Abstract
Objective
Much of the research related to Pediatric Acute Respiratory Distress Syndrome (PARDS) has focused on in-hospital mortality and interventions affecting this outcome. Limited data exists on survivors’ morbidity, hospital disposition and one-year survival. The aim of this study was to determine new morbidity rate, discharge disposition, and one-year mortality for survivors of PARDS.
Design
Secondary analysis of prospective cohort study.
Setting
Quaternary children’s hospital.
Patients
Three-hundred and sixteen mechanically ventilated children with PARDS (Berlin and Pediatric Acute Lung Injury Consensus Conference criteria) between July 2011, and December 2014.
Interventions
None
Measurements and Main Results
We performed secondary analysis of a prospectively recruited cohort of 316 mechanically ventilated children with PARDS between July 2011, and December 2014. Pre-illness and hospital discharge Functional Status Score (FSS) were determined via chart review and factors associated with new morbidity, defined as an increase of FSS of three or more, were analyzed. Demographic variables, PARDS characteristics and ventilator management were tested for association with development of new morbidity, discharge disposition, and one-year mortality. In-hospital mortality of PARDS was 13.3% (42/316). Of 274 survivors to hospital discharge, new morbidity was seen in 63 patients (23%). Discharge to rehabilitation rate was 24.5% (67/274) and associated with development of new morbidity. One-year and three-year mortality of survivors was 5.5% (15 deaths) and 8% (22 deaths), and was associated with baseline FSS, immunocompromised status, PRISM III and organ failures at PARDS onset, but not with PARDS severity.
Conclusion
New morbidity was common after PARDS, and appears to be intermediate phenotype between survival without morbidity and death, making it a useful metric in future interventional and outcome studies in PARDS.
Keywords: Acute Lung Injury, Acute Respiratory Distress Syndrome, Morbidity, Outcome Assessment, Patient Discharge, Pediatrics
INTRODUCTION
Pediatric Acute Respiratory Distress Syndrome (PARDS) causes significant disease burden in pediatric intensive care units (PICU) with a reported prevalence of close to 2%(1) and short-term mortality rates up to 20%(2). In order to better standardize diagnosis, the Pediatric Acute Lung Injury Consensus Conference (PALICC) published diagnostic guidelines in 2015(3). Recent publications have focused on characterizing severity of disease and identifying predictors of short-term mortality.(4, 5)
With improving survival after adult ARDS, long-term outcome studies have begun to shed light on the post-hospital course of these patients. Patients followed up to two years have shown continued functional impairment and worsened health related quality of life.(6, 7) PARDS research, on the other hand, to date, has focused on PICU and hospital mortality, with limited data about survivors’ morbidity, hospital disposition and long-term survival.
In pediatrics, improving mortality rates have also reduced the utility of mortality as an outcome. The Functional Status Score (FSS) was created as an outcome measure for use in pediatrics that can be applied to patients across different ages and developmental stages(8). Assessment of FSS is done by determining patient status across six domains (mental status, sensory functioning, communication, motor functioning, feeding and respiratory status). Each domain is scored from one (normal function) to five (severe dysfunction). Increasing total score represents increasing patient dysfunction.(8) Use of FSS and new morbidity were first detailed in the Trichotomous Outcomes in Pediatric Critical Care (TOPICC) study which supported their use as outcome measures in future interventional trials(9, 10).
The aim of this study was to determine new morbidity rate using change in FSS, hospital discharge disposition, and one- and three-year mortality for survivors of PARDS as well as determining epidemiologic, physiologic and treatment factors associated with these outcomes. In addition, we aimed to assess the utility of FSS as an outcome metric in PARDS.
We hypothesize that new morbidity will be a more common outcome than either in-hospital or long-term mortality. Further, new morbidity will be associated with the same variables as in-hospital mortality. For these reasons, we hypothesize that new morbidity will be a more useful outcome measure for future interventional and follow-up studies of PARDS.
METHODS
Study Design and Patient Selection
This study was approved by the Children’s Hospital of Philadelphia’s (CHOP) Institutional Review Board, and requirement for informed consent waived. The PARDS cohort has been previously described in detail. Briefly, consecutive PICU patients were screened daily for eligibility between July 1, 2011 and December 31, 2014. Children (> 1 month and < 18 years) undergoing invasive mechanical ventilation meeting American-European Consensus Conference (AECC) criteria for acute lung injury (ALI, Pao2/Fio2 ≤ 300 on 2 consecutive arterial blood gases separated by ≥ 1 hour and bilateral parenchymal infiltrates) were included. Exclusion criteria were 1) respiratory failure exclusively from cardiac failure (determined by echocardiography) or fluid overload, 2) exacerbation of underlying chronic respiratory disease, 3) chronic ventilator dependence, 4) mixing cyanotic heart disease, 5) mechanical ventilation for > 7 days before PaO2/FIO2 ≤ 300, and 6) PARDS established outside of the CHOP PICU. Determination of bilateral infiltrates was made independently by a PICU attending and a pediatric radiologist blinded to clinical data; only cases agreed to by both as consistent with AECC-defined ALI met inclusion. Determination of hydrostatic edema (from either heart failure or anuric/oliguric renal failure) as the sole etiology of respiratory failure was made in consultation with the PICU attending using available data. As the study was initiated prior to the 2012 Berlin definition of ARDS, a minimum positive end-expiratory pressure (PEEP) was not specified; however, the CHOP PICU does not utilize PEEP < 5 cmH2O, and all patients met Berlin criteria. Similarly, as data were collected prior to the 2015 PALICC definitions of PARDS, patients were not screened by oxygenation index (OI), and cases diagnosed by non-invasive (SpO2) criteria were not enrolled. However, all children met PARDS criteria by invasive (OI) criteria.
