Abstract
Basement membrane plays a foundational role in the structure and maintenance of many tissues throughout the animal kingdom. In addition to signaling to cells through cell-surface receptors, basement membrane directly influences the development and maintenance of organ shape via its mechanical properties. The mechanical properties of basement membrane are dictated by its composition, geometry, and crosslinking. Distinguishing between the ways the basement membrane influences morphology in vivo poses a major challenge. Drosophila melanogaster, already established as a powerful model for the analysis of cell signaling, has in recent years emerged as a tractable model for understanding the roles of basement membrane stiffness in vivo, in shaping and maintaining the morphology of tissues and organs. In addition to the plethora of genetic tools available in flies, the major proteins found in vertebrate basement membranes are all present in Drosophila. Furthermore, Drosophila has fewer copies of the genes encoding these proteins, making flies more amenable to genetic manipulation than vertebrate models. Because the development of Drosophila organs has been well-characterized, these different organ systems offer a variety of contexts for analyzing the role of basement membrane in development. The developing egg chamber and central nervous system, for example, have been important models for assessing the role of basement membrane stiffness in influencing organ shape. Studies in the nervous system have also shown how basement membrane stiffness can influence cellular migration in vivo. Finally, work in the imaginal wing disc has illuminated a distinct mechanism by which basement membrane can alter organ shape and size, by sequestering signaling ligands. This mini-review highlights the recent discoveries pertaining to basement membrane mechanics during Drosophila development.
Introduction
The basement membrane is a sheet-like extracellular matrix that underlies epithelial cells and endothelial cells and ensheaths muscles, fat and nerves [1]. Ranging from less than 100nm to as much as 10µm thick [2], basement membrane is assembled from core components conserved throughout the animal kingdom: laminin, collagen IV, nidogen, and perlecan [3]. Collagen IV is primarily responsible for bestowing stiffness upon the basement membrane, and the importance of this stiffness is supported by its presence in all metazoans, except some primitive sponges [4]. Notably, collagen IV is present in ctenophores, which is thought to be one of the earliest branching extant animal phyla [5]. In addition to providing structural support to epithelial cells, basement membrane helps direct cellular differentiation and function by signaling via integrin and dystroglycan receptors and by binding to and modulating the diffusion of secreted growth factors [1]. In cultured cells, matrix stiffness affects cellular differentiation [6] and behavior [7]. In vivo, because basement membranes are assembled prior to the completion of embryogenesis [8–10], basement membranes must constantly be modified to accommodate changes within a given tissue as the organism grows and develops over time. Such changes can come in the form of increased deposition or degradation of basement membrane-associated proteins or adjusting the composition of proteins within the basement membrane. All of these possible alterations affect the mechanical stiffness of basement membrane [11]. Understanding the ways in which the mechanical properties of basement membrane affect the development of associated tissues in vivo requires a model organism that provides tools to easily manipulate basement membrane stiffness. One such model organism that has been used successfully for this topic is Drosophila melanogaster.
Drosophila is an excellent model for the study of basement membrane dynamics for several reasons: all of the major basement membrane components are conserved [12], the number of genes coding for each protein are significantly fewer than in mammals (see Table 1), and sophisticated tools have been developed for genetic studies in flies allowing temporal and spatial control of any gene in virtually any cell or tissue. Additionally, there are functional GFP-fusion proteins, expressed by endogenous regulatory sequences, for each of the four major basement membrane proteins in Drosophila, identified either as endogenous gene traps or generated as genomic fragments in BAC transgenes. Finally, the contribution of basement membrane to development can be analyzed at many different stages.
Table 1.
