Summary
The efficiency of treatment of human infections with the unicellular eukaryotic pathogens such as fungi and protozoa remains deeply unsatisfactory. For example, the mortality rates from nosocomial fungemia in critically ill, immunosuppressed or post cancer patients often exceed 50%. A set of six systemic clinical azoles (sterol 14α-demethylase (CYP51) inhibitors) represents the first-line antifungal treatment. All these drugs were discovered empirically, by monitoring their effects on fungal cell growth, though it had been proven that they kill fungal cells by blocking biosynthesis of ergosterol in fungi at the stage of 14α-demethylation of the sterol nucleus. This review briefs the history of antifungal azoles, outlines the situation with the current clinical azole-based drugs, describes the attempts of their repurposing for treatment of human infections with the protozoan parasites that, similar to fungi, also produce endogenous sterols, and discusses the most recently acquired knowledge on the CYP51 structure/function and inhibition. It is our belief that this information should be helpful in shifting from the traditional phenotypic screening to the actual target-driven drug discovery paradigm, which will rationalize and substantially accelerate the development of new, more efficient and pathogen-oriented CYP51 inhibitors.
Keywords: Trypanosoma cruzi, Trypanosoma brucei, Leishmania, amoeba, sterol biosynthesis, antifungal azoles, sterol 14α-demethylase, CYP51, inhibition, crystal structure
INTRODUCTION
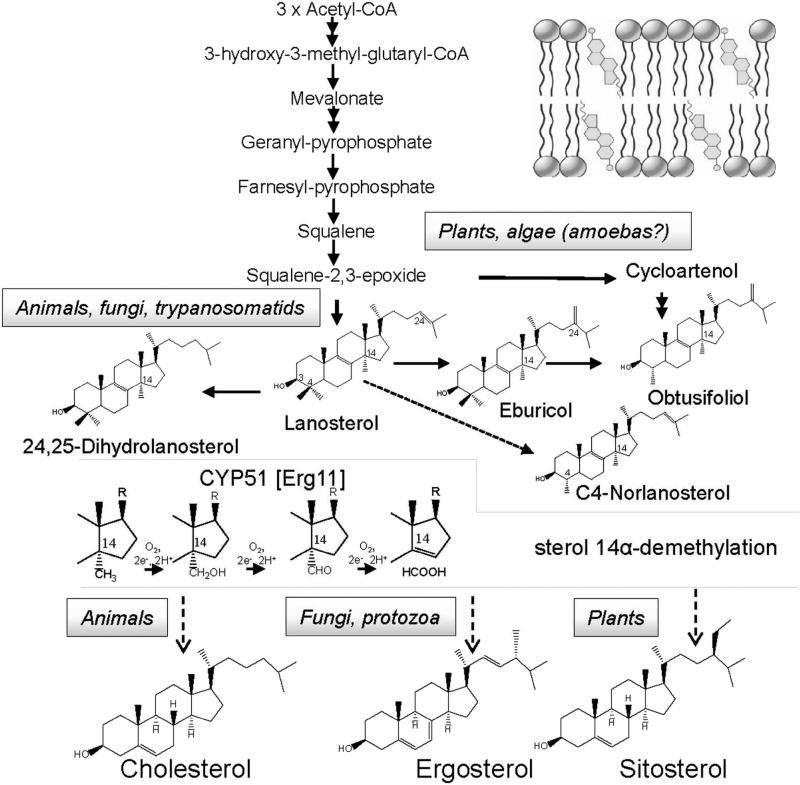
CYP51 (sterol 14α-demethylases) are the most conserved cytochrome P450 enzymes (Lepesheva & Waterman, 2004) across phylogeny that catalyze one, essentially the same stereoselective three step reaction of the oxidative removal of the 14α-methyl group from one or more of the five naturally occurring cyclized sterol precursors, lanosterol, 24,25-dihydrolanosterol, eburicol, obtusifoliol, and C4-norlanosterol (Fig. 1). In eukaryotes, the reaction occurs in the endoplasmic reticulum and is required for biosynthesis of sterols, which serve as essential components of plasma membranes (bulky sterols, cholesterol in humans, ergosterol in fungi) and also as precursors for regulatory molecules that modulate growth, division, differentiation, and development processes (sparking sterols) (Lepesheva & Waterman, 2007; Nes, 2011). Fast sterol production is most crucial for rapidly multiplying cells. It is also important to bear in mind that while humans can consume cholesterol from the diet, blocking of ergosterol production in unicellular human pathogens is lethal.
Figure 1. CYP51 reaction is an essential step upon sterol biosynthesis.
The pathway involves multiple (>30) steps, beginning with the condensation of acetyl-CoA molecules that serve as initial building blocks, and proceeds to squalene, which then forms epoxide and cyclizes into the triterpene sterol skeleton (cycloartenol or lanosterol). These precursors are further modified to produce cholesterol, ergosterol, or sitosterol, which are the major membrane sterols in humans, fungi/protozoa, and plants, respectively. The CYP51 reaction occurs either immediately or soon after squalene cyclization. The 14α-methyl group of the substrate (lanosterol, 24,25-dihydrolanosterol, eburicol, obtusifoliol, and/or C4-norlanosterol) is converted into the alcohol, then into the aldehyde derivative and finally is removed as formic acid with the introduction of the Δ14–15 double bond into the sterol core. The CYP51 reaction includes three consecutive cytochrome P450 catalytic cycles, consuming three molecules of oxygen, 6 electrons and 6 protons. Detailed description of other reactions of the pathway can be found in Nes, 2011. Inset: Sterol molecules are incorporated into membranes with the 3β-OH facing the water interface and the side chain extending into the hydrophobic core to interact with fatty acyl chains of phospholipids and proteins.
Although sterol biosynthesis involves multiple steps, so far only two of them have become the major targets for systemic clinical drugs. Statins (cholesterol lowering agents), which act upstream of the pathway, at the step of mevalonate production, are presently the most frequently prescribed medications (Superko et al., 2012), while azoles, inhibitors of sterol 14α-demethylase, serve as the most widely used antifungals (Denning & Bromley, 2015; Lass-Flörl, 2011) and are under investigation to be repurposed for treatment of human infections with protozoan parasites (Buckner & Urbina, 2012).
A BRIEFING ON THE HISTORY
Chronologically, the use of antifungal azoles was begun long before the mechanism of their action was elucidated. All the drugs were discovered via phenotypic screening, the first of them entering the market more than half a century ago (chlormidazole - 1954; clotrimazole −1958; miconazole −1971 [Janssen Pharmaceutica] (Heeres et al., 2010; Sheehan et al., 1999)). Initially, the azole drugs were mostly applied topically (Sawyer PR, 1975b; Sawyer PR, 1975a), with ketoconazole - 1977 [Janssen Pharmaceutica] becoming the first oral systemic antifungal (Graybill & Craven, 1983).
In 1978, it was reported that azoles kill fungal cells because of the damage to the fungal cell membranes that occurs “as a result of depletion of the major fungal sterol ergosterol” (Van den Bossche et al., 1978). In 1980, it was shown that ergosterol depletion “coincides with the increase of the content of C14-methylated sterol precursors and has to be attributed to an interference with one of the reactions involved in the removal of the 14α-methyl group of lanosterol” (Van den Bossche et al, 1980). By that time it was already observed that in artificial membranes incorporation of lanosterol instead of cholesterol substantially affects the membrane fluidity and permeability (Yeagle et al., 1977).
The fact that lanosterol 14α-demethylation occurs in the microsomal fraction, requires NADPH and molecular oxygen, and is inhibited by CO suggested that “the process must be cytochrome P450 dependent” (Mitropoulos et al., 1976 ; Ohba et al., 1978). This was proven experimentally, when a specific cytochrome P450 enzyme (then named P45014DM) was purified from Saccharomyces cerevisiae (Yoshida & Aoyama, 1984) and shown to catalyze all the three steps of the reaction: the 14α-methyl group hydroxylation, the oxidation of the 14α-methylalcohol to the 14α-methylaldehyde and then elimination of the aldehyde as formic acid with the introduction of the C14-C15 double bond into the sterol core (Aoyama et al., 1984). Moreover, it was noticed that “the activity of yeast P45014DM is 100% inhibited by ketoconazole at a concentration equal to that of the enzyme” (Yoshida & Aoyama, 1987), a feature quite unusual for competitive inhibition.
Later, the orthologous enzyme catalyzing the same reaction was purified from rat hepatic microsomes (Trzaskos et al., 1986). For some time mammalian P45014DM was considered as a potential target for cholesterol-lowering drugs (Frye & Leonard, 1999), but strong inhibitors have never been found. Moreover, it was observed that mammalian cells are much less sensitive to azoles than fungal cells (Van den Bossche, 1988 ; Vanden Bossche, 1985; Vanden Bossche et al., 1987).
WHY THE ENZYME IS CALLED CYP51
Because the first sterol 14α-demethylase protein (Aoyama et al., 1984) and the first sterol 14α-demethylase encoding gene (Kalb et al., 1986) were isolated from yeast, all the catalytically orthologous P450s discovered subsequently were placed into one family and named CYP51, the number that in the updated cytochrome P450 (CYP) nomenclature was initially reserved for fungal sequences (Nelson et al., 1993). The CYP51 family now joins members from all biological kingdoms, including >400 bacteria (actinobacteria and proteobacteria), and this enzyme is regarded as a possible evolutionarily ancestor for all other currently existing cytochromes P450 (Nelson, 1999; Yoshida et al., 2000). Since the number of the CYP51 family members is indefinitely large and there are organisms that have more than one CYP51 gene (e.g. CYP51A and CYP51B in some fungal species (Hargrove et al., 2015; Mellado et al., 2001)) or in T. cruzi strains (Cherkesova et al., 2014), or multiple CYP51 genes in some plants (Lepesheva & Waterman, 2007), attempts to add more labels to the CYP51 name appear to be unreasonable and confusing (e.g. sometimes human CYP51 is called “CYP51A1”, although in fact it is the B-type sterol 14α-demethylase (Hargrove et al., 2016)).
CYP51–TARGETING ANTIFUNGALS
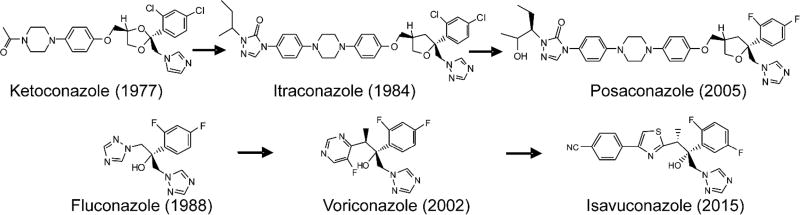
CYP51 inhibitors are broadly used as fungicides in agriculture to protect crops. Amongst them are imidazoles (e.g. imazalil, since 1973 (Janssen)), pyrimidines (fenarimol, since 1971 (Eli Lilly)) and triazoles (triadimefon, since 1973 (Bayer)) (Morton & Staub, 2008). Topical antifungal drugs that are applied in medicine and veterinary to treat localized infections of the skin, hair, or nails (e.g. athlete’s foot, jock itch, ringworm) are predominantly imidazoles (miconazole, clotrimazole, tioconazole, econazole, butaconazole, etc.), with terconazole (1983) and efinaconazole (2013) representing two triazole-based topical antifungals (Patel & Dhillon, 2013; Sheehan et al., 1999). On the contrary, the clinical antifungal azoles that are available for systemic use, except for ketoconazole, are all triazoles (Fig. 2) as it has been proposed that the triazole ring might be generally more advantageous over the imidazole ring of ketoconazole, both in terms of metabolic stability and weaker inhibition of other human cytochromes P450 (Dalvie et al., 2002).
Figure 2. Clinical antifungal azoles used for treatment of systemic human infections.
The whole set is represented by six derivatives of two basic scaffolds, fluconazole and ketoconazole. Ketoconazole is an imidazole, the others are 1,2,4-triazoles.
CLINICAL AZOLE ANTIFUNGALS OF SYSTEMIC USE
Due to some advanced life-saving medical techniques and the increase in the number of immunocompromised patients (both HIV-infected and those who are on steroids, recovering after anticancer chemotherapy or undergoing solid organ and bone marrow transportation), the incidence of systemic (invasive) fungal infections in the past decades has been increasing sharply (De Pascale & Tumbarello, 2015; Gullo, 2009; Kauffman et al., 2007), resulting in global mortality from fungal infections of 1.5 to 2 million people per year, far more than are killed by malaria or tuberculosis (Denning & Bromley, 2015). Yet the number of clinical antifungals available for systemic use remains astonishingly limited. Of the three types of drugs, amphotericin B, a macrolide that removes ergosterol from fungal membrane, is highly toxic, and echinocandins that damage the fungal cell wall have a more limited spectrum of activity, both of them lacking oral formulations (Andes, 2013; Pappas et al., 2016). Therefore, the six clinical azoles presented in Fig. 2 remain the most widely used antifungal medicines.
Ketoconazole (1977)
Ketoconazole (Fig. 2, MW 531, log P 3.54) was developed at Janssen Pharmaceutica as a derivative of miconazole and has served as an oral systemic drug for 40 years (Borelli et al., 1979; Heel et al., 1982; Heeres et al., 2010). The daily dosage is 200 to 400 mg. Ketoconazole displays a broad spectrum of antifungal activity, good oral bioavailability, and is in general well tolerated. Moreover, over the years of use it was reported to be beneficial in anticancer chemotherapy (Lopez-Barcons et al., 2017; Trachtenberg et al., 1983; Trachtenberg & Pont, 1984) or therapy of organ transplant recipients receiving cyclosporine immunosuppression (Carbajal et al., 2004; Chapman et al., 1996). The downside of ketoconazole is a short lifetime in human blood, because the drug is rapidly metabolized in the liver (Dalvie et al., 2002), where it interacts with many drug-metabolizing cytochromes P450, CYP3A4 in particular (Mosca et al., 1985; Zhang et al., 2002). Thus, it was reported that at a single oral dose of 200 mg maximal plasma concentration of ketoconazole (12 µM) was reached in 1 hour, yet in 12 hours only 0.1 µM (less than 1%) of the drug was detectable in the circulation (Huang et al., 1986). The corresponding pharmacokinetic (PK) values reported in mice are: a single oral dose of 40 mg/kg produced maximal plasma concentration of 25 µM 1 hour after administration, the drug being cleared from the circulation within 8 hours (Borelli et al., 1979). Because of the danger of drug-drug interactions, serious hepatotoxicity and the risk of adrenal dysfunction ketoconazole is no longer used for long-term therapy, its systemic use being limited to the cases when patients do not respond to or do not tolerate alternative medications https://www.uptodate.com/contents/pharmacology-of-azoles.
Itraconazole (1984)
Itraconazole (Fig. 2, MW 704, log P 6.77) is the 1,2,4-triazole analog of ketoconazole. The drug was also developed at Janssen Pharmaceutica and became the first triazole-based oral systemic antifungal. The daily dosage is 200 to 400 mg, duration of therapy up to 12 months. Compared to ketoconazole, itraconazole has a wider spectrum of antifungal activity, e.g. it is more efficient in treatment of aspergillosis (Jennings & Hardin, 1993; Tracy et al., 2016). It displays a higher lipophilicity and a lack of endocrine-related side effects (weaker inhibition of human steroidogenic P450s) (Hardin et al., 1988). As a result of its lipophilicity itraconazole has a high affinity to tissues, where its concentration can be a few times higher than in plasma (Heykants et al., 1990; Sheehan et al., 1999), including the brain, though its penetration into the cerebrospinal fluid is very limited (Felton et al., 2014). After a single oral dose of 200 mg peak plasma levels of itraconazole in humans are reached in 3 hours (~0.4 µM), the concentration is low, but steady, the clearance time exceeding 20–30 hours (0.01 µM was reported detectable on day 3 (Hardin et al., 1988)). The corresponding PK values in mice are: a single oral dose of 20 mg/kg produced maximal plasma concentration of 3 µM 1 hour after administration and the drug was still detectable after 8 hours (0.08 µM) (Ishibashi et al., 2007). Absorption of itraconazole can be enhanced by intake of food. It has been reported that after two weeks of therapy combined with the high-fat diet, itraconazole exhibits steady-state kinetics, the average concentration in human blood reaching up to 8 µM, although high range of variability between individual samples was observed (Buchkowsky et al., 2005; Denning et al., 1989). Similar to ketoconazole, itraconazole is extensively metabolized in the liver, predominantly by CYP3A4, and metabolites are excreted into the urine and bile (Bruggemann et al., 2009). Itraconazole is considered safer than ketoconazole, but still can cause different adverse reactions including, gastrointestinal (>10% patients), cardiovascular, dermatologic, hepatic, central nervous system (1–10%) (Tucker et al., 1990). In general, however, the symptoms are mild and can be readily assessed by monitoring the patient clinically (Buchkowsky et al., 2005).
