Abstract
Introduction
Recent work has indicated that there at least two distinct subtypes of psychopathy. Whilst ‘primary’ psychopathy is characterized by low anxiety and thought to result from a genetic predisposition, ‘secondary’ psychopathy is characterized by high anxiety and thought to develop in response to environmental adversity. Primary psychopathy is robustly associated with reduced neural activation to others' emotions and, in particular, distress. However, it has been proposed that the secondary presentation has different neurocognitive correlates.
Methods
Primary (n=50), Secondary (n=100) and Comparison (n=82) groups were drawn from a large volunteer sample (n=1444) using a quartile-split approach across psychopathic trait (affective-interpersonal) and anxiety measures. Participants performed a widely-utilized emotional face processing task during fMRI.
Results
The Primary group showed reduced amygdala and insula activity to fear. The Secondary group did not differ from Comparisons in these regions. Instead, they showed reduced activity compared to Comparisons in several areas including the superior temporal sulcus/inferior parietal lobe, thalamus, pallidum, and substantia nigra. Both groups with psychopathic features also showed reduced activity in response to fear in the anterior cingulate cortex (ACC). During anger processing, the Secondary group exhibited reduced activity in the ACC in comparison with the Primary group.
Conclusions
Distinct neural correlates of fear processing characterize individuals with primary and secondary psychopathic features. The reduced neural response to fear that characterizes individuals with the primary variant of psychopathic traits is not observed in those with the secondary presentation. The neurocognitive mechanisms underpinning secondary psychopathy warrant further, systematic investigation.
Keywords: psychopathy, maltreatment, anxiety, fear, amygdala, fMRI
Introduction
Psychopathy is a personality disorder characterized by criminal and violent behavior (1–3). What distinguishes individuals with psychopathy from their peers with disruptive behavior disorders is their lack of empathy, shallow affect and callous treatment of others. This set of interpersonal and affective traits are captured by Factor 1 of Hare's Psychopathy Checklist – Revised (PCL-R), which dissociates from a second factor (Factor 2) that captures a less aetiologically specific range of antisocial behaviors and lifestyle factors, which are also shared with other individuals with disruptive behavior disorders (4). Psychopathic traits as captured both by Factor 1 and Factor 2 symptoms are continuously distributed in the population and can also be reliably measured in volunteer samples with instruments that have specifically been validated for that purpose (5–9). Individuals diagnosed with criminal psychopathy as well as those with high levels of Factor 1 traits in volunteer samples show attenuated physiological and neural responses to affective stimuli, including stimuli indexing other people's distress (10–14). This blunted reactivity to others' distress is thought, at least in part, to explain why individuals with high levels of Factor 1 traits find it unproblematic to aggress against other people and do not readily empathise with their victims (15).
The original descriptions of psychopathy describe an individual with marked lack of anxiety and consideration for others (16), and indeed, anxiety does appear at odds with the callous and unempathetic presentation that Factor 1 traits describe. Yet accumulating evidence now suggests that these traits (or at least behaviors that can look callous and unempathetic) may co-occur with high levels of anxiety in some individuals (17, 18). Such individuals have been described as presenting with a ‘secondary’ variant of psychopathy, in contrast with the ‘primary’ variant that occurs in the absence of clinically significant anxiety. This primary/secondary distinction has also been drawn in youths presenting with callous-unemotional traits, who are thought to be at risk of developing psychopathy later in life (19, 20). (Note, some studies have employed a different nosological use of ‘secondary’ psychopathy, where the term refers to individuals who present with Factor 2 (lifestyle/antisocial behaviors) but not Factor 1 traits of psychopathy e.g. (21). Here, we use the term ‘secondary’ psychopathy to refer to those individuals who present with psychopathic features and anxiety).
In both childhood and adulthood, those with a secondary presentation do not appear to present with a simple combination of callous/unempathetic and anxious vulnerabilities, but rather show distinct behavioral and psychiatric features not explained by either set of vulnerabilities alone (20, 22–24). Such a secondary presentation has been linked to experiences of significant childhood adversity (23), and is proposed to have a distinct aetiology from the primary, putatively heritable, presentation (25). Secondary psychopathy has therefore been suggested to represent a ‘behavioral phenocopy’ of primary callous and unempathetic traits - arising as an adaptation to environmental adversity (25). In addition to a range of studies indicating heightened levels of childhood maltreatment experience in the secondary group, epigenetic evidence has also provided preliminary support for distinct aetiological pathways to callous-unemotional (Factor 1 type) traits in primary and secondary subtype youths (26). Given the potentially distinct aetiologies and profiles, it appears highly likely that the neural mechanisms underpinning the primary and secondary presentations might differ, yet this has not been investigated to date.
Blunted amygdala response to others' distress, in particular fear, is recognised as a characteristic neurocognitive deficit in primary psychopathy (13). It is thought that this reduced capacity to affectively resonate with others' distress is central to the callous and unempathetic presentation of these individuals (15). However, Factor 1 behavioral presentation in secondary psychopathy may arise from distinct (or partially overlapping) neural mechanisms. Given that both anxiety and maltreatment are typically associated with hyper-responsivity to emotionally evocative stimuli (27, 28) one would assume that individuals with a secondary presentation would share such hyper-responsivity. That is, they would not be expected to share the pattern of hypo-responsivity to emotionally evocative stimuli associated with primary psychopathy presentation. In line with this, behavioral findings have suggested that individuals with secondary psychopathy display greater attentional capture to others' distress than individuals with primary psychopathy (29).
