Abstract
Objective
Extracorporeal membrane oxygenation (ECMO) is increasingly used in the management of severe acute respiratory distress syndrome (ARDS). With ECMO, select patients with ARDS can be managed without mechanical ventilation, sedation, or neuromuscular blockade. Published experience with this approach, specifically with attention to a patient’s respiratory drive following cannulation, is limited.
Designs
We describe our experience with three consecutive patients with severe ARDS supported with right jugular-femoral configuration of veno-venous (VV)-ECMO without therapeutic anticoagulation as an alternative to lung-protective mechanical ventilation (LP-MV). Outcomes are reported including daily respiratory rate (RR), vital capacities (VC), and follow-up pulmonary function testing.
Results
Following cannulation, patients were extubated with 24 hours. With ECMO support, all patients were able to maintain a normal RR and experienced steady improvements in VC. Patients received oral nutrition and ambulated daily. At follow-up, no patients required supplemental oxygen.
Conclusions
Our results suggest that VV-ECMO can provide a safe and effective alternative to LP-MV in carefully selected patients. This approach facilitates participation in physical therapy and avoids complications associated with mechanical ventilation.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation, spontaneous breathing
Introduction
Lung-protective mechanical ventilation (LP-MV) with low tidal volumes remains the mainstay management strategy for patients with acute respiratory distress syndrome (ARDS) (1). Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is a life-sustaining adjunctive modality in ARDS patients with critical hypoxemia or respiratory acidosis despite standard therapies (2). Patients undergoing ECMO traditionally receive sedatives and/or neuromuscular blockade to facilitate continued LP-MV (3). This precludes mobilization, promoting muscular deconditioning, and potentially contributing to the long-term cognitive sequelae of critical illness (4).
Discontinuation of mechanical ventilation may be tolerated in some patients receiving VV-ECMO for ARDS, but the role of this strategy remains uncertain (5). In patients with end-stage lung disease who are being bridged to lung transplantation using VV-ECMO, there is evidence to suggest that spontaneous breathing can facilitate early ambulation and improve transplant outcomes (6). As reduced levels of sedation and increased mobilization may improve long and short-term outcomes in patients with ARDS, a strategy that combines discontinuation of mechanical ventilation, sedation, and paralysis with early ambulation might also prove beneficial in cases where VV-ECMO is used as a bridge to recovery. However, some investigators have suggested that increased respiratory drive following withdrawal of sedation might worsen lung injury (5, 7, 8).
It is known that arterial pH and PaO2 primarily determine respiratory drive. Additionally, respiratory muscle weakness resulting from neuromuscular blockade and sedation, delirium or anxiety, and the presence of an endotracheal tube or tracheostomy tube, can all trigger irregular breathing patterns. We hypothesized that if hypoxemia, hypercapnia, and acidosis could be corrected by VV-ECMO, it would suppress spontaneous respirations and reduce irregular breathing patterns. Furthermore, early and complete separation of the patient from the mechanical ventilation prior to the onset of neurocognitive weakness would allow normal spontaneous breathing patterns. Accordingly, our multidisciplinary respiratory ECMO team extubated carefully selected patients with ARDS on VV-ECMO as an alternative to LP-MV. Here we report our experience with the first 3 patients managed with this approach.
Materials and Methods
At our institution, patients are considered for ECMO if they fail to achieve satisfactory gas exchange (PaO2>55, Oxygen saturations >88, pH>7.2, with plateau pressures less than 35) despite LP-MV, and recruitment maneuvers with neuromuscular blockade. The decision to cannulate is made by a multidisciplinary ECMO team. For patients placed on VV-ECMO (Oxygenator: Quadrox iD adult (7.0), MAQUET Holding B.V. & Co. KG, Germany, Circuit: Rotaflow, MAQUET Holding B.V. & Co. KG, Germany), early extubation is considered if patients are mechanically ventilated for less than 48 hours and have decreasing or no vasopressor requirements. Patients are continued on mechanical ventilation if there is a concern for their ability to protect their airway following extubation or if they had a high secretion burden requiring aggressive bronchial hygiene. In the latter scenario, early tracheostomy is considered within 48 hours of ECMO initiation. While we use a variety of cannulation strategies, all patients in this series were managed with a right internal jugular-femoral vein VV-ECMO configuration. We perform bronchoscopy in all patients prior to extubation and collect bronchoalveolar lavage fluid to help determine the etiology of lung injury (Table 1). Patients are enrolled in a prospective ECMO database approved by the institutional review board (#STU00201798) after obtaining informed consent.