Data Collection and ARDS Management
Patient demographics including PRISM III and pre-illness FSS were recorded at PICU admission. Ventilator settings and Pao2/Fio2 at PARDS onset and 24 hours, and laboratory data and medications for the first 3 days of PARDS, were recorded. FSS scores, an age-independent assessment of pediatric functional status ranging from 6 (good) to 30 (severely abnormal), were recorded for pre-acute illness (baseline) and at hospital discharge.
Absent a standardized ventilator protocol, our institutional practice is to initiate conventional ventilation with a minimum 5 cmh2o of PEEP, and attempt to wean Fio2 to ≤ 0.60. There is no specific target PaO2, but typically PaO2 ≥ 60 mmHg is accepted as long as there is clinical stability. Inability to wean Fio2 prompts PEEP escalation and subsequent efforts to wean Fio2. We exclusively utilize decelerating flow during conventional ventilation (either pressure control or pressure-regulated volume control). Persistently elevated peak inspiratory pressures (PIP ≥ 35 cmh2o), ongoing hypercarbia (PaCO2 ≥ 80), or oxygenation difficulties (inability to wean Fio2 ≤ 0.60 despite increasing PEEP) prompted consideration for changing mode of ventilation, or escalating to extracorporeal membrane oxygenation (ECMO), although actual transition was at the discretion of the attending physician. There was no standardization of ancillary therapies (inhaled nitric oxide [iNO], neuromuscular blockade, corticosteroids), which was at the discretion of the attending physician.
Equations and Definitions
Non-pulmonary organ failures at time of PARDS diagnosis were identified using accepted definitions in children(11). The designation “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, or transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency(12). Severity of illness score used was the PRISM III at 12 hours.
Outcomes
The primary outcome was status at hospital discharge. This outcome was trichotomized to: alive without new morbidity, alive with new morbidity, and dead. New morbidity was defined as increase in FSS (ΔFSS) from pre-illness baseline to discharge of 3 or more points. This degree of change was found to indicate a significant worsening of function in the original description of FSS as an outcome marker by Pollack, M et al.(10) Pre-illness and discharge FSS were obtained via retrospective chart abstraction. Pre-illness FSS was determined to be the individual patient’s FSS before the onset of the acute illness that led to PARDS. The electronic medical record at CHOP is comprehensive of both inpatient and outpatient orders as well as case management and social work notes that were used to aid in the retrospective determination of FSS. Discharge disposition was also recorded, and categorized as discharge to home, to inpatient rehabilitation facility, or to a long-term chronic care facility (CCF). CHOP has an active Rehabilitation Medicine consult service, with significant early involvement by physical, occupational, and speech therapists. Physical and occupational therapists are automatically consulted in the PICU for intubated children and are followed for the duration of the hospital stay. Upon recommendation of therapists, or discretion of the attending physician, Rehabilitation Medicine is consulted for evaluation of appropriateness of inpatient rehabilitation. All-cause mortality at one and three years from the date of PARDS diagnosis was ascertained by review of CHOP electronic medical records and verification with the national Social Security Death Index.
Statistical Analysis
All analyses were performed using STATA 14.2. Data are expressed as percentages or as median [interquartile range, IQR]. Differences between distributions of categorical variables were analyzed by Fisher’s exact test. Continuous variables were compared using Wilcoxon rank-sum test or the Kruskal-Wallis one-way analysis of variance. As the aim of the study was to determine predictors of new morbidity, discharge disposition, and long-term survival, we performed univariate analyses to generate candidate variables for multivariable models without correction for multiple testing.
Multinomial logistic regression was performed to test for independent association between variables and the discharge outcomes of survival with new morbidity or death, using survival without new morbidity as the common reference. Potential confounders were chosen among variables with a univariate association with discharge outcome at p < 0.10. A manual backward stepwise procedure was performed, in which the variable with the least significant p value was removed, and models compared using the likelihood ratio test and their respective Akaike Information Criterion (AIC). This procedure was continued until no further variables could be removed and the AIC was minimized. Model fit was assessed using the Hosmer-Lemeshow extension for multinomial logistic regression.
