Abstract
Objectives
To determine if infrapatellar fat pad (IPFP) signal intensity (SI) measures are predictive of incident radiographic osteoarthritis (iROA) over 4 years in the OA Initiative (OAI) study.
Methods
Case knees (n=355) defined by iROA were matched one-to-one by gender, age and radiographic status with control knees. T2-weighted MR images were assessed at P0 (the visit when iROA was found on radiograph), P-1 (1 year prior to P0) and baseline, and utilized to assess IPFP SI semi-automatically using MATLAB. Conditional logistic regression analyses were used to assess risk of iROA associated with IPFP SI alteration after adjustment for covariates.
Results
Participants were on average 60.2 years old, predominantly female (66.7%) and overweight (mean BMI: 28.3). Baseline IPFP measures including mean value and standard deviation of IPFP SI [Mean(IPFP), sDev(IPFP)] (HR, 95%CI: 5.2, 1.1 to 23.6 and 5.7, 2.2 to 14.5, respectively), mean value and standard deviation of IPFP high SI [Mean(H), sDev(H)] (HR, 95%CI: 3.3, 1.7 to 6.4 and 3.1, 1.3 to 7.7, respectively), median value and upper quartile value of IPFP high SI [Median(H), UQ(H)], and clustering effect of high SI [Clustering factor(H)] were associated with iROA during 4 years. All P-1 IPFP measures were associated with iROA after 12 months. P-0 IPFP SI measures were all associated with ROA.
Conclusions
The quantitative segmentation of high signal in IPFP is confirming previous work based on semiquantitative assessment suggesting its predictive validity. The IPFP high SI alteration could be an important imaging biomarker to predict the occurrence of radiographic OA.
INTRODUCTION
Osteoarthritis (OA) is a chronic disease characterised by articular cartilage loss and osteophyte formation, as well as abnormal changes in other structures within the joint, such as synovitis, menisci damage, ligament tears and infrapatellar fat pad (IPFP) alterations, eventually leading to joint failure and, in some cases, total knee replacement [1].
IPFP is a local fat pad situated inferior to the patella and filling the anterior knee compartment [2]. It performs a buffering and lubricating function in the knee joint, and is extensively vascularized and innervated [3]. It has close interaction with surrounding joint tissues. Sports and trauma can cause IPFP damage including edema, inflammation, synovial proliferation and fibrosis, which may induce pain and restriction of knee movement [4]. Based on the fact that the anatomical cleft within IPFP is lined with synovium [5], high signal intensity (SI) alterations observed on water-sensitive fat suppressed magnetic resonance imaging (MRI) are widely used as a surrogate for synovitis; however, it remains to be determined whether these signals represent inflammation or other pathological changes and whether they play a major role in the early stage of OA [2].
Some MRI studies have reported an association between synovitis measured using IPFP SI alteration and knee pain or cartilage loss in OA patients [6, 7]. Han et al [8] reported that high SI of the IPFP was associated with knee pain, joint structural changes, and knee radiographic OA (ROA) in older adults, suggesting it may serve as an important imaging biomarker in knee OA. A nested case-control study reported that Hoffa-synovitis, where the IPFP SI alteration was assessed on a manual semi-quantitative scale from zero to three [9], was strongly associated with the development of incident ROA (iROA) [10]. However, the reproducibility of this method is not high [9]. It also cannot detect heterogeneity of the signal which might be indicative of ongoing biomechanical perturbation of the region. There is a need for a reliable and valid method to quantify IPFP SI quantitatively. Recently, we developed a semi-automatic, quantitative method to measure SI changes of IPFP. This method is reproducible and has concurrent and clinical construct validity [11], but its predictive validity needs to be examined.
The current nested case-control study is nested within the Osteoarthritis Initiative (OAI) study, which includes data on those who have or those who are at high risk for developing symptomatic knee OA. The aim of this study was to investigate if SI alteration within the IPFP predicts iROA over 4 years, in which IPFP SI was measured using our novel, semi-automatic, quantitative method.
METHODS
Study design and subjects
Participants were selected from the OAI study, which is a multi-center, longitudinal, prospective observational study focusing primarily on knee OA. This study enrolled 4796 participants (aged 45-79 years old) from February 2004 to May 2006, and followed them up for four years. The follow-up included annual clinical assessments and radiological (x-ray and magnetic resonance) images. Our data was from the incidence subcohort, in which participants had characteristics that placed them at increased risk for developing symptomatic knee OA.