Genes encoding basement membrane proteins in mice and flies. Data from Flybase, release FB2017_06 [51]
| Basement membrane protein family |
Mouse genes |
Drosophila genes |
|
|---|---|---|---|
| Structural proteins | Laminin | 11 genes | 4 genes |
| α-chain | Lama1 | Laminin A (LanA) | |
| Lama2 | wing blister (wb) | ||
| Lama3 | |||
| Lama4 | |||
| Lama5 | |||
| β-chain | Lamb1 | LanB1 | |
| Lamb2 | |||
| Lamb3 | |||
| γ-chain | Lamc1 | Laminin B2 (LanB2) | |
| Lamc2 | |||
| Lamc3 | |||
|
| |||
| Collagen IV | 6 genes | 2 genes | |
| Collagen IV α1 | Col4a1 | Collagen IV alpha 1 (Col4A1) | |
| Collagen IV α2 | Col4a2 | Viking (vkg) | |
| Collagen IV α3 | Col4a3 | (not present) | |
| Collagen IV α4 | Col4a4 | (not present) | |
| Collagen IV α5 | Col4a5 | (not present) | |
| Collagen IV α6 | Col4a6 | (not present) | |
|
| |||
| Perlecan | 1 gene | 1 gene | |
| Hspg2 | terribly reduced optic lobes (trol) | ||
|
| |||
| Nidogen | 2 genes | 1 gene | |
| Nid1 | Nidogen/entactin (Ndg) | ||
| Nid2 | |||
|
| |||
| Extracellular BM Modifying Enzymes | Matrix Metalloproteinase | 24 genes | 2 genes |
| Mmp1a, Mmp1b, Mmp2, Mmp3, Mmp7, Mmp8, Mmp9, Mmp10, Mmp11, Mmp12, Mmp13, Mmp14, Mmp15, Mmp16, Mmp17, Mmp19, Mmp20, Mmp21, Mmp23, Mmp24, Mmp25, Mmp27, Mmp28, Mmp29 | Matrix metalloproteinase 1 (Mmp1) | ||
| Matrix metalloproteinase 2 (Mmp2) | |||
|
| |||
| Peroxidasin | 1 gene | 1 gene | |
| Pxdn | Peroxidasin (Pxn) | ||
|
| |||
| Lysyl Oxidase | 5 genes | 2 genes | |
| Lox | Lysyl oxidase-like 1 (Loxl1) | ||
| Loxl1 | Lysyl oxidase-like 2 (Loxl2) | ||
| Loxl2 | |||
| Loxl3 | |||
| Loxl4 | |||
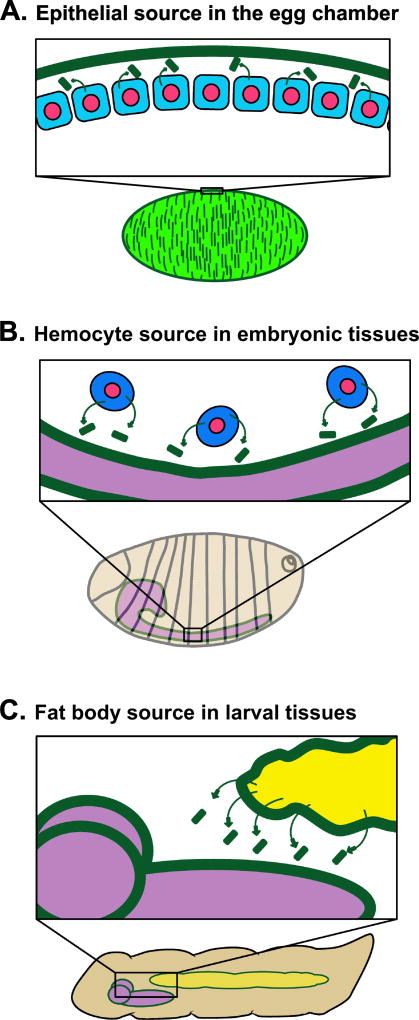
Surprisingly, basement membrane is assembled from different sources at different times during Drosophila development (Figure 1). Here is a brief summary of the dynamics of basement membrane deposition and degradation in Drosophila throughout its life cycle: (1) During oogenesis, each Drosophila egg develops from germ cells surrounded by basement membrane secreted from migratory hemocytes (Drosophila blood cells) and the distant fat body organ [13]. These develop into egg chambers surrounded by a basement membrane secreted from epithelial follicle cells [13–15]. As the egg develops, additional basement membrane is deposited in parallel alignment by neighboring follicle cells, generating patterns in the basement membrane that allow egg shape elongation [16,17]. (2) During embryogenesis, hemocytes secrete basement membrane onto the developing organs in the late embryo, and this matrix deposition is necessary for completion of embryogenesis [18,19]. (3) Once the embryo hatches into a larva, basement membrane proteins are secreted into the hemolymph of the larval open circulatory system, primarily from the fat body, an adipose tissue that functions to regulate metabolism and immunity. These secreted proteins are then incorporated into the basement membranes of growing tissues and organs, such as the central nervous system and the wing disc, as the larva develops from 1st to 3rd instar [20]. (4) When the larva undergoes metamorphosis to take on its adult form, the larval tissues must be broken down as the adult tissues develop from the imaginal tissues. At this time, basement membranes are also broken down, both around the histolyzing larval tissues and around the imaginal discs that are undergoing rapid growth and eversion to create the structures of an adult fly [21,22]. These adult structures will themselves be associated with newly formed basement membrane, though little is known about how this new basement membrane is deposited. These developmental stages offer many opportunities for investigating the assembly and function of basement membranes in shaping and maintaining the organs they encase, as described below.
Figure 1. Cellular sources of basement membrane in Drosophila.
Basement membrane is synthesized and secreted from several different types of cells during Drosophila development.
A) In developing egg chambers, follicle cells (blue) secrete their own basement membrane (green) during egg chamber elongation.
B) During embryogenesis, hemocytes (blue) secrete basement membrane (green) onto developing organs, such as the ventral nerve cord (purple).
C) In larvae, the fat body (yellow) secretes basement membrane proteins (green) into the open circulatory system, which are then deposited onto tissues throughout the body including the central nervous system (purple).