Table 1.
Patient demographics
|
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Etiology of ARDS | PaO2/FiO2 pre ECMO | ECMO duration | Ventilation after ECMO | Anticoagulation | Predicted FeV1% & DLCO% after discharge | |||||
| Patients | Age | Gender | RESP score | (days) | (hours) | Outcome | ||||
| 1 | 29 | M | Viral Pnuemonia | 97 | 6 | 7 | 16 | None | 86 & 84 | Alive |
| 2 | 55 | F | Diffuse Alveolar Hemorrhage | 37 | −2 | 6 | 15 | None | 80 & 31 | Alive |
| 3 | 49 | M | Viral Pnuemonia | 59 | 3 | 5 | 8 | None | 72 & 75 | Alive |
|
|
|
|||||||||
ARDS: Acute respiratory disease syndrome, ECMO: extracorporeal membrane oxygenation,
RESP score: Respiratory ECMO survival protection score (www.respscore.com)
Following extubation, patients in this cohort received either room air or supplemental oxygen administered using nasal cannula to maintain an oxygen saturation ≥ 88%. Patients ambulated daily accompanied by a multidisciplinary team comprised of a physical therapist, skilled nurse, and perfusionist. During ambulation, the ECMO support was maximized including delivered oxygen through the circuit as well as the blood flows. However, the sweep gas was kept the same. All patients received a normal oral diet following a bedside swallowing assessment. Spirometry was measured daily by a respiratory therapist using a Wright Mark 20 Respirometer (Ferraris Medical). The patients also received intermitted hyperinflation therapy (MetaNeb System, Hill-Rom, Chicago, IL) to facilitate secretion clearance. To reduce bleeding complications, patients received only deep vein thrombo-prophylaxis using unfractionated heparin, consistent with our recent reports demonstrating the feasibility of using VV-ECMO without anticoagulation with flow of at least 3.5L/min. (9, 10). Following hospital discharge, full pulmonary function testing was obtained at their first outpatient clinic appointment with thoracic surgery.
Results
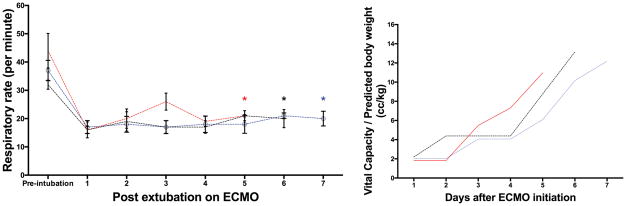
Patient characteristics and outcome parameters are shown in Table 1. During this time, two other patients were considered for respiratory ECMO but were not eligible for early separation from mechanical ventilation. Following extubation on VV-ECMO, the patients demonstrated a physiologic respiratory pattern with an expected increase in respiratory rate during ambulation (Figure 1). All patients demonstrated a progressive improvement in inspiratory capacity, which correlated with an improvement in bilateral opacities seen on chest radiograph. Importantly, during the first 3 days following extubation, the vital capacity corrected by predicted body weight (PBW) remained less than the 6 cc/kg PBW ARDSnet recommendation for tidal volume during the first three days (Figure 1). Hence, potentially injurious excessive tidal volumes were avoided. No patient developed hospital-acquired pneumonia during ECMO support. All patients were discharged from the ICU without the need for reintubation. At the time of their first follow-up clinic visit within 6 weeks of hospital discharge, pulmonary function was preserved except for one isolated decrease in DLco (Table 1) and no patient required supplemental oxygen. During VV-ECMO support, none of these patients experienced thrombotic events including oxygenator change or demonstrated venous thrombosis at the cannulation site.
