Abstract
Background
Parkinsonisms are neurodegenerative disorders characterized pathologically by α-synuclein- (e.g., Parkinson’s, diffuse Lewy body disease, and multiple system atrophy) and/or tau- (e.g., progressive supranuclear palsy, cortical basal degeneration) positive pathology. Using R2* and quantitative susceptibility mapping (QSM), susceptibility changes have been reported in the midbrain of living parkinsonian patients, although the exact underlying pathology of these alterations is unknown. The current study investigated the pathological correlates of these susceptibility MRI measures.
Methods
In vivo MRIs (T1- and T2-weighted, and T2*) and pathology were obtained from 14 subjects enrolled in a Parkinson’s disease biomarker program. We assessed R2* and QSM values in the substantia nigra, semi-quantitative α-synuclein, tau, and iron values, as well as neuronal and glial counts. The data were analyzed using age-adjusted Spearman correlations.
Results
R2* was associated significantly with nigral α-synuclein (r=0.746, p=0.003). QSM correlated significantly with Perls’ (r=0.758, p=0.003), but not with other pathological measurements. Neither measurement correlated with tau or glial cell counts (r≤0.11, p≥0.129).
Conclusions
Susceptibility MRI measurements capture nigral pathologies associated with parkinsonian syndromes. Whereas QSM is more sensitive to iron, R2* may reflect pathological aspects of the disorders beyond iron such as α-synuclein. They may be invaluable tools in diagnosing differential parkinsonian syndromes, and tracking in living patients the dynamic changes associated with the pathological progression of these disorders.
Keywords: susceptibility MRI, substantia nigra, α-synuclein, tau, iron
Introduction
Parkinsonian syndromes (PS) are common and progressive neurodegenerative disorders that encompass a spectrum of disabling movement disorders. Among PS, Parkinson’s disease (PD) is the most prevalent, and is marked pathologically by the loss of dopamine neurons in the substantia nigra (SN) of the basal ganglia (BG) and presence of α-synuclein-positive Lewy bodies. Diffuse Lewy body (DLB) disease also frequently is diagnosed in postmortem studies, and is manifested by a more diffuse spatial pattern of α-synuclein-positive Lewy bodies beyond the SN. Progressive supranuclear palsy (PSP) and multiple system atrophy (MSA) are two other common PS that have neuronal loss in different brain regions including the BG, pons, cerebellum, and related structures. PSP characteristically has tau-positive inclusions in both glia and neurons, whereas MSA has glial cytoplasmic inclusions that are α-synuclein positive. Despite their distinctive pathological signatures, PS cause overlapping motor effects including bradykinesia, rigidity, and/or tremor, probably due to shared dysfunction of BG- and cerebellar-related structures. Currently, there are no in vivo biomarkers to gauge these different pathological processes in brain.1 There are a variety of challenges that have led to a lack of tools to accurately diagnose different PS, to understand their mechanistic underpinnings, and, ultimately, to track disease progression and evaluate potential disease-modifying neuroprotective therapies.
Iron has been implicated in a number of neurodegenerative processes,2 including PD and Alzheimer’s disease (AD). For example, higher iron content has been reported in the SN of PD patients,3, 4 and detected in plaques and tangles in AD.5, 6 Using postmortem brain tissue from six PSP patients and three Controls, Foroutan et al.7 reported that PSP patients had higher iron burden in the cerebral peduncle and SN, a finding confirmed by ex vivo MRI. In addition, ex vivo MRI demonstrated higher R2* in the putamen and globus pallidus (GP) in PSP patients.
Biophysically, susceptibility MRI [R2* and quantitative susceptibility mapping (QSM)] can detect local magnetic susceptibility changes8–11 that are sensitive to metals like iron.12 The apparent transverse relaxation rate (R2*) and QSM have been utilized to detect the distinct pathological patterns in PD, MSA, and PSP.13–20 In cross-sectional studies, both R2* and QSM have been shown to be increased in PD compared to Controls.21–23 Han et al.24 studied 11 PSP, 12 MSA, 15 PD, and 20 Controls, and reported that both PSP and MSA subjects had increased iron content in multiple BG- and cerebellar-related structures, although they also observed that iron levels were significantly different in PSP compared to MSA subjects, particularly in the SN, GP, thalamus, and red nucleus (RN). These results offered potentially exciting evidence that susceptibly MRI may serve as a biomarker for PS despite the fact that the exact pathological correlates of these MRI sequences are unknown.25, 26
In the current study, we investigated whether these susceptibility MRI measurements obtained in vivo from a group of subjects as part of a PD biomarker study correlated with post mortem pathological findings in the SN, the latter the common location for neuronal loss, α-synuclein, or tau pathologies for all major PS.27–30 QSM captures purer susceptibility, and is a more selective marker for tissue iron,8, 31 whereas R2* consists of both susceptibility and the transverse relaxation rate that is, possibly, influenced more by local cellular structural properties.8, 32 Thus, we hypothesized that: 1) QSM may correlate most robustly with iron measurements and tau (which contains iron) and 2) R2* may reflect other PD-related pathological processes such as α-synuclein and neuronal cell loss in additional to iron.