For the first time, we directly compared amygdala response to fear in individuals with high Factor 1 traits and low anxiety (‘Primary’ psychopathic presentation), individuals with high Factor 1 traits and high anxiety (‘Secondary’ psychopathic presentation), and individuals who did not have elevated levels of either trait/symptom domain, drawn from a large volunteer sample with a wide range of behavioral presentation. We predicted that amygdala hypo-reactivity would be related to the primary, but not secondary psychopathic presentation. We also conducted exploratory analyses to investigate whether processing of anger differed in these groups. Anger is a threat emotion with heightened salience to individuals with anxiety and individuals with maltreatment histories (28, 30–32) and disruption to anger processing (heightened amygdala response and lower prefrontal regulatory response) has been proposed to explain increased threat reactive aggression sometimes seen in these populations (33). Given the rich behavioral and psychiatric data available in this sample, we were also able to replicate previous work (conducted in forensic and at-risk samples) regarding the characteristic behavioral and psychiatric profile associated with secondary psychopathy.
Methods and Materials
Participants
Participants were drawn from a dataset of 1144 individuals enrolled into the Duke Neurogenetics Study (DNS), which assessed a range of behavioral and biological traits among non-patient, young adult, student volunteers. Participants were excluded from the DNS in cases of: a) medical diagnosis of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic symptoms, b) use of psychotropic glucocorticoid, or hypolipidemic medication, or c) conditions affecting cerebral blood flow and metabolism. Individuals were not excluded for past or present diagnosis for DSM-IV Axis 1 or select Axis II (antisocial and personality) disorders so that a broad range of behavioral phenotypes related to psychopathology could be assessed. However, none of the participants were taking psychoactive medications for at least 14 days prior to their study enrolment. All participants provided informed consent in accordance with Duke University guidelines, were in good general health and met study inclusion criteria.
Measures
Measures used to create the groups
Psychopathic traits were measured using the Self-Report Psychopathy – Short Form (SRP–SF) (8). The SRP-SF consists of 29 questions divisible into four factors: Interpersonal, affective, lifestyle and antisocial. For the purposes of deriving meaningful subgroups according to Factor 1 traits in this study, interpersonal and affective scales were summed to provide a measure similar to Factor 1 of the PCL-R (4), the current gold standard in assessing psychopathy. Similarly, lifestyle and antisocial facets were summed in order to make a comparative measure to Factor 2 measure of the PCL-R. Anxious disposition was assessed using the 20 question trait measure of the State and Trait Anxiety Inventory (STAIT-T) (34).
Measures of cognitive ability, current functioning and lifetime diagnoses
IQ was measured using full scale scores from the Weschler Abbreviated Scale of Intelligence (WASI) (35). Previous history of maltreatment was assessed using the Child Trauma Questionnaire (CTQ) (36). Current functioning was assessed as follows: aggression was measured using the Buss Perry Aggression Questionnaire (BPAQ) (37); anger was measured using the State-Trait Anger Expression Inventory – trait subscale (STAXI) (38); and impulsivity was measured with the Barratt Impulsiveness Scale (39); perception of own stress was measured with the Perceived Stress Scale (PSS) (40); current anxious and depressive symptomatology were measured with the Mood and Anxiety Symptom Questionnaire (MASQ) (41, 42); alcohol and recreational drug use were assessed with the Alcohol Use Disorders Identification Test (AUDIT) (43) and the Recreational Drug Use (RDU) questionnaire; The Mini-International Neuropsychiatric Interview (MINI) (44) was used to assess lifetime diagnoses of alcohol abuse, substance abuse, major depressive disorder (MDD), generalised anxiety disorder (GAD), bipolar disorder, post-traumatic stress disorder (PTSD), antisocial personality disorder (APSD), borderline personality disorder (BPD), two year risk of suicidality.
Group definition
Previous studies examining psychopathy and anxiety in at-risk and forensic samples have used median split or clustering approaches. To ensure that we captured participants with an elevated psychopathic and anxious trait profile in this high-functioning volunteer sample we adopted a quartile split approach using SRP interpersonal-affective (Factor 1) scores and STAI-T scores to derive Primary, Secondary, and Comparison groups in this sample: i) Comparison if scoring within the bottom quartile for both Factor 1 and anxiety measures (n=82), ii) Primary if scoring within the top quartile for Factor 1 and the lower quartile for anxiety (n=50), and iii) Secondary if scoring within the top quartile for both Factor 1 and anxiety measures (n=100).
