Abstract
Direct Oral Anticoagulants (DOACs) are small molecule inhibitors of the coagulation proteases thrombin and factor Xa that demonstrate comparable efficacy to warfarin for several common indications, while causing less serious bleeding. However, because their targets are required for the normal host-response to bleeding (hemostasis), DOACs are associated with therapy-induced bleeding that limits their use in certain patient populations and clinical situations. The plasma contact factors (factor XII, factor XI, and prekallikrein) initiate blood coagulation in the activated partial thromboplastin time assay. While serving limited roles in hemostasis, pre-clinical and epidemiologic data indicate that these proteins contribute to pathologic coagulation. It is anticipated that drugs targeting the contact factors will reduce risk of thrombosis with minimal impact on hemostasis. Here, we discuss the biochemistry of contact activation, the contributions of contact factors in thrombosis, and novel antithrombotic agents targeting contact factors that are undergoing pre-clinical and early clinical testing.
Keywords: Factor XI, factor XII, prekallikrein, thrombosis, contact activation
1. INTRODUCTION
1.1. Recent Advances in Oral Anticoagulation Therapy.
Direct oral anticoagulants (DOACs) are replacing vitamin K antagonists (VKAs) for treatment and prevention of thrombosis [1–5], particularly for acute venous thromboembolism (VTE) [6–8] and prevention of stroke in non-valvular atrial fibrillation [7,9,10]. VKAs such as warfarin exhibit narrow therapeutic windows and are subject to multiple drug-drug and dietary interactions, as well as pharmacogenetic considerations. As a result, VKAs require laboratory monitoring to maintain patients within a relatively narrow therapeutic window [7,11,12]. DOACs are attractive alternatives to VKAs. Several features of the thrombin inhibitor dabigatran etexilate and the factor Xa (FXa) inhibitors rivaroxaban, apixaban, edoxaban, and betrixaban are appealing, including their safety profiles (notably reduced major bleeding, including intracranial hemorrhage [ICH]) [6], limited drug interactions, and predictable anticoagulant response without monitoring or dose adjustment [2,5,12]. However, DOACs have limitations. Plasma levels may become elevated in patients with renal insufficiency, increasing the bleeding risk. While the anticoagulant effect of VKAs can be reversed quickly with factor concentrates, plasma or vitamin K [13], antidote options for DOACs are limited [14]. The monoclonal IgG idarucizumab is approved for rapid dabigatran neutralization [15,16], but may not be available in all medical centers. Reversal agents for DOACs targeting factor Xa (andexanet alfa and ciraparantag) are under study [17– 19], but are also likely to have limited availability. However, even after considering the complex drug reversal scenarios, use of DOAC is increasing because of the advantages over VKAs [20].
1.2. Alternative Targets for Antithrombotic Therapy.
Clinical trials with DOACs consistently show an ~50% reduction in ICH compared with VKAs [21–23]. However, there remains a 0.1% to 0.3% annual risk of ICH with DOACs [24,25], with a three month ICH-related mortality of ~50% [26,27]. Case-fatality rates for VKA related ICH are similar, with little improvement in prognosis even when reversal agents are available [28]. Furthermore, there are patients who would benefit from antithrombotic therapy who cannot safely receive VKAs or DOACs because they have conditions that predispose to bleeding, or are undergoing procedures in which anticoagulation is not safe. Like the VKAs, DOACs achieve their antithrombotic effects by targeting enzymes required for normal blood clot formation. They will inevitably compromise hemostasis to some extent. There is a clear need for antithrombotic strategies that have minimal impact on physiologic hemostasis. Therapies targeting the procoagulant activities of plasma contact factors have generated interest in this regard [29–32].
The contact protease precursors factor XII (FXII), factor XI (FXI), and prekallikrein (PK), and the co-factor high molecular weight kininogen (HK), are plasma proteins produced in hepatocytes that do not require vitamin K for synthesis. As a group, they initiate coagulation in the activated partial thromboplastin time (aPTT) assay used in clinical practice by a process called contact activation (Fig.1A). While required in the aPTT assay, the contact factors serve, at most, limited roles in physiologic hemostasis [33–35]. Despite this, there is mounting evidence that they make substantive contributions to thrombosis [29–32,36] and consumptive coagulopathies [37]. It is anticipated that inhibition of a contact factor will produce an antithrombotic effect with minimal inhibition of hemostasis. Here, we review the biochemistry of contact activation, data supporting the hypothesis that contact factors contribute to thrombosis, and some novel agents with therapeutic potential that target the contact system.
Figure 1. Contact Activation and Thrombin Generation.
(A) Contact Activation. Proteolytic reactions involved in contact activation are shown on a hypothetical surface represented in gray. Factor (F) XII binding to the surface facilitates autocatalytic conversion of FXII to FXIIa. FXIIa converts prekallikrein (PK) to α-kallikrein, which activates additional FXII and cleaves high-molecular-weight kininogen (HK, yellow arrows), liberating bradykinin (BK). HK also serves as a cofactor in this process by facilitation PK binding to the surface. FXIIa also activates FXI to the protease FXIa. (B) The Classical Coagulation Cascade. FXIa generated in the process described in Panel A triggers plasma coagulation by converting FIX to FIXa. FIXa in turn activates FX to FXa, which then converts prothrombin (F11) to thrombin (F11a). (C) A Current Model of Thrombin Generation Showing Its Relationship to Contact Activation. Reactions within the pink box are the major proteolytic steps involved in thrombin generation at a site of injury. The FVIIa/tissue factor (TF) complex initiates thrombin generation by activating FX and FIX. FXa converts prothrombin (F11) to thrombin (F11a) in the presence of the cofactor FVa, while FIXa sustains FX activation in a FVIIa-dependent manner. In this model, FXI can be activated by thrombin (blue arrow) to FXIa, which sustains coagulation by activating additional FIX. FXI can also be activated by FXIIa, serving as a bridge between contact activation and thrombin generation. There is some evidence that FXIa can also convert FXII to FXIIa (gray arrow). In panels B and C, cofactors are shown in blue ovals. Calcium (Ca2+) and phospholipid (PL) dependent reactions are indicated. For all panels, red arrows indicate the reciprocal activation of FXII and PK, and green arrows the activation of FXI by FXIIa.
2. CONTACT ACTIVATION AND THROMBIN GENERATION
2.1. Contact Activation (Kallikrein-Kinin System)
Contact activation is triggered when blood comes into contact with a variety of artificial and biologic substances or surfaces [33,34]. Purified earths such as silica or kaolin are used to trigger contact activation in the aPTT assay [35]. Polymers of orthophosphate (polyphosphate) [38–40], nucleic acids (DNA or RNA) [41–44], basement membrane components (collagens and laminin) [45,46], ruptured atherosclerotic plaque [47,48], misfolded protein aggregates [49], and microorganism cell membranes/walls [50] are some substances that may promote contact activation in vivo. Surfaces of medical devices such as cardiopulmonary bypass (CPB) circuits [51,52], extracorporeal membrane oxygenators (ECMO) [53], hemodialysis membranes [54], intravenous catheters [55,56], mechanical heart valves [56] and ventricular assist devices (VAD) [57] also induce contact activation. While surfaces/substances that promote contact activation often carry a net negative charge, a broad range of materials facilitate the process.
Contact activation is initiated by binding of FXII to a surface followed by autocatalytic conversion to the protease factor XIIa (FXIIa, Fig.1A) [33–35]. FXIIa catalyzes conversion of PK to the protease α-kallikrein, with HK facilitating PK binding to the surface. α-Kallikrein activates additional FXII. Reciprocal activation of FXII and PK amplifies contact activation (red arrows in Fig.1A). FXII and PK appear to turn over at a basal rate in healthy animals, but the surfaces or cofactors that support this (if any) are not established [58]. We determined that FXII and PK have low levels of intrinsic activity that may initiate contact activation upon surface-binding [59,60]. FXII, PK and HK are collectively referred to as the kallikrein-kinin system (KKS) [33]. The KKS generates pro-inflammatory peptides such as bradykinin through α-kallikrein cleavage of HK. PK may also be activated independently of FXII by the lysosomal protease prolylcarboxy-peptidase (PRCP) [61,62], or by interaction with heat shock protein 90 (HSP90) [63,64].
2.2. Contact Activation and Coagulation
FXIIa promotes coagulation by catalyzing conversion of FXI, a homolog of PK, to the protease factor XIa (FXIa, Fig.1A, green arrow) [65]. FXIa then converts factor IX to factor IXa [65], driving thrombin generation in the aPTT assay (Fig.1B). However, individuals completely lacking FXII, PK or HK do not experience abnormal bleeding, while FXI deficiency may cause a mild to moderate propensity to bleed excessively after trauma [35,66]. These phenotypes indicate that contact activation is not required for hemostasis, and that FXI contributes to hemostasis independently of contact activation.
The KKS appears to contribute to important host-defense and homeostatic processes including the innate response to microorganism invasion, inflammation, and blood pressure regulation [33,34,61]. Its capacity to activate FXI could reflect the importance of thrombin generation and coagulation to one or more functions not directly tied to hemostasis at a site of vascular injury. For example, contact activation-induced coagulation may limit microorganism spread [67,68], a process that falls under the broad umbrella of immunothrombosis [69,70]. Inappropriate triggering of contact-initiated coagulation could contribute to pathologic thrombus formation.
2.3. Tissue Factor-Initiated Coagulation.
The aPTT assay is a useful tool for assessing bleeding and monitoring heparin therapy. However, the sequence of reactions shown in Fig.1B does not reflect hemostasis in vivo. While factor IX deficiency causes severe bleeding (hemophilia B), particularly into soft tissues and joints [71], FXI deficiency causes a milder syndrome involving different tissues [35,72]. Furthermore, as discussed, deficiency of FXII, PK or HK does not compromise hemostasis [35]. These clinical observations are not compatible with the chain of reactions shown in Fig.1B, and have led to revisions in coagulation models.
In current schemes, thrombin generation at an injury site is initiated by a complex comprised of the plasma protease factor VIIa (FVIIa) and the membrane protein tissue factor (TF, Fig.1C). TF is expressed by cells underlying blood vessel endothelium, and is exposed to blood at sites of endothelial damage [73]. The FVIIa/TF complex converts factor X to factor Xa to initiate thrombin generation, and factor IX to factor IXa to sustain thrombin generation. The reactions within the pink box in Fig1.C form the core of the vertebrate thrombin generation mechanism. Complete absence of any single core protein either causes a severe bleeding disorder or is not compatible with life. Note that the model in Fig.1C does not require contact activation to produce thrombin for hemostasis. However, to be complete, it does need to incorporate FXI.
In current coagulation models, FXIa contributes to clot integrity after the clot is formed, primarily by activating factor IX, but perhaps also through activation of factors V, VIII and X, and proteolytic inhibition of tissue factor pathway inhibitor (TFPI) [65,74–76]. The absence of abnormal bleeding in FXII-deficient individuals indicates FXI is activated by FXII-independent processes during the normal response to injury. Thrombin may be the main activator of FXI during hemostasis (Fig.1C, blue arrow) [65,77]. In our current hypothesis, FXI can function as part of contact activation, or independently of it as a component of thrombin generation during hemostasis (Fig.1C). As importantly, FXI forms an interface between the KKS and thrombin generation. Thus, FXI may be involved in more pathways that contribute to thrombosis than other contact factors, and may be a better antithrombotic target for that reason.
2.4. FXI and Hemostasis
As discussed in section 2.3, bleeding in FXI-deficiency is milder and involves different tissues than with deficiency of factor VIII or factor IX (hemophilia A or B). Abnormal bleeding in FXI-deficient patients is usually trauma-induced. The “spontaneous” bleeds characteristic of severe hemophilia A and B are not a feature of FXI deficiency [71,72]. FXI appears to be most important for maintaining clot integrity with injury to tissues with robust fibrinolytic activity, such as the nose, mouth, and urinary tract [35,66,78–82]. Bleeding in other areas is less frequent, and procedures such as circumcision, appendectomy, and orthopedic surgery may be well tolerated without factor replacement [35,66,78,80–82].
While there is disagreement, we feel that there is reasonable support for the conclusion that bleeding is more common and severe in patients with FXI plasma levels <20% of normal than in those with milder deficiency (FXI 20 to 50% of normal) [66,78]. However, symptoms correlate poorly with plasma FXI concentrations [79], and some severely deficient individuals do not experience abnormal bleeding. Several factors could contribute to this conundrum. The predilection for tissue-specific bleeding means some FXI-deficient patients will not experience the type of injury that most often leads to hemorrhage. Also, co-inheritance of other conditions that might otherwise not cause symptoms (e.g. low-normal von Willebrand factor levels), may contribute to bleeding in FXI-deficient patients [83,84]. From available data it seems reasonable to conclude that FXI deficiency exacerbates bleeding in some individuals after injuries, surgical procedures, or childbirth. It may also contribute to menorrhagia [85]. Based on this, we can conclude that a therapeutic strategy that produces the equivalent of severe FXI deficiency will produce symptoms of abnormal hemostasis in some patients.
3. CONTACT FACTORS AND THROMBOSIS
3.1. Mouse Thrombosis Models.
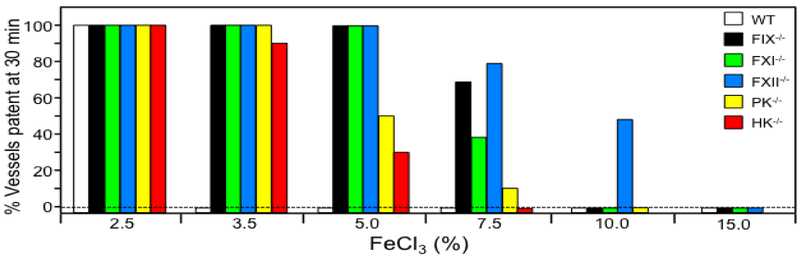
Genetically altered mice have been invaluable in assessing the importance of the contact factors to thrombosis. Fig.2 shows a summary of data from our laboratory testing mice lacking contact factors for resistance to FeCl3-induced arterial thrombosis. Not surprisingly, a higher FeCl3 concentration (more intense injury) is required to induce carotid artery thrombosis in factor IX-deficient mice than in wild type mice [86]. Resistance to thrombosis comes at a price, however, as factor IX deficiency causes a severe bleeding disorder. Counter-intuitively, mice lacking FXI are as resistant to thrombosis as factor IX-deficient mice [86], and factor XII-deficient mice are perhaps more resistant [87], despite the absence of obvious hemostatic abnormalities in animals deficient in FXI or FXII. This implies mechanistic differences between coagulation during thrombosis and hemostasis, and suggests that it is possible to achieve an antithrombotic effect without impairing hemostasis. It also raises the possibility that contact activation-initiated coagulation or something similar to it can promote thrombosis.