Logistic regression was performed to test for independent association between variables and discharge to inpatient rehabilitation versus discharge to home. Given the infrequency of discharge to a CCF, we did not include this outcome in the regression. Potential confounders were selected and the model was built and assessed as described above.
Univariate Cox proportional hazard was used to test for association of variables with one-and three-year survival probability. The proportional hazard assumption, tested by examination of Schoenfeld residuals, held for all variables modeled. Given the low prevalence of death, a multivariable Cox model was not constructed.
RESULTS
Morbidity and PICU Mortality
There were 316 patients diagnosed with PARDS between June 2011 and December 2014 (Figure 1). Of these 316 patients, 274 survived to hospital discharge, for an in-hospital mortality rate of 13.3% (Table 1). The 274 survivors were further subdivided into those that survived without new morbidity (211 patients; 77%) and those with new morbidity (ΔFSS ≥3, 63 patients; 23%). Severity of illness, as measured by PRISM III and non-pulmonary organ failures, as well as severity of PARDS at 24 hours, measured by oxygenation and PIP were significantly different between survivors without new morbidity, survivors with new morbidity, and non-survivors. There was a step-wise increase in each of these severity of illness markers, with survivors without new morbidity demonstrating better values, non-survivors the worst, and survivors with new morbidity having intermediate values (Table 1).
Figure 1.
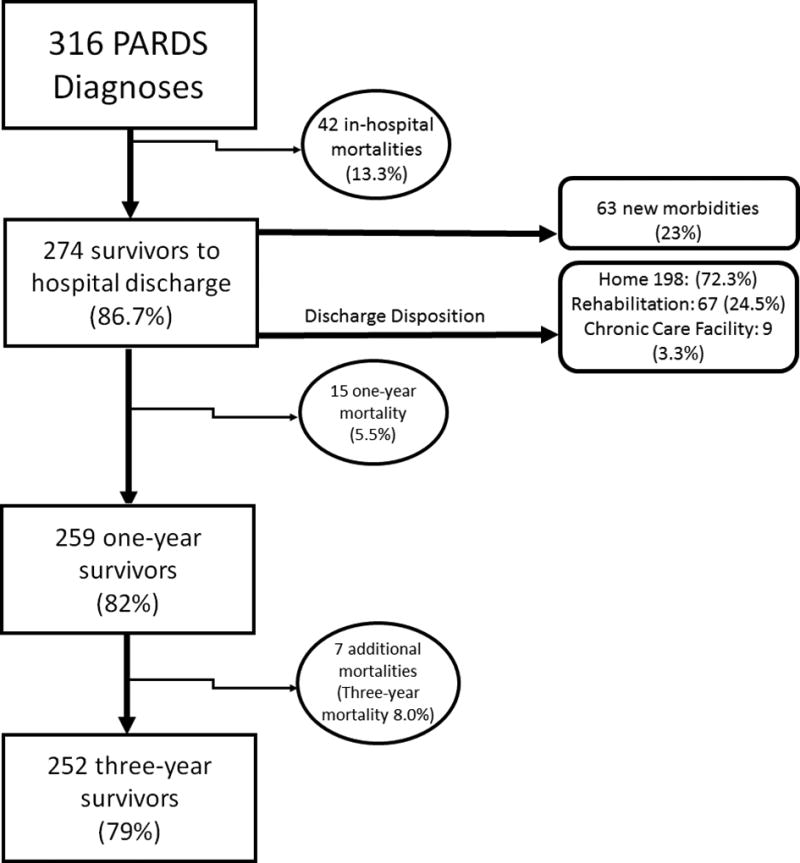
Flow Diagram of Patients Diagnosed with PARDS
Table 1.