Posaconazole (2005)
Posaconazole (Fig. 2, MW 701, log P 5.44) is the itraconazole derivative whose lipophilicity was decreased by the replacement of the two Cl atoms (2,4) in the β-phenyl ring of the molecule with two F atoms. This afforded further elongation of the distal portion of the side chain arm. Posaconazole was synthesized at Schering–Plough (currently Merck). The daily dosage is 800 to 1200 mg, duration of therapy can be more than 1 year (Clark et al., 2015; Keating, 2005). Posaconazole is the broadest spectrum azole antifungal available, with activity against Aspergillus, Candida, Cryptococcus, zygomycota, endemic mycoses, and some agents of hyalohyphomycosis (Kauffman et al., 2007). Yet it is mostly used for prophylaxis in immunosuppressed patients at high risk for invasive fungal infections and for salvage therapy in cancer patients (Clark et al., 2015). Kreiter et al report that after a single oral dose of 400 mg a median posaconazole peak plasma concentration of 0.9 µM was achieved in 10 hours, and the drug was still detectable within 183 hours (Krieter et al., 2004). Li et al found that after a single dose of 800 mg maximal plasma level of posaconazole was reached at 6 hours, varying from 1.8 µM (high-fat meal) to 0.5 µM (fasting) (Li et al., 2010). Because higher posaconazole concentrations are generally associated with improved treatment response [for example, patients with a mean plasma concentration of 1.25 µg/mL had a 75% response rate, whereas patients with mean concentrations of 0.41 to 0.72 µg/mL and 0.13 µg/mL had response rates of 53 and 24%, respectively], a plasma concentration greater than 1.25 µg/ml (1.8 µM) has been suggested as a conservative target (Clark et al., 2015; Walsh et al., 2007). The PK values reported in mice also vary. A single dose of 20 mg/kg produced maximal plasma concentration of 6 µM 8 hours after administration, the drug concentration at 24 hours was 3 µM (Nomeir et al., 2008). A single dose of 40 mg/kg produced maximal plasma concentration of 6 µM 3 hour after administration, the drug concentration at 24 hours decreased to 1.1 µM (Rodriguez et al., 2009). This supports the notion that posaconazole must display saturable oral absorption (Ezzet et al., 2005). Regardless of its relatively low oral bioavailability, posaconazole has broad tissue distribution (Clark et al., 2015), penetrates the blood-brain barrier (Calvo et al., 2010), and in blood cells its concentration can be >10 fold higher than in plasma (Farowski et al., 2010). Given the unpredictable plasma concentrations, and wide interpatient variability associated with the use of oral posaconazole, many experts recommend therapeutic drug monitoring (Clark et al., 2015), although now, when the intravenous formulation of posaconazole has become available (Jeong et al., 2016) it could be helpful in resolving the problem. Unlike ketoconazole or itraconazole, posaconazole undergoes limited hepatic metabolism (primarily via glucuronidation) and is mostly eliminated unchanged via feces (Heeres et al., 2010). It has been reported that posaconazole does not affect human liver P450s such as CYP1A2, 2C8/9, 2D6, 2C19 or 2E1 but still inhibits CYP3A4 (Lass-Flörl, 2011; Wexler et al., 2004). Posaconazole is generally well tolerated, and serious side effects are rare. The most commonly reported adverse reactions is gastrointestinal distress including nausea (7–41%), vomiting (2–6%), abdominal pain (1–5%), and elevated transaminases (2%). Longer duration of treatment did not result in incremental or new side effects, which are generally reversible upon treatment discontinuation (Clark et al., 2015).
Fluconazole (1988)
Fluconazole (Fig. 2, MW 306, log P 0.99) is a water soluble triazole derivative of miconazole, developed at Pfizer (Heeres et al., 2010). Both oral and intravenous formulations are available. The daily dosage varies between 150 – 800 mg (maximum 1600 mg), depending on location and severity of infection; the treatment duration may be > 1 year (Debruyne, 1997; Lass-Flörl, 2011; Pappas et al., 2016). Fluconazole displays excellent oral bioavailability (>90%) and achieves high levels in the cerebrospinal fluid. During the past 30 years it has become and remains the first-line agent for treatment and prophylaxis of all types of invasive candidiasis (Kullberg & Arendrup, 2015; Pappas et al., 2016). Fluconazole is also used to treat blastomycosis (Brick & Agger, 2012) and cryptococcal meningoencephalitis (Perfect et al., 2010). Its spectrum of antifungal activity, however, is mainly limited to yeast (Lass-Flörl, 2011). After a single oral dose of 150 mg the peak plasma concentration of 21 µM was reached in 2 hours; 24 hours after administration it remained 8 µM (Debruyne, 1997). The PK values reported in mice are: a single oral dose of 1 mg/kg resulted in maximal plasma concentration of 2.3 µM 1 hour after administration (Fromtling, 1988), while a 5 mg/kg dose led to the concentration of 23 µM (Louie et al., 1998). Fluconazole is a moderate inhibitor of CYP3A4, CYP2C8/9 and CYP2C19, and interacts with several drugs metabolized by these enzymes [https://www.uptodate.com/contents/pharmacology-of-azoles]. The drug is minimally metabolized, about 80% being excreted unchanged in urine. Because the elimination depends on renal function, dose reduction is mandatory in renal failure (Lass-Flörl, 2011). Overall, fluconazole revealed a good long-term safety (Lass-Flörl, 2011), the side effects are mild and might include headache (2 to 13%), dizziness (1%), skin rash (2%), nausea (2 to 7%), abdominal pain (2 to 6%), vomiting (2 to 5%), diarrhea (2 to 3%), and increased liver enzymes though serious hepatotoxicity is rare. However, the long-term use of fluconazole as a clinical drug has led to the development of resistance in many fungal strains (Morschhauser, 2016).
Voriconazole (2002)
Voriconazole (Fig. 2, MW 349, log P 2.28) is a fluconazole analog, where one of the two triazole rings is replaced with the 5-fluoropyrimidine ring. Although it took Pfizer 14 years, which involved synthesis and testing over 1200 fluconazole analogs before voriconazole was selected (Denning & Bromley, 2015), this modification markedly improved the spectrum of antifungal activity, so that voriconazole not only treats different forms of yeast infections, but is also the agent of choice for invasive aspergillosis (Aperis & Mylonakis, 2006; Denning & Bromley, 2015; Lass-Flörl, 2011): 52% remission, 72% survival (Heeres et al., 2010). Voriconazole is available in both oral and intravenous forms (Heeres et al., 2010). The oral dosing regimen includes a loading dose of 400 mg twice daily for 1 day, followed by 200 mg twice daily, therapy duration is often > 3 months (Elewa et al., 2015; Pappas et al., 2016). The bioavailability after oral administration of voriconazole is > 90% on an empty stomach but it is negatively affected by the presence of food (Heeres et al., 2010), the CSF and CNS penetration is similar to fluconazole (Lass-Flörl, 2011). After a single oral dose of 200 mg the peak plasma concentration of 1.6 µM was reached within 2.4 hours; 24 hours later it decreased to 0.3 µM (Peng & Lien, 2005). After multiple oral doses of 400 mg twice a day the steady state plasma concentrations of voriconazole are reached after 5–7 days of treatment (Bruggemann et al., 2009) and are mainly within the range of 8–17 µM, although high inter- and intrapatient variability is observed (Aperis & Mylonakis, 2006; Elewa et al., 2015), suggesting that therapeutic drug monitoring may be beneficial for optimizing both efficacy and safety (Elewa et al., 2015; Pasqualotto et al., 2010). The PK values in mice are: a single oral dose of 40 mg/kg produced maximal plasma concentration of 31 µM 1 hour after administration, about 15 µM was detectable in 12 hours (Mavridou et al., 2010). Voriconazole is extensively (>98%) metabolized in the liver by N-oxidation, primarily by CYP2C19 and to a lesser extent by CYP3A4 and CYP2C9, its metabolites have little clinical effect and their excretion in urine does not depend on renal function (Aperis & Mylonakis, 2006; Lass-Flörl, 2011). For voriconazole, the main safety issues are the numerous drug interactions and side effects, which include visual disturbances (30%), liver enzyme elevation (13%) skin rush (7%) fever, nausea, vomiting, diarrhea, headache, abdominal pain, and respiratory disorders (<5%) (Elewa et al., 2015; Heeres et al., 2010; Lass-Flörl, 2011).
Isavuconazole (2015)
Isavuconazole (Fig. 2, MW 437, log P 5.24) is produced by Astellas as a water soluble prodrug isavucozonium sulphate (Maertens et al., 2016; Pettit & Carver, 2015). It is the newest antifungal azole approved for clinical systemic use in the USA and Europe. The compound is a derivative of voriconazole and an isomer of ravuconazole (2,5-difluorophenyl vs 2,4-difluorophenyl ring), the drug candidate that has been in clinical trials for a long time, but has not passed them yet, mostly due to the problems with its pharmacokinetics. Isavuconazole has been approved as a broad-spectrum triazole for treatment of invasive aspergillosis and invasive mucormycosis. Both oral and intravenous forms are available and can be switched if necessary without losing bioavailability (Cornely, 2017). After a single oral dose of 200 mg the peak plasma concentration of 6 µM is reached within 3.5 hours; 24 hours after administration the concentration decreases to 0.3 µM, elimination time is extended to 76–104 hours. The standard oral dosing regimen includes a loading dose of 200 mg, followed by maintenance doses of 50 or 100 mg daily, so that the steady state concentration of the drug in plasma remains 6 µM (Pettit & Carver, 2015). The median length of therapy is 180 days (Wilby, 2017). The PK values in mice were reported for subcutaneous administration: a single dose of 13 mg/kg produced maximal plasma concentration of 10 µM in 0.5 hours, the concentration after 8 hours was dropping to 0.5 µM (Warn et al., 2009). Isavuconazole appears to have fewer drug interactions than voriconazole (Pettit & Carver, 2015) and is predominantly metabolized in the liver by CYP3A4 and CYP3A5 followed by UGT glucuronidation, the metabolites are excreted in feces and bile (Rybak et al., 2015). Isavuconazole displays more predictable plasma concentrations and is believed to have improved tolerability (Wilby, 2017). Treatment-emergent adverse events are mild and most commonly included gastrointestinal disorders, pyrexia, hypokalemia, headache, constipation, and cough (Rybak et al., 2015). When compared in the same clinical trials, isavuconazole was shown to be non-inferior to voriconazole, both in terms of efficiency and safety: drug-related treatment-emergent adverse effects were reported in 42% patients receiving isavuconazole and 60% receiving voriconazole (Maertens et al., 2016).
To summarize, though more efficient drugs are clearly needed, so far the 6 clinical azoles represent the safest and most widely used systemic antifungals, the cornerstone of antifungal therapy (Wilby, 2017).
REPURPOSING OF SYSTEMIC ANTIFUNGALS FORHUMAN INFECTIONS WITH PROTOZOA
While many protozoan pathogens (such as Plasmodium, Toxoplasma (Alveolata), or Trichomonas (Excavata)) do not synthesize endogenous sterols, scavenging cholesterol from their hosts, the parasites from the family Trypanosomatidae (order Kinetoplastida) and pathogenic free-living amoebae (e.g. Acanthamoeba (order Centramoebida)) do. These specific endogenous sterols are essential and their functions cannot be fulfilled by human cholesterol.
1. Trypanosomatidae parasites
In Trypanosomatidae, the sterol biosynthesis pathway resembles that in fungi (Fig. 1): squalene-2,3-epoxide is cyclized directly into lanosterol, and the major products are ergosterol and its C24-alkylated analogs. Sequencing of their genomes confirmed the presence of all the required enzymes of the pathway (El-Sayed et al., 2005). There are three major human diseases due to Trypanosomatidae: Trypanosoma cruzi causes Chagas disease (American trypanosomiasis), Trypanosoma brucei causes sleeping sickness (African trypanosomiasis), and Leishmania causes leishmaniasis. Most of the attempts to repurpose antifungal azoles were undertaken on Trypanosoma cruzi and Leishmania.
Trypanosoma cruzi
Trypanosoma cruzi along with its hematophagous triatomine insect vector, also known as kissing bug, were first identified as the origin of human infection by a Brazilian doctor Carlos Chagas in 1908, yet the pathogen has been infecting humans on the American continent for at least 9 000 years (Aufderheide et al., 2004). Chagas disease is a major health problem that is endemic in 21 Latin American countries, with over 25 million people at risk of contracting the disease and more than 10 000 deaths per year [http://www.who.int/chagas/epidemiology]. Furthermore, due to human migration and broadening of the insect vector distribution area, it is now becoming a global health issue (Bern et al., 2011; Perez-Molina & Molina, 2017).
Chagas disease is actually an anthropozoonosis, with >150 mammalian species forming the infection reservoir. T. cruzi is transmitted to humans not only by kissing bugs, but also via blood transfusion, organ transplantation, contaminated food and drinks, breastfeeding, and from mother to child. In mammals, T. cruzi resides both extra- and intracellularly, as non-multiplying bloodstream trypomastigotes that carry the infection throughout the body and multiplying amastigotes, respectively. T. cruzi infects numerous organs and tissues, though damages predominantly the heart, gastrointestinal tract and nervous system. The disease occurs in two phases. The acute phase often manifests with the non-specific symptoms of general inflammation and therefore can pass undiagnosed. Years or sometimes decades later, up to 40% of patients who survive the acute phase develop irreversible cardiomyopathy, arrhythmias, megaviscera, and more rarely, polyneuropathy and stroke, and these frequently lead to death (Bern et al., 2011; Lepesheva, 2013; Perez-Molina & Molina, 2017).
By now it is quite clear that the term Trypanosoma cruzi applies to a genetically highly variable population that represents a pool of more than 70 so called strains, which must be in fact different species (Cherkesova et al., 2014). The strains are now often referred to as belonging to one of the six Distinct Typing Units (DTUs) called TcI-TcVI. Colombiana, TcI (Colombia), Y, TcII (Brazil), Tulahuen, TcVI (Chile) are examples of most relevant strains that cause highly virulent infections (Zingales et al., 2009). Depending on the strain, the disease varies in its progression, mortality rates, the severity of the acute vs chronic stages, tissue tropism, abundance of dormant forms of the parasite, and susceptibility to the treatment (Filardi & Brener, 1987; Martinez-Diaz et al., 2001), which is still limited to two drugs, benznidazole and nifurtimox. Both of them (nifurtimox in particular) are highly toxic, and have low efficacy, especially in the chronic stage (Perez-Molina & Molina, 2017).
The first ever report on the use of antifungal azoles for T. cruzi was on miconazole and econazole, both drugs were shown to inhibit cellular growth of Tulahuen T. cruzi at concentrations of about 20 µM (Docampo et al., 1981). In 1983, it was demonstrated that oral ketoconazole (30 mg/kg) is active in a murine model of Chagas disease, protecting mice from death caused by the Y strain T. cruzi (McCabe et al., 1983). Ketoconazole was also found efficient in preventing death in mice infection with CL, MR, and Tulahuen T. cruzi (60 mg/kg) (McCabe et al., 1984). Similar to fungi, inhibition of T. cruzi multiplication by ketoconazole was accompanied by altered composition of the sterols in the parasite cells: sharp (34-fold) accumulation of 24-methylenedihydrolanosterol (eburicol in Fig. 1) and depletion of ergosterol-like products was observed (Beach et al., 1986). Itraconazole was found even more active, preventing death in mice infected with Y, CL, and Tulahuen T. cruzi at the dosage of 15 mg/kg and apparently leading to the parasitological cure if mice were treated with 150 mg/kg for about 60 days (McCabe et al., 1986). Moreover, in 2013 the outcome of a 20 years of follow-up of treatment of 46 human patients with itraconazole (Apt et al., 1998) was published, concluding that itraconazole prevented the development of ECG abnormalities and cured 33% of patients (Apt et al., 2013). The efficacy of fluconazole, however, was found to be low (Campos et al., 1992), and correlated well with its weak influence on the T. cruzi growth and sterol composition (Goad et al., 1989). Treatment of mice infected with Tulahuen T. cruzi with voriconazole, 40 mg/kg for 30 days, was also not particularly satisfactory, as at the end of the trial it resulted in only 75% survival rate (10% survival rate was observed at these conditions for non-treated animals), with high percentage of mice still having the parasite nests in the myocardium and skeletal muscle (Gulin et al., 2013).
