Abstract
Objective:
There are currently three licensed human papillomavirus (HPV) vaccines that protect against cervical cancer. Here we compare the prevalence of bi-, quadri-, and nonavalent vaccine-related HPV genotypes in a multi-ethnic sample of non-Hispanic white, non-Hispanic black, Hispanic, and Asian women.
Design:
Patients in this analysis (n=419) represent a subset of women with a previous abnormal Pap test participating in a clinical trial. HPV genotyping was conducted using the Roche Linear Array. Prevalent HPV genotypes were grouped according to their inclusion in each of the vaccines: bivalent (16, 18), quadrivalent (16, 18, 6, 11), and nonavalent (16, 18, 31, 33, 45, 52, 58, 6, 11).
Results:
The prevalence of HPV genotypes covered by the bi- and quadrivalent vaccines was lowest among non-Hispanic black (15%) and Hispanic women (20%), compared to non-Hispanic white (38%) and Asian women (38%). Across all racial/ethnic groups, a large proportion of infections (38 to 49%) were with genotypes included in the nonavalent vaccine. However, the prevalence of HPV genotypes not covered by any vaccine was significantly higher among non-Hispanic black (36%) and Hispanic women (42%), compared to non-Hispanic white (24%) and Asian women (16%) (p<0.001). Racial/ethnic differences in HPV genotype prevalence were observed when controlling for demographic and sexual behavior characteristics, as well as when restricting the analysis to women with CIN2+.
Conclusion:
Our data suggest racial/ethnic differences in the prevalence of vaccine-related HPV genotypes. In particular, non-Hispanic black and Hispanic women had the lowest prevalence of HPV genotypes covered by the bi-/quadrivalent vaccines. While a large proportion of their infections were covered by the nonavalent vaccine, non-Hispanic black and Hispanic women also had the highest prevalence of HPV genotypes not covered by any vaccine.
Keywords: Human papillomavirus, race/ethnicity, vaccine, cervical neoplasia
Introduction
Cervical cancer is the second most common malignancy among women worldwide, with the highest burden being among poor, underserved populations (Jemal et al. 2011). While widespread Pap test-based screening has led to a drastic decline in the incidence of cervical cancer in the United States and other developed countries, there continues to be an estimated 12,000 new diagnoses and 4,000 deaths in the USA each year (Howlader et al. 2015). Significant racial/ethnic disparities persist in the incidence of invasive cervical cancer, with incidence rates 19 to 29% higher among non-Hispanic black and Hispanic women, respectively, compared to non-Hispanic whites (Howlader et al. 2015).
Persistent infection by certain types of HPV is a causal and necessary factor for the development of cervical cancer (Walboomers et al. 1999). While there are more than 40 anogenital HPV types, 15 are considered to have a high-risk of carcinogenesis (high-risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82) (Muñoz et al 2003). HR-HPV types 16 and 18 are causally associated with over 70% of cervical cancers (Gillison, Chaturvedi, and Lowy, 2008) and approximately 50% of cervical precancers, i.e., cervical intraepithelial neoplasm type 2, CIN2, or greater (Markowitz et al, 2014). Given the large proportion of cancers caused by HR-HPV types 16 and 18, these genotypes are the primary targets for the first two HPV vaccines that came on the market: Cervarix® (GlaxoSmithKline), a bivalent vaccine that protects against HR-HPV 16 and 18; and Gardasil® (Merck), a quadrivalent vaccine against HR-HPV 16 and 18 as well as low risk types 6 and 11, the genotypes causally associated with 90% of genital warts (Koutsky et al. 2002; Garland et al. 2009)].
In 2014, the Federal Drug Administration (FDA) licensed a new nonavalent vaccine, Gardasil 9® (Merck), which in addition to HPV 16, 18, 6, and 11, protects against high-risk types 31, 33, 45, 52, and 58. These genotypes combined are responsible for an additional 10% of cervical cancer cases in the U.S. (Saraiya et al. 2015) and up to 20% of cervical cancer cases worldwide (Serrano et al. 2014). Additionally, these genotypes account for approximately 25% of cervical precancers (Markowitz et al, 2014). Following the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices’ (ACIP) recommendations for the routine use of a new 9-valent HPV vaccine in girls and boys, the nonavalent vaccine will soon replace the quadrivalent and bivalent vaccine in most practices in the U.S. (Petrosky et al, 2015).