Variables associated with hospital disposition (n=316)1
| Demographic and PARDS variables | Alive without new morbidity (n = 211)2 |
New morbidity (n = 63)3 |
Died (n = 42)4 | p value |
|---|---|---|---|---|
| Age (years) | 3.8 [1.3, 9.9] | 4.7 [1.4, 12.8] | 6.5 [1.6, 15.1] | 0.338 |
| Baseline FSSa | 6 [6, 11] | 6 [6, 8] | 6 [6, 9] | 0.016 |
| Severity of illness | ||||
| PRISM III at 12 hours | 9 [5, 15] | 11 [5, 19] | 16 [11, 30] | < 0.001 |
| Non-pulmonary organ failures | 1 [1, 2] | 2 [1, 3] | 3 [2, 4] | < 0.001 |
| PARDSb etiology (%) | ||||
| Infectious pneumonia | 131 (62) | 28 (44) | 21 (50) | 0.028 |
| Non-pulmonary sepsis | 38 (18) | 12 (19) | 9 (21) | 0.811 |
| Aspiration | 21 (10) | 7 (11) | 4 (10) | 0.940 |
| Trauma | 10 (5) | 10 (16) | 5 (12) | 0.007 |
| Other | 11 (5) | 6 (10) | 3 (7) | 0.347 |
| Co-morbidities (%) | ||||
| History of Prematurity | 34 (16) | 5 (8) | 2 (5) | 0.062 |
| Immunocompromised | 31 (15) | 6 (10) | 24 (57) | < 0.001 |
| Stem cell transplant | 6 (3) | 4 (6) | 14 (33) | < 0.001 |
| PARDS onset | ||||
| PaO2/FIO2 | 160 [118, 215] | 161 [113, 200] | 135 [81, 202] | 0.192 |
| OIc | 10 [6.7, 15.4] | 10.1 [7.9, 15.9] | 11.9 [6.6, 24.7] | 0.197 |
| PIPd | 30 [25, 35] | 30 [27, 35] | 31 [28, 38] | 0.191 |
| PEEPe | 10 [8, 12] | 10 [8, 12] | 10 [8, 12] | 0.837 |
| VT (mL/kg) | 7.6 [6.8, 8.4] | 7.1 [6.2, 7.9] | 7.7 [6.7, 8.3] | 0.078 |
| 24 hours after PARDS onset | ||||
| PaO2/FIO2 | 234 [175, 293] | 208 [158, 267] | 171 [113, 233] | < 0.001 |
| OI | 6.3 [4.7, 9.8] | 7.8 [5.9, 11.8] | 9.2 [6.4, 18.4] | < 0.001 |
| PIP | 26 [24, 30] | 27 [24, 35] | 30 [26, 35] | 0.004 |
| PEEP | 8 [8, 10] | 10 [8, 10] | 10 [8, 12] | 0.068 |
| VT (mL/kg) | 7.4 [6.7, 8.1] | 7.1 [6.3, 8.2] | 7.2 [6.3, 8] | 0.427 |
| Ancillary therapies (%) | ||||
| Inhaled nitric oxide | 65 (31) | 28 (44) | 21 (50) | 0.019 |
| NMBf | 91 (43) | 36 (57) | 19 (45) | 0.143 |
| Corticosteroids | 112 (53) | 37 (59) | 31 (74) | 0.043 |
| Non-conventional ventilator | 66 (31) | 26 (41) | 16 (38) | 0.288 |
| ECMOg | 7 (3) | 5 (8) | 3 (7) | 0.164 |
| Days and type of support | ||||
| Ventilator days | 9 [6, 14] | 15 [8, 39] | 9 [5, 17] | < 0.001 |
| PICU days | 14 [9, 22] | 23 [13, 45] | 15 [6, 26] | < 0.001 |
| Hospital days | 22 [14, 35] | 34 [23, 74] | 15 [6, 26] | < 0.001 |
| New tracheostomy (%) | 6 (3) | 13 (21) | 2 (5) | < 0.001 |
Functional Status Scale,
Pediatric Acute Respiratory Distress Syndrome,
Oxygenation Index,
Peak Inspiratory Pressure,
Positive End Expiratory Pressure,
Neuromuscular Blockade,
Extracorporeal Membrane Oxygenation
Patients diagnosed with PARDS=316,
Surviors to hospital discharge without new morbidity=211,
Patients with FSS≥3(New Morbidity)=63,
In hospital deaths=42. Bracketed [] numbers represent interquartile ranges, Parenthesized () numbers represent percentages.
Dichotomized testing of good outcome (alive without new morbidity) versus poor outcome (new morbidity or death) revealed that poor outcome was associated with PRISM III, non-pulmonary organ failures, immunocompromising co-morbidities, and oxygenation and ventilator pressures 24 hours after PARDS onset (Supplementary Table 1). A diagnosis of pneumonia was associated with survival without new morbidity, whereas PARDS from trauma was associated with a poor outcome. Dichotomized testing of survivors versus non-survivors (Supplementary Table 2) revealed that hospital mortality was associated with PRISM III, immunocompromised status, and worse oxygenation.
In multinomial logistic regression, higher (i.e., worse) baseline FSS and higher (i.e. better) P/F ratio at 24 hours were both associated with lower odds of new morbidity, as well as lower odds for mortality (Table 2). PARDS due to trauma was associated with increased odds of new morbidity, while PRISM III and immunocompromised status were associated with mortality. These results were similar in multinomial logistic regression models with hospital discharge outcomes of either good vs poor as above, or to survivors vs non-survivors (Supplementary Table 3).
Table 2.