Demographic information (age, gender and ethnicity) had been recorded at the first visit. Height and weight were measured twice in light clothing without shoes. BMI (weight/height2, kg/m2) was calculated at the same visit. Inclusion criteria were frequent knee symptoms and frequent medications for knee symptoms. Other screening risk factors were weight, history of knee injury and surgery, bony enlargement of fingers, frequent knee bending, and total knee replacement (TKR) in parent or sibling. Exclusion criteria were bilateral TKR, plans to have bilateral TKR, rheumatoid and inflammatory arthritis, contraindications to 3.0 Tesla MRI, non-ambulatory status, serious comorbid conditions likely to interfere with participation, or plans to relocate and clinical trial participation. Signed consent forms were obtained from all participants.
Cases and controls
Case knees (n=355) were defined by iROA [Kellgren Lawrence grading (KLG)≥2] on the knee radiographs at any assessment after baseline (BL) but prior to the 48-month visit. This sample is all such case knees with available images except for knees that developed ROA by the first follow-up visit (12 months) and were KL = 1 at baseline and KL = 2+ in the contralateral knee. Two knees of a participant could be included if both developed ROA. They were matched one-to-one by gender, age (± 5 years) and radiographic status (KL = 0 or 1 in the index knee, KL = 0 or 1 or 2+ in the contralateral knee) with a control knee. Control knees did not develop incident ROA from BL to 48 months.
Knee injury and surgery history were ascertained by self-report at the enrollment visit (OAI study protocol). Knee injury was defined as a history of injury causing difficulty of walking for at least a week, and surgery was defined as history of any knee surgery such as meniscal and ligamentous repairs. Repetitive knee bending activity was assessed by a questionnaire which including climbing up a total of 10 or more flights of stairs, kneeling for 30 minutes or more, squatting or deep knee bending for 30 minutes or more, moving a heavy (25 pounds or more) object or going into/out of a squat more than 10 times. A 0-5 scale point was used to measure the sum of different activities.
Radiographs
The fixed flexion radiograph was taken in both knees of all participants at baseline and all annual follow-up visits. All participants had bilateral, standing knee films obtained in posteroanterior projection with knees flexed to 20-30 degrees and feet internally rotated 10 degrees. Knee radiographs were read by central readers using standard protocols including KLG and OARSI joint space narrowing grades. ROA was defined as KLG of ≥ 2 [12].
Measurements of SI in IPFP
MR images were assessed at P0 (visit when ROA was found on radiograph), 1 year prior to P0 (P-1), and at OAI BL. Sagittal planes of intermediate weighted images with turbo spin-echo obtained on 3.0-T MRI were utilized to assess IPFP SI semi-automatically using MATLAB (MATLAB X.Y, The MathWorks Inc., Natick, MA, 2000) [11]. The reader manually created an initial lasso around the IPFP by a set of points in sequence near the outer contour of IPFP and it contracted inward to the actual edge of the IPFP automatically (Figure 1a). This new algorithm was easy to distinguish fake edges from real edges and more accurate to identify the IPFP boundary. The high SI regions in IPFP were also obtained subsequently based on the algorithm [11]. It examined the neighbouring pixels of initial seed points to determine whether the pixel neighbours should be added to the area of high intensity signal (Figure 1b, c).
Figure 1. The segmentation of IPFP and high signal intensity measurements on sagittal T2 images using MATLAB.
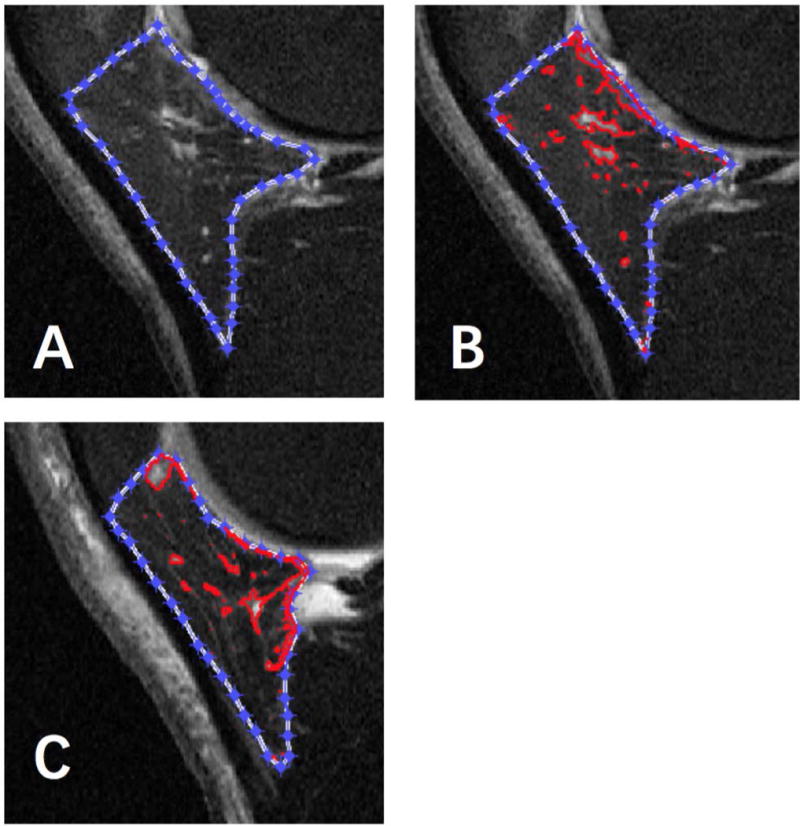
(A) The outer contour of IPFP was contracted inward by software. (B) The high signal intensity region was selected automatically by red circle. (C) The clustering effect of high signal intensity regions on this image was different from image b, which had a lower clustering factor(H).