The distribution of HPV genotypes in a population has implications for vaccine effectiveness as the protective potential of each of the three vaccines may vary depending on the relative frequency of vaccine-preventable genotypes (Castellsague et al. 2008; Pretet et al. 2008). There is evidence of variation in the distribution of HPV genotypes by age (Barnholtz et al. 2009), geographic region (Castellsague et al, 2008; Tjalma et al. 2013; de Sanjose et al. 2010; Li et al. 2011; Guan et al. 2012), and race/ethnicity (Hariri et al. 2012; Niccolai et al. 2013; Vidal et al. 2014). The objective of this analysis is to compare the relative prevalence of vaccine-targeted HPV genotypes among infected non-Hispanic white, non-Hispanic black, Hispanic, and Asian women with a history of a recent abnormal Pap test. Additionally, we evaluate the risk of CIN2 and greater (CIN2+) associated with the genotypes covered by each of the vaccines.
Materials and Methods
Patients in this analysis represent a subset of those participating in a National Institutes of Health-supported clinical trial to evaluate emerging optical technologies for the diagnosis of cervical dysplasia. Recruitment and other study methods have been described in detail elsewhere (Scheurer et al. 2007; Cardenas-Turanzas et al. 2008). Briefly, participants were non-pregnant women, 18 years of age or older, with a history of an abnormal Pap test in the past 12 months. Thus participants may or may not have had a prevalent cytological abnormality at the time of participation, given that some cytological abnormalities may regress in that period of time. Patients were recruited at clinical sites in Houston, Texas, USA; El Paso, Texas, USA; and Vancouver, British Columbia, Canada. As part of the parent trial, participants provided a cervical swab and a biopsy and answered an interviewer-administered questionnaire to ascertain demographic and behavioral risk factor information. The study protocol was reviewed and approved by the Institutional Review Boards of all participating institutions, and all study participants provided written informed consent. Recruitment in Houston and Vancouver (n = 317 of the 419 participants included in this analysis) took place between 2000 and 2003, before the 2006 licensure of the first HPV vaccine. Recruitment in El Paso (n=102) took place between 2012 and 2014, when the bi- and quadrivalent (but not nonavalent) HPV vaccines were available in clinical practice. Among the 102 HPV-positive women in this study who were recruited in El Paso, 19 Hispanic and 2 non-Hispanic white women reported receiving at least one dose of the vaccine.
Cervical swabs were initially tested for HPV infection using Hybrid Capture 2 (HC2, Qiagen). HC2 tests for the presence of 13 high-risk (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and six low-risk types (HPV 6, 11, 40, 42, 43, and 44), without differentiation of the specific types. Test results indicate whether an individual is infected with low-risk types, high-risk types, both, or neither. Genotyping was conducted on a subset of samples (based on date of enrollment in the trial) using the Linear Array HPV test (Roche Diagnostics, Mannheim, Germany) according to manufacturer protocol. The Linear Array is a polymerase chain reaction test designed to detect 37 HPV genotypes including 13 HR-HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, CP6108, and IS39). After the hybridization reaction, test strips were read visually with a reference guide.
In this analysis, prevalent HPV types were grouped according to their inclusion in each of the vaccines: bivalent (16, 18), quadrivalent (16, 18, 6, 11), and nonavalent (16, 18, 31, 33, 45, 52, 58, 6, 11). Women were considered to have genotypes fully covered by a particular vaccine if all type-specific infections detected in their sample were included in the vaccine. Additionally, women with multiple infections were considered to have genotypes partially covered by a vaccine if some, but not all, type-specific infections were included in the vaccine. For example, a participant who tested positive for HPV 16, 33, 52 was considered to have genotypes fully covered by the nonavalent vaccine, while a participant who tested positive for HPV 52, 56, 58, 59, was considered to have genotypes partially covered by the nonavalent vaccine. Women were considered to have genotypes not covered by any vaccine if no types in their sample were included in the vaccine. For example, a participant who tested positive for HPV 35, 39, 40 was considered to have genotypes not covered by any vaccine, while a participant who tested positive for HPV 35, 39, and 45 was considered to have genotypes partially covered by the nonavalent vaccine. Due to sample size considerations, full and partial coverage were grouped into a single category for data analysis purposes. Additionally, results for the bi- and quadrivalent vaccines were grouped given the similarity in the high-risk HPV genotypes covered by the two vaccines.
Histologic specimens were collected from biopsies (2–4 per participant). Histologic diagnosis was based on evaluations by a certified pathologist and represent the histology of the most severe biopsy. For analytical purposes, histologic diagnosis was categorized as ≤ CIN1 (includes normal pathology, HPV associated changes, atypia, and CIN1), and CIN 2+ (includes CIN2, CIN 3, carcinoma in situ [CIS] and invasive cancer).
