Abstract
Proteins are often credited as the macromolecule responsible for performing critical cellular functions, however lipids have recently garnered more attention as our understanding of their role in cell function and human health becomes more apparent. Although cellular membranes are the lipid environment in which many proteins function, it is now apparent that protein and lipid assemblies can be organized to form distinct micro- or nanodomains that facilitate signaling events. Indeed, it is now appreciated that cellular function is partly regulated by the specific spatiotemporal lipid composition of the membrane, down to the nanosecond and nanometer scale. Furthermore, membrane composition is altered during human disease processes such as cancer and obesity. For example, an increased rate of lipid/cholesterol synthesis in cancerous tissues has long been recognized as an important aspect of the rewired metabolism of transformed cells. However, the contribution of lipids/cholesterol to cellular function in disease models is not yet fully understood. Furthermore, an important consideration in regard to human health is that diet is a major modulator of cell membrane composition. This can occur directly through incorporation of membrane substrates, such as fatty acids, e.g., n-3 polyunsaturated fatty acids (n-3 PUFA) and cholesterol. In this review, we describe scenarios in which changes in membrane composition impact human health. Particular focus is placed on the importance of intrinsic lipid/cholesterol biosynthesis and metabolism and extrinsic dietary modification in cancer and its effect on plasma membrane properties.
Introduction
Biological membranes function as a selective barrier within cells and organelles. Although our understanding of the role of genetic elements and proteins in the context of cellular biology has rapidly progressed, knowledge regarding the impact of the plasma membrane on cellular signaling has been modest. Recent advances in techniques used to monitor membrane dynamics, such as super-resolution fluorescence microscopy (Sezgin, 2017), has begun to shed light on how membrane lipid composition modulates cellular function.
In this review, we present current literature describing the composition and function of cellular membranes, beginning with the plasma membrane and concluding with the mitochondrial membrane. We also summarize the latest research implicating dietary bioactives, e.g., n-3 PUFA, capable of modulating the molecular composition and function of cellular membranes. From a cell-extrinsic system perspective, we propose that the reshaping of plasma membrane composition and structure in part underlies the chemoprotective effects of n-3 PUFA. In addition, recent cell-intrinsic mechanisms suggest a fundamental link between lipid-cholesterol biosynthesis and metabolism, plasma membrane properties and cancer biology. Collectively, these tunable processes are crucial for modulating signaling pathways associated with tumorigenesis, which constitute the main cause of cancer mortality.
Cellular lipid classes
Lipid metabolism involves a very large number of metabolic reactions spanning different subcellular compartments in eukaryotic cells, resulting in the formation of a diverse group of chemical compounds (Nielsen, 2009). Lipids can be divided into the following classes: (1) fatty acids (FAs) that mainly serve as intermediates in lipid biosynthesis and their oxygenated metabolites, e.g., eicosanoids; (2) free sterols, e.g., cholesterol, that serve as structural components in membranes; (3) sterol esters that are formed from FAs and sterols and serve as lipid storage compounds, mainly as lipid bodies; (4) triacylglycerols (TAG) that are formed from glycerol and FAs that serve as lipid storage, mainly in lipid bodies; (5) phospholipids/phosphoglycerides (PLs) that are formed from FAs, glycerol and an alcohol moiety, e.g., inositol, serine, choline or ethanolamine, that serve as structural components of membranes; and (6) sphingolipids that contain a sphingosine backbone and a very long chain FA. These phospholipids serve as structural components at the cell surface as well as key signaling roles, e.g., regulation of endocytosis, ubiquitin dependent proteolysis and cell cycle control (Nielsen, 2009; Rohrig and Schulze, 2016). Despite the large chemical variety of lipids, they all have the same key carbon precursor, namely acetyl-CoA, and all initial steps of lipid/cholesterol biosynthesis occur in the cytosol (Rohrig and Schulze, 2016). As illustrated in Figure 1, lipid biosynthesis basically involves two branches from acetyl-CoA, one leading to sterols and the other leading to FAs that serve as building blocks for the biosynthesis of TAG, phospholipids, sterol esters and sphingolipids.
Figure 1. Synthesis of cellular lipids.
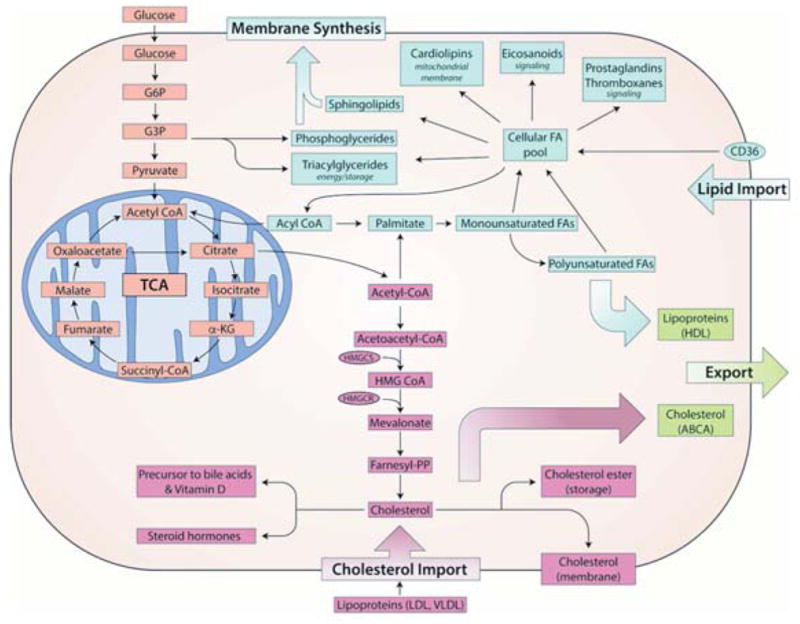
Fatty acid building blocks are derived from exogenous and endogenous (de novo) sources. Glucose uptake can result in the generation of metabolic intermediates which are utilized to generate membrane phospholipids, cholesterol and other lipid moieties. Exogenous lipids can be imported into the cell via CD36, lipoprotein receptors or effluxed via ABCA-related membrane proteins.
Composition of cellular membranes
Eukaryotic cells contain different types of membranes, including the plasma, endosomal, nuclear and mitochondrial membranes. The specific lipid composition of these membranes influences function, and since these membranes carry out vastly different functions, inherently their composition is extremely diverse (van Meer et al., 2008). Their heterogeneous composition is the result of de novo lipid synthesis (Kennedy pathway) and exogenous substrate availability (Lands’ cycle). Unlike proteins that are directly encoded in the genome, lipid composition is the result of an indirect influence of biosynthetic enzymes and exogenous substrate availability.
Structural and cellular function of membrane cholesterol
Cholesterol is an essential component of higher eukaryotic membranes and plays an important role in cell membrane organization, dynamics and function (Bloch, 1983). It is the end product of a sterol biosynthetic pathway involving more than 20 enzymes (Singh et al., 2013). According to the ‘Bloch hypothesis’, the sterol biosynthetic pathway parallels sterol evolution. In other words, the cholesterol biosynthetic pathway has evolved by the process of natural selection to optimize properties of eukaryotic cell membranes for specific biological functions (Hedlund et al., 2011). As an important membrane component, cholesterol helps to generate a semi-permeable barrier between cellular compartments and to regulate membrane fluidity. Cholesterol favors the formation of highly packed and ordered, rigid domains. A common term used for the ordered domains is “lipid-rafts” (Levental and Veatch, 2016; Sezgin et al., 2017). From a functional perspective, the cholesterol / phospholipid composition of cellular membranes is known to influence lipid raft formation and the ability of plasma membrane receptors / signaling proteins to function properly (Griffié et al., 2015; Hou et al., 2016; Phillips et al., 2009).
How does membrane composition modulate cell function?
The plasma membrane is composed of a heterogeneous mixture of lipids/cholesterol and proteins whose distinct organization maintains efficient signal transduction. Lipid rafts, enriched in sphingolipids, cholesterol and associated proteins, are special plasma membrane microdomains with an increased structural order, and are designated liquid ordered domains of plasma membranes (Sezgin et al., 2017). These lipid rafts are believed to be dynamic and small (5–200 nm) membrane microdomains, which play a critical role as sorting platforms for many membrane-associated proteins (Frisz et al., 2013; Hancock, 2006; Kraft, 2013; Levental and Veatch, 2016; Lingwood and Simons, 2010). Importantly, these domains are generally below the resolution of light microscopy (~200 nm). However, using specialized forms of microscopy that rely on non-radiative transfer of energy or single molecule localization, it is now possible to directly observe nanoscale dynamics of membrane lipids in living cells (Eggeling et al., 2009; Sahl et al., 2014). For example, the increased accessibility of super-resolution microscopy techniques has helped resolve the nanoscale organization of membrane proteins (Sezgin, 2017; Wang et al., 2014).
According to the emerging membrane biology picture, protein and lipid nanoclusters can be organized to form domains that are capable of facilitating signaling events (Ariotti et al., 2014; Garcia-Parajo et al., 2014; Zhou and Hancock, 2015). The formation of these nanoclusters is believed to be driven by cortical actin and/or proximal transmembrane proteins (Garcia-Parajo et al., 2014). Currently, protein-protein, lipid-lipid and protein-lipid nanoclusters are considered a predominant feature of the plasma membrane and appear to mediate critical signaling processes (Ariotti et al., 2014), including signal integration and cross talk of the transduction of oncogenic Ras and the epidermal growth factor receptor (EGFR) (Ariotti et al., 2014; Janosi et al., 2012; Zhou et al., 2012) regulated pathways. This is noteworthy, because there is emerging evidence that drugs and select membrane active dietary components, which we term membrane targeted dietary bioactives (MTDBs), e.g., n-3 PUFA, can attenuate Ras and EGFR (Ariotti et al., 2014; Chapkin et al., 2008; Nussinov et al., 2015) activity by modulating nanocluster organization. It also has been suggested that disrupting clustering/dimerization of membrane associated proteins can lead to attenuation of downstream oncogenic signaling and the suppression of tumor growth (Fuentes et al., 2017).
Approximately 90% of cellular cholesterol resides in the plasma membrane (Kim et al., 1991; Lange et al., 1989; Ueland et al., 1986), and increases in cholesterol promote plasma membrane order (Sezgin et al., 2014). This is significant, because highly ordered/rigid lipid rafts are increased in many types of cancer (Li et al., 2006; Patra, 2008) and some multidrug-resistant cancers (Yang et al., 2009; Yi et al., 2013). The term ‘rigidity’ refers to the packing of the lipids in the membrane, and cholesterol favors the production of more tightly pack “rigid” domains. This increased rigidity associated with increased lipid rafts typically facilitates efficient cellular signaling (Simons and Toomre, 2000). Interestingly, multidrug-resistant cells have remodeled their membrane to be in a state that is more rigid and thus receptive to activation (Li et al., 2015; Raghavan et al., 2015). This is consistent with the fact that lung cancer cells have more clustered highly activated membrane receptors, e.g., epidermal growth factor receptor (EGFR), compared to normal lung epithelium.
There is evidence suggesting that disruption of lipid rafts in cancer can lead to increased responsiveness to anti-cancer therapies (Fedida-Metula et al., 2012; Irwin et al., 2011). Additionally, some anti-cancer drugs have beneficial effects through alteration of the protein content of lipid rafts (George and Wu, 2012; Hryniewicz-Jankowska et al., 2014). In colon cancer, lipid rafts have been shown to function in cell death (apoptosis)-mediated signaling (Lacour et al., 2004; Rebillard et al., 2007), cell entry/bioavailability of bioactive compounds (Adachi et al., 2007) and localization of key proteins involved in immune response (Bene et al., 2004). These findings indicate that lipids can no longer be ignored in the structures of membrane complexes, due to their ability to fine-tune and stabilize different signaling interfaces (Barrera et al., 2013; Levental et al., 2016; Lin et al., 2016). For several viruses linked to cancer risk, a dependence on cholesterol for virus entry and/or morphogenesis has also been shown. Cholesterol depletion of virus-infected cells affects integrity of the virus envelope, causes disruption of the viral envelope, and adversely affects virus infectivity (Barman and Nayak, 2007; Imhoff et al., 2007). For this reason, there is considerable interest in identifying alternative strategies for modulating membrane cholesterol homeostasis.