Full multinomial regression model for hospital discharge status
| Patient Variables | Alive with new morbidity versus alive without new morbidity (OR, 95% CI) |
p value |
Dead versus alive without new morbidity (OR, 95% CI) |
p value |
|---|---|---|---|---|
| Baseline FSS | 0.89 (0.81 to 0.97) | 0.012 | 0.84 (0.73 to 0.96) | 0.008 |
| PRISM III at 12 hours | 1.03 (0.99 to 1.06) | 0.166 | 1.11 (1.06 to 1.16) | < 0.001 |
| PARDS etiology | ||||
| Pneumonia | Reference | - | Reference | - |
| Sepsis | 1.51 (0.66 to 3.43) | 0.331 | 0.49 (0.16 to 1.49) | 0.208 |
| Aspiration | 1.51 (0.56 to 4.12) | 0.418 | 0.99 (0.23 to 4.22) | 0.987 |
| Trauma | 3.82 (1.35 to 10.78) | 0.011 | 1.42 (0.34 to 5.92) | 0.631 |
| Other | 2.42 (0.74 to 7.85) | 0.143 | 0.19 (0.03 to 1.39) | 0.102 |
| Immunocompromised | 0.42 (0.15 to 1.13) | 0.084 | 8.31 (3.32 to 20.80) | < 0.001 |
| PaO2/FIO2 at 24 hours (per increase of 25) | 0.88 (0.80 to 0.97) | 0.016 | 0.77 (0.67 to 0.89) | 0.001 |
Hosmer-Lemeshow goodness of fit test with extension for multinomial model p = 0.488 (16 df)
Discharge Disposition
Of the 274 patients discharged from the hospital, 198 (72.3%) were discharged to home; 67 (24.4%) to a rehabilitation facility, and 9 (3.3%) to a CCF (Supplementary Table 4). Patients discharged to a rehabilitation facility had more organ failures, were more likely to have PARDS from trauma, and were more likely to have new morbidity (ΔFSS ≥ 3). In multivariate analysis, ΔFSS ≥ 3 was strongly associated with discharge to rehabilitation (Table 3). Examining the individual domains of FSS (Supplementary Table 5)(8), discharge to rehabilitation was associated with decline in feeding, motor, and respiratory functional domains (Supplemental Figure 1). The increase in FSS for feeding scores was most affected by the addition of tube feedings. Motor function FSS domain increases occurred across all levels of dysfunction; most commonly patients had moderate dysfunction, defined as 2 or more limbs with partial or complete loss of functionality(8). Increases in respiratory FSS scores occurred across all levels of dysfunction. Patients discharged to home had statistically significant increases in the feeding FSS domain. The most common change was addition of some degree of tube feeding. Of the 9 patients discharged to a CCF, 4 of these patients had been in a CCF prior to admission.
Table 3.
Full logistic regression model testing discharge to rehabilitation (versus home)
| Patient variables | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Age (per increase in 1 year) | 1.08 | 1.03 to 1.14 | 0.005 |
| PARDS etiology | |||
| Infectious pneumonia | Reference | - | - |
| Non-pulmonary sepsis | 0.96 | 0.39 to 2.37 | 0.938 |
| Aspiration | 0.68 | 0.21 to 2.22 | 0.522 |
| Trauma | 2.78 | 0.84 to 9.16 | 0.093 |
| Other | 1.50 | 0.34 to 6.72 | 0.594 |
| Immunocompromised | 0.23 | 0.07 to 0.80 | 0.021 |
| ΔFSS ≥ 3 | 11.10 | 5.46 to 22.56 | < 0.001 |
Hosmer-Lemeshow goodness of fit test p = 0.213 (8 df)
Respiratory Outcomes
There was a change from pre-illness to discharge respiratory FSS in 56 patients (20.4% of hospital survivors). Of these, 19 patients underwent tracheostomy placement during the initial hospitalization. In the three-year follow up period, 5 of the 19 patients had undergone tracheostomy decannulation (26%). Of the 56 patients with a change in respiratory FSS, 17 (30.4%) had improvement in their respiratory FSS by three-year follow-up and 10 (17.9%) had returned to a non-morbid FSS of 1. Of these 10 patients, four had their tracheostomies removed, one patient was taken off non-invasive overnight support and five were either weaned off supplemental oxygen, no longer had suctioning requirements, or both. Of the 56 patients with change in respiratory FSS, 7 died within the first year after PARDS (12.5%) and one additional patient died by three year follow up for an overall three-year mortality of 14.3% for those that had a worsened respiratory FSS.
ECMO
Of the 274 patients who survived to hospital discharge, 12 required the use of ECMO during PARDS course. (Supplemental Table 6) Demographics including PRISM III, organ failures, and baseline FSS were not statistically different for the patients requiring ECMO. VA-ECMO was used in 5 of the patients and the remaining 7 received VV-ECMO. Patients who required ECMO had more ventilator days and longer PICU and hospital stays. New morbidity was seen in 5 of the 12 ECMO survivors and discharge to rehabilitation in 4 of the 12, neither of which are statistically significant when comparing to non-ECMO survivors. None of the ECMO subjects died at 1 and 3 years.