Task
We employed a widely adopted blocked perceptual face processing paradigm during which subjects perform a match-to-sample of emotional or neutral faces during the task blocks and geometric shapes during the control blocks. This paradigm has been shown to robustly elicit amygdala reactivity over a wide variety of protocols and populations (45–50). Details for the paradigm version used in the DNS are provided in the supplementary methods
fMRI Analysis
At the first level, regressors for each condition of interest were entered into the general linear model (GLM) from which contrasts of interest (Fear>Shapes, Anger>Shapes) were derived. Contrasts of interest were then individually entered into second level random-effects model to assess group differences in a pairwise manner. Statistical maps were initially produced with an uncorrected threshold of p<0.005. For whole brain analyses, a family-wise error (FWE) correction of p < 0.05 at the cluster level was used to correct for multiple comparisons. To test specific hypotheses about reactivity in the amygdala and OFC these regions of interest (ROIs) were assessed using a voxel-wise small volume corrected FWE p<0.05. A bilateral amygdala ROI was derived from the AAL atlas. A bilateral anterior insula ROI was derived from Deen et al (51). The OFC ROI was a 12mm radius sphere (with areas of non-brain overlap removed) centred on peak activation coordinates from a prior study that found differences in OFC anger activity in individuals with impulsive aggression (52), and consistent with prior work examining OFC activity to anger (53). Due to group differences in sex composition within each group all fMRI analyses controlled for sex. Cohen's d was calculated for cluster maxima from t statistic maps as: d = 2t/√df, where t is the t statistic and df is the degrees of freedom associated with the test.
Behavioral analysis
Binary logistic regressions were planned to analyse categorical data including prior psychiatric diagnoses, however this was not possible in several instances due to complete/quasi-complete separation of data in several variables. For consistency, all categorical data were therefore analysed using chi square tests. Continuous data were analysed using ANOVAs. All behavioral analyses were performed using SPSS v22 (http://www.ibm.com/software/products/en/spss-statistics).
Results
Demographics, current functioning and lifetime psychiatric diagnoses
All demographic information is listed in Supplementary Table 1. Age did not significantly differ between groups. There was a significant difference in the proportion of males and females across groups (X2(2, 229) = 47.16, p < 0.001; % Female: 42% Secondary, 18% Primary, 75% Comparison). There was a trend towards overall group difference in IQ (F(2, 227)=2.68, p=0.070), however group mean IQs were of highly similar levels (Group mean range: 120.57-123.86, see Supplementary Table 1).
Psychopathy scores
In line with previous research, both Primary (p<0.001) and Secondary (p<0.001) groups had higher levels of Factor 1 traits than the Comparison group but importantly did not differ from each other (p=0.710). Both Primary and Secondary groups had higher scores than the Comparison group on Factor 2 of the SRP (all p<0.001), and the Secondary group had higher Factor 2 scores than the Primary group (p=0.028). See Supplementary Table 1 for more detail.
Internalising symptoms
The Primary group did not differ from the Comparison group on current symptomatology of depression (p=1.000) or anxiety (p=1.000) or on diagnoses of major depressive disorder (p = 0.199) or generalised anxiety disorder (p=1.000). The Secondary group differed from both Primary and Comparison groups on depressive and anxious symptoms (all p<0.001). The Secondary group differed from the Primary (p=0.005) and Comparison (p<0.001) groups in depression diagnoses. Additionally, the Secondary group had significantly more diagnoses of generalised anxiety disorder than the Comparison group (p=0.014) with a trend to significance when compared to the primary group (p=0.054). The Secondary group also showed higher diagnoses of bipolar disorder compared to Comparison (p=0.001) and Primary (p=0.039) groups, whilst the Primary and Comparison groups did not differ from each other (p=0.199). See Supplementary Tables 2 and 3 for more detail.
Externalising symptoms
Both the Primary and Secondary group had greater alcohol (both: p<0.001) and substance use (Primary: p=0.002; Secondary: p<0.001) than the Comparison group but did not differ from each other (both: p=1.000). However, results examining lifetime DSM-IV diagnoses differed from this, with the Secondary group more likely to have received a diagnosis of alcohol (p = 0.002) and substance abuse compared to Comparisons (p=0.009) and the Primary group only being more likely to have a lifetime diagnosis of alcohol (p=0.015), but not substance abuse (p = 0.299) compared to Comparisons. The primary and secondary groups did not differ from each other in likelihood of alcohol (p=0.654) or substance abuse diagnoses (p=0.184).
Similar to prior research, both Primary and Secondary groups differed from the Comparison group on aggression, anger, and impulsivity measures (all p<0.001), but also differed from each other (all p<0.001), with the Secondary group being most impaired on all measures. See Supplementary Tables 2 and 3 for more detail.
Suicidality
The Secondary group had heightened risk of suicidality when compared to the Primary (p=0.024) and Comparison (p<0.001) groups. The Primary group had a trend-wise risk of increased suicidality compared to the Comparison group (p=0.068). See Supplementary Tables 2 and 3 for more detail.
Maltreatment history
The Secondary group had more severe history of maltreatment than the Primary or Comparison groups (both: p<0.001) who did not differ from each other (p=0.575). This pattern was observed for each subscale of the CTQ except emotional neglect and sexual abuse (Supplementary table 1). Both Primary (p=0.018) and Secondary (p<0.001) groups had heightened emotional neglect compared to the Comparison group, though the Secondary group still reported significantly higher levels of emotional neglect compared to the primary group (p<0.001). The Secondary group reported higher levels of sexual abuse when compared to Comparisons (p=0.024). The Primary group did not differ from either group (Comparison: p=1.000; Secondary: p=0.389).