The algorithm automatically calculated the SI of the IPFP. Measures of IPFP SI included mean value [Mean(IPFP)] and standard deviation [sDev(IPFP)] of IPFP SI, mean value [Mean(H)] and standard deviation [sDev(H)] of IPFP high SI, median value [Median(H)] and upper quartile value [UQ(H)] of high SI, volume of high SI regions of IPFP [Volume(H)] and the ratio of Volume(H) to volume of whole IPFP [Percentage(H)], and Clustering factor(H) representing clustering effect of high SI.
The sDev(IPFP) was introduced to represent SI variation of whole IPFP. The UQ(H) was used to represent the highest quartile of the signal. The Upper Quartile Value means that the highest quartile cut-point value of the signal. The Volume(IPFP) and Volume(H) were calculated according to the slice thickness and the area on each slice, and the Percentage(H) was used to represent the adjusted quantity of these regions. The clustering regions with high SI in IPFP differed in different patients, which may have different clinical significance. Clustering factor(H) was therefore introduced to represent this clustering effect. The greater the clustering effects, the higher aggregation of the high SI even if they had the same volume of high SI [11].
These SI measures were selected to represent IPFP SI heterogeneity, extent and clustering effect based on the the concurrent validity and the clinical construct validity we previously reported [11]. (Figure 1b, 1c). The ICCs and inter-observer correlation coefficients for all measures are high (>0.90) [11]. Significant correlations were found between the semi-quantitative score and quantitative measures. The Pearson correlation coefficient was list as follows: r =0.30 for Mean (IPFP), r = 0.74 for sDev (IPFP), r =0.58 for Median (H), r = 0.60 for UQ (H), r= 0.19 for Volume (H), r = 0.37 for Percentage (H) and r = 0.49 for Clustering factor (H); all P < 0.001 [11].
Statistical analysis
T, Chi-Squared and Fisher’s Exact tests were used to test the difference between case and control groups. Conditional logistic regression accounting for the correlation of knees in an individual was applied to assess risk of ROA in regard to SI alteration before and after adjustment for covariates measured at baseline. These covariates were self-reported knee injury, self-reported knee surgery, BMI (normal, overweight, obese), and the number of knee bending activities (none, 1-3, 4-5). We rescaled the values of the IPFP measurements by dividing them by 3, 4 or 10 when performing the analyses, in order to make the hazard ratios at the same order of magnitude. Models were run at three time points: baseline, P-1 (one year prior to the iROA) and P0 (concurrent with iROA). Analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC, U.S.A.)
RESULTS
One knee lacked readable MRI data and was removed along with its matched knee, leaving 708 knees. Participants (n = 677) were on average 60.2 years old (SD: 8.6), predominantly female (66.7%), and overweight (mean BMI 28.3, SD: 4.5). Characteristics of the participants are summarized in Table 1. The case and control groups were comparable with respect to age, sex, height and knee bending activities but participants in the case group had higher levels of weight and BMI, and greater proportions of those in the overweight and obese categories. Case knees had higher percentages of baseline knee injury (38.4% vs 19.8%) and knee surgery (15.3% vs 6.8%) than the control knees. The case-defining visit of iROA was 12 months for 119 (33.6%), 24 months for 82 (23.2%), 36 months for 103 (29.1%), and 48 months for 50 (14.1%) knees.
Table 1.