Egg chamber basement membrane: stiffness determines organ shape
Drosophila eggs develop within an egg chamber, consisting of 16 germline cells surrounded by a monolayer of epithelial (follicle) cells [23]. Each egg chamber forms within a smooth sheet-like basement membrane that envelops it [14]. As the egg chamber develops, its volume increases ~5000-fold, and its shape changes from a sphere with an aspect ratio of ~1, to an ellipsoid with an aspect ratio of ~2 [24]. As the egg chamber grows, the basement membrane must expand and remodel to accommodate such a dramatic transformation. However, in recent years it has become apparent that basement membrane remodeling does not merely take place to accommodate egg chamber elongation; rather basement membrane actually drives the process.
Live imaging reveals that the entire Drosophila egg chamber rotates inside its basement membrane covering, around the anterior-posterior axis (A-P axis), until stage 9 of egg development [16]. Rotation results from the follicle epithelial cells collectively crawling on the basement membrane, aligning contractile actin bundles across the tissue so that they are parallel to their migration path [16,25]. During rotation, elongated aggregates of extracellular matrix are deposited beneath the follicle cells, appearing as basement membrane fibrils parallel to the actin bundles [17]. Because egg chamber elongation is co-incident with both rotation and matrix deposition, several labs have used genetic manipulations to investigate the causality among these three phenomena – rotation, basement membrane, and elongation. The existence of a fat2 hypomorphic condition with egg chambers that did not appear to rotate ex vivo but nevertheless elongated suggested that rotation and elongation were independent [26]. However, it was recently determined that egg chambers of the same mutant condition do indeed rotate in vivo, albeit more slowly; this result was enabled by a new tool that identifies the path of a migrating cell by its secreted extracellular matrix [27]. Thus, elongation and rotation remain inseparable, and any perturbation that halts rotation also inhibits elongation.
As the egg chamber rotates, aggregates of collagen IV and other matrix proteins are deposited from follicle cells into the basement membrane, forming aligned fibrils in the direction of cellular migration [14,16,28]. A recent study has shown that these fibrils are secreted from cells basal-laterally into the pericellular space between follicle epithelial cells before being deposited onto the underlying basement membrane [17]. When the integrin βPS subunit myospheroid (mys) or the collagen IV α2 viking (vkg) were mutated, both egg chamber rotation and elongation were disrupted, providing the first evidence that the deposition of this basement membrane influences the elongation of the egg chambers [16], although it was not possible to distinguish whether basement membrane was acting directly by constraining shape or indirectly by promoting rotation. Basement membrane was shown to be the driving force behind egg chamber elongation in an experiment in which the expression of Secreted Protein Acidic and Rich in Cysteine (SPARC) was prolonged into the timeframe when collagen IV fibrils are being deposited [30]. SPARC is thought to solubilize collagen IV, and therefore decrease how much is deposited into the basement membrane [20,29]. The continued expression of SPARC did not disrupt rotation of the follicle epithelium but did result in a substantial decrease in egg chamber elongation, firmly establishing the role of basement membrane in driving elongation independently of rotation [30].
Recently, two studies made a giant leap forward by analyzing the stiffness of this basement membrane at a quantitative level using atomic force microscopy (AFM) [31,32]. AFM measures the compressive modulus, or stiffness, of a material. Here, AFM was used to measure the stiffness of basement membrane when compressed radially from outside the egg chamber toward the center (along the apical-basal axis of the follicle cells). When measured this way, basement membrane stiffness varies along the long (anterior-posterior) axis of a stage 7–8 egg chamber, with stiffness greatest at the center and decreasing towards the poles. Importantly, the basement membrane itself is responsible for creating the tissue stiffness gradient along the anterior-posterior axis, as stiffness is reduced upon collagenase treatment but not upon actin depolymerization with latrunculin A. This anisotropy or difference in stiffness along the anterior-posterior axis is an important driver of elongation, as demonstrated in experiments that separate the anisotropy from absolute stiffness. When overall basement membrane stiffness was decreased by 20% but the anisotropy remained, egg chamber elongation was unchanged. Complementary experiments eliminated the anisotropy by evening out the stiffness along the axis, in one case depleting collagen IV specifically in the middle region of the follicle epithelium, resulting in a 30% decrease in egg chamber elongation. Conversely, when collagen IV deposition was increased, and the anisotropic gradient increased by 20%, the egg chamber became hyper-elongated [31]. These results suggest that as the egg chamber increases in volume, the regions nearer the poles expand radially (along the apical/basal axis of the follicle cells) because of the decreased polar matrix stiffness, whereas the central region is constrained by stiffer matrix. Yet this stiffness anisotropy along the anterior-posterior axis does not appear to account for all the elongation, as egg chambers still elongate 70% of their normal length when stiffness is made constant.
