Abstract
Dynamic macrostructural and microstructural changes take place from the mid-fetal stage to 2 years after birth. Delineating brain structural changes during this early developmental period provides new insights into the complicated processes of both typical brain development and the pathological mechanisms underlying various psychiatric and neurological disorders including autism, attention deficit hyperactivity disorder and schizophrenia. Decades of histological studies have identified strong spatial and functional gradients of maturation in human brain gray and white matter. The recent improvements in magnetic resonance imaging (MRI) techniques, especially diffusion MRI (dMRI), relaxometry imaging, and magnetization transfer imaging (MTI) have provided unprecedented opportunities to non-invasively quantify and map the early developmental changes at whole brain and regional levels. Here, we review the recent advances in understanding early brain structural development during the second half of gestation and the first two postnatal years using modern MR techniques. Specifically, we review studies that delineate the emergence and microstructural maturation of white matter tracts, as well as dynamic mapping of inhomogeneous cortical microstructural organization unique to fetuses and infants. These imaging studies converge into maturational curves of MRI measurements that are distinctive across different white matter tracts and cortical regions. Furthermore, contemporary models offering biophysical interpretations of the dMRI-derived measurements are illustrated to infer the underlying microstructural changes. Collectively, this review summarizes findings that contribute to charting spatiotemporally heterogeneous gray and white matter structural development, offering MRI-based biomarkers of typical brain development and setting the stage for understanding aberrant brain development in neurodevelopmental disorders.
Keywords: diffusion MRI, quantitative MRI, tractography, early development, microstructure, baby brain
1. Introduction
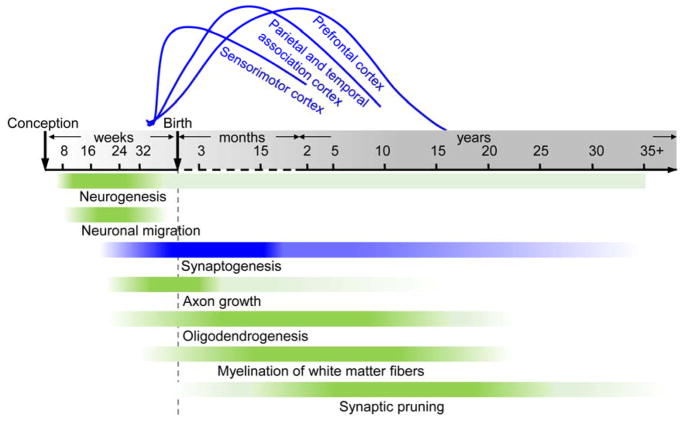
Early brain development, particularly from the mid-fetal stage to 2 years after birth, is probably the most dynamic across the entire lifespan. Striking morphological changes have been observed during fetal development from the 4th post-conceptional week (pcw) to birth during which the brain develops from a tubular structure to a highly complicated yet organized organ with adult-like architecture (e.g. Huang and Vasung, 2014; Silbereis et al., 2016). From birth to 2 years of age, the overall brain size increases dramatically, reaching close to 90% of adult volume by the age of 2 years (Pfefferbaum et al., 1994). The gray matter volume also reaches a lifetime maximum around 2 years of age (Hüppi et al., 1998a; Matsuzawa et al., 2001). These structural changes, in parallel to the brain circuit formation, result from immensely complicated yet precisely regulated molecular and cellular processes (Silbereis et al., 2016), including neurogenesis and neuronal migration (Rakic 1972, 1995; Sidman and Rakic, 1973), synapse formation (Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997), dendritic arborization (Bystron et al., 2008; Sidman and Rakic, 1973), axonal growth, pruning (Innocenti and Price, 2005; Kostović and Jovanov-Milosević, 2006) and myelination (Miller et al., 2012; Yakovlev and Lecours, 1967). These cellular and molecular processes shape the structural and functional architecture of the human brain and underlie the varying rates of maturation of functional systems, from the primary sensorimotor to higher-order cognitive regions. The spatial and functional gradients of these major maturational events in the developing brain are described in Figure 1.
Figure 1.
Timeline of spatiotemporally distinctive human brain maturational processes, including neurogenesis, neuronal migration, synaptogenesis, axon growth, oligodendrogenesis, myelination of white matter fibers and synaptic pruning. Time axis is in post-conceptional weeks (before birth), postnatal months (until 24 months), and postnatal years (after 2 years). The color intensity in each bar corresponds to the rate of developmental changes. The spatial progression across brain regions is illustrated using synaptogenesis (blue bar) as an example. Specifically, the spatial progression of synaptogenesis from primary sensorimotor cortex to higher-order prefrontal cortex is illustrated by the blue curves above the time axis.
Current knowledge of early brain development originated from histological studies. For example, cortical synaptogenesis starts as early as the 18pcw and reaches its peak density in the frontal cortex by the 15th postnatal month, later than synaptogenesis peaks of primary auditory and visual cortices (Huttenlocher and Dabholkar, 1997; Kwan et al., 2012). Similarly, myelination cycles are significantly different across white matter regions and tracts, with varying starting times and durations (Brody et al, 1987; Kinney et al, 1988; Yakovlev and Lecours, 1967). Altered synaptic pruning has been found to be associated with neurodevelopmental disorders such as autism, attention-deficit hyperactivity disorder, and schizophrenia with histological studies (e.g. Feinberg, 1983; McGlashan and Hoffman, 2000; Tang et al., 2014). These major psychiatric disorders have a prominent onset (e.g. Hazlett et al., 2017; Marín, 2016) in early infancy or the fetal period, indicating this early period is likely to be critical to these neurodevelopmental disorders. Despite the significant contribution of histological studies to understanding typical and atypical brain development, such studies are relatively labor-intensive and time-consuming and may not be suitable for surveying the entire brain. Therefore, it is extremely difficult to reveal the global maturation pattern of the white matter or the cerebral cortex with histological approaches alone.
Contemporary magnetic resonance imaging (MRI), capable of surveying the entire brain with usually less than 1-hour scan time, has become a very important tool for understanding the microstructural and macrostructural changes of early developing human brain non-invasively. The MRI images, including diffusion MRI (dMRI), relaxometry MRI (quantitative T1- and T2-maps) and magnetization transfer imaging (MTI), reveal different aspects of gray and white matter microstructural changes as well as myelination processes across the cerebral cortex, subcortical gray matter and major white matter tracts in vivo. In this review, we summarize the macrostructural and microstructural findings in the brains of fetuses, preterm and full-term newborns, and infants less than 2 years of age using MRI approaches, especially dMRI. First, we briefly introduce the basic principles of MRI techniques, emphasizing diffusion tensor imaging (DTI), one of the most widely used dMRI models. Next, we consolidate the current understanding of white matter development, including the emergence pattern of white matter tracts and MRI measurement changes of white matter at both tract and tract group levels. Third, we review findings of unique microstructural organization and heterogeneous maturation patterns across the cortical plate and in different subcortical gray matter nuclei. Finally, we discuss the issues and limitations of studying white and gray matter development in the baby brain using MRI techniques, and suggest avenues that could be explored for future research. Without additional description, the fetal and preterm brain age defined in pcw, postmenstrual week (pmw) or weeks of gestation (wg) was directly adapted from the reviewed studies. The standard age terminology of fetal and infant brain can be found in the policy statement by American Academy of Pediatrics Committee (Engle et al., 2004). We hope this review will become a useful reference for investigations using other modalities such as histochemical, electrophysiological and biobehavioral approaches and facilitate detecting important MRI-based biomarkers for prognosis and treatment monitoring of neurodevelopmental disorders.
2. MRI techniques: Diffusion MRI and beyond
With recent unprecedented technical advances in MRI, significant leaps have been made in understanding early brain development using MRI. Diffusion MRI, especially DTI, has become an effective probe to qualitatively and quantitatively characterize brain tissue microstructure, white matter tract anatomy and the structural connectivity of developing human brain. Other MRI-based techniques including T1 weighted (T1w), T2 weighted (T2w) imaging, relaxometry MRI, and MTI have also been extensively used to study brain development in pediatric populations. The shortening of T1 or T2 relaxation time in brain tissues throughout development, particularly in the white matter, is related to decreases in brain water content and increases in the concentration of macromolecules such as myelin (Barkovich et al., 1988). Based on the difference in T1w and T2w contrasts across brain tissues, volumetric and morphological changes of developing brain gray and white matter can be quantified (e.g. Counsell et al., 2002; Hüppi et al., 1998a). Magnetization transfer ratio (MTR), an index of MTI, has been used to characterize the changes in the brain tissue disorganization associated with axonal loss or demyelination. The following sections provide a brief introduction to the principles of these MRI techniques.
2.1 Diffusion MRI
2.1.1 Basics of diffusion MRI
Diffusion is a Brownian motion process, referring to random movement of water and other small molecules due to thermal collisions. In the human brain, diffusion of water molecules is preferentially along the axons rather than perpendicular to them. dMRI, a non-invasive technique, provides a unique opportunity to measure the diffusional characteristics of the human brain. This is particularly important in cases where the contrast from other imaging methods is not sensitive enough to resolve the boundaries between brain tissues. For example, the contrasts of T1w and T2w images of infant brains are relatively weak due to insufficient myelination. By applying a pair of so-called diffusion gradients to the magnetic field, characterized by b value with units of s/mm2 (LeBihan and Breton, 1985), the diffusion sensitized signal S can be calculated as follows.
| (1) |
where D is the diffusion coefficient with units of mm2/s, S and S0 are the diffusion sensitized and non-diffusion signal. By solving equation (1) in each voxel, the diffusion coefficient (D), known as apparent diffusion coefficient (ADC) in biological tissues, can be obtained. It is noteworthy that one D value reflects diffusion in only one direction, which is along the direction of the appl1ied diffusion gradient.
2.1.2 Diffusion tensor and other advanced diffusion MRI models
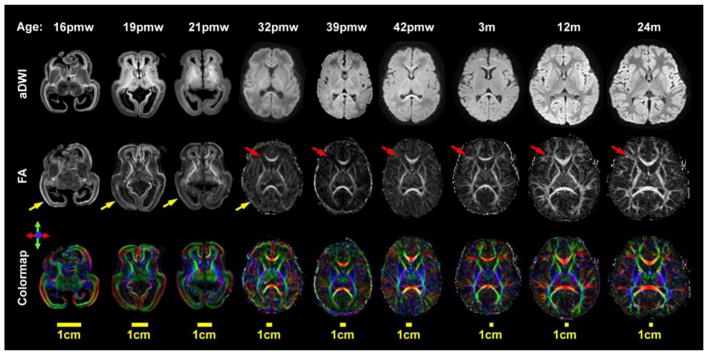
A diffusion profile composed of D value measurements along multiple orientations can be obtained by applying a sufficient number of diffusion gradients along varying directions. We can characterize this diffusion profile with a mathematical model. DTI (Basser et al., 1994) is most widely used among all dMRI models, despite certain limitations (e.g. Jones and Cercignani, 2010; Tournier et al., 2011). DTI characterizes the magnitude, anisotropy and orientation of the diffusion by a three-dimensional ellipsoid, or a rank-2 diffusion tensor, with the underlying assumption that water molecular diffusion follows a Gaussian distribution. After the tensor fitting process, the properties of the three-dimensional ellipsoid such as the length of the longest, middle and shortest axes (called eigenvalues, λ1, λ2, and λ3 respectively) and their orientations (called eigenvectors, ν1, ν2 and ν3, respectively) can be obtained. Once the eigenvalues and eigenvectors are estimated at each image voxel, several DTI-derived metrics can be calculated. Specifically, fractional anisotropy (FA) (Pierpaoli and Basser, 1996), a measurement that characterizes the shape of the three-dimensional ellipsoid, can be calculated with its value ranging from 0 to 1. FA is sensitive to white matter microstructural changes (Beaulieu, 2002). Specifically for the fetal and baby brain, the FA is also sensitive to cerebral cortical microstructural changes. FA is probably the most widely used metric in dMRI studies of baby brains. The orientation of the longest axis of the estimated ellipsoid (primary eigenvector, ν1) can be converted to a color at each pixel (Makris et al., 1997; Pajevic and Pierpaoli, 1999). By combing the intensities of the FA map, a color-encoded orientation map can be obtained. In addition, axial diffusivity (AD), the primary eigenvalue (λ1) of the tensor, quantifies the water diffusion parallel to the primary eigenvector of the diffusion tensor. AD has been thought to describe the axonal integrity of the white matter fiber bundle. Radial diffusivity (RD), usually calculated as the average of the second and the third eigenvalues of the tensor, quantifies the magnitude of diffusion orthogonal to the principal diffusion direction. RD has been thought to reflect the extent of white matter myelination (Song et al., 2005). Although AD and RD have been used to infer these microstructural changes, caution needs to be taken for their interpretations (Wheeler-Kingshott and Cercignani, 2009). Mean diffusivity (MD), calculated as the average of all three eigenvalues, quantifies the average magnitude of water diffusion along all directions. Figure 2 shows DTI-derived maps of fetal and infant brains from the mid-fetal stage to 2-years-old. Furthermore, advanced diffusion MRI models, including but not limited to diffusion kurtosis imaging (DKI) (Jensen et al., 2005) and neurite orientation dispersion and density imaging (NODDI) (Zhang et al., 2012), have been proposed to characterize brain microstructure.