One-Year and Three-Year Mortality
One-year mortality of the 274 survivors to hospital discharge was 5.5% (15 deaths). An additional 7 deaths occurred by three-year follow-up. In this cohort, the 3-year mortality was 8.0% (22 deaths). (Supplemental Table 7) One-and three-year mortality were associated with baseline and discharge FSS, immunocompromised status, PRISM III and organ failures at PARDS admission. One-year mortality was associated with discharge to a CCF. At neither onset of PARDS nor 24 hours after onset were oxygenation or ventilator characteristics (PIP, PEEP, VT) associated with one- or three-year mortality of hospital survivors. Among hospital survivors, the use of neuromuscular blockade was associated with lower one- and three-year mortality.
DISCUSSION
In this study, new morbidity occurs frequently after an episode of PARDS and appears to be an intermediary between intact survival and in-hospital mortality. In-hospital mortality was associated with higher PRISM III scores, more non-pulmonary organ failures and worsened severity of PARDS at 24 hours. New morbidity also had worsening of these variables, but not to the same extent as for those who died. In multinomial regression, improved severity of PARDS at 24 hours and higher baseline FSS (i.e., existing morbidity) were associated with lower rates of both new morbidity and death. By demonstrating that variables associated with in-hospital mortality also have relation to new morbidity and new morbidity exists on an intermediate step along the continuum of intact survival to death, we found that FSS may be a useful outcome measure in PARDS. This expands on and reinforces the previous findings about FSS from Pollack, et al (10) which showed that in addition to increasing PRISM III scores association to mortality, increasing PRISM III scores were associated with development of new morbidity until the highest PRISM III scores, when mortality is most likely. The association described between PRISM III and new morbidity in both our cohort and the original Pollack cohort may suggest that it is a patient’s cumulative physiologic abnormalities that led to new morbidity, and not a single diagnosis or disease process. Severity of PARDS at 24 hours does, however, mirror the PRISM III findings, and the effects on new morbidity and in-hospital mortality may be the overall physiologic perturbations, the PARDS severity, or a combination of these that are responsible for the morbidity effects.. In the Pollack study(10) as well as our PARDS cohort, new morbidity was more likely to be the discharge outcome when compared to mortality. New morbidity is more common than in-hospital mortality, and FSS is dynamic and can be followed over time. Additionally, FSS is universally applicable across all ages of patients admitted to the PICU, which is an important consideration in outcomes measures in pediatric diseases. For these reasons, FSS is a potentially useful and novel outcome for future studies in PARDS.
There were notable differences between patients with new morbidities and in-hospital mortalities. Immunocompromised status was strongly associated with in-hospital mortality. Immunocompromised status in itself may be driving the mortality in these patients and their characteristics of PARDS less important to the in-hospital outcome. When comparing new morbidity or in-hospital mortality to intact survivors, trauma as the etiology of PARDS was associated with new morbidity.
Not surprisingly, new morbidity was also strongly associated with discharge to rehabilitation facility. The greatest increases were seen in the FSS domains for motor functioning, respiratory and feeding. Increases in respiratory dysfunction may be partially explained by new tracheostomy placement, which was also associated with discharge to rehabilitation. Although use of neuromuscular blockade has been shown to cause muscular weakness in adults(13), we did not find an independent association with discharge to rehabilitation in this cohort of pediatric patients.
Development of new morbidities may represent incomplete recovery from PARDS or new complications of the ICU stay and PARDS course, or a combination of both. Better elucidation of the post-hospital PARDS course could be aided by assessing FSS at regular intervals after hospital discharge. In addition, follow-up FSS scores after rehabilitation discharge would be helpful in knowing the effect of the rehabilitation interventions on the dysfunction associated with PARDS.
Patients with higher baseline FSS were less likely to develop new morbidity and were more likely to be discharged to a CCF, which four of nine had also originally presented from, suggesting that this patient population is unique. Development of new morbidity in patients with pre-existing dysfunction across multiple domains of FSS may be increasingly difficult to elucidate, representing a ceiling effect of FSS. For instance, patients that are already on CPAP prior to the acute course would need to have institution of 24 hours per day mechanical ventilation to have their respiratory FSS increase by only one, but a previously healthy patient who requires overnight CPAP at discharge after acute PARDS course would have a three-point increase in respiratory FSS. FSS may not fully capture changes in these patients with highly dysfunctional scores to start. These findings could also suggest that pre-existing dysfunction is the major determinant of their long-term outcome and acute course of PARDS may be less significant a determinant of discharge status.
One- and three-year mortality in survivors of PARDS is most strongly predicted by immunocompromised status and higher baseline FSS, with weaker association with PRISM III score. Neither one-year nor three-year mortality is associated with severity of PARDS. Long term survival after an episode of PARDS seems to be related to the patients underlying comorbidities and less on the severity of the acute illness. This is additionally supported by the fact that discharge to a CCF was strongly predictive of one-year mortality; again however, four of nine patients discharged to CCF had initially presented from one as well, limiting conclusions that can be drawn from this. Aspiration was the only etiology of PARDS associated with one-year mortality. Patients most likely to aspirate are also those that likely have neurologic and feeding comorbidities, supporting that baseline dysfunction and medical comorbidities are important factors in one-year mortality.