Materials and Methods
Subjects
Subjects were enrolled in an ongoing translational study aimed at developing biomarkers for PD and related disorders [the NINDS PD Biomarker Program (PDBP)]. The original cohort was recruited from a tertiary movement disorders clinic patient population or their spouses, and all patients were free of major neurological/medical issues other than PD or a parkinsonian syndrome (such as MSA or PSP). Pathological data were captured from subjects as they died. Fourteen subjects were included in the current analyses because complete in vivo MRI and postmortem pathological data were available by May 2017. Thirteen of the 14 subjects were clinically presumptive for a parkinsonian syndrome, and the other was the spouse of a patient who had chronic diabetes, hypertension, and neuropathy. The demographic and clinical information for the 14 subjects are detailed in Table 1. In vivo MRI brain scans were collected from all subjects as part of the study protocol, in which patients were scanned ‘on’ medication to maximize their comfort.
Table 1.
Demographic and clinical characteristics of study subjects
| Clinical presentation & initial clinical diagnoses* | In vivo MRI | Pathology | MRI-path intervals (mths) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Date (m/y) | Age (y) | Gender | DOI (y)**** | Date (m/y) | Path. Diagn. | SN | |||
| Syn | Tau | |||||||||
| 1 | Diagnosed with PD in 1993 after 2 y of PS** | 01/2013 | 68 | F | 22 | 01/2013 | PD | 1 | 1 | 0 |
| 2 | Diagnosed with PD in 2009 after 2 y history of PS & falls | 01/2013 | 91 | M | 4 | 10/2013 | PSP | 1 | 3 | 9 |
| 3 | Diagnosed with MSA in 2010 after 3 y of PS & falls | 02/2013 | 63 | M | 6 | 03/2014 | MSA | 1 | 1 | 13 |
| 4 | Diagnosed with PSP in 2005 after 3 y of PS & imbalance | 03/2013 | 83 | M | 8 | 05/2014 | PD | 1 | 0 | 14 |
| 5 | Diagnosed with PD in 2002 after 2 y of PS | 10/2013 | 78 | F | 11 | 07/2014 | DLB | 1 | 2 | 9 |
| 6 | Diagnosed with PD in 2006 after 1 y of PS & slurry speech | 10/2013 | 74 | M | 9 | 10/2014 | PSP | 0 | 2 | 12 |
| 7 | Diagnosed with MSA in 2006 after 4 y cognitive decline, PS, & autonomic dysfunction | 04/2013 | 78 | M | 9 | 01/2015 | PD | 1 | 1 | 21 |
| 8 | Diagnosed with PD in 2006 after 5 y PS and 1 y of cognitive complaints | 10/2013 | 75 | M | 9 | 02/2015 | DLB | 1 | 1 | 16 |
| 9 | Diagnosed as MSA in 2011 after 2 y of PS, imbalance, anosmia, and sleep disorder | 06/2014 | 82 | M | 5 | 02/2015 | PSP | 0 | 3 | 8 |
| 10 | Spouse of a patient, had diabetes, hypertension, & neuropathy | 03/2013 | 70 | M | na | 08/2015 | Mild brain atrophy | 0 | 0 | 29 |
| 11 | Diagnosed with PD in 2007 after 3 y PS & cognitive complaints | 02/2014 | 78 | M | 7 | 02/2016 | DLB | 1 | 2 | 24 |
| 12 | Suspected PSP in 2015 after 2 y of backward falling, 20 y of ET | 06/2015 | 83 | F | na | 07/2016 | Mild AD | 0 | 0 | 13 |
| 13 | Diagnosed with PD in 1994 within 1 y of PS | 07/2014 | 69 | M | 20 | 07/2016 | PD | 1 | 0 | 24 |
| 14 | Diagnosed with PD after 2 y of PS | 01/2013 | 73 | M | 3 | 10/2016 | CBG | 0 | 3 | 47 |
Diagnoses were based on the impression of the first neurologist who evaluated the patient for the concerning symptoms. Neurologist may or may not have been a movement disorder specialist.