A possible second mechanism is suggested by measurements of the stiffness of individual matrix fibrils deposited by the rotating follicle epithelium. As expected, these fibrils are stiffer than surrounding fibril-free regions [32]. The alignment of these long parallel fibrils would create another kind of stiffness anisotropy in the 2D plane of the basement membrane: resistance to stretching in one direction (circumferential expansion) but not the other (anterior-posterior elongation). This anisotropy would represent a difference in tensile stiffness in the planar dimensions of the basement membrane, which unfortunately cannot be measured by AFM as it measures compressive stiffness (i.e., resistance to pushing rather than pulling forces); and measures it along a different axis, from the outside in (apical/basal axis) rather than in the basement membrane plane [31,32]. A quantitative analysis of the X-Y stiffness anisotropy generated by the aligned fibers awaits the development of an assay to measure tensile stiffness in the basement membrane plane, one that would likely utilize a sheet of decellularized follicular basement membrane [32].
In addition to its direct role in constraining egg chamber shape, basement membrane also plays an indirect role in increasing egg chamber volume, which is necessary for elongation. Egg chambers develop inside a peristaltic muscle sheath, which rhythmically contracts and propels the egg chambers posteriorly toward the oviduct for laying. Surprisingly, muscle sheath contractions are also important for oocyte yolk uptake from the surrounding hemolymph fluid, and when this muscle function is compromised, the oocyte does not increase in volume as much as wild-type, and egg elongation is reduced [33]. Muscular dystrophy research has illustrated the importance of muscle basement membranes for muscle function, as they distribute actomyosin contractile forces across the muscle surface and thus protect against muscles shredding themselves under the force of contraction [34]. Similarly, egg chamber muscle sheath function requires basement membrane integrity. Thus, when laminin or integrins are depleted from muscle sheaths, muscle contractions are compromised. Without the muscle contractions, egg chambers are smaller in volume and less elongated [33].
Much of basement membrane’s mechanical strength is derived from the significant cross-linking that takes place between collagen IV trimers [35,36]. Since egg chamber elongation is driven by basement membrane stiffness, elongation was used to probe the mechanism of collagen IV crosslinking, resulting in the identification of bromine as a new essential element. Bromine is a required cofactor for the enzyme peroxidasin in the reaction that generates sulfilimine bonds crosslinking collagen IV. This in vitro requirement suggested that bromine is an element essential in animals. To test the bromine requirement in vivo, flies were fed a bromine-free diet. Flies could not survive without bromine, confirming that bromine is an essential element. Interestingly, before they died, female flies laid eggs that were rounder than those that consumed bromine-supplemented food. In addition to decreased aspect ratios, eggs depleted of bromine or with peroxidasin inhibited had significantly smaller volumes than control eggs [37]. These results indicate that bromine-dependent sulfilimine crosslinking is critical for basement membrane function, especially in the sheath muscles required for increases in oocyte volume. Interestingly, when food was supplemented with bromine above physiological levels, elongation was increased above control levels, and this bromine-induced hyper-elongation was dependent on peroxidasin, which catalyzes the collagen IV sulfilimine crosslink. These chemico-genetic experiments provide important in vivo confirmation of the in vitro mechanism of bromide-dependent collagen IV crosslinking; they also illustrate the role of basement membrane stiffness in muscle function and egg chamber elongation [37].
The above studies demonstrate how Drosophila egg chamber development is proving to be a powerful in vivo model for analyzing the role of basement membrane in shaping organs. It is becoming an exceptional model when the mechanical properties of basement membrane are at the center of these questions.
Central nervous system basement membrane: stiffness alters migration
As Drosophila larvae grow from first to third instars, the central nervous system grows along with it. The central nervous system is comprised of two brain lobes and an elongated ventral nerve cord, and it is encased by a basement membrane called the neural lamella, which is important for separating the developing nervous system from the nearby epidermis during embryonic development [38]. This neural lamella is deposited by migrating hemocytes (Drosophila inflammatory cells), and interestingly, the laminin secreted by the hemocytes aids in their migration, as they seem to assemble autonomously a substrate for their migration [18,38]. The relationship between basement membrane composition, stiffness, and organ shape in the ventral nerve cord has been explored in several studies. Changes in matrix composition alter the shape of the ventral nerve cord: specifically, a shorter ventral nerve cord is observed upon the loss of perlecan [20]. This result indicates that matrix stiffness constrains nerve cord elongation, because several studies have determined that perlecan counters basement membrane stiffness. The role of perlecan in softening basement membrane is supported by direct AFM measurement of egg chamber basement membrane stiffness: when perlecan is overexpressed, the basement membrane becomes less stiff [31]. This role of perlecan is also supported by the observation that the same manipulation makes eggs rounder and by several other qualitative phenotypes in which perlecan opposes the action of collagen IV [20,30,39,40]. When perlecan levels are reduced by RNAi in the egg chamber basement membrane, electron microscopy shows that the resulting basement membrane is visibly damaged, and this damage is accompanied by reduction in stiffness measured by AFM [32]. We speculate that perlecan’s role in opposing basement membrane stiffness may be essential for rapid basement membrane expansion without damage.