Statistical analyses were performed in SPSS version 22 (Armonk, NY: IBM Corp). Frequencies and proportions were used to describe the prevalence of HPV genotypes according to their inclusion in each of the three vaccines. Two-sided Χ2 tests (or Fisher’s exact test when the number of cases was small) were used to compare proportions across racial/ethnic groups. Two-sided X2 and Fisher’s exact tests were also used to assess the association between race/ethnicity and vaccine-covered HPV genotypes among women with CIN2+. A p-value below 0.05 was considered statistically significant. Multivariate analyses were conducted using multinomial logistic regression to describe the odds of infection with genotypes covered by a particular vaccine (bi-/quadrivalent, nonavalent, or no vaccine) by race/ethnicity after controlling for demographic and sexual risk behaviors of biologic plausibility (specifically, age, age of sexual debut, and number of lifetime sexual partners). Unadjusted and adjusted odds ratios with a p-value below 0.05 were considered statistically significant.
Results
Cases in this analysis are limited to non-Hispanic white, non-Hispanic black, Hispanic, and Asian women who tested positive for HPV. Of the initial 1052 women enrolled in the parent clinical trial, HPV genotyping was available for 701. Participants with and without genotyping data were similar in regard to demographic and HPV risk behavior characteristics. Among participants with genotyping data, we excluded 266 HPV-negative participants based on the HC2 assay. These included 149 non-Hispanic whites (56% of HPV-negatives), 37 non-Hispanic blacks (14%), 64 Hispanics (24%), and 16 Asians (6%), p<0.001. We also excluded 16 participants whose race/ethnicity was coded as “other.” The final sample was comprised of 419 participants, of whom 206 were non-Hispanic white, 39 non-Hispanic black, 142 Hispanic, and 32 Asian. Analyses utilizing pathology data were further limited to the 380 participants for whom histologic diagnosis was available.
Participants’ demographic and risk behavior characteristics are described in Table 1. The mean and median age of participants was 34 and 32 years, respectively, and there were no statistically significant age differences by race/ethnicity. However, there were statistically significant racial/ethnic differences in regard to other demographic characteristics. Non-Hispanic white and black women were significantly more likely to be U.S.- or Canadian-born (p<0.001); non-Hispanic whites and Asians were significantly more likely to be never married; and Hispanics were almost twice as likely as non-Hispanic blacks and six times as likely as Asians to have less than a high school education. In terms of HPV risk behaviors, non-Hispanic white women were considerably less likely to have at least one pregnancy and significantly more likely to have more than 8 lifetime sexual partners. Age of sexual debut was considerably later among Asian participants, with 41% initiating sexual activity after age 18 years.
Table 1.
Demographic, risk behavior, HPV infection characteristics of study participants, by race/ethnicity.
| Overall n = 419 |
Non- Hispanic white n = 206 |
Non- Hispanic black n = 39 |
Hispanic n = 142 |
Asian n = 32 |
X2 p- value |
||
|---|---|---|---|---|---|---|---|
| Birth Country |
<0.001 | ||||||
| US- and Canada-Born | 70.2% | 92.2% | 88.9% | 48.9% | 12.5% | ||
| Foreign-Born | 28.6% | 7.8% | 11.1% | 51.1% | 87.5% | ||
| Age | 0.501 | ||||||
| ≤ 34 years | 59.2% | 62.6% | 59.0% | 55.3% | 53.1% | ||
| >34 years | 40.8% | 37.4% | 41.0% | 44.7% | 46.9% | ||
| Marital Status |
0.007 | ||||||
| Never Married | 37.8% | 42.4% | 28.2% | 32.6% | 43.8% | ||
| Married/Living like Married | 38.9% | 42.4% | 38.5% | 36.2% | 43.8% | ||
| Divorced/Separated/Widowed | 21.9% | 15.1% | 33.3% | 31.2% | 12.5% | ||
| Education | <0.001 | ||||||
| ≤ High School or GED | 33.2% | 23.4% | 28.2% | 54.6% | 9.4% | ||
| > High School or GED | 66.3% | 76.6% | 71.8% | 45.4% | 90.6% | ||
| Pregnancies | <0.001 | ||||||
| Never Pregnant | 32.9% | 46.6% | 25.6% | 14.9% | 34.4% | ||
| At least 1 Pregnancy | 66.9% | 53.4% | 74.4% | 85.1% | 65.6% | ||
| Number of lifetime sexual partners |
<0.001 | ||||||
| ≤ 8 | 72.1% | 61.7% | 70.3% | 90.1% | 84.4% | ||
| >8 | 25.1% | 38.3% | 29.7% | 9.9% | 15.6% | ||
| Age at first sexual encounter |
<0.001 | ||||||
| ≤ 20 years | 70.4% | 76.1% | 89.5% | 69.0% | 21.9% | ||
| > 20 years | 29.1% | 23.9% | 10.5% | 31.0% | 78.1% | ||
| Number of infections |
0.109 | ||||||
| Single | 53.4% | 57.2% | 43.6% | 54.2% | 37.5% | ||
| Multiple | 46.5% | 42.7% | 56.4% | 45.8% | 62.5% | ||
Table 1 also indicates the prevalence of single and multiple HPV infections. Overall, 53.5% of participants had a single infection and 46.5% had multiple infections. Multiple infections were most common among Asian (62.5%) and African American participants (56.4%), although these differences were not statistically significant.