Parkinsonian symptoms (PS) are defined as bradykinesia, and rest tremor or rigidity;
Abbreviations: AD: Alzheimer’s disease; CBD: Cortical basal degeneration; Diag, Diagnosis; DOI: Duration of illness; DLB: Diffuse Lewy Body Disease; ET: essential tremor; F: Female; M: Male; MSA: multiple system atrophy; mths: months; PD: Parkinson’s disease; PS: parkinsonian symptoms that are defined as bradykinesia, and rest tremor or rigidity; PSP: progressive supranuclear palsy; SN: substantial nigra; Syn: α-synuclein; y: years.
For parkinsonism patients, disease duration was defined as the number of years between the date of death and the date when a parkinsonian syndrome was first diagnosed by a medical professional. Post mortem pathological diagnosis was used as the final diagnosis for each patient. Written informed consent was obtained from all subjects or their legal representative in accordance with the Declaration of Helsinki, and after approval by the Penn State Hershey Medical Center Internal Review Board.
Imaging data acquisition
Brain MRI data acquisition
Brain MRIs were obtained from all participants using a 3.0-Tesla MRI system (Trio; Siemens Magnetom; Erlangen, Germany) with an 8-channel phased-array head coil. A 3D MPRAGE with TR=1540 ms, TE=2.3 ms, and isotropic 1-mm voxels was utilized to acquire T1-weighted images. A fast-spin-echo sequence was used to obtain T2-weighted images with TR/TE=2500/316, FOV=256 mm × 256 mm, matrix=256 × 256, slice thickness=1 mm (with no gap), and slice number=176. T2*-weighted images (for QSM and R2*) were acquired using a multi-gradient-echo sequence with eight echoes (TE ranging from 6.2–49.6 ms and an equal interval of 6.2 ms), TR=55 ms, flip angle=15°, FOV=240 mm, matrix=256 × 256, slice thickness=2 mm, slice number=64, voxel size=0.9 × 0.9 × 2 mm3.
Image data analysis
Estimation of R2* and QSM measures
R2* images were estimated by nonlinear curve fitting of the complex mono-exponential equation (S(TE) = S0e−R2*TEe−i2ωTE) using the Gauss-Newton algorithm. QSM maps were generated by using the morphology-enabled dipole inversion (MEDI) with nonlinear formulation method.33, 34 A head position fixation device installed in the head coil was used to reduce potential head motion. Image quality was evaluated by an MRI technologist during the scan and deemed to be free of severe motion artifacts.
Segmentation of the SN on MR images
The SN was defined manually on T2-weighted images using ITK-SNAP35 by an investigator blinded to subject identification. This region of interest (ROI) was defined as the kidney-shaped band between the red nucleus and cerebral peduncle in axial MRI sections,23, 36, 37 as illustrated in Figure 1A and 1B. The most superior extent of the SN segmentation was defined as one slice inferior to the axial section of the red nucleus showing the largest radius. A total of six slices (from rostral to caudal) were used as the SN ROI.35 Since there were no significant differences between left and right SN MRI measures, they were combined into one SN value. To save time and improve the repeatability of the ROI definition, an atlas-based segmentation (AutoSeg 3.1, Neuro Image Research and Analysis Laboratories, University of North Carolina at Chapel Hill, NC, USA) was used to generate a rough segmentation before manual segmentation.38
Figure 1.
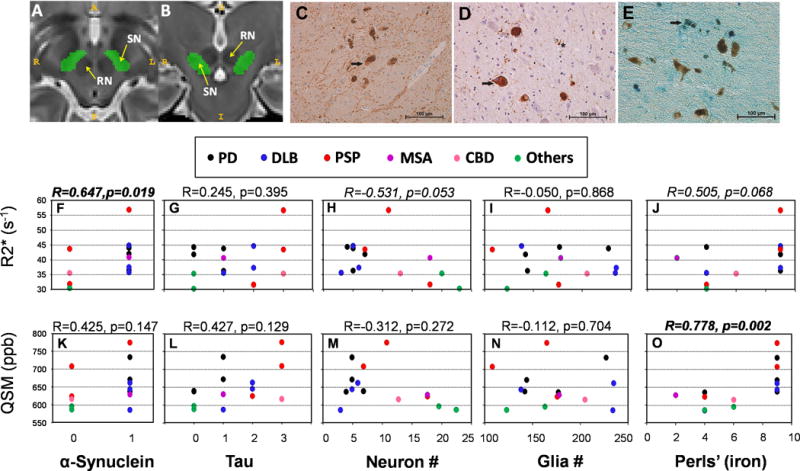
A and B Schematic of substantia nigra segmentation (pars compacta and pars reticulata are lumped together). Illustration of the region of interest (ROI) definition of the substantia nigra in the axial (A) and coronal (B) planes. The yellow arrows indicate the substantia nigra (SN) and red nucleus (RN). C-E: Representative immunostaining images from the substantia nigra pars compacta. α-Synuclein (panel C) immunostaining is a light brown color (denoted by arrow), whereas neuromelanin is a darker shade of brown. Note that α-synuclein immunostaining is not visible in all neuromelanin-containing neurons. Panel D demonstrates tau distribution (light brown) and the presence of helical paired filaments. An arrow denotes a neuron filled with tau and the star indicates a glial cell with tau and filaments. In Panels C and D, hematoxylin is used as a counterstain (blue/purple cells). Perls’ stain for iron is depicted in Panel E. The reaction product is bright blue, with the arrow denoting neuromelanin-positive cells with iron inclusions. F-O: Spearman correlation analysis of raw pathology (α-synuclein, tau, neuronal and glial cell counts, and Perls’ staining) and MRI (R2* and QSM) data. α-Synuclein was either present (1) or absent (0) in the SN. Level of tau staining in the SN was rated semi-quantitatively by our neuropathologist (JWB) such that 0=absence of tau, 1=low levels, 2=moderate levels, 3=ubiquitous levels. Significant findings are in bold-italics, and trends in italics. The diagnosis for each subject is noted above the correlation graphs.