Neurocognitive differences
Neural responses to fear
Consistent with previous studies, in comparison with the Comparison group the Primary group exhibited blunted responses to fearful facial expressions in the left amygdala (SVC: pFWE=0.044) with a trend in the right (SVC: pFWE=0.122), as well as in the bilateral anterior insula (SVC: Left: pFWE=0.012; Right: pFWE=0.038; Table 1; Figure 1). By contrast, the Secondary group showed no reductions in these regions when compared to the Comparison group. The Primary group also showed trend-level reductions in fear responses compared to the Secondary group within the right amygdala (SVC: pFWE=0.055) and bilateral anterior insula (SVC: Left: pFWE=0.063; Right: pFWE=0.070; Table 1; Figure 1).
Table 1. Significant anterior insula and amygdala (SVC) results for Fear > Shapes.
| k | p(FWE-corr) | Cohen' s d | Z | x y z | |
|---|---|---|---|---|---|
| Comparison >Primary | |||||
| Left amygdala | 34 | 0.044 | 0.56 | 3.18 | -22 -2 -14 |
| Left anterior insula | 213 | 0.012 | 0.69 | 3.88 | -38 4 -12 |
| 0.029 | 0.64 | 3.62 | -38 -16 2 | ||
| 0.054 | 0.61 | 3.42 | -34 6 -12 | ||
| 0.056 | 0.61 | 3.41 | -44 6 -6 | ||
| 0.066 | 0.60 | 3.36 | -44 -4 0 | ||
| Right anterior insula | 55 | 0.038 | 062 | 3.53 | 46 0 4 |
| Secondary> Primary | |||||
| Right amygdala | 26 | 0.055 | 0.52 | 3.10 | 30 4 -18 |
| 0.082 | 0.49 | 2.95 | 30 2 -24 | ||
| Left anterior insula | 122 | 0.063 | 0.56 | 3.37 | -38 -16 2 |
| Right anterior insula | 37 | 0.070 | 0.56 | 3.33 | 46 -2 -6 |
| 0.075 | 0.55 | 3.31 | 46 0 4 | ||
| Right anterior insula | 13 | 0.099 | 0.53 | 3.21 | 42 2 -12 |
Figure 1.
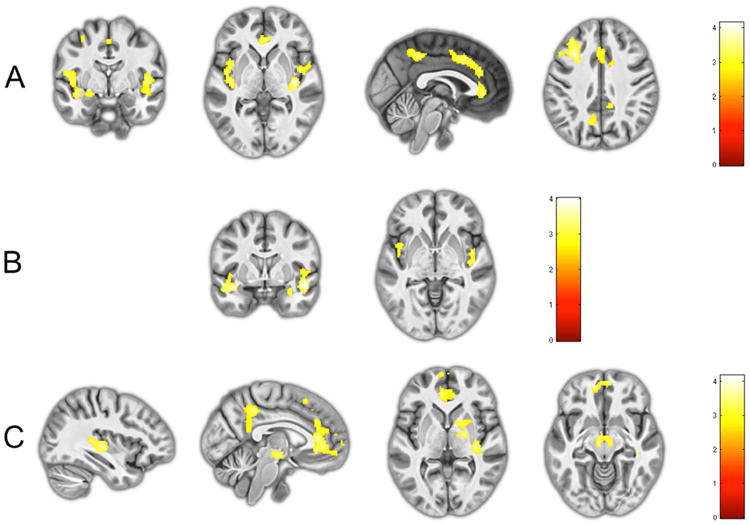
a) Reduced activity in the amygdala (far left), insula (middle left), ACC and PCC (middle right), and dorsolateral prefrontal cortex (far right) in the Primary group compared to the Comparison group during fear processing; b) Trendwise reduced activity in the amygdala (left) and insula (right) in the Primary compared to Secondary group during fear processing; c) Reduced activity in the superior temporal sulcus/inferior parietal lobe (far left), ACC and PCC (middle left), thalamus and globus pallidus (middle right), and susbtantia nigra (far right) in Secondary group compared to the Comparison group during fear processing.
In whole brain analyses the Primary group showed reduced activity in responses to fearful facial expressions in clusters encompassing the insula bilaterally, as well as a frontal cluster extending into the bilateral ACC, and the bilateral precuneus and surrounding regions within the cuneus and cingulate cortex (Table 2; Figure 1). Whole brain analyses also confirmed trends towards reduced activity within the bilateral insula in the Primary compared to the Secondary group (Table 2; Figure 1). Whilst the Secondary group also showed reductions within the ACC compared to the Comparison group, another cluster extending into the left thalamus, bilateral substantia nigra/ventral tegmental area (SN/VTA) and pallidum was also observed that was not seen when comparing Primary and Comparison groups.