The most promising results were produced by posaconazole. The first study was performed using Y and Bertoldo strains (Urbina et al., 1998) and involved both the acute (Y) and chronic (Bertoldo) murine models of Chagas. In the acute model, all the untreated animals died, while 43 doses of posaconazole (25 mg/kg/day) led to a 100% parasitological cure and 100% survival. In the chronic model, the treatment with posaconazole was started 45 to 60 days postinfection (in the Bertoldo strain infection most of the mice survive the acute stage but then deteriorate because of the cardiac conditions), and after 43 doses (15 mg/kg/day) provided 85% protection from death and 75% parasitological cure. The following study involved both immunocompetent and immunocompromised animals. The acute infection was with benznidazole-susceptible CL, partially resistant Y, and highly resistant Colombiana, SC-28, and VL-10 T. cruzi. In the model of chronic infection, the CL, Y, and Colombiana strains were used. The mice were treated with posaconazole (20 mg/kg/day) or benznidazole (100 mg/kg/day). In both cases posaconazole demonstrated better results than benznidazole, especially in immunocompromised hosts (Molina et al., 2000). Posaconazole was also investigated in a murine model of Y T. cruzi infection in combination with the anti-arrhythmic drug, amiodarone and the synergetic effect (100% survival, 80% parasitological cure) was observed (Benaim et al., 2006). In 2010 a successful treatment of chronic Chagas in an immunosuppressed human patient has been reported (Pinazo MJ et al., 2010), and this prompted entering of posaconazole into clinical trials for chronic Chagas disease (Leslie, 2011). The trials named CHAGASAZOL were performed in Spain and included three groups of chronic chagasic patients that were receiving low-dose posaconazole (100 mg twice a day), high-dose posaconazole (400 mg twice a day) or benznidazole (150 mg twice a day) for 60 days (Molina et al., 2014). The results were quite disappointing because, although none of the posaconazole patients had to stop treatment due to side effects (versus 15% of the patients treated with benznidazole, or 32% when the dosage of 200 mg was used (Morillo et al., 2017)), the follow-up rtPCR test demonstrated only 10 and 20 % success rates in the low-dose and high-dose groups, respectively. Later, the same group of authors reported that based on the fact that cure ratio was clearly related to the posaconazole dose, higher doses of the drug and longer treatment time should have produced better results (Molina et al., 2015). In our opinion, this is a very reasonable assumption, because (by analogy with fungal infections) effects of inhibitors of sterol biosynthesis are always slower and strongly depend on the drug exposure, as the endogenous sterols in the parasite cells (including its metabolically quiescent forms) must be exhausted before the drug becomes “cidal” vs “static”. Of special interest is the observation that posaconazole exposure in humans was 5 to 10-fold lower than in animal models (Molina et al., 2015). A prodrug of ravuconazole (E1224, Eisai) was another azole that entered clinical trials for Chagas disease. The trials had a low success rate, and this might well be because of the issues with the compound poor pharmacokinetics, the reason why ravuconazole has never passed clinical trials as antifungal drug. The data on its isomer isavuconazole are not yet available.
In all cases, when the T. cruzi cellular sterols were analyzed after the use of antifungal azoles, accumulation of eburicol (Fig. 1) prevailed, implying that this sterol is the most likely substrate of CYP51 in T. cruzi, and also that the C24 methylation (catalyzed by 24-sterol methyl transferase) should be the first reaction after the squalene-2,3-epoxide cyclization. Some traces of obtusifoliol suggested that even when the 14α-methyl group is still present, partial removal of one of the two methyl groups at the C4-position can occur (see Fig. 1), e.g. (Beach et al., 1986; Urbina et al., 1998).
Leishmania
More than 20 species of trypanosomatids of the genus Leishmania infect humans causing leishmaniasis, a vector-borne disease that is transmitted by sandflies. Sandflies also transmit the parasites to many mammals, such as dogs, rodents, primates, marsupials, bats, etc. (Roque & Jansen, 2014). The disease is spread all over the world, though is more prevalent in the countries with warm climate. The parasite is not found only in Australia and Antarctica. Leishmaniasis is considered endemic in ~90 countries, with 0.7 – 1 million new cases and 20,000 to 30,000 deaths per year [www.who.int/mediacentre/factsheets/fs375/en/].
In the sandfly, Leishmania exists in the form of extracellular promastigote. When the infected sandfly bites humans, promastigotes that reach the puncture wound are phagocytized by macrophages and transform into the obligate intracellular amastigotes that multiply and grow, ultimately rupturing the host cell and infecting new mononuclear phagocytes, including those which circulate in the blood.
Different species of Leishmania are morphologically indistinguishable but cause different types of the disease and therefore are joined into two major groups, one causing cutaneous and mucocutaneous leishmaniasis (e.g. L. major, L. mexicana, L. braziliensis, L. panamensis) and the other causing visceral leishmaniasis also known as kala-azar, or black fever (L. donovani, L. infantum, L. chagasi) (Berman, 1997). Cutaneous leishmaniasis manifests as skin lesions on exposed areas of the body that may start as papules, nodules and end up as ulcers. The ulcers can be dry or wet, localized or disseminated (particularly in immunocompromised patients). Frequently, the lesions are self-healing, though without treatment the process may take more than a year. Mucocutaneous leishmaniasis affects the skin and mucous membranes, causing severe destruction of skin and tissues of the mouth and nasal cavity, complications can irreversibly mutilate the face. Visceral leishmaniasis is the most severe form of the disease, the parasite infects mononuclear phagocyte systems of internal organs, such as spleen, liver, bone marrow and blood. The symptoms include high fever, weight loss, enlargement of the spleen and liver, and anemia. If left untreated, visceral leishmaniasis is fatal in over 95% of cases. Post-Kala-Azar-Dermal-Leishmaniasis (PKDL) develops in some patients alongside but more commonly after apparent cure from visceral leishmaniasis (50% and 5–10% of cases in Sudan and India, respectively) (Zijlstra et al., 2003).
The recommended treatment depends on the type and severity of the disease and includes pentavalent antimonials, pentamidine, miltefosine, amphotericin B and (for cutaneous leishmaniasis) antifungal azoles: ketoconazole, itraconazole, and fluconazole [https//www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html]. Interestingly, the attempts to use antifungal azoles for treatment of leishmaniasis were undertaken even before the information about their effects on Leishmania sterols or animal models of infection became available. This was probably connected with the successful use of amphotericin B (since 1959), which is also the antifungal drug (since 1955) acting through removal of ergosterol from the fungal membranes.
The first case of clinical treatment of leishmaniasis with ketoconazole was reported in 1982. Ketoconazole was administered to six patients (three with cutaneous and three with mucocutaneous) at 200 mg twice a day for three months. The cutaneous lesions disappeared after two weeks, while healing of mucocutaneous infections required 12 weeks. No relapses were seen 3 months after the treatment was completed (Urcuyo & Zaias, 1982). Other examples of successful use of ketoconazole (Jolliffe, 1986; Navin et al., 1992; Saenz et al., 1990), itraconazole (Borelli, 1987; Dogra & Saxena, 1996; Momeni et al., 1996; White et al., 2006), posaconazole (Paniz Mondolfi et al., 2011), and even fluconazole (Alrajhi et al., 2002; Daly et al., 2014; Sousa et al., 2011; Toubiana et al., 2006) for treatment of human cutaneous leishmaniasis are known. The antifungal azoles clearly accelerate the healing process and are tolerated much better than the regular medicine (Raether & Seidenath, 1984). But the question, why they have low efficiency, particularly against visceral leishmaniasis, both in humans (Momeni et al., 1996; Sundar et al., 1990) and in animal models (Al-Abdely et al., 1999), remains unresolved.
Sterol analysis, however, indicates that although azoles inhibit Leishmania cell growth and impair sterol biosynthesis (Berman et al., 1984), they do not stop the pathway at the step of lanosterol, eburicol or even obtusifoliol (Fig. 1). Instead, the C4,C14-methylated zymosterol (C4-norlanosterol), C14-methylated zymosterol (C4-desmethyllanosterol), and C14-methylated fecosterol (C4-desmethylobtusifoliol) accumulate (Beach et al., 1988; Berman et al., 1984; Goad et al., 1985; Lepesheva et al., 2015), indicating that even though the final structure of the ergosterol nucleus cannot be formed (which must still be crucial for sparking sterol functions), the C4-desmethylated precursors are more likely to be at least partially suitable to serve as membrane components, the role which host cholesterol in Leishmania cannot play (Beach et al., 1988). If so, much more efficient azole-based drugs or rather drug combination would be required to produce prompt cytocidal effect in Leishmania (Lepesheva et al., 2015). Promising results of combination therapy using allopurinol and ketoconazole (Halim et al., 1993) or amphotericin B and fluconazole (Horber et al., 1993) (visceral leishmaniasis in human) as well as 81% reduction of the parasite burden in L. donovani infected mice treated with combination of posaconazole and arylimidamide DB766 (Joice et al., 2017) support this notion.
Trypanosoma brucei
Two subspecies of Trypanosoma brucei, T. b. gambiense and T. b. rhodesiense, cause sleeping sickness (human African trypanosomiasis), while the subspecies T. b. brucei causes nagana in cattle but does not infect humans and therefore is often used as a model organism in animal studies. The T. brucei species are transmitted by tsetse fly bites. Wild and domestic animals can host these parasites and thus represent an important reservoir of infection. Sleeping sickness occurs in 36 sub-Saharan Africa countries. Based on the WHO report, approximately 70 million people who live/travel in the endemic area are still at different levels of risk, but, due to increased control, the number of new cases reported in 2014 dropped below 4,000, and the estimated number of actual cases was only about 15,000 [http://www.who.int/trypanosomiasis_african/country/en].
In tsetse fly, T. brucei multiplies as procyclic trypomastigote in the midgut and then as epimastigote in the salivary gland, where it transforms into metacyclic trypomastigote that infects humans. In humans, it transforms into bloodstream trypomastigote, which then invades blood and body fluids, eventually crossing the blood-brain barrier. At all life stages, the parasite remains extracellular. It evades the host immune system via rapid changes of its VSG coat (Pinger et al., 2017).
T. b. gambiense is responsible for 97% of reported cases of sleeping sickness. It is found in western and central Africa and causes a chronic infection: the symptoms of the disease may emerge months or even years after infection, when the central nervous system is affected. T. b. rhodesiense is found in eastern and southern Africa, it causes a rapidly developing acute infection: first symptoms are observed within 1–2 weeks, and death ensues usually within months. The symptoms begin with fever, headache, muscle and joint aches, enlarged lymph nodes and then progress to sleep disturbances, progressive confusion and mental deterioration. Amongst the four drugs that are used to treat sleeping sickness, suramin and pentamidine do not cross the blood-brain barrier and so are only effective at the acute stage of infection. Eflornithine does not work against T. b. rhodesiense, and melarsoprol is extremely toxic causing death in >5% of patients [https://www.cdc.gov/parasites/sleepingsickness/treatment.html]. Untreated, sleeping sickness is usually fatal.
Apparently, amphotericin B is not active against T. brucei, because this parasite can use host cholesterol as a structural component for the membranes. Based on this fact, at some point it was postulated that “bloodstream forms of T. brucei do not synthesize sterols de novo” (Coppens & Courtoy, 2000). As a result, the information on the effects of antifungal azoles on T. brucei/sleeping sickness is very scarce.
Nevertheless, we found that ketoconazole inhibits growth of both procyclic and bloodstream cells of T. brucei in a dose-dependent manner with the EC50 of about 15 µM (Lepesheva et al., 2007). Most recently, the corresponding value of EC50=8.5 µM has been reported for posaconazole (Dauchy et al., 2016), and some inhibitory effect was also produced by itraconazole (Haubrich et al., 2015). Moreover, using highly specific anti-T. brucei CYP51 antibodies for western blot immunoanalysis, we confirmed that, although at a level lower than in procyclic cells, the CYP51 gene is expressed in bloodstream T. brucei (Lepesheva et al., 2010b). These finding were recently reproduced by another research team (Dauchy et al., 2016), which in addition used RNAi to demonstrate the CYP51 gene essentiality in bloodstream T. brucei. Finally, a clear dose-dependent suppression of T. brucei infection in mice was observed after oral administration of an experimental T. brucei CYP51 inhibitor VNI or clotrimazole (Lepesheva et al., 2010b), and posaconazole-eflornithine combination showed substantial improvement in mice survival in the infections with different T. brucei subspecies (Dauchy et al., 2016). These results imply that even if azole-based drugs alone may not be sufficient to cure sleeping sickness, their ability to cause the parasite growth retardation in vivo together with much better safety profile makes this kind of medicine quite attractive for combination therapy.
2. Opportunistic human parasites amongst free-living amoeba
Some details of the sterol biosynthesis pathway in the pathogenic free-living amoebas remain elusive. In 1980s it was reported that Acanthamoeba polyphaga (Raederstorff & Rohmer, 1985) and two species of amoeba of the genus Naegleria (Raederstorff & Rohmer, 1987) synthesize sterols de novo using the pathway that is more typical for photosynthetic organisms so that squalene-2,3-epoxide is cyclized into cycloartenol, which is then converted into 24-methylenecycloartanol and then forms the CYP51 substrate obtusifoliol. Yet in 2017 another team of authors, when analyzing the sterols of Acanthamoeba castellanii failed to identify cycloartenol in this organism and suggested lanosterol as a potential CYP51 substrate (Thomson et al., 2017). Regardless of these discrepancies (or perhaps species-related differences (Thomson et al., 2017)), the products of the pathway appear to be mostly ergosterol-like (see Fig. 1), similar to those in Trypanosomatidae.
Humans can be infected by free-living amoeba species from four genera. Acanthamoeba (e.g., A. polyphaga, A. keratitis, A. culbertsoni, A. hatchetti, A. castellanii) and Balamuthia (B. mandrillaris) belong to the order Centramoebida, Naegleria (N. fowleri) belongs to the order Schizopyrenida, and Sappinia (S. diploidea) belongs to the order Euamoebida. These opportunistic pathogens live in water, soil, and air and cause a variety of severe health complications, particularly in immunocompromised patients and contact lenses wearers. Thus, Acanthamoeba species are causative agents of blinding keratitis, sinus and lung infections, and granulomatous encephalitis. B. mandrillaris may form a skin lesion, or may migrate to the brain and cause granulomatous encephalitis. N. fowleri, or brain-eating amoeba, causes non-opportunistic primary amoebic meningoencephalitis, which is acute, fulminant, and rapidly fatal (Trabelsi et al., 2012), and one case of encephalitis due to S. diploidea has been described (Gelman et al., 2001). The life-cycle of free-living amoeba has two stages: a motile active trophozoite stage and a dormant stress resistant cyst stage with minimal metabolic activity; both of them are infectious. Naegleria also has the third, flagellate stage that can exist in the cerebrospinal fluid. Because the amebic infections are emerging diseases, they are difficult to diagnose clinically, leading to delay in treatment and resulting in a high mortality rate. The treatment usually includes combinations of different drugs, amongst them CDC lists amphotericin B, pentamidine, rifampicin, miltefosine and several antifungal azoles, both systemic (ketoconazole, itraconazole, fluconazole, voriconazole) and topical (miconazole and clotrimazole, for keratitis). Nevertheless, most cases of brain and spinal cord infection with amoeba remain fatal [https://www.cdc.gov/dpdx/freeLivingAmebic/tx.html].
The first clinical use of antifungal azoles for amoeba infection was reported in 1984, when recurrence of the Acanthamoeba keratitis in the second eye transplant was cured with systemic ketoconazole and topical miconazole (Hirst et al., 1984). In 1990, the combination of oral itraconazole and eye drops of miconazole was reported to cure three patients with Acanthamoeba keratitis (Ishibashi et al., 1990). In 1999, a lung transplant patient who had disseminated acanthamoebiasis was successfully cured with a drug combination that included oral itraconazole and ketoconazole cream (Oliva et al., 1999). Combination of corneal cryosurgery with oral fluconazole was found effective in treatment of Acanthamoeba keratitis (Amoils & Heney, 1999). A patient with AIDS diagnosed with granulomatous amebic encephalitis was treated with fluconazole and sulfadiazine, and the single lesion in the brain was surgically excised. No disease relapse was observed (Seijo Martinez et al., 2000). Voriconazole was first used for treatment of disseminated Acanthamoeba infection in in a lung transplant recipient, where it was administered in combination with amphotericin B (Walia et al., 2007). Subsequently, other cases of successful treatment of amoebic keratitis (Arnalich-Montiel et al., 2012; Bang et al., 2010; Tu et al., 2010) and granulomatous amoebic encephalitis (Webster et al., 2012) using voriconazole monotherapy or voriconazole-containing drug combinations were reported.