In contrast to the shorter ventral nerve cord caused by the loss of perlecan, the ventral nerve cord elongates upon the overexpression of perlecan, the loss of collagen IV, or the overexpression of several matrix-cleaving proteases, including the matrix metalloproteinases Mmp2, the ADAM Kuzbanian, and AdamTS-A [20,39,41,42]. Like the perlecan results, these protease results suggest that a loss of matrix stiffness leads to a longer ventral nerve cord and that the basement membrane constrains the length of this organ. It is straightforward to assume that protease cleavage results in a softer matrix, but surprisingly, the inactivation of Mmp2 by expression of its inhibitor Timp or a dominant-negative construct also results in a longer ventral nerve cord, as does the dominant-negative AdamTS-A [39,41]. One possibility is that, in addition to cleaving matrix components directly, these proteases may also activate matrix turnover indirectly by binding to partners that affect this process.
Remarkably, loss-of-function of AdamTS-A or perlecan causes a dramatic cellular phenotype in the central nervous system. In perlecan mutants, neural progenitor cells appear to bulge out of the CNS [20,39] while the loss of AdamTS-A causes them to migrate out of the nervous system and invade other tissues [39]. This aberrant morphology seems to be triggered by increases in matrix stiffness, as the invasion phenotype caused by the loss of AdamTS-A is suppressed by the overexpression of perlecan or loss of function in collagen IV or β-integrin; and when AdamTS-A is reduced, aggregates of basement membrane proteins decorate the normally smooth neural lamella. This aberrant migration is consistent with studies of cultured cells showing that increasing ECM stiffness promotes cell migration [43].
Further evidence for basement membrane stiffness regulating the migration of cells out of the central nervous system comes from a study that used Drosophila eye development as a model for glial tumors. Drosophila retinal glial cells originate in the optic stalk and migrate along it toward the eye disc. In a Drosophila glioma model, ectopic activation of PVR (homolog of PDGF- and VEGF-Receptors) leads to increased glial cell migration in 3rd instar larvae. This glial cell migration depends directly on basement membrane stiffness, and migration requires glial-cell specific Lysyl oxidase (Lox) activity, which crosslinks lysine residues in the ECM and thus increases ECM stiffness. Either chemical inhibition or genetic knock-down of Lox leads to a decrease in glial cell migration. The stiffness of the neural lamella was directly assessed via AFM, confirming that the stiffness of the neural lamella is decreased in the absence of Lox function [44]. Thus in vivo as in cultured cells, matrix stiffness can promote cell migration.
Wing disc basement membrane: distinguishing between the roles of cellular compression and ligand retention
Studies in the Drosophila wing disc have illustrated that basement membrane can have multiple mechanical functions, both altering tissue forces through cellular compression and acting as a physical barrier to ligand diffusion. The wing disc is one of the imaginal discs, specialized insect tissues that contain precursor cells that will develop into adult tissues and organs after metamorphosis. This seemingly simple structure has been studied as a model for tissue morphogenesis for decades [45]. More recently, the wing disc has been an excellent context for interrogating hypotheses about how basement membrane drives the shape of developing tissues, using genetic approaches. Basement membrane surrounds all imaginal discs and is important for determining the shape of the wing disc in the larval stage. For example, depletion of collagen IV by RNAi or collagenase treatment causes the wing disc to expand; conversely depletion of perlecan compresses the wing disc [20]. These observations were used to test the hypothesis that basement-membrane based mechanical signaling alters cellular proliferation, which would lead to changes in the eventual shape of the later adult wing [46]. The depletion of perlecan does appear to increase the compressive forces of the basement membrane around the wing disc, as the apical area of cells in the wing disc decreases dramatically. Contrary to predictions, however, this increase in compression does not affect the size or shape of the later adult wing [40]. The converse experiment, releasing basement membrane constriction in the wing disc, was performed by overexpressing the matrix metalloproteinase Mmp2, which leads to an expanded wing disc made of cells with larger apical area. The mechanical signaling hypothesis predicts that the loss of compression would stimulate cells to proliferate, resulting in a larger adult wing. However, the opposite was observed: adult wings were about 30% smaller. These results suggest that although the basement membrane does influence the final organ size, it is not a mechanically-based signal. Instead, further experiments found that the basement membrane shapes the adult wing by acting as a physical barrier to diffusion of the signaling ligand Dpp, a member of the TGFβ family that is known to bind collagen IV in vivo [47]. Dpp is generated by cells within the wing disc and is retained within the disc environment by the surrounding basement membrane. When this matrix was removed by the expression of Mmp2, Dpp diffused away from the wing disc and was taken up in other distant tissues, with the result that wing disc Dpp signaling was reduced. Thus the reduction in final wing size was attributed to loss of the Dpp signaling ligand without basement membrane to retain it in the wing [40]. In this role, basement membrane acts as a signaling insulator, trapping diffusible signaling molecules in the vicinity of their cellular sources.
This study illustrates the detailed investigation of mechanical-based models of basement membrane function possible in Drosophila. Although it appears that basement membrane stiffness has a profound influence on the associated tissues, great care must be taken to unravel the ways in which basement membrane composition affects morphology, as both mechanical forces and ligand based signaling can be altered by basement membranes.