Table 2.
| p(FWE-corr) | k | Cohen's d | Z | x y z | |
|---|---|---|---|---|---|
| Comparison >Primary | |||||
| Left Cuneus ext. L mid cingulate (MCC) and R Precuneus | <0.001 | 1327 | 0.73 | 4.07 | -14 -68 24 |
| 0.67 | 3.76 | -20 -58 18 | |||
| 0.65 | 3.65 | -6 -42 44 | |||
| Right Precuneus ext. cuneus | 0.065 | 472 | 0.73 | 4.07 | 16 -54 22 |
| 0.59 | 3.33 | 8 -64 20 | |||
| 0.53 | 2.99 | 10 -46 6 | |||
| Left insula ext. postcentral gyrus, rolandic operculum, superior temporal gyrus, amygdala | 0.001 | 1150 | 0.72 | 4.03 | -38 2 -12 |
| 0.70 | 3.9 | -56 -18 18 | |||
| 0.64 | 3.62 | -38 -16 2 | |||
| Right insula ext. rolandic operculum | 0.008 | 746 | 0.72 | 4.02 | 38 -16 0 |
| 0.63 | 3.53 | 46 0 4 | |||
| 0.56 | 3.16 | 58 4 6 | |||
| Left middle frontal ext. L/R ACC, left superior frontal | <0.001 | 2279 | 0.69 | 3.88 | -30 20 36 |
| 0.68 | 3.82 | 8 32 10 | |||
| 0.68 | 3.81 | -4 32 28 | |||
| Secondary> Primary | |||||
| Right superior temporal gyrus ext. insula | 0.073 | 457 | 0.66 | 3.94 | 48 0 -12 |
| 0.55 | 3.31 | 46 0 4 | |||
| 0.55 | 3.29 | 46 -8 -8 | |||
| Left temporal pole ext. insula | 0.086 | 436 | 0.64 | 3.8 | -40 0 -16 |
| 0.56 | 3.39 | -50 4 -16 | |||
| 0.56 | 3.37 | -38 -16 2 | |||
| Comparison >Secondary | |||||
| L/R ACC ext. frontal pole | <0.001 | 1863 | 0.62 | 4.08 | -12 52 -8 |
| 0.57 | 3.74 | -4 36 8 | |||
| 0.54 | 3.55 | -6 32 -4 | |||
| Right superior temporal sulcus, inferior parietal, thalamus, bilateral SN/VTA, and pallidum | 0.003 | 895 | 0.56 | 3.71 | 36 -20 2 |
| 0.54 | 3.58 | 20 -8 2 | |||
| 0.51 | 3.38 | -6 -16 -10 | |||
| Left superior temporal gyrus ext. left supramarginal gyrus | 0.030 | 571 | 0.53 | 3.48 | -46 -28 10 |
| 0.52 | 3.44 | -30 -34 14 | |||
| 0.49 | 3.23 | -42 -36 24 | |||
| L/R MCC ext. posterior cingulate, precunueus | 0.015 | 659 | 0.52 | 3.44 | -2 -42 42 |
| 0.50 | 3.3 | -8 -48 20 | |||
| 0.47 | 3.13 | 8 -44 24 |
Neural responses to anger
Whole brain analyses revealed reduced bilateral ACC activity in response to angry facial expressions in the Secondary group compared to the Primary group (pFWE<0.011; Table 3; Figure 1). Even after SVC, there were no significant differences in OFC activity between any groups during processing of anger.
Table 3. Whole brain results Anger > Shapes.
| p(FWE-corr) | k | Cohen's d | Z | x y z | |
|---|---|---|---|---|---|
| Primary>Secondary | |||||
| L/R ACC | 0.011 | 740 | 0.68 | 4.04 | -26 26 24 |
| 0.61 | 3.64 | 8 32 20 | |||
| 0.59 | 3.52 | -14 30 28 |
Discussion
Here, we provide novel evidence that individuals with secondary psychopathic features are not identical to the primary variant in neural activity to emotional facial expressions. Specifically, when processing fear secondary psychopathic features were associated with reduced activity in the superior temporal sulcus/inferior parietal lobe, pallidum, SN/VTA, and thalamus, while primary psychopathic features were associated with blunted activity in the amygdala and anterior insula. The primary and secondary variants did, however, exhibit some overlap in their neurocognitive presentation, with reduced activity in the anterior cingulate during fear processing observed in both groups in comparison with comparison participants. Contrary to our predictions, we did not find any evidence of altered processing of anger in the secondary psychopathy group when compared to controls.
In line with previous research in incarcerated and volunteer samples, we found that primary psychopathic features are associated with blunted amygdala and insula activity to fear (13 for a review). Importantly, our results extend this body of work to show that these particular neurocognitive hallmarks are observed only in the primary variant. The hypo-active neurocognitive profile in primary psychopathy is consistent with the ‘cold’ clinical presentation of these individuals, who exhibit reduced empathy and correspondingly, a constellation of interpersonal behaviors that show limited regard for others. However, the presence of Factor 1 traits in the absence of this neurocognitive profile in individuals with secondary psychopathy suggest that they may arise via partly distinct mechanisms, though our data do not elucidate what these may be.
