Abstract
Objective
The Society of Critical Care Medicine (SCCM) recommends routine delirium monitoring, based on data in critically ill patients without primary neurological injury. We sought to answer whether there are valid and reliable tools to monitor delirium in neurocritically ill patients, and whether delirium is associated with relevant clinical outcomes [e.g., survival, length of stay (LOS), functional independence, cognition].
Data Sources
We systematically reviewed CINAHL, Web of Science, and PubMed.
Study Selection and Data Extraction
Inclusion criteria allowed any study design investigating delirium monitoring in neurocritically ill patients (e.g., neurotrauma, ischemic and/or hemorrhagic stroke) of any age. We extracted data relevant to delirium tool sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), interrater reliability, and associated clinical outcomes.
Data Synthesis
Among 7 prospective cohort studies and a total of 1,173 patients, delirium was assessed in neurocritically patients using validated delirium tools after considering primary neurological diagnoses and associated complications, finding a pooled prevalence rate of 12–43%. When able to compare against a common reference standard, DSM-IV, the test characteristics showed a sensitivity=62–76%, specificity=74–98%, PPV=63–91%, NPV=70–94%, and reliability kappa=0.64–0.94. Among four studies reporting multivariable analyses, delirium in neurocritically patients was associated with increased hospital LOS (n=3) and ICU LOS (n=1), as well as worse functional independence (n=1) and cognition (n=2), but not survival.
Conclusions
These data from studies of neurocritically ill patients demonstrate that patients with primary neurological diagnoses can meet diagnostic criteria for delirium, and that delirious features may predict relevant untoward clinical outcomes. There is a need for ongoing investigations regarding delirium in these complicated neurocritically ill patients.
Keywords: delirium, encephalopathy, neurological ICU, neurological intensive care unit, neuro-ICU, neuroICU, neurocritical care, neurointensive care unit, neurocritically ill, sepsis, stroke, intracerebral hemorrhage, subarachnoid hemorrhage, ischemic stroke, cognitive dysfunction, head injury, traumatic brain injury, TBI, neurotrauma, trauma, intensive care unit, dementia, intensive care, critical care, neurology, neurosurgery
INTRODUCTION
Delirium is a phenotypic syndrome manifested by the cardinal clinical features of fluctuations in mental status from baseline, inattention, altered level of consciousness, and disorganized thinking that represents acute cerebral dysfunction. Obviously in patients who have primary neurological pathology [e.g., stroke, traumatic brain injury (TBI)], wholesale attribution of such clinical findings to delirium would be inappropriate without first considering the admission diagnostic injury or an extension of this injury. Indeed, it would be clinically dangerous to misattribute a patient’s clinical deterioration to delirium when it was actually due to edema, vasospasm, rebleeding, seizures, and/or ischemia. That is precisely why the study of delirium, an extremely common malady possible in any hospitalized patient, is so difficult. Yet we must acknowledge the literature in medical and surgical critically ill patients showing how predictive delirium is for clinical outcomes including mortality and a long-term dementia-like state (1–4), may also be applicable to the most neurologically vulnerable patients.
Instruments that are used to screen or diagnose delirium (5–8) in settings such as general medical or surgical Intensive Care Unit (ICU) could be adapted to a population of patients who have primary neurological injury. In general critical care, the clinician (i.e., nurse and physician) must consider the very same clinical findings (e.g., fluctuations in mental status and inattention) as potential harbingers of an extention of the primary illness. If after thorough evaluation such findings are considered unlikely, then delirium as a complication of the clinical course would be the consideration. That is how those who have done general ICU investigations to date have handled this area of inquiry. Due to a plethora of data in non-neuro ICU patients, delirium has been considered a “canary in the coal mine” and has triggered clinical teams to consider other dangerous comorbidities such as nosocomial sepsis, metabolic derangements, pharmacological causes, and/or immobilization (9–12). Delirium during critical illness has had associations with survival, length of stay, cost, and long-term cognition (1–4, 13–16), although causation remains unproven, early recognition of delirium remains important.
The Society of Critical Care Medicine’s (SCCM) guidelines for Pain Agitation and Delirium (17) recommend routinely monitoring delirium with the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) (5) or Intensive Care Delirium Screening Checklist (ICDSC) (2) in adult critically ill patients (grade 1B). However, the data were mostly derived from patients in medical, surgical, and cardiovascular ICUs rather than those with primary brain injury (e.g., stroke, neurosurgical resection, traumatic brain injury). For example, it is known that severe disorders of consciousness (e.g., coma) currently preclude delirium assessment, yet clinicians might extend this logic to patients with diseases such as stroke and TBI and not bother to perform delirium monitoring in these ICU patients, even if non-comatose.
Thus, we hypothesize that delirium measured by known tools is often (not always) assessable in those with neurocritical illness (i.e. ICU patients with acute pathoanatomic abnormalities on CT or MRI) and a marker for future adverse outcomes. To paraphrase for clarity, the primary objective of this manuscript is to discuss the hypothesis that delirium is part of the larger risk profile of ongoing brain injury for many patients with primary diagnoses such a stroke or TBI and considered in the landscape of their clinical course. In order to demonstrate that formal scientific inquiry in this area is nascent and stimulate more work in this field, we conducted a systematic review of the literature in neurocritically ill patients related to (a) delirium monitoring and (b) clinical outcomes associated with duration of delirium.
METHODS
Objective
In neurocritically ill patients with delirium versus without delirium [target condition], are there valid and reliable means by which to monitor for delirium [index test], as compared to a psychiatric reference standard when available [reference test]? And, in neurocritically ill patients with delirium versus without delirium, are there altered outcomes (e.g., survival, length of stay, functional independence, cognition)?
Study Eligibility
This review and associated protocol was registered with the PROSPERO international prospective register of systematic reviews (Registration Number: CRD42017074611). Inclusion criteria allowed any type of study design investigating delirium monitoring in neurocritically ill patients of any age. Our definition of neurocritically ill was restricted to and referred to ICU patients with acute intracranial injury (e.g., traumatic brain injury, hemorrhagic stroke), or ischemic stroke. Reference lists of potentially included studies and review articles were also reviewed for additional citations pertinent to this search. Delirium assessments should have occurred at least daily using a delirium screening assessment tool with reporting of rate. When available, the criterion validity data (i.e., sensitivity, specificity, positive/negative predictive values) was captured comparing delirium screening tools against psychiatric standard assessment using the Diagnostic and Statistical Manual (any edition) (18, 19). If validation studies were done, then we also sought associated interrater reliability data (i.e. test-retest stability or kappa), but not those performed in isolation. Only English-language studies and studies published in the peer-reviewed literature were eligible for inclusion. No date restriction was imposed on the search strategy. Exclusion criteria removed editorials, case reports, case-series, lay press articles, abstracts, and reviews.
