Abstract
Exposure to environmental chemicals during periods of renal development from embryogenesis to birth and through childhood can inform critical windows of nephrotoxicity, including changes in childhood blood pressure. This review assessed recent studies that examined the relationship of air pollution, metals, and other organic pollutants with children’s blood pressure outcomes. We restricted this review to peer-reviewed studies published in English between January 2007 and July 2017. We identified a total of 36 articles that estimated associations with childhood blood pressure, of which 14 studies examined the effects of air pollution, 10 examined metals, and 12 examined other organic pollutants including phthalates (n=4), Bisphenol A (n=3), polychlorinated biphenols (n=2), organophosphate pesticides (n=2), or perfluoroalkyl acids (n=1). Similar to the established relationship between tobacco smoke exposure and childhood blood pressure, the majority of studies that examined air pollutants, particularly exposure to PM10 and PM2.5, reported associations with increased childhood blood pressure. The literature reported conflicting evidence for metals, and putative evidence of the effects of exposure to phthalates, Bisphenol A, polychlorinated biphenols, and pesticides. Overall, our review underscores the need for additional studies that assess the impact of nephrotoxicant exposure during early life, particularly the perinatal period, and blood pressure in childhood.
INTRODUCTION
Hypertension (HTN), an important risk factor for cardiovascular disease, affects approximately 30% of adults 18 years of age and older in the U.S. (1). The prevalence of HTN in children, in contrast, is 3.5%, although it ranges from 3.8 to 24.8% in overweight or obese youth populations (2) (reviewed in (3)). Its prevalence is thus rising as child obesity rates increase (2, 4). Of concern, later life HTN is strongly correlated with early life blood pressure (BP) elevation and impaired renal development (5, 6).
The etiology of HTN is complex, and its programming is considered to reflect a complex interplay between genetic predisposition and environment, mediated in part by epigenetic factors. While it is generally accepted that overweight/obesity (7), a sedentary lifestyle (8, 9), and salt intake (10) contribute to HTN, environmental chemical exposures to heavy metals, phthalates, and arsenic are also established risk factors for development of HTN in adult populations, estimated to account for 3–19% of the population attributable risk for high BP (11). Despite the predictive value of childhood BP on HTN later in life, there exist a limited number of studies that examine the effect of environmental chemical exposures on childhood BP.
The developmental origins of health and disease (DOHaD) hypothesis posits that an adverse intrauterine environment programs chronic disease including elevated BP and kidney disease later in life (12). Metanephric kidney development typically begins at 5 weeks’ gestation with the full complement of ~ 1 million nephrons per kidney largely achieved by ~35 weeks gestation (13, 14). Renal blood flow and glomerular filtration, which increase significantly during fetal life, continue to mature after birth, reaching adult levels by 2 years of age (15). Overall, these prenatal and postnatal periods are likely to be critical windows that are susceptible to nephrotoxic environmental chemical exposures predisposing the developing kidney for later life HTN (Figure 1) (16, 17). Although evidence supports this relationship for in utero tobacco exposure and child BP (18, 19), the effects of other highly prevalent environmental nephrotoxicants on renal development and BP are poorly understood. We previously reviewed non-BP effects of environmental chemicals on pediatric kidney function and disease (20).
Figure 1.
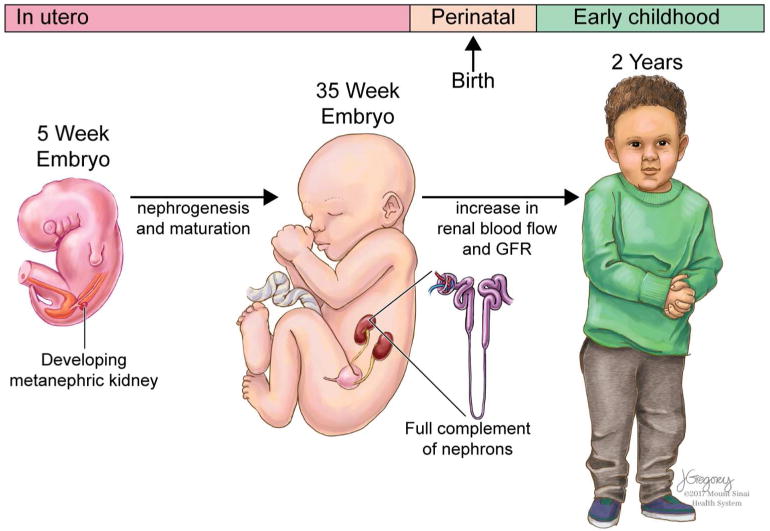
The timing of exposures to environmental chemicals during periods of renal development from embryogenesis to birth and through childhood can inform critical windows of kidney susceptibility. Printed with permission of ©Mount Sinai Health System.
To summarize the state of the literature on the role of environmental chemicals as predictors of childhood BP, we conducted a review of human studies examining exposures during prenatal and postnatal susceptibility windows. We focused on exposure to air pollution, and included a wider range of publication dates and pollutants than previously reported (21, 22). We also included metals and organic contaminants as pervasive and/or emerging contaminants, which have not been reviewed with respect to childhood BP. We present conclusions based on this body of evidence and provide recommendations for future studies.
METHODS
We conducted a literature search to identify human studies that examined the effects of air pollutants [particulate matter (PM10 and PM2.5), nitric oxides (NOx and NO2), ozone (O3), sulfur dioxide (SO2), carbon monoxide (CO), black carbon (BC), and polycyclic aromatic hydrocarbons (PAHs)], nephrotoxic metals [arsenic (As), cadmium (Cd), lead (Pb), or mercury (Hg)], phthalates and other organic contaminants with respect to children’s BP using the following search terms (child* OR infan* OR school OR postnatal OR post-natal OR prenatal OR pre-natal OR fetal OR pregnan* OR in utero) AND (blood pressure OR systolic OR diastolic OR pulse pressure) AND (metal OR “cadmium” [MeSH Terms] OR “arsenic” [MeSH Terms] OR “lead” [MeSH Terms] OR “mercury” [MeSH Terms] OR “BPA” [MeSH Terms] OR “Bisphenol A” [MeSH Terms] OR perfluoroalkyl OR pesticides OR PAH OR polycyclic aromatic hydrocarbon OR phthalate). Among additional contaminants included in the initial search terms including cannabis/marijuana, brominated flame retardants, parabens, dioxins, or furans, we identified no published studies of children’s BP. We used PubMed and Web of Science search engines and restricted the search to peer-reviewed human studies published in English between January 2007 and July 2017. We summarized a total of 14, 10, and 12 studies on air pollution, metals, or phthalates and other organic contaminants, respectively. We note that this manuscript is not a systematic review, which was not possible due to the paucity of peer-reviewed studies for some exposures as well as the heterogeneity of reported exposure and outcome measures.
This review uses updated terminology for pediatric BP as defined in the 2017 report by the American Academy of Pediatrics (AAP) Subcommittee On Screening Management Of High Blood Pressure In Children and Adolescents (2) wherein normal BP, elevated BP (formerly called “preHTN”), Stage 1 HTN and Stage 2 HTN are defined and we refer the reader to the AAP report for details (2). The term “high BP” may refer to both HTN and “elevated BP” together. To further clarify BP nomenclature, we use the term “increased” BP to describe associations with continuous BP (formerly termed “elevated” in some studies). In cases where the former terminology was used in reports herein, we have indicated the authors’ intended definition of “HTN”, “preHTN”, and “elevated BP” and corrected linear regression interpretations to “increased BP” where appropriate.
Epidemiologic evidence of association between air pollution and childhood BP
We identified 14 studies examining the effects of air pollutants on child BP (Table 1). Exposures assessed included PM10 (n=10), PM2.5 (n=8), NOx or NO2 (n=8), O3 (n=5), SO2 (n=3), CO (n=2), BC (n=2), PAHs (n=1), distance to roadway/traffic/point source (n=3), nano-sized ultrafine particles (n=1); eleven studies assessed more than one pollutant. Outdoor air quality was assessed via ambient monitoring, estimates derived with geographic information system (GIS) models (distance to traffic, land-use regression), or satellite/dispersion or other national database estimates. Two studies assessed indoor air quality. Three of the study designs were prospective and 12 were cross-sectional. Among 10 studies of PM10 and 8 studies of PM2.5, four and two reported significant associations with childhood BP, respectively. Five of 7 studies of NO2, four of 5 studies of O3, two of 3 studies of SO2, one of 2 studies of CO, and one of 2 studies of BC reported associations with childhood BP; one study reported evidence of a relationship with nano-sized ultrafine particles (UFP) (particles with a diameter ≤ 100 nm). Two studies examined indoor air pollution and reported no association with children’s BP (23, 24). A single study using school location as proxy for PAH exposure reported an association with childhood BP, but not with urine PAH biomarkers (25). A study using distance to major road as a proxy for ambient PM10 reported higher mean carotid arterial stiffness (derived from BP and ultrasound parameters) among child living closer to the major road (26). A study of road traffic at schools near an airport found no association with child BP (27).
Table 1.