Mitochondria are cholesterol-poor organelles with estimates ranging from 0.5 to 3% of the sterol content found in plasma membranes (Maxfield and Tabas, 2005; Soccio and Breslow, 2004). Interestingly, expression of oncogenic Ras results in the down regulation of the cholesterol transporter ATP-binding cassette transporter A1/cholesterol exporter (ABCA1) (Smith and Land, 2012), a key player in cholesterol metabolism (Figure 1). As a result, cholesterol accumulates in the mitochondria, which inhibits the release of death promoting molecules (Smith and Land, 2012). Similarly, mitochondria from rat or human hepatocellular carcinoma (HC) cells (HCC) or primary tumors from patients with HC exhibit increased mitochondrial cholesterol levels (Montero et al., 2008). Elevated cholesterol in mitochondria membranes may decrease membrane fluidity. This in turn, may inhibit mitochondrial permeability transition (MPT), decreasing the capacity of the pro-cell death/apoptotic Bax protein to oligomerize and penetrate into the mitochondrial outer membrane, which can ultimately promote cancer cell survival (Colell et al., 2003; Montero et al., 2008).
Dietary modulation of the exofacial membrane leaflet
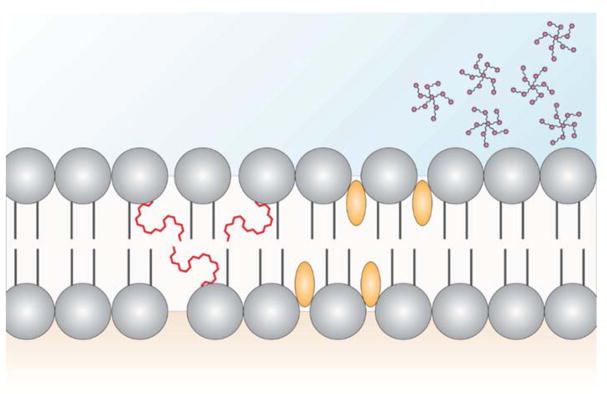
The two lipid bilayers of the plasma membrane each have unique interactions. The cytofacial (inner) leaflet interacts directly with the actin cytoskeleton. The exofacial (outer) leaflet is the site for ligand-receptor interactions, as well as glycosylated protein interactions. Several classes of MTDBs, because of size, hydrophobic/hydrophilic interactions or steric hindrances, do not readily intercalate or incorporate into the phospholipid membrane. Examples, include high oligomer polyphenols, which do not penetrate the membrane but nevertheless alter membrane organization (Erlejman et al., 2004; Fuentes et al., 2017; Verstraeten et al., 2013) (Figure 2). Although intact procyanidins have some systemic biological activity, they are poorly absorbed and pass into the distal intestine (colon) where they are further metabolized by gut microbes to generate monomeric catechin and epicatechin compounds along with other dimers-hexamer species (Choy et al., 2013; Verstraeten et al., 2015; Williamson and Manach, 2005). Interestingly, in the unique anaerobic environment of the gut lumen, microbial metabolites can interact with the apical membranes of colonic epithelial cells (Hou et al., 2016). Additional studies are needed to elucidate the mechanisms by which MTDB’s and select drugs interact at the membrane level.
Figure 2. Potential mechanisms by which bioactive dietary molecules exert chemoprotective effects on the plasma membrane.
Model depicting plasma membrane structure with long-chain n-3 polyunsaturated fatty acids (PUFA) (red lines) incorporated into phospholipids, curcumin (yellow spheroids) intercalating between phospholipids and polyphenols (magenta stars) interacting with the exofacial leaflet. The presence of either molecule may modulate plasma membrane based cellular signaling by disrupting lipid-lipid and lipid-protein interactions.
Clinical impact of lipids and cholesterol in cancer
According to emerging findings in cancer prevention, lipids and membrane biology in part mediate the chemoprotective effects of diet (Fuentes et al., 2017; Hou et al., 2016). With regard to human health, high cholesterol intake is known to increase colorectal cancer risk (Jarvinen et al., 2001). Increased free cholesterol (1.5-fold) and esterified cholesterol (2-fold) in tumor tissue as compared to normal tissue has been reported in human colon cancer patients (Dessì et al., 1994). Interestingly, an inverse relationship between serum cholesterol levels and the risk for colon cancer has been reported (Broitman et al., 1993; Forones et al., 1998). In terms of colon cancer patients, liver metastases are significantly associated with elevated low-density lipoprotein (LDL) cholesterol levels, and LDL receptor (LDLR) expression levels are upregulated and associated with progression of colorectal cancer (Wang et al., 2017a). In addition, epidemiologic studies suggest that statins, a cholesterol synthesis inhibitor, suppress certain malignancies (Hindler et al., 2006), including melanoma (Downs et al., 1998), breast (Boudreau et al., 2004; Cauley et al., 2003), colon (Cardwell et al., 2014; Lakha et al., 2012; Poynter et al., 2005), and prostate cancer (Shannon et al., 2005). Interestingly, elevated mitochondrial cholesterol in cancer cells (Montero et al., 2008) is associated with the suppression of programmed cell death (apoptosis) and the promotion of malignant cell transformation (Smith and Land, 2012) and chemotherapy resistance (Montero et al., 2008). The suppressed apoptosis was restored by cholesterol synthesis inhibition and cholesterol depletion agents establishing a cause-and-effect relationship between mitochondrial membrane cholesterol (membrane order) and membrane-mediated apoptotic signaling (Montero et al., 2008). Further studies are needed to probe the putative link between perturbations in sterol homeostasis and cancer risk.
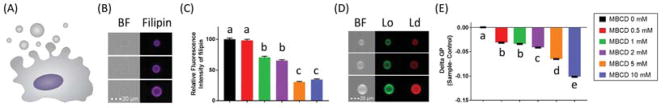
A common method for the assessment of membrane rigidity relies on microscopy based techniques that utilize environmentally sensitive probes such as laurdan or Di-4-ANEPPDHQ. These probes sense packing of the lipid membrane and their fluorescent emission spectra shifts depending on how tightly packed/ordered they are (Owen et al., 2012). As an unpublished example, we have used beta-methyl-cyclodextrin (MBCD) to dose-dependently decreased plasma membrane cholesterol in immortalized mouse colonocyte cells, followed by assessment of membrane order in isolated plasma membrane blebs, termed giant plasma membrane vesicles (GPMVs) (Sezgin et al., 2012) (Figure 3). These data demonstrate that as levels of cholesterol decrease, so does the rigidity of the plasma membrane. This is noteworthy, since membrane homeostasis is essential for maintaining cellular function and cell identity (Derler et al., 2016; Yang et al., 2016). From a therapeutics perspective, there is evidence suggesting that disruption of cholesterol-enriched lipid rafts in cancer can lead to increased responsiveness to anti-cancer therapies (Irwin et al., 2011). For this reason, there is now considerable interest in identifying alternative strategies for lowering cholesterol using nutraceutical and/or pharmaceutical platforms (Table 1).
Figure 3. Relationship between plasma membrane free cholesterol and membrane rigidity.
To highlight the relationship between plasma membrane free cholesterol and plasma membrane rigidity, (A) immortalized young adult mouse colonocyte (YAMC) cells were incubated with varying concentrations of methyl-β-cyclodextrin (MBCD) for 30 min. Cells were then incubated with vesiculation buffer (10 mM HEPES, 150 mM NaCl, 2 mM CaCl2, pH 7.4, 25 mM paraformaldehyde and 2 mM dithiothreitol) for 1 h in order to generate giant plasma membrane vesicles (GPMVs). (B) Representative bright field (BF) and fluorescence images of GPMVs incubated with Filipin (50 mg/mL) for 45 min to stain free cholesterol, were assayed using an imaging based flow cytometry system (Amnis FlowSight). For this purpose, Filipin was excited with a 405-nm laser and emission wavelengths of 430–505 nm were collected. (C) Quantitative data demonstrating the dose dependent decrease of plasma membrane free cholesterol. (D) Representative BF and fluorescence images of GPMVs incubated with the membrane order sensitive probe Di-4-ANEPPDHQ (0.5 μM) were immediately analyzed using imaging based flow cytometry. Di-4-ANEPPDHQ was excited with a 488-nm laser and emission wavelengths of 480–560 nm and 640–745 nm were collected for lipid ordered (Lo, green) and lipid disordered (Ld, red) channels, respectively. (E) General polarization (GP), defined as the integrated fluorescence intensity from the ordered channel minus that of the disordered channel, was normalized by the total intensity (sum of the two channels). Quantification of membrane order is represented as mean GP, normalized to the control (MBCD 0 mM). MBCD treatment, (C) removed free cholesterol and (E) dose-dependently decreased plasma membrane rigidity. In all graphs, data are presented as mean ± SEM for at least 2000 individual vesicles per group. Statistical significance between treatments as indicated by different letters (P<0.05) was determined using 1-way ANOVA and Dunnett’s multiple comparisons test.
Table 1.
Fatty acid and cholesterol metabolism inhibitors in the clinic.
| Targeting lipid and cholesterol synthesis | Reference | ||
|---|---|---|---|
| HMGCR | Statins | Colon cancer | (Cardwell et al., 2014; Holstein et al., 2006; Lakha et al., 2012; Poynter et al., 2005) |
| Hepatocellular carcinoma | (Graf et al., 2008; Kawata et al., 2001) | ||
| Breast cancer | (Garwood et al., 2010) | ||
| Refractory multiple myeloma | (Schmidmaier et al., 2007) | ||
| Brain stem tumors | (López-Aguilar et al., 2008) | ||
| Metastatic colorectal patients | (Lee et al., 2009) | ||
| Targeting lipid mediator | Reference | ||
| COX2 | Celecoxib | Colon | (Wang and Dubois, 2010) |
| Aspirin + Celecoxib | Colon | (Ng et al., 2015) | |
| Celecoxib | Familial adenomatous polyposis (FAP) | (Giardiello et al., 1993; Higuchi et al., 2003; Lynch et al., 2010; Steinbach et al., 2000) | |
| Sulindac | (Giardiello et al., 2002; Labayle et al., 1991; Nugent et al., 1993; West et al., 2010) | ||
Metabolic reprogramming in cancer: Role of lipids and cholesterol in cancer
Cancer cells frequently exhibit specific alterations in their metabolic activity. The best known metabolic abnormality linked to cancer cell biology is the Warburg effect, i.e., (a) increased glycolytic flux, (b) decreased TCA cycle flux and (c) increased utilization of glutamine for anabolic pathways (Baenke et al., 2013). Metabolic reprogramming via alteration in lipid biosynthesis and cholesterol synthesis is also established as a hallmark of cancer (Hanahan and Weinberg, 2011). This metabolic reprogramming supports the increased production of metabolic intermediates for the synthesis of proteins, nucleic acids and lipids, and is a prerequisite for the rapid proliferation of cancer cells (Baenke et al., 2013).