Baseline characteristics of the cases and controls in the study group
| Per Person
|
|||||||
|---|---|---|---|---|---|---|---|
| All (n=677) | Cases (n=322) | Controls (n=355) | p value | ||||
|
| |||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (Years) | 60.2 | (8.6) | 60.3 | (8.7) | 60.1 | (8.4) | 0.8061 |
| BMI | 28.3 | (4.5) | 28.9 | (4.5) | 27.7 | (4.4) | 0.0006 |
| Height (mm) | 1670.0 | (87.9) | 1671.7 | (91.0) | 1668.3 | (85.0) | 0.6114 |
| Weight (kg) | 79.1 | (15.2) | 80.8 | (15.0) | 77.5 | (15.3) | 0.0053 |
| BMI (categorical) | N | % | N | % | N | % | |
| Normal | 167 | (25.0) | 62 | (19.3) | 105 | (30.4) | 0.0031 |
| Overweight | 267 | (40.0) | 135 | (41.9) | 132 | (38.3) | |
| Obese | 233 | (34.9) | 125 | (38.8) | 108 | (31.3) | |
| Sex | N | % | N | % | N | % | |
| 1: Male | 222 | (33.3) | 109 | (33.9) | 113 | (32.8) | 0.8053 |
| 2: Female | 445 | (66.7) | 213 | (66.1) | 232 | (67.2) | |
| # of Knee bending activities (past 30 days) | |||||||
| N | % | N | % | N | % | ||
| None | 63 | (9.5) | 26 | (8.1) | 37 | (10.7) | 0.0631 |
| 1, 2 or 3 | 497 | (74.5) | 234 | (72.7) | 263 | (76.2) | |
| 4 or 5 | 107 | (16.0) | 62 | (19.3) | 45 | (13.0) | |
| Per Knee
|
|||||||
|---|---|---|---|---|---|---|---|
| All (n=708) | Cases (n=354) | Controls (n=354) | p value | ||||
|
| |||||||
| class | N | % | N | % | N | % | |
| 1 (KL 0/0) | 126 | (17.8) | 63 | (17.8) | 63 | (17.8) | 1.0000 |
| 2 (KL 0/1) | 152 | (21.5) | 76 | (21.5) | 76 | (21.5) | |
| 3 (KL 1/1) | 166 | (23.4) | 83 | (23.4) | 83 | (23.4) | |
| 4 (KL 0/2+) | 118 | (16.7) | 59 | (16.7) | 59 | (16.7) | |
| 5 (KL 1/2+) | 146 | (20.6) | 73 | (20.6) | 73 | (20.6) | |
| Baseline knee injury | 206 | (29.1) | 136 | (38.4) | 70 | (19.8) | < 0.0001 |
| Baseline knee surgery | 78 | (11.0) | 54 | (15.3) | 24 | (6.8) | 0.0004 |
Test of group differences by t-test, Chi-Squared, and Fisher’s Exact. BMI, body mass index. KL, Kellgren-Lawrence.
The case group had higher levels of sDev(IPFP), Percentage(H), UQ(H) and Clustering factor(H) than the control group at baseline, 1-year prior ROA and the same time of ROA (Figure 2). The differences between these two groups were all significant except for baseline Percentage(H) (p=0.06).
Figure 2. Comparison of major IPFP SI measures between case and control groups.
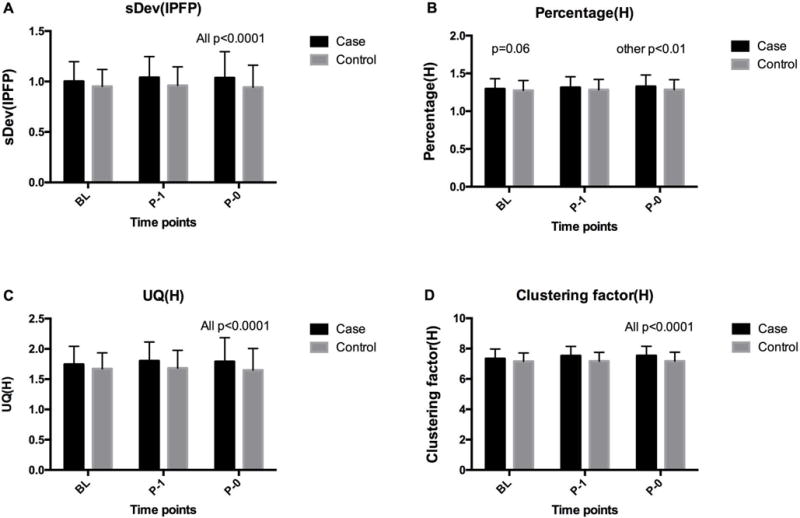
(A) sDev (IPFP), (B) Percentage (H), (C) UQ (H), (D) Clustering factor (H). P0: the visit when iROA was found on radiograph; P-1: 1 year prior to P0; BL: baseline.
Associations between IPFP SI measures at baseline and incident ROA are shown in Table 2. In unadjusted analyses, baseline IPFP measures including sDev(IPFP), Mean (H), sDev(H), Median(H), UQ(H), and Clustering factor(H) were significantly associated with increased iROA during 4 years and these associations remained unchanged after adjustment for BMI, number of knee bending activities, self-reported injury and self-reported knee surgery [HR (95% CI): 5.2 (1.1 to 23.6), 5.7 (2.2 to 14.5), 3.3 (1.7 to 6.4), 3.1 (1.3 to 7.7), 3.2 (1.6 to 6.2), 2.9 (1.6 to 5.2), 1.6 (1.2 to 2.1), respectively]. Baseline Mean(IPFP) was not significantly associated with iROA in univariable analysis, but this association became significant after adjustment for the above covariates. In contrast, baseline Percentage(H) was not significantly associated iROA in both univariable and multivariable analyses. The risk of incidence of ROA of case knees were 1.6 to 5.2 higher than control knees regarding to different IPFP measurements.