Studies assessing exposure to air pollutants and blood pressure outcomes (n=14).
| Authors & Year | Study design, name | Location | Sample sizea | Exposure Measure [Reported level] | BP Evaluation | Confounders | Major Finding(s) |
|---|---|---|---|---|---|---|---|
| Van Rossem et al. 2015 (28) | Prospective cohort Project Viva |
US | 1,131 | PM2.5, NOx, NO2, O3, CO, BC measured ambient monitoring station and averaged by trimester, and 2–90 days before birth. Spatiotemporally resolved estimates for PM2.5 and BC. | SBP and DBP in newborns (30 ± 18 hours after birth) with an automated device, measured 5 times 1-min apart, recorded measurement conditions (i.e sleep/wake, cuff size, arm, position) | Measurement circumstance, birth weight, maternal age, race/ethnicity, SES, education level, third trimester BP, time trend | An IQR increase in BC (0.33 ug/m3) in the 3rd trimester was associated with SBP (1.0 mmHg; 95%CI: 0.2, 1.8). Similar trends observed for mean PM2.5 and BC with higher SBP during the 2–90 day interval before birth. An IQR increase in O3 (13.6 ppb) in the 3rd trimester was negatively associated with SBP (−2.5 mmHg; 95% CI: −4.5, −0.4). Similar trends observed for averages over 2–90 days before birth. Estimates with DBP not reported. |
| Bilenko et al. 2015a (29) | Prospective cohort PIAMA |
Netherlands | 1,147 (subset of 471 non-movers) | Annual average levels of NO2 [median: 21.8 μg/m3, IQR: 7.6] and Cu, Fe, K, Ni, S, Si, V, and Zn components of PM2.5 [median 16.5 μg/m3, IQR: 1.2], PM10 [median: 24.7 μg/m3, IQR 1.0] estimated by Land-Use Regression at birth and at time of BP measure. | SBP and DBP at 12 years with an automated device, sized cuff, on the non-dominant arm while seated, measured 2 times 5-min apart. | Sex, age, height, BMI, cuff size, weight gain 1st yr, breast feeding, maternal smoking in pregnancy, parental smoking in home, physical activity, puberty scale, maternal education, ambient temperature, and room temperature. Stratified by non-movers vs. movers | Among non-movers, an IQR increase in NO2 (7.6 μg/m3) was associated with increased DBP (0.82 mmHg, 95%CI: 0.08,1.57). *Replicated (30). Among non-movers, an IQR increase in iron (119.2 ng/m3), silicon (74.5 ng/m3), and potassium (18.3 ng/m3) in PM10 were associated with increased DBP [0.67 mmHg, 95%CI 0.02, 1.31; 0.85 mmHg, 95%CI: 0.18, 1.52; and 0.75 mmHg, 95%CI: 0.09, 1.41. Marginal associations with iron and silicon in PM2.5 with DBP (p<0.1). |
| Bilenko et al. 2015b (30) | Prospective cohort PIAMA |
Netherlands | 1,432 (471 non-movers) | NO2, PM2.5, and PM10, and PM2.5 absorbance assessed by ambient monitors at home and school for ‘short-term’ (7-day average proceeding BP measure) or ‘long-term’ (annual average calculated by Land-Use Regression). Long-term: NO2 [median, IQR: 9.6, 17.5–25.3 μg/m3] PM2.5 [16.5, 15.6–16.7 μg/m3], PM10, [24.5, 24.0–25 μg/m3] and PM2.5abs [1.2, 1.0–1.3 (10−5/m)] | SBP and DBP at 12 years with an automated device, sized cuff, on the non-dominant arm while seated, measured 2 times 5-min apart. | Sex, age, height, BMI, cuff size, GA at birth, birth weight, weight gain 1st yr, breast feeding, maternal smoking in pregnancy, parental smoking in home, physical activity, puberty scale, maternal education, maternal pregnancy HTN, pneumonia or otitis media in 2 years, ambient temperature, and room temperature. Stratified by non-movers vs. movers, and sex. | Among non-movers, an IQR increase in long-term NO2 (7.8 μg/m3) and PM2.5abs (0.3×10−5/m) were associated with increased DBP (0.83, 95%CI: 0.06, 1.61 and 0.75, 95%CI −0.08, 1.58, respectively), but not SBP. No association with short-term exposures. |
| Zeng et al. 2017 (31) | Cross-sectional SNECCS |
China | 9,354 (4,771 elementary and 4,583 middle school aged children) | PM10 [median: 108.6 μg/m3], SO2 [24.6 μg/m3], NO2 [25.5 μg/m3], and O3 [69.4 μg/m3] 1–5 days preceding BP examine measured by ambient air monitors 2 | SBP and DBP at age 5–17 years using a standard mercury sphygmomanometer after 5 min rest, seated, on the right arm, 3 times at 2-min intervals. Elevated BP defined as either SBP or DBP >95th percentile based on sex, age and height (4th report guides). |
Temperature, age, sex, BMI, breast feeding, birth weight, exercise time, passive smoke exposure, parental education, family income, family history of HTN, and district. | And IQR increase in 5-day mean PM10 (47.4 μg/m3) and O3 (51.4 μg/m3) was associated with increased odds ratio of elevated BP 2.17 (95%CI: 1.61, 2.93) and 2.77 (95%CI: 1.94, 3.95), respectively and increased SBP of 2.07 mmHg (95%CI: 1.71, 2.44) and 3.29 mmHg (95%CI: 2.86, 3.72), respectively. Significant associations also observed for DBP, but not reported. Stratified by sex, an IQR increase in NO2 (18.5 μg/m3) was associated with elevated BP in males (OR: 1.38, 95%CI: 1.00, 1.91), but not females. No associations with SO2 |
| Dong et al. 2015 (33) | Cross-sectional SNECCS |
China | 9,354 Categorized as normal weight (6,311), overweight (1,479) and obese (1,564) | 4-year average PM10, SO2, NO2, and O3 measured at ambient monitoring stations [see below]. | SBP and DBP at age 5–17 years using a standard mercury sphygmomanometer after 5 min rest, seated, on the right arm, 3 times at 2-min intervals. HTN defined as SBP and DBP ≥95th percentile for gender, age and height (4th report guides). |
Age, sex, parental education, LBW, PTB, breast feeding, income, passive smoke exposure, home coal use, exercise time, home size, family HTN history, distance to school, distance between air monitor and school, temperature, and district. | Among normal weight children, an IQR increase in PM10 (30.6 μg/m3), NO2 (13.0 μg/m3), and O3 (46.3 μg/m3) were associated with increased odds of HTN [OR: 1.21 95%CI: 1.07, 1.38; OR: 1.19, 95%CI: 1.07, 1.38; OR: 1.08 95%CI: 1.06, 1.10, respectively. Among normal weight children, a IQR increase of SO2 (23.4 μg/m3) was associated with decreased odds of HTN (0.82 95%CI: 0.72, 0.93). No associations were observed among normal weight children and SBP or DBP. There was evidence of an interaction effect with overweight and obese groups for PM10, SO2, and O3 and both SBP and DBP. |
| Dong et al. 2014 (32) | Cross-sectional SNECCS |
China | 9,354 | 4-year average PM10 [median: 90.4 μg/m3], SO2 [48.4 μg/m3], NO 2 [35.0 μg/m3], O3 [43.8 μg/m3], and CO [1289.5 μg/m3] measured at ambient monitoring stations. | “·” HTN defined as SBP and DBP ≥95th percentile for gender, age and height (4th report guides). |
Age, sex, BMI, parental education, LBW, PTB, breastfeeding, income, passive smoking exposure, home coal use, physical activity, home sq ft per person, family HTN history, and district. Stratified by breast feeding status and sex. | Increases in O3, PM10, CO, SO2, and NO2, were associated with Increased odd ratios for HTN. An IQR increase in O3 (46.3 μg/m3) was associated with an OR for HTN of 1.12 (95%CI: 1.10–1.13). An IQR increase in PM10 (30.6 μg/m3) was associated with an OR for HTN of 1.68 (95%CI: 1.53–1.86). An IQR increase in O3 (46.3 μg/m3) was associated with a 0.50 mmHg increase in SBP (95%CI: 0.43, 0.57) and a 0.58 mmHg increase in DBP (95%CI: 0.52, 0.63 An IQR increase in PM10 (30.6 μg/m3) was associated with a 2.10 mmHg increase in SBP (95%CI: 1.73, 2.47) and a 1.93 increase in DBP (95%CI 1.62, 2.25). An IQR increase in CO (563.4 μg/m3) was associated with a 2.89 mmHg increase in DBP (95%CI: 2.53, 3.24). Effect sizes remained significant and of greater magnitude when restricted to non-breastfed group. |
| Liu et al. 2013 (34) | Cross-sectional GINIplus & LISAplus | Germany | 2368 | ‘Long-term’ (1-year) averages from ambient monitors of traffic-related NO2 [median: 21.5 μg/m3], PM2.5 [median: 13.97 μg/m3] PM10 [median: 21.77 μg/m3], and PM2.5 abs [median: 1.52 × 10−5/m] and for ‘short-term’ (7-day) for NO2 [mean: 27.7 μg/m3] and PM10 [mean: 24.6 μg/m3] | SBP and DBP at 10 years, measured using an automated device after 5 min rest, using a sized cuff, twice on the right arm 2-min apart while seated. | Study site, gender, age, BMI, physical activity, maternal smoking in pregnancy, parental education, parental HTN history, 7-day air pollutant, 7-day temperature. | No associations were observed for NO2, PM2.5 or PM10. Noise was significantly associated with increased DBP. |
| Clark et al. 2012 (27) | Cross-sectional RANCH |
UK | 719 (subset of 276 with NO2) | Traffic-related noise and NO2 at school locations near London Heathrow airport | SBP and DBP at 9–10 years using an automated device with sized cuffs, seated after 5 min of rest three measures on the right arm with 1–2 min intervals (reported in (36)). | Age, gender, employment status, crowding, home ownership, maternal education, chronic illness, language spoken, parental support, classroom window glaze, as well as preterm birth (<36 weeks), parental high BP, low birth weight (<2500 g), cuff size, temperature during testing, and BMI | Among children with air pollution data, no associations were observed for NO2 with SBP or DBP. No associations observed for road traffic noise or aircraft noise with SBP or DBP. |
| Trasande et al. 2015 (25) | Cross-sectional | Saudi Arabia | 184 (males only) | Ambient levels of TSP [mean 444.09 μg/m3] and 14 PAHs [summed mean 36.75 ng/m3] were monitored at 3 school locations at varying proximity to oil refinery. [concentration shown is for closest school] PAH metabolites were measured in urine (1-hydroxypyrene [median: 261.4 ng/g creatinine] and 1-hydroxyphenanthrene [549.6 ng/g creatinine]) |