Figure 2. Ferric chloride-induced carotid artery occlusion.
Carotid artery occlusion was induced in wild type C57Bl/6 mice (WT, white bars), and in mice lacking factor IX (FIX−/−, black), factor XI (FXI−/−, green), factor XII (XII−/−, blue), prekallikrein (PK−/−, yellow), or high molecular weight kininogen (HK−/−, red) by exposing the vessel to varying concentrations of FeCl3 (as indicated at the bottom of the graph) for three minutes. The percent of animals with patent arteries 30 minutes after FeCl3 exposure is shown (n = 10 for each bar).
Resistance to thrombosis is less pronounced in mice lacking PK or HK than in mice lacking factor IX, XI, or XII (Fig.2), consistent with roles for PK and HK in enhancing FXII activation during contact activation, but not being absolutely necessary for it [88]. However, the main effect of PK (and probably HK) on thrombosis may not be due to a defect in FXIIa-mediate thrombin formation (contact activation) [89]. Stavrou et al. showed that reconstituting PK-deficient mice with plasma PK does not restore arterial thrombus formation induced by laser injury [90]. They showed that PK deficiency, probably through reduced BK generation, leads to increased plasma prostacyclin production and reduced tissue factor expression in blood vessels that may inhibit thrombus formation. Thus, PK may influence thrombus formation through effects on blood vessel homeostasis as well as through effects on contact activation.
3.2. Primate Thrombosis Models.
Work with primates has generated results somewhat different from those in mice. Placing collagen-coated vascular grafts into temporary arteriovenous (AV) shunts in baboons results in platelet and fibrin deposition within the collagen-coated portion of the graft, with subsequent clot extension downstream into the uncoated portion of the graft [87]. Using inhibitory antibodies in this model, we noted that inhibition of FXIa produces a greater antithrombotic effect than inhibiting FXII activation, or FXI activation by FXIIa [87,91–93]. The different results with rodent and primate models could reflect mechanistic differences in thrombus formation between species or models. Perhaps thrombin is more important than FXIIa for activating FXI in the primate model than in rodents. But it is also possible that the antibodies used to block FXIa are more effective at neutralizing their target than those used to inhibit FXII/XIIa. In general, the primate studies are in line with human population data (described in the following section) that suggest FXI makes a greater contribution to common thrombotic conditions than does FXII.
3.3. Epidemiologic Data from Human Populations.
The epidemiologic data presented here point to associations between human disease states and laboratory values, but do not establish causation. For the contact factors, data supporting a role in thrombosis in humans is strongest for FXI. Rates of VTE are lower in FXI-deficient individuals than in the general population [94,95], and higher FXI levels are associated with increased VTE risk in the Leiden Thrombophilia Study (LETS) [96] and the Longitudinal Investigation of Thromboembolism Etiology (LITE) study [97]. FXI deficiency is associated with a reduced incidence of stroke [98], while high FXI levels are linked to increased stroke risk in the Atherosclerosis Risk in Communities (ARIC) study [99] and the Risk of Arterial Thrombosis In relation to Oral contraceptives (RATIO) study [100]. Data for myocardial infarction (MI) are mixed. A study of patients with severe FXI deficiency suggested no protective effect [101], however, an analysis from a large health maintenance organization indicated a reduced incidence of MI with mild (FXI activity 30-50%) and moderate to severe deficiency (FXI activity <30%) [95]. FXI levels correlate with MI incidence in the Study of Myocardial Infarction Leiden (SMILE) [102], but not in the ARIC or RATIO studies [99,100]. Taken as a whole, the results support the premise that FXI contributes to thrombosis in humans, but raise the possibility that the contribution varies depending on the vascular bed involved.
Data supporting roles for FXII in VTE, stroke or MI in humans are weaker than for FXI. Indeed, based on anecdotal reports, it was thought for many years that severe FXII deficiency actually predisposed to VTE [103]; however, larger studies did not find such an association [104], and no relationship was identified between VTE and FXII levels in the LETS [105] or LITE [106] studies. Data pointing to a role for FXII in arterial disease is contradictory (reviewed in [29]). Indeed, the SMILE cohort [102] and a large Austrian study [107] showed an as yet unexplained inverse relationship between FXII levels and cardiovascular disease.
Elevated PK levels have been implicated in vascular disease, hypertension and nephropathy in diabetic patients in the Diabetes Control and Complications Trial/Epidemiology and Diabetes Intervention and Complications Study [108,109]. A PK Asn124Ser polymorphism that reduces HK binding was linked to reduced bradykinin and plasma renin concentrations [110,111], and with reduced risks for hypertension and coronary artery disease [112].
3.4. Contact Activation and Artificial Surfaces in Humans.
Data from human populations suggest that FXI is a larger contributor to common thrombotic disorders such VTE, stroke, and MI in humans than are FXII or PK. Supporting this, deficiency of C1-inhibitor, the main plasma regulator of FXIIa and α-kallikrein, leads to bouts of soft tissue swelling (angioedema) from increased bradykinin generation, but has not been linked to thrombosis [113]. However, FXII may be a major driver of thrombosis in certain situations. Plasma markers of contact activation are increased in patients placed on devices known to induce thrombosis, including CPB [114] and ECMO circuits [51,115] and ventricular assist devices [57,116]. Vascular catheters, renal dialysis membranes [54,117] and artificial heart valves [55] induce FXII-dependent plasma coagulation, and their clinical use is also associated with thrombus formation in the absence of systemic anticoagulation.
4. THERAPEUTIC TARGETING OF CONTACT FACTORS
4.1. Rationale.
In 2001 Gruber and Hanson proposed that inhibiting plasma coagulation proteases “upstream” of factor IX (Fig.1B) could produce an antithrombotic effect, and that such an approach would be safer from a bleeding standpoint than conventional anticoagulation [91,118]. In essence, they suggested that it could be possible to dissociate an antithrombotic effect from undesirable anti-hemostatic effects. A substantial body of pre-clinical evidence now supports this premise, particularly for FXI, and therapeutic strategies are being developed with this goal in mind. A phase 2 study using antisense oligonucleotide (ASO) knockdown of FXI has provided proof of concept for the field [119], and ASOs, antibodies and small molecule inhibitors targeting FXI/FXIa have entered phase 1 and 2 evaluation. The following sections discuss therapies under development, with some of the performance characteristic of general approaches presented in Table 1. Tables 2 and 3 list specific inhibitors of FXI(a) and FXII(a), respectively.
Table 1.
Strategies for Therapeutic Inhibition of Contact Factors
| Compound | Mechanism of Action | Onset of Action | Duration of Action | Possible Reversal Strategies |
|---|---|---|---|---|
| Antisense oligonucleotide | Reduces Protein Synthesis | Slow (2-3 weeks) | Effect may last for weeks to months after last dose | FXI replacement (plasma, FXI concentrate) |
| Antibody to precursor | Binds Zymogen and creates a deficiency state | Rapid | Days to Weeks | FXI replacement may saturate the antibody. Hypothetically a recombinant XI lacking the binding site for the IgG inhibitor |
| Antibody to protease form | Binds protease active site, does not bind to zymogen | Rapid | Days to Weeks | Anti-idiotype antibody for neutralization of IgG Inhibitor |
| Small molecule inhibitor | Binds protease active site | Rapid | Typically minutes to hours | Recombinant VIIa. |
Table 2.
Compounds Targeting Factor XI and Factor XIa
| Inhibitor Type | Compound | Mechanism of Action | In Vivo Analyses | Reference |
|---|---|---|---|---|
| Antisense Oligonucletoide | FXI ASO (mouse) | Reduces hepatic synthesis of FXI. | Inhibits arterial and venous thrombosis. | Zhang et al. Blood 2010;116:4684-4692 |
| FXI ASO (rabbit) | Reduces hepatic synthesis of FXI. | Reduces catheter-induced venous thrombosis. | Yau et al. Blood 2014;123:2102-2107 | |
| FXI ASO (cynomolgus monkeys) | Reduces hepatic synthesis of FXI. | No change in bleeding time following partial tail amputation or gum or skin laceration. | Younis et al. Blood 2012;119:2401-2408 | |
| FXI ASO (baboon) | Reduces hepatic synthesis of FXI. | Inhibits thrombus formation in vascular graft | Crosby et al. ATVB 2013; 33:1670 | |
| FXI ASO (human), formerly ISIS-FXI Rx, now IONIS FXI Rx, and BAY 2306001 | Reduces hepatic synthesis of FXI. | Phase I and II trials in humans. Superior to enoxaparin as prophylaxis for VTE in knee replacement surgery. Phase I trials in ESRD. | Liu et al. Blood 2011; 118: Abs 209 Büller et al. N Engl J Med 2015; 372:232-40 Bethune C. et al. Blood 2017;130:1116. | |
| Monoclonal Antibody | O1A6 (aXIMab) | Binds to FXI/XIa Apple 3 domain. Inhibits FXI activation and FXIa activation of factor IX. | Inhibits thrombus formation in baboon AV shunt model. | Tucker et al. Blood 2009;113:936–944. Zhu et al. Blood 2015;126:1494-1592 |
| 14E11 (Xisomab, 3G3) | Binds to FXI Apple 2 domain. Inhibits FXI activation by FXIIa, but does not affect FXI activation by thrombin, or FXIa activation of factor IX. | Prevents arterial occlusion in mouse FeCl3 model. Inhibits thrombus formation in baboon vascular graft model. Reduces consumptive coagulopathy in mouse peritonitis model. | Cheng et al. Blood 2010; 116:3981 Luo et al Infec. Immun. 2012; 80:91-99. Tucker et al. Blood 2012; 119:4762 Leung et al. Trans. Stroke Res. 2012; 381-9 Zhu et al. Blood 2015;126:1494-1592 | |
| C24 and DEF | Reversible FXIa inhibitor. Blocks FXIa active site but not zymogen. DEF has a reversal agent, FXIaiPD. | Inhibits clotting in human blood, prevents FeCl3-induced carotid artery occlusion in mice reconstituted with human FXI and in rabbits. | David et al. Sci Trans Med. 2016;8:353ra112. Ely et al. Structure. 2018;26:1-12. | |
| αFXI-175 & αFXI-203 | FXI Apple domain 4 and domain 2 binding, respectively. | Inhibits FeCl3-induced IVC thrombosis model in mice. alpha FXI-175 inhibits thrombin generation with lower levels of tissue factor. | van Montfoort et al. JTH 2013; 110:1065-1073 | |
| XI-5108 | Binds FXIa light chain. Inhibits FXIa initiated activated FX and FXIa generation. | Reduces thrombin generation, platelet aggregation and fibrin formation in endothelial denuded rabbit jugular vein model. | Takahashi et al. Thromb Res 2010;125:464-470 | |
| BAY 1213790 | FXIa human monoclonal IgG1 binds the FXIa catalytic domain. | Prolongs a PTT in human plasma. Reduces thrombus weight in rabbit FeCl3 arterial thrombosis model. No effect on bleeding times. Phase 1 studies in healthy adult males. Phase 2 trials ongoing in TKA. | Buchmueller et al. Res. Pract. Thromb. Haemost. 2017;1(Suppl. 1). Thomas et al. Res. Pract. Thromb. Haemost. 2017;1(Suppl. 1). NCT03276143 (FOXTROT) | |
| MAA868 | Anti-FXI IgG. | Prolongs aPTT and lowers free FXI in primates for >30 days. Phase 2 studies announced in atrial fibrillation and TKA. | NOVARTIS.com NCT03393434 NCT03393481 | |
| Small Molecule Active site inhibitors | BMS-262084; 4-carboxy-2-azetidinone compound, | Irreversible inhibitor of FXIa active site. Beta lactam arginine. | Inhibits thrombus formation in rabbit AV shunt model. | Wong et al. J Thromb Hameost 2011;32:129-137. |
| BMS-654457. | FXIa active site inhibitor, reversible. | Inhibits ECAT model in rabbits. | Wong et al. J Thromb Thrombolysis. 2015;40:416-423. | |
| BMX-962212. | FXIa parenteral agent, selective, short half-life. | Efficacious in Rabbit AV-shunt thrombosis model. Phase I trial in humans. | Pinto et al. J Med Chem 2017;60(23):9703-9723. Luettgen et al. Stroke. 2017;48:ATMP117. Perera et al. Clin Pharm Ther. 2017; PI-086 | |
| BMS-986177 | Orally bioavailable FXIa inhibitor. | Several phase 1 studies completed with more ongoing No results available. | NCT02608970, NCT02959060, NCT02807909 NCT03341390, NCT02902679, NCT02982707 NCT03362437, NCT03224260, NCT03000673 NCT03196206 | |
| ONO-7750512 | Orally bioavailable FXIa inhibitor. | IC50 of 3.8 nM. Prolongs aPTT but not PT. Inhibits FeCl3 venous thrombosis and mokey AV shunt thrombosis. No change in rabbit model of ear bleeding. | Koyama et al. ISTH. 2015;13(Suppl. 2):389. | |
| ONO-5450598 | Orally available FXIa inhibitor. Greater bioavailability than ONO-7750512. | Inhibits rabbit FeCl3 arterial thrombosis in rabbits. No increase in bleeding combined with clopidogrel. Preclinical models planned. | Ono et al. ISTH 2017. Kouyama et al. ISTH 2017. | |
| ONO-8610539 (ONO-IG-012) | Parenteral FXIa inhibitor. | Inhibits venous FeCl3 thrombosis in rabbits without increasing bleeding. Prevents AV thrombosis in monkeys. Improves cerebral ischemia-perfusion injury in rabbit MCA. | Gohda et al. Blood. 2014; 124:1542. Sakai et al. ISTH. 2015;13(Suppl. 2):OR352. Sakimoto et al. Stroke. 2017;48:AWP286. | |
