Non-alcoholic fatty liver disease (NAFLD) represents a major health problem worldwide because of its high and rising prevalence, its association with cardiovascular disease, and its link with an increased risk of developing cirrhosis and hepatocellular carcinoma (HCC). NAFLD is classified into different degrees, from simple steatosis (overall 20–30% prevalence), which is considered “benign”, to steatohepatitis (NASH: 2–5% prevalence) and fibrosis.1,2 The major risk factors of NAFLD include metabolic syndrome (i.e. obesity, diabetes, hypercholesterolemia and hypertriglyceridemia), sedentary lifestyle, genetic predispositions (e.g. PNPLA3 p.I148M, TM6SF2 p.E167K and MBOAT7 rs641738) and environmental factors (e.g. Western diet).1–3 Indeed, the prevalence of NAFLD/NASH in patients with obesity and/or diabetes increases dramatically.2 The differential diagnosis of NAFLD and NASH is currently available with accurate non-invasive methods based on serum metabolomics and/or imaging approaches,4,5 and the determination and monitoring of liver fat concentration is also possible by magnetic resonance imaging.6 However, the precise determination of hepatocyte ballooning, inflammation and fibrosis still requires histological characterization by liver biopsy. The EASL–EASD–EASO clinical practice guidelines7 recommend a Mediterranean diet and weight loss (7–10%) to obese patients, which have been shown to significantly improve the NAFLD activity score (NAS score).8 However, since lifestyle modifications are often not completely successful, current research is aimed at unravelling the molecular mechanisms that trigger the development and progression of NAFLD. Such advances will aid the ultimate goal of providing new potential targets for pharmacological therapy, as well as discovering biomarkers for prognosis and response to therapy.
Fatty liver is associated with increased hepatocellular death in patients and in experimental models of steatohepatitis. While historically much emphasis was placed on apoptosis (i.e., programmed cell death) and necrosis (i.e., non-programmed cell death) in NAFLD, more recently it has become clear that other types of programmed cell death, such as necroptosis and pyroptosis, may also play a role in NAFLD.9 Notably, there is also crosstalk and/or overlap between the different cell death pathways.10 Apoptosis, a caspase-dependent pathway, is characterized by nuclear condensation and cellular fragmentation into apoptotic bodies, which are phagocytosed and degraded by macrophages.11 This non-lytic pathway has minimal effects on the surrounding cells. In contrast, lytic cell death is highly inflammatory, and includes not only necrosis but also the programmed cell death pathways necroptosis12 and pyroptosis.13 Pyroptosis, the most recently described form of programmed cell death is downstream of inflammasome activation. While pyroptosis is morphologically similar to necrosis (i.e., it leads to membrane rupture and/or pore formation), it is also dependent on caspase activation, similar to apoptosis.14
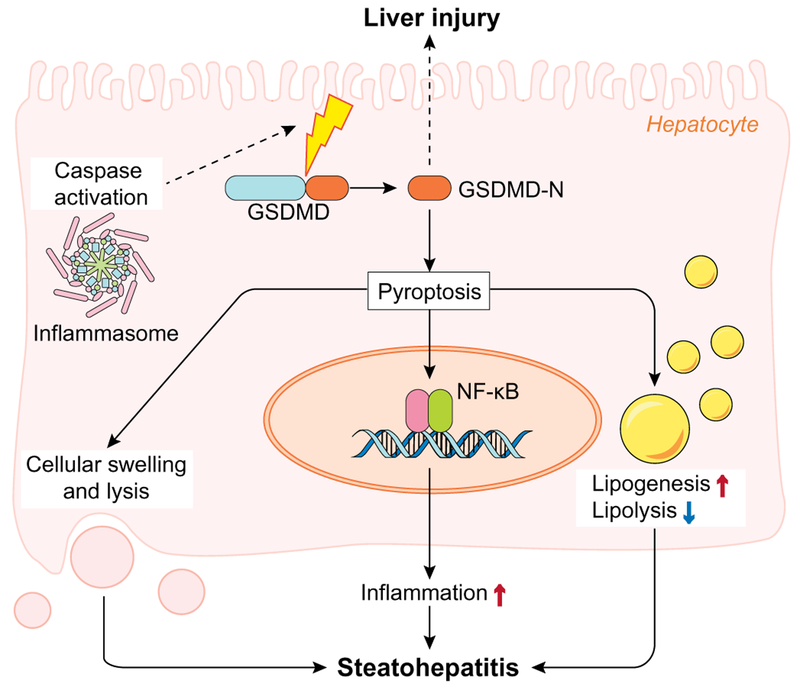
A major executor of pyroptosis is gasdermin D (GSDMD),15,16 which is a generic substrate of inflammatory caspases. GSDMD acts as a pyroptosis executor via its caspase-cleaved gasdermin-N domain (GSDMD-N) that triggers pyroptosis and causes inter-leukin (IL)-1β release. Recently, Khanova et al. demonstrated a crucial role of GSDMD-induced pyroptosis in the pathogenesis of alcoholic hepatitis.17 However, the role of GSDMD during NAFLD pathogenesis and the underlying mechanism(s) were still unknown. In the current issue of Journal of Hepatology, Xu et al. showed that protein levels of GSDMD and its