Concluding remarks
The studies discussed in this review illustrate how Drosophila offers an in vivo model to manipulate basement membrane mechanics and to discern the contributions of basement membrane mechanics to development. The contributions of these studies include a greater understanding of the mechanism by which basement membrane shapes organs, influences cellular migration, and facilitates cell signaling. One important conclusion from these collected Drosophila basement membrane studies is that basement membrane stiffness is not uniform. Even within one organ system, the egg chamber, stiffness reproducibly varies by 300% across a region that is only 13 cells and 100 µm wide [31]. This observation leads to intriguing questions about how the stiffness is determined and to what extent cells can alter it. Clearly altering the ratios of collagen IV and perlecan can affect dramatic changes in stiffness. In addition to changing basement membrane composition, another mechanism that could alter stiffness is changing basement membrane crosslinking. Indeed, the extent of sulfilimine crosslinking of collagen IV molecules varies between tissues in a stereotyped manner, with mammalian placental basement membrane about 50% more crosslinked than glomerular basement membrane, and sulfilimine crosslinking is essential for organ shape and viability [37]. Importantly, Drosophila glial cells migrating over neural lamella use Lox-based matrix crosslinking to alter the stiffness of that basement membrane about two-fold. Expression of lox is under control of an integrin-based signaling system, indicating that the cell senses matrix stiffness and then increases it [44]. Thus, in this tumor model, cells regulate matrix stiffness in a dynamic manner, a finding that suggests cells may dynamically alter stiffness under normal physiological or developmental conditions as well. Indeed, an analysis of basement membrane stiffness in mosaic egg chambers where some follicle cells have been genetically manipulated to secrete less collagen IV suggests that basement membrane stiffness is rapidly altered by follicle cells [32]. It will be interesting to discover how relative and absolute levels of basement membrane stiffness are set during development and to discover how those set-points are changed in response to developmental and environmental cues.
Finally, while this review has focused on the contributions of Drosophila to our understanding of basement membrane mechanics, it is important to acknowledge that C. elegans, the other major invertebrate model organism, is also revolutionizing our understanding of basement membrane biology. Notable work in C. elegans has illuminated the intricacies of basement membrane invasion [48], identified new structures in basement membrane architecture [49], and has added to our knowledge of the basement membrane’s role in nervous system development [50]. As the genetic tools in both Drosophila and C. elegans continue to evolve, there is no doubt that both model systems will continue play an essential role in the field.
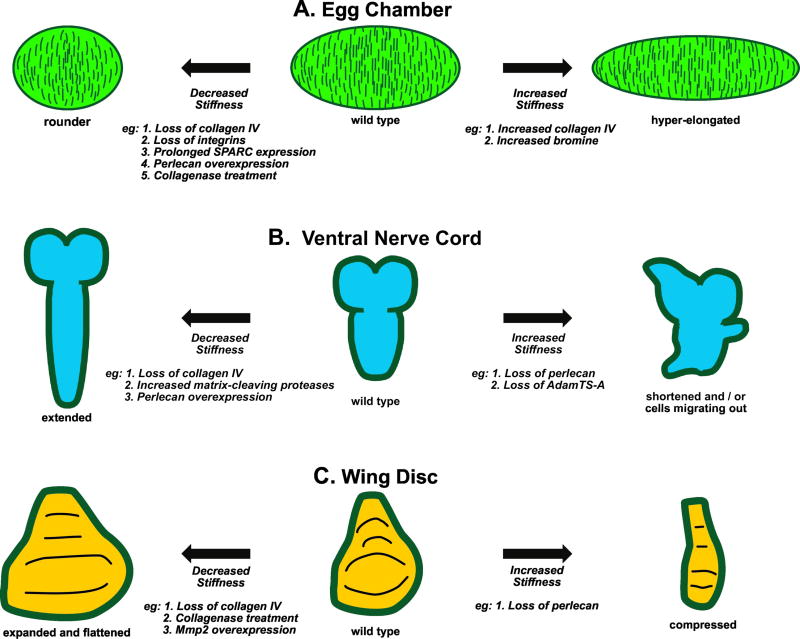
Figure 2. Basement membrane stiffness alters organ shape.
All Drosophila organs are surrounded by a continuous sheet of basement membrane.
A) The basement membrane surrounding wild-type developing egg chambers does not have uniform stiffness, but rather is more stiff in the middle and less stiff toward the poles. Perturbations that decrease the stiffness lead to rounded egg chambers, whereas those that increase the stiffness leads to hyper-elongated egg chambers. See text for details.
B) The larval central nervous system is composed of two brain lobes (top) and an elongated ventral nerve cord. Perturbations that soften the basement membrane allow hyper-elongation of the ventral nerve cord, whereas those that generate stiffer basement membrane result in compaction of the ventral nerve cord and mobilize neural progenitor cells to migrate out of the central nervous system, as described in the text.
C) The larval wing disc is the precursor of adult wing and notum tissues, and it has a characteristic shape. Softer basement membrane allows the wing disc to expand and flatten, whereas stiffer basement membrane compresses it, as described in the text.