| Aryl boronic acid derivative | Irreversible inhibitor of FXIa active site. | Not tested in animals. | Lazarova et al. Bioorgan Med Chem Lett 2006; 16:5022–5027. | |
| Chloro- and amino-quinalone derivatives Compound 13 | Inhibitor of FXIa active site. FXIa IC50 of 1.0nM. | Not tested in animals. | Fjellstroöm et al. PLoS One 2015;10:e0113705 | |
| Compound 1 | Small molecule inhibitor of FXIa. | Inhibits rabbit arterial thrombosis model and cerebral microembolic signals. | Wang et al. J Pharm Exp Ther 2017; 360:476-483. | |
| Phenylimidazole. Compound 13. | FXIa active site inhibitor. | Inhibits carotid artery thrombosis model in rabbits without prolonging bleeding time. | Hu et al. ACS Med. Chem Lett. 2015; 6:590-595. | |
| Macrocyclic Compound 16 | Active site inhibition. Potent anticoagulant activity in vitro clotting assay. | No in vivo data. | Corte et al. J Med Chem 2017;60:1060-1075. | |
| Phenylalanine derived diamides. Compound 21. | Irreversible FXIa active site inhibitor. | Dose-dependent reduction in thrombosis in rabbit carotid arterial thrombosis model. | Smith II et al. Bioorganic and Medicinal Chemistry Letters. 2016; 26:472-478 | |
| Peptidomimetic: Compound 36, 32 | Selective FXIa inhibitor | Prevents rat model of venous thrombosis | Lin et al. J of Med Chem 2006; 49:7781-7791 | |
| Ketoarginine Peptidomimetic | Irreversible inhibitor of FXIa active site. | Inhibits thrombus formation in a rat venous thrombosis model. | Deng et al. Bioorgan Med Chem Lett 2006; 16:3049 | |
| Phenylimidazoles | Reversible inhibitor of FXIa active site. | Inhibits thrombus formation in rabbit AV shunt model | Hangeland et al. J Med Chem 2014; 57:9915 | |
| Pyridine, Pyridinone | FXIa active site inhibitors. In vitro anticoagulant activity. Excellent selectivity (except PK and trypsin) | Not tested in animals | Corte et al. Bioorg. Med. Chem. Lett. 25 (2015): 925-930 | |
| Tetrahydroquinalone derivative | Reversible inhibitor of FXIa active site. | Inhibits thrombus formation in rabbit AV shunt model. | Quan et al. J Med Chem 2014;57;955 | |
| Allosteric inhibitors | Sulfated Pentagalloylglucoside (SPGG) | Non-competitive inhibitor of FXIa. Binds to charged residues on the FXI/XIa catalytic domain producing allosteric effect on activity. | Testing underway in rodents | Al-Horani et al. J Med Chem 2013;56:867-878. Al-Horani and Desai. J Med Chem 2014;57:4805-4818. Al-Horani et al. Throm Res 2015;136:379-387 |
| Monosulfated benzofurans | May bind to the FXIa A3 domain, producing an allosteric effect on activity. | Not tested in animals. | Argade et al. J Med Chem 2014;57:3559 | |
| Natural Inhibitors | AcaNAP 10 -nematode anticoagulant peptide 10 | Inhibitor of FXIa and FVIIa/TF from dog hookworm Ancylostoma caninum. | No in vivo studies. Prolongs PT/aPTT | Deng et al. Biochem & Biophys Res Comm. 2010;392:155-159. Gan et al. FEBS Letters. 2009;583:1976-1980. |
| Clavatadine A | Bromine containing compound from marine sponge S. clavata. FXIa active site inhibitor. | Not tested in animals. | Buchanan et al. J Med Chem 2008;51:3583-3587. Conn et al. J Nat Prod 2015;78:120-124. | |
| Ir-CPI | Serine protease from tick Ixodes ricinus. Inhibits FXI, FXII & PK. | Inhibits murine venous thrombosis model, interferes with arterial thrombosis in mice. | Decrem. J Exp Med. 2009; 23 81-2395 | |
| Boophilin | Kunitz-type FXI and kallikrein inhibitor from midgut of tick, Rhipicephalus microplus | Inhibits FeCl3 carotid artery occlusion; increases bleeding by tail transection | Assumpcao et al. PLoS Neg Trop Dis 2016; 10(1) | |
| Protease nexin-2 Kunitz-domain | Inhibits FXIa active site | Inhibits FeCl3 carotid artery thrombosis in mice and decreases ischemic tissue in middle cerebral artery occlusion model. | Wu et al. Blood 2012; 120:671-677. | |
| rFasxiator | Kunitz-type FXIa inhibitor, from venom of Bungarus fasciatus, the banded krait snake. Recombinant mutant selected. | Inhibits FeCl3 induced murine carotid artery thrombosis | Chen et al. JTH 2015;13:248-261 | |
| Desmolaris | Kunitz-type FXIa active site inhibitor from the salivary gland of the vampire bat Desmodus rotundus. | Inhibits arterial thrombosis in mice. | Ma et al. Blood 2013; 122:4094-4106. | |
| Bikunin / Urinary trypsin inhibitor; MR1007 | Fusion protein with anti-CD14 antibody and bikunin. Inhibits LPS-induced cytokine production and factor XIa. | Improved survival in rabbit endotoxemia and sepsis models. | Nakamura et al. Eur. J. Pharm. 2017;802:60-68. Nakamura et al. J Biophys Chem. 2012;3:132-141. | |
| Aptamers | FELIAP – Factor ELeven Inhibitory APtamer | Inhibits FXIa S2366 cleavage, FIX activation and complex formation with antithrombin | No animal model | Donkor et al. Sci Rep. 2017; 18;7(1):2102. |
Table 3.
Compounds Targeting Factor XII and Factor XIIa.
| Inhibitor Type | Compound | Mechanism of action | In vivo Analysis | Reference |
|---|---|---|---|---|
| Antisense Oligonucleotides | FXII ASO (mouse) | Reduces hepatic synthesis of FXII. | Inhibits arterial and venous thrombosis. | Revenko et al. Blood 2011; 118:5302 |
| FXII ASO (rabbit) | Reduces hepatic synthesis of FXII. | Reduces catheter-induced venous thrombosis. | Yau et al. Blood 2014; 123:2102 | |
| Monoclonal Antibodies | 15H8 | Binds to the FXII heavy chain and inhibits FXII conversion to FXIIa | Inhibits thrombus formation in baboon AV shunt model. Inhibits arterial thrombosis in mice. | Matafonov et al. Blood 2014;123:1739 |
| CSL 3F7 | Binds to the active site of FXIIa. | Inhibit thrombus formation in rabbit ECMO model. | Larsson et al. Sci Transl Med 2014;6:222ra17 Worm Ann Transl Med 2015;3:247 | |
| DO6 (599C-X181-DO6) | FXIIa IgG from human antibody phage display library binds active site. Reduces reciprocal FXII activation by PK. | Reduces fibrin formatin in human blood flowing through collagen coated tubes. | Kokoye et al. Thromb Res. 2016; 140:118-24. | |
| Small Molecule Inhibitors | FXII304 | Inhibitor of FXIIa cleavage of a chromogenic substrate-mechanisms not certain. | Not tested in animals. | Baeriswyl et al. J Med Chem 2013; 56:3742-3746 |
| FXII618 | Inhibitor of FXIIa. | Not tested in vivo. | Midden dorp et al. J Med Chem. 2017. | |
| FXII516 | FXIIa active site inhibitor. | Not tested in vivo. | Baeriswyl ACS Chem Bio 2015;10:1861-70 | |
| 3-carboxymide coumarins, COU254 | Selective FXIIa inhibitor. | No change in acute stroke in mice with intraluminal filament method. | Bouckaert et al. Eu J Med Chem 2016; 110:181-194 Kraft Exp Transl Stroke Med 2010;5 | |
| H-D-Pro-Phe-Arg-chloromethylketone (PCK) | Irreversible inhibitor of activated FXIIa and PK mediated FXII activation. | Protects against ischemic damage in transient MCA occlusion in WT mice (similar to FXII −/− mice) | Kleinschnitz et al J Exp Med 2006; 203: 513-518 | |
| Natural inhibitors | Ayadualin | Lutzomyia ayacuchensis (sand fly) salivary gland cDNA. Inhibits FXII activation to FXIIa. | None | Kato. Biochimie. 2015;112:49-56 |
| Dimiconin | Triatoma dimidiate (kissing bug). Inhibits FXII activation to FXIIa. | None | Ishimaru J Exp Bio 2012;3597-3602 | |
| Ir-CPI | Serine protease from tick Ixodes ricinus. Inhibits FXI, FXII & PK. | Inhibits murine venous thrombosis model, interferes with arterial thrombosis in mice. | Decrem. J Exp Med. 2009; 2381-2395 | |
| Infestin-4, rHA-Infestin-4, rHA-Infestin-4 (mutant) | Inhibitor of FXIIa from the hematophagus insect Triatoma infestan. Recombinant protein fused to albumin. | Inhibits arterial thrombosis in mice. Inhibits AV shunt thrombosis in rats and rabbits. Decreased occlusion rates in mechanically-induced arterial and FeCl3-induced venous thrombosis model in mice. Protects rabbits from FeCl3-induced arterial thrombosis and stasis-prompted venous thrombosis. | Hagedorn et al. Circulation 2010; 121:1510 Xu et al. Thromb Haemost 2014; 111:694 Krupka PLoS One 2016; 27:11 Barbieri et al. J Pharm Exp Ther. 2017; 360:466-475. May et al. BJH. 2016; 173:769-778 Campos et al. Acta Cryst. 2012; 68:695-702 | |
| Aptamers | R4cXII-1 | Inhibits FXII autoactivation and FXIIa activation of FXI. | Not tested in animals. | Woodruff et al. J Thromb Haemost 2013;11:1362 |
4.2. Antisense Approaches.
4.2.1. Antisense Oligonucleotides (ASOs).
ASOs termed gapmers are short (~20 base pair) DNA sequences that are antisense partners for the mRNA of a protein of interest [120,121]. Their plasma stability and cellular uptake is enhanced by 2′-methoxyethyl modified DNA wings attached to the ends of the antisense sequence. After subcutaneous administration, ASOs are taken up by certain cell types, including hepatocytes. This makes them ideal for targeting coagulation proteases, most of which are synthesized in the liver. The ASO forms a heterodimer with its target mRNA, and the complex is degraded by an RNAse H-dependent process. The result is specific reduction in synthesis of the target protein and a decrease in its plasma concentration. Carefully selected ASOs are highly specific for a target protein, and the effect on plasma level can last for weeks to months after the last dose [120,121]. The deficiency state created by ASOs can be reversed by infusion of plasma or factor concentrate. ASOs take two to four weeks to reduce proteins to a desired level, so they are most appropriate for long-term therapy, and not for situations requiring rapid anticoagulation.
4.2.2. Pre-clinical studies with ASOs.
ASOs specific for FXI, FXII, and PK have been tested in mice, and produce antithrombotic effects in arterial and venous thrombosis models that are comparable to those observed in gene-deleted mice [58,122]. Interestingly, reducing plasma FXII results in an increase in the plasma level of PK, and reducing PK leads to increased FXII, consistent with these proteins reciprocally activating each other at a basal level in healthy animals [58]. ASOs that reduced plasma levels of FXI, FXII, or HK increased the time to jugular vein occlusion induced by placement of an intravenous catheter in rabbits [123].
The ASO ISIS 416858 (now IONIS-FXI Rx or BAY2306001) reduced plasma FXI concentration in a dose-dependent manner in cynomolgous monkeys and baboons [124]. An antithrombotic effect was observed in the baboon AV shunt model (section 3.2) starting when the plasma FXI level was reduced to ~50% of normal and reaching maximum effect at ~20% of normal. This dose-response is consistent with observations with ASO-treated mice [122]. Rodents and primates treated with FXI ASOs did not demonstrate abnormal hemostasis in response to tail, gum, or skin laceration, even though some animals had FXI levels <5% of normal.
4.2.3. Pre-clinical studies with small interfering RNAs (siRNAs).
siRNAs are another antisense-based approach for post-translational gene silencing [125]. A model of spontaneous venous thrombosis in mice was developed using siRNA-mediated reductions of the coagulation regulatory proteins antithrombin and protein C [126]. When an siRNA that reduces FXII was studied in this model, surprisingly, there was an acceleration of thrombosis [127]. This suggests that FXII may serve a previously unrecognized regulatory (antithrombotic) function. It must be noted that the results of this study run counter to those from other studies on FXII and thrombosis in mice. While the reasons for this are not established, an unidentified off-target effect of the siRNA is a possibility.
4.2.4. Phase 1 and 2 studies with ASOs.
Results of a phase 1 trial in healthy volunteers treated with IONIS-FXI Rx were reported in 2011 [128]. Subjects received 50 to 300 mg of ASO subcutaneously three times during the first week of treatment, and then once weekly for four weeks. A dose response was noted, with 200 or 300 mg doses consistently reducing FXI levels to 20% of normal. Some subjects had barely detectable levels (<5% of normal). Reduced plasma FXI was accompanied by prolongation of the aPTT, with the maximum effect achieved after 2 to 3 weeks of treatment. Excessive bleeding was not noted, and most adverse reactions were related to mild irritation at the ASO injection sites.
IONIS-FXI Rx has been compared to enoxaparin for VTE prophylaxis in patients undergoing total knee arthroplasty [119]. A 200 mg ASO regimen reduced plasma FXI to 38% of normal (average), while the 300 mg regimen reduced it to ~20%. 200 mg ASO reduced the incidence of thrombus formation (27%) to a similar degree as standard enoxaparin therapy (30%) as determined by venography performed 8 to 12 days post-surgery. The 300 mg ASO regiment was superior to enoxaparin, with thrombi detected in only 4% of patients. In addition to the striking reduction in incidence, clots in patients treated with 300 mg ASO were much smaller than in those on enoxaparin or 200 mg ASO. The study was not powered to assess differences in bleeding, however, there was a trend toward less clinically relevant bleeding with ASOs (3%) than with enoxaparin (8%). An important feature of the study was that patients treated with ASOs underwent major orthopedic surgery while under the full effect of the drug (nadir FXI levels) with little evidence of abnormal hemostasis [119]. FXI levels were still reduced several weeks after the last dose, indicating this approach is well suited for long-term prophylaxis.