Pathology data acquisition and quantification
The brain was removed and placed in 10% buffered formalin for 48 hours prior to dissection and sample collection. All subjects were assessed using a standard neurodegenerative autopsy protocol, including extensive sampling of the brain according to published guidelines.39 Tissue sections were formalin-fixed for an additional 24 hours prior to processing and paraffin embedding.
SN blocks were cut into 5 μm thick slices for pathological diagnoses and stained with hematoxylin and eosin (H&E), phospho-α-synuclein (hereafter referred to as α-synuclein), and tau, all using standard pathological procedures.39 Thioflavin S was used to assess neuritic plaques (see Figure 1C and 1D for representative images of α-synuclein- and tau-positive stains, and typical plaques/tangles). SN α-synuclein was evaluated on a scale of 0 or 1, with 0 representing the absence of any staining and 1 the presence of staining. Tau SN pathology was assessed using a semi-quantitative approach whereby sections were coded on a scale from 0-3, with 0 representing no tau, 1 representing low tau levels, 2 representing moderate tau levels, and 3 representing ubiquitous tau levels. Final pathological diagnoses were obtained according to the intensity and distribution of neuronal cell loss, α-synuclein, tau, and neuritic plaques based on published guidelines.39 SN iron staining was evaluated according to the method of Perls. Briefly, 30 μm thick SN sections were heated in a 50°C oven for 20 minutes prior to de-paraffinizing in xylenes, followed by hydration through an ethanol gradient. Sections were washed in distilled water, which was followed by incubation in freshly prepared Perls’ solution, consisting of 10% potassium ferrocyanide with 10% hydrochloric acid in a 2:1 ratio, for 30 minutes. Sections were washed with distilled water, dehydrated in 100% ethanol, and then placed in xylenes prior to coverslipping. A representative image of Perls’ staining is seen in Figure 1E. Iron extent and intensity were evaluated using a grading scale from 0–4 in which 0 represented absence of iron staining, 1 denoted rare cell staining, 2 represented moderate/frequent cell staining, 3 represented heavy cell staining, and 4 represented >90% cell staining. A composite score for iron staining was calculated by multiplying intensity by extent. Cell counts for neurons and glia were obtained using 5 high powered fields (HPF, 400x) within the SN (see Figure 1D for representative images of SN neurons and glia).
Statistical analysis
Initial descriptive correlations between raw MRI (R2* and QSM) and pathology (α-synuclein, tau, neuronal and glial counts, and iron levels) measurements were assessed using the Spearman correlation coefficient without adjustment for potential confounders. Since there were only two groups for α-synuclein (presence or absence), Spearman correlation analysis for α-synuclein is equivalent to two-sample Wilcoxon rank sum test.
We then conducted a multiple regression analysis using the stepwise selection method to explore which clinical and pathological data contributed significantly to the susceptibility MRI measurements. These analyses indicated that age was an important factor for both the R2* and QSM measures. As a result, we adjusted for age when we conducted our partial Spearman correlation analyses assessing the relationship between MRI and pathology measurements. Significance was defined as p<0.05. All statistical analyses were performed using SAS 9.3 with two-sided α values (SAS Institute Inc., Cary, NC, USA).