Figure 2.
Diffusion MRI (dMRI) contrast in the fetal and infant brain from 16 postmenstrual weeks (pmw) to 2 years (24 months) of age. From top to bottom, axial slices of the averaged diffusion weighted images (aDWI), fractional anisotropy (FA) maps and color-encoded diffusion orientation maps are shown. Yellow arrows indicate high FA values in the cortical plate, and red arrows indicate high FA values in the white matter regions during this early developing period. This figure is generated with the data from Huang lab.
2.1.3 Diffusion MRI based tractography
Tractography based on dMRI has been used to reconstruct white matter pathways three-dimensionally and map the structural connections of the human brain. The results are highly dependent upon the dMRI models and parameters used. DTI-based deterministic tractography refers to the techniques of connecting the primary eigenvectors to reconstruct the pathways of white matter bundles. Streamline propagation methods (Mori et al., 1999; Jones et al., 1999) have been widely used. Since DTI only reveals one dominant fiber orientation in each voxel, it oversimplifies the underlying complex neural structures at voxels containing crossing or kissing fiber bundles. More advanced dMRI tractography methods, including Q-ball (e.g. Tuch et al., 2002), diffusion spectrum imaging (DSI in e.g. Wedeen et al., 2008), constrained spherical deconvolution (CSD in e.g. Tournier et al., 2012), and ball and stick (e.g. Behrens et al., 2007), have been proposed to resolve complex fiber architecture in a given voxel. It is beyond the scope of this review to describe these advanced tractography methods in depth. Reviews of dMRI-based tractography can be found in the literature (e.g. Behrens and Jbabdi, 2009; Jbabdi and Johansen-Berg, 2011).
2.2 Beyond Diffusion MRI: Other quantitative MRI approches
2.2.1 Approaches of quantifying myelination based on T1 and T2 relaxometry
The longitudinal relaxation time (T1) characterizes the proton interactions with its environment (“spin-lattice” interactions), while the transverse relaxation time (T2) characterizes the interactions between protons (“spin-spin” interactions). Both T1 and T2 are sensitive to local chemical and magnetic environment. Although sensitive to relaxation times, anatomical T1w and T2w images only provide qualitative information on their variations across brain tissues. Indeed it is challenging to compare T1w and T2w signal intensities across individuals because of the variability of exams related to scan acquisition settings, head size and positioning within the coil. And it is challenging to compare T1w and T2w signal intensities across brain regions of individual subject too because of B0 and B1 field inhomogeneity, particularly when phased-array surface coils are used. Recently, computing the ratio between T1w and T2w image intensities has been proposed to map myelination differences across cortical areas (Glasser and Van Essen, 2011). This strategy enables one to benefit from the anatomical images that are routinely acquired in the clinical setting, and to improve the localization of heavily myelinated regions in contrast to lightly myelinated ones. The contrast to noise in the ratio between T1w and T2w image is increased because the myelin content covaries with T1w and T2w intensity, but in opposite directions. The ratio between T1w and T2w image is also more reliable than T1w or T2w image alone, as some aspects of spatial inhomogeneity in signal intensity can be ameliorated. Specifically, the bias related to the sensitivity profile of the radio frequency receiver coil (B1−) can be corrected with the ratio, since it is the same in T1w and T2w images. Variations due to transmit field (B1+) biases are not corrected but minimized as they are correlated between (but not the same in) both images, and these variations might be less problematic in scanners with good B1+ homogeneity (Glasser and Van Essen, 2011).
Nonetheless, the best approach to measure reliable differences across individuals (e.g. in infants during development) or across brain regions within the same individual is to map T1 and T2 relaxation time constants quantitatively. This requires the acquisition of several images with different parameter settings (different inversion times to compute T1, or different echo times to compute T2), and the estimation of absolute T1 and T2 values by fitting the model of the expected signal to the actual measurements in each voxel.
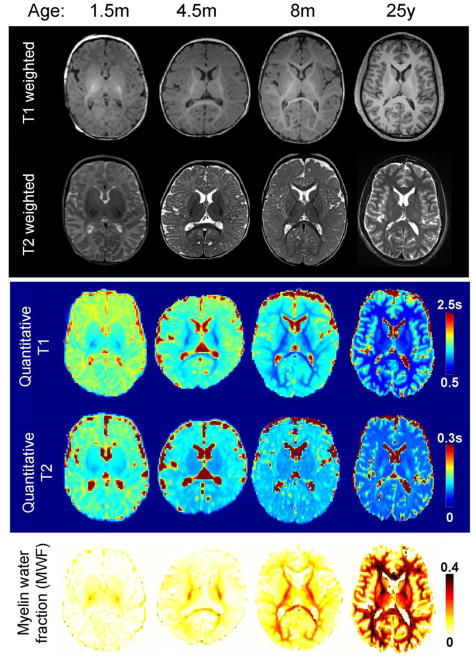
Maps of the fraction of water related to myelin, sometimes called the “myelin water fraction” (MWF), can be obtained with T1 and T2 measurements. T1 and T2 strongly depend on the water concentration. At least two distinct pools of water molecules contribute to the MR T1 and T2 signal in brain tissues: water located within the myelin sheath (showing relatively short T1 and T2 relaxation times), vs intra-axonal, intra-cellular and interstitial water (i.e. water outside of the myelin sheath, showing longer T1 and T2). Approaches based on “multi-component relaxation” (MCR) analyses further provide direct information about the myelin content, by modeling different pools of water molecules in each voxel (Spader, et al. 2013). These pools can be distinguished from measured MR signals, on the basis of different relaxometric properties (T1 and/or T2) and of specific exchange relationships (Beaulieu, et al. 1998; Menon, et al. 1991; Whittall, et al. 1997). Such decomposition is supposed to provide valuable information about tissue microstructure. Although the exact number of pools to be modelled is still debated (Deoni, et al., 2012), a consistent pool of water related to myelin is the MWF. Unlike relaxation times, MWF is independent of the magnetic field strength, but its computation is highly sensitive to both the acquisition protocol and the post-processing model, making direct comparisons across studies challenging. An example of T1w, T2w, quantitative T1 and T2 time constant maps, and MWF in representative baby brains at 1.5-, 4.5- and 8-months-old is illustrated in Figure 3.
Figure 3.
Anatomical images of the developing brain. From top to bottom, T1 weighted, T2 weighted images, quantitative maps of T1, T2 relaxation times (in seconds) and myelin water fraction (MWF) of representative subjects at different ages, infant of 1.5 month (m), 4.5m and 8m and a young adult (25 years), are shown.
2.2.2 Approaches based on magnetization transfer
Other MR quantitative parameters relying on myelin amount have been proposed in the recent years. The MTR, an index of MTI, informs about the ratio between free water and water with restricted motion bound to macromolecules such as proteins and lipids (McGowan, 1999). This technique appears to be sensitive to myelin-associated macromolecules, but also to the macromolecular density of axonal cytoskeleton components such as microtubules and neurofilaments (Nossin-Manor, et al. 2013). While MTR is generally thought to directly reflect the myelin amount (Kucharczyk, et al. 1994), it might also depend on the tight organization and close package of non-myelinated fibers (Nossin-Manor, et al. 2013).
3. Maturation of white matter from middle fetal stage to 2-years-old
The brain can be divided into white and gray matter. Gray matter can be regarded as information processing hubs, and white matter acts as a long-range communication and transmission system. For several decades, the architecture of white matter has been delineated with histology of postmortem brains. The recent advances of MRI of developing brains shed light on important questions such as when and how certain connections emerge at the beginning of life and what are the maturational trajectories of white matter tracts in typical development. Especially with dMRI, the emergence and maturation processes of fetal and infant brains’ white matter tracts have been elucidated (e.g. Dubois et al., 2006, 2008; Huang et al., 2006, 2009; Hüppi et al., 1998b; Kasprian et al., 2008, 2013; Kolasinski et al., 2013; Miller et al., 2014; Neil et al., 1998; Partridge et al., 2004; Takahashi et al., 2011; Xu et al., 2012).