Table 2.
Associations between IPFP signal intensity measures at baseline and incident radiographic OA
| Univariable | Multivariable* | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Mean (IPFP) | 3.8 (0.9, 16.4) | 5.2 (1.1, 23.6) |
| sDev (IPFP) | 5.2 (2.1, 12.9) | 5.7 (2.2, 14.5) |
| Mean (H) | 2.9 (1.5, 5.6) | 3.3 (1.7, 6.4) |
| sDev (H) | 2.6 (1.1, 6.3) | 3.1 (1.3, 7.7) |
| Median (H) | 2.9 (1.5, 5.5) | 3.2 (1.6, 6.2) |
| UQ (H) | 2.7 (1.5, 4.7) | 2.9 (1.6, 5.2) |
| Percentage (H) | 2.8 (1.0, 8.4) | 2.7 (0.9, 8.2) |
| Clustering factor (H) | 1.7 (1.3, 2.1) | 1.6 (1.2, 2.1) |
N=708.
Adjustment for BMI, number of knee bending activities, self-reported injury and self-reported knee surgery. IPFP: infrapatellar fat pad; HR: hazard ratio; Mean (IPFP), mean value of IPFP intensity; sDev (IPFP), standard deviation of IPFP signal intensity; Mean (H), mean value of IPFP high intensity; sDev (H), standard deviation of IPFP high signal intensity; Median (H), median value of high signal intensity region; UQ(H), upper quartile value of high signal intensity region; Percentage (H): ratio of volume of high signal intensity region/whole IPFP volume; Clustering factor(H): clustering factor of high signal intensity. Significant differences at p<0.05 are shown in bold.
Associations between IPFP SI measures 1-year prior and iROA are shown in Table 3. All P-1 IPFP measures were significantly and positively associated with iROA after 12 months before and after adjustment for BMI, number of knee bending activities, self-reported injury, and self-reported knee surgery (HR (95% CI): 12.6 (2.8 to 57.2); 8.1 (3.2 to 20.4); 5.1 (2.6 to 9.9); 2.8 (1.1 to 6.7); 4.8 (2.5 to 9.2); 4.0 (2.2 to 7.2); 5.0 (1.6 to 15.7); 2.7 (2.0 to 3.7)). The hazard ratios were larger than those at baseline.
Table 3.
Associations between IPFP signal intensity measures 1 year prior and incident radiographic OA
| Univariable | Multivariable* | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Mean (IPFP) | 9.4 (2.4, 40.5) | 12.6 (2.8, 57.2) |
| sDev (IPFP) | 8.5 (3.4, 21.1) | 8.1 (3.2, 20.4) |
| Mean (H) | 5.1 (2.6, 9.8) | 5.1 (2.6, 9.9) |
| sDev (H) | 2.8 (1.2, 6.8) | 2.8 (1.1, 6.7) |
| Median (H) | 4.8 (2.5, 9.2) | 4.8 (2.5, 9.2) |
| UQ (H) | 4.0 (2.3, 7.2) | 4.0 (2.2, 7.2) |
| Percentage (H) | 4.8 (1.5, 15.3) | 5.0 (1.6, 15.7) |
| Clustering factor (H) | 2.7 (2.0, 3.6) | 2.7 (2.0, 3.7) |
N=658.
Adjustment for BMI, knee bending activities, self-reported injury and self-reported knee surgery. IPFP: infrapatellar fat pad; HR: hazard ratio; Mean (IPFP), mean value of IPFP intensity; sDev (IPFP), standard deviation of IPFP signal intensity; Mean (H), mean value of IPFP high intensity; sDev (H), standard deviation of IPFP high signal intensity; Median (H), median value of high signal intensity region; UQ(H), upper quartile value of high signal intensity region; Percentage (H): ratio of volume of high signal intensity region/whole IPFP volume; Clustering factor(H): clustering factor of high signal intensity. Significant differences at p<0.05 are shown in bold.
Associations between IPFP SI measures and ROA assessed at the same time are shown in Table 4. Similar to the P-1 IPFP measures, all P-0 IPFP measures were significantly and positively associated with concurrent ROA in unadjusted analyses and after adjustment for the covariates (HR (95% CI), 8.8 (2.0, 39.0); 5.7 (2.5, 12.9); 3.2 (1.7, 6.0); 2.8 (1.3, 6.4); 3.5 (1.9, 6.6); 3.0 (1.7, 5.3); 9.7 (2.9, 32.5); 2.6 (1.9, 3.5)).