Search Methods and Data Extraction
With the assistance of an experienced medical librarian, we systematically searched Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, and PubMed from the National Center for Biotechnology Information (Supplementary Table 1). The search was not restricted by date. Reference lists of potentially included studies and review articles were reviewed for additional citations pertinent to this search. All of the abstracts of the studies identified by our search were independently examined by two authors who determined the eligibility of each study. A third author resolved any disagreements by consensus at each step as needed in the review process. Then, two authors reviewed the titles and abstracts of all remaining eligible studies to determine which required further inclusion. Two authors then retrieved and reviewed the manuscripts of the remaining articles and used data abstraction forms to collect the relevant study information. Captured data included study time period, sample size, subject sex characteristic, eligibility criteria, severity of illness markers, as well as delirium tool utilized, delirium prevalence, reference standard for delirium assessment with test characteristics (if present) (18). Note, the term prevalence was conservatively chosen as a more inclusive epidemiologic term encompassing old and new cases of delirium, although some articles reported incidence without clarifying how new cases were distinguished. We did not plan for a quantitative synthesis or meta-analysis given the anticipated heterogeneity of this emerging literature, delirium tools, and reference standards. For cohort studies, selection of the exposed and nonexposed groups, the comparability of the groups, the assessment of the outcomes, and the adequacy of follow-up was addressed using the Newcastle-Ottawa Scale.
RESULTS
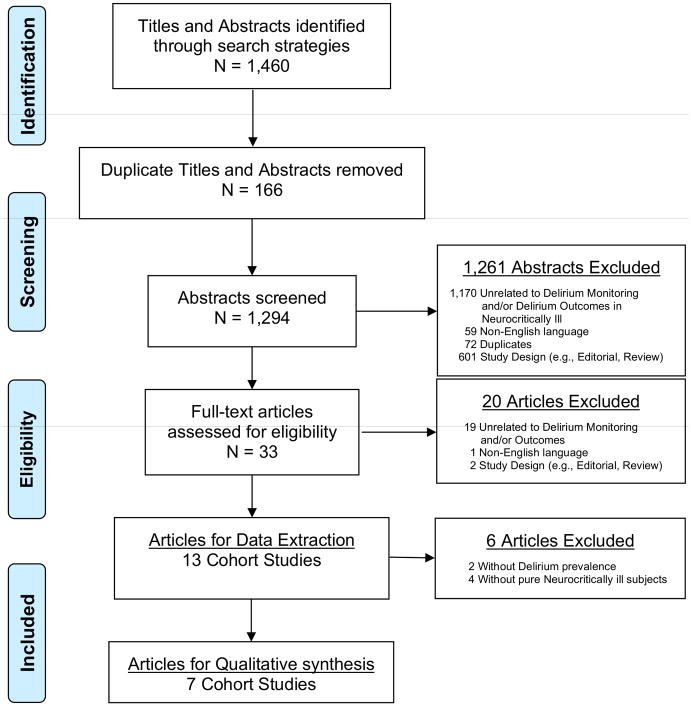
A total of 1460 relevant citations were screened from our search strategies (CINAHL, n=128; WoS, n=888; PubMed, n=441; reference lists, n=3), while 166 duplicates were excluded. Twelve hundred and sixty-one were excluded after title and abstract review because they did not meet inclusion criteria (Figure 1). A total of 33 citations were reviewed at the manuscript level and we excluded 20 of those. Of these excluded articles, nineteen were unrelated to our review (15, 19–36), one was written in a non-English language (37), one was an editorial, and one was a review (19, 31). During data extraction, two articles were further excluded as they failed to provide outcome data relevant to delirium test characteristics or complications of delirium (38, 39). Of the remaining were eleven articles (40–50) without overlapping data, four final articles were excluded due to the ICU cohorts not exclusively composed of neurocritically ill patients (40–42, 50), thus leaving 7 articles for qualitative synthesis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram for Systematic Review Phases of Delirium Monitoring in Neurocritical Care
Qualitative Synthesis
Descriptive statistics were extracted from the 7 prospective cohort designs, representing 5 single center studies, and 2 dual center studies (Table 1 and Supplementary Table 2). In total, 1,173 subjects were represented across studies with a range from 61–527 (median 108) subjects per study. Sex characteristics were unclear or not stated for three of the cohorts. One study involved trauma and traumatic brain injury patients, and six studies involved stroke patients and no trauma patients. Five studies did not state whether mechanical ventilation was affecting the study population, with the remaining two studies having mechanical ventilation rates from 7–66% (median 36%). Severity of illness was broadly defined and either used the Injury Severity Scale (score of 23.3 among 1 study), Glasgow Coma Scale (score range 13.9–14.5 with median 14 among 3 studies), National Institutes of Health Stroke Scale (score range 3–9 with median 8 among 5 studies), and/or the Acute Physiology and Chronic Health Evaluation II (score of 11.5 among 1 study); severity of illness was unclassified in 1 study.
Table 1.