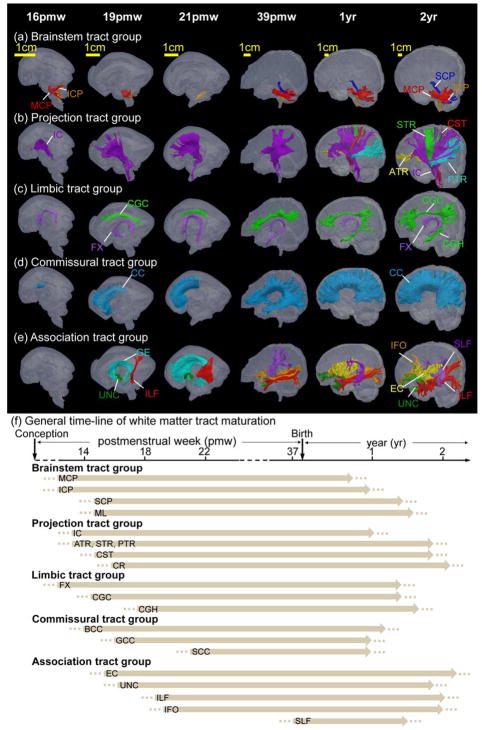
3.1 Delineating emergence of white matter tracts with DTI tractography
The major white matter tracts in the human brain can be categorized into five functional categories: limbic, commissural, projection, association and brainstem tract groups (Wakana et al., 2004, 2007). Figures 4a–4e show the emergence of these developing white matter tracts of the five tract groups in the baby brain from the mid-fetal stage to 2-years-old. As all tracts were three-dimensionally reconstructed with DTI tractography, it is noteworthy that Figure 4 only demonstrates the timeline from the perspective of DTI tractography and is restricted by factors that limit DTI tractography (see e.g. Jbabdi and Johansen-Berg, 2011 for review). Thus, Figure 4 may not accurately reflect the exact emergence time of white matter tracts. Histological delineation is complementary to DTI tractography, but is restricted by the factors such as local coverage with small blocks and difficulty for three-dimensional reconstruction. In addition, it is labor-intensive. With fetal brain DTI studies (e.g. postmortem brain in Huang et al., 2006, 2009, Ouyang et al., 2015, Song et al., 2017, Takahashi et al., 2011; in utero fetuses in Kasprian et al., 2008, 2013), a heterogeneous emergence pattern of white matter across different tracts and tract groups was observed. Specifically, in the limbic tract group, the fornix (FX) probably emerges earlier than 13 weeks of gestational age (wg), and the cingulum bundle in cingulate gyrus (CGC) and cingulate bundle in hippocampus (CGH) appear around 19wg. In the commissural tract group, the body of the corpus callosum (BCC) emerges around 15wg, followed by the genu (GCC, before 19wg) and splenium (SCC, after 19wg) of the corpus callosum. In the projection fiber tracts, the anterior corona radiata (ACR) probably emerges before 13wg, followed by the superior portion (SCR) around 15wg and the posterior portion (PCR) after 19wg. In the association tract group, the external capsule (EC) emerges before 13wg, followed by the appearance of the uncinate fasciculus (UNC) around 15wg, and the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFO) around 19wg. The exact time for the emergence of the superior longitudinal fasciculus (SLF) is not clear. The observations from dMRI tractography suggest that the SLF may emerge during the third trimester (Huang et al., 2006, 2009; Miller et al., 2014; Takahashi et al., 2011). Histological studies show such long-range cortico-cortical fibers emerge around 33–35pcw (Kostovic and Jovanov-Milosevic, 2006; Kostovic and Rakic, 1990), although the SLF is not prominently visible with dMRI-based tractography even at birth using current techniques. The white matter tracts that emerge earlier (e.g. the brainstem tracts) may play important roles in supporting the basic functions for life. Conversely, the later emerging tracts such as the SLF may contribute more to higher-level brain functions. Playing a critical role in language function, the SLF has been demonstrated to mature late (e.g. Zhang et al., 2007). The approximate emergence pattern of white matter tracts from a suite of dMRI tractography studies (Huang et al., 2006, 2009; Kasprian et al., 2008, 2013; Kolasinski et al., 2013; Miller et al., 2014; Takahashi et al., 2011; Xu et al., 2012) and histological atlases (Bayer and Altman, 2004, 2005) is summarized in Figure 4f.
Figure 4.
Diffusion MRI tractography of the white matter tracts in developing fetal and infant brain from 16 postmenstrual weeks (pmw) to 2 years. (a) Brainstem tracts including inferior, middle, superior cerebellar peduncle (ICP, MCP and SCP) and medial lemniscus (ML); (b) Projection tracts including interior capsule (IC), corona radiata (CR), corticospinal tract (CST), anterior, superior and posterior thalamic radiation (ATR, STR and PTR); (c) Limbic tracts including cingulum bundle in the cingulate cortex (CGC), cingulum bundle in the temporal cortex (CGH) and fornix (FX); (d) Commissural tracts including body, genu and splenium of corpus callosum (BCC, GCC and SCC); (e) Association tracts including fibers in external capsule (EC), inferior longitudinal fasciculus (ILF), inferior occipitofrontal fasciculus (IFO), superior longitudinal fasciculus (SLF) and uncinate fasciculus (UNC). Ganglionic eminence (GE), a transient fetal brain structure and well traced with DTI tractography, is also included in this tract group. (f) General timeline of white matter maturation across different tracts and tract groups. Dotted lines indicate that white matter tracts emerge at these ages, though to a relatively minor degree, and arrows indicate that overall white matter tracts are formed with continuous maturational processes such as myelination and axonal packing thereafter. This figure is generated with the data from Huang lab.
3.2 The heterogeneous microstructural changes of white matter tracts and tract groups with DTI measurements
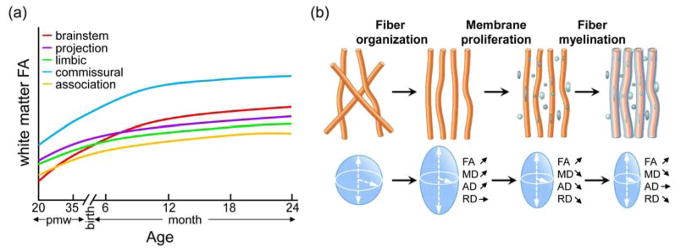
Significant microstructural changes of white matter tracts take place during the fetal stage (Kersbergen et al., 2014) and the first two years of life (Geng et al., 2012; Hermoye et al., 2006; Mishra et al., 2013; Mukherjee et al., 2001; Yu et al., 2014). A general pattern of age-dependent FA increase and diffusivity (i.e. MD, AD and RD) decrease has been found in most baby brain white matter tracts (e.g. 28–43wg in Berman et al., 2005; 30–40wg in Kersbergen et al., 2014; 1–4 month in Dubois et al., 2008; 0–2 year in Geng et al., 2012). Within this general pattern, the maturational rates of different white matter tracts are spatiotemporally heterogeneous. Linear (Berman et al., 2005; Dubois et al., 2006, 2008), exponential (Lebel et al., 2008; Mukherjee et al., 2001; Yu et al., 2017) and logarithmic (e.g. Yu et al., 2014) curves have been used to fit the age-dependent changes of DTI-derived metric measurements of white matter tracts based on the studied age range. Linear curves were usually used for characterizing white matter microstructural changes in a relatively short period while exponential or logarithmic curves were used in longer developmental periods. Schematic maturational curves of FA values for the five white matter tract groups from the mid-fetal stage to 2-years-old are illustrated in Figure 5a based on previous studies (28–43wg in Berman et al., 2005; 30–40wg in Kersbergen et al., 2014; 1–4 month in Dubois et al., 2008; 0–2 year in Gao et al., 2009; Geng et al., 2012; Hermoye et al., 2006; Mukherjee et al., 2001, 2002; Yu et al., 2017). The increasing FA values in all tract groups probably indicate the widespread myelination process across white matter regions. The maturational pattern of different tract groups is heterogeneous (Figure 5a). As shown in the sketch plot in Figure 5a, during the age from 20pmw to 2-years-old the FA values of commissural tracts are the highest among the five tract groups, while the association tracts are the lowest. These high FA values in commissural tracts could be due to the higher level of axonal density, and the low level of fiber orientation dispersion, while the lower FA value in association tracts may be related to lower axonal density and greater fiber orientation dispersion, as shown by NODDI (Chang et al., 2015), as well as the crossing fibers in these regions. To further characterize the temporal changes of the white matter tracts in the early developing brain, a three-stage maturational pattern from 0 to 3 years: an initial rapid maturation stage, a relatively slow maturation in the middle, and a final plateaued maturation stage, was proposed in a cross-sectional study (Yu et al., 2017). Based on this three-stage pattern, it was found that FA percentage increases of the commissural and brainstem tract groups from 0 to 2 years were higher than those of the projection, limbic and association tract groups in the same developmental period. In addition, it was found that the association tract group is the last one to reach the plateau stage, suggesting the maturation of the association tracts continues for a longer time than other tract groups. Developmental asynchrony across different white matter tracts has also been confirmed by a longitudinal study (Sadeghi et al., 2013) of the early developing brain with measurements of FA, MD, AD and RD.
Figure 5.
Developmental trajectories of fractional anisotropy (FA) across white matter tracts from the mid-fetal stage to 2 years (a) and the biophysical model of white matter maturation (b). In (a), sketch plots show age-dependent white matter FA changes in five tract groups, namely, brainstem, projection, limbic, commissural and association tract groups from 20 postmenstrual weeks (pmw) to 2 years. Time axis in (a) is in postmenstrual weeks (pmw) before birth and postnatal months until 24 months. In (b), the biophysical model interprets the changes of DTI-derived metrics (i.e. FA, mean/axial/radial diffusivity: MD, AD and RD) (adapted with permission from Dubois et al., 2008 and Qiu et al., 2015).
White matter microstructural changes are governed by a suite of complicated but synchronized developmental processes. Strongest correlations between homologous tracts in the left and right hemispheres were found with correlation analysis of tract-based measurements of MD, FA, AD and RD in the normal adult brain (Wahl et al., 2010). The differences of inter-tract correlations between neonates and children around puberty were investigated using hierarchical clustering analysis (Mishra et al., 2013). It was suggested that inhomogeneous but organized myelination processes may contribute to a reshuffled inter-tract correlation pattern and strengthening of the correlation of homologous tracts from neonates to children around puberty. In a different developmental age range of 7 to 25 years, synchronous changes of cortical thickness and corresponding white mater microstructure has also been observed (Jeon et al., 2015). Although DTI-derived measurements of white matter microstructure may offer significant information about brain development, caution needs to be taken when interpreting the results. FA and diffusivity measurements are at most indirect indicators of myelination (Beaulieu, 2002; Song et al., 2005), axon packing (Mädler, et al., 2008) or axon density (Klawiter et al., 2011). For example, a high FA value in the corpus callosum that is already observed in the preterm brain may reflect the higher level of axonal packing than other white matter regions and a low FA value in the EC may reflect its fanning geometry.
3.3 Advanced dMRI techniques to assess white matter maturation
As introduced in section 2.1.2, a variety of advanced dMRI techniques have also been used to characterize white matter maturation. In addition to the single “shell” of diffusion-weighted orientation measurements needed for DTI at b values typically ranging from b=600–1300 s/mm2, these more advanced dMRI methods require one or more additional shells at higher b values to probe the non-Gaussian component of water diffusion in biological tissues. Specifically, DKI uses this multi-shell dMRI acquisition to characterize white matter tract development in the infant brain from 0 to 5 years old with an exponential curve (Paydar et al., 2014)., Similar to FA change patterns, the mean kurtosis (MK) values, defined as the average kurtosis over all diffusion directions in DKI, of the commissural tracts (SCC and GCC) were higher than the MK of the projection (PLIC and ALIC) and the association tracts (EC). Age-dependent increases of both FA and MK may reflect the myelination of white matter. However, MK was detected to continue to change even as FA has reached its plateau in some white matter regions, suggesting that MK may be a more sensitive measurement to quantify certain microstructural changes of biological tissues (Paydar et al., 2014). DTI and DKI are statistical descriptions of the diffusion-weighted signal, with DTI encompassing the second moment (Gaussian variance) with a rank-2 tensor and DKI including also the fourth moment (non-Gaussian kurtosis) with a rank-4 tensor. This sets them apart from biophysical compartment modeling approaches such as AxCaliber (Assaf et al., 2008), NODDI (Zhang et al., 2012) and white matter tract integrity (WMTI; Jelescu et al., 2015) that explicitly use the multi-shell dMRI data to estimate biologically meaningful properties of the tissue such as the relative volume fractions of free water (CSF), extracellular space and intracellular space, as well as the overall geometry of neurite orientations. These NODDI metrics have been applied to differentiate myelinated fibers (e.g. PLIC) from not yet myelinated ones (e.g. ALIC) in neonate brains (Kunz et al., 2014). With WMTI and NODDI metrics from the multi-shell dMRI acquisition also used for DKI (Fieremans et al., 2011), non-linear increases in both intra-axonal volume fraction and tortuosity of the extra-axonal space were found in GCC, SCC and PLIC in infants from 0 to 3 years old (Jelescu et al., 2015). These increases likely result from active myelination combined with the known asynchrony of fiber development.