Most recently, direct inhibition of the Acanthamoeba castellanii CYP51 activity with voriconazole, itraconazole and fluconazole was studied in vitro, showing that, unlike fluconazole, voriconazole and itraconazole are highly potent inhibitors that could not be displaced by the enzyme substrate during the reaction. In cellular experiments, however, the potency of voriconazole was found more than 10-fold higher in comparison with itraconazole, which the authors suggest might be due to the poor uptake of the bulky itraconazole molecule into the cells. In this study obtusifoliol was experimentally confirmed as the preferred amoeba CYP51 substrate (Lamb et al., 2015). This is in full agreement with the amoeba CYP51 sequences (see Fig. 3, the signature residue is marked with black circle).
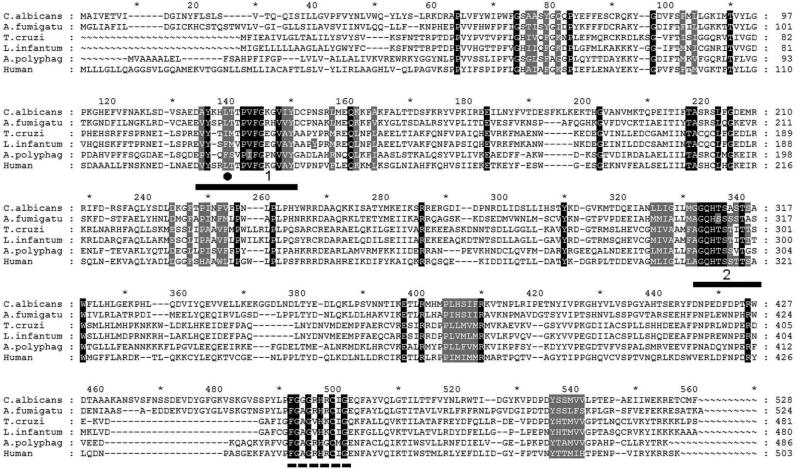
Figure 3. Amino acid sequence alignment of eukaryotic CYP51.
The alignment was performed using >200 proteins. The sequences of two fungal (C. albicans and A. fumigatus), three protozoan: two Trypanosomatidae (T. cruzi and L. infantum) and amoeba (A. polyphaga), and human CYP51s are displayed as examples. The residues conserved in >99% CYP51 family members are in black, the phyla-specific residues that form the surface of the substrate binding cavity are in gray. The residue that defines the CYP51 substrate preferences is marked with black circle (●): F- C4-monomethylated sterols, L/I – C4-dimethylated sterols). Two CYP51 family signatures are underlined, the P450 signature, involving the heme-coordinating cysteine, is marked with the dashed line.
CYP51 STRUCTURE/FUNCTION AND INHIBITION
In the absence of structure/functional information on the target CYP51 enzymes, the use of phenotypic screening remained the only option, and the development of new drugs was slow and had low efficiency. With the advances in the molecular biology techniques, sequencings of the genomes of many human pathogens have been performed finally enabling inclusion of the CYP51 enzymes into the drug discovery process.
CYP51 sequences
The CYP51 enzymes from different phyla have very low amino acid sequence identity (Fig. 3). For example, the average sequence identity between trypanosomatid and fungal CYP51s is around 25%, the identity between the amoeba and trypanosomatid CYP51s is 33%, and even the identity between the C. albicans and A. fumigatus CYP51 sequences is only 46%. The average length of a eukaryotic CYP51 polypeptide chain is about 500 residues, and yet only less than 40 of them are completely conserved across the phyla (Lepesheva & Waterman, 2007). Because of this low sequence identity, attempts to repurpose the antifungal azoles for treatment of protozoan infections may not be the best solution, especially since the set of systemic antifungal azoles is rather limited and the efficacy of treatment of systemic fungal infections is quite unsatisfactory (Denning & Bromley, 2015). New, better CYP51 inhibitors are needed.
The CYP51 genes from the species of interest can now be cloned, the proteins can be heterologously expressed in bacteria (E. coli), purified and characterized. The fact that the enzyme is in its functionally active state can be verified by the CO-spectrum, because as a Cys–coordinated hemoprotein, upon the iron reduction CYP51 produce a characteristic absorbance maximum around 450 nm after binding of carbon monoxide (Omura & Sato, 1964).
CYP51 spectral response to ligand binding
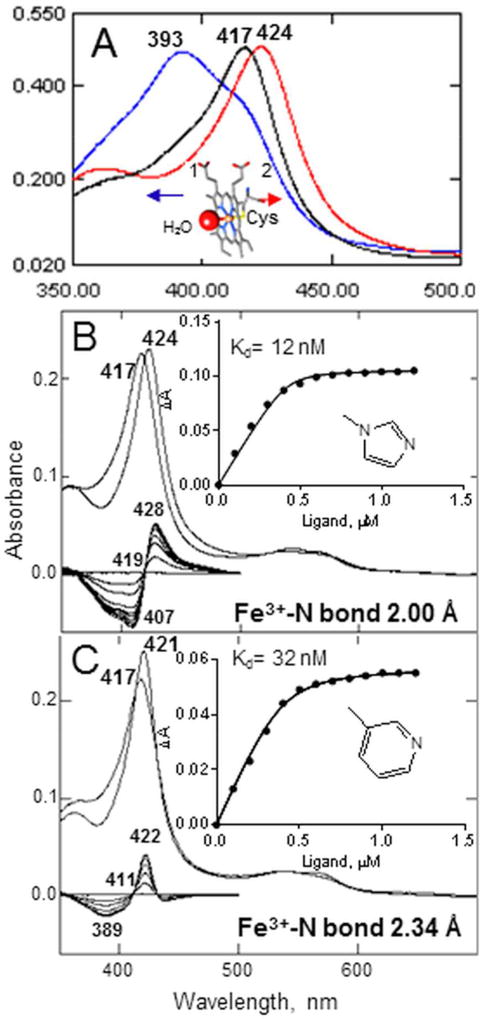
When expressed in bacteria, CYP51 enzymes display the Soret band maximum at 417 nm (Fig. 4A). This means that the purified protein is in the substrate-free form, with the heme iron in the oxidized (Fe3+) low-spin hexa-coordinated state and a water molecule playing the role of the sixth (axial) ligand, bound to the iron on the distal side of the heme plane, the side that forms the catalytic surface of the CYP51 substrate binding cavity (Hargrove et al., 2012a). When the substrate (a substrate analog) enters the CYP51 binding cavity, it expels the water molecule from the iron coordination sphere. The iron becomes penta-coordinated high-spin, and the Soret band maximum shifts to 393 nm, producing the type 1 spectral response. When a ligand stronger than water (e.g. a heterocyclic ring nitrogen) coordinates to the CYP51 heme iron, the Soret band maximum shifts to the right, producing the type 2 spectral response, the length of the shift inversely correlating with the length of the Fe-N coordination bond (Hargrove et al., 2013) (Fig. 4B, C).
Figure 4. CYP51 binding ligands can be identified by spectral titration.
A. Absolute absorbance spectra of water-bound (black), obtusifoliol-bound (blue, type 1 response), and azole-bound (red, type 2 response) T. brucei CYP51.The Soret band maxima are marked. Inset: the water-bound heme iron. B, C. Type 2 response of T. cruzi CYP51 to the binding of imidazole-based VNI [PDB code 3gw9] (B) and, pyridine-based UDO [PDB code 3zg3] (C). Absolute (top) and difference (bottom) absorption spectra. The P450 concentration ~0.4 µM, the optical path length 5 cm. Insets: the titration curves, prepared in Prism.
This feature can and has been used in optical high-throughput screening (HTS) for new CYP51 binding ligands, e.g. (Konkle et al., 2009; Lepesheva et al., 2008), and the apparent binding affinities of the HTS hits can be estimated by spectral titration. The results, however, have to be taken with caution because the low Kds do not necessarily mean strong enzyme inhibition (Fig. 5), and therefore tight binding ligands with the apparent Kds< 1 µM (close to the average values calculated for the CYP51-substrate complex formation) have to be further tested as inhibitors of the CYP51 activity in the reconstituted enzyme reaction in vitro (Lepesheva et al., 2008; Lepesheva et al., 2007).
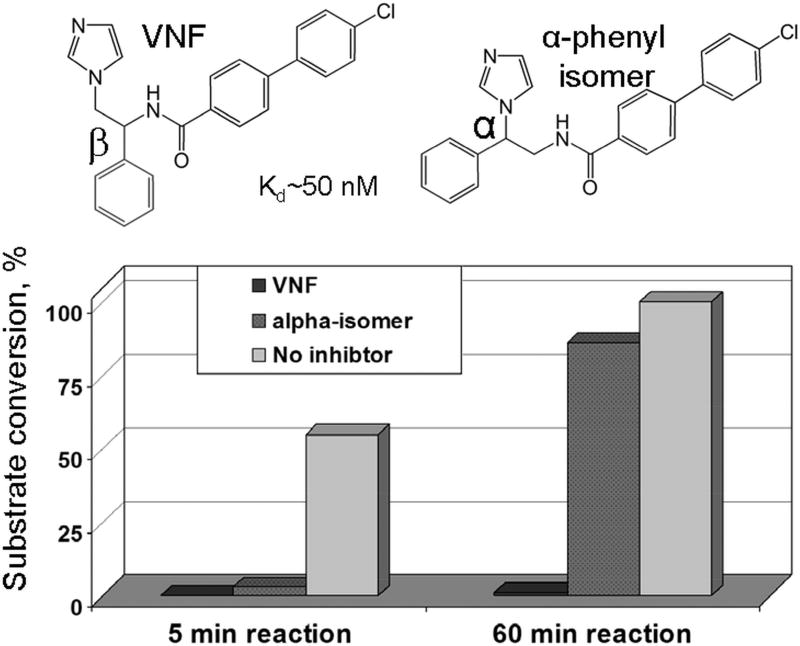
Figure 5. Low spectral Kds do not necessarily mean strong inhibition of the CYP51 activity.
While both VNF and it’s α-phenyl isomer display high spectral binding affinity and comparable inhibitory effects on the initial rate of reaction (5 min), VNF is not replaced in the CYP51 active site with the substrate overtime (60 min). The reaction mixture contained 1 µM T. cruzi CYP51, 1 µM inhibitor, and 50 µM substrate.
CYP51 inhibition
By definition, antifungal azoles are supposed to work as competitive reversible inhibitors. Indeed, many of them (for example fluconazole), similar to the α-phenyl isomer of VNF in Fig. 5, strongly affect the initial turnover number but are being replaced by the substrate in the CYP51 active site during the course of the reaction (Lepesheva et al., 2007). These kind of drugs can be useful (provided they have good pharmacokinetic properties), yet their efficacy is always going to be limited, because they cannot completely block sterol biosynthesis in a pathogen, the feature required for the “cidal” vs “static” inhibitory effect.
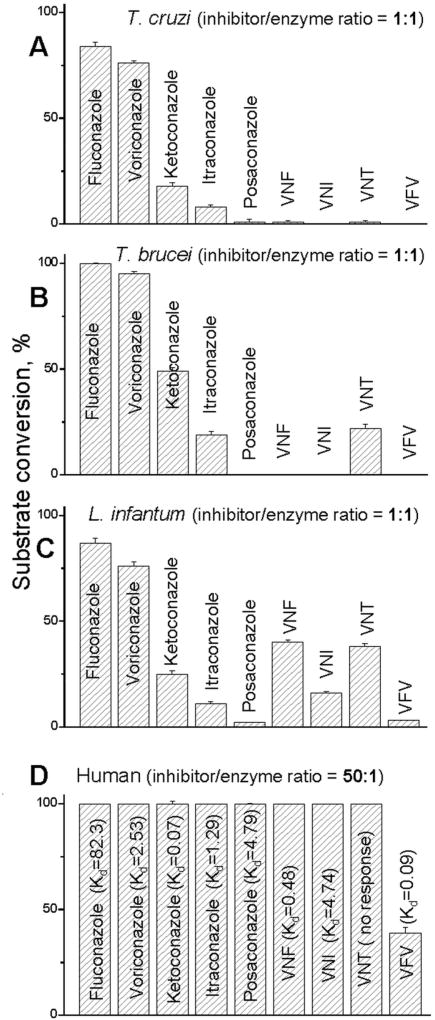
The questions why some of the compounds completely block the CYP51 activity at 1/1 molar ratio inhibitor/enzyme (Lepesheva et al., 2008; Lepesheva et al., 2007; Yoshida & Aoyama, 1987) (Fig. 6A, B, C) and cannot be replaced in the enzyme binding cavity by the substrate, thus acting as functionally irreversible inhibitors, and why this is never the case for human CYP51 (Fig. 6D) had remained enigmatic until the CYP51 crystal structures have been determined.
Figure 6.
Inhibitory effects of systemic clinical antifungal azoles and experimental inhibitors on the activity of (A) T. cruzi, (B) T. brucei, (C) L. infantum, and (D) human CYP51 orthologs; 60 min reaction. The results are presented as means ± SEM. In all experiments the P450 concentration was 0.5 µM, the concentration of the sterol substrates (A, eburicol; B, C, obtusifoliol; D, lanosterol) was 50 µM. The values of the apparent spectral Kds for human CYP51 are given in µM.
CYP51 structures and structural basis for the enzyme druggability
We have characterized and solved the structures of CYP51s from three trypanosomatid parasites (T. brucei (Lepesheva et al., 2004), T. cruzi (Lepesheva et al., 2006) and L. infantum (Hargrove et al., 2011)), in the ligand-free form (Lepesheva et al., 2010b) and in complexes with different inhibitors, including clinical azoles fluconazole and posaconazole (Hargrove et al., 2011; Lepesheva et al., 2010a) and experimental compounds, heme-coordinated imidazole- (Andriani et al., 2013; Buckner, 2012; Friggeri et al., 2014; Lepesheva et al., 2010a; Lepesheva et al., 2010b), triazole- (Lepesheva et al., 2015), pyridine- (Hargrove et al., 2013), and tetrazole-based (Hoekstra et al., 2016) as well as the substrate analog MCP (Hargrove et al., 2012b), CYP51s from two fungal human pathogens (A. fumigatus (Hargrove et al., 2017b; Hargrove et al., 2015) and C. albicans (Hargrove et al., 2017a)) and the CYP51 counterpart from human (Hargrove et al., 2016). Comparative structural analysis has led us to the following conclusions.
CYP51 enzymes preserve their conserved catalytic function regardless of the extremely low amino acid sequence identity across phylogeny by maintaining strict similarity at the secondary and tertiary structural levels (Lepesheva & Waterman, 2011). For example, nine of the invariant CYP51 family residues (shown in black in Fig. 3) are glycines or prolines that separate the major secondary structural elements, thus maintaining their length and location within the CYP51 molecule. Most of the other invariant family residues either form the heme binding sequence (P450 signature, dashed line in Fig. 3) or are located within the substrate binding cavity, although the surface of the cavity is mostly formed by the residues that are phyla-specific (grey in Fig. 3) (Hargrove et al., 2017a; Lepesheva & Waterman, 2011).
All the inhibitors, including the substrate analog MCP, bind in the CYP51 active site without causing any substantial rearrangements in the protein backbone, as if “freezing” the enzyme in its substrate-free conformation (Fig. 7). The inhibitors simply occupy the available space and acquire the shape of the CYP51 substrate binding cavity. Because large-scale conformational rearrangements in the protein moiety are very likely to be required upon the CYP51 catalysis (binding of the physiological substrate and/or formation of the specific complex with the electron donor protein cytochrome P450 reductase), it appears that the stronger this “freezing” effect is, the stronger the inhibitory potency must be. In addition to the formation of the Fe-N coordination bond (which affects the iron redox potential), this freezing effect is achieved through multiple interactions between the inhibitor molecule and the protein residues. Some of these interactions have a greater impact than others.