SBP and DBP at 10–14 years using a standard cuff sphygmomanometer and DynaPulse instrument. BP Z-scores were calculated according to age, height and sex (4th report). PreHTN defined as z-score SBP or DBP >90th percentile. |
BMI z-score, SES, smokers in home (yes/no), use of incense, and age. | Proximity to oil refinery was associated with a 0.55 mmHg (95%CI: 0.26, 0.84) increase in z-score DBP and a 0.56 mmHg (95%CI: 0.16, 0.96) increase in SBP z-score. Significant associations were also observed with continuous SBP and DBP. A 4.35-fold odds of preHTN (95%CI: 1.42, 13.3) was associated with attending school closest the refinery compared to the background school. Urine biomarkers were not significantly associated with BP outcomes. |
| Baumgart-ner et al. 2012 (24) | Cross-sectional | China | 180 | Indoor 24.hr PM2.5 [range: 14–393 μg/m3] and BC [range: 2.0–9.6 μg/m3] measured with personal monitors in rural households (reported in (39)) | SBP and DBP at 5–14 years using an automated device. | Sex, age, height, BMI, passive smoking, SES, salt intake, MSG use, physical activity, day of week, time & day of BP measure | No significant associations observed for indoor PM2.5 or BC with SBP or DBP. |
| Sughis et al. 2012 (23) | Cross-sectional | Pakistan | 166 Categorized as low- (n=73) or high- (n=93) traffic areas | Indoor and outdoor PM10 [mean ‘low’ vs. high’ 223.0 vs. 728.6 μg/m3] and PM2.5 [28.5 vs. 183 μg/m3] measured with ambient monitors at school sites. Exposure categorized by site. | SBP and DBP at 8–12 years using an automated device after 5 min rest, single-sized cuff, seated, measured 5 times. PreHTN defined as 120–139 mmHg SBP and 80–89 mmHg DBP. |
Age, gender, height, weight, SES, passive smoke exposure, urinary Na, and creatinine. | Higher mean SBP (p<0.0001) and DBP (p<0.0038) observed in areas with high traffic pollution vs. low. Increased, and marginally significant odds ratio of elevated BP (2.56: 95%CI: 0.96, 6.78). |
| Pieters et al. 2015 (37) | Longitudinal HEAPS |
Belgium | 130 | Ambient monitors of nano-sized ultrafine particles (UFP) [range: 2,020–17,701 particles/cm3], PM2.5 [2–53 μg/m3], PM10 {5–64 μg/m3], and PMcoarse [1.34 μg/m3] in school playgrounds. PMcoarse defined as PM10 – PM2.5 fraction. |
SBP and DBP measured twice, ~6 months apart, at 6–12 years of age using an automated device following 5 min of rest, while seated, with a pediatric cuff, 5–7 measures. | Sex, age, height, weight, parental education, SES, fish consumption, heart rate, school, day of week, season, wind speed, humidity, and ambient temperature day of exam. | An IQR increase in nano-size UFP of 20–30, 30–50, and 70–100 nm was associated with 6.35 mmHg (95%CI: 1.56, 11.47), 1.18 mmHg (95%CI: 0.05, 2.31), and 0.86 mmHg (95%CI: 0.05, 1.68) increase in SBP, respectively. An IQR increase in total UFP (1,666/cm3) associated with a 0.79 mmHg (95%CI: 0.07, 1.51) increase in SBP. No associations with DBP. No association with PM measures. |
| Calderon et al. 2007 (38) | Cross-sectional | Mexico | 81 Categorized by residence in NE Mexico City (n=19), SW Mexico City (n=40), Polotitlán (n=22, ref.) | Ambient PM10, PM2.5, and O3 measured over 1-, 2-, and 7-day periods preceding blood draw exam (not BP exam). | Systolic pulmonary arterial pressure (PAP) at 6–13 years using Doppler echocardiography in supine left position, with caffeine avoidance for 24-hrs before exam. Mean pulmonary arterial pressure (MPAP) calculated as 0.61*SPAP + 2 mmHg (65). |
None. | Average MPAP was higher in both NE (p<0.01) and SW (p<0.05) Mexico City compared to Polotitlán. Among all children, increased plasma endothelin.1 levels were associated with higher MPAP (p<0.0001). An increase in 7-day mean PM2.5 was positively correlated with endothelin-1 levels (p=0.03). |
| Ianuzzi et al. 2010 (26) | Cross-sectional | Italy | 52 Categorized by distancetertiles as farthest (n=17), middle (n=17), or shortest (n=18) distance from major road. | Distance to major road as a proxy from PM10; ambient PM10 monitoring farthest (>1 km) from road [median: 10 μg/m3], middle distance (300m – 1km) [22 μg/m3], or closest (<300 m) to the trafficked roads [40 μg/m3] | SBP and DBP at 6–14 years of age was measured using a standard sphygmomanometer following 10 min rest, seated, with a sized cuff, three times with 1-min intervals, to the nearest 2 mmHg. Mean arterial pressure (MAP) calculated as DBP + 1/3 (SBP.DBP). Carotid arterial parameters measured using ultrasound. Stiffness was calculated as [(ln(SBP)-ln(DBP)]/(Systolic diam. – Diastolic diam.)/Diastolic Diam. |
Age, gender, and BMI. | No association observed with MAP. Mean carotid arterial stiffness was higher in both groups nearer to road vs. farthest (p<0.008). |
Represents the number of subjects included in analyses of exposure and BP if this was different from the overall study number and reported in the original article.
Abbreviations:
PIAMA: Prevention and Incidence of Asthma and Mite Allergy
SENCCS: Seven Northeastern Cities Chinese Children’s Study
GINIplus: German Infant Nutritional Intervention plus environmental and genetic influences on allergy development study
LISAplus: Lifestyle-Related factors on the Immune System and the Development of Allergies in Childhood plus the influence of traffic emissions and genetics
RANCH: Road Traffic and Aircraft Noise Exposure and Children’s Cognition and Health
MSG: monosodium glutamate
HEAPS: Health Effects of Air Pollution in Antwerp Schools
Among the 3 prospective cohort studies, all identified significant associations with exposure to air pollution (PM2.5 and other pollutants) and child BP. In a prospective study of 1,131 infants born in the US, higher mean PM2.5 and BC in the third trimester (90-days before birth) were associated with higher SBP in newborns ~30 hours after birth (28). Ozone (O3) was associated with lower SBP, and no significant associations were observed for NOx, NO2, or CO averages during the third trimester. The observed significant third trimester findings with PM2.5, BC, and O3 were bolstered by estimates of “moving averages” calculated from 2 to 7, 14, 30, 60, and 90 days before birth. No significant relationships were observed with 1st trimester pollutant averages; however, putative relationships in the second trimester were observed for NOx and CO with lower SBP. The study did not report associations with DBP. Two studies conducted in the PIAMA birth cohort in the Netherlands (29, 30) reported associations between PM2.5 and increased DBP at 12 years. In a subset of 471 children who did not move since birth, long-term NO2 and PM2.5 (reported as annual averages at participants’ home and school addresses estimated by land use regression) were associated with increased DBP (30). No associations were observed with short-term (7-day average preceding BP measure) exposure. In a concurrent study, the composition of PM2.5 and PM10 (estimated as annual averages at participants’ home addresses), reported that increased DBP was associated with levels of iron, silicon, and potassium in PM10, and showed marginal associations (p<0.1) with iron and silicon in PM2.5 (29). The authors attributed part of the observed effect to NO2 (a marker of traffic exhaust emissions), and noted that the possible association with iron suggested non-exhaust emissions were present.
Among the 10 cross-sectional studies, 8 reported significant relationships between exposure to air pollution and children’s BP. Three of these studies were based in a large cross-sectional study of 9,354 children ages 5–17 years old in China, the Seven Northeastern Cities Chinese Children’s Study (SNECCS) cohort (31–33). The studies assessed the effects of long-term (32, 33) or short-term exposure (31). Four-year average concentrations of PM10, SO2, NO2, O3, and CO were calculated from monitoring stations. One analysis reported the increased odds of childhood HTN with O3 and PM10. Increased DBP was associated with O3 PM10, and CO; increased SBP was associated with O3 and PM10 (32). These relationships were attenuated when stratified by children who were breastfed versus those who were not. Another analysis conducted in the SNECCS cohort reported the interaction effects of obesity and air pollutant exposure on BP outcomes, and found significant relationships between NO2, O3 and PM10 with the odds of HTN that were consistently larger effect sizes among overweight/obese children compared to normal weight children (33). The findings reported evidence of an interactive effect with child weight and exposure to PM10, SO2, and O3 for both SBP and DBP. A third study assessed short-term effects using 5-day mean exposures to PM10, SO2, NO2, and O3, and reported increased odds of elevated BP (defined as either SBP or DBP > 95th percentile) for both O3 and PM10 (31). SBP and DBP were positively associated with all four pollutants at various lag times (31). When stratified by sex the relationship between elevated BP and NO2 was observed only in males. Another large cross-sectional study of 2,368 German children at 10 years of age exposed to air and noise pollution due to traffic (34, 35) found no association between NO2, PM2.5, or PM10 and BP overall, however, when restricted to the 605 participants with noise information a significant relationship with NO2 and increased DBP was observed (34).