3.4 Other quantitative MRI approaches to assess white matter maturation
3.4.1 Approaches based on T1 and T2 relaxometry
T1 and T2 decrease with developmental processes, more strongly in white matter than in grey matter because of the myelination (Barkovich, 2000; Prayer and Prayer, 2003). Assessing the maturation of regions is first possible based on visual inspection of T1w and T2w images, which show an abrupt increase in myelinated white matter in preterm brain between 35 and 41pcw (Hüppi et al., 1998a). To identify mature from immature regions, T1w contrast is generally preferred during the first 6–8 postnatal months, when brain images show an “infantile pattern” (with a reversal of the normal adult contrasts), while T2w contrast is preferred between 6 and 14 months, when images show an iso-intense or early-adult pattern (Barkovich et al., 1992; van der Knaap and Valk, 1990; Paus et al., 2001; Dubois et al., 2014a).
The age-related decreases in T1 and T2 values might be further quantified in the developing brain. Both T1 and T2 values of brain tissue drop in parallel with the decrease in water concentration, nevertheless their time courses are different, and two distinct mechanisms can be distinguished: the change in water compartmentalization (Matsumae et al., 2001), and the increase of protein and lipid content (Barkovich et al., 1988; Kucharczyk et al., 1994). T1 shortening starts during the “pre-myelination” state, while T2 shortening correlates temporally with the chemical maturation of the myelin sheath (Barkovich et al., 1988; Baumann and Pham-Dinh, 2001; Poduslo and Jang, 1984). T1 and T2 drops are particularly rapid over the two first years (Engelbrecht et al., 1998; Haselgrove et al., 2000), and age-related changes are generally modelled with exponentials functions (Leppert et al., 2009). Because of differences in the maturation timeline, the specific time-course of these patterns is brain-region dependent.
Regarding measurement of the myelin water fraction, a strong increase is observed during infancy (Kulikova et al., 2016), and follows a nonlinear growth pattern until toddlerhood (Dean et al., 2014; Deoni et al., 2012). Consistent with histological studies of myelination, the spatiotemporal pattern of myelination progression is demonstrated (Deoni et al., 2011, 2012), showing successive increases in the cerebellum, pons, and internal capsule, caudo-cranially from the splenium of the corpus callosum and optic radiations, to the occipital and parietal lobes, and then to the genu of the corpus callosum and to the frontal and temporal lobes.
Altogether MRI parameters provide complementary information on multiple maturational processes (Deoni et al., 2013; Lancaster et al., 2003) and it is only by comparing these parameters that one can hope to outline comprehensive patterns of white matter microstructure (Dubois et al., 2014a, 2016b; Qiu et al., 2015). This makes it possible to differentiate bundles based on their maturation patterns, as recently highlighted during the preterm period (Nossin-Manor et al., 2013, 2015). Furthermore, DTI-derived measurements might be combined with T1 and T2 by considering an original measure of maturation based on the computation of the Mahalanobis distance comparing infants’ individual data with a group of adults, taking into account the possible correlations between MRI measurements (Kulikova et al., 2015). This measure confirms the asynchrony of white matter maturation in infants between 3 and 21 postnatal weeks, and provides a quantitative evaluation in weeks of the developmental differences between white matter bundles. Clustering brain voxels over a group of infants on the basis of T1, T2 and DTI-derived measurements, has also uncovered four white matter regions with different compactness and maturation (Lebenberg et al., 2015). The spatial distribution of these clusters was anatomically relevant while the approach was free from any anatomical hypothesis.
3.4.2 Magnetization transfer
MTR increases during white matter maturation (Kucharczyk et al., 1994, Nossin-Manor et al., 2013, 2015) with an exponential time course (Engelbrecht et al., 1998; van Buchem et al., 2001). Differences across regions are observed, with a relatively mature stage at around 13 months and 16 months in the occipital and frontal white matter respectively, and at 18 months and 19 months in the splenium and genu of the corpus callosum (Xydis et al., 2006).
The different MRI measurements seem to capture different properties of white matter maturation, even if some studies have suggested some developmental relationships such as relative inverse correlations between MTR and T2 (Engelbrecht et al., 1998) or MTR and T1 (Nossin-Manor et al., 2013). Notably, a study using the cuprizone mouse model of demyelination has shown that the bound pool fraction (ƒ) from MTI is the best indicator of the myelin sheath fraction, while T1 relates to the fraction of myelinated axons, and AD from DTI relates to the fraction of non-myelinated cells (Thiessen et al., 2013). These latter two measurements might thus characterize the tissue micro-architecture rather than myelination.
Complementary to myelin-sensitive and dMRI techniques, a composite framework has been proposed recently to estimate the g-ratio (Stikov et al., 2015), i.e. the ratio between the inner and outer diameters of the myelin sheath, which is thought to be a fundamental characteristic of white matter fibers. In the adult brain, the approach relies on MTI to estimate the myelin content and on NODDI to assess the intra-axonal fraction. Similar approaches using MWF instead of MTI have been tested to quantify myelination in the developing brain of newborns (Melbourne et al., 2016), infants and children (Dean et al., 2016). However, like other MRI measurements, MWF is a voxel-wise aggregate index that merges the properties of distinct fibers that may be crossing or fanning at the voxel level.
3.5 Biophysical models to interpret DTI measurement changes
To better understand the cellular processes underlying white matter maturation in terms of axonal growth, organization and myelination, biophysical models have been proposed to link these cellular processes to the DTI-derived measurement changes during early brain development. For example, a model including different major maturational changes taking place in the tissue (fiber organization, membrane proliferation and fiber myelination as shown in Figure 5b) was proposed as a biophysical interpretation for the changes of DTI-derived measurements (i.e. FA, MD, RD and AD) during white matter development (see details in Dubois et al., 2008; Nossin-Manor et al., 2015). Specifically, the axonal or fiber organization stage would be expected to be associated predominantly with an increase in AD without affecting RD, leading to an increase in both FA and MD. The membrane proliferation or “pre-myelination” stage, characterized with an expansion of immature oligodendroglia cells and their processes, would be expected to be associated with a reduction in all three diffusivity indices due to the increased membrane density and the decreased water content in brain tissue; meanwhile FA would increase. The third stage, myelination of white matter fibers, is associated with a decreasing RD and a little changed AD, which drive FA increases and MD decreases. In places where different tracts are crossing, the situation may require more complex interpretation if these tracts mature with different timelines (Dubois et al, 2014, 2016b). These DTI-based models could be tested with the newer multi-shell dMRI-based models (e.g. DKI and NODDI) that may measure free water content, axonal density and fiber orientation dispersion (see section 7 for details).
4. Microstructural maturation of gray matter from middle fetal stage to 2-years-old
Gray matter also develops rapidly in the fetal and infant stages. It has been shown with structural MRI that the cortical gray matter volume in the human brain increases more than 4-fold in the short period of the 3rd trimester (Hüppi et al., 1998a; Limperopoulos et al., 2005) and around 1.5-fold in the first two years of postnatal life (Knickmeyer et al., 2008). Because of its sensitivity to microstructural changes of organized cortical tissue unique in the fetal and infant brain, dMRI offers insight into the development of not only white matter but also cortical cytoarchitecture. With spatiotemporal mapping of FA of the cerebral cortex, dMRI also sheds light on the dynamics of neuronal processes across the early developing cerebral cortex.
4.1 Microstructural organization in the cortical plate in fetal and preterm human brain and its DTI quantification
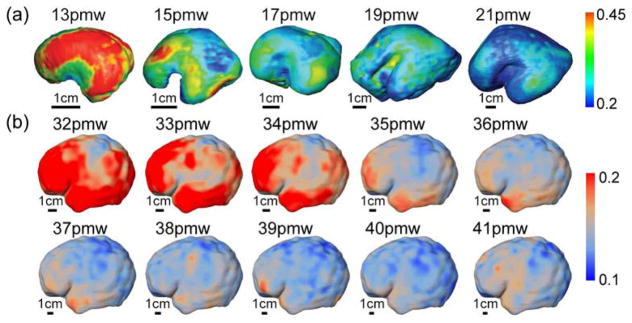
In the fetal and preterm human brain, the cerebral wall is characterized by a laminar organization (e.g. Kostović et al., 2002; Kostović and Vasung, 2009) with some transient compartments lacking their direct counterparts in the adult brain. The outermost layer of this cerebral wall is known as the cortical plate. During cortical development, the majority of cortical neurons are generated near the cerebral ventricles and migrate towards the cortical surface along a radially arranged scaffolding of glial cells. They arrive at their final destination in the fetal cortical plate and assume a well-defined columnar structure (Rakic 1972, 1995; Sidman and Rakic, 1973). In this immature cortex, water molecules preferentially diffuse along the radial glial scaffold and radially oriented apical dendrites instead of perpendicular to them. The highly organized radial architecture of the immature cerebral cortex of fetal and preterm brains is characterized by high FA values as well as by an organized radial orientation of diffusion tensor primary eigenvectors. Unlike the adult human brain, where the FA of the cerebral cortex is low, the FA values of the cortical plate are high in fetal and preterm development (indicated by yellow arrows in Figure 2). With high-resolution postmortem DTI, the cerebral wall of fetal of 13–21wg has been subdivided into cortical plate, subplate and inner layer (Huang et al., 2009, 2013). During 13–21wg, FA values of the cortical plate are much higher than those of the subplate, a transient compartment located beneath the cortical plate. Compared to the cortical FA of the adult brain, relatively high FA values (0.2–0.3) were also found in cortical regions of preterm neonates in the 3rd trimester using in vivo DTI (e.g. 26wg in McKinstry et al., 2002; 25–27pmw in Maas et al., 2004; 25wg in deIpolyi et al., 2005; 27pcw in Ball et al., 2013). Figure 6 shows the cortical FA maps during the 2nd (13–21pmw) and the 3rd (32–41pmw) trimesters. It is clear from Figure 6 that the FA distribution across the cortical regions at a given fetal or preterm age is heterogeneous. A relatively high FA at the frontal cortex compared with primary sensorimotor cortex can be appreciated. This distribution (e.g. Ball et al., 2013; Huang et al., 2013; deIpolyi et al., 2005; Yu et al., 2016) suggests that the frontal cortex is relatively more immature, having less dendritic arborization, synaptic formation, or cellular differentiation in the fetal and preterm brains. Visualization of the primary eigenvectors of the diffusion tensor also demonstrates radial (orthogonal to the cortical surface) organization in the immature cerebral cortex (Huang et al., 2009, 2010; McKinstry et al., 2002; deIpolyi et al., 2005).
Figure 6.
Mapping of FA onto the cortical surface from 13 to 21 postmenstrual weeks (pmw) in the 2nd trimester (a) (adapted with permission from Huang et al., 2013) and the cortical surface from 32 to 41pmw in the 3rd trimester (b) (adapted with permission from Jeon et al., 2016).