Individual Study Inclusion, Sample Size, and Severity of Illness for this Systematic Review of Delirium Monitoring in the Neurocritically Ill
| Author (Reference) Time Period |
Total N (N of Male Sex) | Inclusion | % Mech Vent | Severity of Illness |
|---|---|---|---|---|
|
Frenette (43) Unspecified |
61 (47) | Two centers, Trauma patients with mild/moderate TBI, Age>=18y, admitted to ICU>48h | 65.5% | Mean ISS=23.3±9.4; Median GCS=14 (IQR 3); Mean APACHEII=11.5±6.4; 28% isolated TBI; 72% polytrauma and TBI |
|
Kostalova (44) 01/2009 – 03/2010 |
100 (53) | One center, Stroke patients in ICU with cerebral infarction or intracerebral haemorrhage; Assessable <=24h of stroke; Approval of patient or surrogate | Not stated | Mean NIHSS=8.8, and Mean GCS=13.9 |
|
Lees (45) 04/2012 – 06/2012 |
108 (Uncleara) | One center, Stroke patients in ICU (ischemia and hemorrhage) | Not Stated | Median NIHSS=3 (IQR: 1–5); 38% with History of Stroke |
|
Mitasova (46) 1/2009 – 1/2010 |
129 (72) | One center, Stroke patients in ICU (with ischemia or hemorrhage), Assessment<=24h of stroke; Approval of patient or surrogate | 7% | Median NIHSS=9.0 (no IQR); GCS=14.5 (mean/median not specified) |
|
Naidech (47) 12/2009 – 04/2013 |
98 (Unclearb) | One center, Intracerebral hemorrhage by CT (neurologist-verified) in Neuro/Spine ICU | Not stated | Not stated for cohort; but Delirium group with median NIHSS=8 (IQR:3–15), and No delirium group with median NIHSS=6 (IQR:2–16) |
|
Oldenbeuving (48) 1y period, unspecified |
527 (288) | Two centers, Stroke (neurologic deficit of sudden onset >24h; ischemic or hemorrhagic) in ICU, Age>18y | Not stated | Median NIHSS=5 (IQR:0–36); 11% hemorrhage stroke |
|
Rosenthal (49) 12/2009 – 10/2014 |
150 (Unclearc) | One center, Intracerebral hemorrhage by CT (neurologist-verified) in Neuro/Spine ICU | Not stated | Unclearc |
N, Sample Size of Cohort assessed for Delirium; % Mech Vent, Percent Mechanically Ventilated of Sample
AIS, Abbreviated Injury Score; APACHE II, Acute Physiology and Chronic Health Evaluation II score; GCS, Glasgow Coma Scale score; h, Hours; ICU, Intensive Care Unit; IQR, Interquartile Range; ISS, Injury Severity Scale score; N, Sample Size; NIHSS, National Institutes of Health Stroke Scale score; TBI, Traumatic Brain Injury; y, Years
Men represented 55 of 111 subjects with cognitive assessment data; without sex further specified, 108 had 4AT data relevant to this review
Women represented 52 of 114 subjects; without sex further specified 98 in this study were assessable for delirium
Women represented 82 of 174 subject with long-term follow-up and Of 174 subjects, NIHSS=11 (IQR not provided); Median GCS=13.5 (IQR:8–15); however without sex or severity of illness further specified, 150 subjects in this study were assessable for delirium
Delirium was assessed most commonly by the Confusion Assessment Method-ICU (CAM-ICU, 5 studies) with a prevalence rate of 24–43% (median 29%) when reported (Table 2). Other tools used were the Intensive Care Delirium Screening Checklist (ICDSC, prevalence not reported), 4-A Test (4AT, prevalence 27%), and Confusion Assessment Method (prevalence 12%). Four studies used a reference standard (n=61–129, median=104), mostly commonly the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, 3 of 4 studies with a 28–46% prevalence with median 37% when reported), and the Confusion Assessment Method (CAM, 1 study, 11% prevalence). Two studies used the CAM-ICU tool against a DSM-IV reference standard with sensitivities ranging 62–76% (median 69%), specificities ranging 74–98% (median 86%), positive predictive values (PPV) ranging 63–91% (median 77%), and negative predictive values (NPV) ranging 70–94% (median 82%). The ICDSC against a DSM-IV reference standard reported 64% sensitivity, 79% specificity, 74% PPV, and 69% NPV, while the 4-AT against a CAM reference standard reported 100% sensitivity, 82% specificity, 43% PPV, and 100% NPV. Reliability was assessed in two studies using the CAM-ICU with kappa range from 0.64–0.94 (median 0.79). The risk of bias was predominantly low (Table 3).
Table 2.
Delirium Prevalance and Delirium Tool Psychometrics for this Systematic Review of Delirium Monitoring in the Neurocritically Ill
| Author (Reference) | Delirium Prevalencea | Delirium Tool (ReferenceStandard) | Sens | Spec | PPV | NPV | Kappa |
|---|---|---|---|---|---|---|---|
| Frenette (43) | Not stated for Tool (45.9% by Ref Std) | CAM-ICU (DSM-IV) ICDSC (DSM-IV) |
62% (95%CI: 44–76) 64% (95%CI: 49–77) |
74% (95%CI: 59–85) 79% (95%CI: 63–89) |
63% (95%CI: 45–78) 74% (95%CI: 55–87) |
70% (95%CI: 55–82) 69% (95%CI: 54–81) |
0.64 0.68 |
| Kostalova (44) | 43% (Not stated for Ref Std) | CAM-ICU (DSM-IV) | - | - | - | - | |
| Lees (45) | 27% (11% by Ref Std) | 4AT (CAM) | 100% (95%CI: 74–100) | 82% (95%CI: 72–89) | 43% (No CI) | 100% (No CI) | |
| Mitasova (46) | 24% (28% by Ref Std) | CAM-ICU, Czech (DSM-IV) | 76% (95%CI: 55–91) | 98% (95%CI: 93–100) | 91% (95%CI: 70–99) | 94% (95%CI: 88–98) | 0.94 (95% CI: 0.83–1.0) |
| Naidech (47) | 27% | CAM-ICU (N/A) | - | - | - | - | |
| Oldenbeuving (48) | 11.8%; 95% CI: 9.0–15.1 | CAM (N/A) | - | - | - | - | |
| Rosenthal (49) | 30% | CAM-ICU (N/A) | - | - | - | - |
4AT, 4-A Test; CAM-ICU, Confusion Assessment Method-ICU; CAM, Confusion Assessment Method; CI, Confidence Interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ICDSC, Intensive Care Delirium Screening Checklist; Kappa, unit of interrater reliability; NPV, Negative Predictive Value; PPV, Positive Predictive Value; Ref Std, Reference Standard Delirium Assessment; Sens, Sensitivity; Spec, Specificity
The term prevalence was conservatively chosen as a more inclusive epidemiologic term encompassing old and new cases of delirium; although some articles reported incidence without clarifying how new cases were distinguished
Table 3.
Risk of Bias Assessment for Cohort Studies (based on modified Newcastle-Ottawa scale) of Delirium Monitoring in the Neurocritically Ill
| Author (Reference) | Selection | Comparability | Outcome | Total Score |
|---|---|---|---|---|
| Frenette (43) | *** | * | *** | 7/9 |
| Kostalova (44) | *** | * | *** | 7/9 |
| Lees (45) | *** | * | ** | 6/9 |
| Mitasova (46) | *** | ** | *** | 8/9 |
| Naidech (47) | *** | * | *** | 7/9 |
| Oldenbeuving (48) | *** | * | *** | 7/9 |
| Rosenthal (49) | *** | * | *** | 7/9 |
Stars are awarded for each quality item. Higher Total Stars/Score means Lower Risk of Bias.
Across four studies (Table 4), the occurrence of delirium was studied with different outcomes ranging mortality, ICU length of stay, hospital length of stay, disposition, and neuropsychological outcomes (e.g., disability, cognition, health-related quality of life). None of these studies reported delirium completely with test characteristics with a reference standard (above), and none reported all of these outcome domains. Four studies used multivariable analysis to associate delirium with selected outcome measures. For example, delirium independently prolonged ICU length of stay by a median 2.1 days (95%CI:1.1–4.5; P=0.03) after adjusting for age, admission National Institutes of Health Stroke Scale, and any benzodiazepine exposure (47). Similarly, delirium independently prolonged hospital length of stay in three studies [HR=1.63, 95% CI:1.11–2.38, P=0.013 (46); median 2.1d longer, 95%CI:1.5–8.3; P=0.004 (47); median 5.4d longer, 95% CI:2.1–8.6, P<0.001 (48)]. Also, delirium independently was associated with worse functional independence [by Barthel Index (50)] and cognition in two studies [by Quality of Life in Neurological Disorders metric (47).