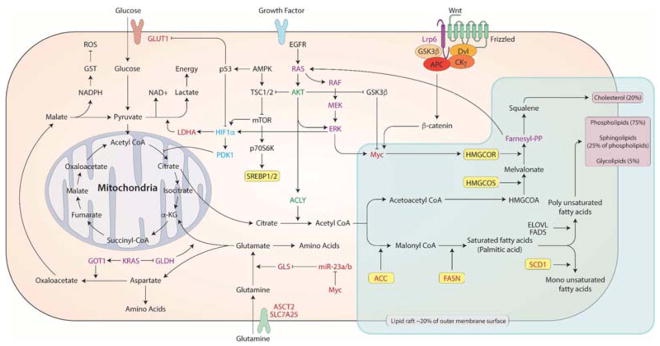
Many of the predominant mutations observed in cancer also control tumor cell metabolism, leading to the theory that oncogene and tumor suppressor networks mediate the metabolic shift in cancer (Vogelstein and Kinzler, 2004). For example, it has been demonstrated that mutations in major oncogenes, e.g.,phosphoinositide-3-kinase (PI3K)/AKT, KRAS and MYC (Arora et al., 2015; Bost et al., 2016; Slaninova et al., 2016), mediate metabolic shifts in cancer cells and activate de novo FA synthesis (Hsieh et al., 2015; Manning and Cantley, 2007; Sears et al., 2000). Figure 4 summarizes how oncogenes modulate metabolic shifts involving FA de novo synthesis, which is tightly associated with plasma membrane properties. Collectively, these observations support the cancer link between metabolic reprogramming, FA/cholesterol de novo synthesis and alterations in plasma membrane-mediated signaling pathways.
Figure 4. Potential molecular mechanism of metabolic reprogramming and membrane fatty acids synthesis.
Oncogenic Myc (red), Ras (violet), HIF-1 (blue), PI3K/AKT (green) which have common roles in metabolic reprogramming also increase cholesterol/lipid synthesis which may affect plasma membrane order altering membrane mediated signaling pathways.
Highly proliferative cancer cells satisfy lipid/cholesterol needs by either increasing the uptake of exogenous lipids and lipoproteins or by chronically upregulating their endogenous (de novo) biosynthetic pathways (Accioly et al., 2008; Qiu et al., 2015; Yue et al., 2014). A plethora of studies have confirmed the importance of FA biosynthesis as a contributory feature of early stage cancer development (Baenke et al., 2013), cancer cell growth and survival (Currie et al., 2013; Rohrig and Schulze, 2016; Santos and Schulze, 2012). Excessive lipids and cholesterol in cancer cells are stored in lipid droplets (LDs). This is significant because elevated LDs and stored-cholesteryl ester content in tumors (Accioly et al., 2008; Guillaumond et al., 2015; Qiu et al., 2015; Yue et al., 2014) are considered to be hallmarks of cancer aggressiveness (Abramczyk et al., 2015; Bozza and Viola, 2010; de Gonzalo-Calvo et al., 2015; Yue et al., 2014).
As noted above, in cancer cells, carbon must be diverted from energy production to FA/cholesterol membrane biosynthesis. The bulk of cell membrane lipids are PLs, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE), in addition to other lipids, such as sterols, and sphingolipids. All of these lipids are derived in part from acetyl CoA, and many contain FA. The FA building blocks are derived from either exogenous sources or from de novo FA synthesis. Thus, it is important to understand how lipid/cholesterol biosynthesis is enhanced in cancer because most normal human cells prefer exogenous sources whereas tumors synthesize FA de novo (Currie et al., 2013; Medes et al., 1953; Ookhtens et al., 1984; Santos and Schulze, 2012) and often exhibit a shift toward FA synthesis (Ookhtens et al., 1984). From a mechanistic perspective, it is interesting to note that in many cases, the endogenous synthesis of lipids relays physiological / pathophysiological cues that cannot be mimicked by chemically identical exogenous lipids (Wei et al., 2016).
Oncogene-transformed cancer cells require elevated levels of cholesterol to support their rapid growth (Gabitova et al., 2015). In addition to enhanced de novo cholesterol synthesis (Pitroda et al., 2009; Silvente-Poirot and Poirot, 2014), the process of cholesterol uptake is also tightly regulated by the signaling activity of the epidermal growth factor receptor (EGFR), which is increased in cancer (Guo et al., 2011). Although cancer cells activate de novo FA synthesis, Ras-driven tumors also require the uptake of essential FAs through the nonselective endocytic uptake of extracellular lipids and proteins driven by membrane-lipid and cytoskeletal remodeling, termed macropinocytosis (Bohdanowicz and Grinstein, 2013; Commisso et al., 2013; Lim and Gleeson, 2011; Salloum et al., 2014). Ras driven tumors are so dependent on macropinocytosis that it is now exploited as a delivery mechanism of oncogene targeted therapeutics (Kamerkar et al., 2017).
Membrane cholesterol and stem cells
In preclinical studies, diet-induced obesity (DIO), perturbed cellular lipid homeostasis, which was associated with enhanced Lgr5+ cell stemness (Beyaz et al., 2016). This is noteworthy, because intestinal Lgr5+ stem cells are the cells-of-origin of intestinal cancer (Barker et al., 2007, 2009). This is consistent with recent work from our lab indicating that DIO promotes colonic Lgr5+ stem cell expansion during cancer initiation (DeClercq et al., 2015). Interestingly, the inactivation of cholesterol efflux transporters (ABCA1 and ABCG1) increase the number of circulating hematopoietic stem cells (HSPCs) (Feng et al., 2012; Gomes et al., 2010; Yvan-Charvet et al., 2007) and activate inflammatory cells (Lang and Cimato, 2014), suggesting that the accumulation of cellular membrane cholesterol may directly regulate aspects of stem cell biology. From a mechanistic perspective, the Wnt/β-catenin pathway maintains a stem cell/progenitor phenotype in many cell types (Pinto and Clevers, 2005). Interestingly, recent evidence indicates that cholesterol selectively activates canonical Wnt signaling (Sheng et al., 2014). This is noteworthy, because Wnt positively regulates cholesterol uptake for growth of cancer cells (Scott et al., 2015). Collectively, these findings suggest that perturbations in lipid homeostasis may play a critical role in stem cell signaling, thereby modulating cancer risk.
Impact of dietary fatty acids on plasma membrane composition and structure
Diet can play an impactful role in modifying the plasma membrane. The idea that dietary lipids can influence membrane structure is intuitive, since the plasma membrane itself is composed of phospholipids. Phospholipids are amphiphilic molecules, typically composed of two hydrophobic fatty acid tails and a hydrophilic head. In general, dietary lipids are transported as circulating lipoproteins containing triglycerides and phospholipids. Cells subsequently take up circulating lipids and use them for energy or the synthesis and remodeling of membranes. Animal and plant fats and oils are composed of fatty acids of different chain lengths (14–24 carbons), which contain different amounts of unsaturation (1–6 double bonds). Not only the chain length and amount of unsaturation, but also the position of the double bonds in relation to the methyl end of FA (n-9 vs n-6 vs n-3) impact its biophysical properties. Mammalian cells can endogenously produce most fatty acids, however, they lack the desaturase enzymes necessary to produce n-6 or n-3 fatty acids de novo (Chapkin, 2007). Therefore, these lipids must be obtained via the diet and are considered essential. For example, linoleic acid (LA), an n-6 PUFA is highly enriched in a common staple of the western-diet, vegetable oils. In comparison, n-3 PUFA, e.g., alpha-linoleic acid (ALA), eicosapentaenoic acid (EPA, 20:5Δ5,8,11,14,17), and docosahexaenoic acid (DHA, 22:6Δ4,7,10,13,16,19) are less prevalent in the western-diet.
There is evidence that the consumption of marine or algal oils, containing EPA and/or DHA, may reduce colon cancer risk in humans (Cockbain et al., 2014; Courtney et al., 2007; Hall et al., 2008; Song et al., 2016; West et al., 2010) (Table 2). EPA and DHA appear to be ideally suited to work either alone or in combination with chemoprotective drugs (Vaughan et al., 2013). For example, it was recently demonstrated that EPA reduced rectal polyp number and size in patients with familial adenomatous polyposis (FAP) (Cockbain et al., 2012; West et al., 2010). Most impressive is the fact that fish oil derived n-3 PUFA suppressed FAP to a degree similar to the selective COX-2 inhibitor celecoxib. Ongoing clinical trials (Clinical Trials.gov) are currently examining the effects of EPA on subjects at high risk of CRC (NCT02069561); the combinatory role of EPA and DHA in reducing rectal cancer risk (NCT02534389), and the combinatory role of EPA and NSAIDs on polyp recurrence in the colon (NCT01070355; ISRCTN05926847) (Cockbain et al., 2014; Hull et al., 2013). Collectively, these data indicate that n-3 PUFA hold promise as chemoprevention agents. Hence, establishing a causal role of n-3 PUFA in colon cancer prevention would have a major translational impact because these dietary nutrients are safe, well tolerated (Lien, 2009), relatively inexpensive and provide numerous health benefits (Rizos and Elisaf, 2013). In addition, the ingestion of n-3 PUFA in combination with other membrane-active agents with complementary anti-tumor action, e.g., curcumin (Altenburg et al., 2011) and drugs (Morland et al., 2016), may improve their efficacy in colon cancer prevention/therapy. This combination strategy utilizing n-3 PUFA and curcumin is also under clinical investigation as a prevention mechanism for type 2 diabetes (Thota et al., 2016). However, before a drug-nutrient combination approach can be adopted, it is imperative that the molecular mechanisms of action be rigorously probed.
Table 2.
Clinical trials examining the chemoprotective effects of n-3 PUFA.
| Bioactive compound | Daily dose (n, duration of treatment) | Patient type | Beneficial biological effect | Reference |
|---|---|---|---|---|
| EPA | 2 g EPA-FFA (enteric-coated formulation of EPA) (n = 55, 6 months) | Familial adenomatous polyposis (FAP) | 22.4% reduction in polyp number and a 29.8% decrease in the sum of polyp diameters. | (West et al., 2010) |
| n-3 PUFA | 2.18 g (n = 52, 3 months) | Range of malignant cancers | Decreased cancer-induced weight loss in pediatric patients. | (Bayram et al., 2009) |
| n-3 PUFA | 4 g = 2520 mg EPA + 1680 mg DHA (n = 20, 3 months) | Postmenopausal breast cancer survivors | Bone resorption was inhibited in the fish oil responders compared to placebo. | (Hutchins-Wiese et al., 2014) |
| LOVAZA | 840 mg n-3 PFA = 465 mg EPA + 375 mg DHA (n = 180, 6 months) | Acute myocardial infarction | Reduction of adverse left ventricular remodeling and serum biomarkers of systemic inflammation. | (Heydari et al., 2016) |
| Lovaza | 3360 mg = 1860 mg of EPA + 1500 mg of DHA ethyl esters (n = 36, 6 months) | Breast cancer | Cytologic atypia and Ki-67 were decreased with an increase in the ratio of EPA DHA to AA in erythrocyte phospholipids. | (Fabian et al., 2015) |
| DHASCO | 1.8 g DHA (n = 25, 5 months) | Breast cancer | DHA during chemotherapy was devoid of adverse side effects and can improve the outcome of chemotherapy. | (Bougnoux et al., 2009) |
| n-3 PUFA | 2 g and 4 g EPA (n = 171~175, 4 and 8 wks) | Lung or gastrointestinal cancer | Mean weight was increased by 1.2 kg with 2 g EPA and by no significant increase with 4g EPA. | (Fearon et al., 2006) |
| n-3 PUFA or celecoxib | 6 g fish oil (1.8 g n-3 PUFA) or 6 g fish oil + 400 mg celecoxib (n = 10~12, 6wks). | Lung cancer | Symptoms associated with a Systemic Immune-Metabolic Syndrome (SIMS) was ameliorated by n-3 fatty acids + celecoxib. | (Cerchietti et al., 2007) |
| n-3 PUFA | 2.04 g EPA and 1.36 g of DHA (n = 13, 66 days) | Inoperable nonsmall cell lung cancer | C-reactive protein and IL-6 was decreased by 22 days and 66 days. | (Finocchiaro et al., 2012) |
| n-3 PUFA | Food frequency questionnaire with 22 years of followup (n = 22071) | Colorectal cancer | Intake of n-3 PUFA and fish decreased the risk of colorectal cancer development in dose dependent manner. | (Hall et al., 2008) |
| n-3 PUFA | 2.2 g EPA + 240–500 mg DHA (n = 15, 6 wks) | Lung cancer | Fish oil intake resulted in increased chemotherapy efficacy and contributed to increased survival. | (Murphy et al., 2011) |
With respect to the molecular mechanism of n-3 PUFA action, there is a growing body of in vitro and in vivo evidence indicating that n-3 PUFA reshape plasma membrane domains. For example, dietary EPA and DHA are rapidly incorporated into cells (Katan et al., 1997), primarily into membrane phospholipids at the sn-2 position (Chapkin et al., 1991; Williams et al., 2012). The presence of long chain n-3 PUFA in membrane phospholipids imparts unique biophysical properties which have been linked to alterations in plasma membrane structure and function (Levental et al., 2016; Ma et al., 2004; Seo et al., 2006; Williams et al., 2012). For example, DHA is known to increase membrane fluidity, ion permeability, fatty acid exchange, and suppress resident protein function (Hou et al., 2015, 2016; Stillwell and Wassall, 2003), including the inhibition of epidermal growth factor receptor (EGFR) signaling in tumor bearing mice by disassociating EGFR from lipid rafts (Turk et al., 2012). Interestingly, n-3 PUFA are also known to promote differences in membrane rigidity between raft and non-raft domains (Levental et al., 2016; Lin et al., 2016), likely driven by repulsive forces between n-3 PUFA and cholesterol (Wang et al., 2017b). From a systemic perspective, membrane order is also increased in T cell plasma membranes from fish oil fed mice or transgenic mice that produce n-3 PUFA (Kim et al., 2008, 2014). Similarly, B cells isolated from mice fed n-3 PUFA-enriched diet exhibit an increase in membrane order in cross-linked cells relative to non-cross-linked (Rockett et al., 2012). This is in contrast to the decrease in membrane order reported in Jurkat cells treated with EPA and DHA (Kim et al., 2010; Zech et al., 2009). A possible explanation for the differences reported in these studies is that malignant transformed Jurkat cell lines may be inherently distinct from primary T-cells with respect to specific plasma membrane properties. Precisely how these diet mediated changes in cell membrane order modulate cell function remains to be determined.