Table 4.
Associations between IPFP signal intensity measures at the same time and incident radiographic OA
| Univariable | Multivariable* | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Mean (IPFP) | 6.4 (1.5, 26.6) | 8.8 (2.0, 39.0) |
| sDev (IPFP) | 5.5 (2.4, 12.4) | 5.7 (2.5, 12.9) |
| Mean (H) | 3.0 (1.6, 5.7) | 3.2 (1.7, 6.0) |
| sDev (H) | 2.8 (1.2, 6.6) | 2.8 (1.3, 6.4) |
| Median (H) | 3.2 (1.7, 6.1) | 3.5 (1.9, 6.6) |
| UQ (H) | 2.9 (1.6, 5.1) | 3.0 (1.7, 5.3) |
| Percentage (H) | 9.8 (3.0, 31.4) | 9.7 (2.9, 32.5) |
| Clustering factor (H) | 2.7 (2.0, 3.6) | 2.6 (1.9, 3.5) |
N=666.
Adjustment for BMI, physical activities, self-reported injury and self-reported knee surgery. IPFP: infrapatellar fat pad; HR: hazard ratio; Mean (IPFP), mean value of IPFP intensity; sDev (IPFP), standard deviation of IPFP signal intensity; Mean (H), mean value of IPFP high intensity; sDev (H), standard deviation of IPFP high signal intensity; Median (H), median value of high signal intensity region; UQ(H), upper quartile value of high signal intensity region; Percentage (H): ratio of volume of high signal intensity region/whole IPFP volume; Clustering factor(H): clustering factor of high signal intensity. Significant differences at p<0.05 are shown in bold.
DISCUSSION
To the best of our knowledge, this is the first study to demonstrate that IPFP SI alterations are associated with iROA using a novel method to measure IPFP SI alterations quantitatively. We found that except for Percentage(H), the baseline IPFP SI measures were all significantly associated with iROA during 4 years. All IPFP measures at P-1 predicted iROA after 12 months and at P-0 were associated with concurrent iROA. These findings suggest that our quantitative measurements of IPFP SI alterations have predictive validities. IPFP SI alterations, which can be regarded as an important imaging marker similarly as bone marrow lesions, cartilage defects, mensical tears and effusion-synovitis, may play a role in the pathogenesis of early OA.
Usually IPFP SI alteration was assessed semi-quantitatively (0-3), with a grade of ≥1 termed as Hoffa synovitis [9, 13], even though IPFP SI alteration may also represent other pathological changes such as vascular neoformation, oedema, or fibrosis [4]. The roles of IPFP SI alteration or Hoffa synovitis in knee OA remain unclear. A 2.6-year longitudinal study reported that baseline IPFP SI was positively associated with knee pain when going up/down stairs, cartilage defects and bone marrow lesions, while negatively associated with lateral tibial cartilage volume in older adults [8]. A case-control study using a semi-quantitative method (grade 0-3) found that the baseline Hoffa-synovitis was associated with incident ROA over 4 years [10]. The results from Multicenter Osteoarthritis Study (MOST) showed that Hoffa’s synovitis was an independent cause of incident knee OA during 84 months follow-up [14]. In contrast, a 30-month follow-up study reported that Hoffa synovitis did not predict cartilage loss in participants at high risk of knee OA [15]. Similar results were found in symptomatic knee OA patients, in which IPFP SI changes were not associated with cartilage loss at 15-30 month follow-up, but significantly associated with pain change assessed by visual analogue scale (VAS) [6]. Although these findings were not all consistent, they suggest that IPFP SI alteration was potentially a biomarker of knee OA development; however, the semi-quantitative assessment was insensitive for change which would not be an ideal outcome measure for interventions.
Other studies have focused on the special region of IPFP, the superolateral Hoffa fat pad (SHFP), based on the hypothesis that SHFP edema (grade 0-3) was caused by friction between the patellar tendon and the lateral femoral condyle and by patellofemoral joint malalignment [16,17]. Results based on the MOST study were shown that SHFP hyperintensity were siganificantly associated with cartilage damage and BMLs in the lateral PFJ, and the worsening BMLs in the medial PFJ. It may be a local marker of PFJ structural damage. Edema in superolateral Hoffa’s fat pad may be an important indicator of underlying patellofemoral maltracking or impingement in younger, symptomatic patients [18]. Our current study designed to include older rather younger adults, so we focused on SI in whole rather than regional IPFP.
Our newly-developed quantitative method has concurrent and clinical construct validity to measure IPFP SI alterations, when compared with the semi-quantitative method and examing the associations with joint structural outcomes, respectively [11]. This measurement was also reproducible with high ICCs. However, this study was preliminary and was limited by a small sample size and the cross-sectional design, and significant associations between IPFP measures and structural changes in knee OA patients were not all consistent [11]. Furthermore, the predictive validity of this new method was not reported. We presented IPFP SI measures which represent aspects of SI but they are highly correlated. Future work would explore if a composite score of these measures can be established for a valid IPFP assessment in OA studies.