Table 4.
Delirium Associations with Outcomes: Qualitative Results from a Systematic Review of Delirium Monitoring in the Neurocritically Ill
| Ref | Death | ICU LOS | Hospital LOS | Disposition and Neuropsychological Outcomes |
|---|---|---|---|---|
| (46) | Unchanged 6-month mortality (HR=1.22; 95%CI: 0.48–2.98; P=0.668)a | - | Increased length of hospital stay (HR=1.63; 95% CI: 1.11–2.38; P=0.013)b | - |
| (47) | - | Longer ICU LOS (mean 2.1d longer; 95%CI: 1.1–4.5; P=0.03)c | Longer hospital LOS (mean 3.5d longer; 95%CI: 1.5–8.3; P=0.004)d | Poor mRS at 28d (OR=8.7, 95%CI: 1.4–52.5; P=0.018)e Worse HRQoL (domains of applied cognition–executive function and fatigue)f |
| (48) | Unchanged in-hospital mortality (OR=2.0; 95%CI: 0.8–5.1, P<0.001)g | - | Longer hospital LOS by 5.4d (95% CI: 2.1–8.6, P<0.001)h | Worse 1 mo. BI (median BI 20.0 vs. 7.5, P<0.001)i Unfavorable outcome (OR=2.0; 95%CI 1.0–4.0, P<0.001)j |
| (49) | - | - | - | Worse cognitive function HRQoL at 28d and 1yk |
BI, Barthel Index; d, Days; HRQoL, Health-related Quality of Life using Neuro-QOL (Quality of Life in Neurological Disorders) metric; ICU, Intensive Care Unit; IQR, Interquartile range; LOS, Length of Stay; mo., Months; mRS, modified Rankin Scale; Ref, Reference; vs., Versus
Multivariable cox regression model shown in table was adjusted for age, gender, prestroke dementia, National Institutes of Health Stroke Scale at admission, first-day Sequential Organ Failure Assessment, and aphasia; Univariate data showed 6-month mortality with Delirium: 23.6% vs. No Delirium: 14.9%
Multivariable cox regression model using time-dependent covariate analysis was adjusted for age, gender, prestroke dementia, National Institutes of Health Stroke Scale at admission, first-day Sequential Organ Failure Assessment, and aphasia; Univariate data showed Hospital Length of Stay with Delirium:18.0d vs. No Delirium:12.0d
Multivariable data shown in table was adjusted for age, admit National Institutes of Health Stroke Scale, and any benzodiazepine exposure; Univariate data showed ICU Length of Stay with Delirium: 7.0d (IQR: 3.4–10.2) vs. No Delirium: 2.3d (IQR: 1.1–6.7)
Multivariable data shown in table was adjusted for age, admit National Institutes of Health Stroke Scale, and any benzodiazepine exposure; Univariate data showed hospital Length of Stay with Delirium: 13.2d (IQR:7.9–24.2) vs. No Delirium: 6.4d(IQR:4.0–13.2)
Multivariable data shown in table demonstrated an increased odds of poor outcome at 28 days of mRS>3 vs. mRS<2, and was adjusted for admission National Institutes of Health Stroke Scale and age
Multivariable data shown in table was adjusted for the National Institutes of Health Stroke Scale, age, benzodiazepine exposure, and time to follow-up
Multivariable data shown in table was adjusted for age, IQCODE score, and severity of stroke (NIHSS score); Univariate data showed in-hospital mortality for Delirium: 19.4% vs. No Delirium: 6.5%
Multivariable data shown in table was adjusted for age, Informant Questionnaire on Cognitive Decline in the Elderly score, and stroke severity; Univariate showed showed a longer hospital stay with Delirium:23.7d vs. No Delirium:13.9d
Univariate analysis only
Multivariable data shown in table defined unfavorable outcome at 1 month as dead or BI <12 (BI range 0 –20) for those with delirium, and the OR was corrected for age, National Institutes of Health Stroke Scale, and Informant Questionnaire on Cognitive Decline in the Elderly score; Univariate data showed unfavorable outcome with Delirium:66.7% vs. No Delirium:21.3%
Multivariable summary shown in table that controlled for age, National Institutes of Health Stroke Scale, time of assessment, and multiple comparisons; 28d NeuroQOL T-scores for delirium with agitation 20.9±7.3, delirium without agitation 30.4±16.5, agitation without delirium 36.6±17.5, and neither agitated nor delirious 40.3±15.9; P=0.03) and at 1 year (P=0.006); agitation defined as Richmond Agitation-Sedation Scale score>=2
DISCUSSION
These data from the first systematic review of delirium monitoring in the neurocritically ill patient show that, in these subsets of patients in a research setting, it is possible to measure delirium in adult ICU patients with mild-moderate stroke (ischemic and/or hemorrhagic) or neurotrauma with existing delirium instruments. Important caveats include of course that the delirium prevalence rates and test characteristics were variable, as expected, likely due to diversity of patient populations and severity of illness, and also due to performance variations that exist depending on tool application (e.g., approach to determination of baseline mental status and attention testing) in this challenging and understudied population. In this nascent field, it is important that in four studies, the identification of delirium in neurocritically ill patients independently predicted poor clinical outcomes including longer length of stay, and worse functional recovery and cognition. Among 7 prospective cohort studies and a total of 1,173 neurocritically ill subjects, delirium was assessable using a myriad of tools (e.g., CAM-ICU, ICDSC, 4-AT) with a pooled prevalence rate of 12–43% and often validated against the DSM-IV This work shows monitoring delirium in the neurocritically ill is relevant, has potential to improve ICU prognostics for this population, needs integration into ICU delirium guidelines, and requires further research.
We warn the readership that it is important to use the delirium monitoring information as a complement to the neurological examination and to expand the differential diagnosis when there is a change in the neurological exam. In this complicated patient population, it is critical to acknowledge that a positive screen for delirium may be due to the underlying neurological disease or its sequaelae (e.g., edema, vasospasm, seizures, rebleeding, ischemia) requiring very different treatments than delirium, and often with urgent or emergent time pressure to avoid further brain damage. Only once these have been ruled out as a cause for the neurological decline, should delirium be considered as a differential diagnosis.