While sample size considerations precluded statistical analysis of individual HPV genotypes by race/ethnicity, genotype-specific data are presented in Supplemental Table 1 for descriptive purposes. HPV 52 was the genotype with the highest frequency of infection, followed by HPV 16. As shown in Figure 1, the prevalence of samples with genotypes partially or completely covered by the bi- and quadrivalent vaccines was significantly higher among non-Hispanic white (38.3%) and Asian women (37.5%) compared to non-Hispanic black (15.4%) and Hispanic women (19.7%). The overall prevalence of samples with genotypes covered by the nonavalent but not the bi-/quadrivalent vaccine was 39.4%. A high proportion of women with genotypes covered by the nonavalent vaccine was observed across all racial/ethnic groups, particularly among non-Hispanic black and Asian women (48.7% and 46.9%, respectively). However, the proportion of women infected with HPV genotypes not covered by any vaccine was highest among the racial/ethnic groups with the lowest prevalence of genotypes covered by the bi- and quadrivalent vaccines. Specifically, more Hispanic (42.3%) and non-Hispanic black women (35.9%) had samples in which none of the prevalent genotypes were covered by any vaccine, compared to non-Hispanic white (24.3%) and Asian women (15.6%). Non-vaccine HPV types were primarily high-risk (68.2%, data not shown).
Figure 1.
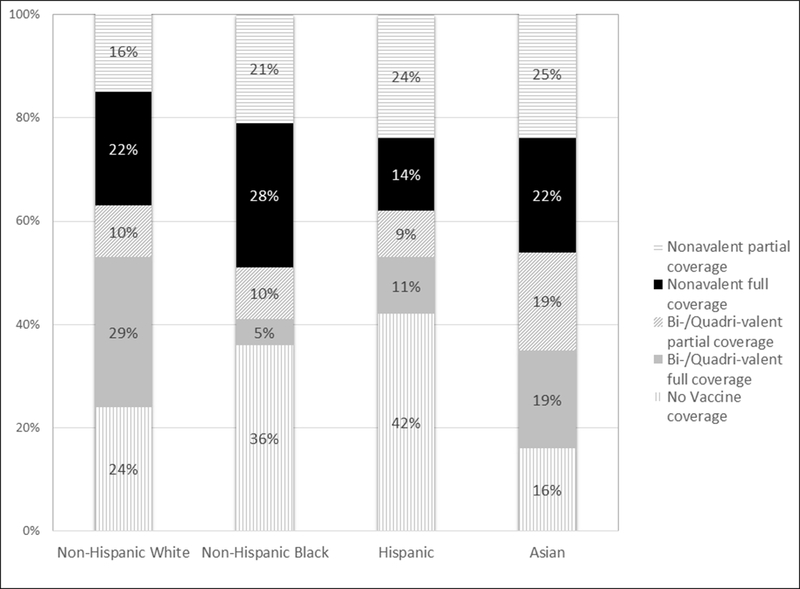
Distribution of prevalent HPV genotypes among Non-Hispanic White, Non-Hispanic black, Hispanic, and Asian study participants (n=419), according to vaccine coverage (p<0.001).
Footnotes to Figure 1:
Participants were considered ‘not covered’ if no types in their sample were included in any vaccine.
Other genotypes in sample considered to have partial vaccine coverage are described in Supplemental Table 1.
