistry and Molecular Biology; 2004. Plasma Membrane Ca2+ ATPase 4 Is Required for Sperm Motility and Male Fertility; pp. 28220–28226. [DOI] [PubMed] [Google Scholar]
- Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(17):6823–8. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Spina FA, Puga LC, Romarowski A, Vitale AM, Falzone TL, Krapf D, Hirohashi N, Buffone MG. Developmental Biology. 2. Vol. 411. Elsevier; 2016. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct; pp. 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stival C, del Puga Molina LC, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm Capacitation and Acrosome Reaction in Mammalian Sperm. Advances in anatomy, embryology, and cell biology. 2016;220:93–106. doi: 10.1007/978-3-319-30567-7_5. [DOI] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471(7338):382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Su Y-H, Vacquier VD. A flagellar K(+)-dependent Na(+)/Ca(2+) exchanger keeps Ca(2+) low in sea urchin spermatozoa. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(10):6743–8. doi: 10.1073/pnas.102186699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS. Control of hyperactivation in sperm. Human Reproduction Update. 2008;14(6):647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Dai X. Intracellular calcium reaches different levels of elevation in hyperactivated and acrosome-reacted hamster sperm. Molecular reproduction and development. 1995;42(3):325–33. doi: 10.1002/mrd.1080420310. [DOI] [PubMed] [Google Scholar]
- Tao J, Du J, Critser ES, Critser JK. Assessment of the acrosomal status and viability of human spermatozoa simultaneously using flow cytometry. Human reproduction (Oxford, England) 1993;8(11):1879–85. doi: 10.1093/oxfordjournals.humrep.a137953. [DOI] [PubMed] [Google Scholar]
- Tateno H, Krapf D, Hino T, Sánchez-Cárdenas C, Darszon A, Yanagimachi R, Visconti PE. Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18543–8. doi: 10.1073/pnas.1317113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Flores VV, Picazo-Juárez G, Hernández-Rueda Y, Darszon A, González-Martínez MT, Gonzlez-Martínez MT. Human reproduction. 10. Vol. 26. Oxford University Press; 2011. Sodium influx induced by external calcium chelation decreases human sperm motility; pp. 2626–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño CL, Felix R, Castellano LE, Gutiérrez C, Rodríguez D, Pacheco J, López-González I, Gomora JC, Tsutsumi V, Hernández-Cruz A, Fiordelisio T, Scaling AL, Darszon A. Expression and differential cell distribution of low-threshold Ca2+channels in mammalian male germ cells and sperm. FEBS Letters. 2004;563(1–3):87–92. doi: 10.1016/S0014-5793(04)00257-1. [DOI] [PubMed] [Google Scholar]
- Treviño CL, Serrano CJ, Beltrán C, Felix R, Darszon A. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Letters. 2001;509(1):119–125. doi: 10.1016/S0014-5793(01)03134-9. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121(4):1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Krapf D, de la Vega-Beltrán JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian journal of andrology. 2011;13(3):395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121(4):1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Westenbroek RE, Xu T, Hille B, Babcock DF. CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. The Journal of biological chemistry. 2000;275(28):21210–7. doi: 10.1074/jbc.M002068200. [DOI] [PubMed] [Google Scholar]
- White DR, Aitken RJ. Relationship Between Calcium, Cyclic AMP, ATP, and lntracellular pH and the Capacity of Hamster Spermatozoa to Express Hyperactivated Motility. Gamete Res. 1989;177:163–177. doi: 10.1002/mrd.1120220205. [DOI] [PubMed] [Google Scholar]
- Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp UB, Weyand I. Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. The Journal of cell biology. 1998;142(2):473–84. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reproductive biology and endocrinology: RB&E. 2009;7:119. doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. In: Mammalian fertilization. Neill JD, KE, editors. New York: Raven Press; 1994. [Google Scholar]
- Zhou R, Shi B, Chou KCK, Oswalt MD, Haug A. Changes in intracellular calcium of porcine sperm during in vitro incubation with seminal plasma and a capacitating medium. Biochemical and biophysical research communications. 1990;172(1):47–53. doi: 10.1016/s0006-291x(05)80171-8. [DOI] [PubMed] [Google Scholar]
- Zoppino FCM, Halón ND, Bustos MA, Pavarotti MA, Mayorga LS. Recording and sorting live human sperm undergoing acrosome reaction. Fertility and Sterility. 2012;97(6):1309–1315. doi: 10.1016/j.fertnstert.2012.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative movie showing the [Ca2+]i response obtained before and after non-capacitating TYH medium addition. At the end, 10 μM Ionomycin (Iono) was added as a viability control.
Representative movie showing the [Ca2+]i response before and after TYH+HCO3− addition. At the end, 10 μM Ionomycin (Iono) was added as a viability control.