Results
Subject description
Demographic, clinical information, date of MRI, time interval between MRI and death, postmortem data collection, and pathological diagnosis for the 14 subjects are presented in Table 1. In vivo MRI imaging was obtained in patients with an average disease duration of 9.1±5.8 years (range 3-22 y) and at a mean age of 76.1±7.4 years (range 63-91 y). The average time interval from in vivo MR imaging to death was 17.1±11.5 months (range 0-47 months). Pathological diagnoses included PD (n=4), DLB (n=3), PSP (n=3), MSA (n=1) and CBD (n=1). Two subjects did not have parkinsonism diagnoses, but pathology of one showed mild atrophy, whereas the other had mild Alzheimer-related changes.
Relationship between raw susceptibility imaging measures and nigral pathology
The scatter plot of raw pathology and MRI data, along with unadjusted Spearman correlation results, are presented in Figure 1F–O. α-Synuclein (presence or absence) was associated significantly with R2* values (R=0.647, p=0.019, Figure 1F) and Perls’ staining (iron) was correlated significantly with QSM values (Figure 1O). The associations of R2* with neuronal cell counts (R=−0.531, p=0.053, Figure 1H), iron staining (Perls’; R=0.505, p=0.068, Figure 1J), glial cell count (R=−0.050, p=0.868), and tau (R=0.245, p=0.395) were not statistically significant.
Multiple regression analyses with stepwise selection
To identify which clinical and pathological data contributed to the MRI measures, we conducted a multiple regression analysis using the stepwise selection method. Age (p=0.006) and α-synuclein (p=0.012) were identified to be associated significantly with R2*, whereas iron staining (Perls’; p=0.031) was identified to be associated potentially with QSM.
Partial Spearman correlation analyses
As seen in Table 2, SN α-synuclein was associated significantly with R2* values (R=0.747, p=0.003) using partial Spearman correlation analysis adjusted for age. The relationship was strengthened compared to analysis of the raw data (Figure 1). The associations between R2* and SN neuron cell counts (R=−0.511, p=0.075) did not reach statistical significance, similar to the raw data analysis (Figure 1). Adjustment for age weakened the relationship between iron and R2* values (R=0.443, p=0.129), but did not influence the strong QSM-iron association (R=758, p=0.003)
Table 2.
Spearman partial correlation coefficients adjusted for age (N=14)
| α-Synuclein | Tau | Neuron # | Glia # | Perls’ | ||
|---|---|---|---|---|---|---|
| R2* | ||||||
| R | 0.746 | 0.172 | −0.511 | −0.066 | 0.443 | |
| P value | 0.003 | 0.575 | 0.075 | 0.831 | 0.129 | |
| QSM | ||||||
| R | 0.460 | 0.389 | −0.275 | −0.124 | 0.758 | |
| P value | 0.114 | 0.189 | 0.364 | 0.687 | 0.003 |
Spearman partial correlation analyses for all pathological variables analyzed using all subjects after adjustment for age.
Abbreviations: QSM, quantitative susceptibility mapping
Multiple regression and partial Spearman correlation analyses excluding subjects without parkinsonism diagnoses
The purpose of the study was to investigate whether MRI captures pathological changes associated with parkinsonian syndromes, as this information may guide clinical diagnosis of disease and clinical research (such as enrolling subjects for clinical trials). Thus, we purposely included two subjects without a pathological diagnosis of parkinsonism in the design of our analyses. It may be useful, however, to determine whether MRI can capture changes within parkinsonian patients. We thus excluded the two subjects without a pathological diagnosis of a parkinsonism, and reanalyzed the correlation data. The results remained largely unchanged. Namely, multiple regression analyses identified that age was associated significantly with R2* (p=0.011) but not with QSM (p=0.094). α-Synuclein (p=0.091) and iron (p=0.070) did not reach statistical significance for either R2* or QSM. For the partial Spearman correlation adjusted for age, α-synuclein remained significantly correlated with R2* values (R=0.603, p=0.049, Supplemental Table 1) and Perls’ staining was associated significantly with QSM values (R=0.775, p=0.005).
Discussion
The results from the current study demonstrate that R2* and QSM may capture different pathological processes in the SN of PS patients. Specifically, as expected, R2* and QSM correlated positively with iron, whereas R2* was associated with pathological processes beyond iron such as α-synuclein. Together, these data support that susceptibility imaging may reflect one or a combination of nigral pathologies. Larger studies are warranted to test these hypotheses more rigorously, and may assist in the diagnosis of different PS, subject selection for clinical trials, and tracking in vivo progression (see companion paper40).
SN susceptibility measures and iron measurements
Traditionally, R2* and QSM have been utilized to reflect iron accumulation in tissue,8, 21, 31, 41–43 and iron is known to accumulate in the SN of PD patients, particularly in the later stages.21, 23, 44, 45 It has been suggested that QSM is a better tissue iron marker because it captures purer susceptibility.8, 31 Indeed, in a recent study of 13 postmortem subjects, Langkammer et al.46 reported that QSM in gray matter structures was strongly and linearly correlated with iron content measured by inductively-coupled plasma mass spectrometry (r=0.84). This is consistent with our data showing that the association between iron and QSM is stronger than that between iron and R2*. Together, these data support that susceptibility MRI, particularly QSM, may mark iron accumulation, potentially tracking its changes in patients with different disease stages (see companion paper40).