Diet can also impact plasma membrane structure in a less intuitive manner. Specifically, many foods contain amphiphilic molecules that can directly interact with the plasma membrane (Fuentes et al., 2017). For example, turmeric (Curcuma longa Linn) extracts, including curcumin (diferuloylmethane), a yellow color pigment of turmeric, insert deep into the membrane in a trans-bilayer orientation, anchored by hydrogen bonding to the phosphate group of lipids in a manner analogous to cholesterol (Figure 2) (Hung et al., 2008; Ingolfsson et al., 2007). The combinatorial membrane interactions of n-3 PUFA and curcumin may explain the beneficial synergism observed in various studies (Altenburg et al., 2011; Kim et al., 2016; Saw et al., 2010; Siddiqui et al., 2013).
n-3 PUFA, mitochondrial membranes and programmed cell death (apoptosis)
It is well known that dietary n-3 PUFA supplementation alters cell plasma membrane phospholipid composition and downstream signaling events, which can result in the amelioration of various disease processes (Calder, 2015; van den Elsen et al., 2012; Hou et al., 2016; McMurray et al., 2011) In addition to targeting the plasma membrane, mitochondrial membrane composition and the function of mitochondria can also be modulated by exogenous lipid species (Herbst et al., 2014; Ting et al., 2015) Non-esterified fatty acids enter cells via fatty acid transporters and passive diffusion, and undergo activation by select enzymes to become fatty acyl-CoAs (Glatz and Luiken, 2017; Jay and Hamilton, 2016). The fatty acyl-CoAs have multiple fates, which include esterification into membrane polar phospholipids and neutral lipids, as well as serving as an ATP source via oxidative pathways (Jump, 2002). There is growing evidence linking n-3 PUFA supplementation with dramatic alterations in mitochondrial phospholipid membrane composition with favorable changes in mitochondrial function (Calder, 2015; Chapkin et al., 2002, 2014; Stanley et al., 2012). We have shown that animals fed fish oil, enriched in DHA and EPA, have higher levels of colonic apoptosis, mediated in part via elevated mitochondrial proton leak and enhanced reactive oxygen species (ROS)(Bancroft et al., 2003; Cho et al., 2014; Fan et al., 2011, 2009; Hong et al., 2005; Kim et al., 2016; Sanders et al., 2004). The chemoprotective effects of n-3 PUFA are seen at both the initiation and post-initiation stages of carcinogenesis (Davidson et al., 2004). In addition, in vivo studies have shown that n-3 PUFA and fermentable fiber co-treatment alter mitochondrial membrane cardiolipin mass, membrane potential, enhance mitochondrial membrane phospholipid oxidation, cytosolic levels of cytochrome C, promoted mitochondrial proton leak, and apoptosis in colonocytes (Fan et al., 2016; Kolar et al., 2007; Ng et al., 2005).
Mitochondria are key organelles of the intrinsic apoptotic pathway, mediating cell death in pathological and stress conditions (Dingeldein et al., 2017; Green et al., 2014). Cardiolipin is a diphosphatidylglycerol lipid, synthesized exclusively in the mitochondria. It is predominantly found in the inner and, to a lesser extent, outer mitochondrial membranes (Hatch, 2004; Houtkooper and Vaz, 2008; Mejia et al., 2014). Cardiolipin is required for mitochondrial structural integrity and for the proper function of the electron transport chain. Cardiolipin allows cytochrome C to anchor to the mitochondrial inner membrane and thereby, regulates its release from the mitochondria. The peroxidation of cardiolipin due to an increase in oxidative stress can lead to the detachment of cytochrome C from the mitochondrial membrane and its release into the cytosol, thereby inducing apoptosis (Montero et al., 2010). With respect to exogenous lipids in vitro, n-3 PUFA incubation with human fetal colon HFC and adenocarcinoma HCT-116 colon cell lines significantly modified the composition of a wide range of cardiolipin species (Hofmanova et al., 2017). Since PUFA residues have been considered a source of oxygenated mediators (Schlame and Greenberg, 2017; Tyurina et al., 2014), it is not surprising that DHA-enriched human colonic adenocarcinoma HT-29 cells which contain cardiolipin up to 48 mol%, produced 4-fold more ROS than did cells enriched with other n-6 and n-9 fatty acids (Watkins et al., 1998). Similarly, T24/83 human bladder cancer cells incubated with EPA exhibited an enrichment in mitochondrial cardiolipin EPA content, which was associated with decreased mitochondrial membrane potential, increased lipid peroxide, ROS generation, caspase-3 activation, apoptosis, and decreased cell proliferation (Colquhoun, 2009).
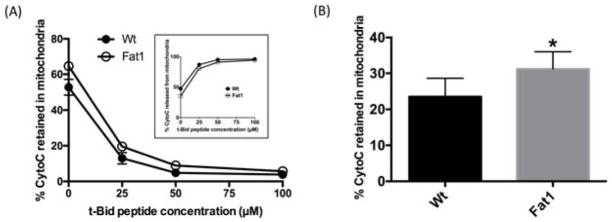
Given the critical nature of apoptosis in cancer prevention, we and others further explored the previously described link between n-3 PUFA, mitochondrial cardiolipin, and mediators of apoptosis (Agnihotri et al., 2016; Chapkin et al., 2002; Hofmanova et al., 2017; Hong et al., 2002; Sharma et al., 2016). These dietary studies using preclinical models provide evidence that n-3 PUFA modulate mitochondria cardiolipin molecular species in a dose dependent manner, augmenting apoptosis and thereby attenuating carcinogenesis (Agnihotri et al., 2016; Chapkin et al., 2002; Hong et al., 2002; Shaikh et al., 2015; Sharma et al., 2016). In a series of complementary unpublished experiments, we demonstrated that n-3 PUFA can promote t-Bid / cardiolipin mediated mitochondrial cytochrome C release in a “cell free” in vitro system as originally described (Chandra and Tang, 2009; Taneja et al., 2001). In these experiments, mitochondria were isolated from wild type or Fat1 transgenic mice. These mice express the C. elegans n-3 fatty acid desaturase transgene which converts n-6 to n-3 fatty acids (Kang et al., 2004). This results in a mouse model which contains n-3 PUFA enriched membranes in the absence of dietary n-3 PUFA. Mitochondria from colonic mucosa were subsequently incubated with various doses of exogenous recombinant t-Bid in order to see if n-3 PUFA are capable of modulating cytochrome C release (a biomarker of apoptosis). As expected, exogenous t-Bid peptide dose-dependently decreased mitochondrial cytochrome C (Figure 5). Interestingly, the overall % of retained cytochrome C in mitochondria was elevated in Fat1 mice, indicating a reduction in cytochrome C release. Adding to a growing body of evidence that n-3 PUFA alters mitochondrial membrane composition and function, in a recent finding, we demonstrated that dietary n-3 PUFA from fish oil are not directly incorporated into mitochondrial cardiolipin in vivo (Fan et al., 2016). Instead, fish oil indirectly influenced colonocyte cardiolipin molecular species with a combined carbon number of C68 or greater, suggesting some form of compensatory regulation. Collectively, these findings suggest that the modulatory effect of fish oil feeding on cardiolipin / t-Bid induced apoptosis is not the result of a direct effect of n-3 PUFA on cardiolipin remodeling.
Figure 5. Percentage of Cytochrome C retained in the mitochondria following t-Bid incubation.
Colonic mucosa mitochondria isolated from Fat1 (n-3 PUFA enriched) and wild type (Wt) mice were incubated with various doses of t-Bid peptide (EDIIRNIARHLAQVGDSMDR) (0, 25, 50, 100 μM) for 30 min. Both cytochrome C (CytoC) retained in mitochondria and released into the cytosol were measured by ELISA. (A) Data are presented as percentage of Cytochrome C retained in the mitochondria (mean±SE, n=5 mice). Inset: Percentage of Cytochrome C released from mitochondria. (B) Pooled data of percentage of Cytochrome C retained in mitochondria from all t-Bid peptide (0 – 100 μM) incubations (mean±SE, n=25 mice), *p<0.05.
In summary, we now know that dietary n-3 PUFA have pleiotropic effects on promoting intrinsic apoptosis including cardiolipin remodeling, increasing mitochondrial membrane lipid peroxidation, decreasing MMP, increasing mitochondrial proton leak, ROS, intramitchondrial Ca2+, t-Bid / bcl-2 ratio, cytochrome C release, all of which contribute to a reduction in the number of cells exhibiting DNA damage, which is linked to a reduction in cancer risk.
n-3 PUFA synergy with other dietary bioactives
Although the chemoprotective effects of n-3 PUFA on human health are well documented (Abel et al., 2014; D’Eliseo and Velotti, 2016; Pelliccia et al., 2013; Serini et al., 2014; Yates et al., 2014), it now appears that the combination of n-3 PUFA with other dietary bioactive compounds may have synergistic beneficial effects. For example, in vivo studies have shown the strongest pro-apoptotic / anti-carcinogenic effect was observed following the combined feeding of dietary fish oil and highly fermentable fiber, which enhances butyrate (a 4 carbon short chain fatty acid) concentrations in the colonic lumen (Cho et al., 2011, 2012; Sanders et al., 2004; Shah et al., 2016; Turk et al., 2012; Vanamala et al., 2008). We have shown the combination of DHA and butyrate trigger mitochondrial Ca2+ loading, potentiate mitochondrial lipid oxidation and the dissipation of MMP which contribute to the induction of intrinsic mitochondrial-mediated apoptotic pathway in colonocytes (Kolar et al., 2011, 2007; Ng et al., 2005). Similar synergetic apoptotic effects of DHA and butyrate have been described in human colon cancer cell lines, e.g., HCT-116, HT-29, and CaCo-2 (Hofmanova et al., 2009, 2012; Hossain et al., 2006).