In the multivariable analysis (Table 2), race/ethnicity remained associated with prevalent vaccine-related HPV genotypes after controlling for age, age of sexual debut, and number of lifetime partners. Specifically, non-Hispanic black and Hispanic women were significantly more likely than non-Hispanic white women to have prevalent HPV genotypes not covered by any of the vaccines (OR=1.3 and 1.22 for non-Hispanic blacks and Hispanics, respectively). Non-Hispanic blacks were more likely than non-Hispanic whites to have genotypes covered by the nonavalent versus bi-/quadrivalent vaccine (OR=1.46), while Hispanics were less likely to have nonavalent vaccine-covered genotypes (OR=0.77).
Table 2.
Multinomial logistic regression describing the unadjusted and adjusted odds ratios (OR) for the association between race/ethnicity and prevalent HPV genotypes by vaccine coverage. Genotypes with bi-/quadrivalent vaccine coverage are specified as the base outcome.
| Genotypes by vaccine coverage |
OR (unadjusted) |
p-value | OR (adjusteda) |
p-value | |
|---|---|---|---|---|---|
| Bi-/quadri valent vaccine |
Base outcome | ||||
| Nonavalent vaccine coverage |
Non-Hispanic White |
Reference group | |||
| Non-Hispanic Black |
1.17 | 0.017 | 1.46 | 0.007 | |
| Hispanic | 0.68 | 0.016 | 0.77 | 0.009 | |
| Asian | 0.25 | 0.553 | 0.11 | 0.511 | |
| No vaccine coverage |
Non-Hispanic White |
Reference group | |||
| Non-Hispanic Black |
1.30 | 0.012 | 1.60 | 0.005 | |
| Hispanic | 1.22 | <0.001 | 1.35 | <0.001 | |
| Asian | 0.42 | 0.457 | 0.39 | 0.511 | |
Adjusted odds ratios are adjusted for age, age of sexual debut, and number of lifetime partners.
Table 3a indicates the distribution of HPV genotypes by histologic diagnosis. Among women with a CIN2+ diagnosis, 38.0% had genotypes covered by the bi-/quadrivalent vaccine. An additional 46.0% had genotypes covered by the nonavalent but not the bi-/quadrivalent vaccine. However, 16.0% of CIN2+ diagnoses occurred among women with HPV genotypes not covered by any vaccine. In stratified analyses (Table 3b), CIN2+ diagnoses were significantly more prevalent among non-Hispanic white women and Asian women (57.2% and 66.7%, respectively) compared to non-Hispanic Black and Hispanic women (32.4% and 21.2%, respectively).
Table 3a.
Prevalence of HPV genotypes covered by the bi-, quadri-, and nonavalent vaccines by histological diagnosis.
| N=380a (%) |
≤ CIN 1 N=217 |
CIN 2± N=163 |
X2 P-Value |
|
|---|---|---|---|---|
| <0.001 | ||||
| Bi-/Quadrivalent coverage | 115 (27.4%) | 24.4% | 38.0% | |
| Nonavalent coverage | 146 (34.8%) | 32.7% | 46.0% | |
| Not covered by any vaccine | 119 (28.4%) | 42.9% | 16.0% |
Histology data are missing for 39 of the 419 cases included in the overall analysis.
bParticipants were considered ‘not covered’ if no types in their sample were included in any vaccine.
We conducted exploratory analyses restricted to women with CIN2+ histology (Table 4). While the sample size is small and precludes any statistically significance, we observed patterns similar to those found in the larger sample. In particular, the prevalence of samples with genotypes partially or completely covered by the bi-/quadrivalent vaccines was highest among non-Hispanic white women (45.8%) and lower among non-Hispanic black (8.3%), Hispanic (25.0%), and Asian women (31.3%). The proportion of women with genotypes covered by the nonavalent vaccine was highest among non-Hispanic blacks (75.0%), followed by Hispanics (57.1%). Interestingly, the prevalence of samples with HPV genotypes not covered by any vaccine was similar across all racial/ethnic groups (15.0%, 16.7%, 17.9%, and 18.8% among non-Hispanic whites, non-Hispanic blacks, Hispanics, and Asians, respectively).
Table 4.