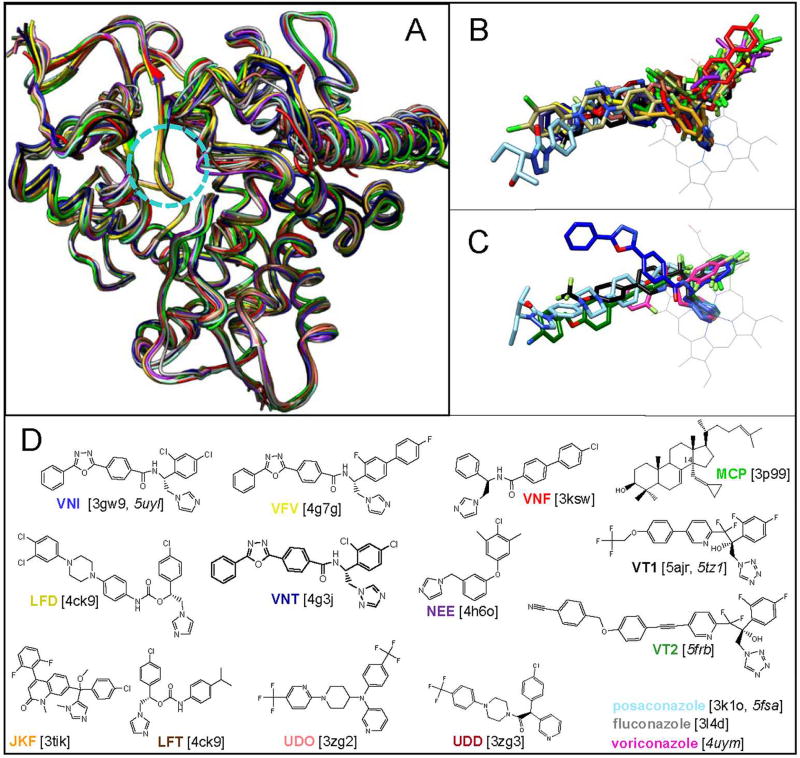
Figure 7. Binding of inhibitors does not cause any large-scale rearrangements in the backbone of the CYP51 molecule.
A, B. Superimposed protozoan CYP51 structures, A: protein chains of ligand-free T. brucei (black) and inhibitor-bound T. brucei, T. cruzi and L. infantum CYP51. The substrate entry is circled. B: inhibitors bound in the protozoan CYP51 active site. C. Inhibitors bound in the fungal CYP51 active site. The heme is depicted in grey. D. Formulas of the inhibitors (posaconazole, voriconazole and fluconazole are shown in Fig. 2). The color code of each crystal structure corresponds to the color of the inhibitor name (PDB ID) beneath the formulas. The correspondent PDB codes of the crystal structures are shown in brackets, the codes of fungal structures are italicized.
Hydrogen bonds
H-bonds are at least one order of magnitude stronger than Van der Waals contacts. For example, we found that the carboxamide fragment of an experimental inhibitors VNI (Lepesheva et al., 2010b) and its derivative VFV (Lepesheva et al., 2015) in the protozoan CYP51 structures forms two H-bonds that connect two functionally essential regions of the CYP51 molecule, the B’ and I helices (Fig. 8A). On the other hand, VNI that acts as a highly potent functionally irreversible inhibitor of protozoan CYP51s is a rather moderate reversible inhibitor of fungal enzymes (Hargrove et al., 2012a). Accordingly, in the structure of A. fumigatus CYP51 VNI is oriented differently (see Fig. 7C) and does not form any H-bonds with the protein (Hargrove et al., 2015). Another example, the tetrazole-based investigational antifungal drug candidates from Viamet (VT1 and VT2 in Fig 7D) form the H-bond with the fungal-specific histidine (His374 in A. fumigatus and His377 in C. albicans CYP51, respectively) (Hargrove et al., 2017a; Hargrove et al., 2017b). Both of these compounds act as functionally irreversible inhibitors of the A. fumigatus and C. albicans enzymes (Hargrove et al., 2017a; Hargrove et al., 2017b). Besides, they have also been found effective in vivo against isolates of C. glabrata and C. krusei, which were clinically resistant to other antifungal treatments (Schell et al., 2017). Comparison between fluconazole and voriconazole as inhibitors of A. fumigatus CYP51 is probably even more impressive. The two molecules differ only in the composition of a single ring, a smaller triazole ring in fluconazole (weak reversible inhibitor) and a larger 5-fluoropyrimidine ring in voriconazole (see Fig. 2). The pyrimidine ring of voriconazole forms the H-bond with A. fumigatus CYP51 Y122, and voriconazole, regardless of its small size, is one of the most potent inhibitors of A. fumigatus (Hargrove et al., 2015).
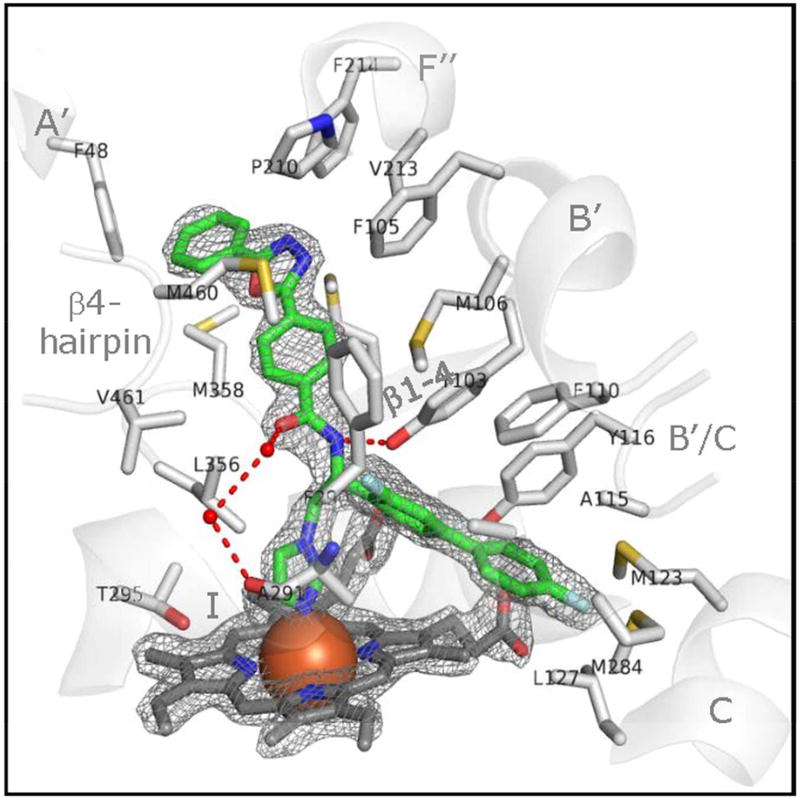
Figure 8. VFV bound in the CYP51 active site.
The 2Fo-Fc electron density map around the heme and the inhibitor is contoured at 2.0 σ and shown as grey mesh. The carbon atoms of VFV are green, the carbon atoms of 22 CYP51 residues that form Van der Waals contacts with the inhibitor are light grey, and the carbon atoms of the heme are dark grey. The atoms of oxygen, nitrogen, and sulfur are read, blue, and yellow, respectively. The heme iron is presented as an orange sphere. The active site-defining secondary structural elements (semitransparent cartoon) are labeled for clarity. The H-bonds between the enzyme and inhibitor are displayed as red dashes.
Surface binding subsite around the substrate entrance
Posaconazole represents an example of the compound that acts as functionally irreversible inhibitor for all tested CYP51s from human pathogens. In the CYP51 structures, its long arm is exposed above the protein surface, forming multiple interactions around the substrate entry (Hargrove et al., 2017a; Lepesheva et al., 2010a). It appears that these interactions prevent opening of the substrate access channel. A similar effect was displayed by a potent experimental inhibitor LFD (shown in Fig. 7D), although the compound was only tested as an inhibitor of and co-crystallized with T. cruzi CYP51 (Friggeri et al., 2014).
The C/I-helices area
The helices C and I (they contact the biphenyl arm of VFV in Fig. 8) form the deepest portion of the CYP51 substrate binding cavity, which is unique for sterol 14α-demethylases and is not present in other P450s. This area supports the aliphatic arm of the sterol molecule (Hargrove et al., 2012b), and the compounds that bind here (VNF and NEE being two examples, seen in Fig. 7B), regardless of their small size, still cannot be replaced by the substrate in the CYP51 reaction (Andriani et al., 2013; Lepesheva et al., 2010a). Both of these compounds were found to produce strong antiparasitic effect in T. cruzi cells, although unfortunately, NEE was not active in mouse models of Chagas disease and VNF has never been tested in vivo. Deeper insights into this phenomenon might help select small but potent CYP51 inhibitors with e.g. the enhanced ability to penetrate the blood-brain barrier.
CYP51 structure-based drug design
The fact that no conformational changes in the backbone of the CYP51 substrate binding cavity are involved in inhibitor binding makes this enzyme an attractive target for structure-based drug design. Indeed, our first attempts on the VNI scaffold modification (VNT and VFV in Fig. 7) strongly support this notion as all three compounds superimpose perfectly in the protozoan CYP51 active site (Lepesheva et al., 2015).
The reasons for the modifications were as follows. Although we found that VNI is a highly promising molecule (it cured, with 100% efficiency and 100% survival, both the acute and chronic Chagas disease in mice infected with Tulahuen T. cruzi (Villalta et al., 2013)), the compound was less potent as inhibitor of CYP51 from Leishmania infantum (Fig. 6C) and CYP51A from the Y strain T. cruzi (Cherkesova et al., 2014).
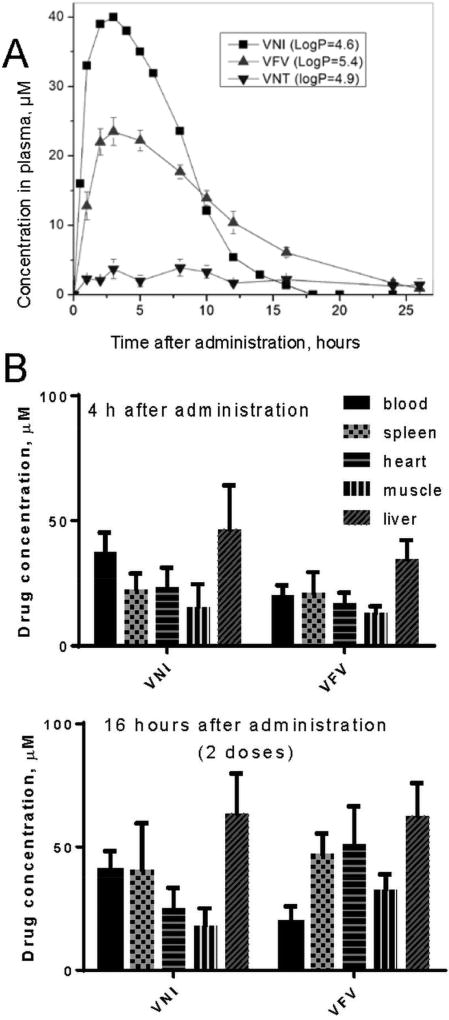
VNT was made to test the general belief that replacement of the metabolically more vulnerable imidazole ring with the triazole ring may have a positive effect on the compound pharmacokinetics. VFV was made with the goal to broaden the antiprotozoan spectrum of activity by filling the deepest (CYP51-specific) portion of the substrate binding cavity with the additional aromatic ring. Both derivatives retained their potency to inhibit the activity of Tulahuen T. cruzi CYP51 in vitro (Fig. 6A) and were more active than posaconazole in cellular experiments, killing Tulahuen T. cruzi amastigotes within cardiomyocytes with the EC50 values within 1 nM (Lepesheva et al., 2015). In the same experiments posaconazole displayed the EC50 of 5 nM (Villalta et al., 2013). Furthermore, both compounds showed weaker inhibitory effect on CYP3A4 (the IC50 405, 2900 and 3600 nM for VNI, VNT and VFV respectively; the corresponding values for ketoconazole and posaconazole are 8 and 120 nM (Hargrove et al., 2012a)). The life time of VNT in mice plasma was indeed longer, however its concentration did not exceed 5 µM, thus being about 8-fold lower than the maximal plasma concentration of VNI (Fig. 10). Because of this and also since VNT was too selective for Tulahuen T. cruzi CYP51 (Fig. 6B, C), it was not tested in vivo. VFV, on the other hand, met our expectations. It cured Tulahuen T. cruzi infection in mice and was found more potent than VNI in the mouse model of visceral leishmaniasis: 89% vs 60% reduction of the parasite burden (Lepesheva et al., 2015). It also showed better results in curing the Y strain T. cruzi infection in mice (Guedes-da-Silva et al., 2017). Moreover, after a single oral dose of 25 mg/kg, VFV displayed a longer clearance time in mouse plasma (Fig. 10A) and revealed higher than VNI affinities to tissues (Fig. 10B). Non-toxic, non-mutagenic, VNI and VFV are promising new drug candidates for entering clinical trials.
Figure 10. Pharmacokinetics of VNI and derivatives.
A. Plasma concentration curves after a single oral dose of 25 mg/kg. B. VNI and VFV tissue distribution 4 hours after administration (single dose) and 16 hours after administration (2 doses).
Human CYP51 is naturally resistant to inhibition
There is still a general concern that inhibitors of pathogenic CYP51s might actually affect the human counterpart. This is, however, a controversial issue, because inhibitors of human CYP51 could potentially serve as cholesterol-lowering agents. Nevertheless, all the attempts to use human CYP51 as a drug target have failed, and none of the known antifungal azoles or experimental inhibitors of CYP51 from human pathogens inhibit the activity of human CYP51 (Fig. 6D) (Hargrove et al., 2016). We believe that this natural resistance of human CYP51 to inhibition results from the higher flexibility of the substrate binging cavity. Unlike the microbial CYP51 structures, human CYP51 displays the loop-like (low-energy) area in the middle portion of the I-helix (the core helix in the P450 structural fold). The structure suggests that this loop-like area is formed because of the long sequence of polar residues (-HTSSTTS-, CYP51 signature 2 area in Fig. 3), which is found in all animal CYP51 sequences but is interrupted by at least one or two hydrophobic residues in the CYP51 enzymes from other phyla. In the human structure, the OH groups of these polar residues disrupt the normal main chain α-helical H-bonding (Hargrove et al., 2016). The notion of higher flexibility of the human CYP51 active site is supported by structural dynamic simulation experiments (Yu et al., 2016), together suggesting that all animal CYP51 enzymes must be naturally resistant to inhibition.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The fact that CYP51 had served blindly but quite successfully as a drug target for more than half a century addresses the question of the enzyme druggability. However, better and more efficient drugs are badly needed. Current progress in understanding the CYP51 structure/function has made it possible to include the target enzyme into the drug discovery process, thus making it quite straightforward. Optical high throughput screening as well as virtual screening of large libraries of drug-like molecules can be used to identify novel inhibitory scaffolds. The tight-binding ligands can be selected by spectral titration, and those of them that act as functionally irreversible inhibitors in the reconstituted CYP51 reaction should proceed to cellular experiments and/or animal models. The issues of toxicity, bioavailability and pharmacokinetics can be resolved rationally by minor, structure-guided optimization of the molecules. It is our strong belief that a larger set of more efficient, pathogen-oriented CYP51 inhibitors will help to reduce the mortality rates of fungemias, cure Chagas disease and amoebiasis, and must be effective in combination therapy for visceral leishmaniasis and sleeping sickness. Team efforts from academia and pharmaceutical companies are highly advisable to accelerate the process.
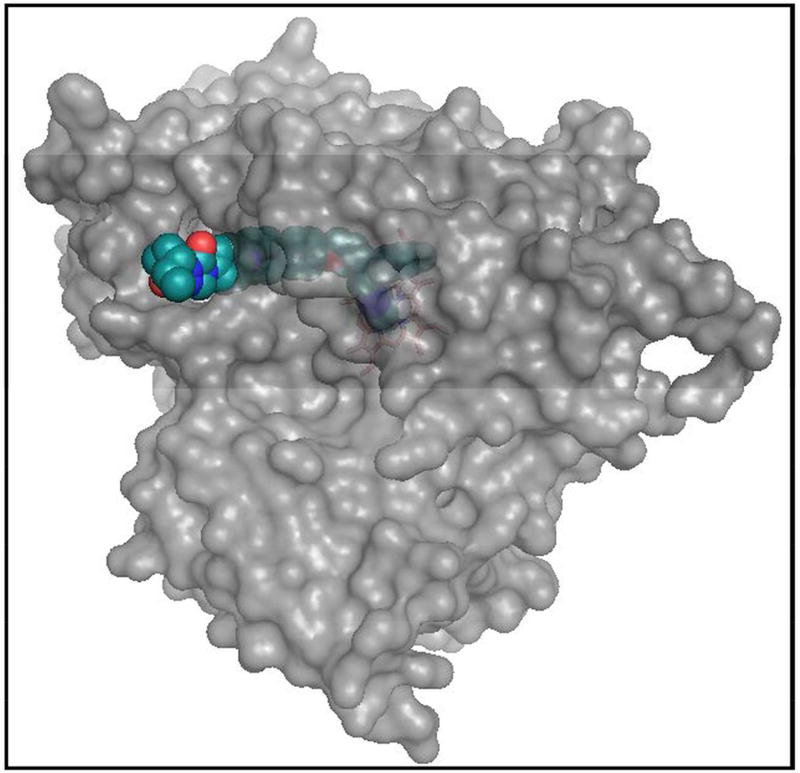
Figure 9. Surface representation of T. cruzi CYP51 bound to posaconazole.