Based on prior research we expected that individuals with secondary psychopathic features would show increased neural activity to others' distress. In prior studies, individuals with secondary callous/unemotional traits have shown increased attentional capture to distressing stimuli compared to their low anxiety counterparts (though it is noteworthy that in this sample too, the secondary group did not differ from comparisons) (29), and higher startle modulation to aversive images when compared to primary and comparison groups (54). The absence of any differences between the secondary group and comparisons in amygdala activity to fear in the present study are not what one might have expected given the startle modulation data by Kimonis et al. (54). However, several methodological differences should be noted when considering these findings. Firstly, previous studies have used distressing and aversive (including threatening and victim related) scenes that are intended to elicit robust behavioral responses, and may therefore represent more affectively salient stimuli than fearful facial expressions used in the present study. The use of fearful facial expressions afforded ready comparability with prior data on psychopathy/psychopathic traits in adults and enabled us to confirm that those with the primary variant presented in line with data from prior studies using such stimuli. Further studies with a variety of paradigms will be important to more comprehensively examine the neurocognitive deficits associated with the secondary variant. Secondly, differences between these studies may also reflect sampling strategies. Both previous studies used samples of juvenile offenders, contrasting to the approach we have adopted with student volunteers, which is likely to have captured a less severe secondary group. Though these factors may explain the inconsistencies between behavioral and neural measures of emotional responsiveness, it is noteworthy that the behavioral and psychological profile of the secondary subtype is highly consistent with prior studies, suggesting that we did accurately capture primary and secondary groups. Another possibility is that whilst there is increased behavioral emotional reactivity in secondary individuals, this is determined by some other neural factor than amygdala hyper-activity.
Our analyses revealed several commonalities and differences in fear processing between the two groups that may in part underpin the secondary presentation. Firstly, we show that both primary and secondary variants showed reduced activity in the ACC during fear processing, compared to the comparisons. The ACC has been variously associated with the acquisition, expression and extinction of fear conditioning, as well as the regulation of emotional conflict and the top down regulation of emotion (55). It may be that the reduced ACC activity reflects an impairment in a common mechanism for both groups; however, it is equally possible that ACC activity reflects different cognitive processes in the primary and secondary variants (e.g. relative impairments in conditioning to vs. regulation of distress stimuli). This question needs to be investigated systematically in the future and (for example) related to the psychophysiological profile of the subtypes. Secondly, we show reduced activity in the SN/VTA, pallidum, and thalamus in response to fear that was unique to the secondary group. The VTA sends dopaminergic projections to the amygdala, where dopamine signalling plays an important role in the acquisition of fear responses (56). Similarly the thalamus has been linked to the acquisition and expression of fear conditioning (57, 58). The role of pallidum in such a model is less clear, though it sends extensive projections to the thalamus (59) and is also linked to the amygdala (60). Although purely speculative, a possible explanation for the presentation of Factor 1 traits in maltreated individuals may be abnormal development of fear conditioning responses. Future studies may seek to address how different components of fear conditioning are affected in primary and secondary psychopathy by using experimental paradigms that afford inference regarding this. Thirdly, superior temporal sulcus/temporoparietal junction activity was also observed to be hypoactive in the secondary group. These regions have been tightly linked to perspective taking and inferring others mental states and emotions (61), and may reflect another mechanism by which blunted affect to others' emotions could be instantiated in individuals with Factor 1 traits. However, future studies are required to systematically examine how these processes are altered in secondary psychopathy. Finally, we hypothesised that atypical or impaired anger processing within the OFC may play a role in the development of callous aggressive behaviors in the secondary group. We did not find evidence for this hypothesis in the current sample, but it would be interesting to explore whether such a difference is observed in forensic or at-risk groups.
In line with prior research into primary and secondary variants of psychopathy (20, 22, 23, 29, 54, 62–64), our volunteer sample of individuals with high psychopathic personality traits showed largely similar increases in externalising symptoms such as substance and alcohol abuse when compared with comparison participants. However, the primary and secondary groups differed significantly on several dimensions. Notably, the secondary group showed markedly higher maltreatment history and internalising symptoms, as well as heightened levels of suicidality and aggression, than either the primary or comparison groups. Although the present work suggests a highly similar behavioral phenotype can be captured in volunteer samples to those in offending settings, additional studies are required to replicate our neurocognitive findings in forensic and vulnerable samples. Indeed, a study in forensic groups with high levels of maltreatment may provide a more stringent test of any effects of maltreatment on brain function and reactive aggression.
Although our data suggest several possibilities about the neurocognitive mechanisms underpinning Factor 1 traits in the presence of anxiety, it is important not to rely on reverse inference and future studies must use methods that can directly interrogate a number of different processes. For instance, hypoactive fear processing in the thalamus, SN/VTA and ACC suggest abnormalities in fear conditioning may play a role in the development of Factor 1 traits in the presence of anxiety, possibly in response to environmental trauma. Assessing how different aspects of fear conditioning are altered in primary and secondary individuals, and particularly perhaps in response to maltreatment, is therefore warranted.