Adjunct chemotherapeutic potential of n-3 PUFA
Triggering the mitochondrial intrinsic apoptosis pathway resulting in cancer-cell suicide is a potential option for cancer therapy (Fulda et al., 2010; Weinberg and Chandel, 2015). However, the therapeutic effects of drugs that act on mitochondria have not been remarkable in clinical trials possibly because of concerns related to insufficient drug delivery to the mitochondria and side effects due to long-term toxicity. Use of adjuvant anti-cancer agents in combination with chemotherapeutic elements may therefore provide an ideal strategy to enhance the efficacy of drug application. As noted above, due to the amphiphilic nature of n-3 PUFA, these lipids are readily incorporated into the lipid bilayer of cells, especially tumor cells. Therefore, n-3 PUFA may have utility to increase the therapeutic efficacy of anticancer drugs which modulate biological membranes (Merendino et al., 2013; Sorensen et al., 2014; Wang et al., 2012). Benefits of dietary n-3 PUFA adjunct to FDA approved drugs in cancer treatment or prevention have been reported. For example, DHA combination sensitized colon cancer cells to non-steroidal anti-inflammatory drug (sulindac sulfide)-induced apoptosis, leading to enhanced growth suppression of human colon cancer xenografts (Lim et al., 2012). This noteworthy, because NSAIDs are one of the most efficacious classes of drugs used to reduce the risk of colon cancer. n-3 PUFA also have been shown to enhance the susceptibility of human colorectal cancer cells to 5-fluorouracil, a popular colon cancer chemotherapeutic agent (Calviello et al., 2005; Granci et al., 2013; Jordan and Stein, 2003; Vasudevan et al., 2014; Zhuo et al., 2009). Similar pro-apoptotic effects have been observed in colon carcinoma mouse models (Rani et al., 2017). Beyond colon cancer, adjuvant chemotherapy using n-3 PUFA has been documented in various disease types, including adjunct with celecoxib in mammary carcinoma (Negi et al., 2016), tamoxifen in breast cancer (Manni et al., 2015; Wu et al., 2015), cisplatin in lung cancer (Zajdel et al., 2014), gemcitabine in breast and liver cancer (Li et al., 2014); doxorubicin, vincristine and fludarabine in leukemia (Fahrmann and Hardman, 2013).
Clinical impact of n-3 PUFA
There are more than 550 clinical trials registered in the ClinicalTrials.gov investigating the effect of fish oil on various chronic diseases, implying the potential clinical impact of n-3 PUFA in human health. For example, EPA (2 g/d for 3 mo) has been shown to reduce colonic crypt cell proliferation and increase apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas (Courtney et al., 2007). No tolerable upper limit has been set for EPA and DHA, although the US Food and Drug Administration recognizes doses of up to 3 g/day as safe and the European Safety Union up to 5 g/day as safe (Azzi et al., 2005). Side effects of fish oil supplements or EPA + DHA ethyl esters include fishy burps, dyspepsia, gas, and diarrhea (Browning et al., 2012; Yates et al., 2014). Importantly, human studies using doses as high as 17.6 g/day EPA+DHA have been performed with no serious side effects (Skarke et al., 2015). Alternatively, n-3 PUFA can be administered through intravenous lipid emulsions as part of a parenteral nutrition regimen (Klek, 2016). n-3 PUFA emulsion-based parenteral nutrition alleviates the inflammatory reactions and reduces the rate of inflammatory complications for patients recovering from surgical resection of gastric tumors (Wei et al., 2014). EPA is incorporated rapidly into colonic mucosa and the colonic muscular layer in patients given 3 g of n-3 PUFA daily for 7 days before surgery for colorectal cancer (Sorensen et al., 2014), which supports claims related to the clinical benefits of n-3 PUFA. Additional clinical studies are needed to assess the effect of EPA and/or DHA dose and duration on key molecular targets, e.g., cell plasma membranes and related phenotypic outcomes.
Summary
Advances in quantitative lipidomics has begun to shed light on the importance of plasma membrane composition and architecture in relation to chronic disease prevention. Here we have summarized literature regarding the interactions of membrane lipids and diet in terms of cancer biology. Since membrane lipid composition influences cellular function, future research is merited in order to elucidate how both endogenous and exogenous lipids, e.g., dietary n-3 PUFA, modulate cell membrane structure / function and reduce chronic disease risk.
Supplementary Material
Acknowledgments
We would like to thank Rachel C. Wright for generation of summary diagrams.
This work was supported by National Institutes of Health grants R35CA197707 and P30ES023512, the Cancer Prevention Research Institute of Texas (CPRIT) and funds from the Allen Endowed Chair in Nutrition & Chronic Disease Prevention. Natividad Fuentes is a recipient of a Predoctoral Fellowship in Pharmacology/Toxicology from the PhRMA Foundation and the National Science Foundation Texas A&M University System Louis Stokes Alliance for Minority Participation (TAMUS LSAMP) Bridge to the Doctorate Fellowship (HRD-1249272).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel S, Riedel S, Gelderblom WC. Dietary PUFA and cancer. Proc Nutr Soc. 2014;73:361–367. doi: 10.1017/S0029665114000585. [DOI] [PubMed] [Google Scholar]
- Abramczyk H, Surmacki J, Kopec M, Olejnik AK, Lubecka-Pietruszewska K, Fabianowska-Majewska K. The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst. 2015;140:2224–2235. doi: 10.1039/c4an01875c. [DOI] [PubMed] [Google Scholar]
- Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008;68:1732–1740. doi: 10.1158/0008-5472.CAN-07-1999. [DOI] [PubMed] [Google Scholar]
- Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- Agnihotri N, Sharma G, Rani I, Renuka, Bhatnagar A. Fish oil prevents colon cancer by modulation of structure and function of mitochondria. Biomed Pharmacother. 2016;82:90–97. doi: 10.1016/j.biopha.2016.04.045. [DOI] [PubMed] [Google Scholar]
- Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z, Jo Davisson V, Siddiqui RA. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti N, Fernández-Rojo MA, Zhou Y, Hill MM, Rodkey TL, Inder KL, Tanner LB, Wenk MR, Hancock JF, Parton RG, et al. Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling. J Cell Biol. 2014;204:777–792. doi: 10.1083/jcb.201307055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Singh S, Bhatt AN, Pandey S, Sandhir R, Dwarakanath BS. Interplay Between Metabolism and Oncogenic Process: Role of microRNAs. Transl Oncogenomics. 2015;7:11–27. doi: 10.4137/TOG.S29652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A, Brigelius-Flohe R, Kelly F, Lodge JK, Ozer N, Packer L, Sies H. On the opinion of the European Commission “Scientific Committee on Food” regarding the tolerable upper intake level of vitamin E (2003) Eur J Nutr. 2005;44:60–62. doi: 10.1007/s00394-005-0549-8. [DOI] [PubMed] [Google Scholar]
- Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft LK, Lupton JR, Davidson LA, Taddeo SS, Murphy ME, Carroll RJ, Chapkin RS. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free Radic Biol Med. 2003;35:149–159. doi: 10.1016/s0891-5849(03)00240-5. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Barman S, Nayak DP. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J Virol. 2007;81:12169–12178. doi: 10.1128/JVI.00835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Zhou M, Robinson CV. The role of lipids in defining membrane protein interactions: insights from mass spectrometry. Trends Cell Biol. 2013;23:1–8. doi: 10.1016/j.tcb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Bayram I, Erbey F, Celik N, Nelson JL, Tanyeli A. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy-related weight loss in children. Pediatr Blood Cancer. 2009;52:571–574. doi: 10.1002/pbc.21852. [DOI] [PubMed] [Google Scholar]
- Bene L, Bodnar A, Damjanovich S, Vamosi G, Bacso Z, Aradi J, Berta A, Damjanovich J. Membrane topography of HLA I, HLA II, and ICAM-1 is affected by IFN-gamma in lipid rafts of uveal melanomas. Biochem Biophys Res Commun. 2004;322:678–683. doi: 10.1016/j.bbrc.2004.07.171. [DOI] [PubMed] [Google Scholar]
- Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch KE. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14:47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Bohdanowicz M, Grinstein S. Role of phospholipids in endocytosis, phagocytosis and macropinocytosis. Physiol Rev. 2013;93:69–106. doi: 10.1152/physrev.00002.2012. [DOI] [PubMed] [Google Scholar]
- Bost F, Decoux-Poullot AG, Tanti JF, Clavel S. Energy disruptors: rising stars in anticancer therapy? Oncogenesis. 2016;5:e188. doi: 10.1038/oncsis.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100:2308–2316. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101:1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fat Acids. 2010;82:243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Broitman SA, Cerda S, Wilkinson Jth. Cholesterol metabolism and colon cancer. Prog Food Nutr Sci. 1993;17:1–40. [PubMed] [Google Scholar]
- Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Calviello G, Di Nicuolo F, Serini S, Piccioni E, Boninsegna A, Maggiano N, Ranelletti FO, Palozza P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother Pharmacol. 2005;55:12–20. doi: 10.1007/s00280-004-0846-6. [DOI] [PubMed] [Google Scholar]
- Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J Clin Oncol. 2014;32:3177–3183. doi: 10.1200/JCO.2013.54.4569. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, Cummings SR, Bauer DC. Lipid-Lowering Drug Use and Breast Cancer in Older Women. A Prospective Study. J Women’s Heal. 2003;12:749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- Cerchietti LCA, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59:14–20. doi: 10.1080/01635580701365068. [DOI] [PubMed] [Google Scholar]
- Chandra D, Tang DG. Detection of apoptosis in cell-free systems. Methods Mol Biol. 2009;559:65–75. doi: 10.1007/978-1-60327-017-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin R. In Fatty Acids in Foods and Their Health Implications. 3. CRC Press; 2007. Reappraisal of the Essential Fatty Acids; pp. 675–691. [Google Scholar]
- Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res. 1991;32:1205–1213. [PubMed] [Google Scholar]
- Chapkin RS, Hong MY, Fan YY, Davidson LA, Sanders LM, Henderson CE, Barhoumi R, Burghardt RC, Turner ND, Lupton JR. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta-Biomembr. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin RS, DeClercq V, Kim E, Fuentes NR, Fan YYY. Mechanisms by Which Pleiotropic Amphiphilic n-3 PUFA Reduce Colon Cancer Risk. Curr Color Cancer Rep. 2014;10:442–452. doi: 10.1007/s11888-014-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Kim H, Turner ND, Mann JC, Wei J, Taddeo SS, Davidson LA, Wang N, Vannucci M, Carroll RJ, et al. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J Nutr. 2011;141:1029–1035. doi: 10.3945/jn.110.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp Biol Med. 2012;237:1387–1393. doi: 10.1258/ebm.2012.012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR, et al. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Exp Biol Med. 2014;239:302–310. doi: 10.1177/1535370213514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy YY, Jaggers GK, Oteiza PI, Waterhouse AL. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J Agric Food Chem. 2013;61:121–127. doi: 10.1021/jf301939e. [DOI] [PubMed] [Google Scholar]
- Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A, Loadman PM, Toogood GJ, Hull Ma. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014;63:1760–1768. doi: 10.1136/gutjnl-2013-306445. [DOI] [PubMed] [Google Scholar]
- Colell A, Garcia-Ruiz C, Lluis JM, Coll O, Mari M, Fernandez-Checa JC. Cholesterol impairs the adenine nucleotide translocator-mediated mitochondrial permeability transition through altered membrane fluidity. J Biol Chem. 2003;278:33928–33935. doi: 10.1074/jbc.M210943200. [DOI] [PubMed] [Google Scholar]