The long arm of the inhibitor protrudes above the entrance into the substrate access channel.
Acknowledgments
FINANCIAL SUPPORT
Financial support was provided by the National Institutes of Health Grant R01 GM067871 (to G.I.L.).
ETHICAL STANDARDS
Not applicable
Footnotes
CONFLICT OF INTEREST
None
References
- Al-Abdely HM, Graybill JR, Loebenberg D, Melby PC. Efficacy of the triazole SCH 56592 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous and visceral leishmaniases. Antimicrobial Agents and Chemotherapy. 1999;43:2910–2914. doi: 10.1128/aac.43.12.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. New England Journal of Medicine. 2002;346:891–895. doi: 10.1056/NEJMoa011882. [DOI] [PubMed] [Google Scholar]
- Amoils SP, Heney C. Acanthamoeba keratitis with live isolates treated with cryosurgery and fluconazole. American Journal of Ophthalmology. 1999;127:718–720. doi: 10.1016/s0002-9394(98)00426-7. [DOI] [PubMed] [Google Scholar]
- Andes D. Optimizing antifungal choice and administration. Current Medical Research and Opinions. 2013;29(Suppl 4):13–18. doi: 10.1185/03007995.2012.761135. [DOI] [PubMed] [Google Scholar]
- Andriani G, Amata E, Beatty J, Clements Z, Coffey BJ, Courtemanche G, Devine W, Erath J, Juda CE, Wawrzak Z, Wood JT, Lepesheva GI, Rodriguez A, Pollastri MP. Antitrypanosomal lead discovery: identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth. Journal of Medicinal Chemistry. 2013;56:2556–2567. doi: 10.1021/jm400012e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama Y, Yoshida Y, Sato R. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. II. Lanosterol metabolism by purified P-450(14)DM and by intact microsomes. Journal of Biological Chemistry. 1984;259:1661–1666. [PubMed] [Google Scholar]
- Aperis G, Mylonakis E. Newer triazole antifungal agents: pharmacology, spectrum, clinical efficacy and limitations. Expert Opinion on Investigational Drugs. 2006;15:579–602. doi: 10.1517/13543784.15.6.579. [DOI] [PubMed] [Google Scholar]
- Apt W, Aguilera X, Arribada A, Pérez C, Miranda C, Sánchez G, Zulantay I, Cortés P, Rodriguez J, Juri D. Treatment of chronic Chagas’ disease with itraconazole and allopurinol. American Journal of Tropical Medicine and Hygiene. 1998;59:133–138. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- Apt W, Arribada A, Zulantay I, Rodríguez J, Saavedra M, Muñoz A. Treatment of Chagas’ disease with itraconazole: electrocardiographic and parasitological conditions after 20 years of follow-up. Journal of Antimicrobial Chemotherapy. 2013 doi: 10.1093/jac/dkt135. [DOI] [PubMed] [Google Scholar]
- Arnalich-Montiel F, Martin-Navarro CM, Alio JL, Lopez-Velez R, Martinez-Carretero E, Valladares B, Pinero JE, Lorenzo-Morales J. Successful monitoring and treatment of intraocular dissemination of acanthamoeba. Archives of Ophthalmology. 2012;130:1474–1475. doi: 10.1001/archophthalmol.2012.2376. [DOI] [PubMed] [Google Scholar]
- Aufderheide AC, Salo W, Madden M, Streitz J, Buikstra J, Guhl F, Arriaza B, Renier C, Wittmers LE, Fornaciari G, Allison M. A 9,000-year record of Chagas’ disease. Proceedings of National Academy of Sciences of the USA. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Edell E, Eghrari AO, Gottsch JD. Treatment with voriconazole in 3 eyes with resistant Acanthamoeba keratitis. American Journal of Ophthalmology. 2010;149:66–69. doi: 10.1016/j.ajo.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Beach DH, Goad LJ, Holz GG. Effects of ketoconazole on sterol biosynthesis by Trypanosoma cruzi epimastigotes. Biochemical and Biophysical Research Communications. 1986;136:851–856. doi: 10.1016/0006-291x(86)90410-9. [DOI] [PubMed] [Google Scholar]
- Beach DH, Goad LJ, Holz GG., Jr Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Molecular and Biochemical Parasitology. 1988;31:149–162. doi: 10.1016/0166-6851(88)90166-1. [DOI] [PubMed] [Google Scholar]
- Benaim G, Sanders JM, Garcia-Marchan Y, Colina C, Lira R, Caldera AR, Payares G, Sanoja C, Burgos JM, Leon-Rossell A, Concepcion JL, Schijman AG, Levin M, Oldfield E, Urbina JA. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. Journal of Medicinal Chemistry. 2006;49:892–899. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clinical Infectious Diseases. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- Berman JD, Holz GG, Jr, Beach DH. Effects of ketoconazole on growth and sterol biosynthesis of Leishmania mexicana promastigotes in culture. Molecular and Biochemical Parasitology. 1984;12:1–13. doi: 10.1016/0166-6851(84)90039-2. [DOI] [PubMed] [Google Scholar]
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clinical Microbiology Reviews. 2011;24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli D. A clinical trial of itraconazole in the treatment of deep mycoses and leishmaniasis. Reviews of Infectious Diseases. 1987;9(Suppl 1):S57–63. doi: 10.1093/clinids/9.supplement_1.s57. [DOI] [PubMed] [Google Scholar]
- Borelli D, Bran JL, Fuentes J, Legendre R, Leiderman E, Levine HB, Restrepo A, Stevens DA. Ketoconazole, an oral antifungal: laboratory and clinical assessment of imidazole drugs. Postgraduate Medical Journal. 1979;55:657–661. doi: 10.1136/pgmj.55.647.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick KE, Agger WA. Successful treatment of brainstem blastomycosis with fluconazole. Clinical Medicine and Research. 2012;10:72–74. doi: 10.3121/cmr.2011.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clinical Infectious Diseases. 2009;48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- Buchkowsky SS, Partovi N, Ensom MH. Clinical pharmacokinetic monitoring of itraconazole is warranted in only a subset of patients. Therapeutic Drug Monitoring. 2005;27:322–333. doi: 10.1097/01.ftd.0000150135.22645.ea. [DOI] [PubMed] [Google Scholar]
- Buckner F, Bahia MT, Suryadevara PK, White KL, Shackleford DM, Chennamaneni NK, Hulverson MA, Laydbak JU, Chatelain E, Scandale I, Verlinde CL, Charman SA, Lepesheva GI, Gelb MH. Pharmacological characterization, structural studies, and in vivo activity of anti-Chagas disease lead compounds derived from tipifarnib. Antimicrobial Agents and Chemotherapy. 2012;56:4914–4921. doi: 10.1128/AAC.06244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner FS, Urbina JA. Recent developments in sterol 14-demethylase inhibitors for Chagas disease. International Journal for Parasitology, Drugs and Drug Resistance. 2012;2:236–242. doi: 10.1016/j.ijpddr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E, Pastor FJ, Rodríguez MM, Mayayo E, Salas V, Guarro J. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrobial Agents and Chemotherapy. 2010;54:919–923. doi: 10.1128/aac.01284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos R, Amato Neto V, Moreira AA, de Souza HB, Okumura M, Pinto PL, Braz LM, Silva MF, Matsubara L. [Evaluation of the therapeutic activity of fluconazole in acute experimental infection caused by Trypanosoma cruzi] Revista do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. 1992;47:174–175. [PubMed] [Google Scholar]
- Carbajal H, Soltero L, Rodriguez-Montalvo C, Valdes A. Cyclosporine and low-dose ketoconazole in renal transplant recipients: a single-center experience. Transplantation. 2004;77:1038–1040. doi: 10.1097/01.tp.0000122343.51904.c3. [DOI] [PubMed] [Google Scholar]
- Chapman SA, Lake KD, Solbrack DF, Milfred SK, Marshall PS, Kamps MA. Considerations for using ketoconazole in solid organ transplant recipients receiving cyclosporine immunosuppression. Journal of Transplant Coordination. 1996;6:148–154. doi: 10.7182/prtr.1.6.3.660840145v3g3631. [DOI] [PubMed] [Google Scholar]
- Cherkesova TS, Hargrove TY, Vanrell MC, Ges I, Usanov SA, Romano PS, Lepesheva GI. Sequence variation in CYP51A from the Y strain of Trypanosoma cruzi alters its sensitivity to inhibition. FEBS Letters. 2014;588:3878–3885. doi: 10.1016/j.febslet.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NM, Grim SA, Lynch JP., 3rd Posaconazole: use in the prophylaxis and treatment of fungal infections. Seminars in Respiratory and Critical Care Medicine. 2015;36:767–785. doi: 10.1055/s-0035-1562902. [DOI] [PubMed] [Google Scholar]
- Coppens I, Courtoy PJ. The adaptative mechanisms of Trypanosoma brucei for sterol homeostasis in its different life-cycle environments. Annual Review of Microbiology. 2000;54:129–156. doi: 10.1146/annurev.micro.54.1.129. [DOI] [PubMed] [Google Scholar]
- Cornely OA. Isavuconazole: is there a need for a new antifungal? Journal of Antimicrobial Chemotherapy. 2017;72:i2–i4. doi: 10.1093/jac/dkx027. [DOI] [PubMed] [Google Scholar]
- Dalvie D, Kalgutkar A, Khojasteh-Bakht S, Obach R, O’Donnell J. Biotransformation reactions of five-membered aromatic heterocyclic rings. Chemical Research in Toxicology. 2002;15:269–299. doi: 10.1021/tx015574b. [DOI] [PubMed] [Google Scholar]
- Daly K, De Lima H, Kato H, Sordillo EM, Convit J, Reyes-Jaimes O, Zerpa O, Paniz-Mondolfi AE. Intermediate cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis successfully treated with fluconazole. Clinical and Experimental Dermatology. 2014;39:708–712. doi: 10.1111/ced.12359. [DOI] [PubMed] [Google Scholar]
- Dauchy FA, Bonhivers M, Landrein N, Dacheux D, Courtois P, Lauruol F, Daulouede S, Vincendeau P, Robinson DR. Trypanosoma brucei CYP51: Essentiality and targeting therapy in an experimental model. PLoS Neglected Tropical Diseases. 2016;10:e0005125. doi: 10.1371/journal.pntd.0005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascale G, Tumbarello M. Fungal infections in the ICU: advances in treatment and diagnosis. Current Opinion in Critical Care. 2015;21:421–429. doi: 10.1097/mcc.0000000000000230. [DOI] [PubMed] [Google Scholar]
- Debruyne D. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clinical Pharmacokinetics. 1997;33:52–77. doi: 10.2165/00003088-199733010-00005. [DOI] [PubMed] [Google Scholar]
- Denning DW, Bromley MJ. How to bolster the antifungal pipeline. Science. 2015;347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- Denning DW, Tucker RM, Hanson LH, Stevens DA. Treatment of invasive aspergillosis with itraconazole. American Journal of Medicine. 1989;86:791–800. doi: 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- Docampo R, Moreno SN, Turrens JF, Katzin AM, Gonzalez-Cappa SM, Stoppani AO. Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1981;3:169–180. doi: 10.1016/0166-6851(81)90047-5. 0166-6851(81)90047-5 [pii] [DOI] [PubMed] [Google Scholar]
- Dogra J, Saxena VN. Itraconazole and leishmaniasis: a randomised double-blind trial in cutaneous disease. International Journal of Parasitology. 1996;26:1413–1415. doi: 10.1016/s0020-7519(96)00128-2. [DOI] [PubMed] [Google Scholar]
- El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran A-N, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ, Caler E, Cerqueira GC, Branche C, Haas B, Anupama A, Arner E, Åslund L, Attipoe P, Bontempi E, Bringaud F, Burton P, Cadag E, Campbell DA, Carrington M, Crabtree J, Darban H, da Silveira JF, de Jong P, Edwards K, Englund PT, Fazelina G, Feldblyum T, Ferella M, Frasch AC, Gull K, Horn D, Hou L, Huang Y, Kindlund E, Klingbeil M, Kluge S, Koo H, Lacerda D, Levin MJ, Lorenzi H, Louie T, Machado CR, McCulloch R, McKenna A, Mizuno Y, Mottram JC, Nelson S, Ochaya S, Osoegawa K, Pai G, Parsons M, Pentony M, Pettersson U, Pop M, Ramirez JL, Rinta J, Robertson L, Salzberg SL, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Tammi MT, Tarleton R, Teixeira S, Van Aken S, Vogt C, Ward PN, Wickstead B, Wortman J, White O, Fraser CM, Stuart KD, Andersson B. The Genome Sequence of Trypanosoma cruzi, Etiologic Agent of Chagas Disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- Elewa H, El-Mekaty E, El-Bardissy A, Ensom MHH, Wilby KJ. Therapeutic drug monitoring of voriconazole in the management of invasive fungal infections: a critical review. Clinical Pharmacokinetics. 2015;54:1223–1235. doi: 10.1007/s40262-015-0297-8. [DOI] [PubMed] [Google Scholar]
- Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clinical Pharmacokinetics. 2005;44:211–220. doi: 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- Farowski F, Cornely OA, Vehreschild JJ, Hartmann P, Bauer T, Steinbach A, Ruping MJ, Muller C. Intracellular concentrations of posaconazole in different compartments of peripheral blood. Antimicrobial Agents and Chemotherapy. 2010;54:2928–2931. doi: 10.1128/aac.01407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clinical Microbiology Reviews. 2014;27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Friggeri L, Hargrove TY, Rachakonda G, Williams AD, Wawrzak Z, Di Santo R, De Vita D, Waterman MR, Tortorella S, Villalta F, Lepesheva GI. Structural basis for rational design of inhibitors targeting Trypanosoma cruzi sterol 14α-demethylase: two regions of the enzyme molecule potentiate its inhibition. Journal of Medicinal Chemistry. 2014;57:6704–6717. doi: 10.1021/jm500739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling RA. Overview of medically important antifungal azole derivatives. Clinical Microbiology Reviews. 1988;1:187–217. doi: 10.1128/cmr.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye LL, Leonard DA. Lanosterol analogs: dual-action inhibitors of cholesterol biosynthesis. Critical Reviews in Biochemistry and Molecular Biology. 1999;34:123–140. doi: 10.1080/10409239991209246. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Rauf SJ, Nader R, Popov V, Borkowski J, Chaljub G, Nauta HW, Visvesvara GS. Amoebic encephalitis due to Sappinia diploidea. Jama. 2001;285:2450–2451. doi: 10.1001/jama.285.19.2450. [DOI] [PubMed] [Google Scholar]
- Goad LJ, Berens RL, Marr JJ, Beach DH, Holz GG., Jr The activity of ketoconazole and other azoles against Trypanosoma cruzi: biochemistry and chemotherapeutic action in vitro. Molecular and Biochemical Parasitology. 1989;32:179–189. doi: 10.1016/0166-6851(89)90069-8. [DOI] [PubMed] [Google Scholar]
- Goad LJ, Holz GG, Jr, Beach DH. Sterols of ketoconazole-inhibited Leishmania mexicana mexicana promastigotes. Molecular and Biochemical Parasitology. 1985;15:257–279. doi: 10.1016/0166-6851(85)90089-1. [DOI] [PubMed] [Google Scholar]
- Graybill JR, Craven PC. Antifungal agents used in systemic mycoses. Activity and therapeutic use. Drugs. 1983;25:41–62. doi: 10.2165/00003495-198325010-00003. [DOI] [PubMed] [Google Scholar]
- Guedes-da-Silva FH, Batista DG, Da Silva CF, De Araujo JS, Pavao BP, Simoes-Silva MR, Batista MM, Demarque KC, Moreira OC, Britto C, Lepesheva GI, Soeiro MN. Antitrypanosomal activity of sterol 14alpha-demethylase (CYP51) inhibitors VNI and VFV in the Swiss mouse models of Chagas disease induced by the Trypanosoma cruzi Y strain. Antimicrobial Agents and Chemotherapy. 2017:61. doi: 10.1128/aac.02098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulin JEN, Eagleson MA, Postan M, Cutrullis RA, Freilij H, Bournissen FG, Petray PB, Altcheh J. Efficacy of voriconazole in a murine model of acute Trypanosoma cruzi infection. Journal of Antimicrobial Chemotherapy. 2013;68:888–894. doi: 10.1093/jac/dks478. [DOI] [PubMed] [Google Scholar]
- Gullo A. Invasive fungal infections. Drugs. 2009;69:65–73. doi: 10.2165/11315530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Halim MA, Alfurayh O, Kalin ME, Dammas S, al-Eisa A, Damanhouri G. Successful treatment of visceral leishmaniasis with allopurinol plus ketoconazole in a renal transplant recipient after the occurrence of pancreatitis due to stibogluconate. Clinical Infectious Diseases. 1993;16:397–399. doi: 10.1093/clind/16.3.397. [DOI] [PubMed] [Google Scholar]