This encouraging data are limited regarding the reliability of delirium tools (2 studies, 0.64–0.94, median 0.79) in a neurocritical care population. However, another two studies have corroborating reliability data on delirium monitoring in the neurologically injured patient, which were excluded from our review’s eligibility criteria (i.e., studies that did not measure delirium incidence or prevalence). Soja et al. (39) implemented delirium monitoring in a trauma ICU with a subset of patients with TBI, representing over one-third of observations. An expert evaluator performed 1,011 random CAM-ICU assessments within 1h of the bedside nurse’s assessments. Overall agreement (kappa) between nurses and expert evaluator was 0.75 (0.667–0.829; p < 0.0001) in TBI patients, attesting to the ease of delirium monitoring in patients with polytrauma. Also, Yu (38) evaluated 151 patients from neurological, neurosurgical and trauma ICUs. In the 439 assessments performed by bedside staff and researchers, pain and sedation were always assessable with excellent inter-rater reliability (intraclass correlation= 0.86). Patients were sufficiently alert for delirium screening 75% of the time, and delirium screening items had good concordance. Importantly, each additional ICDSC item present, in proportion to the total ICDSC score, was associated with a 10% increase in ICU length of stay. Ultimately, clinicians should feel confident that delirium tools have solid reliability in the neurocritical care setting.
Past ICU data show that delirium is independently associated with increased mortality, length of stay, cost of care, accelerated or acquired dementia-like cognitive impairment, and the inability to return to independent living (2, 3, 16, 51–58). Now, the literature is showing a similar pattern in the neurocritically ill population, with the notable exception of lack of association of delirium with mortality, which may be unique to this population and/or due to better statistical risk-adjustment methods compared to past work. We hope this work sheds light and provides structure to the care of the complex neurocritically ill patient given the utility and prognostics associated with delirium monitoring.
Unaddressed challenges for delirium monitoring in the neurocritically ill
The neurocritically ill patient population is obviously more challenging than general medical and general surgical patients with respect to delirium assessment (59). Some are not testable for delirium due to decreased level of consciousness (i.e., coma) because of the primary neurological injury or due to deep sedation (Richmond Agitation-Sedation Scale<−3) for high intracranial pressures. Stroke patients, for example, present with a high prevalence of cognitive and communication deficits such as aphasia that can make delirium assessments especially challenging (46). We accept that it is not possible to do delirium assessments on receptive aphasic patients, but expressive aphasic patients can follow commands and indicate answers to questions with head nods or hand movements, thus can complete tests of attentiveness. Purely aphasic (expressive or receptive) patients do not have impaired arousal, however aphasia often times is not an isolated finding. Level of arousal will depend on the degree of comorbid brain injury along with other covariates such as psychoactive medications, sleep deficits, degree of agitation. Psychiatric disorders, such as depression or catatonia, are other confounders that may mimic some hypoactive delirium symptoms in their most severe forms (60). Overall, it remains unclear which proportions of hypoactive or hyperactive delirium exist in neurocritical populations or subpopulations.
Similarly, nonconvulsive status epilepticus may also mimic some features of delirium and can only be diagnosed with an electroencephalogram (EEG). An unproven approach is that seizures should remain on the differential diagnosis when underlying neurological abnormalities or common risk factors of delirium do not explain a patient’s neurological exam. Furthermore, patients afflicted by both blindness and deafness are often pose challenges for delirium assessments, neurologic examination, and ICU care. Despite these difficulties, there is growing evidence that it is possible to assess delirium using tools such as the CAM-ICU or ICDSC in many (38) neurologically injured patients, provided that serial assessments are conducted to detect fluctuations in relationship to the post-injury (new) baseline status determined after the admission primary brain injury lesion.
Future areas for delirium research in neurocritically ill patients
There have been broader and well-done reviews relevant to delirium in stroke (59, 61), and our work is uniquely limited by our focus on the critically ill patient affected by primary neurologic conditions. Additionally, we acknowledge our review excluded four studies of impure neurocritically populations (40–42, 50). We note that there is no study validating any delirium tool in the neurocritically ill against the current psychiatric gold-standard for delirium, the DSM-5 (62), which formally excludes coma from inattention (i.e. delirium) assessments, but broadly classifies anything else as inattention. Also, the DSM-5 does not specify whether to measure or include those with pre-existing impaired cognition (i.e. delirium superimposed on dementia) (63), further complicating delirium assessments in neurocritically ill populations. Within any delirium tool, it is unclear which subfeatures (e.g., fluctuating mental status, inattention) are more prevalent in stroke and/or neurotrauma. The stroke and neurotrauma delirium data are not entirely comparable, as neither population has been studied under a single delirium protocol or framework. We also designed our review to be broad, inclusive of any age, yet we find no children have been studied in either stroke or neurotrauma population. Although assessment tools for the child have been created, such as the pediatric or preschool delirium assessment (pCAM-ICU, psCAM-ICU), or Cornell Assessment for Pediatric Delirium (CAP-D)] (64–66), none of these have specifically been assessed for reliability and validity for the neurocritically ill child. Another significant knowledge gap appears to be that delirium prognostics are being inconsistently reported across studies, and no study provides comprehensive associations with clinical outcomes. We also acknowledge that there are no data available to the guide specific treatment of delirium among neurologic ICU patients.
Even within the stroke or neurotrauma studies are heterogeneous populations of neurocritically ill patients, using research and clinical environments, employing different diagnostic tools, and claiming wide ranges about the assessable proportion of subjects. Also, the characteristics of any tool can be influenced by factors such as education and training of the rater (50, 67, 68). Further research is needed and has started on well-defined and homogenous subgroups of neurologically injured patients (69), like the PRospective Observational POLIsh Study on post-stroke delirium (PROPOLIS). Given the heterogeneity among patients, future studies should expand our nascent understanding of the prevalence and long-term neuropsychiatric implications (e.g., cognitive impairment, mood disorders) of both duration and pattern of time spent in a delirious state in populations like stroke and trauma. The methodological rigor of such investigations must be high (e.g., biostatistical design must include time-varying covariates).
Although there are no acute imaging correlates for delirium seen acutely or in-hospital, MRI and other techniques offer promise to uncover the hidden consequences of this secondary acute brain dysfunction. Neuroimaging of ICU cohorts with delirium is being pursued but is still in its infancy. The VISIONS MRI studies (70, 71) showed that medical and surgical ICU survivors with delirium were more likely to have brain atrophy in the prefrontal cortex and hippocampus as well as white matter abnormalities demonstrated via fractional anisotropy and diffusion tensor imaging. This suggests that there is indeed microstructural damage, and this cohort did prove to have subsequent cognitive impairment manifested by executive dysfunction and memory deficits. In patients with intracerebral hemorrhage, hematomas in specific locations are more likely to manifest delirium symptoms (72). Moving forward, it will be important to study the hypothesis that quantifiable delirium parameters predict some portion of the long-term neuroimaging and clinical deficits seen in survivors of neurocritical illness.