Prevalence of HPV genotypes covered by the bi-, quadri-, and nonavalent vaccine among participants with a CIN2± diagnosis, by race/ethnicity.
| N=163 (%) |
Non-Hispanic White N=118 |
Non-Hispanic Black N=13 |
Hispanic N=30 |
Asian N=21 |
Fisher’s exact test P-value |
|
|---|---|---|---|---|---|---|
| 0.117 | ||||||
| Bi-/Quadrivalent coverage | 62 (38.0%) | 15.0% | 16.7% | 17.9% | 18.8% | |
| Nonavalent coverage | 75 (46.0%) | 45.8% | 8.3% | 25.0% | 31.3% | |
| Not covered by any vaccine | 26 (16.0%) | 39.3% | 75.0% | 57.1% | 50.0% |
bParticipants were considered ‘not covered’ if no types in their sample were included in any vaccine.
Discussion
Our data suggest racial/ethnic differences in the proportion of women with prevalent HPV genotypes covered by the available HPV vaccines. Non-Hispanic black and Hispanic women had the lowest prevalence of HPV types covered by the bi-/quadrivalent vaccines (15% and 20%, respectively). These findings support the work of Vidal et al., who similarly found a lower proportion of HPV 16 and 18 infections among African American women compared to non-Hispanic whites (Vidal et al. 2014).
Women in our sample were those with a history of an abnormal Pap test in the past 12 months and thus included those with ≤CIN 1 and those with CIN2+. Non-Hispanic black and Hispanic women were significantly less likely to have a CIN2+ diagnosis compared to non-Hispanic white and Asian women, which may partly explain the lower prevalence of HPV 16 and 18 infections observed in these groups. However, in our exploratory analyses restricted to women with CIN2+, we similarly found that the proportion of women with genotypes covered by the bi-/quadrivalent vaccine was highest among non-Hispanic white women, while the proportion of those with genotypes covered by the nonavalent vaccine was highest among non-Hispanic black and Hispanic women. While these exploratory analyses lack statistical power, they suggest that racial/ethnic differences are observed in both cases of high and low-grade dysplasia.
Across all groups, particularly among non-Hispanic black (49%) and Asian women (47%), we observed a high proportion of infection with genotypes covered by the nonavalent vaccine. This suggests that the phasing in of the nonavalent vaccine into clinical practices will provide the greatest level of enhanced protection among one of the racial/ethnic groups with the highest burden of HPV-associated cervical cancer, non-Hispanic black women. In 2004–2008, the age-adjusted incidence of HPV-associated cervical cancer among non-Hispanic blacks was 9.9 per 100,000, compared to 7.4 per 100,000 among non-Hispanic whites (Centers for Disease Control and Prevention, 2012). The age-adjusted incidence among Hispanic women is 11.3 per 100,000.
Despite the additional genotypes covered by the nonavalent vaccine, a large proportion of non-Hispanic black and Hispanic women were infected with genotypes not covered by any vaccine. Specifically, 36% and 42% of non-Hispanic black and Hispanic women, respectively, had at least one prevalent HPV genotype that was not covered by any vaccine. The increased odds of being infected with a genotype not covered by any vaccine remained statistically significant after controlling for key demographic (age) and sexual behavior characteristics (age of sexual debut and number of lifetime sexual partners). Genotypes not covered by the existing vaccines are generally considered of lesser public health significance, given that the majority of invasive cervical cancers are caused by vaccine-preventable HPV types, particularly HPV 16 and 18. However, it is worth mentioning that the non-vaccine genotypes in our sample were associated with 16% of CIN2+ histology and that the proportion was similar across all racial/ethnic groups. While CIN2+ disease does not always progress to cervical cancer, its diagnosis does often require significant therapeutic intervention. In 2002, the estimated cost of treating CIN2/3 among insured women in the U.S. was $450 million dollars (Insinga, Glass, and Rush, 2004).
The strengths of our study include the racial/ethnic diversity of participants, the availability of histological diagnoses, and the inclusion of women with and without prevalent cytological abnormalities. Previous studies examining racial/ethnic differences in HPV genotypes have focused on women with high grade cervical lesions (Hariri et al. 2012; Niccolai et al. 2013) and cytological abnormalities (Vidal et al. 2014), precluding their ability to account for HPV infection with a lower potential to progress. Additionally, most participants (95%) were recruited prior to the licensure of the first HPV vaccine; thus our participants represent a largely vaccine-naïve sample. Our findings should nonetheless be interpreted in the context of our study’s limitations. The sample size is small for certain racial/ethnic groups (particularly non-Hispanic black and Asian women) and even more so for racial/ethnic and histological group combinations (particularly non-Hispanic black and Asian women with CIN2+ histology). This limited our power to detect differences in HPV vaccine genotype across racial/ethnic groups among women with high-grade dysplasia. Also, the lack of a probability-based sample limits the generalizability of our findings. Despite these limitations, the current analyses provide valuable data regarding differences in the distribution of HPV genotypes among the four main racial/ethnic groups in the U.S.