pyroptosis-inducing fragment GSDMD-N were increased in liver tissues of human NAFLD/NASH compared to normal controls.18 Moreover, the authors demonstrated that GSDMD-N levels correlated with the NAFLD activity score (NAS) and fibrosis. In line with these results, in an experimental model of NAFLD, Gsdmd−/− mice fed a methionine and choline deficient diet (MCD) were protected from steatohepatitis and fibrosis, suggesting a causal role for GSDMD in NAFLD. Mechanistically, the authors showed that GSDMD induced the expression of pro-inflammatory cytokines (IL-1β, TNF-α and MCP-1), caused activation of the NF-κB signaling pathway and subsequent macrophage recruitment. Moreover, in MCD-fed Gsdmd−/− mice, the phosphorylation of p65 was partially enhanced by transfer of WT macrophages, indicating an important role in the pathogenic effects of GSDMD in steatohepatitis (Fig. 1).
Fig. 1.
Schematic depiction of hepatocellular mechanisms of gasdermin D-induced pyroptosis during NASH. Caspase-cleaved gasdermin D (GSDMD) acts as a pyroptosis executor directly by causing an increase in proinflammatory cytokines, and indirectly by activating of the NF-κB signaling pathway and subsequent macrophage recruitment. GSDMD also contributes to steatohepatitis via increased lipogenesis and decreased lipolysis. NASH, non-alcoholic steatohepatitis.
The literature suggests that although steatosis can occur without a significant inflammatory component (i.e., simple steatosis vs. steatohepatitis), steatosis also appears to play a significant role in the progression of severe stages of NAFLD.19 Increasing evidence has also identified crosstalk between steatosis and cell death signaling (e.g., autophagy).20 In this context, the finding that GSDMD contributes to lipogenesis, not only indirectly but also via direct signaling, yields a novel mechanistic insight. Specifically, Gsdmd−/− mice were protected from steatosis via downregulation of the lipogenic gene Srebp1c and induction of lipolytic genes, including Ppara, Aco, Lcad, Cyp4a10 and Cypa14. Importantly, overexpression of the GSDMD-N domain could spontaneously induce liver injury even without a secondary factor, indicating that GSDMD-N-induced pyroptosis is a crucial mechanism involved in the pathogenesis of steatohepatitis. Moreover, the finding that GSDMD expression increases with NASH severity may yield important clues for future work.
This study contributes to our knowledge of the role of inflammation and pyroptosis in the development and progression of NAFLD. However, new questions arise and need to be addressed in the future. For instance, the correlation of hepatic GSDMD and GSDMD-N expression with the NAS score and fibrosis index should be validated in a larger and independent cohort of patients, and their expression should also be compared to advanced stages of disease, such as NAFLD-cirrhosis and/or NAFLD-HCC. This information could contribute to determine their prognostic value and the appropriate disease stage for potential therapeutic interventions. Moreover, the determination of the expression and role of GSDMD/GSDMD-N in steatotic livers from lean NAFLD, diabetes or alcoholic steatohepatitis (ASH) patients may provide knowledge on their etiopathogenic role. Meanwhile, the fact that a proportion of patients with NAFLD and NASH present with similar hepatic GSDMD and GSDMD-N expression levels as healthy controls, suggests that their overexpression may be associated with genetic predispositions associated with the pathogenesis of NAFLD/NASH.3 Finally, future studies should characterize the molecular mechanisms that trigger the overexpression of GSDMD and GSDMD-N in NAFLD/NASH and their potential therapeutic regulatory value.