IONIS-FXI Rx has been tested in a phase 2 multicenter study in patients with end stage renal disease (ESRD) on dialysis [129]. All patients received standard heparin therapy during dialysis. The ASO did not accumulate over the 12 weeks of the study. Reducing FXI activity to <40% of normal reduced the number of severe clotting events in the dialysis circuit. No major or clinically relevant non-major bleeding occurred. Minor bleeding at AV fistula sites occurred mostly in the group receiving the highest (300 mg) ASO dose, but bleeding did not correlate with FXI level. Based on the results of this study safety, a phase 2 dose evaluation study of IONIS-FXI RX administered over 26 weeks to patients with ESRD is planned [130].
4.3. Antibodies.
A properly selected antibody combines high specificity for a target with a long plasma half-life. IgGs that bind precursors of contact proteases essentially produce a factor-deficient state. It can be difficult to titrate the drug effect because of the broad normal plasma ranges of the targets and, realistically, such IgGs will be used to saturate a target, producing maximum inhibition. IgGs targeting active proteases will not engage the target until it is activated. These IgGs typically bind at the protease active site, and must compete with physiologic substrates for binding. It may be easier to titer their effects as inhibition will not be complete, except at very high IgG concentrations. Because of their long half-lives and tendency to equilibrate across the intra- and extravascular spaces, it may be difficult to reverse the inhibitory effect of an antibody in a short period of time. Here we discuss IgGs that bind FXI, FXII, PK, and their active forms.
4.3.1. Anti-FXI IgG.
Several monoclonal IgGs that bind FXI have been tested in pre-clinical models. IgG O1A6 (aXIMab) was raised against human FXI in wild type mice, and binds to the FXI apple 3 (A3) domain [92], blocking an exosite required for factor IX binding [131]. It also inhibits FXI activation by FXIIa [132]. O1A6 is a potent antithrombotic in FXI-deficient mice reconstituted with human FXI and in the baboon AV shunt model [92]. van Montfoort et al. described two monoclonal anti-human IgGs, αFXI-175 (A2 domain) and αFXI-203 (A4 domain), that reduce venous thrombus size in FXI-deficient mice reconstituted with human FXI [133].
IgG 14E11 was raised by immunizing FXI-deficient mice with mouse FXI [87]. It binds to the A2 domain of FXI from most mammalian species, and interferes with FXI activation by FXIIa [87]. It does not inhibit FXI activation by thrombin, nor FXIa activation of factor IX, in vitro. It may, therefore, interfere less with hemostasis than O1A6. The antithrombotic effect of 14E11 is comparable to O1A6 in mice. While also inhibiting thrombus formation in the baboon model, it is less potent that O1A6 [87]. 14E11 also demonstrated beneficial effects in several infection/sepsis models. It improved survival in mice after ligation and puncture of the cecum (CLP) [37] and after infection with Listeria monocytogenes [134]. A humanized version of 14E11 (ABO22, Xisomab 3G3, Aronora Inc.) protected baboons from death after a lethal injection of heat inactivated Staphylococcus aureus [135]. In the CLP model, 14E11 blunted the early cytokine response to injury, and reduced activation of contact factors [37]. Similar effects were noted in baboons after S. aureus infusion [135]. 14E11 also reduced tissue injury in cerebral [136] and myocardial ischemia-reperfusion models in mice [137]. 3G3 has completed phase 1 safety and tolerability evaluations in healthy adults (NCT03097341) [138,139].
In preclinical studies, a single saturating dose of the anti-FXI IgG MAA868 (Novartis Pharma) prolonged the aPTT in primates for >30 days. Phase 2 trials are planned to compare the efficacy of MAA868 to enoxaparin for VTE prophylaxis in knee replacement surgery (NCT03393481) and to measure its effects on D-dimer and other biomarkers of thrombogenesis in comparison to apixaban in patients with atrial fibrillation (NCT03398434) [140,141].
4.3.2. Anti-FXIa IgG.
David et al. described humanized IgGs from a phage display library with high affinity for FXIa but not zymogen FXI [142]. C24 prevented FeCl3-induced carotid artery occlusion in FXI-deficient mice reconstituted with human FXI. DEF is similar to C24, but with a modified Fc region to avoid platelet or complement activation. In rabbits, DEF prevented thread-induced inferior vena cava thrombosis without inducing bleeding. Its effects can be reversed with the anti-idiotype IgG revC4 [142]. A structure of DEF in complex with a recombinant inactivated FXIa protease domain (FXIaiPD), revealed that the DEF light chain occludes the S1 substrate binding site of FXIa, while the heavy chain interacts with other sites on the protease surface [143]. FXIaiPD binds DEF with picomolar affinity, and can be used to saturate free DEF [143], reversing its inhibitory effects in vitro in a manner similar to that by which andexanet alfa (an inactive factor Xa catalytic domain) reverses the effects of DOACs targeting factor Xa [17].
The human monoclonal IgG1 BAY1213790 (Bayer Pharma) binds to the FXIa active site, and produces a dose-dependent reduction in thrombus weight in a rabbit FeCl3 arterial thrombosis model without increasing ear or gum bleeding times [144]. Healthy volunteers receiving a single infusion of BAY 1213790 in a dose-escalation study experienced prolongation of the aPTT in a dose-dependent manner without increased bleeding time [145]. A phase 2 trial (FOXTROT) is enrolling patients to compare BAY1213790 with enoxaparin or apixaban in prevention of venous thrombosis in patients undergoing elective knee replacement (NCT03276143) [146].
4.3.3. Anti-FXII-IgG.
In the baboon AV shunt model, the anti-FXII-IgG 15H8 produces an antithrombotic effect similar in potency to anti-FXI IgG 14E11 [87,93,147], but less potent than IgG O1A6 [92,93]. 15H8 binds to the FXII non-catalytic heavy chain, a part of the protein required for surface-dependent conversion of FXII to FXIIa. It has little effect on FXIIa activity. The weaker effect of 15H8 relative to O1A6 could reflect the importance of thrombin-mediated FXI activation to thrombosis in primates, which would be blocked by O1A6, but not 15H8. Alternatively, the inability of 15H8 to block FXIIa activity may render it a suboptimal FXII inhibitor, and an antibody that binds to the catalytic domain, or that neutralizes FXIIa activity may be more effective.
4.3.4. Anti-FXIIa-IgG.
Laarson et al. described CSL 3F7, an IgG isolated from a phage display library that binds to the FXIIa active site. It interacts poorly with the precursor FXII [148]. In rabbits undergoing extracorporeal membrane oxygenation (ECMO), 3F7 was comparable to heparin in its ability to prevent thrombotic occlusion of the oxygenator circuit [148]. This study elegantly demonstrates the importance of FXIIa and contact activation to thrombus formation when blood comes into contact with an artificial (non-biologic) surface.
4.3.5. Anti-kallikrein IgG.
Inhibitors of kallikrein have been developed primarily for treating angioedema, and have not been studied extensively for their effects on thrombosis. In an ex vivo flow model with human blood, surface-induced thrombin generation and fibrin formation are reduced by an IgG to the kallikrein active site (559A-M202-H03, Dyax), but to a lesser extent than an IgG directed against the FXIIa active site (599C-X181-D06, Dyax) [88]. A related anti-kallikrein IgG (DX-2930, Dyax) has been evaluated in phase 1b studies for angioedema. DX-2930 reduces plasma levels of cleaved HK and the frequency of angioedema episodes in patients with C1-INH deficiency [149,150]. It has not been tested for antithrombotic effects.
4.4. Small Molecule Active Site Inhibitors.
Several synthetic small molecule inhibitors of FXIa have entered, or will soon enter, phase 1 and phase 2 trials. Most are administered parenterally, but progress is being made on orally available agents. The duration of the effects are generally much shorter than those of ASOs or antibodies, making them useful for situations where a brief anti-thrombotic effect is needed. Orally available drugs would ideally have sufficiently long plasma half-lives to permit once or twice a day dosing. Specificity can be an issue with small molecules. The catalytic domain of FXIa belongs to the large family of trypsin-like proteases, and small molecule inhibitors to FXIa may show cross-reactivity with other group members. For example, several FXIa inhibitors have activity against α-kallikrein, a homolog of FXIa. An off-target effect involving α-kallikrein may actually be advantageous when it comes to treating thrombosis or reducing inflammation.
4.4.1. Parenteral FXIa inhibitors.
Small molecule inhibitors that bind to the FXIa active site have been prepared on a variety of scaffolds (Table 2). Here we discuss agents that are furthest along in development. BMS-654457 (Bristol-Myers Squibb) is a competitive, reversible, parenterally administered FXIa inhibitor (Ki 0.2 nM) that prolongs the aPTT of human and rabbit plasma, with no effect on the PT or platelet aggregation [151]. It has some activity against plasma kallikrein, which may contribute to aPTT prolongation, and perhaps to efficacy in vivo. BMS-654457 prolongs the aPTT in rabbits in a dose-dependent manner, and prevents vessel occlusion in an electrically induced carotid artery thrombosis (ECAT) model. Unlike warfarin or dabigatran, BMS-654457 had no significant effect on bleeding time in the ECAT model [151].
BMS-962212 is a reversible parenterally administered FXIa inhibitor (Ki 0.7 nM for human FXIa). Like BMS-654457, it prolongs the aPTT and reduces thrombus weight in the rabbit ECAT model without increasing bleeding [152,153]. Results of phase 1 trials examining pharmacodynamics, pharmacokinetics, and safety in humans were recently reported [154]. BMS-962212 was administered to healthy volunteers for two hours or five days as a continuous infusion, and exhibited mean plasma half-lives ranging from 2 to 8.6 hours depending on the dose. There were no dose-limiting toxicities, and the most common adverse event was mild pain and/or erythema at the infusion site. Steady state was attained after approximately two hours of infusion, coinciding with prolongation of the PTT and decreased plasma FXI activity [154].
ONO-8610539 (Ono Pharmaceutical) is a parenterally administered FXIa inhibitor with similar effects on the aPTT to BMS-654457. It has been studied in several preclinical models. ONO-8610539 prevents FeCl3-induced inferior vena cava thrombosis in rabbits without compromising hemostasis [155], protects against wire-induced AV shunt thrombosis in cynomolgus monkeys [156], and prevents photothrombotic middle cerebral artery occlusion in rabbits [157].
Lin et al. used the structure of known thrombin inhibitors to develop peptidomimetics with FXIa inhibitory activity. X-ray crystal structures indicate that the inhibitor Compound 32 interacts with the S1 region of the FXIa active site [158]. Compound 32 has much greater affinity for FXIa than FXa or thrombin. Despite having greater activity for human FXIa than rat FXIa (based on PTT assays), Compound 32 was efficacious in a rat model of venous thrombosis at high concentrations without increasing bleeding [158].
4.4.2. Oral FXIa inhibitors.
Two orally available FXIa inhibitors have been described [138]. ONO-5450598 has shown promising results in preclinical studies in rabbits and monkeys. It has a Ki of 2 nM for human FXIa and a 10-fold higher Ki for kallikrein. In rabbits treated with clopidogrel, ONO-5450598 was compared to rivaroxaban for prevention of FeCl3-induced arterial thrombosis. At higher doses, ONO-5450598 was comparable or superior to rivaroxaban in prolonging time to vessel occlusion, with significantly less bleeding [159]. It is effective in a monkey AV shunt thrombosis model but has not yet entered phase 1 studies [160]. The orally available FXIa inhibitor BMS-986177 has completed several phase 1 pharmacokinetic and pharmacodynamics studies, with additional studies currently recruiting patients [161,162].
4.5. Other Compounds that Inhibit Contact Factors.
While ASOs, monoclonal antibodies, and small molecule inhibitors directed at contact factors are farthest along in development, several other types of interesting inhibitors have been described, some of which have been tested in pre-clinical models.
4.5.1. Sulfated pentagalloyl glucopyranoside.
In general, inhibitors of FXIa either bind to or block the protease active site (e.g. BAY1213790 or BMS-654457), or block a site remote from the active site (an exosite) required for engaging other macromolecules (e.g. aXIMab). SPPG2, a sulfated pentagalloyl B-D-glucopyranoside appears to act by a distinct mechanism. SPGG2 is a non-saccharide glycosaminoglycan mimetic that binds to an anion binding site on the FXIa catalytic domain (Lys529, Arg530 and Arg532) that is not directly involved in interactions with substrates [163,164]. The molecule appears to induce conformational changes in the protease domain that interfere with active site function. SPGG2 prolongs the APTT of normal plasma, but has not been tested in animals. Because its interaction with FXIa is largely charge based, its effect can be partially reversed with the polycation protamine.
4.5.2. RNA and DNA aptamers.
Aptamers are short single-stranded oligonucleotides (ssDNA or ssRNA) that fold into structures that bind a target of interest. They are usually selected from pools of random oligonucleotides. Woodruff et al. prepared two ssRNA aptamers (11.16 and 12.7) to FXIa using Systemic Evolution of Ligands by Exponential Enrichment (SELEX) [165]. The aptamers are non-competitive inhibitors of FXIa cleavage of small chromogenic substrates and of activation of factor IX. Both aptamers interact with anion binding sites on FXIa, possibly through the positively charged phosphate backbones of the aptamers. Donkor et al. described Factor ELeven Inhibitory APtamer (FELIAP) [166], an 80 nucleotides ssDNA aptamer that binds with nanomolar affinity near the FXIa active site, competitively inhibiting substrate engagement. Because inhibition is partial, micromolar concentrations are required to inhibit nanomolar FXIa, and structural optimization is required to improve potency before testing in animals [166].
Woodruff et al. also described an RNA aptamer (R4cXII-1) with high affinity for the non-catalytic heavy chain of FXII and FXIIa [167]. R4cXII-1 prevents FXII autoactivation and FXIIa activation of FXI, effectively blocking contact activation-induced thrombin generation in vitro. Interestingly, it does not block FXIIa activation of PK. Steen Burrell et al. reported on an RNA aptamer (Kall1-T4) that binds to PK and kallikrein with subnanomolar affinities [168]. Kall1-T4 prolongs plasma clotting in aPTT assays and reduces HK cleavage by kallikrein.