Highlights.
Cellular sources of basement membrane proteins change during Drosophila development.
Anisotropic stiffness of basement membrane drives Drosophila egg chamber elongation.
Perlecan counteracts collagen IV in stiffening the basement membrane
Excessive basement membrane stiffness promotes cellular migration out of the Drosophila central nervous system.
Basement membrane degradation around the wing disc leads to the loss of Dpp and smaller adult wings.
Drosophila is a powerful model for in vivo analysis of basement membrane function and stiffness.
Acknowledgments
The authors wish to thank Nick Ferrell for discussions of stiffness measurements, Gautam Bhave for guidance on crosslinking, Heather Broihier for help with the nervous system, Patrick Page-McCaw for discussion of enzymatic modifications, and anonymous reviewers for many helpful suggestions that strengthened this manuscript. This work was supported by the National Institutes of Health R21AR072510 to APM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yurchenco PD. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harbor Perspectives in Biology. 2011;3:1–27. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adhesion & Migration. 2014;7:64–71. doi: 10.4161/cam.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO. The evolution of metazoan extracellular matrix. The Journal of Cell Biology. 2012;196:671–679. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, G B, Yin VP, et al. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. U.S.A. 2014;111:331–336. doi: 10.1073/pnas.1318499111/-/DCSupplemental/pnas.201318499SI.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, et al. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. Elife. 2017;6:1–24. doi: 10.7554/eLife.24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 8.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 9.Borchiellini C, Coulon J, Le Parco Y. The function of type IV collagen during Drosophila muscle development. Mechanisms of Development. 1996;58:179–191. doi: 10.1016/S0925-4773(96)00574-6. [DOI] [PubMed] [Google Scholar]
- 10.Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, et al. Perlecan Maintains the Integrity of Cartilage and Some Basement Membranes. The Journal of Cell Biology. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RT. Mechanical properties of basement membrane in health and disease. Matrix Biology. 2017;57–58:366–373. doi: 10.1016/j.matbio.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO, Zhao Q. The evolution of cell adhesion. The Journal of Cell Biology. 2000;150:F89–96. doi: 10.1083/jcb.150.2.F89. [DOI] [PubMed] [Google Scholar]
- 13.Van De Bor V, Zimniak G, Papone L, Cerezo D, Malbouyres M, Juan T, et al. Companion Blood Cells Control Ovarian Stem Cell Niche Microenvironment and Homeostasis. Cell Reports. 2015;13:546–560. doi: 10.1016/j.celrep.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. Journal of Cell Science. 1991;100:781–788. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- 15.Denef N, Chen Y, Weeks SD, Barcelo G, Schüpbach T. Crag Regulates Epithelial Architecture and Polarized Deposition of Basement Membrane Proteins in Drosophila. Developmental Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isabella AJ, Horne-Badovinac S. Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Developmental Cell. 2016;38:47–60. doi: 10.1016/j.devcel.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsubayashi Y, Louani A, Dragu A, Sánchez-Sánchez BJ, Serna-Morales E, Yolland L, et al. A Moving Source of Matrix Components Is Essential for De Novo Basement Membrane Formation. Current Biology. 2017;27:3526–3534. doi: 10.1016/j.cub.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fessler JH, Fessler LI. Drosophila extracellular matrix. Annu. Rev. Cell Biol. 1989;5:309–339. doi: 10.1146/cellbio.1989.5.issue-1;page:string:Article/Chapter. [DOI] [PubMed] [Google Scholar]
- 20.Pastor-Pareja JC, Xu T. Shaping Cells and Organs in Drosophila by Opposing Roles of Fat Body-Secreted Collagen IV and Perlecan. Developmental Cell. 2011;21:245–256. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page-McCaw A, Serano J, Santé JM, Rubin GM. Drosophila Matrix Metalloproteinases Are Required for Tissue Remodeling, but Not Embryonic Development. Developmental Cell. 2003;4:95–106. doi: 10.1016/S1534-5807(02)00400-8. [DOI] [PubMed] [Google Scholar]
- 23.Duhart JC, Parsons TT, Raftery LA. The repertoire of epithelial morphogenesis on display: Progressive elaboration of Drosophila egg structure. Mechanisms of Development. 2017;148:18–39. doi: 10.1016/j.mod.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Bilder D, Haigo SL. Expanding the Morphogenetic Repertoire: Perspectives from the Drosophila Egg. Developmental Cell. 2012;22:12–23. doi: 10.1016/j.devcel.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cetera M, Juan GRR-S, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, et al. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nature Communications. 2014;5:1–12. doi: 10.1038/ncomms6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aurich F, Dahmann C. A Mutation in fat2 Uncouples Tissue Elongation from Global Tissue Rotation. Cell Reports. 2016;14:2503–2510. doi: 10.1016/j.celrep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 27.Chen D-Y, Crest J, Bilder D. A Cell Migration Tracking Tool Supports Coupling of Tissue Rotation to Elongation. Cell Reports. 2017;21:559–569. doi: 10.1016/j.celrep.2017.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, et al. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahab J, Baratta C, Scuric B, Godt D, Venken KJT, Ringuette MJ. Loss of SPARC dysregulates basal lamina assembly to disrupt larval fat body homeostasis in Drosophila melanogaster. Developmental Dynamics. 2015;244:540–552. doi: 10.1002/dvdy.24243. [DOI] [PubMed] [Google Scholar]