Use of neuromuscular blockade was associated with lower one-year mortality in univariate analysis. This is an interesting result, worthy of further investigation in the setting of adult ARDS studies having shown benefit of cisatricurium on in-hospital mortality rates(2). This association was found in univariate analysis limiting the conclusions that should be drawn from this. In addition, there was no standard practice as to which patients received neuromuscular blockade and this finding may be a reflection of severity of disease. Our data did not show an impact on in-hospital outcomes and the effect on one year outcomes at this stage remains speculative, and requires further investigation and confirmation before any conclusions can be made.
The limitations of this study are that it is a single center study and PARDS etiologies, mortality and morbidity rates may differ across institutions. Additionally, specific ventilator management at our center may limit generalizability. However, this is a large cohort of patients meeting the current definition for PARDS, that all underwent ventilatory strategies well accepted in current practice, with a cohort and in-hospital mortality similar to previously described cohorts at other centers(14–16). FSS was determined retrospectively via chart review and not by prospective observation and chart review as it was in the original paper describing FSS(4). The integration of early physical therapy services and an active rehabilitative medicine team with an associated inpatient rehabilitation unit at our center may contribute to the frequency of this discharge disposition. The availability of rehabilitation centers that provide inpatient pediatric care may be limited in some locations and the services provided may not be as integrated into the PICU disposition algorithm limiting the conclusions that can be drawn from this single center cohort to the more general PICU discharge process. The ready availability of rehabilitation services at CHOP may represent the higher end of the frequency of utilization of these services after surviving PARDS. One-year mortality remains a significant burden, but in this single center study may not capture enough patients to show differences in outcomes for patients exposed to ancillary therapies. Finally, aspects of patient selection and management may further limit generalizability. Only intubated PARDS patients with bilateral infiltrates on chest radiograph, who had an arterial line and two consecutive P/F ≤ 300 were included. This cohort does not include patients managed with non-invasive positive pressure ventilation for PARDS or diagnosed via non-invasive measures. Our cohort, for these reasons, may be more representative of Berlin ARDS than PALICC PARDS criteria. For these reasons, findings on new morbidities and discharge dispositions may not reflect that of the PARDS, which is inclusive of more patients, but is typically less sick overall (17). The use of non-conventional ventilation strategies, particularly high-frequency percussive ventilation, is not available at all institutions. Institutional use of inhaled nitric oxide was also higher than use at many other centers. These adjunctive management strategies may be associated with both short- and long term outcomes, but current association is unknown due to lack of specific trials of these therapies. However, our study has several strengths. This is a large, prospective PARDS cohort from a large PICU, with detailed data collection and close follow-up, making it the one of the first PARDS cohorts capable of addressing our aims. While the single center nature may impact generalizability, PARDS etiologies and severity were similar to others(14–16, 18–20), and the single center nature may favorably limit heterogeneity in ventilator management and use of ancillary therapies. A large multicenter cohort with a variety of ventilation strategies, access to adjunctive therapies and different access to rehabilitation medicine inpatient teams and follow up rehabilitation units is needed to replicate our findings. The present study suggests that measuring FSS would be an appropriate framework for such a study.
CONCLUSION
In this large, single-center cohort study, new morbidity appears to be an intermediate phenotype between survival without morbidity and death, and is more common than in-hospital mortality, suggesting that FSS and new morbidity may be useful outcome measures for future studies in PARDS.
Supplementary Material
Supplemental Figure 1: Baseline and discharge FSS for hospital survivors discharged to (A) home (n = 198) and to (B) rehabilitation (n = 67). Survivors discharged to home had worsening in Feeding scores, whereas survivors discharged to rehabilitation had deterioration in Motor, Feeding, and Respiratory scores. P values represent results of a Wilcoxon rank-sum test
Acknowledgments
Funding Source: NIH K12-HL109009; NIH K23-HL136688 (NY)
Dr. Neal J. Thomas reports personal fees from Therabron and CareFusion, and grants from the Federal Drug Association, all outside of the submitted work.
Abbreviations
- ARDS
acute respiratory distress syndrome
- PARDS
pediatric acute respiratory distress syndrome
- CCF
chronic care facility
- PICU
pediatric intensive care unit
- FSS
Functional Status Scale
- PRISM
Pediatric Risk of Mortality
- PALICC
Pediatric Acute Lung Injury Consensus Conference
- OI
oxygenation index
- ECMO
extracorporeal membrane oxygenation
- PIP
Peak Inspiratory Pressure
Footnotes
Financial Disclosure: The remaining authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no potential conflict of interest to disclose.