- Hardin TC, Graybill JR, Fetchick R, Woestenborghs R, Rinaldi MG, Kuhn JG. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrobial Agents and Chemotherapy. 1988;32:1310–1313. doi: 10.1128/aac.32.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Friggeri L, Wawrzak Z, Qi A, Hoekstra WJ, Schotzinger RJ, York JD, Guengerich FP, Lepesheva GI. Structural analyses of Candida albicans sterol 14alpha-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. Journal of Biological Chemistry. 2017a;292:6728–6743. doi: 10.1074/jbc.M117.778308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Friggeri L, Wawrzak Z, Sivakumaran S, Yazlovitskaya EM, Hiebert SW, Guengerich FP, Waterman MR, Lepesheva GI. Human sterol 14alpha-demethylase as a target for anticancer chemotherapy: towards structure-aided drug design. Journal of Lipid Research. 2016;57:1552–1563. doi: 10.1194/jlr.M069229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Garvey EP, Hoekstra WJ, Yates CM, Wawrzak Z, Rachakonda G, Villalta F, Lepesheva GI. Crystal structure of the new investigational drug candidate VT-1598 in complex with Aspergillus fumigatus sterol 14alpha-demethylase provides insights into its broad-spectrum antifungal activity. Antimicrobial Agents and Chemotherapy. 2017b:61. doi: 10.1128/aac.00570-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Kim K, de Nazaré Correia Soeiro M, da Silva CF, da Gama Jaen Batista D, Batista MM, Yazlovitskaya EM, Waterman MR, Sulikowski GA, Lepesheva GI. CYP51 structures and structure-based development of novel, pathogen-specific inhibitory scaffolds. International Journal of Parasitology. Drugs and Drug Resistance. 2012a;2:178–186. doi: 10.1016/j.ijpddr.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Alexander PW, Chaplin JH, Keenan M, Charman SA, Waterman MR, Chatelain E, Lepesheva GI. Complexes of Trypanosoma cruzi sterol 14α-demethylase (CYP51) with two pyridine-based drug candidates for Chagas disease: structural basis for pathogen selectivity. Journal of Biological Chemistry. 2013;288:31602–31615. doi: 10.1074/jbc.M113.497990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Lamb DC, Guengerich FP, Lepesheva GI. Structure-functional characterization of cytochrome P450 sterol 14α-demethylase (CYP51B) from Aspergillus fumigatus and molecular basis for the development of antifungal drugs. Journal of Biological Chemistry. 2015;290:23916–23934. doi: 10.1074/jbc.M115.677310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Liu J, Nes WD, Waterman MR, Lepesheva GI. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14alpha-demethylase (CYP51) from Leishmania infantum. Journal of Biological Chemistry. 2011;286:26838–26848. doi: 10.1074/jbc.M111.237099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Liu J, Waterman MR, Nes WD, Lepesheva GI. Structural complex of sterol 14α-demethylase (CYP51) with 14α-methylenecyclopropyl-Δ7-24, 25-dihydrolanosterol. Journal of Lipid Research. 2012b;53:311–320. doi: 10.1194/jlr.M021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich BA, Singha UK, Miller MB, Nes CR, Anyatonwu H, Lecordier L, Patkar P, Leaver DJ, Villalta F, Vanhollebeke B, Chaudhuri M, Nes WD. Discovery of an ergosterol-signaling factor that regulates Trypanosoma brucei growth. Journal of Lipid Research. 2015;56:331–341. doi: 10.1194/jlr.M054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heel RC, Brogden RN, Carmine A, Morley PA, Speight TM, Avery GS. Ketoconazole: a review of its therapeutic efficacy in superficial and systemic fungal infections. Drugs. 1982;23:1–36. doi: 10.2165/00003495-198223010-00001. [DOI] [PubMed] [Google Scholar]
- Heeres J, Meerpoel L, Lewi P. Conazoles. Molecules. 2010;15:4129–4188. doi: 10.3390/molecules15064129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heykants J, Van Peer A, Lavrijsen K, Meuldermans W, Woestenborghs R, Cauwenbergh G. Pharmacokinetics of oral antifungals and their clinical implications. British Journal of Clinical Practice. Supplement. 1990;71:50–56. [PubMed] [Google Scholar]
- Hirst LW, Green WR, Merz W, Kaufmann C, Visvesvara GS, Jensen A, Howard M. Management of Acanthamoeba keratitis. A case report and review of the literature. Ophthalmology. 1984;91:1105–1111. doi: 10.1016/s0161-6420(84)34200-2. [DOI] [PubMed] [Google Scholar]
- Hoekstra WJ, Hargrove TY, Wawrzak Z, da Gama Jaen Batista D, da Silva CF, Nefertiti AS, Rachakonda G, Schotzinger RJ, Villalta F, Soeiro Mde N, Lepesheva GI. Clinical Candidate VT-1161’s antiparasitic effect in vitro, activity in a murine model of Chagas disease, and structural characterization in complex with the target enzyme CYP51 from Trypanosoma cruzi. Antimicrobial Agents and Chemotherapy. 2016;60:1058–1066. doi: 10.1128/aac.02287-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horber FF, Lerut JP, Reichen J, Zimmermann A, Jaeger P, Malinverni R. Visceral leishmaniasis after orthotopic liver transplantation: impact of persistent splenomegaly. Transplant International. 1993;6:55–57. doi: 10.1007/BF00336642. [DOI] [PubMed] [Google Scholar]
- Huang YC, Colaizzi JL, Bierman RH, Woestenborghs R, Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrobial Agents and Chemotherapy. 1986;30:206–210. doi: 10.1128/aac.30.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Uchida K, Nishiyama Y, Yamaguchi H, Abe S. Oral administration of itraconazole solution has superior efficacy in experimental oral and oesophageal candidiasis in mice than its intragastric administration. Journal of Antimicrobial Chemotherapy. 2007;59:317–320. doi: 10.1093/jac/dkl472. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Matsumoto Y, Kabata T, Watanabe R, Hommura S, Yasuraoka K, Ishii K. Oral itraconazole and topical miconazole with debridement for Acanthamoeba keratitis. American Journal of Ophthalmology. 1990;109:121–126. doi: 10.1016/s0002-9394(14)75974-4. [DOI] [PubMed] [Google Scholar]
- Jennings TS, Hardin TC. Treatment of aspergillosis with itraconazole. Annals of Pharmacotherapy. 1993;27:1206–1211. doi: 10.1177/106002809302701011. [DOI] [PubMed] [Google Scholar]
- Jeong W, Haywood P, Shanmuganathan N, Lindsay J, Urbancic K, Ananda-Rajah MR, Chen SC, Bajel A, Ritchie D, Grigg A, Seymour JF, Peleg AY, Kong DC, Slavin MA. Safety, clinical effectiveness and trough plasma concentrations of intravenous posaconazole in patients with haematological malignancies and/or undergoing allogeneic haematopoietic stem cell transplantation: off-trial experience. Journal of Antimicrobial Chemotherapy. 2016;71:3540–3547. doi: 10.1093/jac/dkw322. [DOI] [PubMed] [Google Scholar]
- Joice AC, Yang S, Farahat AA, Meeds H, Feng M, Li J, Boykin DW, Wang MZ, Werbovetz KA. Antileishmanial efficacy and pharmacokinetics of DB766-azole combinations. Antimicrobial Agents and Chemotherapy. 2017 doi: 10.1128/aac.01129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe DS. Cutaneous leishmaniasis from Belize--treatment with ketoconazole. Clinical and Experimental Dermatology. 1986;11:62–68. doi: 10.1111/j.1365-2230.1986.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Kalb VF, Loper JC, Dey CR, Woods CW, Sutter TR. Isolation of a cytochrome P-450 structural gene from Saccharomyces cerevisiae. Gene. 1986;45:237–245. doi: 10.1016/0378-1119(86)90021-1. [DOI] [PubMed] [Google Scholar]
- Kauffman CA, Malani AN, Easley C, Kirkpatrick P. Posaconazole. Nature Reviews Drug Discovery. 2007;6:183–184. doi: 10.1038/nrd2270. [DOI] [PubMed] [Google Scholar]
- Keating GM. Posaconazole. Drugs. 2005;65:1553–1567. doi: 10.2165/00003495-200565110-00007. discussion 1568-1559. [DOI] [PubMed] [Google Scholar]
- Konkle ME, Hargrove TY, Kleshchenko YY, von Kries JP, Ridenour W, Uddin MJ, Caprioli RM, Marnett LJ, Nes WD, Villalta F, Waterman MR, Lepesheva GI. Indomethacin amides as a novel molecular scaffold for targeting Trypanosoma cruzi sterol 14α-demethylase. Journal of Medicinal Chemistry. 2009;52:2846–2853. doi: 10.1021/jm801643b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieter P, Flannery B, Musick T, Gohdes M, Martinho M, Courtney R. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrobial Agents and Chemotherapy. 2004;48:3543–3551. doi: 10.1128/aac.48.9.3543-3551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg BJ, Arendrup MC. Invasive Candidiasis. New England Journal of Medicine. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- Lamb DC, Warrilow AG, Rolley NJ, Parker JE, Nes WD, Smith SN, Kelly DE, Kelly SL. Azole antifungal agents to treat the human pathogens Acanthamoeba castellanii and Acanthamoeba polyphaga through inhibition of sterol 14alpha-demethylase (CYP51) Antimicrobial Agents and Chemotherapy. 2015;59:4707–4713. doi: 10.1128/aac.00476-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections. Drugs. 2011;71:2405–2419. doi: 10.2165/11596540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lepesheva G, Hargrove T, Kleshchenko Y, Nes W, Villalta F, Waterman M. CYP51: a major drug target in the cytochrome P450 superfamily. Lipids. 2008;43:1117–1125. doi: 10.1007/s11745-008-3225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI. Design or screening of drugs for the treatment of Chagas disease: what shows the most promise? Expert Opinion on Drug Discovery. 2013;8:1479–1489. doi: 10.1517/17460441.2013.845554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Hargrove TY, Anderson S, Kleshchenko Y, Furtak V, Wawrzak Z, Villalta F, Waterman MR. Structural insights into inhibition of sterol 14 alpha-demethylase in the human pathogen Trypanosoma cruzi. Journal of Biological Chemistry. 2010a;285:25582–25590. doi: 10.1074/jbc.M110.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Hargrove TY, Rachakonda G, Wawrzak Z, Pomel S, Cojean S, Nde PN, Nes WD, Locuson CW, Calcutt MW, Waterman MR, Daniels JS, Loiseau PM, Villalta F. VFV as a new effective CYP51 structure-derived drug candidate for Chagas disease and visceral leishmaniasis. Journal of Infectious Diseases. 2015;212:1439–1448. doi: 10.1093/infdis/jiv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Nes WD, Zhou W, Hill GC, Waterman MR. CYP51 from Trypanosoma brucei is obtusifoliol-specific. Biochemistry. 2004;43:10789–10799. doi: 10.1021/bi048967t. [DOI] [PubMed] [Google Scholar]
- Lepesheva GI, Ott RD, Hargrove TY, Kleshchenko YY, Schuster I, Nes WD, Hill GC, Villalta F, Waterman MR. Sterol 14 alpha-demethylase as a potential target for antitrypanosomal therapy: enzyme inhibition and parasite cell growth. Chemistry Biology. 2007;14:1283–1293. doi: 10.1016/j.chembiol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Park HW, Hargrove TY, Vanhollebeke B, Wawrzak Z, Harp JM, Sundaramoorthy M, Nes WD, Pays E, Chaudhuri M, Villalta F, Waterman MR. Crystal structures of Trypanosoma brucei sterol 14 alpha-demethylase and implications for selective treatment of human infections. Journal of Biological Chemistry. 2010b;285:1773–1780. doi: 10.1074/jbc.M109.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Waterman MR. CYP51--the omnipotent P450. Molecular and Cellular Endocrinology. 2004;215:165–170. doi: 10.1016/j.mce.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochimica Biophysica Acta. 2007;1770:467–477. doi: 10.1016/j.bbagen.2006.07.018. S0304-4165(06)00214-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Waterman MR. Structural basis for conservation in the CYP51 family. Biochimica Biophysica Acta. 2011;1814:88–93. doi: 10.1016/j.bbapap.2010.06.006. S1570-9639(10)00164-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Zaitseva NG, Nes WD, Zhou W, Arase M, Liu J, Hill GC, Waterman MR. CYP51 from Trypanosoma cruzi: a phyla-specific residue in the B’ helix defines substrate preferences of sterol 14alpha-demethylase. Journal of Biological Chemistry. 2006;281:3577–3585. doi: 10.1074/jbc.M510317200. [DOI] [PubMed] [Google Scholar]
- Leslie M. Drug developers finally take aim at a neglected disease. Science. 2011;333:933–935. doi: 10.1126/science.333.6045.933. [DOI] [PubMed] [Google Scholar]
- Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clinical Pharmacokinetics. 2010;49:379–396. doi: 10.2165/11319340-000000000-00000. 310.2165/11319340-000000000-000000000. [DOI] [PubMed] [Google Scholar]
- Lopez-Barcons L, Maurer BJ, Kang MH, Reynolds CP. P450 inhibitor ketoconazole increased the intratumor drug levels and antitumor activity of fenretinide in human neuroblastoma xenograft models. International Journal of Cancer. 2017;141:405–413. doi: 10.1002/ijc.30706. [DOI] [PubMed] [Google Scholar]
- Louie A, Drusano GL, Banerjee P, Liu QF, Liu W, Kaw P, Shayegani M, Taber H, Miller M|H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrobial Agents and Chemotherapy. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee D-G, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann A-H, Zeiher B, Ullmann AJ. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. The Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Diaz RA, Escario JA, Nogal-Ruiz JJ, Gomez-Barrio A. Biological characterization of Trypanosoma cruzi strains. Memorias do Instituto Oswaldo Cruz. 2001;96:53–59. doi: 10.1590/s0074-02762001000100006. [DOI] [PubMed] [Google Scholar]
- Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrobial Agents Chemotherapy. 2010;54:4758–4764. doi: 10.1128/aac.00606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe RE, Araujo FG, Remington JS. Ketoconazole protects against infection with Trypanosoma cruzi in a murine model. American Journal of Tropical Medicine and Hygiene. 1983;32:960–962. doi: 10.4269/ajtmh.1983.32.960. [DOI] [PubMed] [Google Scholar]
- McCabe RE, Remington JS, Araujo FG. Ketoconazole inhibition of intracellular multiplication of Trypanosoma cruzi and protection of mice against lethal infection with the organism. Journal of Infectious Diseases. 1984;150:594–601. doi: 10.1093/infdis/150.4.594. [DOI] [PubMed] [Google Scholar]
- McCabe RE, Remington JS, Araujo FG. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. American Journal of Tropical Medicine and Hygiene. 1986;35:280–284. doi: 10.4269/ajtmh.1986.35.280. [DOI] [PubMed] [Google Scholar]
- Mellado E, Diaz-Guerra TM, Cuenca-Estrella M, Rodriguez-Tudela JL. Identification of two different 14-{alpha} sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. Journal of Clinical Microbiology. 2001;39:2431–2438. doi: 10.1128/jcm.39.7.2431-2438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K, Gibbons G, BE R. Lanosterol 14alpha-demethylase. Similarity of the enzyme system from yeast and rat liver. Steroids. 1976;6:821–829. doi: 10.1016/0039-128x(76)90141-0. [DOI] [PubMed] [Google Scholar]
- Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A. Randomized Trial of Posaconazole and Benznidazole for Chronic Chagas’ Disease. New England Journal of Medicine. 2014;370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- Molina I, Salvador F, Sa´nchez-Montalva´ A. The use of posaconazole against Chagas disease. Current Opinion in Infectious Diseases. 2015;5:397–407. doi: 10.1097/QCO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- Molina J, Martins-Filho O, Brener Z, Romanha AJ, Loebenberg D, Urbina JA. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrobial Agents and Chemotherapy. 2000;44:150–155. doi: 10.1128/aac.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni AZ, Jalayer T, Emamjomeh M, Bashardost N, Ghassemi RL, Meghdadi M, Javadi A, Aminjavaheri M. Treatment of cutaneous leishmaniasis with itraconazole. Randomized double-blind study. Archives of Dermatology. 1996;132:784–786. [PubMed] [Google Scholar]