In a secondary analysis of a subsample of 276 children age 9- and 10-years in the UK attending school near a major airport, no association was observed between NO2 levels and child BP with or without adjustment for noise (27), and the study replicated previous findings that aircraft and road traffic noise were not associated with child BP (36). In a cross-sectional study of 184 adolescent males ages 10–14 in Saudi Arabia, increased SBP and DBP and odds of ‘preHTN’ were associated with attendance at schools located closest to an oil refinery, compared to a school location further from the refinery (25). Concentrations of PM10 and PAH metabolites were measured including total hydroxyphenanthrenes and 1-hydroxypyrene, however no relationships were identified. In a longitudinal study of 130 children in Belgium ages 6–12 years, increased total ultrafine particle fraction as well as fraction of nano-sized (20–30 nm) ultrafine particles was associated with increased SBP. No effects were identified for nano-sized fractions with diameter >100nm (PM0.1–2.5), nor PM2.5, PMcourse, or PM10 (37). In a cross-sectional study of 81 Mexican children 6–13 years of age, 7-day average PM2.5 and O3 were measured/estimated based on residence in Polotitlan (n=22, control), Northeast (n=19) or Southwest Mexico City (n=40). Doppler echocardiography was used to measure systolic pulmonary arterial pressure (PAP) and diastolic PAP, and calculate mean pulmonary arterial pressure (MPAP) (38). MPAP was increased in NE and SW Mexico City locations compared to Polotitlan, and the 7-day mean PM2.5 was associated with MPAP. The assessment of MPAP, rather than systemic BP, provides insight into potential pulmonary effects of PM2.5, but may be less informative for developmental renal effects.
In addition to two studies discussed above (25, 27), three of the cross-sectional studies of indoor air quality or pollution assessed by proxy measures (e.g. distance, location) reported no association between exposure to air pollution and children’s BP. A cross-sectional study of 240 Chinese study children 10 years of age, identified no association between 24-hour indoor PM2.5 or BC exposure (24), using previously reported assessment techniques (39). A cross-sectional study of 179 Pakistani children 8–12 years of age measured indoor and outdoor PM10 and PM2.5 at schools located in geographic regions with low (n=79) and high (n=93) air pollution (23). Although direct associations were not reported for indoor or outdoor PM measures, the authors observed higher SBP and DBP in areas with high pollution compared to controls (both indoor and outdoor levels were increased in ‘high’ pollution area). Finally, a cross-sectional analysis of 52 Italian children, which assessed distance to major road as a proxy for PM2.5 exposure, reported no significant association with MAP, or BP, however the authors could not adjust for covariates (26).
Epidemiologic evidence of association between metals and childhood BP
We identified 10 studies that examined the effects of selected nephrotoxic metals comprised of As (n=2), Pb (n=2) Cd (n=4), and Hg (n=3) on child BP (Table 2). One study examined both Cd and As and one study examined Cd and Pb.
Table 2.
Studies assessing exposure to nephrotoxic metals and children’s blood pressure outcomes (n=10).
| Authors & Year | Study design, name | Location | Sample sizea | Exposure Measure [Reported level] | BP Evaluation | Covariates | Major Finding |
|---|---|---|---|---|---|---|---|
| Hawkesworth et al. 2013 (40) | Prospective cohort MINIMat |
Bangladesh | 1887 | Maternal urinary As (8th [median: 83 μg/L] and 30th week [83 μg/L)) and urinary Cd (week 8 [0.63 μg/L] Child U-As at 18 mo [34 μg/L]. |
SBP and DBP at 4.5 years using an automated device, after 5 min rest, while seated, in triplicate with 1-min intervals. | Child’s sex, age, height, SES, season of birth, maternal early pregnancy BP. | Each 1 mg/L increase in pregnancy U-As was associated with 3.69 mmHg (95%CI: 0.74, 6.63) increase in SBP and 2.91 (95%CI: 0.41, 5.42) increase in DBP. Child U-As at 18 mo was associated with 8.25 mmHg (95%CI: 1.37, 15.1) increase SBP. No associations observed with U-Cd. |
| Skroder et al. 2015 (41) | Cross-sectional MINIMat |
Bangladesh | 1,356 | Children’s urine iAs [Mean: 54 μg/L, range: 16–343 μg/L] and urine Cd [Mean: 0.22 μg/L, range: 0.0078–0.63 μg/L] at ~5 years of age, collected ~8 mo after BP evaluation | SBP and DBP at 4.4–5.4 years using an automated device, after 5 min rest, while seated, in triplicate with 1-min intervals. HTN classified according to height and age 1996 report. | Sex, birth weight, season of birth, age, weight for age z-score, maternal early pregnancy BMI, parity and SES; additionally adjusted for arsenic, cadmium and selenium. Stratified by sex. | No observed significant associations. A moderate association was observed for each 0.5 ug/L increase in U-Cd and a 0.60 (95%CI: −0.16, 1.4) increase DBP (p=0.12). |
| Skroder et al. 2016 (43) | Prospective cohort MINIMat |
Bangladesh | 1,511 or 713 (with Pb data at 14 or 30 weeks) | Maternal blood erythrocyte Pb at gestational weeks 14 [median: 73 μg/kg, range: 1.6–368] and 30 [85 μg/kg; range: 19–370] | SBP and DBP at 4.5 years using an automated device, after 5 min rest, while seated, in triplicate with 2–3 min intervals. HTN classified according to height and age 1996 report. | Gender, birth weight, season of birth, age, height for age z-score, maternal early pregnancy BMI, parity, SES, and supplementation group. Stratified by sex. |
No observed associations. |
| Zhang et al. 2012 (42) | Prospective cohort ELEMENT |
Mexico | 457 | Maternal tibia Pb median: 9.3 μg/g] and patella Pb [11.6 μg/g] measured 1-mo postpartum, and cord blood Pb [mean: 5.51 μg/dL] | SBP and DBP at 10 years using a standard sphygmomanometer after 5 min rest, on the left arm, with a sized cuff. | Maternal education, smoke during pregnancy, caloric intake, calcium and iron intake, infant birth order, gestational age, weight at birth, and child age, height, and BMI. Stratified by sex. |
And IQR increase in maternal tibia Pb (13ug/g) was associated with 2.11 mmHg (95%CI: 0.69, 3.52) increased SBP and 1.60 mmHg (95%CI: 0.28, 2.91) DBP in girls, but not boys. No associations observed with patella or cord blood Pb. |
| Swaddiwudhipong et al. 2015 (46) | Cross-sectional | Thailand | 594 (301 vs. 293 Cd-exposed vs. comparison) | Urinary Cd [geometric mean: 0.57 ug/L vs. 0.39 μg/L comparison] | SBP and DBP measured twice on the right arm while seated. | Age, sex, blood lead | No association between continuous or tertiled Cd with BP. |
| Cao et al. 2009 (44) | Prospective cohort (Secondary analysis of RCT) TLC |
US | 441 (combined 223 placebo and 218 succimer groups) | Blood Cd at 2 years [Mean: 0.21 μg/L] | SBP and DBP measured at 2, 5 and 7 years using an automated device, while seated, measured up to three times. | None reported for the relationship with BP. | No association with SBP or DBP at 2, 5 or 7 years. |
| Kelishadi et al. 2013 (45) | Case-control CASPIAN-III |
Iran | 320 (160 with metabolic syndrome vs. 160 controls) | Blood Cd [Mean MetS: 10.09 ug/L; Mean Control: 9.97 ug//L] | SBP and DBP at 10–18 years measured using a protocol reported in (4th report 2004) | None | No significant association with DBP or SBP. |
| Kalish et al. 2014 (48) | Prospective Cohort Project Viva |
US | 1,103 | Second trimester blood erythrocyte Hg [mean: 4.0 ng/g] | SBP and DBP at early (median 3.2 yrs) and mid-childhood (median 7.7 yrs) using an automated device with a sized cuff, arm and position noted, up to 5 times in 1-min intervals | Measurement conditions (position, sleep/wake state, arm), child age, sex, BMI z-score, fetal growth z-score, maternal age, race/ethnicity, education, marital status, pre-pregnancy BMI, smoking status, and second trimester BP second trimester fish consumption | No association with SBP observed at age 3 or 7 years, comparing Q4 to Q1. |
| Thurston et al. 2007 (47) | Prospective cohort SCDS |
Seychelles | 644 and 559 (age 12 and 15) | Total Hg assessed in maternal hair during pregnancy [mean: ~6.6 ppm for male offspring, and 7.0 for girls] | SBP and DBP at 12 and 15 years using an automated device, with a sized cuff following a few min of rest, while seated, measured in duplicate. | Maternal HTN during pregnancy, child sex, birth weight, age, BMI, height. | A 1-ppm increase in prenatal meHg was associated with a 0.17 mmHg increase in DBP at age 15 years among boys (p<0.02), but not girls. No associations observed at age 12 years. |
| Valera et al. 2012 (49) | Prospective Cohort | Canada, Nunavik Inuit | 226 | Cord blood Hg [median: 81.5 nmol/L], blood at 11 years [14.5 nmol/L], and hair at 11 years [4.75 nmol/g] | SBP and DBP at 11 years using a mercury sphygmomanometer after 5 min rest, while seated, measured in triplicate (averaged last 2 measures). | Age, sex, birth weight, BMI, height, total cord n-3 fatty acids, PBC 153, cord blood lead, selenium and maternal smoking in pregnancy | No association observed with SBP or DBP for either blood Hg assessment time point or hair levels at age 11. |
Represents the number of subjects included in analyses of exposure and BP if this was different from the overall study number and reported in the original article.