4.5.3. Kunitz-type protease inhibitors.
Kunitz-type inhibitors are small (50 to 60 amino acid) protein sequences typically constrained by three disulfide bonds. While often part of a larger protein, isolated Kunitz domains such as aprotinin (bovine pancreatic trypsin inhibitor or traysylol) have been used as scaffolds for developing more specific inhibitors. Protease nexin-2 (PN2), an isoform of the beta-amyloid protein precursor, contains a Kunitz domain that is a potent FXIa inhibitor [169]. PN2 was identified as the major FXIa inhibitor in platelet α-granules [169]. Over-expressing PN2 in mice reduces thrombus formation [170], and recombinant versions of PN2 are antithrombotic in mice without apparent compromise of hemostasis [171]. While there are reports that PN2 inhibits several coagulation proteases (factor VIIa, factor IXa, factor Xa) [172], other work indicates high specificity for FXIa [173], and doses of PN2 that prevent carotid artery occlusion and ischemic stroke in mice do not increase bleeding [171].
FXIa interacts with a number of other Kunitz-type inhibitors. Puy et al. showed that FXIa inhibits the anticoagulant activity of TFPI by cleaving within the active sites of TFPI’s second and third Kunitz domains [76]. This suggests that TFPI initially engages the FXIa active site through its Kunitz domains. The TFPI homolog TFPI-2 inhibits FXIa and kallikrein via its first Kunitz domain [174,175]. Desmolaris is a homolog of TFPI lacking the Kunitz-1 domain that is expressed in the salivary gland of the vampire bat Desmodus rotundus [176]. Its inhibition of FXIa is enhanced by heparin. It also inhibits kallikrein, and reduces thrombus formation in mice without increasing injury-induced bleeding. Fasxiator is a Kunitz-type FXIa inhibitor isolated from the venom of the banded krait snake (Bungarus fasiatus) [177]. Modified versions of fasxiator have subnanomolar affinities for FXIa. The modified molecule prolonged time to thrombus formation in a mouse arterial thrombosis model.
4.5.4. Kazal-type protease inhibitors.
Kazal domains are small protein sequences containing a short alpha-helix, a beta-fold and three disulfide bonds that often appear in tandem arrays. Infestin is a Kazal-type protease inhibitor isolated from the midgut of a hematophagous insect, the kissing bug Triatoma infestans. Infestin domains inhibit several coagulation proteases, with the fourth demonstrating activity against FXIIa, and some effect on plasmin and factor Xa [178]. A recombinant protein comprised of the fourth Kazal-type domain linked to human albumin reduces thrombus formation in animal venous and arterial thrombosis models, and inhibits surface induced thrombosis [179–183]. Subsequent modifications have made it more selective for FXIIa than the native protein without loss of antithrombotic potency [178].
5. FUTURE DIRECTIONS
Phase 1 and 2 studies are underway to test the safety and efficacy of several compounds that target FXI(a). While epidemiologic data for disorders such as VTE and ischemic stroke suggest that FXI is a better target than FXII for common thrombotic conditions, they do not establish that this will be the case in all situations. The spectrum of thrombo-inflammatory disorders amenable to manipulation of the contact system remains to be established. Larger trials comparing strategies targeting contact factors to standard therapies will ultimately establish effectiveness and safety, however, available information permits informed speculation on situations where drugs targeting contact proteins may be useful.
5.1. Which Contact Factor is the Best Therapeutic Target?
FXI appears to be a more important contributor to common thrombotic conditions such as VTE and stroke than other contact factors. This may relate to the protein’s dual role as part of a thrombin-induced feedback circuit that sustains coagulation independently of FXII, and as a link between the KKS and thrombin generation. As TF likely contributes to many thrombotic events in humans [184,185], blocking FXIa generated by the TF-initiated thrombin feedback pathway shown in Fig.1C may be an important component of the antithrombotic effect of a FXIa inhibitor. In this case, there is likely to be an advantage to therapeutic targeting of FXI over FXII as a FXIIa inhibitor would only block one source of FXI activation.
The most attractive feature of strategies directed at FXII is safety. A specific FXIIa inhibitor should not increase bleeding risk, nor aggravate trauma-induced bleeding. A possible downside, as suggested by epidemiologic data, could be poor efficacy for prevention of common disorders such as VTE, stroke or MI. Even if this is the case, FXII appears to be a major driver of thrombosis when blood is exposed to artificial surfaces that promote contact activation. Pre-clinical and in vitro data indicate that FXII/XIIa inhibitors will effectively limit thrombus formation triggered by such devices. Another potential advantage of a factor XIIa inhibitor over a FXIa inhibitor in this setting is that the former would limit prekallikrein activation, reducing bradykinin formation and the associated inflammatory response.
Kallikrein inhibitors are being developed for treatment or prevention of angioedema. While designed to block kallikrein cleavage of HK, they could be used to interfere with FXII-PK reciprocal activation, reducing FXIIa generation. Pre-clinical and ex vivo data suggest that kallikrein inhibitors alone would have a relatively weak antithrombotic effect compared to a FXIIa inhibitor. However, combining a FXIIa and a kallikrein inhibitor could produce a synergistic effect that may have a larger effect on FXII activation than targeting either FXIIa or kallikrein alone.
5.2. What Conditions are Most Suitable for Treatment with a Contact Factor Inhibitor?
In humans, plasma FXI levels are most closely linked with VTE risk, and it makes sense to test FXI/FXIa inhibitors for primary or secondary prophylaxis to prevent VTE. As demonstrated by ASO trials in humans and non-human primates, lowering plasma FXI levels to ~20% of normal has a potent antithrombotic effect with little compromise of hemostasis, even during orthopedic surgery. As primary prophylaxis, a drug targeting FXI could have a better safety profile than heparins or DOACs. This may allow prophylaxis to be used in more patients across a broader range of clinical situations, including patients with bleeding propensities. Exceptions would likely be those undergoing surgery on the nasopharynx, mouth or urinary tract.
FXI may be a particularly attractive target for secondary prevention of VTE. The usefulness of extended anticoagulation beyond the typical 3 to 6 months for unprovoked deep vein thrombosis or pulmonary embolus has been debated [186,187]. The advantage of preventing relatively rare deaths with extended prophylaxis may be offset partly by increased major bleeding. However, there is benefit to preventing more common complications such as post-phlebitic syndrome and pulmonary hypertension. Low doses of FXa-targeting DOACs have shown efficacy and safety in this scenario [188]. FXIa inhibitors may be effective here as well, while potentially lowering the bleeding risk even further.
Inhibitors of FXI or FXII may be useful in preventing stroke in patients with atrial fibrillation who do not receive treatment with warfarin or a DOAC because of a perceived high risk for bleeding. A retrospective study of more than 90,000 patients with acute ischemic stroke and prior atrial fibrillation determined that 30% were not on antithrombotic therapy, and ~40% received an anti-platelet agent only [189]. Two-thirds had no documented reason as to why anticoagulation was withheld, but for those with a documented reason, bleeding (16%) and falls (10%) were cited most often. Many of these untreated patients are at high risk of stroke [190], and could benefit from treatments that carry a lower risk of bleeding than warfarin or DOACs. Patients with atrial fibrillation and severe renal impairment may be excellent candidates for therapy targeting the contact system. The benefits of warfarin therapy may be offset by adverse side effects in this group, and DOACs have not been thoroughly evaluated. Patients with renal failure, regardless of whether or not they have atrial fibrillation, have high rates of cardiovascular events that account for about half of the group mortality [117,191]. This may be another setting where a FXI or FXII inhibit may have a superior risk-benefit ratio to current anticoagulants.
Perhaps the setting where justification for testing FXI or FXII inhibitors is clearest is in patients on extracorporeal circuits (cardiopulmonary bypass, ECMO) or with indwelling mechanical devices (heart valves, VADs, central venous catheters), where there is a high risk for thrombus-induced device failure and thromboembolism in the absence of anticoagulation. Many patients on extracorporeal circuits or with indwelling devices are at increased risk for bleeding, even as they require antithrombotic therapy. The situation with VADs illustrates the dilemma. VAD patients are typically treated with warfarin and an anti-platelet agent to prevent thrombus formation within the device. At the same time, the turbulent flow created by the VAD leads to loss of large von Willebrand factor multimers, causing a defect in platelet function (acquired von Willebrand syndrome) that contributes to a bleeding rate of >10%. Therapy directed at FXI or FXII may reduce thrombin generation on the device surface, while causing less impairment of hemostasis than warfarin, lowering the impact of the acquired von Willebrand syndrome
At this point, it is difficult to draw firm conclusions regarding the intensities of the anticoagulant effects produced by FXI or FXII inhibitors relative to those produced by warfarin or DOACs. Given this, it is difficult to speculate on whether targeting a contact factor will be useful for treatment of acute thrombosis. Until we better understand the manner in which FXI and FXII contribute to thrombosis, it seems prudent to limit clinical trials to prophylactic situations.
5.3. Is a Reversal Agent Needed for FXI and FXIa Inhibitors?
FXI is required for hemostasis in some individuals [72], and some patients receiving FXI inhibitors may experience therapy-related bleeding during surgery on certain tissues (nose, oropharynx, urinary tract) or after trauma. While most hemorrhage in FXI-deficient patients involves injury to vulnerable tissues, “spontaneous” bleeding in the form of menorrhagia can be a problem in FXI-deficient women [85]. Furthermore, a substantial number of patients who may receive therapy with a FXIa inhibitor will be elderly, with conditions that predispose to bleeding even when they are not anticoagulated. This raises the question of whether or not reversal agents are necessary to deal with bleeding in patients on FXIa inhibitors.
For FXI ASO-based therapy, factor replacement with plasma or FXI concentrate will rapidly (although temporarily) reverse the effect of the ASO. Such an approach may also work with anti-FXI IgGs, which could be saturated with exogenous FXI. Some FXI-deficient patients develop alloantibody inhibitors to FXI after replacement therapy [192]. Antifibrinolytic therapy or recombinant factor VIIa (NovoSeven, Novo Nordisk Inc.) have been used successfully to treat bleeding episodes or prevent bleeding with invasive procedures in such patients [193–195]. These strategies could be used to treat bleeding in patients receiving anti-FXIa antibodies or small molecule FXIa inhibitors, or to prepare patients on such agents for invasive procedures. However, they may not be deemed suitable for certain patients, such as those at high risk for thromboembolism. A reversal agent that does not directly impact other parts of the coagulation mechanism (e.g. anti-idiotype IgG for anti-FXIa antibodies, or a decoy such as FXIaiPD [section 4.3.2]) is desirable. However, it is important to recognize that many patients who will be receiving FXIa inhibitors may be predisposed to bleeding independently of the medication. Given this, while reversal agents may be developed that negate the effects of FXI/XIa inhibitors on the aPTT, it may be difficult to demonstrate their efficacy in terms of changes in bleeding.
5.4. Monitoring Strategies.
An advantage of DOACs targeting thrombin and factor Xa is that fixed doses are used for most patients, and testing to establish that a patient is in a therapeutic range is not usually necessary. However, it is now clear that it is useful to have the ability to measure drug concentration in some situations (acute changes in kidney or liver function, extreme body weight, unexpected bleeding, thrombosis in the face of apparently adequate therapy) [196]. Complete FXII deficiency is not associated with an obvious abnormal phenotype. Unlike the current DOACs, a therapy targeting this protein could be designed to block all or almost all activity. In essence, there would be no therapeutic range for a FXII/FXIIa inhibitor, and the goal would be to make sure enough compound was on board to produce a desired clinical effect. Drugs targeting FXI or FXIa present a different challenge, as they may contribute to bleeding. Pre-clinical and clinical studies suggest that reducing plasma FXI concentration to 20% of normal with ASOs may achieve a maximum anti-thrombotic effect. Given the clinical impression that patients with severe FXI deficiency (<20% of normal) may bleed more than those with mild deficiency, there may be advantages to adjusting therapy based on a therapeutic range.
Drugs targeting FXI, FXII or their active forms prolong the aPTT and, hypothetically, this assay could be used for monitoring in a manner similar to heparin. Such an approach would likely be most accurate for ASO-based treatments or antibodies that bind the zymogen of a target because they cause either true deficiency (ASOs) or the equivalent of a deficiency state (antibodies to zymogens). Indeed, specific measurements of FXI activity based on the aPTT could be used in ASO-treated individuals. However, most small molecule inhibitors and some antibodies will be inhibitors of FXIa and FXIIa. Their effects on the aPTT are likely to vary based on the version of the assay used, and drug features such affinity for the target and on and off rates. Case in point, rivaroxaban has a greater effect on most prothrombin time assays than apixaban, even though both target factor Xa and have comparable therapeutic effects. Specific chromogenic assays based on the ability of drug in patient plasma to inhibit a known amount of added FXIa or FXIIa could be used to determine drug concentration, similar to factor Xa-based assays for measuring heparins and DOACs. However, such assays have not been developed, and would probably not be widely available. From a practical standpoint, if monitoring is deemed necessary for FXIa or FXIIa inhibitors, it will probably initially be done with the aPTT or a modification of it.
5.5. Multi-drug Therapy with FXI or FXII Inhibitors.
Several studies using combination drug therapy have demonstrated improved antithrombotic efficacy at the cost of significantly increased bleeding. Adding the DOAC rivaroxaban to standard antiplatelet therapy in the ATLAS ACS-TIMI 46 trial reduced death from cardiovascular disease, MI, and stroke compared with standard therapy, but significantly increased major bleeding [197]. Superimposing the PAR-1 inhibitor vorapaxar onto standard antiplatelet therapy (aspirin and a P2Y12 inhibitor) in patients with acute coronary syndromes in the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial, was associated with increased intracranial bleeding in patients with a prior history of stroke [198]. Because of their limited contributions to hemostasis, addition of a drug targeting FXI(a), FXII(a) or PK/kallikrein to pre-existing anticoagulation or antiplatelet therapy may avoid the pitfall of increased bleeding. Indeed, even if FXI and FXII inhibitors do not provide the same level of antithrombotic activity as inhibitors of thrombin or factor Xa, they may produce a synergistic or additive antithrombotic effect when superimposed on current agents without further compromise of hemostasis.
6. SUMMARY
The demonstration that FXI reduction can protect patients undergoing knee replacement surgery from venous thromboembolism provides proof of concept for the notion that a useful antithrombotic effect can be produced in humans by targeting a contact factor. Future efforts will be directed at establishing the spectrum of clinical scenarios in which FXI and contact activation contribute to thrombosis, and determining which components of the contact system are the best targets for each clinical situation. If drugs targeting contact proteases prove to be effective antithrombotics in humans, their safety profiles could increase the number of patients with indications for anticoagulation who actually receive therapy, and widen the spectrum of clinical conditions in which antithrombotic therapy can be safely administered.