In conclusion, our data suggest that while the nonavalent vaccine may confer additional protection to non-Hispanic black and Hispanic women, a higher proportion of women from these racial/ethnic groups may be infected with HPV genotypes not covered by any of the available vaccines. This is disconcerting given that non-Hispanic black and Hispanic women are the racial/ethnic groups that carry the highest burden of cervical cancer in the U.S. The ability of the HPV vaccines to reduce racial/ethnic disparities in cervical cancer incidence will rely on the effectiveness of public health campaigns to promote vaccine uptake among a large proportion of the population and eliminate disparities in vaccine series initiation and completion. While in 2014, non-Hispanic black and Hispanic adolescent girls (aged 13–17 years) were more likely than non-Hispanic white adolescents to initiate and complete the vaccine series, only 60% of adolescent females nationwide received at least one dose of the vaccine and only 40% completed the three-dose series (Reagan-Steiner et al. 2014). Without enhanced vaccine uptake it is likely that racial/ethnic disparities in cervical cancer incidence will persist into the future.
Supplementary Material
Table 3b.
HPV genotypes covered by the bi-, quadri-, and nonavalent vaccine by histological diagnosis and race/ethnicity.
| All (%) | Non-Hispanic White | Non-Hispanic Black | Hispanic | Asian | X2 or Fisher’s exact test P-value |
|
|---|---|---|---|---|---|---|
| All HPV Types | N=380a | N=206 | N=39 | N=142 | N=32 | <0.001 |
| ≤ CIN 1 | 217 (57.2%) | 42.8% | 67.6% | 78.8% | 33.3% | |
| CIN 2+ | 163 (42.8%) | 57.2% | 32.4% | 21.2% | 66.7% | |
| Bi-/Quadrivalent coverage | N=125 (32.9%) | N=79 (38.3%) | N=6 (15.4%) | N=28 (19.7%) | N=12 (37.5%) | 0.003c |
| ≤ CIN 1 | 53 (46.1%) | 34.7% | 80.0% | 73.1% | 44.4% | |
| CIN 2+ | 62 (53.9%) | 65.3% | 20.0% | 26.9% | 55.6% | |
| Nonavalent coverage | N=165 (43.4%) | N=77 (37.4%) | N=19 (48.7%) | N=54 (38.0%) | N=15 (46.9%) | 0.003 |
| ≤ CIN 1 | 71 (48.6%) | 36.4% | 50.0% | 68.6% | 27.3% | |
| CIN 2+ | 75 (51.4%) | 63.6% | 50.0% | 31.4% | 72.7% | |
| Not covered by any vaccineb | N=129 (33.9%) | N=50 (24.3%) | N=14 (35.9%) | N=60 (42.3%) | N=5 (15.6%) | 0.001c |
| ≤ CIN 1 | 93 (78.2%) | 65.2% | 85.7% | 90.9% | 25.0% | |
| CIN 2+ | 26 (21.8%) | 34.8% | 14.3% | 9.1% | 75.0% |
Histology data are missing for 39 of the 419 cases included in the overall analysis.
Participants were considered ‘not covered’ if no types in their sample were included in any vaccine.
P-value based on Fisher’s exact test.
Acknowledgements
Our research team is saddened by the loss of our colleague, Dr. Karen Adler-Storthz. She was a pioneer in the field of HPV-associated cancers, and her guidance and friendship will be missed.
Funding: This research is supported by a grant from the National Institutes of Health (Grant P01CA082710, PI: M. Follen).
Footnotes
Conflicts of interest: None of the authors have any potential conflicts of interest to disclose.