Abbreviations:
MINIMat: Maternal and Infant Nutrition Interventions, Matlab
iAs: inorganic arsenic
ELEMENT: Early Life Exposures in Mexico to Environmental Toxicants
TLC: Treatment of Lead-Exposed Children
CASPIAN-III: Childhood and adolescence surveillance and prevention of adult non-communicable disease
SCDS: Seychelles Child Development Study
Of the 2 studies examining early life As exposure, one identified a significant relationship with childhood BP, for both prenatal and postnatal As exposure (40). In a prospective cohort study of 1,887 mother-baby pairs in Bangladesh, an increase in pregnancy urine-As (U-As) (average measure at week 8 and 30 of gestation) was associated with increased SBP and DBP at 4.5 years of age. Additionally, children’s U-As at 18 months was associated with an increase in SBP at 4.5 years. In a subsequent cross-sectional study of 1,356 children aged 4.5 years from the same population, no association was observed with U-As levels (reported as the sum of inorganic, methylarsonic acid, and dimethylarsonic metabolites) measured approximately 8 months following BP assessment (41). Although U-As typically reflects ongoing exposure, the study aimed to capture spot urine measures as a proxy for consistent As exposure through drinking water, as well as chronic Cd exposure (discussed below).
Of the 2 studies examining early life Pb exposure, both were conducted in prospective birth cohorts, however, only one identified a significant relationship with childhood BP (42). In a prospective birth cohort of 457 mother-child pairs in Mexico City, an increase in maternal tibia Pb was associated with increased SBP and DBP in girls, but not boys. No associations were observed with patella Pb or cord blood Pb levels. In a prospective cohort of 1,511 dyads in Bangladesh, no association was observed with SBP or DBP at 4.5 years with maternal blood Pb measured at 14 and 30 weeks gestation (43).
Among the 4 studies examining early life Cd exposure, none reported a significant relationship with childhood BP (41, 44–46). Among these studies, 2 measured urine Cd (U-Cd), in either maternal (41) or children’s samples (46), and two measured blood Cd in children’s samples (44, 45). Two studies were prospective (including a secondary analysis of a randomized control trial), and two were cross-sectional (including a case-control design measured at two time points). The prospective study of 1,356 Bangladeshi children reported no association with maternal U-Cd levels in pregnancy (41). In a prospective cohort (secondary analysis of randomized control trial data) of 223 control and 218 US children who received succimer treatment, blood Cd measured at 2 years of age was not associated with BP at 2, 5, or 7 years (44). A decrease in SBP was observed 1-week following succimer treatment, although the authors note the fall in BP may be attributable to reduction in Pb burden and there was no clear association with Cd and BP. In a cross-sectional study of 594 Thai school-age children randomly selected from 10 Cd-contaminated schools and 3 outside the contamination area, no significant association was found for U-Cd when analyzed as a continuous variable or categorized by tertile with SBP or DBP (46). Blood Pb was measured in this study but the relationship with BP was not reported. Lastly, in a case-control study of 320 children from Iran (160 normal weight, 160 cases with metabolic syndrome), blood Cd measured at 10 and 18 years showed a positive, but not significant association with DBP. The analysis did not include adjustment for covariates, and did not report the method of BP measurement. Notably, the Cd levels were relatively high among the control group (mean was 10.09 μg/L for cases and 9.97 μg/L for controls), and the analysis included linear regression across all cases and controls and reported null associations for the relationship between blood Cd and DBP or SBP.
We identified three studies examining early life Hg exposure, and only one reported an association with childhood BP. All 3 studies were prospective cohorts. A prospective cohort study of 645 and 561 children in the Seychelles that assessed maternal hair Hg and followed children to 12 and 15 years, respectively, reported that prenatal maternal Hg levels were associated with increased DBP among 15-year-old boys but not girls (47). No associations were observed among 12-year-old children. A large prospective cohort study of 1,103 mother-child pairs in the US, found no association with maternal blood erythrocyte Hg measured during the second trimester and SBP at 3.2 or 7.7 years (48). Another prospective cohort study of 226 Nunavik Inuit children, measured cord blood Hg, as well as blood and hair Hg at 11 years of age, and reported no association with Hg exposure at any time point and BP at 11 years (49). Notably, the studies above reported lower Hg levels than a 1999 study (outside the range of years reviewed herein) conducted of 917 children in the Faroe Islands (50). The study identified a significant relationship between cord blood Hg and SBP at 7 years, and we note that Hg levels were 2–3x higher in cord or maternal hair levels than those reported in (49) and (47), which may account for the lack of statistically significant findings in the later studies. Additionally, the selection of appropriate biomarker matrices for nephrotoxicity may have affected the results (i.e. hair vs. urine).
Epidemiologic evidence of association between phthalates and other organic compounds and childhood BP
We identified 12 studies that examined the effects of other potential environmental nephrotoxicants on child BP, comprised of phthalates (n=4), Bisphenol A (PBA) (n=3), polychlorinated biphenols (PCBs) (n=2), pesticides (n=4, two also measured PCBs) and flame retardants (n=1) (Table 3). The studies were either cross-sectional (n=6) or prospective (n=6) in design. Studies of phthalates, pesticides, and BPA used urinary levels of the parent compound or metabolites, whereas PCBs and flame retardants were measured in blood.
Table 3.
Studies assessing exposure to phthalates (n=4), bisphenol A (n=3), polycholinated byphenols (n=2), pesticides (n=3, one also measured PCBs), and flame retardants/perfluoroalkyl chemicals (n=1) and children’s blood pressure outcomes (n=12).
| Authors & Year | Study design, name | Location | Sample sizea | Exposure Measure [Reported level] | BP Evaluation | Covariates | Major Finding |
|---|---|---|---|---|---|---|---|
| Valvi et al. 2015 (51) | Prospective cohort INMA |
Spain | 379 | Maternal urine MBzP, MEHP, MEHHP, MEOHP, MECPP, MEP, MiBP, and MEP in the first and third trimester of pregnancy; creatinine-adjusted and converted to molar sums for: ΣDEHPm [median: 95.1 μg/g creatinine], ΣHMWPm [112 μg/g creatinine], and ΣLMWPm [472 μg/g creatinine] | SBP and DBP at age 4 and 7 using an automated device after 5 min rest, with a sized cuff, on the right arm, Age- and height-specific z-scores, stratified by sex. High BP defined as any BP z-score ≥90th percentile. |
Child sex and age, maternal country of origin, age at delivery, parity, education, social class, pre-pregnancy BMI, and smoking in pregnancy. | Across all ages, ΣHMWPm were associated with lower SBP z-scores in girls (adjusted β = –0.39; 95%CI: –0.65, –0.12 for the 2nd tertile and –0.28; –0.55, –0.01 for the 3rd tertile of exposure), but not boys. Associations with ΣHMWPm were driven by DEHP. ΣLMWPm were associated with lower SBP z-scores in girls (adjusted β = –0.23; 95% CI: –0.50, –0.04 in 2nd tertile and –0.40; –0.66, –0.12 in 3rd tertile of exposure), but not boys. Associations with ΣLMWPm were driven by MEP. No association observed with DBP z-scores. |
| Tran et al. 2017 (52) | Prospective cohort CHAMACOS |
US | 258 | Maternal urine assessed for 11 phthalate metabolites at ~13 and 26 weeks’ gestation (LMW: MEP, MBP, MiBP; ΣDEHP: MEHP, MEHHP, MEOHP, MECPP; HMW: MBzP, MCPP, MCOP, MCNP) and 8-isoprostane creatinine-adjusted. | SBP and DBP at age 5, 9, and 14 years using an automated device after rest. | BMI, sex, and creatinine | Prenatal MEOHP and MEHHP were associated with DBP at 14 years (betas not reported). Cross-sectionally, SBP and DBP were positively associated with 8-isoprostane at 14 years (SBP beta: 0.008 95%CI: 0.002, 0.015; DBP beta: 0.011 95%CI: 0.001, 0.02). Isoprostane at 14 years was positively associated with MEHP, MECPP and ΣDEHP at 12 weeks of gestation. The effect of 13 week MEOHP and MEHHP on 14-year DBP mediated by 14-year 8-isoprotane was not significant. |
| Trasande et al. 2013 (54) | Cross-sectional NHANES (2003–2008) |