It is interesting to note that increases in QSM (and possibly R2*) occur only after a certain threshold of iron positivity is detected by Perls’ staining (Figure 1O). The exact reasons for this are unclear, but may suggest that susceptibility MRI is insensitive to lower tissue iron levels. Our current quantification of iron staining is based on a categorical grading scale rated by an experienced neuropathologist. Moreover, we did not have sufficient data to explore this threshold or to compare QSM (and R2) across each level of Perls’ staining (as would be possible with a larger sample size). Future studies are need ed that have better tissue iron quantification to delineate the detection threshold for susceptibility MRI, as well as to utilize larger sample sizes to explore these relationships.
R2* also may reflect α-synuclein
The pathological hallmark of PD and MSA is the presence of α-synuclein. Currently, in vivo methods to reflect α-synuclein pathology are sorely needed. Biofluid assays suggest α-synuclein decreases in cerebral spinal fluid samples from PD patients,47 although this measure does not capture spatial information or differentiate PD from parkinsonism disorders. The current study provides the first data suggesting that R2* may reflect this key nigral pathology in living patients. The exact reason why R2* captured α-synuclein is unclear, but may be related to oligomers within Lewy bodies and neurites (reviewed in48) that interfere with cellular function, altering the transverse relaxation properties. Neuroimaging that can reflect the pattern of α-synuclein pathology not only will help differentiate PS, it also may help in staging and differentiating PS because of the spatial and temporal pattern of the pathology.
Potential implications for clinical practice and translational research
Although extensive literature, including our own, suggested that several MRI modalities may gauge pathology associated with neurodegenerative processes in parkinsonian syndromes21, 49–51, the current study is the first demonstration of this phenomenon in this population. Longitudinal studies of these MRI measurements may have important implications for understanding dynamic changes, and guiding the mechanistic understanding of parkinsonism-related pathologies as they unfold in living people (see companion paper40).
Parkinsonian syndromes cause overlapping motor effects including bradykinesia, rigidity, and/or tremor at rest, probably due to shared dysfunction of basal ganglia and related structures. This makes differential diagnoses among parkinsonism patients challenging clinically, especially in early disease stages. A recent study by Adler and colleagues1 reports that upon first visit to a movement disorder center, of patients with a possible or probable PD diagnosis and a disease duration less than five years, only 53% had neuropathologically-confirmed PD. The clinical diagnoses after the initial neurologist evaluation for our patients largely were incorrect (see Table 1), and even worse than Alder’s report. This probably is due to the fact that most of our patients first were evaluated by a general neurologist rather than a movement disorder specialist; this underscores the challenge of diagnosing parkinsonian syndromes in a clinical setting. Misdiagnosing patients not only has a negative impact on patient management and consulting, it also affects disease-modifying drug trials that typically focus on early-stage PD patients.52, 53 Confirmation of the current findings may lead to improved MRI biomarker-guided diagnoses in early parkinsonian patients.
Limitations of our study and future directions
Our study has several limitations. First, although the sample size is relatively large for a pathology study, our study is still very small and has modest statistical power for studying relationships like R2*-iron, R2*-neuronal cell count, and QSM-tau. Second, neuronal and glial cell counts were assessed on single SN sections, similar to previous human studies.54, 55 Cell counting at the midportion of the SN has been reported to provide a good estimate of neuronal loss in the SN.56, 57 This method, however, may be biased by brain tissue shrinkage and/or uneven neurodegeneration across the region. Previous studies indicate that PD SN neuronal loss is greatest in the midportion, and this may overestimate cell loss using the single section as opposed to a stereological approach.56 Third, the exact form of iron detected using MRI approaches is unknown.8 Langkammer et al.46 postulated that most of the iron in the brain is bound to ferritin based on theoretical considerations of ferritin magnetism. Perls’ staining measures total iron and cannot differentiate the form of iron. Thus, further studies are needed to determine whether there are differential correlations between individual susceptibility metrics and distinct forms of iron. Fourth, as standardized pathological procedures, ROIs such as the SN are carved out and pathological evaluations are performed on these individual regions. This process does not preserve the spatial information and renders us unable to separate different nigral regions such as the pars compacta or reticulata as can be done for in vivo imaging study (see companion paper40). Future studies should focus on voxel-based MRI-pathological correlations with 3-D histological data. Finally, the current study was limited to the SN, but it is known that pathology in PS is more widespread. As such, evaluation of other regions is necessary in order to gain a more holistic view of MRI-pathology correlates, such as a recent study demonstrating α-synuclein pathology in cerebellum-related structures in PD and DLB patients.58
Supplementary Material
Acknowledgments
We express gratitude to all of the participants who volunteered for this study and the study personnel who contributed to its completion.