4.2 Heterogeneous microstructural changes across the cortical regions assessed by DTI during fetal and preterm development
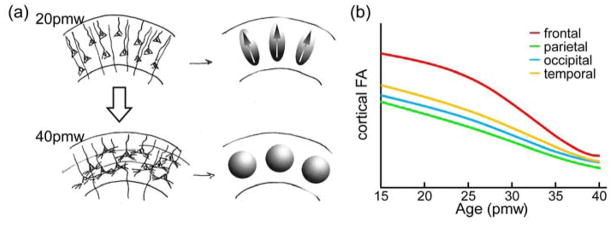
The maturation pattern of the developing cortical plate is characterized by a reduction in both FA and MD, in contrast to the increase in anisotropy and decline in MD of white matter during early development. From 13 to 21wg, the FA measurements of the overall cortical plate undergo a significant decrease as shown in Figure 6a, with the highest value being more than 0.5 at 13wg dropping to around 0.25–0.3 in most cortical areas at 21wg (Huang et al., 2013). Cortical FA decrease has been reported in the developing brain in the 3rd trimester with in vivo DTI of preterm neonates (e.g. 27–38pcw in Ball et al., 2013; 25–38wg in deIpolyi et al., 2005; 27–42wg in Eaton-Rosen et al., 2017; 32–41pmw in Jeon et al., 2016; 26–36wg in McKinstry et al., 2002; 26–40pmw in Smyser et al., 2016, 20–35pmw and 35–40pmw in Yu et al., 2016). For slightly older neonates (38–45pcw in Ball et al., 2013; 36–41wg in McKinstry et al., 2002), no significant FA changes were found in any cortical areas. Cortical FA maps in 32–41pmw are demonstrated in Figure 6b. While neuronal migration is essentially complete by 26pcw (Kostović et al., 2002; Sidman and Rakic, 1973), the subsequent cortical maturation processes involving synaptogenesis and synapse pruning, dendritic arborization, myelination of intracortical white matter, and axonal growth (i.e. Bystron et al., 2008; Huttenlocher and Dabholkar, 1997; Kostović and Jovanov-Milosević, 2006; Marin-Padilla, 1992) disrupt the pronounced radial organization of radial glial scaffold and result in the FA decrease (Figure 7a) (McKinstry et al., 2002). Such cortical FA decreases have also been documented in early developing animal brains (e.g. Huang et al., 2008; Kroenke et al., 2007, 2009; Mori et al., 2001; Sizonenko et al., 2007; Takahashi et al., 2010; Thornton et al., 1997). Besides reductions in FA, a continuous reduction of MD values was found in the cortical regions of neonates from 26–46pmw (27–46pcw in Ball et al., 2013; 26–40pmw in Smyser et al., 2016). The steady decrease of cortical MD could be associated with a dramatic decrease in water content and an increase in tissue density of the cortex secondary to increases in neurite number, cellular complexity, and synapse formation (Dobbing and Sands, 1973). As water content decreases and tissue density increases, barriers to motion move closer, leading to a decrease in MD values.
Figure 7.
Biophysical model of disruption of radial glial scaffold and associated fractional anisotropy (FA) decrease (a) and distinctive spatiotemporal FA decreases across different cortical regions from 15 to 40 postmenstrual weeks (pmw) (b). In (a), upper left panel demonstrates highly organized radial glial fibers, pyramidal neurons with prominent, radially oriented apical dendrites in a 20pmw brain cortical plate, resulting in the diffusion ellipsoids with high FA values and the primary axes oriented radially (upper right panel); lower left panel demonstrates prominent basal dendrites for the pyramidal cells and thalamocortical afferents disrupting the organized radial organization in a 40pmw brain cortical plate, resulting in the diffusion ellipsoids with low FA values (adapted with permission from McKinstry et al., 2002).
Though both FA and MD decrease globally during cortical maturation, the rate of maturation is asynchronous across cortical areas, with the maturation of primary motor and sensory regions preceding that of the association and prefrontal areas, as summarized in the sketch plots in Figure 7b. A sharper FA decrease was found in the frontal cortex than the perirolandic and occipital cortices from preterm brains of 27 to 38pcw (Ball et al., 2013). Similar findings were reported in preterm brains during 35–40pmw, with FA decreasing in the frontal cortical regions faster than in primary cortices (Yu et al., 2016). This differentiated developmental pattern of cortical microstructure among various regions is consistent with neuroanatomical, physiological and functional findings, including regional cerebral glucose utilization with positron emission tomography (PET) (Chugani and Phelps, 1986; Chugani et al., 1987), regional cerebral blood flow (CBF) with arterial spin labeling (ASL) MRI (e.g. Ouyang et al., 2017a) and cortical functional connectivity with resting-state functional MRI (e.g. Cao et al., 2017; Doria et al., 2010). An exploratory correlation between the cortical microstructure and blood flow demonstrated that a sharper FA decrease in the frontal cortex was significantly correlated with a more rapid CBF increase in the same region in the preterm brains of 32 to 45pmw (Ouyang et al., 2017a). This study provides preliminary evidence on the association between cortical microstructural changes and elevated regional CBF. From a microscopic view, synaptogenesis and synaptic elimination have been observed to have distinct regional variations with an earlier beginning in the primary sensorimotor regions, and later start in association regions such as the prefrontal cortex (Huttenlocher and Dabholkar, 1997). From a macroscopic-connectomic view, the rapid increase of functional connectivity in preterm neonates aged 31–41pmw was predominantly found in the primary sensorimotor and visual regions (Cao et al., 2017). Taken together, the regionally asynchronous pattern of cortical microstructural changes observed with DTI during fetal and infant brain development may interact with functional and physiological maturational processes in the cortex observed with other approaches. The heterogeneous maturation pattern of cortical FA and MD may be used to infer the complicated but precisely organized cellular and molecular processes during cortical maturation.
4.3 Other quantitative MRI approaches to assess the maturational changes across cortical regions during the preterm period and infancy
A few studies have aimed to characterize maturational changes in the developing cortex throughout infancy. Based on T2w signal, differences are observed across regions of the linguistic network from 1 to 4 months old, with delayed maturation of the ventral superior temporal sulcus when compared with Heschl gyrus, planum temporale and the inferior frontal region (Leroy et al., 2011). In 12- to19-months-old infants, the T1w intensity and contrast significantly increase with age in the left lateral temporal regions involved in lexico-semantic processing, suggesting that neurophysiological processes supporting cognitive behaviors may develop before microstructural maturation is complete within associative cortices (Travis et al., 2013). So far, the T1w/T2w ratio has only been computed from 8 years of age, showing that intra-cortical maturation is ongoing until the late 30s (Grydeland et al., 2013).
Regarding multi-parametric approaches (DTI and relaxometry), a recent study evaluating the cortical microstructural properties in preterm born infants scanned at term equivalent age, suggested differential maturation across regions (Friedrichs-Maeder et al., 2017). At term, MD and T1 are lower in the primary sensorimotor cortices than in secondary processing areas, which in turn are lower than in higher order tertiary areas (Friedrichs-Maeder et al., 2017). From 1 year of age, T1 decreases while MWF increases in most cortical regions; however the developmental pattern still varies across primary and associative cortices (Deoni et al., 2015). All these microstructural changes observed within the infant cortical regions with distinct MRI measurements probably reflect a complex interplay between several mechanisms, such as the development of dendritic arborization, the proliferation of glial cells, and/or the myelination of intra-cortical fibers.
4.4 Developmental changes in deep grey matter assessed by DTI and other quantitative MRI approaches during the preterm period
Marked microstructural changes are also observed in central grey nuclei throughout development. Pioneering DTI studies have shown that MD values strongly decrease in preterm brains with age between 30 and 40wg (Neil et al., 1998) and in infant brains from 1 day to 4 years (Mukherjee et al., 2001), while anisotropy in the central grey nuclei increases but to a lesser extent than in white matter tracts (Mukherjee et al., 2001). Much like the cortical regions, the maturational pattern differs across nuclei (e.g. the caudate head and lentiform nucleus vs the thalamus) (Mukherjee et al., 2001). More specifically in the thalamus, RD decreases with age in all substructures from 36 to 43pmw, while AD decreases in all except the right thalamo-postcentral and parietal and occipital substructures (Poh et al., 2015). In the basal ganglia (caudate, putamen, globus pallidus), AD and RD decrease while FA increases over the same period (Qiu et al., 2013). These microstructural changes observed with DTI suggest that membrane proliferation and fiber myelination are intense in the developing deep grey matter of the baby brain.
A few additional studies have used other quantitative approaches to characterize the maturation of the central grey nuclei. During the preterm period, T1 measures decrease gradually with age in the thalamus and lentiform nucleus, simultaneously with white matter regions (Schneider et al., 2016). Comparing T1, MTR and DTI measures seem particularly relevant to fully characterize the microstructural properties and the maturational patterns of deep grey matter regions such as the globus pallidus, putamen, thalamus and ventolateral thalamic nucleus (Nossin Manor et al., 2013). For instance, this latter structure shows a high concentration of myelin-associated macromolecules and a low water content (high MTR and low T1), low diffusivities (low MD and RD), and low directionality and coherence (low FA and AD). During the first post-natal months, T2 strongly decreases in the thalamus, caudate nucleus and putamen (Bültmann et al., 2017). Finally, combined observations from 27 to 58wg have shown that the intra-axonal fraction from NODDI and the fraction of water related to the myelin increase in the thalamus while T2 decreases, suggesting that these microstructural changes are not solely due to myelination (Melbourne et al., 2016). Taken together, these studies suggest that disentangling microstructural processes, and studying maturational patterns across white matter, cortical and deep grey regions, requires the consideration of multi-parametric MRI approaches.
5. Connectome of early developing brain
In addition to studies of gray and white matter microstructure, it is also important to understand the connectivity between maturing brain regions in order to elucidate the development first of sensory and motor processing and subsequently of higher-order cognitive and behavioral functions. Exciting new advances in defining the developmental changes of whole-brain connectivity have been achieved by applying graph theory to diffusion tractography of white matter, the “structural connectome”, and resting state fMRI of gray matter, the “functional connectome”. In network analysis of the structural connectome, the gray matter regions represent the “nodes” and the white matter connections between different nodes represent the “edges”. It is beyond the scope of this article to comprehensively review this rapidly evolving field, but the studies thus far demonstrate dramatic increases in the strength, efficiency and integration of the structural and functional brain networks during fetal (Jakab et al., 2014; Thomason et al., 2014, 2015; Schopf et al., 2012; Song et al., 2017; van den Heuvel and Thomason 2016) and neonatal (Ball et al., 2014; Brown et al., 2014; Cao et al., 2017; Fransson et al., 2007; Huang et al., 2015; Smyser et al., 2010; Tymofiyeva et al., 2012, 2013; van den Heuvel et al., 2015) development using traditional graph metrics, with both “small-world” and “rich club” organization already established at the earliest gestational ages investigated. Recent studies of the anatomic embedding of the structural connectome in adults show the highest density of connectome edges within the periventricular white matter (Owen et al., 2015, 2016), which may overlap with the “periventricular crossroads” of crossing commissural, association and projection fiber pathways that have been identified in studies of fetal development (Judas et al., 2005). A promising new advance is the discovery that the structural connectome can be decomposed into canonical spatial eigenmodes that characterize information transmission throughout the network and that are perturbed in neurodevelopmental disorders (Wang et al., 2017). These eigenmodes of the white matter network can explain functional activity and functional connectivity (Abdelnour et al., 2014; Robinson et al., 2016) and can thereby reproduce the known resting state fMRI networks (Atasoy et al., 2016). Brain network eigenmodes therefore provide a natural bridge between the structural and functional connectomes. Their development during fetal and neonatal life remains an area for future investigation.