US | 2,447 | Spot urine levels converted to molar sums of ΣLMWP (comprised of MEP, MBP, MiBP), ΣHMWP (comprised of MECPP, MEHHP, MEOHP, MEHP, MBzP), and ΣDEHP (comprised of MEHP, MECPP, MEHHP, MEOHP). | SBP and DBP 8–19 years using a standard sphygmomanometer, following 5 min rest, in triplicate. Age- and height z-scores calculated according to 4th report. PreHTN defined as ≥90th percentile age and height z-score stratified by sex. |
Urine creatinine, BMI, race/ethnicity, age, caregiver education, poverty-income ratio, sex, serum cotinine, caloric intake, and TV watching | Each log-unit (~3-fold) increase in ΣDEHP was associated with a 0.041 SD unit increase in SBP z-score (p=0.047). ΣDEHP relationships with SBP z-score driven by MEHP, MBP, MEHHP, and MEOHP (all p<0.05). MEP associated with increased odds of preHTN (OR: 1.20, p=0.04). No association observed with ΣLMWP or ΣHMWP. |
| Trasande et al. 2015 (53) | Cross-sectional NHANES (2009.2012) |
US | 1,329 | Urine phthalates converted to molar sums: ΣLMW, ΣHMW, ΣDEHP, (ΣDINP) and (ΣDIDP). | SBP and DBP at 8–19 years using a standard sphygmomanometer, following 5 min rest, in triplicate. Age- gender- and height-standardized z-scores calculated according to 4th report. PreHTN defined as ≥90th percentile age and height z-score stratified by sex. |
Urine creatinine, BMI, race/ethnicity, age, poverty-income ratio, gender, serum cotinine, caloric intake, and physical activity. | A 1-log unit increase in ΣHMW, ΣDINP, ΣDIDP, and ΣDEHP associated with ~0.10 SD increase in SBP z-score (p<0.05). No association observed with ΣLMWP. Associations with SBP z-score driven by MCOP, MNP, MCNP, MEHHP, MECPP (p<0.05). A 1-log unit increase in ΣDEHP associated with a 0.09 SD increase in DBP z-score (p=0.04). Associations with DBP z-score driven by MEHHP, MEOHP (p<0.05). |
| Bae et al. 2017 (55) | Prospective cohort EDC |
Korea | 486 | BPA after 8 hr fasting in maternal urine at 20 weeks gestation [geometric mean: 1.2 μg/g creatinine] and child urine at age 4 [geometric mean: 3.3 μg/g creatinine]. | DBP and SBP at 4 years using an automated device following 10 min rest, while seated, on the same arm, with a pediatric cuff, measured twice in 5 min interval. PP calculated as SBP-DBP. |
Age, sex, height, weight, gestational age, birth weight, parental history of HTN, paternal education, exposure to environmental tobacco, pulmonary/enteric infection, physical activity (min/week). Stratified by sex. | A 1-log unit of prenatal BPA was associated with 7.9 mmHg increase in DBP (p=0.0001), at BPA levels above an identified threshold level of 4.5 μg/g creatinine (n=41). A 1-log unit of prenatal BPA was associated with 8.0 mmHg decrease in PP (p=0.0015), at BPA levels above a threshold level of 5.0 μg/g creatinine (n=31). Among boys, similar relationships were observed. Among girls, significant U-shaped relationships were observed for SBP above and below a 0.7 μg/g creatinine threshold (p<0.05). A negative relationship below a 0.9 μg/g creatinine threshold with DBP (p=0.03). |
| Vafeiadi et al. 2016 (56) | Prospective cohort RHEA | Greece | 500 (4-year cross section) 235 (prenatal and 2.5 years) |
BPA in maternal urine in first of pregnancy trimester [geometric mean: 1.2 μg/g creatinine, n=235], and child urine at 2.5 [geometric mean: 5.1 μg/g creatinine n=235] and 4 years [geometric mean: 1.9 μg/g creatinine n=500] | SBP and DBP at 4 years using an automated device, with a sized cuff, following 5 min rest, while seated, measured 5 times in 1-min intervals. | Maternal education, age, pre-pregnancy BMI, employment status during pregnancy, child sex, z-score birth weight for gestational age, breastfeeding status, height z-score, time spent watching TV and energy intake at 4 years. | No significant associations observed with any exposure time point. |
| Khalil et al. 2014 (57) | Cross-sectional | US | 39 obese/overweight children | Urine BPA [median: 1.82 μg/g creatinine] | SBP and DBP at 3–8 years using an automated device with 2 cuff sizes. | Age, ethnicity | Among males (n=17), a 1-unit increase in log BPA was associated with 4.01 mmHg increase in DBP (p=0.01). Associations with SBP not reported. |
| Vafeiadi et al. 2016 (58) | Prospective Cohort Rhea |
Greece | 427 | POPs (PCBs, DDE [median: 1981.2 pg/mL], and HCB [median: 82.5 pg/mL]) in first trimester maternal serum ΣPCB [median: 319.4 pg/mL] comprised of congeners 118, 138, 153, 156, 170 and 180. |
SBP and DBP at 4 years using an automated device with a sized cuff, after 5 min rest, while seated, measured 5 times in 1-min intervals. | Maternal serum triglycerides and cholesterol, age, pre-pregnancy BMI, parity, education, smoking status during pregnancy, breastfeeding duration, child sex, age, birth weight and gestational age. | A 10-fold increase in HCB was associated with a 4.34 mmHg (95%CI: 0.63, 8.05) increase in SBP. A 10-fold increased in DDE was associated with higher DBP (1.79 mmHg 95%CI: 0.13, 3.46) ΣPCB was not associated with SBP or DBP. |
| Lee et al. 2016 (59) | Prospective Ewha Birth & Growth Cohort |
Korea | 158 | POPs [32 PCBs and 19 OCPs] in blood 7 PCBs (52, 101, 118, 138, 153, 156, and 180) and 5 OCPs (HCB, trans-Nonachlor, β-HCH, p,p′-DDT and p,p′-DDE) were considered “detectable” and analyzed individually. “Marker PCBs” category comprised of 6 congeners (28, 52, 101, 138, 153, 180) and ΣPCB was comprised of all 32 congeners. |
BP at 7–9 years and after 1-year follow-up using an automated device with a sized cuff, after rest, in a “stable” position, measured twice in 5-min intervals. MAP was calculated as [(SBP-DBP)/3]+DBP. |
Sex, age, household income, and change in BMI | PCB 138 and 153 were associated with an increase in relative change in 1-year DBP (3.01: 95%CI 0.20–5.82 and 2.33: 95%CI: 0.21, 4.45). ΣPCB and “marker PCB” were associated with an increase in relative change in 1-year DBP (4.84: 95%CI 1.11, 8.57 and 4.44 95%CI: 0.85, 8.03). No association with organochlorine pesticides associated with DBP. No associations observed with SBP. |
| Suarez. Lopez et al. 2013 (61) | Cross-sectional ESPINA |
Ecuador | 271 A subset of 138 reported cohabitation with a flower plantation worker. | Secondary pesticide exposure defined as living with a flower worker [mean no. of workers at home: 1.64], duration of cohabitation with a flower worker [mean: 5.3 years], and number of “bad practices” likely to result in home contamination with pesticides [mean: 2.9]. Erythrocyte AChE activity was measured by finger prick as a proxy for exposure to pesticides that inhibit cholinesterase activity [mean: 3.14 U/mL]. |
SBP and DBP at 4–9 years with a pediatric aneroid sphygmomanometer, following 3–5 min rest, while seated, both feet on floor, measured twice according to AHA (Pickering 2005). BP z-scores for age, sex and height for age for calculated according to Fourth Report (2005) |
Age, sex, race, height. for-age z-score, exam date, heart rate, number of smokers in home, residence to nearest flower plantation, pesticide use in household, and pesticide use by neighbors. | Each year longer duration of cohabitation with a flower worker and increase in number of bad practices were associated with a 0.32 mmHg (95%CI: −0.64, −0.02) and 0.82 mmHg (95%CI: −1.45, −0.20) decrease in SBP. Living with flower worker was associated with a 1.72 mmHg decrease in SBP (95%CI: −3.53, 0.08; p=0.06). A 1-U/mL decrease in AChE activity was associated with a 2.86 mmHg decrease in SBP (95%CI: −5.20, −0.53) and a 2.89 mmHg decrease in DBP (−5.00, −0.78). Similar results observed with BP z-scores. |
| Harari et al. 2010 (60) | Cross-sectional | Ecuador | 87 | Prenatal exposure assessed by interview of maternal/paternal occupational exposure. Children’s spot urine assessed for 6 organophosphate metabolites, individually and summed as Σdimethyl, Σdiethyl, or Σall molar concentrations. Erythrocyte AChE activity measured by finger prick. |
SBP and DBP at 6–8 years using a standard sphygmomanometer, while seated with a child-sized cuff, measured twice. | Age, sex, race, BMI, stunting, and cohort | Prenatal maternal occupational pesticide exposure was associated with a 3.6 mmHg (95%CI: −0.1, 7.2) increase in SBP (p ≤ 0.05) compared to those without maternal exposure. No associations observed with paternal occupational exposure or cross-sectional children’s exposure. |
| Geiger et al. 2014 (62) | Cross-sectional NHANES (1999–2000, 2003–2008) |
US | 1,655 | PFOS and PFOA in serum | SBP and DBP at 12–18 years using a standard sphygmomanometer, following 5 min rest while seated, measured 3 times. HTN defined as BP ≥ 95th percentile adjusted for age, height, and sex according to 1996 report. |
Age, sex, race/ethnicity, BMI, household income, physical activity, total cholesterol, and serum cotinine. | No association was observed for SBP or DBP. |
Represents the number of subjects included in analyses of exposure and BP if this was different from the overall study number and reported in the original article.