CONCLUSIONS
Data from adult neurocritical care investigations indicate that tools are available for delirium monitoring in stroke patients, as well as neurotrauma patients. In such patients, the clinical information is a complement to the neurological examination. In this case, delirium tools serve to expand the differential diagnosis. Delirium tools are to be used only after first considering the underlying admission neurologic diagnosis. The value of the delirium tool, therefore, rests both in earlier detection of well-known causes of an abnormal neurological exam in this population (e.g., edema, vasospasm, seizures, rebleeding, ischemia), as well as adding common causes of delirium that might not be considered early enough (e.g., sepsis or sedatives) into the daily diagnostic and therapeutic conversations for these high-risk patients. We hope this work provides the reader with a clinically applicable framework for neurologically critically ill patients that considers delirium as a manifestation of secondary brain injury potentially superimposed on major neurologic deficits seen after primary brain injury. It is incumbent on the medical field to generate more data and advance our understanding so that we may develop specific preventative and treatment strategies that will allow us to serve our neurologically injured patients better tomorrow than we do today.
Supplementary Material
Acknowledgments
Funding:
Federal sources included the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC, Nashville, TN) and the National Institutes of Health AG027472, AG035117, HL111111, GM120484 (Bethesda, MD). EWE has conducted CME activities sponsored by Abbott, Hospira, and Orion. PPP has received a research grant from Hospira Inc, in collaboration with the NIH.
Footnotes
Author Contributions:
Review Structure: MBP, PL, AM, PPP, EWE
Literature Search: MBP, PL
Data Sheets: MBP, PL, PPP, EWE
Title and Abstract Screening: MBP, PPP, EWE
Full Text Screening: MBP, PPP, EWE
Data Extraction: MBP, PPP, EWE
Bias Assessments: MBP, PPP, EWE
Systematic Review Coordination: MBP, PL, EWE
Critical Revisions to Manuscript: All
Copyright form disclosure: Dr. Patel’s institution received funding from National Institutes of Health (NIH) HL111111 and NIH GM120484; he received funding from Pfizer/Hospira (education presentation); and he disclosed that funding was provided by federal sources including the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC, Nashville, TN) and the National Institutes of Health AG027472, AG035117, HL111111, GM120484 (Bethesda, MD). Drs. Patel and Ely received support for article research from the NIH. Ms. Klein’s institution received funding from Hill Rom Co. Dr. Naidech received support for article research from AHRQ K18 HS023437. Dr. Pun received funding from the Society of Critical Care Medicine, the American Association of Critical Care Medicine, and the France Foundation to provide continuing education. Dr. John disclosed other support from CSL Behring (speaker). Dr. Pandharipande’s institution received funding from Hospira. Dr. Ely’s institution received funding from NIH and VA funding, and he received funding from Orion Laboratories, Abbott Laboratories, and Pfizer. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Salluh JI, Wang H, Schneider EB, et al. Outcome of Delirium in Critically Ill Patients: Systematic Review and Meta-Analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Girard TD, Jackson JC, et al. Long-Term Cognitive Impairment after Critical Illness. New England Journal of Medicine. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a Predictor of Long-Term Cognitive Impairment in Survivors of Critical Illness. Critical Care Medicine. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta S, Cook D, Devlin JW, et al. Prevalence, Risk Factors, and Outcomes of Delirium in Mechanically Ventilated Adults. Critical Care Medicine. 2015;43(3):557–566. doi: 10.1097/CCM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Inouye SK, Bernard GR, et al. Delirium in Mechanically Ventilated Patients: Validity and Reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: Evaluation of a New Screening Tool. Intensive care medicine. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 7.Brummel NE, Vasilevskis EE, Han JH, et al. Implementing Delirium Screening in the ICU: Secrets to Success. Critical Care Medicine. 2013;41(9):2196–2208. doi: 10.1097/CCM.0b013e31829a6f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ely EW, Margolin R, Francis J, et al. Evaluation of Delirium in Critically Ill Patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Critical Care Medicine. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Bleck TP, Smith MC, Pierre-Louis SJ, et al. Neurologic Complications of Critical Medical Illnesses. Critical Care Medicine. 1993;21(1):98–103. doi: 10.1097/00003246-199301000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Young GB, Wijdicks EFM. Disorders of Consciousness. Edinburgh; New York: Elsevier; 2008. [Google Scholar]
- 11.Eidelman LA, Putterman D, Putterman C, et al. The Spectrum of Septic Encephalopathy. Definitions, Etiologies, and Mortalities. JAMA. 1996;275(6):470–473. [PubMed] [Google Scholar]
- 12.Bleck TP. Sepsis on the Brain. Critical Care Medicine. 2002;30(5):1176–1177. doi: 10.1097/00003246-200205000-00047. [DOI] [PubMed] [Google Scholar]
- 13.van den Boogaard M, Schoonhoven L, Evers AW, et al. Delirium in Critically Ill Patients: Impact on Long-Term Health-Related Quality of Life and Cognitive Functioning. Critical Care Medicine. 2012;40(1):112–118. doi: 10.1097/CCM.0b013e31822e9fc9. [DOI] [PubMed] [Google Scholar]
- 14.Gunther ML, Morandi A, Krauskopf E, et al. The Association between Brain Volumes, Delirium Duration, and Cognitive Outcomes in Intensive Care Unit Survivors: The Visions Cohort Magnetic Resonance Imaging Study*. Critical Care Medicine. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lat I, McMillian W, Taylor S, et al. The Impact of Delirium on Clinical Outcomes in Mechanically Ventilated Surgical and Trauma Patients. Critical Care Medicine. 2009;37(6):1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 16.Milbrandt EB, Deppen S, Harrison PL, et al. Costs Associated with Delirium in Mechanically Ventilated Patients. Critical care medicine. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 17.Barr J, Fraser GL, Puntillo K, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Critical Care Medicine. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 18.Macaskill PGC, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJBPM, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 10. The Cochrane Collaboration; 2010. [Google Scholar]