7. RESEARCH AGENDA.
Identify the plasma contact factor that is the most appropriate target for specific situations.
Establish whether or not a reversal strategy is required for drugs targeting factor XI.
Establish if monitoring will be necessary for drugs targeting contact factors and, if so, determine what the best monitoring assay would be.
Study the therapeutic effects of drugs targeting contact factors when superimposed on current drug therapy.
ACKNOWLEDGMENTS
The authors wish to acknowledge support from awards HL81326, HL58837 and HL140025 (D. Gailani); HL101972, GM116184, and AI088937 (A. Gruber); and HL101972 and GM116184 (O. McCarty) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE (CONFLICTS OF INTEREST)
B. Tillman has no conflicts of interest to report. A. Gruber and OHSU have financial interest in Aronora, Inc., a company that is developing antithrombotic agents. O. McCarty has no conflicts of interest to report. D. Gailani is a consultant for several pharmaceutical companies (Bayer, Bristol-Myers Squibb, Dyax, Instrument Laboratory, Ionis, and Ono) and receives consultant fees or research support.
REFERENCES
- [1].Enriquez A, Baranchuk A, Redfearn D, Simpson C, Abdollah H, Michael K. Dabigatran for the prevention and treatment of thromboembolic disorders. Expert Rev Cardiovasc Ther 2015;13:529–40. [DOI] [PubMed] [Google Scholar]
- [2].Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol 2015;35:1056–65. [DOI] [PubMed] [Google Scholar]
- [3].Ferreira JL, Wipf JE. Pharmacologic Therapies in Anticoagulation. Med Clin North Am 2016;100:695–718. [DOI] [PubMed] [Google Scholar]
- [4].Chan NC, Eikelboom JW, Weitz JI. Evolving Treatments for Arterial and Venous Thrombosis: Role of the Direct Oral Anticoagulants. Circ Res 2016;118:1409–24. [DOI] [PubMed] [Google Scholar]
- [5].Czuprynska J, Patel JP, Arya R. Current challenges and future prospects in oral anticoagulant therapy. Br J Haematol 2017;178:838–51. [DOI] [PubMed] [Google Scholar]
- [6].van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014;124:1968–75. [DOI] [PubMed] [Google Scholar]
- [7].Black SA, Cohen AT. Anticoagulation strategies for venous thromboembolism: moving towards a personalised approach. Thromb Haemost 2015;114:660–9. [DOI] [PubMed] [Google Scholar]
- [8].Piran S, Schulman S. Management of venous thromboembolism: an update. Thromb J 2016;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Verheugt FWA, Granger CB. Oral anticoagulants for stroke prevention in atrial fibrillation: current status, special situations, and unmet needs. Lancet Lond Engl 2015;386:303–10. [DOI] [PubMed] [Google Scholar]
- [10].Senoo K, Lip GYH. Comparative efficacy and safety of the non-vitamin K antagonist oral anticoagulants for patients with nonvalvular atrial fibrillation. Semin Thromb Hemost 2015;41:146–53. [DOI] [PubMed] [Google Scholar]
- [11].Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:160S–198S. [DOI] [PubMed] [Google Scholar]
- [12].Baker WL, Johnson SG. Pharmacogenetics and oral antithrombotic drugs. Curr Opin Pharmacol 2016;27:38–42. [DOI] [PubMed] [Google Scholar]
- [13].Yates SG, Sarode R. New strategies for effective treatment of vitamin K antagonist-associated bleeding. J Thromb Haemost JTH 2015;13 Suppl 1:S180–186. [DOI] [PubMed] [Google Scholar]
- [14].Ruff CT, Giugliano RP, Antman EM. Management of Bleeding With Non-Vitamin K Antagonist Oral Anticoagulants in the Era of Specific Reversal Agents. Circulation 2016;134:248–61. [DOI] [PubMed] [Google Scholar]
- [15].Glund S, Stangier J, Schmohl M, Gansser D, Norris S, van Ryn J, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet Lond Engl 2015;386:680–90. [DOI] [PubMed] [Google Scholar]
- [16].Pollack CV, Reilly PA, Weitz JI. Dabigatran Reversal with Idarucizumab. N Engl J Med 2017;377:1691–2. [DOI] [PubMed] [Google Scholar]
- [17].Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med 2015;373:2413–24. [DOI] [PubMed] [Google Scholar]
- [18].Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso M, Brown K, et al. Use of PER977 to Reverse the Anticoagulant Effect of Edoxaban. N Engl J Med 2014;371:2141–2. [DOI] [PubMed] [Google Scholar]
- [19].Costin JC, Laulicht B, Bakhru S, Steiner S. PER977 REVERSES LOW MOLECULAR WEIGHT HEPARIN IN ADDITION TO IIA AND XA NEW ORAL ANTICOAGULANTS. J Am Coll Cardiol 2015;65:A2056. [Google Scholar]
- [20].Keita I, Aubin-Auger I, Lalanne C, Aubert J-P, Chassany O, Duracinsky M, et al. Assessment of quality of life, satisfaction with anticoagulation therapy, and adherence to treatment in patients receiving long-course vitamin K antagonists or direct oral anticoagulants for venous thromboembolism. Patient Prefer Adherence 2017;11:1625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- [22].Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- [23].Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- [24].Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet Lond Engl 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- [25].Steiner T, Weitz JI, Veltkamp R. Anticoagulant-Associated Intracranial Hemorrhage in the Era of Reversal Agents. Stroke 2017;48:1432–1437. [DOI] [PubMed] [Google Scholar]
- [26].Hart RG, Diener H-C, Yang S, Connolly SJ, Wallentin L, Reilly PA, et al. Intracranial Hemorrhage in Atrial Fibrillation Patients During Anticoagulation With Warfarin or Dabigatran: The RE-LY Trial. Stroke 2012;43:1511–7. [DOI] [PubMed] [Google Scholar]
- [27].Hankey GJ, Stevens SR, Piccini JP, Lokhnygina Y, Mahaffey KW, Halperin JL, et al. Intracranial Hemorrhage Among Patients With Atrial Fibrillation Anticoagulated With Warfarin or Rivaroxaban: The Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation. Stroke 2014;45:1304–12. [DOI] [PubMed] [Google Scholar]
- [28].Dowlatshahi D, Butcher KS, Asdaghi N, Nahirniak S, Bernbaum ML, Giulivi A, et al. Poor Prognosis in Warfarin-Associated Intracranial Hemorrhage Despite Anticoagulation Reversal. Stroke 2012;43:1812–7. [DOI] [PubMed] [Google Scholar]
- [29].Key NS. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematol Am Soc Hematol Educ Program 2014;2014:66–70. [DOI] [PubMed] [Google Scholar]
- [30].Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost 2015;13:1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].JI Weitz, Fredenburgh JC. Factors XI and XII as Targets for New Anticoagulants. Front Med 2017;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schmaier AH. Antithrombotic potential of the contact activation pathway. Curr Opin Hematol 2016;23:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost 2016;14:28–39. [DOI] [PubMed] [Google Scholar]
- [34].Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost JTH 2016;14:427–37. [DOI] [PubMed] [Google Scholar]
- [35].Gailani D, Wheeler AP, Neff AT. Rare Coagulation Factor Deficiencies Hematol. Basic Princ. Pract 7th ed., Philadelphia, PA: Elsevier/Saunders; 2018, p. 2034–50. [Google Scholar]
- [36].van Montfoort ML, Meijers JCM. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematol Am Soc Hematol Educ Program 2014;2014:60–5. [DOI] [PubMed] [Google Scholar]
- [37].EI Tucker, Verbout NG, Leung PY, Hurst S, McCarty OJT, Gailani D, et al. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood 2012;119:4762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009;139:1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood 2011;118:6963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Morrissey JH, Smith SA. Polyphosphate as modulator of hemostasis, thrombosis, and inflammation. J Thromb Haemost JTH 2015;13 Suppl 1:S92–97. doi:10.1111/jth.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A 2007;104:6388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SHC, Weitz JI, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol 2014;34:1977–84. [DOI] [PubMed] [Google Scholar]
- [43].Vu TT, Leslie BA, Stafford AR, Zhou J, Fredenburgh JC, Weitz JI . Histidine-rich glycoprotein binds DNA and RNA and attenuates their capacity to activate the intrinsic coagulation pathway. Thromb Haemost 2016;115:89–98. [DOI] [PubMed] [Google Scholar]
- [44].Ivanov I, Shakhawat R, Sun M-F, Dickeson SK, Puy C, McCarty OJT, et al. Nucleic acids as cofactors for factor XI and prekallikrein activation: Different roles for high-molecular-weight kininogen. Thromb Haemost 2017;117:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van der Meijden PEJ, Munnix ICA, Auger JM, Govers-Riemslag JWP, Cosemans JMEM, Kuijpers MJE, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood 2009;114:881–90. [DOI] [PubMed] [Google Scholar]
- [46].White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, et al. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost JTH 2010;8:1295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van Montfoort ML, Kuijpers MJE, Knaup VL, Bhanot S, Monia BP, Roelofs JJTH, et al. Factor XI regulates pathological thrombus formation on acutely ruptured atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2014;34:1668–73. [DOI] [PubMed] [Google Scholar]
- [48].Kuijpers MJE, van der Meijden PEJ, Feijge MAH, Mattheij NJA, May F, Govers-Riemslag J, et al. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol 2014;34:1674–80. [DOI] [PubMed] [Google Scholar]
- [49].Maas C, Govers-Riemslag JWP, Bouma B, Schiks B, Hazenberg BPC, Lokhorst HM, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest 2008;118:3208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Frick I-M, Björck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost 2007;98:497–502. [PubMed] [Google Scholar]
- [51].Wendel HP, Jones DW, Gallimore MJ. FXII levels, FXIIa-like activities and kallikrein activities in normal subjects and patients undergoing cardiac surgery. Immunopharmacology 1999;45:141–4. [DOI] [PubMed] [Google Scholar]
- [52].Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011;113:1319–33. [DOI] [PubMed] [Google Scholar]
- [53].Worm M, Köhler EC, Panda R, Long A, Butler LM, Stavrou EX, et al. The factor XIIa blocking antibody 3F7: a safe anticoagulant with anti-inflammatory activities. Ann Transl Med 2015;3:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Frank RD, Weber J, Dresbach H, Thelen H, Weiss C, Floege J. Role of contact system activation in hemodialyzer-induced thrombogenicity. Kidney Int 2001;60:1972–81. [DOI] [PubMed] [Google Scholar]
- [55].Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost JTH 2015;13 Suppl 1:S72–81. [DOI] [PubMed] [Google Scholar]
- [56].Yau JW, Stafford AR, Liao P, Fredenburgh JC, Roberts R, Brash JL, et al. Corn trypsin inhibitor coating attenuates the prothrombotic properties of catheters in vitro and in vivo. Acta Biomater 2012;8:4092–100. [DOI] [PubMed] [Google Scholar]
- [57].Himmelreich G, Ullmann H, Riess H, Rosch R, Loebe M, Schiessler A, et al. Pathophysiologic role of contact activation in bleeding followed by thromboembolic complications after implantation of a ventricular assist device. ASAIO J Am Soc Artif Intern Organs 1992 1995;41:M790–794. [DOI] [PubMed] [Google Scholar]
- [58].Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood 2011;118:5302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ivanov I, Matafonov A, Sun M-F, Cheng Q, Dickeson SK, Verhamme IM, et al. Proteolytic properties of single-chain factor XII: a mechanism for triggering contact activation. Blood 2017;129:1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ivanov I, Matafonov A, Sun M-F, Gailani D. Proteolytic activity in single-chain prekallikrein. Res Pract Thromb Haemost 2017:OC151. [Google Scholar]
- [61].Schmaier AH. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regul Integr Comp Physiol 2003;285:R1–13. [DOI] [PubMed] [Google Scholar]
- [62].Wang J, Matafonov A, Madkhali H, Mahdi F, Watson D, Schmaier AH, et al. Prolylcarboxypeptidase independently activates plasma prekallikrein (fletcher factor). Curr Mol Med 2014;14:1173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Joseph K, Tholanikunnel BG, Kaplan AP. Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII. Proc Natl Acad Sci U S A 2002;99:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Joseph K, Tholanikunnel BG, Bygum A, Ghebrehiwet B, Kaplan AP. Factor XII-independent activation of the bradykinin-forming cascade: Implications for the pathogenesis of hereditary angioedema types I and II. J Allergy Clin Immunol 2013;132:470–5. [DOI] [PubMed] [Google Scholar]
- [65].Mohammed BM, Matafonov A, Ivanov I, Sun M-F, Cheng Q, Dickeson SK, et al. An update on factor XI structure and function. Thromb Res 2018;161:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Duga S, Salomon O. Congenital factor XI deficiency: an update. Semin Thromb Hemost 2013;39:621–31. [DOI] [PubMed] [Google Scholar]
- [67].Nitzsche R, Rosenheinrich M, Kreikemeyer B, Oehmcke-Hecht S. Streptococcus pyogenes triggers activation of the human contact system by streptokinase. Infect Immun 2015;83:3035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Seweryn K, Karkowska-Kuleta J, Wolak N, Bochenska O, Kedracka-Krok S, Kozik A, et al. Kinetic and thermodynamic characterization of the interactions between the components of human plasma kinin-forming system and isolated and purified cell wall proteins of Candida albicans. Acta Biochim Pol 2015;62:825–35. [DOI] [PubMed] [Google Scholar]
- [69].Gaertner F, Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol 2016;28:561–9. [DOI] [PubMed] [Google Scholar]
- [70].Keragala CB, Draxler DF, McQuilten ZK, Medcalf RL. Haemostasis and innate immunity - a complementary relationship: A review of the intricate relationship between coagulation and complement pathways. Br J Haematol 2017. [DOI] [PubMed] [Google Scholar]
- [71].Carcao M, Moorehead P, Lillicrap D. Hemophilia A and B In: Hoffman H, Benz EJ, Silberstein LS. Heslop HE, Weitz JI, Anastasi J, Salama ME, Abutalib SA eds. Hematology, Basic Principles and Practice, 7th ed Philadelphia, PA: Elsevier-Saunders; 2018:2001–2022., n.d. [Google Scholar]