INMA: Infancia y Medio Ambiente (Environment and Childhood)
CHAMACOS: Center for Health Assessment of Mothers and Children of Salinas
EDC: Environment and Development of Children
ESPINA: Estudio de la Exposición Secundaria a Plaguicidas en Infantes, Niños y Adolescentes (Secondary Exposure to Pesticides among Infants, Children and Adolescents Study)
DINP: di-isononyl phthalate
DIDP: di-isodecyl phthalate
DDE: dichlorodiphenyl-dichloroethylene
HCB: hexachlorobenzene
NHANES: National Health and Nutrition Examination Survey
PFOA: perfluorooctanoic acid
PFOS: perfluorooctane sulfonate
Of the 4 studies examining early life phthalate exposure, all reported a significant relationship with subsequent childhood BP, for both prenatal and postnatal exposure. In a prospective study of 379 mother-child pairs in Spain, maternal phthalate exposure (averaged from first and third trimester urine measures, grouped by high or low molecular weight), showed significantly lower SBP z-score among girls comparing the 2nd and 3rd tertiles to the first tertile of summed molar concentrations of high molecular weight phthalates (HMWP) and comparing only the 3rd tertile to first for summed molar concentrations of low molecular weight phthalates (LMWP) (51). In another prospective cohort study of 258 children in the US, prenatal levels of mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) measured in maternal urine (at 12 and 20 weeks) were associated with DBP at 14 years of age, but not at 5 or 9 years of age (52). This study did not directly report estimates of the association between phthalates and SBP or DBP. Associations were also noted in two large cross-sectional studies in the US (53, 54). Among 2,447 adolescents participating in NHANES 2003–2008, grouped urine phthalates and metabolite molar sums (HMWP, LMWP, and diethylhexyl phthalate [DEHP]) were assessed in relation to SBP z-score and ‘preHTN’ (54). An increase in DEHP metabolites was associated with a significantly higher SBP z-score. Individual metabolites including MEHP, MBP, MEHHP, and MEOHP were associated with increased SBP z-score and MEP was associated with increased odds of preHTN. No association was observed with the LMWP or HMWP groups. In a subsequent study of 1,329 adolescents participating in NHANES 2009–2012, molar sums of HMWP and urinary DEHP metabolites as well as the parent compound for its replacements, di-isononyl phthalate (DINP) and di-isodecyl phthalate (DIDP), were each associated with increased SBP z-score (53). No associations were observed with the LMWP group. An increase in summed DEHP metabolites was also associated with increased DBP z-score. This study further identified the significant metabolites within each group and we refer the reader to Table 3 and the original article for details.
Among 3 studies examining early life BPA exposure, 2 were conducted in prospective cohorts and one was a cross-sectional study. Two reported significant associations with childhood BP. In a prospective cohort of 486 mother-child pairs in Korea, higher prenatal BPA exposure (measured at 20 weeks gestation) was associated with a significant increase in DBP at levels above an identified threshold of 4.5 μg BPA per g creatinine (55). Additionally, for prenatal BPA levels above 5.0 μg/g creatinine, a relationship with decreased pulse pressure was observed. Among girls, a U-shaped relationship with SBP was identified above and below the level of 0.7 μg BPA/g creatinine. However, in a prospective cohort study of 500 mother-child pairs in Greece, urine BPA (measured during the 1st trimester, as well as at 2.5 and 4 years) was not associated with child BP at 4 years (56). Lastly, a limited cross-sectional study of 39 obese or overweight children ages 3–8 years in the US, urinary BPA adjusted for creatinine was associated with elevated DBP among males, but not girls (57). No associations were observed with SBP.
Among 2 studies examining early life PCB exposure, (a persistent organic pollutant banned in the 1970s) one reported evidence of association with BP. In a prospective cohort study of 427 Greek children, the persistent organic pollutant hexachlorobenzene (HCB) was associated with higher SBP, and p,p′-DDE, a metabolite of DDT, was associated with higher DBP at 4 years of age (58). No association was observed for a group of summed PCBs comprised of six congeners. In a cross-sectional study of 158 Korean children aged 7–9 at baseline, the sum of 32 measured PCBs and two individual PCBs (congeners 138 and 153) were associated with significantly higher DBP at 1-year follow-up, after adjustment for sex, age, household income, and BMI (59). No associations were identified with SBP or DBP and 19 organochlorines measured.
Among 4 studies examining early life organochlorine compound exposure, three reported significant associations with BP. The prospective cohort study by Lee et al. (described above) reported no association with organocholorine pesticides (59), whereas the study by Vefaidi et al. (described above) (58), and two cross-sectional studies from the ESPINA cohort in Ecuador reported associations (60, 61). In a cross-sectional examination of 87 children, prenatal pesticide exposure assessed by parental occupation interview were marginally associated with higher SBP at 6–8 years among a subset of 69 (60); no relationships were observed with urine organophosphate metabolites. In a follow-up study of 271 children, duration of living with a flower worker as a proxy for exposure as well as number of “bad practices” likely to result in home contamination were significantly associated with lower SBP (61).
Finally, we identified a single study examining early life exposure to perfluoroalkyl flame retardants with childhood BP. A cross-sectional analysis of 1,655 children participating in NHANES in years 1999–2000 and 2003–2008, reported no association with serum PFOA or PFOS and continuous BP measure or HTN (62).
DISCUSSION
Summary and Recommendations for Future Directions
There is a growing body of literature supporting adverse cardiorenal effects of air pollution exposure. For metals, the literature is sparse in children, but suggests that As, Hg and Pb may affect childhood BP. Among the persistent organic pollutants there is limited but consistent evidence that phthalates and pesticide exposure may alter childhood BP. There is mixed evidence for BPA and PCB effects, and there is no current evidence to support an association between childhood PFC exposure and BP. There remain many gaps in our knowledge, including the importance of developmental susceptibility windows (i.e. the role of exposure timing in predicting effects), appropriate study design such as prospective studies that can avoid the issue of reverse causality (in which renal damage alters biomarker levels - a potential issue in cross-sectional studies), more standardized measurement and definition of BP outcomes, need for more complex statistical modeling approaches that factor in developmental trajectories or mixed exposure, and consideration of genetic susceptibility and epigenetic mediation as a way to define susceptible subpopulations and mechanisms of action. Future studies should also consider possible effects of emerging contaminants given that over 100,000 chemicals are registered for industrial use in the U.S. yet only a handful have been studied with regards to renal toxicity. Additional attention is also needed to examine potential maternal BP effects from exposures in pregnancy. Incorporating these considerations into new studies will enable the research community to address existing limitations and enhance our understanding of early life environmental exposures on BP. Given the heterogeneity of reported exposures and outcomes in the peer-reviewed studies summarized herein, we also recommend that exposure-specific systematic reviews and meta-analyses are needed in the advancing field of developmental exposures and childhood BP.
Conclusions
This review of the recent literature suggests that early life exposure to air pollutants, metals, and organic pollutants may contribute to changes in childhood BP. Limited evidence suggests that prenatal exposure may be more deleterious to BP than postnatal exposures and requires additional investigation. Because childhood BP tracks with adult BP and HTN (63, 64), it is imperative that hypertensive children are identified early for intervention and potential environmental, behavioral, and biological risk factors.
Acknowledgments
This work was supported in part by funding from the Children’s Research Foundation and NIH: K99ES027508, P30ES23515, P30DK079307 (The Pittsburgh Center for Kidney Research), U2CES026561 R01ES013744, R01ES14930, R01ES020268, and R01ES021357. The illustrations in Figure 1 were created by Jill Gregory, Department of Academic Medical Illustration, Icahn School of Medicine at Mount Sinai.
Footnotes
Competing Financial Interests:
There were no competing financial interests.
Category: Regular review
References
- 1.Yoon SS, Carroll MD, Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011-2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 2.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017 doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 3.Rao G. Diagnosis, Epidemiology, and Management of Hypertension in Children. Pediatrics. 2016;138 doi: 10.1542/peds.2015-3616. [DOI] [PubMed] [Google Scholar]
- 4.Rosner B, Cook NR, Daniels S, Falkner B. Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988–2008. Hypertension. 2013;62:247–254. doi: 10.1161/HYPERTENSIONAHA.111.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MH, Kang DR, Kim HC, Ahn SV, Khaw KT, Suh I. A 24-year follow-up study of blood pressure tracking from childhood to adulthood in Korea: the Kangwha Study. Yonsei Med J. 2014;55:360–366. doi: 10.3349/ymj.2014.55.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorey ES, Pantaleon M, Weir KA, Moritz KM. Adverse prenatal environment and kidney development: implications for programing of adult disease. Reproduction. 2014;147:R189–198. doi: 10.1530/REP-13-0478. [DOI] [PubMed] [Google Scholar]
- 7.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 8.Gidding SS, Barton BA, Dorgan JA, et al. Higher self-reported physical activity is associated with lower systolic blood pressure: the Dietary Intervention Study in Childhood (DISC) Pediatrics. 2006;118:2388–2393. doi: 10.1542/peds.2006-1785. [DOI] [PubMed] [Google Scholar]
- 9.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Zhang Z, Kuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611–619. doi: 10.1542/peds.2011-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiue I, Hristova K. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens Res. 2014;37:1075–1081. doi: 10.1038/hr.2014.121. [DOI] [PubMed] [Google Scholar]
- 12.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes–a global concern. Nat Rev Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 13.Benz K, Amann K. Maternal nutrition, low nephron number and arterial hypertension in later life. Biochim Biophys Acta. 2010;1802:1309–1317. doi: 10.1016/j.bbadis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Solhaug MJ, Bolger PM, Jose PA. The developing kidney and environmental toxins. Pediatrics. 2004;113:1084–1091. [PubMed] [Google Scholar]