- 30.Isabella AJ, Horne-Badovinac S. Dynamic regulation of basement membrane protein levels promotes egg chamber elongation in Drosophila. Developmental Biology. 2015;406:212–221. doi: 10.1016/j.ydbio.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crest J, Diz-Muñoz A, Chen D-Y, Fletcher DA, Bilder D. Organ sculpting by patterned extracellular matrix stiffness. Elife. 2017;6:1–16. doi: 10.7554/eLife.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chlasta J, Milani P, Runel G, Duteyrat JL, Arias L, Lamire LA, et al. Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development. 2017;144:4350–4362. doi: 10.1242/dev.152652. [DOI] [PubMed] [Google Scholar]
- 33.Andersen D, Horne-Badovinac S. Influence of ovarian muscle contraction and oocyte growth on egg chamber elongation in Drosophila. Development. 2016;143:1375–1387. doi: 10.1242/dev.131276/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durbeej M. Current Topics in Membranes. Elsevier Ltd; 2015. Laminin-alpha 2 Chain-Deficient Congenital Muscular Dystrophy: Pathophysiology and Development of Treatment; pp. 31–60. [DOI] [PubMed] [Google Scholar]
- 35.Bhave G, Colon S, Ferrell N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. American Journal of Physiology-Renal Physiology. 2017;313:F596–F602. doi: 10.1152/ajprenal.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanacore R, Ham A-JL, Voehler M, Sanders CR, Conrads TP, Veenstra TD, et al. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture. Cell. 2014;157:1380–1392. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Sánchez BJ, Urbano JM, Comber K, Dragu A, Wood W, Stramer B, et al. Drosophila Embryonic Hemocytes Produce Laminins to Strengthen Migratory Response. Cell Reports. 2017;21:1461–1470. doi: 10.1016/j.celrep.2017.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skeath JB, Wilson BA, Romero SE, Snee MJ, Zhu Y, Lacin H. The extracellular metalloprotease AdamTS-A anchors neural lineages in place within and preserves the architecture of the central nervous system. Development. 2017;144:3102–3113. doi: 10.1242/dev.145854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma M, Cao X, Dai J, Pastor-Pareja JC. Basement Membrane Manipulation in Drosophila Wing Discs Affects Dpp Retention but Not Growth Mechanoregulation. Developmental Cell. 2017;42:97–106.e4. doi: 10.1016/j.devcel.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Meyer S, Schmidt I, Klämbt C. Glia ECM interactions are required to shape the Drosophila nervous system. Mechanisms of Development. 2014;133:105–116. doi: 10.1016/j.mod.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Borchiellini C, Coulon J, Le Parco Y. The function of type IV collagen during embryogenesis. Roux's Arch Dev Biol. 1996;205:468–475. doi: 10.1007/BF00377228. [DOI] [PubMed] [Google Scholar]
- 43.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. U.S.A. 2012;109:10334–10339. doi: 10.1073/pnas.1118073109/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SN, Jeibmann A, Halama K, Witte HT, Wälte M, Matzat T, et al. ECM stiffness regulates glial migration in Drosophila and mammalian glioma models. Development. 2014;141:3233–3242. doi: 10.1242/dev.106039. [DOI] [PubMed] [Google Scholar]
- 45.Beira JV, Paro R. The legacy of Drosophila imaginal discs. Chromosoma. 2016:1–20. doi: 10.1007/s00412-016-0595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eder D, Aegerter C, Basler K. Forces controlling organ growth and size. Mechanisms of Development. 2017;144:53–61. doi: 10.1016/j.mod.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 48.Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: the anchor cell breaches the barrier. Current Opinion in Cell Biology. 2011;23:589–596. doi: 10.1016/j.ceb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrissey MA, Keeley DP, Hagedorn EJ, McClatchey STH, Chi Q, Hall DH, et al. B-LINK: A Hemicentin, Plakin, and Integrin- Dependent Adhesion System that Links Tissues by Connecting Adjacent Basement Membranes. Developmental Cell. 2014;31:319–331. doi: 10.1016/j.devcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin J, Liang J, Ding M. Perlecan Antagonizes Collagen IV and ADAMTS9/GON-1 in Restricting the Growth of Presynaptic Boutons. Journal of Neuroscience. 2014;34:10311–10324. doi: 10.1523/JNEUROSCI.5128-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gramates LS, Marygold SJ, Santos GD, Urbano J-M, Antonazzo G, Matthews BB, et al. FlyBase at 25: looking to the future. Nucleic Acids Research. 2017;45:D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]