- Colquhoun A. Mechanisms of action of eicosapentaenoic acid in bladder cancer cells in vitro: alterations in mitochondrial metabolism, reactive oxygen species generation and apoptosis induction. J Urol. 2009;181:1885–1893. doi: 10.1016/j.juro.2008.11.092. [DOI] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Color Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- Currie E, Schulze A, Zechner R, Walther TC, Farese RV., Jr Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eliseo D, Velotti F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J Clin Med. 2016;5 doi: 10.3390/jcm5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Wang N, Lupton JR, Carroll RJ, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClercq V, McMurray DN, Chapkin RS. Obesity promotes colonic stem cell expansion during cancer initiation. Cancer Lett. 2015;369:336–343. doi: 10.1016/j.canlet.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derler I, Jardin I, Stathopulos PB, Muik M, Fahrner M, Zayats V, Pandey SK, Poteser M, Lackner B, Absolonova M, et al. Cholesterol modulates Orai1. channel function. Sci Signal. 2016;9:ra10-ra10. doi: 10.1126/scisignal.aad7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessì S, Batetta B, Pulisci D, Spano O, Anchisi C, Tessitore L, Costelli P, Baccino FM, Aroasio E, Pani P, et al. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73:253–258. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dingeldein APG, Pokorna S, Lidman M, Sparrman T, Sachl R, Hof M, Grobner G. Apoptotic Bax at Oxidatively Stressed Mitochondrial Membranes: Lipid Dynamics and Permeabilization. Biophys J. 2017;112:2147–2158. doi: 10.1016/j.bpj.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM, Jr, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schönle A, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- van den Elsen L, Garssen J, Willemsen L. Long chain N-3 polyunsaturated fatty acids in the prevention of allergic and cardiovascular disease. Curr Pharm Des. 2012;18:2375–2392. doi: 10.2174/138161212800165960. [DOI] [PubMed] [Google Scholar]
- Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res. 2004;38:1311–1320. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- Fabian CJ, Kimler BF, Phillips TA, Box JA, Kreutzjans AL, Carlson SE, Hidaka BH, Metheny T, Zalles CM, Mills GB, et al. Modulation of Breast Cancer Risk Biomarkers by High-Dose Omega-3 Fatty Acids: Phase II Pilot Study in Premenopausal Women. Cancer Prev Res (Phila) 2015;8:912–921. doi: 10.1158/1940-6207.CAPR-14-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrmann JF, Hardman WE. Omega 3 fatty acids increase the chemo-sensitivity of B-CLL-derived cell lines EHEB and MEC-2 and of B-PLL-derived cell line JVM-2 to anti-cancer drugs doxorubicin, vincristine and fludarabine. Lipids Heal Dis. 2013;12:36. doi: 10.1186/1476-511X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YYY, Ran Q, Toyokuni S, Okazaki Y, Callaway ES, Lupton JR, Chapkin RS. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev Res. 2011;4:1267–1274. doi: 10.1158/1940-6207.CAPR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Zhan Y, Aukema HM, Davidson LA, Zhou L, Callaway E, Tian Y, Weeks BR, Lupton JR, Toyokuni S, et al. Proapoptotic effects of dietary (n-3) fatty acids are enhanced in colonocytes of manganese-dependent superoxide dismutase knockout mice. J Nutr. 2009;139:1328–1332. doi: 10.3945/jn.109.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Vaz FM, Chapkin RS. Dietary fat and fiber interactively modulate apoptosis and mitochondrial bioenergetic profiles in mouse colon in a site-specific manner. Eur J Cancer Prev. 2016 doi: 10.1097/CEJ.0000000000000263. doi:10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon KCH, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, Murray GD. Double-blind, placebo-controlled. randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–3407. doi: 10.1200/JCO.2005.04.5724. [DOI] [PubMed] [Google Scholar]
- Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012;33:740–750. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- Feng Y, Schouteden S, Geenens R, Van Duppen V, Herijgers P, Holvoet P, Van Veldhoven PP, Verfaillie CM. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PLoS One. 2012;7:e47286. doi: 10.1371/journal.pone.0047286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro C, Segre O, Fadda M, Monge T, Scigliano M, Schena M, Tinivella M, Tiozzo E, Catalano MG, Pugliese M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr. 2012;108:327–333. doi: 10.1017/S0007114511005551. [DOI] [PubMed] [Google Scholar]
- Forones NM, Falcao JB, Mattos D, Barone B. Cholesterolemia in colorectal cancer. Hepatogastroenterology. 1998;45:1531–1534. [PubMed] [Google Scholar]
- Frisz JF, Lou K, Klitzing HA, Hanafin WP, Lizunov V, Wilson RL, Carpenter KJ, Kim R, Hutcheon ID, Zimmerberg J, et al. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc Natl Acad Sci U S A. 2013;110:E613–22. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes NR, Salinas ML, Kim E, Chapkin RS. Emerging role of chemoprotective agents in the dynamic shaping of plasma membrane organization. Biochim Biophys Acta. 2017;1859:1668–1678. doi: 10.1016/j.bbamem.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Gabitova L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz LE, et al. Endogenous Sterol Metabolites Regulate Growth of EGFR/KRAS-Dependent Tumors via LXR. Cell Rep. 2015;12:1927–1938. doi: 10.1016/j.celrep.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Parajo MF, Cambi A, Torreno-Pina JA, Thompson N, Jacobson K. Nanoclustering as a dominant feature of plasma membrane organization. J Cell Sci. 2014;127:4995–5005. doi: 10.1242/jcs.146340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, Flowers CI, Garber J, Lesnikoski BA, Hwang ES, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119:137–144. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George KS, Wu S. Lipid raft: A floating island of death or survival. Toxicol Appl Pharmacol. 2012;259:311–319. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, Piantadosi S, Garrett E, Geiman DE, Hubbard W, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz JFC, Luiken JJFP. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–26. doi: 10.1016/j.biochi.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Gomes AL, Carvalho T, Serpa J, Torre C, Dias S. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 2010;115:3886–3894. doi: 10.1182/blood-2009-08-240580. [DOI] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D, Lopez-Vilaro L, Nasarre L, Perez-Olabarria M, Vazquez T, Escuin D, Badimon L, Barnadas A, Lerma E, Llorente-Cortes V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. 2015;15:460. doi: 10.1186/s12885-015-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf H, Jüngst C, Straub G, Dogan S, Hoffmann RT, Jakobs T, Reiser M, Waggershauser T, Helmberger T, Walter A, et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion. 2008;78:34–38. doi: 10.1159/000156702. [DOI] [PubMed] [Google Scholar]
- Granci V, Cai F, Lecumberri E, Clerc A, Dupertuis YM, Pichard C. Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br J Nutr. 2013;109:1188–1195. doi: 10.1017/S000711451200308X. [DOI] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science (80- ) 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffié J, Burn G, Owen DM. The Nanoscale Organization of Signaling Domains at the Plasma Membrane. Current Topics in Membranes. 2015:125–165. doi: 10.1016/bs.ctm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2015;112:2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Chavarro JE, Lee IMM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GM. Cell biology of cardiac mitochondrial phospholipids. Biochem Cell Biol. 2004;82:99–112. doi: 10.1139/o03-074. [DOI] [PubMed] [Google Scholar]
- Hedlund PO, Johansson R, Damber JE, Hagerman I, Henriksson P, Iversen P, Klarskov P, Mogensen P, Rasmussen F, Varenhorst E, et al. Significance of pretreatment cardiovascular morbidity as a risk factor during treatment with parenteral oestrogen or combined androgen deprivation of 915 patients with metastasized prostate cancer: evaluation of cardiovascular events in a randomized trial. Scand J Urol Nephrol. 2011;45:346–353. doi: 10.3109/00365599.2011.585820. [DOI] [PubMed] [Google Scholar]
- Herbst EA, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski A, Heigenhauser GJ, Spriet LL, Holloway GP. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol. 2014;592:1341–1352. doi: 10.1113/jphysiol.2013.267336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, et al. Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction: The OMEGA-REMODEL Randomized Clinical Trial. Circulation. 2016;134:378–391. doi: 10.1161/CIRCULATIONAHA.115.019949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Iwama T, Yoshinaga K, Toyooka M, Taketo MM, Sugihara K. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor on rectal polyps in familial adenomatous polyposis patients. Clin Cancer Res. 2003;9:4756–4760. [PubMed] [Google Scholar]
- Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11:306–315. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- Hofmanova J, Vaculova A, Koubkova Z, Hyzd’alova M, Kozubik A. Human fetal colon cells and colon cancer cells respond differently to butyrate and PUFAs. Mol Nutr Food Res. 2009;53(Suppl 1):S102–13. doi: 10.1002/mnfr.200800175. [DOI] [PubMed] [Google Scholar]
- Hofmanova J, Ciganek M, Slavik J, Kozubik A, Stixova L, Vaculova A, Dusek L, Machala M. Lipid alterations in human colon epithelial cells induced to differentiation and/or apoptosis by butyrate and polyunsaturated fatty acids. J Nutr Biochem. 2012;23:539–548. doi: 10.1016/j.jnutbio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Hofmanova J, Slavik J, Ovesna P, Tylichova Z, Vondracek J, Strakova N, Vaculova AH, Ciganek M, Kozubik A, Knopfova L, et al. Dietary fatty acids specifically modulate phospholipid pattern in colon cells with distinct differentiation capacities. Eur J Nutr. 2017;56:1493–1508. doi: 10.1007/s00394-016-1196-y. [DOI] [PubMed] [Google Scholar]
- Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ. Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol. 2006;57:155–164. doi: 10.1007/s00280-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- Hong MY, Bancroft LK, Turner ND, Davidson LA, Murphy ME, Carroll RJ, Chapkin RS, Lupton JR. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr Cancer. 2005;52:166–175. doi: 10.1207/s15327914nc5202_7. [DOI] [PubMed] [Google Scholar]
- Hossain Z, Konishi M, Hosokawa M, Takahashi K. Effect of polyunsaturated fatty acid-enriched phosphatidylcholine and phosphatidylserine on butyrate-induced growth inhibition, differentiation and apoptosis in Caco-2 cells. Cell Biochem Funct. 2006;24:159–165. doi: 10.1002/cbf.1202. [DOI] [PubMed] [Google Scholar]
- Hou TY, Barhoumi R, Fan YY, Rivera GM, Hannoush RN, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress CD4(+) T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamem.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TY, Davidson LA, Kim E, Fan YY, Fuentes NR, Triff K, Chapkin RS. Nutrient-Gene Interaction in Colon Cancer, from the Membrane to Cellular Physiology. Annu Rev Nutr. 2016;36:543–570. doi: 10.1146/annurev-nutr-071715-051039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniewicz-Jankowska A, Augoff K, Biernatowska A, Podkalicka J, Sikorski AF. Membrane rafts as a novel target in cancer therapy. Biochim Biophys Acta. 2014;1845:155–165. doi: 10.1016/j.bbcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Hull Ma, Sandell AC, Montgomery Aa, Logan RF, Clifford GM, Rees CJ, Loadman PM, Whitham D. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized cont. Trials. 2013;14:237. doi: 10.1186/1745-6215-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung WCC, Chen FYY, Lee CCC, Sun Y, Lee MTT, Huang HW. Membrane-thinning effect of curcumin. Biophys J. 2008;94:4331–4338. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins-Wiese HL, Picho K, Watkins BA, Li Y, Tannenbaum S, Claffey K, Kenny AM. High-dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in postmenopausal breast cancer survivors on aromatase inhibitors: a pilot study. Nutr Cancer. 2014;66:68–76. doi: 10.1080/01635581.2014.847964. [DOI] [PubMed] [Google Scholar]