- [72].Wheeler AP, Gailani D. Why factor XI deficiency is a clinical concern. Expert Rev Hematol 2016;9:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Brummel-Ziedens K, Mann KG. Molecular basis of blood coagulation In: Hoffman H, Benz EJ, Silberstein LS. Heslop HE, Weitz JI, Anastasi J, Salama ME, Abutalib SA eds. Hematology, Basic Principles and Practice, 7th ed Philadelphia, PA: Elsevier-Saunders; 2018:1885–1905., n.d. [Google Scholar]
- [74].Whelihan MF, Orfeo T, Gissel MT, Mann KG. Coagulation procofactor activation by factor XIa. J Thromb Haemost JTH 2010;8:1532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Matafonov A, Cheng Q, Geng Y, Verhamme IM, Umunakwe O, Tucker EI, et al. Evidence for factor IX-independent roles for factor XIa in blood coagulation. J Thromb Haemost JTH 2013;11:2118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, et al. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood 2015;125:1488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Matafonov A, Sarilla S, Sun M, Sheehan JP, Serebrov V, Verhamme IM, et al. Activation of factor XI by products of prothrombin activation. Blood 2011;118:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI Deficiency in Ashkenazi Jews in Israel. N Engl J Med 1991;325:153–8. [DOI] [PubMed] [Google Scholar]
- [79].Mumford AD, Ackroyd S, Alikhan R, Bowles L, Chowdary P, Grainger J, et al. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors’ Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol 2014;167:304–26. [DOI] [PubMed] [Google Scholar]
- [80].Santoro C, Di Mauro R, Baldacci E, De Angelis F, Abbruzzese R, Barone F, et al. Bleeding phenotype and correlation with factor XI (FXI) activity in congenital FXI deficiency: results of a retrospective study from a single centre. Haemoph 2015;21:496–501. [DOI] [PubMed] [Google Scholar]
- [81].Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemoph 2006;12:490–3. [DOI] [PubMed] [Google Scholar]
- [82].James P, Salomon O, Mikovic D, Peyvandi F. Rare bleeding disorders - bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Haemoph 2014;20 Suppl 4:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ragni MV, Sinha D, Seaman F, Lewis JH, Spero JA, Walsh PN. Comparison of bleeding tendency, factor XI coagulant activity, and factor XI antigen in 25 factor XI-deficient kindreds. Blood 1985;65:719–24. [PubMed] [Google Scholar]
- [84].Bolton-Maggs PH, Patterson DA, Wensley RT, Tuddenham EG. Definition of the bleeding tendency in factor XI-deficient kindreds--a clinical and laboratory study. Thromb Haemost 1995;73:194–202. [PubMed] [Google Scholar]
- [85].Kadir RA, Economides DL, Lee CA. Factor XI deficiency in women. Am J Hematol 1999;60:48–54. [DOI] [PubMed] [Google Scholar]
- [86].Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu M-Y, Smith PL, et al. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost JTH 2005;3:695–702. [DOI] [PubMed] [Google Scholar]
- [87].Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun M -f, et al. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood 2010;116:3981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kokoye Y, Ivanov I, Cheng Q, Matafonov A, Dickeson SK, Mason S, et al. A comparison of the effects of factor XII deficiency and prekallikrein deficiency on thrombus formation. Thromb Res 2016;140:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Schmaier AH. Plasma Prekallikrein: Its Role in Hereditary Angioedema and Health and Disease. Front Med 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stavrou EX, Fang C, Merkulova A, Alhalabi O, Grobe N, Antoniak S, et al. Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood 2015;125:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood 2003;102:953–5. [DOI] [PubMed] [Google Scholar]
- [92].El Tucker, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJT, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood 2009;113:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Matafonov A, Leung PY, Gailani AE, Grach SL, Puy C, Cheng Q, et al. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood 2014;123:1739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost 2011;105:269–73. [DOI] [PubMed] [Google Scholar]
- [95].Preis M, Hirsch J, Kotler A, Zoabi A, Stein N, Rennert G, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood 2017;129:1210–5. [DOI] [PubMed] [Google Scholar]
- [96].Li Y, Bezemer ID, Rowland CM, Tong CH, Arellano AR, Catanese JJ, et al. Genetic variants associated with deep vein thrombosis: the F11 locus. J Thromb Haemost JTH 2009;7:1802–8. [DOI] [PubMed] [Google Scholar]
- [97].Folsom AR, Tang W, Roetker NS, Heckbert SR, Cushman M, Pankow JS. Prospective study of circulating factor XI and incident venous thromboembolism: The Longitudinal Investigation of Thromboembolism Etiology (LITE). Am J Hematol 2015;90:1047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood 2008;111:4113–7. [DOI] [PubMed] [Google Scholar]
- [99].Suri MFK, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis Basel Switz 2010;29:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Siegerink B, Govers-Riemslag JWP, Rosendaal FR, Ten Cate H, Algra A. Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the Risk of Arterial Thrombosis in relation to Oral contraceptives (RATIO) case-control study. Circulation 2010;122:1854–61. [DOI] [PubMed] [Google Scholar]
- [101].Salomon O, Steinberg DM, Dardik R, Rosenberg N, Zivelin A, Tamarin I, et al. Inherited factor XI deficiency confers no protection against acute myocardial infarction. J Thromb Haemost JTH 2003;1:658–61. [DOI] [PubMed] [Google Scholar]
- [102].Doggen CJM, Rosendaal FR, Meijers JCM. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood 2006;108:4045–51. [DOI] [PubMed] [Google Scholar]
- [103].Halbmayer WM, Mannhalter C, Feichtinger C, Rubi K, Fischer M. The prevalence of factor XII deficiency in 103 orally anticoagulated outpatients suffering from recurrent venous and/or arterial thromboembolism. Thromb Haemost 1992;68:285–90. [PubMed] [Google Scholar]
- [104].Zeerleder S, Schloesser M, Redondo M, Wuillemin WA, Engel W, Furlan M, et al. Reevaluation of the incidence of thromboembolic complications in congenital factor XII deficiency--a study on 73 subjects from 14 Swiss families. Thromb Haemost 1999;82:1240–6. [PubMed] [Google Scholar]
- [105].Koster T, Rosendaal FR, Briët E, Vandenbroucke JP. John Hageman’s factor and deep-vein thrombosis: Leiden thrombophilia Study. Br J Haematol 1994;87:422–4. [DOI] [PubMed] [Google Scholar]
- [106].Cushman M, O’Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood 2009;114:2878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Endler G, Marsik C, Jilma B, Schickbauer T, Quehenberger P, Mannhalter C. Evidence of a U-shaped association between factor XII activity and overall survival. J Thromb Haemost JTH 2007;5:1143–8. [DOI] [PubMed] [Google Scholar]
- [108].Jaffa AA, Durazo-Arvizu R, Zheng D, Lackland DT, Srikanth S, Garvey WT, et al. Plasma prekallikrein: a risk marker for hypertension and nephropathy in type 1 diabetes. Diabetes 2003;52:1215–21. [DOI] [PubMed] [Google Scholar]
- [109].Jaffa MA, Luttrell D, Schmaier AH, Klein RL, Lopes-Virella M, Luttrell LM, et al. Plasma Prekallikrein Is Associated With Carotid Intima-Media Thickness in Type 1 Diabetes Diabetes 2016;65:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Katsuda I, Maruyama F, Ezaki K, Sawamura T, Ichihara Y. A new type of plasma prekallikrein deficiency associated with homozygosity for Gly104Arg and Asn124Ser in apple domain 2 of the heavy-chain region. EurJ Haematol 2007;79:59–68. [DOI] [PubMed] [Google Scholar]
- [111].Biswas N, Maihofer AX, Mir SA, Rao F, Zhang K, Khandrika S, et al. Polymorphisms at the F12 and KLKB1 loci have significant trait association with activation of the renin-angiotensin system. BMC Med Genet 2016;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gittleman HR, Merkulova A, Alhalabi O, Stavrou EX, Veigl ML, Barnholtz-Sloan JS, et al. A Cross-sectional Study of KLKB1 and PRCP Polymorphisms in Patient Samples with Cardiovascular Disease. Front Med 2016;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Reshef A, Zanichelli A, Longhurst H, Relan A, Hack CE. Elevated D-dimers in attacks of hereditary angioedema are not associated with increased thrombotic risk. Allergy 2015;70:506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Boisclair MD, Lane DA, Philippou H, Esnouf MP, Sheikh S, Hunt B, et al. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood 1993;82:3350–7. [PubMed] [Google Scholar]
- [115].Wendel HP, Scheule AM, Eckstein FS, Ziemer G. Haemocompatibility of paediatric membrane oxygenators with heparin-coated surfaces. Perfusion 1999;14:21–8. [DOI] [PubMed] [Google Scholar]
- [116].Koster A, Loebe M, Hansen R, Potapov EV, Noon GP, Kuppe H, et al. Alterations in coagulation after implantation of a pulsatile Novacor LVAD and the axial flow MicroMed DeBakey LVAD. Ann Thorac Surg 2000;70:533–7. [DOI] [PubMed] [Google Scholar]
- [117].Ekdahl KN, Soveri I, Hilborn J, Fellström B, Nilsson B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat Rev Nephrol 2017. [DOI] [PubMed] [Google Scholar]
- [118].Gruber A, Hanson S. Antithrombotic factor XI antibody inhibition of the intrinsic pathway. Blood 2001;98:42a. [Google Scholar]
- [119].Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015;372:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kastelein JJP, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 2006;114:1729–35. [DOI] [PubMed] [Google Scholar]
- [121].Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 2010;50:259–93. [DOI] [PubMed] [Google Scholar]
- [122].Zhang H, Lowenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood 2010;116:4684–92. [DOI] [PubMed] [Google Scholar]
- [123].Yau JW, Liao P, Fredenburgh JC, Stafford AR, Revenko AS, Monia BP, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood 2014;123:2102–7. [DOI] [PubMed] [Google Scholar]
- [124].Crosby JR, Marzec U, Revenko AS, Zhao C, Gao D, Matafonov A, et al. Antithrombotic Effect of Antisense Factor XI Oligonucleotide Treatment in Primates. Arterioscler Thromb Vasc Biol 2013;33:1670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Grijalvo S, Alagia A, Jorge AF, Eritja R. Covalent Strategies for Targeting Messenger and Non-Coding RNAs: An Updated Review on siRNA, miRNA and antimiR Conjugates. Genes 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Safdar H, Cheung KL, Salvatori D, Versteeg HH, Laghmani EH, Wagenaar GTM, et al. Acute and severe coagulopathy in adult mice following silencing of hepatic antithrombin and protein C production. Blood 2013;121:4413–6. [DOI] [PubMed] [Google Scholar]
- [127].Heestermans M, Salloum-Asfar S, Salvatori D, Laghmani EH, Luken BM, Zeerleder SS, et al. Role of platelets, neutrophils, and factor XII in spontaneous venous thrombosis in mice. Blood 2016;127:2630–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [128].Liu Q, Bethune C, Dessouki E, Grundy J, Monia BP, Bhanot S. ISIS-FXIRx, A Novel and Specific Antisense Inhibitor of Factor XI, Caused Significant Reduction in FXI Antigen and Activity and Increased aPTT without Causing Bleeding in Healthy Volunteers. Blood 2011;118:209–209. [Google Scholar]
- [129].Bethune C, Walsh M, Jung B, Yu R, Geary RS, Bhanot S. Pharmacokinetics and Pharmacodynamics of Ionis-FXIRx, an Antisense Inhibitor of Factor XI, in Patients with End-Stage Renal Disease on Hemodialysis. Blood 2017;130:1116–1116. [Google Scholar]
- [130].A Study of ISIS 416858 Administered Subcutaneously to Patients With End-Stage Renal Disease on Hemodialysis - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03358030 (accessed January 19, 2018).
- [131].Sun Y, Gailani D. Identification of a factor IX binding site on the third apple domain of activated factor XI. J Biol Chem 1996;271:29023–8. [DOI] [PubMed] [Google Scholar]
- [132].Kravtsov DV, Matafonov A, Tucker EI, Sun M-F, Walsh PN, Gruber A, et al. Factor XI contributes to thrombin generation in the absence of factor XII. Blood 2009;114:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].van Montfoort ML, Knaup VL, Marquart JA, Bakhtiari K, Castellino FJ, Hack CE, et al. Two novel inhibitory anti-human factor XI antibodies prevent cessation of blood flow in a murine venous thrombosis model: Thromb Haemost 2013;110:1065–73. [DOI] [PubMed] [Google Scholar]
- [134].Luo D, Szaba FM, Kummer LW, Johnson LL, Tucker EI, Gruber A, et al. Factor XI-deficient mice display reduced inflammation, coagulopathy, and bacterial growth during listeriosis. Infect Immun 2012;80:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Silasi R, Keshari RS, van Rensburg WJ, Regmi G, Lupu C, Lortenz CU, et al. Inhibition of Factor XI Activation by Factor XIIa Blocks Coagulopathy and Provides Organ Protection and Survival Benefit in a Baboon Model of S. aureus Sepsis. Res Pract Thromb Haemost 2017;1:115. [Google Scholar]
- [136].Leung PY, Hurst S, Berny-Lang MA, Verbout NG, Gailani D, Tucker EI, et al. Inhibition of Factor XII-Mediated Activation of Factor XI Provides Protection Against Experimental Acute Ischemic Stroke in Mice. Transl Stroke Res 2012;3:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lorentz CU, Verbout NG, Cao Z, Liu L, Hinds MT, McCarty OJT, et al. Factor XI contributes to myocardial ischemia-reperfusion injury in mice. Blood Adv 2018;2:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gómez-Outes A, García-Fuentes M, Suárez-Gea ML. Discovery methods of coagulation-inhibiting drugs. Expert Opin Drug Discov 2017;12:1195–205. [DOI] [PubMed] [Google Scholar]
- [139].Safety and Tolerability Study of Xisomab 3G3 in Healthy Adult Subjects - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03097341 (accessed January 19, 2018).
- [140].Prevention of Thromboembolic Events in Total Knee Replacement Patients - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03393481 (accessed January 19, 2018).
- [141].Efficacy and Safety of MAA868 in Patients With Atrial Fibrillation - Full Text View- ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03398434 (accessed January 19, 2018).