In summary, the study by Xu et al. provides the first insights of the relevant role of gasdermin D in the development and progression of obese-related NAFLD by promoting liver lipogenesis, inflammation and pyroptosis. These data point to gasdermin D as a potential biomarker of disease progression and a therapeutic target that deserves future attention.
Financial support
Spanish Ministries of Economy and Competitiveness [J.M. Banales (FIS PI12/00380, FIS PI15/01132 and Miguel Servet Programme CON14/00129)] cofinanced by “Fondo Europeo de Desarrollo Regional” (FEDER); “Instituto de Salud Carlos III” [CIBERehd: J.M. Banales], Spain; “Diputación Foral Gipuzkoa” (J.M. Banales: DFG15/010, DFG16/004), BIOEF (Basque Foundation for Innovation and Health Research: EiTB Maratoia BIO15/CA/016/BD to J.M. Banales); Department of Health of the Basque Country (J.M. Banales: 2,013111173), and AECC Scientific Foundation (J.M. Banales). National Institutes of Health: K01 DK096042, R03 DK107912 to Juliane I Beier. Juliane I Beier was also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113226 and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number P50AA024337. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
References
- [1].Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- [2].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assess ment of prevalence, incidence and outcomes. Hepatology 2016;64(1): 73–84. 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- [3].Krawczyk M, Jimenez-Aguero R, Alustiza JM, Emparanza JI, Perugorria MJ, Bujanda L, et al. PNPLA3 p. I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg Obes Relat Dis 2016;12:1838–1846. [DOI] [PubMed] [Google Scholar]
- [4].Barr J, Vazquez-Chantada M, Alonso C, Perez-Cormenzana M, Mayo R, Galan A, et al. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated with the progression of nonalcoholic fatty liver disease. J Proteome Res 2010;9:4501–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gallego-Duran R, Cerro-Salido P, Gomez-Gonzalez E, Pareja MJ, Ampuero J, Rico MC, et al. Imaging biomarkers for steatohepatitis and fibrosis detection in non-alcoholic fatty liver disease. Sci Rep 2016;12:31421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jimenez-Aguero R, Emparanza JI, Beguiristain A, Bujanda L, Alustiza JM, Garcia E, et al. Novel equation to determine the hepatic triglyceride concentration in humans by MRI: diagnosis and monitoring of NAFLD in obese patients before and after bariatric surgery. BMC Med 2014;26:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- [8].Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829–846. [DOI] [PubMed] [Google Scholar]
- [9].Eguchi A, Wree A, Feldstein AE. Biomarkers of liver cell death. J Hepatol 2014;60:1063–1074. [DOI] [PubMed] [Google Scholar]
- [10].Mazzolini G, Sowa JP, Canbay A. Cell death mechanisms in human chronic liver diseases: a far cry from clinical applicability. Clin Sci (Lond) 2016;130:2121–2138. [DOI] [PubMed] [Google Scholar]
- [11].Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol 2017;17:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 2015;265:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 2005;73:1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015;526:666–671. [DOI] [PubMed] [Google Scholar]
- [16].Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015;526:660–665. [DOI] [PubMed] [Google Scholar]
- [17].Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis. Hepatology 2017. 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol 2018;68:773–782. [DOI] [PubMed] [Google Scholar]
- [19].Petta S, Grimaudo S, Camma C, Cabibi D, Di Marco V, Licata G, et al. IL28B and PNPLA3 polymorphisms affect histological liver damage in patients with non-alcoholic fatty liver disease. J Hepatol 2012;56:1356–1362. [DOI] [PubMed] [Google Scholar]
- [20].Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature 2009;458:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]