Funding Information: This work was supported in part by the National Institute of Neurological Disease and Stroke (NS060722 and NS082151 to XH), the Penn State-Hershey Medical Center General Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), and the Penn State College of Medicine Translational Brain Research Center All analyses, interpretations, and conclusions are those of the authors and not the research sponsors.
Footnotes
Financial Disclosure/Conflict of Interest: The authors report no conflicts of interest.
Author Roles
1) Research project: A: Conception, B: Organization, C: Execution
2) Statistical analysis: A: Design, B: Execution, C: Review and critique
3) Manuscript preparation: A: Writing the first draft, B: Review and critique
Mechelle M. Lewis: 1A, 1B, 1C, 2A, 2C, 3A, 3B
Guangwei Du: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Jennifer Baccon: 1B, 1C, 2C, 3B
Amanda M. Snyder: 1B, 1C, 2C, 3B
Ben Murie: 1C, 2C, 3B
Felicia Cooper: 1C, 2C, 3B
Christopher Sica: 1B, 1C, 2C, 3B
Richard B. Mailman: 1A, 1B, 2C, 3B
James Connor: 1B, 1C, 2C, 3B
Xuemei Huang: 1A, 1B, 1C, 2A, 2C, 3B
References
- 1.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–412. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li K, Reichmann H. Role of iron in neurodegenerative diseases. J Neural Transm (Vienna) 2016;123:389–399. doi: 10.1007/s00702-016-1508-7. [DOI] [PubMed] [Google Scholar]
- 3.Riederer P, Sofic E, Rausch WD, et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 4.Sofic E, Riederer P, Heinsen H, et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- 5.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 7.Foroutan P, Murray ME, Fujioka S, et al. Progressive supranuclear palsy: high-field-strength MR microscopy in the human substantia nigra and globus pallidus. Radiology. 2013;266:280–288. doi: 10.1148/radiol.12102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Eskreis-Winkler S, Deh K, Gupta A, et al. Multiple sclerosis lesion geometry in quantitative susceptibility mapping (QSM) and phase imaging. J Magn Reson Imaging. 2015;42:224–229. doi: 10.1002/jmri.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson N, Wu J, Zhang Q, et al. Age and sex related differences in subcortical brain iron concentrations among healthy adults. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweitzer AD, Liu T, Gupta A, et al. Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. AJR Am J Roentgenol. 2015;204:1086–1092. doi: 10.2214/AJR.14.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Spincemaille P, Liu Z, et al. Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blain CR, Barker GJ, Jarosz JM, et al. Measuring brain stem and cerebellar damage in parkinsonian syndromes using diffusion tensor MRI. Neurology. 2006;67:2199–2205. doi: 10.1212/01.wnl.0000249307.59950.f8. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti G, Rizzo G, Barbagallo G, et al. Diffusivity of cerebellar hemispheres enables discrimination of cerebellar or parkinsonian multiple system atrophy from progressive supranuclear palsy-Richardson syndrome and Parkinson disease. Radiology. 2013;267:843–850. doi: 10.1148/radiol.12120364. [DOI] [PubMed] [Google Scholar]
- 15.Prodoehl J, Li H, Planetta PJ, et al. Diffusion tensor imaging of Parkinson’s disease, atypical parkinsonism, and essential tremor. Mov Disord. 2013;28:1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Focke NK, Helms G, Pantel PM, et al. Differentiation of typical and atypical Parkinson syndromes by quantitative MR imaging. AJNR Am J Neuroradiol. 2011;32:2087–2092. doi: 10.3174/ajnr.A2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Han YH, Kang BM, Mun CW, Lee SJ, Baik SK. Quantitative assessment of subcortical atrophy and iron content in progressive supranuclear palsy and parkinsonian variant of multiple system atrophy. J Neurol. 2013;260:2094–2101. doi: 10.1007/s00415-013-6951-x. [DOI] [PubMed] [Google Scholar]
- 18.Barbagallo G, Sierra-Pena M, Nemmi F, et al. Multimodal MRI assessment of nigro-striatal pathway in multiple system atrophy and Parkinson disease. Mov Disord. 2015 doi: 10.1002/mds.26471. [DOI] [PubMed] [Google Scholar]
- 19.Planetta PJ, Ofori E, Pasternak O, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain. 2015 doi: 10.1093/brain/awv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa JH, Santos AC, Tumas V, et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn Reson Imaging. 2015;33:559–565. doi: 10.1016/j.mri.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology. 2008;70:1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- 22.Peran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133:3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- 23.Du G, Lewis MM, Styner M, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han YH, Lee JH, Kang BM, et al. Topographical differences of brain iron deposition between progressive supranuclear palsy and parkinsonian variant multiple system atrophy. J Neurol Sci. 2013;325:29–35. doi: 10.1016/j.jns.