In conclusion, we provide novel evidence that individuals presenting with Factor 1 traits differ in their neurocognitive presentation depending on the presence or absence of concurrent anxiety. Whilst individuals with primary psychopathic features show blunted affective responses to others distress that has come to be seen as a central neurocognitive phenotype of psychopathy, this pattern is not observed in individuals with a secondary psychopathic presentation. Whilst individuals high on Factor 1 traits appear to present with reduced ACC activity to fear independent of anxiety levels, those with the high-anxiety secondary variant of these traits appear to show additional abnormalities in circuitry that plays a role in fear learning and regions associated with mentalization. We do not, however, find amygdala hyper-activity to fear in the secondary group. Though this is unexpected, it is also not clear how such a hyper-activity could be understood alongside the callous disregard for others that is characterised by Factor 1 traits. Whilst future work will be required to directly assess any mechanisms underpinning Factor 1 traits in the presence of anxiety, and whether these mechanisms do, as proposed, stem for childhood maltreatment, the present study shows a critical divergence in the neurocognitive presentation of these two groups.
Supplementary Material
Supplementary Table S1. Demographics, SRP & maltreatment scores
Supplementary Table S2. Individual functioning
Supplementary Table S3. Lifetime psychiatric diagnoses
Supplementary Table S4. Ranges and distributions of key variables across and within groups
Supplementary Table S5. Correlation matrix between key variables across sample (n = 1144)
Acknowledgments
We thank all members of the Laboratory of NeuroGenetics for their assistance in conducting the Duke Neurogenetics Study, which was supported by Duke University and NIH grant R01DA033369. A.R.H. is further supported by NIH grant R01AG049789. E.V. is a Royal Society Wolfson Research Merit Award holder and British Academy Mid-Career Fellow.
Footnotes
Declaration of Conflicts of Interest: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hare RD, McPherson LM. Violent and aggressive behavior by criminal psychopaths. Int J Law Psychiatry. 1984;7:35–50. doi: 10.1016/0160-2527(84)90005-0. [DOI] [PubMed] [Google Scholar]
- 2.Serin RC. Psychopathy and Violence in Criminals. J Interpers Violence. 1991;6:423–431. [Google Scholar]
- 3.Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annu Rev Clin Psychol. 2008;4:217–46. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- 4.Hare RD. The Hare Psychopathy Checklist-Revised (Hare PCL-R) Toronto: Multi Health Systems Inc; 2003. [Google Scholar]
- 5.Salekin RT, Trobst KK, Krioukova M. Construct validity of psychopathy in a community sample: a nomological net approach. J Pers Disord. 2001;15:425–41. doi: 10.1521/pedi.15.5.425.19196. [DOI] [PubMed] [Google Scholar]
- 6.Neumann CS, Hare RD. Psychopathic traits in a large community sample: links to violence, alcohol use, and intelligence. J Consult Clin Psychol. 2008;76:893–9. doi: 10.1037/0022-006X.76.5.893. [DOI] [PubMed] [Google Scholar]
- 7.Mahmut MK, Menictas C, Stevenson RJ, Homewood J. Validating the factor structure of the Self-Report Psychopathy scale in a community sample. Psychol Assess. 2011;23:670–8. doi: 10.1037/a0023090. [DOI] [PubMed] [Google Scholar]
- 8.Paulhus DL, Neumann CS, Hare RD. In: Manual for the Self-Report Psychopathy Scale. Systems MH, editor. 2015. [Google Scholar]
- 9.Neumann CS, Pardini D. Factor Structure and Construct Validity of the Self-Report Psychopathy (SRP) Scale and the Youth Psychopathic Traits Inventory (YPI) in Young Men. J Pers Disord. 2014;28:419–433. doi: 10.1521/pedi_2012_26_063. [DOI] [PubMed] [Google Scholar]
- 10.Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: startle reflex modulation. J Abnorm Psychol. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- 11.Benning SD, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–62. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Soc Neurosci. 2013;8:122–35. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seara-Cardoso A, Viding E. Functional Neuroscience of Psychopathic Personality in Adults. J Pers. 2015;83:723–37. doi: 10.1111/jopy.12113. [DOI] [PubMed] [Google Scholar]
- 14.Seara-Cardoso A, Sebastian CL, Viding E, Roiser JP. Affective resonance in response to others' emotional faces varies with affective ratings and psychopathic traits in amygdala and anterior insula. Soc Neurosci. 2016;11:140–52. doi: 10.1080/17470919.2015.1044672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair JR. The neurobiology of psychopathic traits in youths. Nat Rev Neurosci. 2013;14:786–99. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleckley HM. The Mask of Sanity. Oxford, England: Mosby; 1941. [Google Scholar]
- 17.Skeem J, Johansson P, Andershed H, Kerr M, Louden JE. Two subtypes of psychopathic violent offenders that parallel primary and secondary variants. J Abnorm Psychol. 2007;116:395–409. doi: 10.1037/0021-843X.116.2.395. [DOI] [PubMed] [Google Scholar]
- 18.Kahn RE, Frick PJ, Youngstrom EA, Kogos Youngstrom J, Feeny NC, Findling RL. Distinguishing primary and secondary variants of callous-unemotional traits among adolescents in a clinic-referred sample. Psychol Assess. 2013;25:966–78. doi: 10.1037/a0032880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viding E, McCrory EJ. Developmental risk for psychopathy. In: Thapar A, Pine DS, Leckman JF, Scott S, Snowling MJ, Taylor E, editors. Rutter's Child Adolesc Psychiatry. Chichester, UK: Wiley-Blackwell; 2015. [DOI] [Google Scholar]