- 15.Rubin MI, Bruck E, Rapoport M, Snively M, McKay H, Baumler A. Maturation of Renal Function in Childhood: Clearance Studies. J Clin Invest. 1949;28:1144–1162. doi: 10.1172/JCI102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun M, Li S, Sun D, et al. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: the Bogalusa Heart Study. J Hypertens. 2015;33:266–274. doi: 10.1097/HJH.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juonala M, Magnussen CG, Raitakari OT. Parental smoking produces long-term damage to vascular function in their children. Curr Opin Cardiol. 2013;28:569–574. doi: 10.1097/HCO.0b013e3283642882. [DOI] [PubMed] [Google Scholar]
- 19.Banderali G, Martelli A, Landi M, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med. 2015;13:327. doi: 10.1186/s12967-015-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng LY, Sanders AP, Saland JM, Wright RO, Arora M. Environmental exposures and pediatric kidney function and disease: A systematic review. Environ Res. 2017;158:625–648. doi: 10.1016/j.envres.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens. 2014;32:2130–2140. doi: 10.1097/HJH.0000000000000342. discussion 2141. [DOI] [PubMed] [Google Scholar]
- 22.Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. Air Pollution Exposure and Blood Pressure: An Updated Review of the Literature. Curr Pharm Des. 2016;22:28–51. doi: 10.2174/1381612822666151109111712. [DOI] [PubMed] [Google Scholar]
- 23.Sughis M, Nawrot TS, Ihsan-ul-Haque S, Amjad A, Nemery B. Blood pressure and particulate air pollution in schoolchildren of Lahore, Pakistan. BMC Public Health. 2012;12:378. doi: 10.1186/1471-2458-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgartner J, Zhang Y, Schauer JJ, Ezzati M, Patz JA, Bautista LE. Household air pollution and children’s blood pressure. Epidemiology. 2012;23:641–642. doi: 10.1097/EDE.0b013e3182593fa9. [DOI] [PubMed] [Google Scholar]
- 25.Trasande L, Urbina EM, Khoder M, et al. Polycyclic aromatic hydrocarbons, brachial artery distensibility and blood pressure among children residing near an oil refinery. Environ Res. 2015;136:133–140. doi: 10.1016/j.envres.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iannuzzi A, Verga MC, Renis M, et al. Air pollution and carotid arterial stiffness in children. Cardiol Young. 2010;20:186–190. doi: 10.1017/S1047951109992010. [DOI] [PubMed] [Google Scholar]
- 27.Clark C, Crombie R, Head J, van Kamp I, van Kempen E, Stansfeld SA. Does traffic-related air pollution explain associations of aircraft and road traffic noise exposure on children’s health and cognition? A secondary analysis of the United Kingdom sample from the RANCH project. Am J Epidemiol. 2012;176:327–337. doi: 10.1093/aje/kws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rossem L, Rifas-Shiman SL, Melly SJ, et al. Prenatal air pollution exposure and newborn blood pressure. Environ Health Perspect. 2015;123:353–359. doi: 10.1289/ehp.1307419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilenko N, Brunekreef B, Beelen R, et al. Associations between particulate matter composition and childhood blood pressure–The PIAMA study. Environ Int. 2015;84:1–6. doi: 10.1016/j.envint.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Bilenko N, van Rossem L, Brunekreef B, et al. Traffic-related air pollution and noise and children’s blood pressure: results from the PIAMA birth cohort study. Eur J Prev Cardiol. 2015;22:4–12. doi: 10.1177/2047487313505821. [DOI] [PubMed] [Google Scholar]
- 31.Zeng XW, Qian ZM, Vaughn MG, et al. Positive association between short-term ambient air pollution exposure and children blood pressure in China-Result from the Seven Northeast Cities (SNEC) study. Environ Pollut. 2017;224:698–705. doi: 10.1016/j.envpol.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 32.Dong GH, Qian ZM, Trevathan E, et al. Air pollution associated hypertension and increased blood pressure may be reduced by breastfeeding in Chinese children: the Seven Northeastern Cities Chinese Children’s Study. Int J Cardiol. 2014;176:956–961. doi: 10.1016/j.ijcard.2014.08.099. [DOI] [PubMed] [Google Scholar]
- 33.Dong GH, Wang J, Zeng XW, et al. Interactions Between Air Pollution and Obesity on Blood Pressure and Hypertension in Chinese Children. Epidemiology. 2015;26:740–747. doi: 10.1097/EDE.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Fuertes E, Tiesler CM, et al. The associations between traffic-related air pollution and noise with blood pressure in children: results from the GINIplus and LISAplus studies. Int J Hyg Environ Health. 2014;217:499–505. doi: 10.1016/j.ijheh.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Markevych I, Thiering E, Fuertes E, et al. A cross-sectional analysis of the effects of residential greenness on blood pressure in 10-year old children: results from the GINIplus and LISAplus studies. BMC Public Health. 2014;14:477. doi: 10.1186/1471-2458-14-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Kempen E, van Kamp I, Fischer P, et al. Noise exposure and children’s blood pressure and heart rate: the RANCH project. Occup Environ Med. 2006;63:632–639. doi: 10.1136/oem.2006.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pieters N, Koppen G, Van Poppel M, et al. Blood Pressure and Same-Day Exposure to Air Pollution at School: Associations with Nano-Sized to Coarse PM in Children. Environ Health Perspect. 2015;123:737–742. doi: 10.1289/ehp.1408121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calderon-Garciduenas L, Vincent R, Mora-Tiscareno A, et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect. 2007;115:1248–1253. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumgartner J, Schauer JJ, Ezzati M, et al. Patterns and predictors of personal exposure to indoor air pollution from biomass combustion among women and children in rural China. Indoor Air. 2011;21:479–488. doi: 10.1111/j.1600-0668.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- 40.Hawkesworth S, Wagatsuma Y, Kippler M, et al. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int J Epidemiol. 2013;42:176–185. doi: 10.1093/ije/dys215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skroder H, Hawkesworth S, Kippler M, et al. Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic–potential alleviation by selenium. Environ Res. 2015;140:205–213. doi: 10.1016/j.envres.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Zhang A, Hu H, Sanchez BN, et al. Association between prenatal lead exposure and blood pressure in children. Environ Health Perspect. 2012;120:445–450. doi: 10.1289/ehp.1103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skroder H, Hawkesworth S, Moore SE, Wagatsuma Y, Kippler M, Vahter M. Prenatal lead exposure and childhood blood pressure and kidney function. Environ Res. 2016;151:628–634. doi: 10.1016/j.envres.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Cao Y, Chen A, Radcliffe J, et al. Postnatal cadmium exposure, neurodevelopment, and blood pressure in children at 2, 5, and 7 years of age. Environ Health Perspect. 2009;117:1580–1586. doi: 10.1289/ehp.0900765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelishadi R, Askarieh A, Motlagh ME, et al. Association of blood cadmium level with cardiometabolic risk factors and liver enzymes in a nationally representative sample of adolescents: the CASPIAN-III study. J Environ Public Health. 2013;2013:1428–6. doi: 10.1155/2013/142856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaddiwudhipong W, Mahasakpan P, Jeekeeree W, et al. Renal and blood pressure effects from environmental cadmium exposure in Thai children. Environ Res. 2015;136:82–87. doi: 10.1016/j.envres.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Thurston SW, Bovet P, Myers GJ, et al. Does prenatal methylmercury exposure from fish consumption affect blood pressure in childhood? Neurotoxicology. 2007;28:924–930. doi: 10.1016/j.neuro.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalish BT, Rifas-Shiman SL, Wright RO, et al. Associations of prenatal maternal blood mercury concentrations with early and mid-childhood blood pressure: a prospective study. Environ Res. 2014;133:327–333. doi: 10.1016/j.envres.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valera B, Muckle G, Poirier P, Jacobson SW, Jacobson JL, Dewailly E. Cardiac autonomic activity and blood pressure among Inuit children exposed to mercury. Neurotoxicology. 2012;33:1067–1074. doi: 10.1016/j.neuro.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Sorensen N, Murata K, Budtz-Jorgensen E, Weihe P, Grandjean P. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology. 1999;10:370–375. [PubMed] [Google Scholar]
- 51.Valvi D, Casas M, Romaguera D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123:1022–1029. doi: 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran V, Tindula G, Huen K, et al. Prenatal phthalate exposure and 8-isoprostane among Mexican-American children with high prevalence of obesity. J Dev Orig Health Dis. 2017;8:196–205. doi: 10.1017/S2040174416000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trasande L, Attina TM. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension. 2015;66:301–308. doi: 10.1161/HYPERTENSIONAHA.115.05603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr. 2013;163:747–753 e741. doi: 10.1016/j.jpeds.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bae S, Lim YH, Lee YA, Shin CH, Oh SY, Hong YC. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension. 2017;69:367–374. doi: 10.1161/HYPERTENSIONAHA.116.08281. [DOI] [PubMed] [Google Scholar]
- 56.Vafeiadi M, Roumeliotaki T, Myridakis A, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. doi: 10.1016/j.envres.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Khalil N, Ebert JR, Wang L, et al. Bisphenol A and cardiometabolic risk factors in obese children. Sci Total Environ. 2014;470–471:726–732. doi: 10.1016/j.scitotenv.2013.09.088. [DOI] [PubMed] [Google Scholar]
- 58.Vafeiadi M, Georgiou V, Chalkiadaki G, et al. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother-Child Cohort (Crete, Greece) Environ Health Perspect. 2015;123:1015–1021. doi: 10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HA, Park SH, Hong YS, Ha EH, Park H. The Effect of Exposure to Persistent Organic Pollutants on Metabolic Health among KOREAN Children during a 1-Year Follow-up. Int J Environ Res Public Health. 2016;13 doi: 10.3390/ijerph13030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harari R, Julvez J, Murata K, et al. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect. 2010;118:890–896. doi: 10.1289/ehp.0901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suarez-Lopez JR, Jacobs DR, Jr, Himes JH, Alexander BH. Acetylcholinesterase activity, cohabitation with floricultural workers, and blood pressure in Ecuadorian children. Environ Health Perspect. 2013;121:619–624. doi: 10.1289/ehp.1205431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geiger SD, Xiao J, Shankar A. No association between perfluoroalkyl chemicals and hypertension in children. Integr Blood Press Control. 2014;7:1–7. doi: 10.2147/IBPC.S47660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toschke AM, Kohl L, Mansmann U, von Kries R. Meta-analysis of blood pressure tracking from childhood to adulthood and implications for the design of intervention trials. Acta Paediatr. 2010;99:24–29. doi: 10.1111/j.1651-2227.2009.01544.x. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. doi: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]