In this study, we found that all IPFP measures assessed at the time of incidence were significantly associated with iROA, further confirming clinical construct validity of this method. Furthermore, we found that IPFP measures assessed 12 months prior to iROA were all significantly associated with iROA, and measures assessed at baseline were mostly [except for Percentage (H)] associated with iROA, suggesting the predictive validity of this quantitative measure. The more heterogeneous SI of whole IPFP, higher SI quantity and more clustering of high SI, the more likely they are to predict incident OA. The associations for baseline IPFP measures were not as strong as those for P-1 IPFP measures, indicating that IPFP SI measures would be more strongly associated with iROA in a fixed, shorter time interval (1 year) than in variable time intervals (1-4 years).
The underlying mechanisms for the association between IPFP SI alteration and iROA remain to be elucidated. IPFP SI alteration can be a sign of synovial inflammation [6, 20]. Synovial tissues from early OA patients were characterized by increased mononuclear cell infiltration and over-production of proinflammatory cytokines [21]. These cytokines diffused into cartilage through the synovial fluid and influenced cartilage metabolism by producing proteases and other catabolic factors such as nitric oxide (NO), causing other structural changes associated with the disease process [22] and thereafter ROA.
IPFP has been identified as a potential source of cytokines and adipokines [23-25]. In vitro studies have demonstrated that IPFP has an anabolic phenotype and the factors secreted by IPFP can influence cartilage metabolism and mesenchymal stem cells (MSCs)-derived cartilage repair [4, 26, 27]. IPFP is also enriched with immune cells towards a proinflammatory phenotype [25, 27], which can be influenced by the proinflammatory environment in the joint [23]. Activated immune cells (e.g., macrophages) produced various growth factors, cytokines and enzymes that enhanced osteophyte formation, mitigated cartilage breakdown by MMP activity, induced joint effusion by vasodilation and might influence subchondral bone metabolism [4]. We recently reported that serum IL-17 level was positively, and serum adiponectin was negatively, associated with IPFP SI alteration in knee OA patients [28], suggesting that association between IPFP SI alteration and iROA may be through dysregulated cytokines and adipokines.
Our study was unique as we looked at multiple time points prior to the diagnosis of ROA in a well-designed nested case-control study, matched by gender, age, and baseline radiographic disease status in both knees, which ensured maximal comparability of baseline characteristics between cases and controls. There were also some potential limitations. First, the SI alterations in IPFP on non-enhanced MRI were sensitive but nonspecific in detecting inflammatory changes as compared with contrast enhanced MRI images in OA [7]; however, non-enhanced MRI is more economical and less invasive, and the SI changes on non-enhanced MRI have been widely used as a synovitis surrogate and are correlated with chronic synovitis [13]. Second, pathological examinations were unable to be performed in our epidemiological study so the pathological changes associated with IPFP high SI alteration are unknown. Third, our new method only included the high SI alterations. While the low signal alterations may also be associated with the outcomes of knee OA [28], further modifications to our technique to identify them were needed in the future. Last, the case group had higher percentages of obesity, surgery and injury than the control group, which could influence our results; however, we have added them as potential confounders into the analysing models and therefore our findings should not be greatly affected by these factors.
The quantitative segmentation of high signal in IPFP is confirming previous work based on semiquantitative assessment of IPFP high SI suggesting its predictive validity. These findings emphasise the importance of IPFP pathology to the structural pathogenesis of OA. The quantitative measures of IPFP signal intensity is sensitive for changes and could be ideal endpoints for intervention. Targeting inflammation or synovitis may have the potential to delay knee OA development.
Significance and Innovation.
We provided a novel quantitative method to measure infrapatellar fat pad (IPFP) signal intensity alterations.
It demonstrates that IPFP signal intensity alterations are associated with incidence of radiographic osteoarthritis using this novel quantitative method.
Acknowledgments
Special thanks go to the subjects who made this study possible, the OAI investigators, staff and participants.
Funding statement
This study and image acquisition were fund by the OAI study. The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Institute on Aging (NIA) lead this initiative at the National Institutes of Health (NIH). Private funding partners included GlaxoSmithKline, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, and Pfizer. Private-sector funding for the OAI is being managed by the Foundation for the National Institutes of Health.
Footnotes
PROF. DAVID J HUNTER (Orcid ID: 0000-0003-3197-752X)
Conflict of interest statement
The authors declare that they have no competing interests.