- [142].David T, Kim YC, Ely LK, Rondon I, Gao H, OBrien P, et al. Factor XIa-specific IgG and a reversal agent to probe factor XI function in thrombosis and hemostasis. Sci Transl Med 2016;8:353ra112–353ra112. [DOI] [PubMed] [Google Scholar]
- [143].Ely LK, Lolicato M, David T, Lowe K, Kim YC, Samuel D, et al. Structural Basis for Activity and Specificity of an Anticoagulant Anti-FXIa Monoclonal Antibody and a Reversal Agent. Struct Lond Engl 1993 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Buchmueller A, Wilmen A, Strassburger J, Schmidt MV, Laux V. The anti-factor XIa antibody BAY 1213790 is a novel anticoagulant that shows strong antithrombotic efficacy without an increased risk of bleeding in rabbit models. Res Pr Thromb Haemost 2017;1. [Google Scholar]
- [145].Thomas D, Thelen K, van der Mey D, Schwers S, Schiffer S, Unger S, et al. First evaluation of the safety, pharmacokinetics and pharmacodynamics of BAY 1213790, a full human IgG1 antibody targeting coagulation factor XIa, in healthy young men. Res Pr Thromb Haemost 2017;1. [Google Scholar]
- [146].A Study to Investigate Different Doses of BAY1213790 to Prevent Blood Clots in Patients Undergoing Elective Total Knee Replacement Surgery - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03276143 (accessed January 3, 2018).
- [147].Zhu S, Travers RJ, Morrissey JH, Diamond SL. FXIa and platelet polyphosphate as therapeutic targets during human blood clotting on collagen/tissue factor surfaces under flow. Blood 2015;126:1494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Larsson M, Rayzman V, Nolte MW, Nickel KF, Björkqvist J, Jämsä A, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transi Med 2014;6:222ra17. [DOI] [PubMed] [Google Scholar]
- [149].Kenniston JA, Faucette RR, Martik D, Comeau SR, Lindberg AP, Kopacz KJ, et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem 2014;289:23596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Banerji A, Busse P, Shennak M, Lumry W, Davis-Lorton M, Wedner HJ, et al. Inhibiting Plasma Kallikrein for Hereditary Angioedema Prophylaxis. N Engl J Med 2017;376:717–28. [DOI] [PubMed] [Google Scholar]
- [151].Wong PC, Quan ML, Watson CA, Crain EJ, Harpel MR, Rendina AR, et al. In vitro, antithrombotic and bleeding time studies of BMS-654457, a small-molecule, reversible and direct inhibitor of factor XIa. J Thromb Thrombolysis 2015;40:416–23. [DOI] [PubMed] [Google Scholar]
- [152].Pinto DJP, Orwat MJ, Smith LM, Quan ML, Lam PYS, Rossi KA, et al. Discovery of a Parenteral Small Molecule Coagulation Factor XIa Inhibitor Clinical Candidate (BMS-962212). J Med Chem 2017;60:9703–23. [DOI] [PubMed] [Google Scholar]
- [153].Luettgen JM, Wong PC, Perera V, Wang Z, Russo C, Chen W, et al. Abstract TMP117: Preclinical and Early Clinical Characterization of a Parenterally Administered Direct Factor XIa Inhibitor. Stroke 2017;48:ATMP117–ATMP117. [Google Scholar]
- [154].Perera V, Luettgen JM, Wang Z, Frost CE, Yones C, Russo C, et al. First in human study to assess safety, pharmacokinetics and pharmacodynamics of BMS-962212, a direct, reversible, small molecule factor XIa inhibitor in non-Japanese and Japanese healthy subjects. Br J Clin Pharmacol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gohda M, Sakai M, Tanaka K, Hagio T, Suzuki K, Koyama S, et al. Discovery of a Novel, Potent, Selective and Injectable Small Molecule Inhibitor of Blood Coagulation Factor XIa, ONO-8610539: in Vitro and in Vivo Pharmacological Profiles. Blood 2014;124:1542–1542.25190747 [Google Scholar]
- [156].Sakai M, Hagio T, Koyama S, Gohda M, Suzuki K, Ono T, et al. Antithrombotic effect of ONO-8610539, a new, potent and selective small molecule factor XIa inhibitor, in a monkey model of ateriovenous shunt. Int Soc Thromb Haemost 2015;13:230–1. [Google Scholar]
- [157].Sakimoto S, Hagio T, Yonetomi Y, Ono T, Koyama S, Hashimoto A, et al. Abstract WP286: ONO-8610539, an Injectable Small-Molecule Inhibitor of Blood Coagulation Factor XIa, Improves Cerebral Ischemic Injuries Associated with Photothrombotic Occlusion of Rabbit Middle Cerebral Artery. Stroke 2017;48:AWP286–AWP286. [Google Scholar]
- [158].Lin J, Deng H, Jin L, Pandey P, Quinn J, Cantin S, et al. Design, Synthesis, and Biological Evaluation of Peptidomimetic Inhibitors of Factor XIa as Novel Anticoagulants. J Med Chem 2006;49:7781–91. [DOI] [PubMed] [Google Scholar]
- [159].Inc MG. ONO-5450598, an Orally Available Small-molecule Inhibitor of… by Takehiro Ono n.d. http://academy.isth.org/isth/2017/berlin_eposters/188083/takehiro.ono.ono-5450598.an.orally.available.small-molecule.inhibitor.of.html (accessed January 25, 2018).
- [160].Inc MG. Discovery of ONO-5450598, a Highly Orally Bioavailable Small… by Sho Kouyama n.d. http://academy.isth.org/isth/2017/berlin_eposters/188080/sho.kouyama.discovery.of.ono-5450598.a.highly.orally.bioavailable.small.html (accessed January 25, 2018).
- [161].To Evaluate the Safety and Pharmacokinetics of BMS-986177 in Healthy Japanese Participants - Full Text View- ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03224260 (accessed January 19, 2018).
- [162].An Investigational Study to Assess the Effect of BMS-986177 on Aspirin in Healthy Participants - Full Text View- ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT03341390 (accessed January 19, 2018).
- [163].Al-Horani RA, Ponnusamy P, Mehta AY, Gailani D, Desai UR. Sulfated Pentagalloylglucoside Is a Potent, Allosteric, and Selective Inhibitor of Factor XIa. J Med Chem 2013;56:867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Al-Horani RA, Gailani D, Desai UR. Allosteric inhibition of factor XIa. Sulfated non-saccharide glycosaminoglycan mimetics as promising anticoagulants. Thromb Res 2015;136:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Woodruff RS, Ivanov I, Verhamme IM, Sun M-F, Gailani D, Sullenger BA. Generation and characterization of aptamers targeting factor XIa. Thromb Res 2017;156:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Donkor DA, Bhakta V, Eltringham-Smith LJ, Stafford AR, Weitz JI, Sheffield WP. Selection and characterization of a DNA aptamer inhibiting coagulation factor XIa. Sci Rep 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Woodruff RS, Xu Y, Layzer J, Wu W, Ogletree ML, Sullenger BA. Inhibiting the intrinsic pathway of coagulation with a factor XII-targeting RNA aptamer. J Thromb Haemost JTH 2013;11:1364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Steen Burrell K-A, Layzer J, Sullenger BA. A kallikrein-targeting RNA aptamer inhibits the intrinsic pathway of coagulation and reduces bradykinin release. J Thromb Haemost JTH 2017;15:1807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Smith RP, Higuchi DA, Broze GJ. Platelet coagulation factor XIa-inhibitor, a form of Alzheimer amyloid precursor protein. Science 1990;248:1126–8. [DOI] [PubMed] [Google Scholar]
- [170].Xu F, Davis J, Miao J, Previti ML, Romanov G, Ziegler K, et al. Protease nexin-2/amyloid beta-protein precursor limits cerebral thrombosis. Proc Natl Acad Sci U S A 2005;102:18135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wu W, Li H, Navaneetham D, Reichenbach ZW, Tuma RF, Walsh PN. The kunitz protease inhibitor domain of protease nexin-2 inhibits factor XIa and murine carotid artery and middle cerebral artery thrombosis. Blood 2012;120:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Mahdi F, Rehemtulla A, Van Nostrand WE, Bajaj SP, Schmaier AH. Protease nexin-2/Amyloid beta-protein precursor regulates factor VIIa and the factor VIIa-tissue factor complex. Thromb Res 2000;99:267–76. [DOI] [PubMed] [Google Scholar]
- [173].Navaneetham D, Sinha D, Walsh PN. Mechanisms and specificity of factor XIa and trypsin inhibition by protease nexin 2 and basic pancreatic trypsin inhibitor. J Biochem (Tokyo) 2010;148:467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Vadivel K, Ponnuraj S-M, Kumar Y, Zaiss AK, Bunce MW, Camire RM, et al. Platelets contain tissue factor pathway inhibitor-2 derived from megakaryocytes and inhibits fibrinolysis. J Biol Chem 2014;289:31647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Vadivel K, Kumar Y, Ogueli GI, Ponnuraj SM, Wongkongkathep P, Loo JA, et al. S2’-subsite variations between human and mouse enzymes (plasmin, factor XIa, kallikrein) elucidate inhibition differences by tissue factor pathway inhibitor -2 domainl-wiId-type, Leu17Arg-mutant and aprotinin. J Thromb Haemost 2016;14:2509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Ma D, Mizurini DM, Assumpção TC, Li Y, Qi Y, Kotsyfakis M, et al. Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood 2013;122:4094–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Chen W, Carvalho LPD, Chan MY, Kini RM, Kang TS. Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J Thromb Haemost 2015;13:248–61. [DOI] [PubMed] [Google Scholar]
- [178].Campos ITN, Souza TACB, Torquato RJS, De Marco R, Tanaka-Azevedo AM, Tanaka AS, et al. The Kazal-type inhibitors infestins 1 and 4 differ in specificity but are similar in three-dimensional structure. Acta Crystallogr D Biol Crystallogr 2012;68:695–702. [DOI] [PubMed] [Google Scholar]
- [179].Hagedorn I, Schmidbauer S, Pleines I, Kleinschnitz C, Kronthaler U, Stoll G, et al. Factor XIIa Inhibitor Recombinant Human Albumin Infestin-4 Abolishes Occlusive Arterial Thrombus Formation Without Affecting Bleeding. Circulation 2010;121:1510–7. [DOI] [PubMed] [Google Scholar]
- [180].Xu Y, Cai T-Q, Castriota G, Zhou Y, Hoos L, Jochnowitz N, et al. Factor XIIa inhibition by Infestin-4: in vitro mode of action and in vivo antithrombotic benefit: Thromb Haemost 2013;111:694–704. [DOI] [PubMed] [Google Scholar]
- [181].May F, Krupka J, Fries M, Thielmann I, Pragst I, Weimer T, et al. FXIIa inhibitor rHA-Infestin-4: Safe thromboprotection in experimental venous, arterial and foreign surface-induced thrombosis. Br J Haematol 2016;173:769–78. [DOI] [PubMed] [Google Scholar]
- [182].Krupka J, May F, Weimer T, Pragst I, Kleinschnitz C, Stoll G, et al. The Coagulation Factor XIIa Inhibitor rHA-Infestin-4 Improves Outcome after Cerebral Ischemia/Reperfusion Injury in Rats. PloS One 2016;11:e0146783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Barbieri CM, Wang X, Wu W, Zhou X, Ogawa AM, O’Neill K, et al. Factor XIIa as a Novel Target for Thrombosis: Target Engagement Requirement and Efficacy in a Rabbit Model of Microembolic Signals. J Pharmacol Exp Ther 2017;360:466–75. [DOI] [PubMed] [Google Scholar]
- [184].Tatsumi K, Mackman N. Tissue Factor and Atherothrombosis. J Atheroscler Thromb 2015;22:543–9. [DOI] [PubMed] [Google Scholar]
- [185].Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, et al. Venous thrombosis. Nat Rev Dis Primer 2015;1:15006. [DOI] [PubMed] [Google Scholar]
- [186].Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:1794–801. [DOI] [PubMed] [Google Scholar]
- [187].Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- [188].JI Weitz, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N Engl J Med 2017;376:1211–22. [DOI] [PubMed] [Google Scholar]
- [189].Xian Y, O’Brien EC, Liang L, Xu H, Schwamm LH, Fonarow GC, et al. Association of Preceding Antithrombotic Treatment With Acute Ischemic Stroke Severity and In-Hospital Outcomes Among Patients With Atrial Fibrillation. JAMA 2017;317:1057–67. [DOI] [PubMed] [Google Scholar]
- [190].Redfors B, Gray WA, Lee RJ, Ellenbogen KA, Bonafede M, Ben-Yehuda O. Patients With Atrial Fibrillation Who Are Not on Anticoagulant Treatment Due to Increased Bleeding Risk Are Common and Have a High Risk of Stroke. JACC Clin Electrophysiol 2017:483. [DOI] [PubMed] [Google Scholar]
- [191].Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis 2010;55:S1–420, A6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Salomon O, Zivelin A, Livnat T, Dardik R, Loewenthal R, Avishai O, et al. Prevalence, causes, and characterization of factor XI inhibitors in patients with inherited factor XI deficiency. Blood 2003;101:4783–8. [DOI] [PubMed] [Google Scholar]
- [193].Livnat T, Tamarin I, Mor Y, Winckler H, Horowitz Z, Korianski Y, et al. Recombinant activated factor VII and tranexamic acid are haemostatically effective during major surgery in factor XI-deficient patients with inhibitor antibodies. Thromb Haemost 2009;102:487–92. [DOI] [PubMed] [Google Scholar]
- [194].Setty S, Reddell A, England A, Gomez K, Kadir R. The role of recombinant factor VIIa for obstetric block in women with severe factor XI deficiency. Haemoph 2011;17:906–9. [DOI] [PubMed] [Google Scholar]
- [195].Schulman S, Németh G. An illustrative case and a review on the dosing of recombinant factor VIIa in congenital factor XI deficiency. Haemoph 2006;12:223–7. [DOI] [PubMed] [Google Scholar]
- [196].Lippi G, Favaloro EJ. Laboratory monitoring of direct oral anticoagulants (DOACs)-The perfect storm? Ann Transl Med 2017;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 2009;374:29–38. [DOI] [PubMed] [Google Scholar]
- [198].Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20–33. [DOI] [PubMed] [Google Scholar]