6. Current issues and limitations
There are several issues arising from studying the structural development of white and gray matter of the fetal and infant brain using MRI techniques. First, motion-induced artifacts are probably the most prominent among all of the unsolved problems in baby MRI. In general, MRI scans require the subject to remain still in the scanner. In certain clinical settings, this issue can sometimes be circumvented with sedation. However, sedation is not preferred by either the clinicians or the parents, as it involves cumbersome setup, careful monitoring of the baby, and has potential complications related to sedation. In research of typically developing infants, the subjects are usually scanned during natural sleep. However, even small motion during the sleep can make it very challenging to acquire MR images without any motion artifacts. A few techniques (e.g. Gholipour et al., 2010; Kim et al., 2010; Dubois et al., 2014b; Cordero-Grande et al., 2018) have been developed to correct motion retrospectively based on the post-processing of the images. While the retrospective analysis plays an important role, research on prospective aspects of fetal and infant brain MRI during acquisition, including adopting novel MR sequences (i.e. self-navigated MR sequences, see Tisdall et al., 2012) and specific hardware devices (e.g. KinetiCor device for monitoring motion), could potentially further improve motion correction. Second, given the smaller size of the baby brain, higher resolution of MR images compared to that of adult brains is needed to delineate brain structures with similar anatomical detail to that of adult brains. This higher resolution imaging entails lower signal-to-noise ratio, making it more difficult to reduce the scan time and the likelihood of subject motion. Advances in data acquisition using simultaneous multiple-slice acquisition (Sotiropoulos et al., 2013) could dramatically improve the resolution to isotropic 1–1.5mm in DTI. Third, dMRI has its complications and intrinsic limitations. The advanced dMRI sequences with multiple shells and many diffusion-encoded directions make it more difficult to reduce the scan time and minimize motion. The artifacts from dMRI acquisition (e.g. those related to magnetic gradient imperfections, eddy currents, etc.) and complications associated with various non-DTI models are not elaborated here. The prominent limitations related to the most widely used DTI model for fetal and infant populations must be recognized when interpreting DTI-related results. As DTI-derived measurements can be only used to infer the microstructure and cannot directly quantify the axonal density, axonal packing or myelin level (Wheeler-Kingshott and Cercignani, 2009), caution needs to be taken when interpret age-dependent or pathology-related DTI parameter value changes. FA is also affected by crossing-fiber issues at imaging resolution of isotropic 2mm widely used in most fetal and infant DTI research. By retrospectively processing the diffusion weighted images, the approach towards correcting lower FA at the crossing-fiber regions (e.g. Mishra et al. 2015) could alleviate the bias of FA measurements at these regions. A well-known limitation associated with DTI tractography is the potential false-negative tractography results as DTI tractography is not able to resolve fiber topography in regions of crossing fibers (see e.g. Jbabdi and Johansen-Berg, 2011; Jones et al., 2013 for review). This limitation directly affects the interpretation of the emergence of certain white matter tracts using dMRI tractography (e.g. Huang et al., 2009, Miller et al., 2014; Takahashi et al., 2011).
7. Conclusion and future directions
We have consolidated the findings on early brain development from fetuses to infants using MR imaging techniques, focusing especially on dMRI. First, the maturational process of major white matter fiber bundles in most of the period from mid-fetal to 2-years-old is characterized by an increased FA and a decreased MD, whereas that of cortical gray matter is characterized by a decrease in both FA and MD. Second, maturation patterns of DTI-derived measurements reflect the cellular and molecular processes. For example, the increase of FA of white matter is related to myelination and axonal packing, whereas the decrease of FA of cortical gray matter is related to synaptogenesis and dendritic arborization that disrupt the radial organization of the developing cortex. Alternative quantitative MRI approaches could provide complementary information to the dMRI findings and open the door for future multi-modality studies. Third, the early development of white and gray matter is spatiotemporally heterogeneous Specifically, myelination begins earlier in projection and commissural fiber tracts than that in the association fiber tracts, and the multi-modal associative cortices (e.g. prefrontal regions) are shown to mature later than uni-modal associative cortices, which in turn mature later than primary sensory and motor cortices, consistent with past histologic studies.
Future research could include development of novel MR imaging techniques in both data acquisition and post-processing for motion correction in baby MRI. More sophisticated diffusion models (e.g. DKI, NODDI) are needed to overcome the limitations of the diffusion tensor model. After comprehensive validation of these models, they may provide information about microstructural changes in white matter and cortical regions in addition to those discovered through DTI. Further, complementary to the current literature which mostly focuses on long-range white matter fiber development, more investigations are expected to improve our understanding of how short-range white matter fibers mature in this early brain developmental period (see e.g. Ouyang et al., 2016; Phillips et al., 2013; and Ouyang et al., 2017b for review). Methodologies for performing reliable tractography of short-range white matter fibers or white matter bundles across different maturational stages are needed (Dubois et al., 2016a, 2016b). Another interesting direction for future research would be to investigate the relationships between brain structural and cognitive/behavioral development, which would particularly benefit from longitudinal studies that allow tracking of developmental trajectories of white matter and/or gray matter. Establishing normative developmental charts of structure, function and behavior is not only essential for understanding the complicated and rapid early development, but also critical for revealing alterations caused by neuropathology for early diagnosis and intervention. Finally, data sharing could significantly facilitate the understanding of the complicated structural and functional dynamics during the fetal and infant stages. The recent release of multi-modality neonate MRI from ERC-sponsored developing human connectome project (dHCP) (www.developingconnectome.org) and NIMH-sponsored fetal brain development (www.brainmrimap.org) could potentially expand our knowledge of baby brain development.
Highlights.
Dramatic microstructural changes take place in developing fetal and infant brain
Diffusion and other MRI modalities effectively quantify these developmental changes
Emergence of different white matter tracts were delineated with dMRI tractography
Cerebral cortical microstructural changes were quantified with dMRI metrics
4D spatiotemporal patterns of brain structural development were delineated
Acknowledgments
We thank Dr. Jeffrey Neil for the comments and suggestions. We thank the lab member, Michelle Slinger, for contribution to writing.
Footnotes
8. Funding and Disclosure: National Institutes of Health (MH09235, MH092535-S1 and HD086984). Grants from the Fondation de France, Fyssen Foundation, funding from the European Union’s Horizon 2020 Framework Program for Research and Innovation under Grant Agreement No. 604102 (Human Brain Project HBP’s ramp-up phase).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelnour F, Voss HU, Raj A. Network diffusion accurately models the relationship between structural and functional brain connectivity networks. Neuroimage. 2014;90:335–347. doi: 10.1016/j.neuroimage.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59(6):1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy S, Donnelly I, Pearson J. Human brain networks function in connectome-specific harmonic waves. Nat Commun. 2017;7:10340. doi: 10.1038/ncomms10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci USA. 2013;110(23):9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, Merchant N, Robinson EC, Ogundipe E, Rueckert D, Edwards AD, Counsell SJ. Rich-club organization of the newborn human brain. Proc Natl Acad Sci USA. 2014;111(20):7456–7461. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 2000;21(6):1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1):173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Lyon G, Evrard P. Formation, maturation, and disorders of white matter. AJNR Am J Neuroradiol. 1992;13(2):447–461. [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. The human brain during the third trimester. Boca Raton, FL: CRC; 2004. [Google Scholar]
- Bayer SA, Altman J. The human brain during the second trimester. Boca Raton, FL: CRC; 2005. [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Fenrich FR, Allen PS. Multicomponent water proton transverse relaxation and T 2-discriminated water diffusion in myelinated and nonmyelinated nerve. Magn Reson Imag. 1998;16(10):1201–1210. doi: 10.1016/s0730-725x(98)00151-9. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Jbabdi S. MR Diffusion Tractography. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI: from Quantitative Measurement to in vivo Neuroanatomy. Elsevier Academic Press; New York: 2009. pp. 333–351. [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27(4):862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I An autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46(3):283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Miller SP, Booth BG, Andrews S, Chau V, Poskitt KJ, Hamarneh G. Structural network analysis of brain development in young preterm neonates. Neuroimage. 2014;101:667–680. doi: 10.1016/j.neuroimage.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Bültmann E, Spineli LM, Hartmann H, Lanfermann H. Measuring in vivo cerebral maturation using age-related T2 relaxation times at 3 T. Brain Dev. 2017 doi: 10.1016/j.braindev.2017.07.011. doi.org/10.1016/j.braindev.2017.07.011 in press. [DOI] [PubMed]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, Chalak L, Bi Y, Rollins N, Dong Q, Huang H. Early development of functional network segregation revealed by connectomic analysis of the preterm human brain. Cereb Cortex. 2017;27(3):1949–1963. doi: 10.1093/cercor/bhw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Owen JP, Pojman NJ, Thieu T, Bukshpun P, Wakahiro ML, Berman JI, Roberts TP, Nagarajan SS, Sherr EH, Mukherjee P. White Matter Changes of Neurite Density and Fiber Orientation Dispersion during Human Brain Maturation. PLoS One. 2015;10(6):e0123656. doi: 10.1371/journal.pone.0123656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;4:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Cordero-Grande L, Hughes EJ, Hutter J, Price AN, Hajnal JV. Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging. Magn Reson Med. 2018;79(3):1365–1376. doi: 10.1002/mrm.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell SJ, Maalouf EF, Fletcher AM, Duggan P, Battin M, Lewis HJ, Herlihy AH, Edwards AD, Bydder GM, Rutherford MA. MR imaging assessment of myelination in the very preterm brain. AJNR Am J Neuroradiol. 2002;23(5):872–881. [PMC free article] [PubMed] [Google Scholar]
- Dean DC, O’muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, Han M, Deoni SC. Modeling healthy male white matter and myelin development: 3 through 60 months of age. Neuroimage. 2014;84:742–752. doi: 10.1016/j.neuroimage.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, O’muircheartaigh J, Dirks H, Travers BG, Adluru N, Alexander AL, Deoni SC. Mapping an index of the myelin g-ratio in infants using magnetic resonance imaging. Neuroimage. 2016;132:225–237. doi: 10.1016/j.neuroimage.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deIpolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage. 2005;27(3):579–586. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, O’muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63:1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, Remer J, Dirks H, O’Muircheartaigh J. Cortical maturation and myelination in healthy toddlers and young children. Neuroimage. 2015;115:147–161. doi: 10.1016/j.neuroimage.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Matthews L, Kolind SH. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn Reson Med. 2013;70(1):147–154. doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48(10):757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, Larkman DJ. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Adibpour P, Poupon C, Hertz-Pannier L, Dehaene-Lambertz G. MRI and M/EEG studies of the white matter development in human fetuses and infants: Review and Opinion. Brain Plasticity. 2016b;2(1):49–69. doi: 10.3233/BPL-160031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014a;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Perrin M, Mangin JF, Cointepas Y, Duchesnay E, Le Bihan D, Hertz-Pannier L. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Dubois J, Kulikova S, Hertz-Pannier L, Mangin JF, Dehaene-Lambertz G, Poupon C. Correction strategy for diffusion-weighted images corrupted with motion: application to the DTI evaluation of infants’ white matter. Magn Reson imag. 2014b;32(8):981–992. doi: 10.1016/j.mri.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Dubois J, Poupon C, Thirion B, Simonnet H, Kulikova S, Leroy F, Hertz-Pannier L, Dehaene-Lambertz G. Exploring the early organization and maturation of linguistic pathways in the human infant brain. Cereb Cortex. 2016a;24(5):2283–2298. doi: 10.1093/cercor/bhv082. [DOI] [PubMed] [Google Scholar]