Copyright form disclosure: Dr. Keim received support for article research from the National Institutes of Health (NIH). Dr. Thomas’ institution received funding from GeneFluidics, and he received funding from CareFusion and Therabron. Dr. Yehya’s institution received funding from the NIH/National Heart, Lung, and Blood Institute, and he received support for article research from the NIH. Dr. Watson has disclosed that he does not have any potential conflicts of interest.
Contributors’ Statement Page
Dr. Keim assisted in the conceptualization of the study, preformed the majority of the data collection and drafted the initial manuscript.
Dr. Watson and Thomas contributed their expertise in long term outcome studies, reviewed the data and assisted in the revision of the manuscript.
Dr. Yehya originally conceptualized the study, assisted in data collection, preformed the initial data analysis and contributed to the initial manuscript and review and revision of the final manuscript.
References
- 1.Kneyber MCJ, Brouwers AGA, Caris JA, et al. Acute respiratory distress syndrome: Is it underrecognized in the pediatric intensive care unit? Intensive Care Med. 2008;34:751–754. doi: 10.1007/s00134-008-1029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerman JJ, Akhtar SR, Caldwell E, et al. Incidence and outcomes of pediatric acute lung injury. [Internet] Pediatrics. 2009;124:87–95. doi: 10.1542/peds.2007-2462. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19564287. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. [Internet] Pediatr Crit Care Med. 2015;16:428–39. doi: 10.1097/PCC.0000000000000350. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehya N, Servaes S, Thomas NJ. Characterizing Degree of Lung Injury in Pediatric Acute Respiratory Distress Syndrome [Internet] Crit Care Med. 2015;43:937–946. doi: 10.1097/CCM.0000000000000867. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003246-201505000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Yehya N, Servaes S, Thomas NJ, et al. Corticosteroid exposure in pediatric acute respiratory distress syndrome [Internet] Intensive Care Med. 2015;41:1658–1666. doi: 10.1007/s00134-015-3953-4. Available from: http://link.springer.com/10.1007/s00134-015-3953-4. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AM, Tansey CM, Tomlinson G, et al. Two-Year Outcomes, Health Care Use, and Costs of Survivors of Acute Respiratory Distress Syndrome [Internet] Am J Respir Crit Care Med. 2006;174:538–544. doi: 10.1164/rccm.200505-693OC. Available from: http://www.atsjournals.org/doi/abs/10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS. Recovery and Long-Term Outcome in Acute Respiratory Distress Syndrome [Internet] Crit Care Clin. 2011;27:685–704. doi: 10.1016/j.ccc.2011.04.003. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124:e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. [Internet] Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8706448. [DOI] [PubMed] [Google Scholar]
- 10.Pollack MM, Holubkov R, Funai T, Berger JT, Clark AE, Meert K, Berg RA, Carcillo J, Wessel DL, Moler F, Dalton H, Newth CJ, Shanley T, Harrison RE, Doctor A, Jenkins TL, Tamburro RDJ. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015:1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 12.Yehya N, Topjian AA, Thomas NJ, et al. Improved Oxygenation 24 Hours After Transition to Airway Pressure Release Ventilation or High-Frequency Oscillatory Ventilation Accurately Discriminates Survival in Immunocompromised Pediatric Patients With Acute Respiratory Distress Syndrome* [Internet] Pediatr Crit Care Med. 2014;15:e147–e156. doi: 10.1097/PCC.0000000000000069. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00130478-201405000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kress JP, Hall JB. ICU-Acquired Weakness and Recovery from Critical Illness. [Internet] N Engl J Med. 2014;371:288–290. doi: 10.1056/NEJMc1406274. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25014703. [DOI] [PubMed] [Google Scholar]
- 14.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 15.Khemani RG, Markovitz BP, Curley MAQ. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest. 2009;136:765–771. doi: 10.1378/chest.09-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand - A prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 17.Khemani RG, Wilson DF, Esteban A, et al. Evaluating the Berlin definition in pediatric ARDS. Intensive Care Med. 2013;39:2213–2216. doi: 10.1007/s00134-013-3094-6. [DOI] [PubMed] [Google Scholar]
- 18.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 19.López-Fernández Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. [Internet] Crit Care Med. 2012;40:3238–45. doi: 10.1097/CCM.0b013e318260caa3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22990455. [DOI] [PubMed] [Google Scholar]
- 20.Yehya N, Bhalla AK, Thomas NJ, et al. Alveolar Dead Space Fraction Discriminates Mortality in Pediatric Acute Respiratory Distress Syndrome [Internet] Pediatr Crit Care Med. 2015:1. doi: 10.1097/PCC.0000000000000613. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00130478-900000000-98900. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Baseline and discharge FSS for hospital survivors discharged to (A) home (n = 198) and to (B) rehabilitation (n = 67). Survivors discharged to home had worsening in Feeding scores, whereas survivors discharged to rehabilitation had deterioration in Motor, Feeding, and Respiratory scores. P values represent results of a Wilcoxon rank-sum test