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Tuite P. Magnetic resonance imaging as a potential biomarker for Parkinson’s disease. Translational research: the journal of laboratory and clinical medicine. 2016;175:4–16. doi: 10.1016/j.trsl.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Heim B, Krismer F, De Marzi R, Seppi K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J Neural Transm (Vienna) 2017;124:915–964. doi: 10.1007/s00702-017-1717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 28.Padovani A, Borroni B, Brambati SM, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006;77:457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 2014 doi: 10.1002/mrm.25358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duyn JH, Schenck J. Contributions to magnetic susceptibility of brain tissue. NMR Biomed. 2017;30 doi: 10.1002/nbm.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2013;69:467–476. doi: 10.1002/mrm.24272. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Xu W, Spincemaille P, Avestimehr AS, Wang Y. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE Trans Med Imaging. 2012;31:816–824. doi: 10.1109/TMI.2011.2182523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Vaillancourt DE, Spraker MB, Prodoehl J, Zhou XJ, Little DM. Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiol Aging. 2012;33:35–42. doi: 10.1016/j.neurobiolaging.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massey LA, Yousry TA. Anatomy of the substantia nigra and subthalamic nucleus on MR imaging. Neuroimaging Clin N Am. 2010;20:7–27. doi: 10.1016/j.nic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Vachet C, Rumple A, et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front Neuroinform. 2014;8:7. doi: 10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du G, Lewis MM, Sica C, et al. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov Disord. 2018;XX:XXX–XXX. doi: 10.1002/mds.27318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham JM, Paley MN, Grunewald RA, Hoggard N, Griffiths PD. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain. 2000;123(Pt 12):2423–2431. doi: 10.1093/brain/123.12.2423. [DOI] [PubMed] [Google Scholar]
- 42.Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2010;257:455–462. doi: 10.1148/radiol.10100495. [DOI] [PubMed] [Google Scholar]
- 43.Walsh AJ, Wilman AH. Susceptibility phase imaging with comparison to R2 mapping of iron-rich deep grey matter. Neuroimage. 2011;57:452–461. doi: 10.1016/j.neuroimage.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson’s disease. Mov Disord. 2012;27:1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He L, Lee EY, Sterling NW, et al. The Key Determinants to Quality of Life in Parkinson’s Disease Patients: Results from the Parkinson’s Disease Biomarker Program (PDBP) J Parkinsons Dis. 2016;6:523–532. doi: 10.3233/JPD-160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62:1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosborough K, Patel N, Kalia LV. alpha-Synuclein and Parkinsonism: Updates and Future Perspectives. Curr Neurol Neurosci Rep. 2017;17:31. doi: 10.1007/s11910-017-0737-y. [DOI] [PubMed] [Google Scholar]
- 49.Chan LL, Rumpel H, Yap K, et al. Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du G, Lewis MM, Styner M, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. The New England journal of medicine. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 53.Marras C, McDermott MP, Rochon PA, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64:87–93. doi: 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 54.Colloby SJ, McParland S, O’Brien JT, Attems J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain. 2012;135:2798–2808. doi: 10.1093/brain/aws211. [DOI] [PubMed] [Google Scholar]
- 55.Kraemmer J, Kovacs GG, Perju-Dumbrava L, Pirker S, Traub-Weidinger T, Pirker W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord. 2014;29:1767–1773. doi: 10.1002/mds.25975. [DOI] [PubMed] [Google Scholar]
- 56.Ma SY, Collan Y, Roytta M, Rinne JO, Rinne UK. Cell counts in the substantia nigra: a comparison of single section counts and disector counts in patients with Parkinson’s disease and in controls. Neuropathol Appl Neurobiol. 1995;21:10–17. doi: 10.1111/j.1365-2990.1995.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 57.Parkkinen L, O’Sullivan SS, Collins C, et al. Disentangling the relationship between lewy bodies and nigral neuronal loss in Parkinson’s disease. J Parkinsons Dis. 2011;1:277–286. doi: 10.3233/JPD-2011-11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seidel K, Bouzrou M, Heidemann N, et al. Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies. Ann Neurol. 2017;81:898–903. doi: 10.1002/ana.24937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