- 19.Agarwal S. Risk Factors for Delirium in Older Trauma Patients Admitted to the Surgical Intensive Care Unit (Vol 77, Pg 944, 2014) Journal of Trauma and Acute Care Surgery. 2015;78(1):211–211. doi: 10.1097/TA.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BJ, Reilly JP, Shashaty MGS, et al. Admission Plasma Levels of the Neuronal Injury Marker Neuron-Specific Enolase Are Associated with Mortality and Delirium in Sepsis. Journal of Critical Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bigatello LM, Amirfarzan H, Haghighi AK, et al. Effects of Routine Monitoring of Delirium in a Surgical/Trauma Intensive Care Unit. The Journal Of Trauma And Acute Care Surgery. 2013;74(3):876–883. doi: 10.1097/TA.0b013e31827e1b69. [DOI] [PubMed] [Google Scholar]
- 22.Blondell RD, Powell GE, Dodds HN, et al. Admission Characteristics of Trauma Patients in Whom Delirium Develops. American journal of surgery. 2004;187(3):332–337. doi: 10.1016/j.amjsurg.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Branco BC, Inaba K, Bukur M, et al. Risk Factors for Delirium in Trauma Patients: The Impact of Ethanol Use and Lack of Insurance. The American Surgeon. 2011;77(5):621–626. [PubMed] [Google Scholar]
- 24.Ceriana P, Fanfulla F, Mazzacane F, et al. Delirium in Patients Admitted to a Step-Down Unit: Analysis of Incidence and Risk Factors. Journal of Critical Care. 2010;25(1):136–143. doi: 10.1016/j.jcrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Colombo R, Corona A, Praga F, et al. A Reorientation Strategy for Reducing Delirium in the Critically Ill. Results of an Interventional Study Minerva Anestesiologica. 2012;78(9):1026–1033. [PubMed] [Google Scholar]
- 26.Guenther U, Popp J, Koecher L, et al. Validity and Reliability of the Cam-Icu Flowsheet to Diagnose Delirium in Surgical Icu Patients. Journal of Critical Care. 2010;25(1):144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Ingalls NK, Armstrong B, Hester M, et al. The Fog of War: Delirium Prevalence in a Combat Intensive Care Unit. Military Medicine. 2016;181(3):209–212. doi: 10.7205/MILMED-D-15-00064. [DOI] [PubMed] [Google Scholar]
- 28.Kozak HH, Uguz F, Kilinc I, et al. Delirium in Patients with Acute Ischemic Stroke Admitted to the Non-Intensive Stroke Unit: Incidence and Association between Clinical Features and Inflammatory Markers. Neurologia I Neurochirurgia Polska. 2017;51(1):38–44. doi: 10.1016/j.pjnns.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S, Cook D, Devlin JW, et al. Prevalence, Risk Factors, and Outcomes of Delirium in Mechanically Ventilated Adults. Critical care medicine. 2015;43(3):557–566. doi: 10.1097/CCM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 30.Otter H, Martin J, Basell K, et al. Validity and Reliability of the Dds for Severity of Delirium in the Icu. Neurocritical Care. 2005;2(2):150–158. doi: 10.1385/NCC:2:2:150. [DOI] [PubMed] [Google Scholar]
- 31.Rahme RJ, Pines AR, Welz M, et al. Improving Neurosurgical Outcomes in the Intensive Care Unit: Could Dexmedetomidine Make a Difference in Ventilator Free Days, Neurological Monitoring, and Outcomes? World Neurosurgery. 2016;94:556–558. doi: 10.1016/j.wneu.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 32.Reddy DRS, Singh TD, Guru PK, et al. Identification of Acute Brain Failure Using Electronic Medical Records. Journal of Critical Care. 2016;34:12–16. doi: 10.1016/j.jcrc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson BR, Mueller EW, Henson K, et al. An Analgesia-Delirium-Sedation Protocol for Critically Ill Trauma Patients Reduces Ventilator Days and Hospital Length of Stay. The Journal of Trauma. 2008;65(3):517–526. doi: 10.1097/TA.0b013e318181b8f6. [DOI] [PubMed] [Google Scholar]
- 34.Ryosuke T, Yasutaka O, Ayumi S, et al. Delirium and Coma Evaluated in Mechanically Ventilated Patients in the Intensive Care Unit in Japan: A Multi-Institutional Prospective Observational Study. Journal of Critical Care. 2014;29(3):472e471–475. doi: 10.1016/j.jcrc.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Serpa Neto A, Slooter AJ. Delirium Detection in Stroke Patients. Critical Care Medicine. 2012;40(7):2266–2267. doi: 10.1097/CCM.0b013e31824fc115. author reply 2267–2268. [DOI] [PubMed] [Google Scholar]
- 36.van Rijsbergen MWA, Oldenbeuving AW, Nieuwenhuis-Mark RE, et al. Delirium in Acute Stroke: A Predictor of Subsequent Cognitive Impairment? A Two-Year Follow-up Study. Journal of the Neurological Sciences. 2011;306(1–2):138–142. doi: 10.1016/j.jns.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Bajo BR, Bueno JCR, Moral MS, et al. Incidence and Predictive Factors of Delirium in Hospitalised Neurological Patients. Neurologia. 2013;28(6):356–360. doi: 10.1016/j.nrl.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Yu A, Teitelbaum J, Scott J, et al. Evaluating Pain, Sedation, and Delirium in the Neurologically Critically Ill-Feasibility and Reliability of Standardized Tools: A Multi-Institutional Study. Critical Care Medicine. 2013;41(8):2002–2007. doi: 10.1097/CCM.0b013e31828e96c0. [DOI] [PubMed] [Google Scholar]
- 39.Soja SL, Pandharipande PP, Fleming SB, et al. Implementation, Reliability Testing, and Compliance Monitoring of the Confusion Assessment Method for the Intensive Care Unit in Trauma Patients. Intensive Care Medicine. 2008;34(7):1263–1268. doi: 10.1007/s00134-008-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angles EM, Robinson TN, Biffl WL, et al. Risk Factors for Delirium after Major Trauma. American Journal Of Surgery. 2008;196(6):864–869. doi: 10.1016/j.amjsurg.2008.07.037. discussion 869–870. [DOI] [PubMed] [Google Scholar]
- 41.Bryczkowski SB, Lopreiato MC, Yonclas PP, et al. Risk Factors for Delirium in Older Trauma Patients Admitted to the Surgical Intensive Care Unit. The Journal Of Trauma And Acute Care Surgery. 2014;77(6):944–951. doi: 10.1097/TA.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 42.Duceppe MA, Williamson DR, Elliott A, et al. Modifiable Risk Factors for Delirium in Critically Ill Trauma Patients. Journal of Intensive Care Medicine. 2017 doi: 10.1177/0885066617698646. 885066617698646. [DOI] [PubMed] [Google Scholar]
- 43.Frenette AJ, Bebawi ER, Deslauriers LC, et al. Validation and Comparison of Cam-Icu and Icdsc in Mild and Moderate Traumatic Brain Injury Patients. Intensive Care Medicine. 2016;42(1):122–123. doi: 10.1007/s00134-015-3964-1. [DOI] [PubMed] [Google Scholar]
- 44.Kostalova M, Bednarik J, Mitasova A, et al. Towards a Predictive Model for Post-Stroke Delirium. Brain Injury. 2012;26(7–8):962–971. doi: 10.3109/02699052.2012.660510. [DOI] [PubMed] [Google Scholar]