- Imhoff H, von Messling V, Herrler G, Haas L. Canine distemper virus infection requires cholesterol in the viral envelope. J Virol. 2007;81:4158–4165. doi: 10.1128/JVI.02647-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolfsson HI, Koeppe RE, Andersen OS. Curcumin is a Modulator of Bilayer Material Properties†. Biochemistry. 2007;46:10384–10391. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- Irwin ME, Mueller KL, Bohin N, Ge Y, Boerner JL. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2011;226:2316–2328. doi: 10.1002/jcp.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janosi L, Li Z, Hancock JF, Gorfe AA. Organization, dynamics, and segregation of Ras nanoclusters in membrane domains. Proc Natl Acad Sci U S A. 2012;109:8097–8102. doi: 10.1073/pnas.1200773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen R, Knekt P, Hakulinen T, Rissanen H, Heliovaara M, Järvinen R, Knekt P, Hakulinen T, Rissanen H, Heliövaara M. Dietary fat, cholesterol and colorectal cancer in a prospective study. Br J Cancer. 2001;85:357–361. doi: 10.1054/bjoc.2001.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay AG, Hamilton JA. The enigmatic membrane fatty acid transporter CD36: New insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot Essent Fat Acids. 2016 doi: 10.1016/j.plefa.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Jordan A, Stein J. Effect of an omega-3 fatty acid containing lipid emulsion alone and in combination with 5-fluorouracil (5-FU) on growth of the colon cancer cell line Caco-2. Eur J Nutr. 2003;42:324–331. doi: 10.1007/s00394-003-0427-1. [DOI] [PubMed] [Google Scholar]
- Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277:8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504–504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–2022. [PubMed] [Google Scholar]
- Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y, et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886–891. doi: 10.1054/bjoc.2000.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Davidson LA, Zoh RS, Hensel ME, Salinas ML, Patil BS, Jayaprakasha GK, Callaway ES, Allred CD, Turner ND, et al. Rapidly cycling Lgr5+ stem cells are exquisitely sensitive to extrinsic dietary factors that modulate colon cancer risk. Cell Death Dis. 2016;7:e2460. doi: 10.1038/cddis.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Maxwell K, Hajjar DP, Berliner JA. Beta-VLDL increases endothelial cell plasma membrane cholesterol. J Lipid Res. 1991;32:1125–1131. [PubMed] [Google Scholar]
- Kim W, Fan YYY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010;49:250–261. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Barhoumi R, McMurray DN, Chapkin RS. Dietary fish oil and DHA down-regulate antigen-activated CD4+ T-cells while promoting the formation of liquid-ordered mesodomains. Br J Nutr. 2014;111:254–260. doi: 10.1017/S0007114513002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klek S. Omega-3 Fatty Acids in Modern Parenteral Nutrition. A Review of the Current Evidence. J Clin Med. 2016;5:34. doi: 10.3390/jcm5030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar S, Barhoumi R, Jones CK, Wesley J, Lupton JR, Fan YY, Chapkin RS. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca(2)(+) loading and apoptosis in colonocytes. Cancer. 2011;117:5294–5303. doi: 10.1002/cncr.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar SS, Barhoumi R, Callaway ES, Fan YY, Wang N, Lupton JR, Chapkin RS. Synergy between docosahexaenoic acid and butyrate elicits p53-independent apoptosis via mitochondrial Ca(2+) accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G935–43. doi: 10.1152/ajpgi.00312.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft ML. Plasma membrane organization and function: moving past lipid rafts. Mol Biol Cell. 2013;24:2765–2768. doi: 10.1091/mbc.E13-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- Lakha F, Theodoratou E, Farrington SM, Tenesa A, Cetnarskyj R, Din FVN, Porteous ME, Dunlop MG, Campbell H. Statin use and association with colorectal cancer survival and risk: case control study with prescription data linkage. BMC Cancer. 2012;12:487. doi: 10.1186/1471-2407-12-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JK, Cimato TR. Cholesterol and hematopoietic stem cells: inflammatory mediators of atherosclerosis. Stem Cells Transl Med. 2014;3:549–552. doi: 10.5966/sctm.2013-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y, Swaisgood MH, Ramos BV, Steck TL. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem. 1989;264:3786–3793. [PubMed] [Google Scholar]
- Lee J, Jung KH, Park YS, Ahn JB, Shin SJ, Im SA, Oh DY, Shin DB, Kim TW, Lee N, et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: a multicenter phase II study. Cancer Chemother Pharmacol. 2009;64:657–663. doi: 10.1007/s00280-008-0913-5. [DOI] [PubMed] [Google Scholar]
- Levental I, Veatch SL. The Continuing Mystery of Lipid Rafts. J Mol Biol. 2016;428:4749–4764. doi: 10.1016/j.jmb.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Lorent JH, Lin X, Skinkle AD, Surma MA, Stockenbojer EA, Gorfe AA, Levental I. Polyunsaturated Lipids Regulate Membrane Domain Stability by Tuning Membrane Order. Biophys J. 2016;110:1800–1810. doi: 10.1016/j.bpj.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Qin J, Tian C, Cao J, Fida G, Wang Z, Chen H, Qian Z, Chen WR, Gu Y. The targeting mechanism of DHA ligand and its conjugate with Gemcitabine for the enhanced tumor therapy. Oncotarget. 2014;5:3622–3635. doi: 10.18632/oncotarget.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang X, Cao D. Nanoparticle hardness controls the internalization pathway for drug delivery. Nanoscale. 2015;7:2758–2769. doi: 10.1039/c4nr05575f. [DOI] [PubMed] [Google Scholar]
- Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1105–1107. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien EL. Toxicology and safety of DHA. Prostaglandins Leukot Essent Fat Acids. 2009;81:125–132. doi: 10.1016/j.plefa.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- Lim SJ, Lee E, Lee EH, Kim SY, Cha JH, Choi H, Park W, Choi HK, Ko SH, Kim SH. Docosahexaenoic acid sensitizes colon cancer cells to sulindac sulfide-induced apoptosis. Oncol Rep. 2012;27:2023–2030. doi: 10.3892/or.2012.1706. [DOI] [PubMed] [Google Scholar]
- Lin X, Lorent JH, Skinkle AD, Levental KR, Waxham MN, Gorfe AA, Levental I. Domain Stability in Biomimetic Membranes Driven by Lipid Polyunsaturation. J Phys Chem B. 2016;120:11930–11941. doi: 10.1021/acs.jpcb.6b06815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science (80- ) 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- López-Aguilar E, Sepúlveda-Vildósola AC, Betanzos-Cabrera Y, Rocha-Moreno YG, Gascón-Lastiri G, Rivera-Márquez H, Wanzke-del-Angel V, Cerecedo-Díaz F, de la Cruz-Yañez H. Phase II Study of Metronomic Chemotherapy with Thalidomide, Carboplatin-Vincristine-Fluvastatin in the Treatment of Brain Stem Tumors in Children. Arch Med Res. 2008;39:655–662. doi: 10.1016/j.arcmed.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Lynch PM, Ayers GD, Hawk E, Richmond E, Eagle C, Woloj M, Church J, Hasson H, Patterson S, Half E, et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol. 2010;105:1437–1443. doi: 10.1038/ajg.2009.758. [DOI] [PubMed] [Google Scholar]
- Ma DWL, Seo J, Davidson La, Callaway ES, Fan YYY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- Manni A, El-Bayoumy K, Skibinski CG, Thompson HJ, Santucci-Pereira J, Bidinotto LT, Russo J. Combination of Antiestrogens and Omega-3 Fatty Acids for Breast Cancer Prevention. Biomed Res Int. 2015;2015:638645. doi: 10.1155/2015/638645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- McMurray DN, Bonilla DL, Chapkin RS. n-3 Fatty acids uniquely affect antimicrobial resistance and immune cell plasma membrane organization. Chem Phys Lipids. 2011;164:626–635. doi: 10.1016/j.chemphyslip.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medes G, Thomas A, Weinhouse S. Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res. 1953;13:27–29. [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia EM, Nguyen H, Hatch GM. Mammalian cardiolipin biosynthesis. Chem Phys Lipids. 2014;179:11–16. doi: 10.1016/j.chemphyslip.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Merendino N, Costantini L, Manzi L, Molinari R, D’Eliseo D, Velotti F. Dietary omega -3 polyunsaturated fatty acid DHA: a potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. doi: 10.1155/2013/310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero J, Morales A, Llacuna L, Lluis JM, Terrones O, Basanez G, Antonsson B, Prieto J, Garcia-Ruiz C, Colell A, et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68:5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- Montero J, Mari M, Colell A, Morales A, Basanez G, Garcia-Ruiz C, Fernandez-Checa JC. Cholesterol and peroxidized cardiolipin in mitochondrial membrane properties, permeabilization and cell death. Biochim Biophys Acta. 2010;1797:1217–1224. doi: 10.1016/j.bbabio.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland SL, Martins KJB, Mazurak VC. n-3 polyunsaturated fatty acid supplementation during cancer chemotherapy. J Nutr Intermed Metab. 2016;5:107–116. [Google Scholar]
- Murphy RA, Mourtzakis M, Chu QSC, Baracos VE, Reiman T, Mazurak VC. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer. 2011;117:3774–3780. doi: 10.1002/cncr.25933. [DOI] [PubMed] [Google Scholar]
- Negi AK, Bhatnagar A, Agnihotri N. Fish oil augments celecoxib mediated alteration in apoptotic pathway in the initiation phase of 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis. Biomed Pharmacother. 2016;79:9–16. doi: 10.1016/j.biopha.2016.01.032. [DOI] [PubMed] [Google Scholar]
- Ng K, Meyerhardt JA, Chan AT, Sato K, Chan JA, Niedzwiecki D, Saltz LB, Mayer RJ, Benson AB, Schaefer PL, et al. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst. 2015;107:345. doi: 10.1093/jnci/dju345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, Lupton JR, Chapkin RS. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. Systems biology of lipid metabolism: from yeast to human. FEBS Lett. 2009;583:3905–3913. doi: 10.1016/j.febslet.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- Nussinov R, Jang H, Tsai CJ. Oligomerization and nanocluster organization render specificity. Biol Rev Camb Philos Soc. 2015;90:587–598. doi: 10.1111/brv.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookhtens M, Kannan R, Lyon I, Baker N. Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol. 1984;247:R146–53. doi: 10.1152/ajpregu.1984.247.1.R146. [DOI] [PubMed] [Google Scholar]
- Owen DM, Rentero C, Magenau A, Abu-Siniyeh A, Gaus K. Quantitative imaging of membrane lipid order in cells and organisms. Nat Protoc. 2012;7:24–35. doi: 10.1038/nprot.2011.419. [DOI] [PubMed] [Google Scholar]
- Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Pelliccia F, Marazzi G, Greco C, Franzoni F, Speziale G, Gaudio C. Current evidence and future perspectives on n-3 PUFAs. Int J Cardiol. 2013;170:S3–7. doi: 10.1016/j.ijcard.2013.06.044. [DOI] [PubMed] [Google Scholar]
- Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Pitroda SP, Khodarev NN, Beckett MA, Kufe DW, Weichselbaum RR. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci U S A. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter JN, Gruber SB, Higgins PDR, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the Risk of Colorectal Cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- Qiu B, Ackerman D, Sanchez DJ, Li B, Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B, Simon MC. HIF2alpha-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015;5:652–667. doi: 10.1158/2159-8290.CD-14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V, Vijayaraghavalu S, Peetla C, Yamada M, Morisada M, Labhasetwar V. Sustained Epigenetic Drug Delivery Depletes Cholesterol-Sphingomyelin Rafts from Resistant Breast Cancer Cells, Influencing Biophysical Characteristics of Membrane Lipids. Langmuir. 2015;31:11564–11573. doi: 10.1021/acs.langmuir.5b02601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani I, Sharma B, Kumar S, Kaur S, Agnihotri N. Apoptosis mediated chemosensitization of tumor cells to 5-fluorouracil on supplementation of fish oil in experimental colon carcinoma. Tumour Biol. 2017;39:1010428317695019. doi: 10.1177/1010428317695019. [DOI] [PubMed] [Google Scholar]
- Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann M, Kaina B, et al. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865–7874. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- Rizos EC, Elisaf MS. Current evidence and future perspectives of omega-3 polyunsaturated fatty acids for the prevention of cardiovascular disease. Eur J Pharmacol. 2013;706:1–3. doi: 10.1016/j.ejphar.2013.02.050. [DOI] [PubMed] [Google Scholar]
- Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–685. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- Sahl SJ, Leutenegger M, Hell SW, Eggeling C. High-Resolution Tracking of Single-Molecule Diffusion in Membranes by Confocalized and Spatially Differentiated Fluorescence Photon Stream Recording. ChemPhysChem. 2014;15:771–783. doi: 10.1002/cphc.201301090. [DOI] [PubMed] [Google Scholar]
- Salloum D, Mukhopadhyay S, Tung K, Polonetskaya A, Foster DA. Mutant ras elevates dependence on serum lipids and creates a synthetic lethality for rapamycin. Mol Cancer Ther. 2014;13:733–741. doi: 10.1158/1535-7163.MCT-13-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Wang N, Spinka CM, Carroll RJ, Turner ND, Chapkin RS, et al. An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J Nutr. 2004;134:3233–3238. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- Saw CLL, Huang Y, Kong ANN. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem Pharmacol. 2010;79:421–430. doi: 10.1016/j.bcp.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Schlame M, Greenberg ML. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim Biophys Acta - Mol Cell Biol Lipids. 2017;1862:3–7. doi: 10.1016/j.bbalip.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidmaier R, Baumann P, Bumeder I, Meinhardt G, Straka C, Emmerich B. First clinical experience with simvastatin to overcome drug resistance in refractory multiple myeloma. Eur J Haematol. 2007;79:240–243. doi: 10.1111/j.1600-0609.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- Scott CC, Vossio S, Vacca F, Snijder B, Larios J, Schaad O, Guex N, Kuznetsov D, Martin O, Chambon M, et al. Wnt directs the endosomal flux of LDL-derived cholesterol and lipid droplet homeostasis. EMBO Rep. 2015;16:741–752. doi: 10.15252/embr.201540081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- Serini S, Fasano E, Celleno L, Cittadini A, Calviello G. Potential of long-chain n-3 polyunsaturated fatty acids in melanoma prevention. Nutr Rev. 2014;72:255–266. doi: 10.1111/nure.12093. [DOI] [PubMed] [Google Scholar]
- Sezgin E. Super-resolution optical microscopy for studying membrane structure and dynamics. J Phys Condens Matter. 2017;29:273001. doi: 10.1088/1361-648X/aa7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E, Kaiser HJJ, Baumgart T, Schwille P, Simons K, Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 2012;7:1042–1051. doi: 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Sadowski T, Simons K. Measuring Lipid Packing of Model and Cellular Membranes with Environment Sensitive Probes. Langmuir. 2014;30:8160–8166. doi: 10.1021/la501226v. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MS, Kim E, Davidson LA, Knight JM, Zoh RS, Goldsby JS, Callaway ES, Zhou B, Ivanov I, Chapkin RS. Comparative effects of diet and carcinogen on microRNA expression in the stem cell niche of the mouse colonic crypt. Biochim Biophys Acta. 2016;1862:121–134. doi: 10.1016/j.bbadis.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh SR, Kinnun JJ, Leng X, Williams Ja, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta. 2015;1848:211–219. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–325. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- Sharma G, Rani I, Bhatnagar A, Agnihotri N. Apoptosis-Mediated Chemoprevention by Different Ratios of Fish Oil in Experimental Colon Carcinogenesis. Cancer Invest. 2016;34:220–230. doi: 10.1080/07357907.2016.1183023. [DOI] [PubMed] [Google Scholar]
- Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian W, Cui Y, Choi JC, Doh J, Han JK, et al. Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling. Nat Commun. 2014;5:4393. doi: 10.1038/ncomms5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui RA, Harvey KA, Walker C, Altenburg J, Xu Z, Terry C, Camarillo I, Jones-Hall Y, Mariash C. Characterization of synergistic anti-cancer effects of docosahexaenoic acid and curcumin on DMBA-induced mammary tumorigenesis in mice. BMC Cancer. 2013;13:418. doi: 10.1186/1471-2407-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvente-Poirot S, Poirot M. Cancer. Cholesterol and cancer, in the balance. Science (80- ) 2014;343:1445–1446. doi: 10.1126/science.1252787. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Singh P, Saxena R, Srinivas G, Pande G, Chattopadhyay A. Cholesterol biosynthesis and homeostasis in regulation of the cell cycle. PLoS One. 2013;8:e58833. doi: 10.1371/journal.pone.0058833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarke C, Alamuddin N, Lawson JA, Ferguson JF, Reilly MP, FitzGerald GA. Bioactive products formed in humans from fish oils. J Lipid Res. 2015;56:1808–1820. doi: 10.1194/jlr.M060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaninova V, Krafcikova M, Perez-Gomez R, Steffal P, Trantirek L, Bray SJ, Krejci A. Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open Biol. 2016;6:150155. doi: 10.1098/rsob.150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:580–590. doi: 10.1016/j.celrep.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio RE, Breslow JL. Intracellular cholesterol transport. Arter Thromb Vasc Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- Song M, Zhang X, Meyerhardt JA, Giovannucci EL, Ogino S, Fuchs CS, Chan AT. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut. 2016 doi: 10.1136/gutjnl-2016-311990. gutjnl-2016-311990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen LS, Rasmussen HH, Aardestrup IV, Thorlacius-Ussing O, Lindorff-Larsen K, Schmidt EB, Calder PC. Rapid incorporation of omega-3 fatty acids into colonic tissue after oral supplementation in patients with colorectal cancer: a randomized, placebo-controlled intervention trial. JPEN J Parenter Enter Nutr. 2014;38:617–624. doi: 10.1177/0148607113491782. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2012;15:122–126. doi: 10.1097/MCO.0b013e32834fdaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Stillwell W, Wassall SR. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- Taneja N, Tjalkens R, Philbert MA, Rehemtulla A. Irradiation of mitochondria initiates apoptosis in a cell free system. Oncogene. 2001;20:167–177. doi: 10.1038/sj.onc.1204054. [DOI] [PubMed] [Google Scholar]
- Thota RN, Acharya SH, Abbott KA, Garg ML. Curcumin and long-chain Omega-3 polyunsaturated fatty acids for Prevention of type 2 Diabetes (COP-D): study protocol for a randomised controlled trial. Trials. 2016;17:565. doi: 10.1186/s13063-016-1702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting HC, Chao YJ, Hsu YH. Polyunsaturated fatty acids incorporation into cardiolipin in H9c2 cardiac myoblast. J Nutr Biochem. 2015;26:769–775. doi: 10.1016/j.jnutbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS One. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Domingues RM, Tyurin VA, Maciel E, Domingues P, Amoscato AA, Bayir H, Kagan VE. Characterization of cardiolipins and their oxidation products by LC–MS analysis. Chem Phys Lipids. 2014;179:3–10. doi: 10.1016/j.chemphyslip.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland PM, Refsum H, Male R, Lillehaug JR. Disposition of endogenous homocysteine by mouse fibroblast C3H/10T1/2 Cl 8 and the chemically transformed C3H/10T1/2 MCA Cl 16 cells following methotrexate exposure. J Natl Cancer Inst. 1986;77:283–289. [PubMed] [Google Scholar]
- Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, Ford JR, Braby LA, Chapkin RS, Turner ND, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan A, Yu Y, Banerjee S, Woods J, Farhana L, Rajendra SG, Patel A, Dyson G, Levi E, Maddipati KR, et al. Omega-3 fatty acid is a potential preventive agent for recurrent colon cancer. Cancer Prev Res. 2014;7:1138–1148. doi: 10.1158/1940-6207.CAPR-14-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan VC, Hassing MR, Lewandowski PA. Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer. 2013;108:486–492. doi: 10.1038/bjc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten SV, Jaggers GK, Fraga CG, Oteiza PI. Procyanidins can interact with Caco-2 cell membrane lipid rafts: involvement of cholesterol. Biochim Biophys Acta. 2013;1828:2646–2653. doi: 10.1016/j.bbamem.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Verstraeten SV, Fraga CG, Oteiza PI. Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance. Food Funct. 2015;6:32–41. doi: 10.1039/c4fo00647j. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li P, Xuan J, Zhu C, Liu J, Shan L, Du Q, Ren Y, Ye J. Cholesterol Enhances Colorectal Cancer Progression via ROS Elevation and MAPK Signaling Pathway Activation. Cell Physiol Biochem. 2017a;42:729–742. doi: 10.1159/000477890. [DOI] [PubMed] [Google Scholar]
- Wang C, Yu Y, Regen SL. Lipid Raft Formation: Key Role of Polyunsaturated Phospholipids. Angew Chemie Int Ed. 2017b;56:1639–1642. doi: 10.1002/anie.201611367. [DOI] [PubMed] [Google Scholar]
- Wang J, Luo T, Li S, Zhao J. The powerful applications of polyunsaturated fatty acids in improving the therapeutic efficacy of anticancer drugs. Expert Opin Drug Deliv. 2012;9:1–7. doi: 10.1517/17425247.2011.618183. [DOI] [PubMed] [Google Scholar]
- Wang YY, Gao J, Guo X, Tong T, Shi X, Li L, Qi M, Wang YY, Cai M, Jiang J, et al. Regulation of EGFR nanocluster formation by ionic protein-lipid interaction. Cell Res. 2014;24:959–976. doi: 10.1038/cr.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res. 1998;39:1583–1588. [PubMed] [Google Scholar]
- Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Wang W, Chen J, Yang D, Yan R, Cai Q. A prospective, randomized, controlled study of omega-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumors. Nutr J. 2014;13:25. doi: 10.1186/1475-2891-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NJ, Clark SK, Phillips RKS, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, Wassall SR. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93. intervention studies. Am J Clin Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- Wu S, Guo Y, Wu Y, Zhu S, He Z, Chen YQ. Omega-3 free fatty acids inhibit tamoxifen-induced cell apoptosis. Biochem Biophys Res Commun. 2015;459:294–299. doi: 10.1016/j.bbrc.2015.02.103. [DOI] [PubMed] [Google Scholar]
- Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chaerkady R, Beer MA, Mendell JT, Pandey A. Identification of miR-21 targets in breast cancer cells using a quantitative proteomic approach. Proteomics. 2009;9:1374–1384. doi: 10.1002/pmic.200800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Yi JS, Mun DG, Lee H, Park JS, Lee JW, Lee JS, Kim SJ, Cho BR, Lee SW, Ko YG. PTRF/cavin-1 is essential for multidrug resistance in cancer cells. J Proteome Res. 2013;12:605–614. doi: 10.1021/pr300651m. [DOI] [PubMed] [Google Scholar]
- Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel A, Wilczok A, Latocha M, Tarkowski M, Kokocinska M, Dzierzewicz Z. Polyunsaturated fatty acids potentiate cytotoxicity of cisplatin in A549 cells. Acta Pol Pharm. 2014;71:1060–1065. [PubMed] [Google Scholar]
- Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Harder T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–476. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hancock JF. Ras nanoclusters: Versatile lipid-based signaling platforms. Biochim Biophys Acta. 2015;1853:841–849. doi: 10.1016/j.bbamcr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cho KJ, Plowman SJ, Hancock JF. Nonsteroidal anti-inflammatory drugs alter the spatiotemporal organization of Ras proteins on the plasma membrane. J Biol Chem. 2012;287:16586–16595. doi: 10.1074/jbc.M112.348490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Z, Zhang L, Mu Q, Lou Y, Gong Z, Shi Y, Ouyang G, Zhang Y. The effect of combination treatment with docosahexaenoic acid and 5-fluorouracil on the mRNA expression of apoptosis-related genes, including the novel gene BCL2L12, in gastric cancer cells. Vitr Cell Dev Biol Anim. 2009;45:69–74. doi: 10.1007/s11626-008-9154-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.