- Eaton-Rosen Z, Scherrer B, Melbourne A, Ourselin S, Neil JJ, Warfield SK. Investigating the maturation of microstructure and radial orientation in the preterm human cortex with diffusion MRI. NeuroImage. 2017;162:65–72. doi: 10.1016/j.neuroimage.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Engelbrecht V, Rassek M, Preiss S, Wald C, Mödder U. Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. AJNR Am J Neruoradiol. 1998;19(10):1923–1929. [PMC free article] [PubMed] [Google Scholar]
- Engle WA American academy of pediatrics committee on fetus newborn. Age terminology during the perinatal period. Pediatrics. 2004;114:1362–1364. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. NeuroImage. 2011;58:177–188. doi: 10.1016/j.neuroimage.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs-Maeder CL, Griffa A, Schneider J, Hüppi PS, Truttmann A, Hagmann P. Exploring the role of white matter connectivity in cortex maturation. PloS One. 2017;12(5):e0177466. doi: 10.1371/journal.pone.0177466. doi.org/10.1371/journal.pone.0177466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith J, Jewells V, Gilmore J. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol. 2009;30:290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, Gerig G, Gilmore JH. Quantitative tract-based white matter development from birth to age 2 years. Neuroimage. 2012;61:542–557. doi: 10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A, Estroff JA, Warfield SK. Robust super-resolution volume reconstruction from slice acquisitions: application to fetal brain MRI. IEEE Trans Med Imaging. 2010;29(10):1739–1758. doi: 10.1109/TMI.2010.2051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1-and T2-weighted MRI. J Neurosci. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci. 2013;33(47):18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J, Moore J, Wang Z, Traipe E, Bilaniuk L. A method for fast multislice T1 measurement: feasibility studies on phantoms, young children, and children with Canavan’s disease. J Magn Reson Imag. 2000;11(4):360–367. doi: 10.1002/(sici)1522-2586(200004)11:4<360::aid-jmri3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Huang H. Structure of the fetal brain: what we are learning from DTI. Neuroscientist. 2010;16:634–649. doi: 10.1177/1073858409356711. [DOI] [PubMed] [Google Scholar]
- Huang H, Jeon T, Sedmak G, Pletikos M, Vasung L, Xu X, Yarowsky P, Richards LJ, Kostović I, Šestan N, Mori S. Coupling diffusion imaging with histological and gene expression analysis to examine the dynamics of cortical areas across the fetal period of human brain development. Cereb Cortex. 2013;23(11):2620–2631. doi: 10.1093/cercor/bhs241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Vasung L. Gaining insight of fetal brain development with diffusion MRI and histology. Int J Dev Neurosci. 2014;32:11–22. doi: 10.1016/j.ijdevneu.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, Rollins N, Gong G, Cheng H, Peng Y, Dong Q, He Y. Development of human brain structural networks through infancy and childhood. Cereb Cortex. 2015;25(5):1389–1404. doi: 10.1093/cercor/bht335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29:4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Yamamoto A, Hossain MA, Younes L, Mori S. Quantitative cortical mapping of fractional anisotropy in developing rat brains. J Neurosci. 2008;28(6):1427–1433. doi: 10.1523/JNEUROSCI.3194-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PC, Mori S. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33(1):27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998a;43(2):224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998b;44(4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6(12):955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schöpf V, Langs G. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front Hum Neurosci. 2014;8:852. doi: 10.3389/fnhum.2014.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Johansen-Berg H. Tractography: where do we go from here? Brain Connect. 2011;1(3):169–183. doi: 10.1089/brain.2011.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelescu IO, Veraart J, Adisetiyo V, Milla SS, Novikov DS, Fieremans E. One diffusion acquisition and different white matter models: how does microstructure change in human early development based on WMTI and NODDI? Neuroimage. 2015;107:242–256. doi: 10.1016/j.neuroimage.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jeon T, Mishra V, Ouyang M, Chen M, Huang H. Synchronous changes of cortical thickness and corresponding white matter microstructure during brain development accessed by diffusion MRI tractography from parcellated cortex. Front Neuroanat. 2015;9:158. doi: 10.3389/fnana.2015.00158. doi.org/10.3389/fnana.2015.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon T, Sotiras A, Ouyang M, Chen M, Chalak L, Davatzikos C, Huang H. Spatiotemporal dynamics and patterns of cortical mean kurtosis and fractional anisotropy in the preterm brains. Proceedings of the 24th Annual Meeting of ISMRM; Singapore. 2016; 2016. Abstract 1997. [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42(1):37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Judas M, Rados M, Jovanov-Milosevic N, Hrabac P, Stern-Padovan R, Kostovic I. Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol. 2005;26:2671–2784. [PMC free article] [PubMed] [Google Scholar]
- Kail R. Processing time declines exponentially during childhood and adolescence. Dev Psychol. 1991;27:259. [Google Scholar]
- Kasprian G, Brugger PC, Schöpf V, Mitter C, Weber M, Hainfellner JA, Prayer D. Assessing prenatal white matter connectivity in commissural agenesis. Brain. 2013;136:168–179. doi: 10.1093/brain/aws332. [DOI] [PubMed] [Google Scholar]
- Kasprian G, Brugger PC, Weber M, Krssák M, Krampl E, Herold C, Prayer D. In utero tractography of fetal white matter development. Neuroimage. 2008;43:213–224. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Kersbergen KJ, Leemans A, Groenendaal F, van der Aa NE, Viergever MA, de Vries LS, Benders MJ. Microstructural brain development between 30 and 40 weeks corrected age in a longitudinal cohort of extremely preterm infants. Neuroimage. 2014;103:214–224. doi: 10.1016/j.neuroimage.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Kim K, Habas PA, Rousseau F, Glenn OA, Barkovich AJ, Studholme C. Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation. IEEE Trans Med Imaging. 2010;29(1):146–158. doi: 10.1109/TMI.2009.2030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski J, Takahashi E, Stevens AA, Benner T, Fischl B, Zöllei L, Grant PE. Radial and tangential neuronal migration pathways in the human fetal brain: anatomically distinct patterns of diffusion MRI coherence. Neuroimage. 2013;79:412–422. doi: 10.1016/j.neuroimage.2013.04.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milosević N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Radoš M, Hrabač P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12(5):536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostović I, Vasung L. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Pernatol. 2009;33:220–233. doi: 10.1053/j.semperi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cereb Cortex. 2009;19(12):2916–2929. doi: 10.1093/cercor/bhp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 2007;27(46):12506–12515. doi: 10.1523/JNEUROSCI.3063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczyk W, Macdonald PM, Stanisz GJ, Henkelman R. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology. 1994;192(2):521–529. doi: 10.1148/radiology.192.2.8029426. [DOI] [PubMed] [Google Scholar]
- Kulikova S, Hertz-Pannier L, Dehaene-Lambertz G, Buzmakov A, Poupon C, Dubois J. Multi-parametric evaluation of the white matter maturation. Brain Struct Func. 2015;220(6):3657–3672. doi: 10.1007/s00429-014-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova S, Hertz-Pannier L, Dehaene-Lambertz G, Poupon C, Dubois J. A new strategy for fast MRI-based quantification of the myelin water fraction: application to brain imaging in infants. PloS one. 2016;11(10):e0163143. doi: 10.1371/journal.pone.0163143. doi.org/10.1371/journal.pone.0163143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz N, Zhang H, Vasung L, O’brien KR, Assaf Y, Lazeyras F, Alexander DC, Hüppi PS. Assessing white matter microstructure of the newborn with multi-shell diffusion MRI and biophysical compartment models. Neuroimage. 2014;96:288–299. doi: 10.1016/j.neuroimage.2014.03.057. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Šestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139(9):1535–1546. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Andrews T, Hardies LJ, Dodd S, Fox PT. Three-pool model of white matter. J Magn Reson Imag. 2003;17(1):1–10. doi: 10.1002/jmri.10230. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E. Imagerie de diffusion in-vivo par resonance magnetique nucleaire. Cr Acad Sci (Paris) 1985;301:1109–1112. [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebenberg J, Poupon C, Thirion B, Leroy F, Mangin JF, Dehaene-Lambertz G, Dubois J. Clustering the infant brain tissues based on microstructural properties and maturation assessment using multi-parametric MRI. IEEE ISBI. 2015 Apr;:148–151. [Google Scholar]
- Leppert IR, Almli CR, McKinstry RC, Mulkern RV, Pierpaoli C, Rivkin MJ, Pike GB. T2 relaxometry of normal pediatric brain development. J Magn Reson Imag. 2009;29(2):258–267. doi: 10.1002/jmri.21646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Glasel H, Dubois J, Hertz-Pannier L, Thirion B, Mangin JF, Dehaene-Lambertz G. Early maturation of the linguistic dorsal pathway in human infants. J Neurosci. 2011;31(4):1500–1506. doi: 10.1523/JNEUROSCI.4141-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- Maas LC, Mukherjee P, Carballido-Gamio J, Veeraraghavan S, Miller SP, Partridge SC, Henry RG, Barkovich AJ, Vigneron DB. Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage. 2004;22(3):1134–1140. doi: 10.1016/j.neuroimage.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Mädler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination?: Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Res Imag. 2008;26:874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Makris N, Worth AJ, Papadimitriou GM, Stakes JW, Caviness VS, Kennedy DN, Pandya DN, Kaplan E, Sorensen AG, Wu O, Reese TG. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;42(6):951–962. doi: 10.1002/ana.410420617. [DOI] [PubMed] [Google Scholar]
- Marín O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med. 2016;22(11):1229–1238. doi: 10.1038/nm.4225. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Comp Neurol. 1992;321:223–240. doi: 10.1002/cne.903210205. [DOI] [PubMed] [Google Scholar]
- Matsumae M, Kurita D, Atsumi H, Haida M, Sato O, Tsugane R. Sequential changes in MR water proton relaxation time detect the process of rat brain myelination during maturation. Mech Ageing Dev. 2001;122(12):1281–1291. doi: 10.1016/s0047-6374(01)00265-2. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex. 2001;11(4):335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. JAMA Psychiatry. 2000;57(7):637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGowan JC. The physical basis of magnetization transfer imaging. Neurology. 1999;53:S3–S7. [PubMed] [Google Scholar]
- McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, Almli CR, Shiran SI, Conturo TE, Neil JJ. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12(12):1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- Melbourne A, Eaton-Rosen Z, Orasanu E, Price D, Bainbridge A, Cardoso MJ, Kendall GS, Robertson NJ, Marlow N, Ourselin S. Longitudinal development in the preterm thalamus and posterior white matter: MRI correlations between diffusion weighted imaging and T2 relaxometry. Hum Brain Mapp. 2016;37(7):2479–2492. doi: 10.1002/hbm.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Rusinko MS, Allen PS. Multiexponential proton relaxation in model cellular systems. Magn Reson Med. 1991;20:196–213. doi: 10.1002/mrm.1910200204. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Šestan N, Wildman DE, Lipovich L. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Aiona K, Arnold JM. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Cheng H, Gong G, He Y, Dong Q, Huang H. Differences of inter-tract correlations between neonates and children around puberty: a study based on microstructural measurements with DTI. Front Hum Neurosci. 2013;7:721. doi: 10.3389/fnhum.2013.00721. doi.org/10.3389/fnhum.2013.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Guo X, Delgado MR, Huang H. Toward tract-specific fractional anisotropy (TSFA) at crossing-fiber regions with clinical diffusion MRI. Magn Reson Med. 2015;74(6):1768–1779. doi: 10.1002/mrm.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Itoh R, Zhang J, Kaufmann WE, van Zijl P, Solaiyappan M, Yarowsky P. Diffusion tensor imaging of the developing mouse brain. Magn Reson Med. 2001;46(1):18–23. doi: 10.1002/mrm.1155. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR Imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR, Akbudak E, Aronovitz JA, Miller JP, Lee BC, Conturo TE. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209(1):57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R, Card D, Morris D, Noormohamed S, Shroff MM, Whyte HE, Taylor MJ, Sled JG. Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T 1 imaging. Neuroimage. 2013;64:505–516. doi: 10.1016/j.neuroimage.2012.08.086. [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R, Card D, Raybaud C, Taylor MJ, Sled JG. Cerebral maturation in the early preterm period-A magnetization transfer and diffusion tensor imaging study using voxel-based analysis. Neuroimage. 2015;112:30–42. doi: 10.1016/j.neuroimage.2015.02.051. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Jeon T, Mishra V, Du H, Wang Y, Peng Y, Huang H. Global and regional cortical connectivity maturation index (CCMI) of developmental human brain with quantification of short-range association tracts. Proc SPIE9788, Meidcal Imaging 2016: Biomedical Applications in Molecular, Structural, and Functional Imaging. 2016:97881B. doi: 10.1117/12.2218029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang A, Jeon T, Sunkin SM, Pletikos M, Sedmak G, Sestan N, Lein ES, Huang H. Spatial mapping of structural and connectional imaging data for the developing human brain with diffusion tensor imaging. Methods. 2015;73:27–37. doi: 10.1016/j.ymeth.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Liu P, Jeon T, Chalak L, Heyne R, Rollins NK, Licht DJ, Detre JA, Roberts TP, Lu H, Huang H. Heterogeneous increases of regional cerebral blood flow during preterm brain development: Preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. NeuroImage. 2017a;147:233–242. doi: 10.1016/j.neuroimage.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Kang H, Detre JA, Roberts TP, Huang H. Short-range connections in the developmental connectome during typical and atypical brain maturation. Neurosci Biobehav Rev. 2017b;83:109–122. doi: 10.1016/j.neubiorev.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Chang YS, Mukherjee P. Edge density imaging: mapping the anatomic embedding of the structural connectome within the white matter of the human brain. Neuroimage. 2015;109:402–417. doi: 10.1016/j.neuroimage.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Wang MB, Mukherjee P. Periventricular white matter Is a nexus for network connectivity in the human brain. Brain Connect. 2016;6(7):548–557. doi: 10.1089/brain.2016.0431. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med. 1999;42(3):526–540. [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paydar A, Fieremans E, Nwankwo J, Lazar M, Sheth H, Adisetiyo V, Helpern J, Jensen J, Milla S. Diffusional kurtosis imaging of the developing brain. AJNR Am J Neuroradiol. 2014;35:808–814. doi: 10.3174/ajnr.A3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phillips OR, Clark KA, Luders E, Azhir R, Joshi SH, Woods RP, Mazziotta JC, Toga AW, Narr KL. Superficial white matter: effects of age, sex, and hemisphere. Brain Connect. 2013;3(2):146–159. doi: 10.1089/brain.2012.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo SE, Jang Y. Myelin development in infant brain. Neurochem Res. 1984;9(11):1615–1626. doi: 10.1007/BF00964595. [DOI] [PubMed] [Google Scholar]
- Poh JS, Li Y, Ratnarajah N, Fortier MV, Chong YS, Kwek K, Saw SM, Gluckman PD, Meaney MJ, Qiu A. Developmental synchrony of thalamocortical circuits in the neonatal brain. Neuroimage. 2015;116:168–176. doi: 10.1016/j.neuroimage.2015.03.039. [DOI] [PubMed] [Google Scholar]
- Prayer D, Prayer L. Diffusion-weighted magnetic resonance imaging of cerebral white matter development. Eur J Radiol. 2003;45(3):235–243. doi: 10.1016/s0720-048x(02)00312-1. [DOI] [PubMed] [Google Scholar]
- Qiu A, Fortier MV, Bai J, Zhang X, Chong YS, Kwek K, Saw SM, Godfrey KM, Gluckman PD, Meaney MJ. Morphology and microstructure of subcortical structures at birth: a large-scale Asian neonatal neuroimaging study. Neuroimage. 2013;65:315–323. doi: 10.1016/j.neuroimage.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Qiu A, Mori S, Miller MI. Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol. 2015;66:853–876. doi: 10.1146/annurev-psych-010814-015340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci USA. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PA, Zhao X, Aquino KM, Griffiths JD, Sarkar S, Mehta-Pandejee G. Eigenmodes of brain activity: Neural field theory predictions and comparison with experiment. Neuroimage. 2016;142:79–98. doi: 10.1016/j.neuroimage.2016.04.050. [DOI] [PubMed] [Google Scholar]
- Sadeghi N, Prastawa M, Fletcher PT, Wolff J, Gilmore JH, Gerig G. Regional characterization of longitudinal DT-MRI to study white matter maturation of the early developing brain. Neuroimage. 2013;68:236–247. doi: 10.1016/j.neuroimage.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Kober T, Graz MB, Meuli R, Hüppi PS, Hagmann P, Truttmann AC. Evolution of T1 relaxation, ADc, and fractional anisotropy during early brain maturation: a serial imaging study on preterm infants. AJNR Am J Neuroradiol. 2016;37(1):155–162. doi: 10.3174/ajnr.A4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöpf V, Kasprian G, Brugger PC, Prayer D. Watching the fetal brain at ‘rest’. Int J Dev Neurosci. 2012;30(1):11–17. doi: 10.1016/j.ijdevneu.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous System. Neuron. 2016;89:248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Sizonenko SV, Camm EJ, Garbow JR, Maier SE, Inder TE, Williams CE, Neil JJ, Huppi PS. Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI. Cereb Cortex. 2007;17(11):2609–2617. doi: 10.1093/cercor/bhl168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser TA, Smyser CD, Rogers CE, Gillespie SK, Inder TE, Neil JJ. Cortical gray and adjacent white matter demonstrate synchronous maturation in very preterm infants. Cereb Cortex. 2016;26(8):3370–3378. doi: 10.1093/cercor/bhv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Mishra V, Ouyang M, Peng Q, Slinger M, Liu S, Huang H. Human fetal brain connectome: structural network development from middle fetal stage to birth. Front Neurosci. 2017;11:561. doi: 10.3389/fnins.2017.00561. doi.org/10.3389/fnins.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 2013;80:125–143. doi: 10.1016/j.neuroimage.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spader HS, Ellermeier A, O’Muircheartaigh J, Dean DC, III, Dirks H, Boxerman JL, Cosgrove GR, Deoni SC. Advances in myelin imaging with potential clinical application to pediatric imaging. Neurosurg Focus. 2013;34(4):E9. doi: 10.3171/2013.1.FOCUS12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stikov N, Campbell JS, Stroh T, Lavelée M, Frey S, Novek J, Nuara S, Ho MK, Bedell BJ, Dougherty RF, Leppert IR. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage. 2015;118:397–405. doi: 10.1016/j.neuroimage.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Dai G, Rosen GD, Wang R, Ohki K, Folkerth RD, Galaburda AM, Wedeen VJ, Ellen Grant P. Developing neocortex organization and connectivity in cats revealed by direct correlation of diffusion tractography and histology. Cereb Cortex. 2010;21(1):200–211. doi: 10.1093/cercor/bhq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Folkerth RD, Galaburda AM, Grant PE. Emerging cerebral connectivity in the human fetal brain: an MR tractography study. Cereb Cortex. 2011;22:455–464. doi: 10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83(5):1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiessen JD, Zhang Y, Zhang H, Wang L, Buist R, Del Bigio MR, Kong J, Li XM, Martin M. Quantitative MRI and ultrastructural examination of the cuprizone mouse model of demyelination. NMR in Biomed. 2013;26(11):1562–1581. doi: 10.1002/nbm.2992. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Brown JA, Dassanayake MT, Shastri R, Marusak HA, Hernandez-Andrade E, Yeo L, Mody S, Berman S, Hassan SS, Romero R. Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One. 2014;9(5):e94423. doi: 10.1371/journal.pone.0094423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Grove LE, Lozon TA, Vila AM, Ye Y, Nye MJ, Manning JH, Pappas A, Hernandez-Andrade E, Yeo L, Mody S. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev Cogn Neurosci. 2015;11:96–104. doi: 10.1016/j.dcn.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JS, Ordidge RJ, Penrice J, Cady EB, Amess PN, Punwani S, Clemence M, Wyatt JS. Anisotropic water diffusion in white and gray matter of the neonatal piglet brain before and after transient hypoxia-ischaemia. Magn Reson Imag. 1997;15(4):433–440. doi: 10.1016/s0730-725x(96)00378-5. [DOI] [PubMed] [Google Scholar]
- Tymofiyeva O, Hess CP, Ziv E, Tian N, Bonifacio SL, McQuillen PS, Ferriero DM, Barkovich AJ, Xu D. Towards the “baby connectome”: mapping the structural connectivity of the newborn brain. PLoS One. 2012;7(2):e31029. doi: 10.1371/journal.pone.0031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymofiyeva O, Hess CP, Ziv E, Lee PN, Glass HC, Ferriero DM, Barkovich AJ, Xu D. A DTI-based template-free cortical connectome study of brain maturation. PLoS One. 2013;8(5):e63310. doi: 10.1371/journal.pone.0063310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68(2):389–399. doi: 10.1002/mrm.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int J Imag Sys Tech. 2012;22(1):53–66. [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65(6):1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Curran MM, Torres C, Leonard MK, Brown TT, Dale AM, Elman JL, Halgren E. Age-related changes in tissue signal properties within cortical areas important for word understanding in 12- to 19-month-old infants. Cereb Cortex. 2013;24(7):1948–1955. doi: 10.1093/cercor/bht052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52:1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- van Buchem MA, Steens SC, Vrooman HA, Zwinderman AH, McGowan JC, Rassek M, Engelbrecht V. Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: a preliminary study. AJNR Am J Neuroradiol. 2001;22(4):762–766. [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology. 1990;31(6):459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, de Vries LS, Benders MJ. The neonatal connectome during preterm brain development. Cereb Cortex. 2015;25(9):3000–3013. doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MI, Thomason ME. Functional connectivity of the human brain in utero. Trends Cogn Sci. 2016;20(12):931–939. doi: 10.1016/j.tics.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P. Microstructural correlations of white matter tracts in the human brain. Neuroimage. 2010;51(2):531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wang MB, Owen JP, Mukherjee P, Raj A. Brain network eigenmodes provide a robust and compact representation of the structural connectome in health and disease. PLoS Comput Biol. 2017;13(6):e1005550. doi: 10.1371/journal.pcbi.1005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, Pandya DN, Hagray matterann P, D’Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About ‘axial’ and ‘radial’ diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Whittall KP, Mackay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37(1):34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- Xu G, Takahashi E, Folkerth RD, Haynes RL, Volpe JJ, Grant PE, Kinney HC. Radial coherence of diffusion tractography in the cerebral white matter of the human fetus: neuroanatomic insights. Cerebral Cortex. 2012;24:579–592. doi: 10.1093/cercor/bhs330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydis V, Astrakas L, Zikou A, Pantou K, Andronikou S, Argyropoulou MI. Magnetization transfer ratio in the brain of preterm subjects: age-related changes during the first 2 years of life. Eur Radiol. 2006;16(1):215–220. doi: 10.1007/s00330-005-2796-8. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell Science; 1967. pp. 3–70. [Google Scholar]
- Yu Q, Kang H, Peng Q, Ouyang M, Slinger M, Peng Y, Fang F, Huang H. Differentiated maturation of white matter tracts in early developing brain aged 0–3 years. Proceedings of the 25th Annual Meeting of ISMRM; Hawaii, USA. 2017; 2017. Abstract 1267. [Google Scholar]
- Yu Q, Ouyang A, Chalak L, Jeon T, Chia J, Mishra V, Sivarajan M, Jackson G, Rollins N, Liu S, Huang H. Structural development of human fetal and preterm brain cortical plate based on population-averaged templates. Cereb Cortex. 2016;26(11):4381–4391. doi: 10.1093/cercor/bhv201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Peng Y, Mishra V, Ouyang A, Li H, Zhang H, Chen M, Liu S, Huang H. Microstructure, length, and connection of limbic tracts in normal human brain development. Front Aging Neurosci. 2014;6:228. doi: 10.3389/fnagi.2014.00228. doi.org/10.3389/fnagi.2014.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Evans A, Hermoye L, Lee SK, Wakana S, Zhang W, Donohue P, Miller MI, Huang H, Wang X, van Zijl PC, Mori S. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage. 2007;38(2):239–247. doi: 10.1016/j.neuroimage.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]