- 45.Lees R, Corbet S, Johnston C, et al. Test Accuracy of Short Screening Tests for Diagnosis of Delirium or Cognitive Impairment in an Acute Stroke Unit Setting. Stroke (00392499) 2013;44(11):3078–3083. doi: 10.1161/STROKEAHA.113.001724. [DOI] [PubMed] [Google Scholar]
- 46.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke Delirium Incidence and Outcomes: Validation of the Confusion Assessment Method for the Intensive Care Unit (Cam-Icu) Critical Care Medicine. 2012;40(2):484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 47.Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral Hemorrhage and Delirium Symptoms. Length of Stay, Function, and Quality of Life in a 114-Patient Cohort. American Journal Of Respiratory And Critical Care Medicine. 2013;188(11):1331–1337. doi: 10.1164/rccm.201307-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oldenbeuving AW, de Kort PL, Jansen BP, et al. Delirium in the Acute Phase after Stroke: Incidence, Risk Factors, and Outcome. Neurology. 2011;76(11):993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal LJ, Francis BA, Beaumont JL, et al. Agitation, Delirium, and Cognitive Outcomes in Intracerebral Hemorrhage. Psychosomatics. 2017;58(1):19–27. doi: 10.1016/j.psym.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine Use of the Confusion Assessment Method for the Intensive Care Unit: A Multicenter Study. American Journal Of Respiratory And Critical Care Medicine. 2011;184(3):340–344. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]
- 51.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, Risk Factors and Consequences of Icu Delirium. Intensive Care Medicine. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 52.Ouimet S, Riker R, Bergeron N, et al. Subsyndromal Delirium in the Icu: Evidence for a Disease Spectrum. Intensive Care Medicine. 2007;33(6):1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 53.Ely EW, Shintani A, Truman B, et al. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 54.Pisani MA, Kong SY, Kasl SV, et al. Days of Delirium Are Associated with 1-Year Mortality in an Older Intensive Care Unit Population. American Journal Of Respiratory And Critical Care Medicine. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium Duration and Mortality in Lightly Sedated, Mechanically Ventilated Intensive Care Patients. Critical Care Medicine. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 56.Ely EW, Gautam S, Margolin R, et al. The Impact of Delirium in the Intensive Care Unit on Hospital Length of Stay. Intensive Care Medicine. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolters AE, van Dijk D, Pasma W, et al. Long-Term Outcome of Delirium During Intensive Care Unit Stay in Survivors of Critical Illness: A Prospective Cohort Study. Critical Care (London, England) 2014;18(3):R125. doi: 10.1186/cc13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein Klouwenberg PM, Zaal IJ, Spitoni C, et al. The Attributable Mortality of Delirium in Critically Ill Patients: Prospective Cohort Study. BMJ. 2014;349:g6652. doi: 10.1136/bmj.g6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klimiec E, Dziedzic T, Kowalska K, et al. Knowns and Unknowns About Delirium in Stroke: A Review. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2016;29(4):174–189. doi: 10.1097/WNN.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 60.Skrobik Y. Delirium in Patients with Stroke: The Dark Side of the Moon? Critical care medicine. 2012;40(2):676–677. doi: 10.1097/CCM.0b013e31823878c4. [DOI] [PubMed] [Google Scholar]
- 61.Carin-Levy G, Mead GE, Nicol K, et al. Delirium in Acute Stroke: Screening Tools, Incidence Rates and Predictors: A Systematic Review. Journal of Neurology. 2012;259(8):1590–1599. doi: 10.1007/s00415-011-6383-4. [DOI] [PubMed] [Google Scholar]
- 62.Association. AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 63.Morandi A, Davis D, Bellelli G, et al. The Diagnosis of Delirium Superimposed on Dementia: An Emerging Challenge. J Am Med Dir Assoc. 2017;18(1):12–18. doi: 10.1016/j.jamda.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith HA, Boyd J, Fuchs DC, et al. Diagnosing Delirium in Critically Ill Children: Validity and Reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Critical Care Medicine. 2011;39(1):150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: A Valid, Rapid, Observational Tool for Screening Delirium in the Picu*. Critical Care Medicine. 2014;42(3):656–663. doi: 10.1097/CCM.0b013e3182a66b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith HA, Gangopadhyay M, Goben CM, et al. The Preschool Confusion Assessment Method for the Icu: Valid and Reliable Delirium Monitoring for Critically Ill Infants and Children. Critical Care Medicine. 2016;44(3):592–600. doi: 10.1097/CCM.0000000000001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neto AS, Nassar AP, Jr, Cardoso SO, et al. Delirium Screening in Critically Ill Patients: A Systematic Review and Meta-Analysis. Critical care medicine. 2012;40(6):1946–1951. doi: 10.1097/CCM.0b013e31824e16c9. [DOI] [PubMed] [Google Scholar]
- 68.Devlin JW, Marquis F, Riker RR, et al. Combined Didactic and Scenario-Based Education Improves the Ability of Intensive Care Unit Staff to Recognize Delirium at the Bedside. Critical Care (London, England) 2008;12(1):R19. doi: 10.1186/cc6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klimiec E, Dziedzic T, Kowalska K, et al. Prospective Observational Polish Study on Post-Stroke Delirium (Propolis): Methodology of Hospital-Based Cohort Study on Delirium Prevalence, Predictors and Diagnostic Tools. BMC Neurology. 2015;15:94. doi: 10.1186/s12883-015-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunther ML, Morandi A, Krauskopf E, et al. The Association between Brain Volumes, Delirium Duration, and Cognitive Outcomes in Intensive Care Unit Survivors: The Visions Cohort Magnetic Resonance Imaging Study*. Critical Care Medicine. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morandi A, Rogers BP, Gunther ML, et al. The Relationship between Delirium Duration, White Matter Integrity, and Cognitive Impairment in Intensive Care Unit Survivors as Determined by Diffusion Tensor Imaging: The Visions Prospective Cohort Magnetic Resonance Imaging Study. Critical Care Medicine. 2012;40(7):2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naidech AM, Polnaszek KL, Berman MD, et al. Hematoma Locations Predicting Delirium Symptoms after Intracerebral Hemorrhage. Neurocritical Care. 2016;24(3):397–403. doi: 10.1007/s12028-015-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.