References
- Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. “Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity.” Cancer Causes and Control 2009;20:1129–1138. [DOI] [PubMed] [Google Scholar]
- Cardenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, Adler-Storthz K, Benedet JL, Beck JR, Follen M, Cantor SB. “The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings.” Cancer Epidemiology, Biomarkers, and Prevention 2008;17(10):2865–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Klaustermeier J, Carrilho C, Albero G, Sacarlal J, Quint W, Kleter B, Lloveras B, Ismail MR, de San Jose S, Bosch FX, Alonso P, Menendez C. “Vaccine-related HPV genotypes in women with and without cervical cancer in Mozambique: burden and potential for prevention.” International Journal of Cancer 2008;122(8):1901–1904. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. “Human papillomavirus-associated cancers - United States, 2004–2008.” Morbidity and Mortality Weekly Report 2012;61:258–261. [PubMed] [Google Scholar]
- de Sanjose S, Quint WG, Alemany L et al. “Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study.” Lancet Oncology 2010;11:1048–1056. [DOI] [PubMed] [Google Scholar]
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. “Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine.” Journal of Infectious Disease 2009;199(6):805–814. [DOI] [PubMed] [Google Scholar]
- Gillison ML, Chaturvedi AK, Lowy DR. “HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women.” Cancer 2008;113(10 Suppl):3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, Clifford GM.. “Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer.” International Journal of Cancer 2012; 131:2349–2359. [DOI] [PubMed] [Google Scholar]
- Hariri S, Unger ER, Powell SE, Bauer HM, Bennet NM, Bloch KC, Niccolai LM, Schafer S, Steinau M, Markowitz LE. “Human papillomavirus genotypes in high grade cervical lesions in the United States.” Journal of Infectious Disease 2012;206:1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- Insinga RP, Glass AG, Rush BB. “The health care costs of cervical human papillomavirus--related disease.” American Journal of Obstetrics and Gynecology 2004;191(1):114–120. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer Journal for Clinicians 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. “A controlled trial of a human papillomavirus type 16 vaccine.” New England Journal of Medicine 2002;347(21):1645–1651. [DOI] [PubMed] [Google Scholar]
- Li N, Franceschi S, Howell-Jones R, Snijders PJF, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancer worldwide: variation by geographical region, histological type and year of publication. International Journal of Cancer 2011;128:927–935. [DOI] [PubMed] [Google Scholar]
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Unger ER. “Human papillmavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP).” Morbidity and Mortality Weekly Report Recommendation Reports 2014;63(RR-05):1–30 [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. “Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer.” New England Journal of Medicine 2003;348(6):518–527. [DOI] [PubMed] [Google Scholar]
- Niccolai LM, Russ CR, Julian PJ, Hariri S, Sinard J, Meek JI, McBride V, Markowitz LE, Unger ER, Hadler JL, Sosa LE. “Individual and geographic disparities in human papillomavirus types 16/18 in high-grade cervical lesions.” Cancer 2013;119(6):3052–8. [DOI] [PubMed] [Google Scholar]
- Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. “Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommentations of the Advisory Committee on Immunization Practices.” Morbidity and Mortality Weekly Report 2015;64(11):300–4. [PMC free article] [PubMed] [Google Scholar]
- Pretet JL, Jacquard AC, Carcopino X, Charlot JF, Bouhour D, Kantelip B, Soubeyrand B, Leocmach Y, Mougin C, Riethmuller D. “Human papillomavirus (HPV) genotype distribution in invasive cervical cancers in France: EDITH study.” International Journal of Cancer 2008;122(2):428–432. [DOI] [PubMed] [Google Scholar]
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD., Singleton, Curtis R, MacNeil J, Markowitz LE, Stokley SS. “National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years--United States, 2014.” Morbidity and Mortality Weekly Report 2015;64(29):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiya M, Unger ER, Thompson TD, et al. “US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines.” Journal National Cancer Institute 2015;107(6):djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurer ME, Guillaud M, Tortolero-Luna G, McAulay C, Follen M, Adler-Storthz K. “Human papillomavirus-related cellular changes measured by cytometric analysis of DNA ploidy and chromatin texture.” Cytometry Part B Clinical Cytometry 2007;72(5):324–331. [DOI] [PubMed] [Google Scholar]
- Serrano B, Alemany L, Ruiz PA, Tous S, Lima MA, Bruni L, Jain A, Clifford GM, Qiao YL, Weiss T, Bosch FX, de Sanjosé S. “Potential impact of a 9-valent HPV vaccine in HPV-related cervical disease in 4 emerging countries (Brazil, Mexico, India and China).” Cancer Epidemiology 2014;38(6):748–756. [DOI] [PubMed] [Google Scholar]
- Tjalma WA, Fiander A, Reich O, et al. “Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe.” International Journal of Cancer 2013;132(4):854–867. [DOI] [PubMed] [Google Scholar]
- Vidal AC, Smith JS, Valea F, Bentley R, Gradison M, Yarnall KS, Ford A, Overcash F, Grant K, Murphy SK, Hoyo C. “HPV genotypes and cervical intraepithelial neoplasia in a multiethnic cohort in the southeastern USA.” Cancer Causes and Control 2014; 25:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. “Human papillomavirus is a necessary cause of invasive cervical cancer worldwide.” Journal of Pathology 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