- 20.Fanti KA, Demetriou CA, Kimonis ER. Variants of callous-unemotional conduct problems in a community sample of adolescents. J Youth Adolesc. 2013;42:964–79. doi: 10.1007/s10964-013-9958-9. [DOI] [PubMed] [Google Scholar]
- 21.Dean AC, Altstein LL, Berman ME, Constans JI, Sugar CA, McCloskey MS. Secondary psychopathy, but not primary psychopathy, is associated with risky decision-making in noninstitutionalized young adults. Pers Individ Dif. 2013;54:272–277. doi: 10.1016/j.paid.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimonis ER, Skeem JL, Cauffman E, Dmitrieva J. Are secondary variants of juvenile psychopathy more reactively violent and less psychosocially mature than primary variants? Law Hum Behav. 2011;35:381–91. doi: 10.1007/s10979-010-9243-3. [DOI] [PubMed] [Google Scholar]
- 23.Kimonis ER, Fanti KA, Isoma Z, Donoghue K. Maltreatment profiles among incarcerated boys with callous-unemotional traits. Child Maltreat. 2013;18:108–21. doi: 10.1177/1077559513483002. [DOI] [PubMed] [Google Scholar]
- 24.Cecil CAM, McCrory EJ, Barker ED, Guiney J, Viding E n.d. Characterising youth with callous-unemotional traits and concurrent anxiety: Evidence for a high-risk clinical groupe. Eur Child Adolesc Psychiatry. doi: 10.1007/s00787-017-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viding E, Kimonis ER . n.d. Callous-Unemotional Traits. In: Patrick CJ, editor. Handb Psychopathy. 2nd [Google Scholar]
- 26.Cecil CAM, Lysenko LJ, Jaffee SR, Pingault JB, Smith RG, Relton CL, et al. Environmental risk, Oxytocin Receptor Gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol Psychiatry. 2014;19:1071–7. doi: 10.1038/mp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21:R947–8. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 28.McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry. 2013;202:269–76. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- 29.Kimonis ER, Frick PJ, Cauffman E, Goldweber A, Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Dev Psychopathol. 2012;24:1091–103. doi: 10.1017/S0954579412000557. [DOI] [PubMed] [Google Scholar]
- 30.Bishop SJ, Duncan J, Lawrence AD. State Anxiety Modulation of the Amygdala Response to Unattended Threat-Related Stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 32.McCrory EJ, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blair RJR. The Neurobiology of Impulsive Aggression. J Child Adolesc Psychopharmacol. 2016;26:4–9. doi: 10.1089/cap.2015.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spielberger C. State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 35.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Pearson Education; 1999. [Google Scholar]
- 36.Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26:327–32. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- 37.Buss AH, Perry MP. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 38.Spielberger CD. The State-Trait Anger Expression Inventory-2 (STAXI-2): Professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1999. [Google Scholar]
- 39.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 41.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 42.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 43.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–32. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998:22. 33-57. [PubMed] [Google Scholar]
- 45.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 46.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 47.Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5 HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–3. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- 48.Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, et al. Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19:2499–507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am J Psychiatry. 2007;164:1613–4. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deen B, Pitskel NB, Pelphrey KA. Three Systems of Insular Functional Connectivity Identified with Cluster Analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 54.Kimonis ER, Fanti KA, Goulter N, Hall J. Affective startle potentiation differentiates primary and secondary variants of juvenile psychopathy. Dev Psychopathol. 2017;29:1149–1160. doi: 10.1017/S0954579416001206. [DOI] [PubMed] [Google Scholar]
- 55.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 57.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519:455–9. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Yim CY, Mogenson GJ. Response of ventral pallidal neurons to amygdala stimulation and its modulation by dopamine projections to nucleus accumbens. J Neurophysiol. 1983;50:148–61. doi: 10.1152/jn.1983.50.1.148. [DOI] [PubMed] [Google Scholar]
- 61.Frith CD, Frith U. The Neural Basis of Mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Tatar JR, Cauffman E, Kimonis ER, Skeem JL. Victimization History and Posttraumatic Stress: An Analysis of Psychopathy Variants in Male Juvenile Offenders. J Child Adolesc Trauma. 2012;5:102–113. [Google Scholar]
- 63.Vaughn MG, Edens JF, Howard MO, Smith ST. An Investigation of Primary and Secondary Psychopathy in a Statewide Sample of Incarcerated Youth. Youth Violence Juv Justice. 2009;7:172–188. [Google Scholar]
- 64.Lee Z, Salekin RT, Iselin AMR. Psychopathic traits in youth: is there evidence for primary and secondary subtypes? J Abnorm Child Psychol. 2010;38:381–93. doi: 10.1007/s10802-009-9372-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Demographics, SRP & maltreatment scores
Supplementary Table S2. Individual functioning
Supplementary Table S3. Lifetime psychiatric diagnoses
Supplementary Table S4. Ranges and distributions of key variables across and within groups
Supplementary Table S5. Correlation matrix between key variables across sample (n = 1144)