References
- 1.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15:225. doi: 10.1186/ar4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y-P, Li S-Z, Yuan F, Xia J, Yu X, Liu X, et al. Infrapatellar fat pad may be with tendon repairing ability and closely related with the developing process of patella Baja. Med Hypotheses. 2011;77:620–23. doi: 10.1016/j.mehy.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJVM, Van Offel JF, Verhaar JAN, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18:876–82. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Patel SJ, Kaplan PA, Dussault RG, Kahler DM. Anatomy and clinical significance of the horizontal cleft in the infrapatellar fat pad of the knee: MR imaging. Am J Roentgenol. 1998;170:1551–55. doi: 10.2214/ajr.170.6.9609172. [DOI] [PubMed] [Google Scholar]
- 6.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–03. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa’s Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. Am J Roentgenol. 2009;192:1696–00. doi: 10.2214/AJR.08.2038. [DOI] [PubMed] [Google Scholar]
- 8.Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75:1783. doi: 10.1136/annrheumdis-2015-208360. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–02. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75:390–95. doi: 10.1136/annrheumdis-2014-205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu M, Chen Z, Han W, Zhu Z, Jin X, Hunter DJ, et al. A novel method for assessing signal intensity within infrapatellar fat pad on MR images in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2016;24:1883–89. doi: 10.1016/j.joca.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fujii T, et al. What comes first?: Multi-tissue involvement leading to radiographic osteoarthritis: MRI-based trajectory analysis over 4 years in the Osteoarthritis Initiative. Arthritis rheumatol. 2015;67:2085–96. doi: 10.1002/art.39176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crema MD, Felson DT, Roemer FW, Niu J, Marra MD, Zhang Y, et al. Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced mri and association with pain. the most study Osteoarthritis Cartilage. 2013;21:413–18. doi: 10.1016/j.joca.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24:458–64. doi: 10.1016/j.joca.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study: A Longitudinal Multicenter Study of Knee Osteoarthritis. Ann Rheum Dis. 2011;70:1804–09. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widjajahakim R, Roux M, Jarraya M, Roemer FW, Neogi T, Lynch JA, Stefanik, et al. Relationship of Trochlear Morphology and Patellofemoral Joint Alignment to Superolateral Hoffa Fat Pad Edema on MR Images in Individuals with or at Risk for Osteoarthritis of the Knee: The MOST Study. Radiology. 2017;162342 doi: 10.1148/radiol.2017162342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campagna R, Pessis E, Biau DJ, Guerini H, Feydy A, Thevenin FS, et al. Is Superolateral Hoffa Fat Pad Edema a Consequence of Impingement between Lateral Femoral Condyle and Patellar Ligament? Radiology. 2012;263:469–474. doi: 10.1148/radiol.12111066. [DOI] [PubMed] [Google Scholar]
- 18.Subhawong TK, Eng J, Carrino JA, Chhabra A. Superolateral Hoffa’s Fat Pad Edema: Association With Patellofemoral Maltracking and Impingement. AJR American journal of roentgenology. 2010;195:1367–1373. doi: 10.2214/AJR.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarraya M, Guermazi A, Felson DT, Roemer FW, Nevitt MC, Torner J, Lewis CE, Stefanik JJ. Is superolateral Hoffa’s fat pad hyperintensity a marker of local patellofemoral joint disease? The MOST study. Osteoarthritis Cartilage. 2017;S1063-4584(17):31039–7. doi: 10.1016/j.joca.2017.05.020. ii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballegaard C, Riis RGC, Bliddal H, Christensen R, Henriksen M, Bartels EM, et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: A cross-sectional study. Osteoarthritis Cartilage. 2014;22:933–40. doi: 10.1016/j.joca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Benito M, Veale D, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–67. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier J-P, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheumatol. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62:108–12. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production Osteoarthritis Cartilage. 2006;14:690–95. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–57. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 26.Wei W, Rudjito E, Fahy N, Verhaar JA, Clockaerts S, Bastiaansen-Jenniskens YM, et al. The infrapatellar fat pad from diseased joints inhibits chondrogenesis of mesenchymal stem cells. Eur Cells Mater. 2015;30:303–14. doi: 10.22203/ecm.v030a21. [DOI] [PubMed] [Google Scholar]
- 27.Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: An important source of interleukin-6 and its soluble receptor. Arthritis Rheumatol. 2009;60:3374–77. doi: 10.1002/art.24881. [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Xu J, Cai J, Zheng S, Han W, Antony B, et al. Serum levels of interleukin-17 and adiponectin are associated with infrapatellar fat pad volume and signal intensity alternation in patients with knee osteoarthritis. Arthritis Res Ther. 2016;18:193. doi: 10.1186/s13075-016-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Hypointense signals in the infrapatellar fat pad assessed by magnetic resonance imaging are associated with knee symptoms and structure in older adults: a cohort study. Arthritis Res Ther. 2016;18:234. doi: 10.1186/s13075-016-1130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