Figure 1. Respiratory pattern of study patients.
The graph on the left demonstrates the daily respiratory rate of the patients (mean ± standard deviation). The star (*) indicates the day each patient was separated from VV-ECMO. The graph on the right illustrates the daily vital capacity of each patient divided by the predicted body weight.
Discussion
Our observations suggest that carefully selected patients with ARDS can be safely supported only with VV-ECMO, thus avoiding complications associated with mechanical ventilation including ventilator-induced lung injury. Reduced levels of sedation associated with this strategy facilitated participation in respiratory and physical therapy. Avoiding anticoagulation reduced the risk of bleeding and the need for laboratory monitoring of anticoagulation. Interestingly, each patient was hypotensive requiring vasopressors while intubated, but their hemodynamic parameters normalized quickly following discontinuation of mechanical ventilation. This improvement may have resulted from a reduction in intrathoracic pressure or the restoration of sympathetic tone with the removal of positive pressure ventilation and sedation, respectively. This reduction of vasopressor requirements could be from normalization of pH resulting from correction of global oxygen debt and amelioration of hypercarbia with support of VV-ECMO.
Our results are consistent with a report from investigators at Hannover Medical Center in Germany who used VV-ECMO in lieu of mechanical ventilation in six patients (11). In their cohort of carefully selected patients, three successfully weaned from VV-ECMO without the need for any mechanical ventilation. The patients who failed this strategy appear to have received longer duration of mechanical ventilation potentially predisposing them to neuromuscular decline. Together, these findings contribute to increasing equipoise with respect to the need for continued mechanical ventilation in patients with ARDS receiving VV-ECMO support. While the pathobiology of neuromuscular and cognitive impairment in ARDS survivors is incompletely understood, a strong evidence base supports the short- and long-term benefits of early physical therapy and avoidance of deep sedation for critically ill patients (12). Early liberation from mechanical ventilation for patients on VV-ECMO facilitates rapid incorporation of these principles into the care of patients who might otherwise receive prolonged sedation and neuromuscular blockade. As controversy persists regarding the risks and benefits of spontaneous breathing on ECMO, robust clinical trials to help inform these decisions are urgently needed (13). Additionally, we suggest that clinical trials comparing conventional ARDS management with early extubation on VV-ECMO should include protocols to determine whether low-tidal volume ventilation can be achieved during spontaneous breathing by optimizing gas exchange using the ECMO circuit.
Our patients had a favorable Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) suggesting a higher likelihood of survival with ECMO (14). Factors such as duration of mechanical ventilation, use of nitric oxide, bicarbonate infusion adversely impact the RESP score and reduce the likelihood of survival upon initiation of ECMO for the same severity of ARDS. Hence, we postulate that by instituting ECMO early before additional complications associated with poor survival developed, we were able achieve favorable outcomes. It is certainly possible that the favorable risk profile of our cohort achieved by earlier intervention of ECMO, once recruitment maneuvers failed, may in part explain why our experience differs from a published report where only a small subset of patients with ARDS successfully tolerated spontaneous breathing (15). Additionally, we used this strategy on patients with low secretion burden and without therapeutic levels of anticoagulation which avoided causes of failure observed in prior series. However, this report only demonstrates an association between our management approach and favorable patient outcomes. A randomized clinical trial will be required to determine the true clinical benefit of early extubation for patients with ARDS on VV-ECMO. Finally, we did not utilize esophageal pressure monitoring to approximate transpulmonary pressure in our patients. While the clinical utility of this technique is uncertain in extubated patients, it may provide important insight into respiratory effort and changes in transpulmonary pressure during spontaneous breathing in the imminent future (16, 17).