- Morillo CA, Waskin H, Sosa-Estani S, Del Carmen Bangher M, Cuneo C, Milesi R, Mallagray M, Apt W, Beloscar J, Gascon J, Molina I, Echeverria LE, Colombo H, Perez-Molina JA, Wyss F, Meeks B, Bonilla LR, Gao P, Wei B, McCarthy M, Yusuf S. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: The STOP-CHAGAS trial. Journal of American College of Cardiology. 2017;69:939–947. doi: 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Morschhauser J. The development of fluconazole resistance in Candida albicans - an example of microevolution of a fungal pathogen. Journal of Microbiology. 2016;54:192–201. doi: 10.1007/s12275-016-5628-4. [DOI] [PubMed] [Google Scholar]
- Morton V, Staub T. A short history of fungicides. 2008 doi10.1094/APSnetFeature-2008-0308. [Google Scholar]
- Mosca P, Bonazzi P, Novelli G, Jezequel AM, Orlandi F. In vivo and in vitro inhibition of hepatic microsomal drug metabolism by ketoconazole. British Journal of Experimental Pathology. 1985;66:737–742. [PMC free article] [PubMed] [Google Scholar]
- Navin TR, Arana BA, Arana FE, Berman JD, Chajon JF. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. Journal of Infectious Diseases. 1992;165:528–534. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- Nelson DR. Cytochrome P450 and the individuality of species. Archives of Biochemistry and Biophysics. 1999;369:1–10. doi: 10.1006/abbi.1999.1352. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, Gonzalez FJ, Coon MJ, Gunsalus IC, Gotoh O, et al. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA and Cell Biology. 1993;12:1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Nes WD. Biosynthesis of cholesterol and other sterols. Chemical Reviews. 2011;111:6423–6451. doi: 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomeir AA, Pramanik BN, Heimark L, Bennett F, Veals J, Bartner P, Hilbert M, Saksena A, McNamara P, Girijavallabhan V, Ganguly AK, Lovey R, Pike R, Wang H, Liu YT, Kumari P, Korfmacher W, Lin CC, Cacciapuoti A, Loebenberg D, Hare R, Miller G, Pickett C. Posaconazole (Noxafil, SCH 56592), a new azole antifungal drug, was a discovery based on the isolation and mass spectral characterization of a circulating metabolite of an earlier lead (SCH 51048) Journal of Mass Spectrometry. 2008;43:509–517. doi: 10.1002/jms.1341. [DOI] [PubMed] [Google Scholar]
- Ohba M, Sato R, Yoshida Y, Nishino T, Katsuki H. Involvement of cytochrome P-450 and a cyanide-sensitive enzyme in different steps of lanosterol demethylation by yeast microsomes. Biochemical and Biophysical Research Communications. 1978;85:21–27. doi: 10.1016/S0006-291X(78)80005-9. [DOI] [PubMed] [Google Scholar]
- Oliva S, Jantz M, Tiernan R, Cook DL, Judson MA. Successful treatment of widely disseminated acanthamoebiasis. Southern Medical Journal. 1999;92:55–57. doi: 10.1097/00007611-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for Its hemoprotein nature. Journal of Biological Chemistry. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Paniz Mondolfi AE, Stavropoulos C, Gelanew T, Loucas E, Perez Alvarez AM, Benaim G, Polsky B, Schoenian G, Sordillo EM. Successful treatment of Old World cutaneous leishmaniasis caused by Leishmania infantum with posaconazole. Antimicrobial Agents and Chemotherapy. 2011;55:1774–1776. doi: 10.1128/aac.01498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clinical Infectious Diseases. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualotto AC, Xavier MO, Andreolla HF, Linden R. Voriconazole therapeutic drug monitoring: focus on safety. Expert Opin Drug Saf. 2010;9:125–137. doi: 10.1517/14740330903485637. [DOI] [PubMed] [Google Scholar]
- Patel T, Dhillon S. Efinaconazole: first global approval. Drugs. 2013;73:1977–1983. doi: 10.1007/s40265-013-0152-x. [DOI] [PubMed] [Google Scholar]
- Peng LW, Lien YH. Pharmacokinetics of single, oral-dose voriconazole in peritoneal dialysis patients. American Journal of Kidney Disease. 2005;45:162–166. doi: 10.1053/j.ajkd.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Perez-Molina JA, Molina I. Chagas disease. Lancet. 2017 doi: 10.1016/s0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clinical Infectious Diseases. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit NN, Carver PL. Isavuconazole: a new option for the management of invasive fungal infections. Annals of Pharmacotherapy. 2015;49:825–842. doi: 10.1177/1060028015581679. [DOI] [PubMed] [Google Scholar]
- Pinazo MJ, Espinosa G, Gállego M, López-Chejade PL, Urbina JAJG. Successful treatment with posaconazole of a ratient with chronic Chagas disease and systemic lupus erythematosus. American Journal of Tropical Medicine and Hygiene. 2010;82:583–587. doi: 10.4269/ajtmh.2010.09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinger J, Chowdhury S, Papavasiliou FN. Variant surface glycoprotein density defines an immune evasion threshold for African trypanosomes undergoing antigenic variation. Nature Communications. 2017;8:828. doi: 10.1038/s41467-017-00959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raederstorff D, Rohmer M. Sterol biosynthesis de nova via cycloartenol by the soil amoeba Acanthamoeba polyphaga. Biochemical Journal. 1985;231:609–615. doi: 10.1042/bj2310609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raederstorff D, Rohmer M. Sterol biosynthesis via cycloartenol and other biochemical features related to photosynthetic phyla in the amoeba Naegleria lovaniensis and Naegleria gruberi. European Journal of Biochemistry. 1987;164:427–434. doi: 10.1111/j.1432-1033.1987.tb11075.x. [DOI] [PubMed] [Google Scholar]
- Raether W, Seidenath H. Ketoconazole and other potent antimycotic azoles exhibit pronounced activity against Trypanosoma cruzi, Plasmodium berghei and Entamoeba histolytica in vivo. Zeitschrift Fur Parasitenkunde. 1984;70:135–138. doi: 10.1007/BF00929583. [DOI] [PubMed] [Google Scholar]
- Rodriguez MM, Pastor FJ, Calvo E, Salas V, Sutton DA, Guarro J. Correlation of In Vitro Activity,serum levels, and in vivo efficacy of posaconazole against Rhizopus microsporus in a murine disseminated infection. Antimicrobial Agents and Chemotherapy. 2009;53:5022–5025. doi: 10.1128/aac.01026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque ALR, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. International Journal for Parasitology: Parasites and Wildlife. 2014;3:251–262. doi: 10.1016/j.ijppaw.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak JM, Marx KR, Nishimoto AT, Rogers PD. Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy. 2015;35:1037–1051. doi: 10.1002/phar.1652. [DOI] [PubMed] [Google Scholar]
- Saenz RE, Paz H, Berman JD. Efficacy of ketoconazole against Leishmania braziliensis panamensis cutaneous leishmaniasis. The American Journal of Medicine. 1990;89:147–155. doi: 10.1016/0002-9343(90)90292-l. [DOI] [PubMed] [Google Scholar]
- Sawyer PRBR, Pinder RM, Speight TM, Avery GS. Miconazole: a review of its antifungal activity and therapeutic efficacy. Drugs. 1975a;9:406–423. doi: 10.2165/00003495-197509060-00002. [DOI] [PubMed] [Google Scholar]
- Sawyer PRBR, Pinder RM, Speight TM, Avery Clotrimazole: a review of its antifungal activity and therapeutic efficacy. Drugs. 1975b;9:424–447. doi: 10.2165/00003495-197509060-00003. [DOI] [PubMed] [Google Scholar]
- Schell WA, Jones AM, Garvey EP, Hoekstra WJ, Schotzinger RJ, Alexander BD. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against Candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrobial Agents and Chemotherapy. 2017:61. doi: 10.1128/aac.01817-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijo Martinez M, Gonzalez-Mediero G, Santiago P, Rodriguez De Lope A, Diz J, Conde C, Visvesvara GS. Granulomatous amebic encephalitis in a patient with AIDS: isolation of acanthamoeba sp. Group II from brain tissue and successful treatment with sulfadiazine and fluconazole. Journal of Clinical Microbiology. 2000;38:3892–3895. doi: 10.1128/jcm.38.10.3892-3895.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clinical Microbiology Reviews. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AQ, Frutuoso MS, Moraes EA, Pearson RD, Pompeu MM. High-dose oral fluconazole therapy effective for cutaneous leishmaniasis due to Leishmania (Vianna) braziliensis. Clinical Infectious Diseases. 2011;53:693–695. doi: 10.1093/cid/cir496. [DOI] [PubMed] [Google Scholar]
- Sundar S, Kumar K, Singh VP. Ketoconazole in visceral leishmaniasis. The Lancet. 1990;336:1582–1583. doi: 10.1016/0140-6736(90)93358-V. [DOI] [PubMed] [Google Scholar]
- Superko HR, Momary KM, Li Y. Statins personalized. Medical Clinics of North America. 2012;96:123–139. doi: 10.1016/j.mcna.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Thomson S, Rice CA, Zhang T, Edrada-Ebel R, Henriquez FL, Roberts CW. Characterisation of sterol biosynthesis and validation of 14alpha-demethylase as a drug target in Acanthamoeba. Scientific Reports. 2017;7:8247. doi: 10.1038/s41598-017-07495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana J, Armengaud JB, Dupouy Camet J, Gendrel D. Oral fluconazole treatment for extensive cutaneous leishmaniasis in an 11-year-old child. The Pediatric Infectious Diseases Journal. 2006;25:1083–1084. doi: 10.1097/01.inf.0000242968.36675.64. [DOI] [PubMed] [Google Scholar]
- Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, Makni F, Ayadi A. Pathogenic free-living amoebae: epidemiology and clinical review. Pathologie Biologie (Paris) 2012;60:399–405. doi: 10.1016/j.patbio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J, Halpern N, Pont A. Ketoconazole: a novel and rapid treatment for advanced prostatic cancer. The Journal of Urology. 1983;130:152–153. doi: 10.1016/s0022-5347(17)51007-1. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J, Pont A. Ketoconazole therapy for advanced prostate cancer. Lancet. 1984;2:433–435. doi: 10.1016/s0140-6736(84)92909-x. [DOI] [PubMed] [Google Scholar]
- Tracy M, Okorie C, Foley E, Moss R. Allergic bronchopulmonary aspergillosis. Journal of Fungi. 2016;2:17. doi: 10.3390/jof2020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskos J, Kawata S, Gaylor JL. Microsomal enzymes of cholesterol biosynthesis. Purification of lanosterol 14 alpha-methyl demethylase cytochrome P-450 from hepatic microsomes. Journal of Biological Chemistry. 1986;261:14651–14657. [PubMed] [Google Scholar]
- Tu EY, Joslin CE, Shoff ME. Successful treatment of chronic stromal acanthamoeba keratitis with oral voriconazole monotherapy. Cornea. 2010;29:1066–1068. doi: 10.1097/ICO.0b013e3181cbfa2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RM, Haq Y, Denning DW, Stevens DA. Adverse events associated with itraconazole in 189 patients on chronic therapy. Journal of Antimicrobial Chemotherapy. 1990;26:561–566. doi: 10.1093/jac/26.4.561. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Payares G, Contreras LM, Liendo A, Sanoja C, Molina J, Piras M, Piras R, Perez N, Wincker P, Loebenberg D. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrobial Agents and Chemotherapy. 1998;42:1771–1777. doi: 10.1128/aac.42.7.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuyo FG, Zaias N. Oral ketoconazole in the treatment of leishmaniasis. International Journal of Dermatology. 1982;21:414–416. doi: 10.1111/j.1365-4362.1982.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Van den Bossche H, editor. Mode of action of pyridine, pyrimidine and azole antifungals. Ellis Horwood; Chichester: 1988. pp. 79–119. [Google Scholar]
- Van den Bossche H, Willemsens G, Cools W, Lauwers WF, Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chemico-Biological Interactions. 1978;21:59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H. Biochemical targets for antifungal azole derivatives: hypothesis on the mode of action. In: McGinnis MR, editor. Current topics in medical mycology. Vol. 1. Springer-Verlag; New York: 1985. pp. 313–351. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H, Marichal P, Gorrens J, Bellens D, Verhoeven H, Coene M-C, Lauwers W, Janssen PAJ. Interaction of azole derivatives with cytochrome P-450 isozymes in yeast, fungi, plants and mammalian cells. Pesticide Science. 1987;21:289–306. doi: 10.1002/ps.2780210406. [DOI] [Google Scholar]
- Villalta F, Dobish MC, Nde PN, Kleshchenko YY, Hargrove TY, Johnson CA, Waterman MR, Johnston JN, Lepesheva GI. VNI cures acute and chronic experimental Chagas disease. Journal of Infectious Diseases. 2013;208:504–511. doi: 10.1093/infdis/jit042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia R, Montoya JG, Visvesvera GS, Booton GC, Doyle RL. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transplant Infectious Diseases. 2007;9:51–54. doi: 10.1111/j.1399-3062.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clinical Infectious Diseases. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrobial Agents and Chemotherapy. 2009;53:3453–3461. doi: 10.1128/aac.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D, Umar I, Kolyvas G, Bilbao J, Guiot M-C, Duplisea K, Qvarnstrom Y, Visvesvara GS. Treatment of granulomatous amoebic encephalitis with voriconazole and miltefosine in an immunocompetent soldier. American Journal of Tropical Medicine and Hygiene. 2012;87:715–718. doi: 10.4269/ajtmh.2012.12-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D, Courtney R, Richards W, Banfield C, Lim J, Laughlin M. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. European Journal of Pharmaceutical Sciences. 2004;21:645–653. doi: 10.1016/j.ejps.2004.01.005. [DOI] [PubMed] [Google Scholar]
- White JM, Salisbury JR, Jones J, Higgins EM, Vega-Lopez F. Cutaneous leishmaniasis: three children with Leishmania major successfully treated with itraconazole. Pediatric Dermatology. 2006;23:78–80. doi: 10.1111/j.1525-1470.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Wilby KJ. A Review of the clinical pharmacokinetics and pharmacodynamics of isavuconazole. Eur J Drug Metabolism and Pharmacokinetics. 2017 doi: 10.1007/s13318-017-0445-7. [DOI] [PubMed] [Google Scholar]
- Yeagle PL, Martin RB, Lala AK, Lin HK, Bloch K. Differential effects of cholesterol and lanosterol on artificial membranes. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:4924–4926. doi: 10.1073/pnas.74.11.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Aoyama Y. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. I. Purification and spectral properties. Journal of Biological Chemistry. 1984;259:1655–1660. [PubMed] [Google Scholar]
- Yoshida Y, Aoyama Y. Interaction of azole antifungal agents with cytochrome P-45014DM purified from Saccharomyces cerevisiae microsomes. Biochemical Pharmacology. 1987;36:229–235. doi: 10.1016/0006-2952(87)90694-0. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Aoyama Y, Noshiro M, Gotoh O. Sterol 14-demethylase P450 (CYP51) provides a breakthrough for the discussion on the evolution of cytochrome P450 gene superfamily. Biochemical and Biophysical Research Communications. 2000;273:799–804. doi: 10.1006/bbrc.2000.3030. 10.1006/bbrc.2000.3030S0006-291X(00)93030-4 [pii] [DOI] [PubMed] [Google Scholar]
- Yu X, Nandekar P, Mustafa G, Cojocaru V, Lepesheva GI, Wade RC. Ligand tunnels in T. brucei and human CYP51: Insights for parasite-specific drug design. Biochimica and Biophysica Acta. 2016;1860:67–78. doi: 10.1016/j.bbagen.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF, Sellers EM. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metabolism and Disposition. 2002;30:314–318. doi: 10.1124/dmd.30.3.314. [DOI] [PubMed] [Google Scholar]
- Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infectious Diseases. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MR, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memorias do Instituto Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]