In conclusion, our results suggest that VV-ECMO can provide a safe and effective alternative to LP-MV in carefully selected patients. This approach may facilitate participation in physical therapy and avoids complications associated with mechanical ventilation. However, larger studies are necessary to validate our observations in this report.
Acknowledgments
The authors would like to thank Ms. Elena Susan for administrative assistance in the submission of this manuscript. AB is supported by National Institutes of Health HL125940 and matching funds by the Thoracic Surgery Foundation.
Footnotes
Copyright form disclosure: Dr. Bharat’s institution received funding from National Institutes of Health (NIH) HL125940 and NIH matching funds by the Thoracic Surgery Foundation. Drs. Bharat, Singer, and Budinger received support for article research from the NIH. Drs. Singer and Budinger’s institutions received funding from the NIH. Dr. Singer’s institution also received funding from the Francis Family Foundation. Dr. Cajigas’ institution received funding from United Therapeutics (research study) and Actelion (research study). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Acute Respiratory Distress Syndrome N. Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 3.Marhong JD, Telesnicki T, Munshi L, et al. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11:956–961. doi: 10.1513/AnnalsATS.201403-100BC. [DOI] [PubMed] [Google Scholar]
- 4.Langer T, Santini A, Bottino N, et al. “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care. 2016;20:150. doi: 10.1186/s13054-016-1329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauri T, Cambiaghi B, Spinelli E, et al. Spontaneous breathing: a double-edged sword to handle with care. Ann Transl Med. 2017;5:292. doi: 10.21037/atm.2017.06.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–768. doi: 10.1164/rccm.201109-1599OC. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Nakahashi S, Nakamura MAM, et al. Volume-controlled Ventilation Does Not Prevent Injurious Inflation during Spontaneous Effort. Am J Respir Crit Care Med. 2017;196:590–601. doi: 10.1164/rccm.201610-1972OC. [DOI] [PubMed] [Google Scholar]
- 8.Spahija J, de Marchie M, Albert M, et al. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010;38:518–526. doi: 10.1097/CCM.0b013e3181cb0d7b. [DOI] [PubMed] [Google Scholar]
- 9.Bharat A, DeCamp MM. Veno-arterial extracorporeal membrane oxygenation without therapeutic anticoagulation for intra-operative cardiopulmonary support during lung transplantation. J Thorac Dis. 2017;9:E629–E631. doi: 10.21037/jtd.2017.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasko J, Prasad SM, Dell DO, et al. Therapeutic anticoagulation-free extracorporeal membrane oxygenation as a bridge to lung transplantation. J Heart Lung Transplant. 2016;35:947–948. doi: 10.1016/j.healun.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Wiesner O, Hadem J, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in patients with acute respiratory distress syndrome. Intensive Care Med. 2013;39:2056–2057. doi: 10.1007/s00134-013-3052-3. [DOI] [PubMed] [Google Scholar]
- 12.Kress JP. Sedation and mobility: changing the paradigm. Crit Care Clin. 2013;29:67–75. doi: 10.1016/j.ccc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Fan E, Brodie D, Slutsky AS. Fifty Years of Research in ARDS. Mechanical Ventilation during Extracorporeal Support for Acute Respiratory Distress Syndrome. For Now, a Necessary Evil. Am J Respir Crit Care Med. 2017;195:1137–1139. doi: 10.1164/rccm.201702-0292ED. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 15.Crotti S, Bottino N, Ruggeri GM, et al. Spontaneous Breathing during Extracorporeal Membrane Oxygenation in Acute Respiratory Failure. Anesthesiology. 2017;126:678–687. doi: 10.1097/ALN.0000000000001546. [DOI] [PubMed] [Google Scholar]
- 16.Sahetya SK, Brower RG. The promises and problems of transpulmonary pressure measurements in acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22:7–13. doi: 10.1097/MCC.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauri T, Langer T, Zanella A, et al. Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensive Care Med. 2016;42:2101–2103. doi: 10.1007/s00134-016-4470-9. [DOI] [PubMed] [Google Scholar]