Abstract
Objective
Currently, HIV pre-exposure prophylaxis (PrEP) is not covered by health insurance in the Netherlands. We examined time trends in use of PrEP, characteristics of PrEP users, PrEP eligibility and intention to use PrEP among HIV-negative men who have sex with men (MSM) participating in the Amsterdam Cohort Studies (ACS).
Design
Prospective cohort study.
Methods
We used data from four 6-monthly questionnaire waves, collected between 2015–2017. PrEP use over time was examined in logistic regression models using generalized estimating equations. Using descriptive statistics, we compared PrEP users before first-time initiation to non-PrEP-users. We used national guidelines to assess PrEP eligibility.
Results
We included 687 MSM. Median age was 40 (IQR 33–47) years in 2015. Recent PrEP use was reported by 57/687 (8%) MSM. PrEP use increased over calendar time (P<0.001) to 7% in 2017. PrEP users did not differ from non-PrEP users in socio-demographic characteristics, but reported a significantly higher median number of casual sex partners, more often reported condomless anal sex and chemsex with casual partners, and more often had an sexually transmitted infection in the preceding 6 months (all P<0.05). PrEP eligibility increased over time, but the effect was not statistically significant (P = 0.075). PrEP eligibility criteria were met by 149/460 (32%) at wave 4, of whom 31/149 (21%) reported use of PrEP. The proportion with a high intention to use PrEP was greater among eligible than non-eligible MSM (51% vs. 24%, P<0.001).
Conclusion
PrEP use increased over time but remained under 10%, even though 32% met the eligibility criteria, of whom 51% had a high intention to use PrEP. This suggests that a large proportion of Dutch MSM at risk could benefit from PrEP.
Introduction
Despite proven efficacy and approval of pre-exposure prophylaxis (PrEP) for HIV prevention among men who have sex with men (MSM) in Europe, PrEP is not yet covered by health insurance in most countries, including the Netherlands [1,2]. In the Netherlands, MSM at high risk for HIV currently obtain PrEP free-of-charge only if participating in one of the two PrEP studies at the Public Health Service of Amsterdam: the Amsterdam PrEP project (AMPrEP), a demonstration project, initiated in 2015, to assess the uptake of daily and event-driven PrEP among HIV-negative MSM and transgender persons at increased risk for HIV infection [3], and the international multicenter DISCOVER study, which started enrolment in Amsterdam in 2017 and is aimed at evaluating the efficacy and safety of emtricitabine and tenofovir alafenamide for PrEP [4]. Alternatively, Dutch MSM can obtain PrEP by out-of-pocket purchase abroad or online or through friends or on a doctor’s prescription, which became legal after emtricitabine/tenofovir disoproxil was approved by the European Medicines Agency for the use of PrEP in July 2016 [5]. The price of 30 tablets decreased from 550 euro for the brand to around 50 euro for generics, which have been available since the beginning of 2018 [6]. A community-initiative of gay men has been informing MSM about PrEP access since 2015. This initiative organized self-importation and support for self-obtaining PrEP from the beginning of 2017 up to the availability of the generic product.
PrEP-related additional care (e.g. renal function and hepatitis C virus testing) is not routinely offered at sexually transmitted infections (STI) clinics in the Netherlands and capacity for quarterly visits, as is advised in professional guidelines, is limited. Although primary care providers can offer full testing, knowledge and willingness to prescribe PrEP varies across providers. Moreover, Dutch insurance systems have an obligatory deductible excess, meaning that a minimum of the first 385 euro of health care costs, including laboratory testing ordered by primary care providers, are out-of-pocket. This might cause reticence to visit a primary care provider.
Political support for PrEP has been patchy, with only some cities providing financial resources for health care for self-obtainers. In October 2016, the Minister of Health requested an advice on implementation of PrEP from the Health Council. The report was published in March 2018 and stated that the high burden of the HIV epidemic justifies implementation of PrEP, that a fee for the users can be considered, and that adequate monitoring of those who use PrEP is essential, in addition to national surveillance [7]. In July 2018, the Minister of Health decided to partially reimburse PrEP for 6,500 MSM at substantial risk for HIV within a research setting for a period of 5 years from 2019 onwards [8].
To further inform decision-making on full PrEP implementation and reimbursement, as well as resources needed, in the Netherlands, scientific data are needed on the number of MSM who would be eligible for PrEP, the current intention to use PrEP among MSM, and the characteristics of MSM who self-obtain PrEP [9].
The Amsterdam Cohort Studies (ACS) on HIV was initiated in 1984, and is an open, prospective cohort study among MSM [10]. PrEP awareness and the intention to use PrEP among MSM participating in the ACS was previously investigated between 2012 and 2013 [11]. In that period, 54% reported being aware of PrEP, but only 13% reported a high intention to use daily PrEP. In more recent years, PrEP awareness has likely increased. The added option of event-driven use of PrEP (i.e. PrEP use before and after sex) may have resulted in a higher PrEP uptake and intention to use PrEP. In this current study, we determined PrEP eligibility and intention to use PrEP among MSM participating in the ACS between 2015 and 2017. We moreover tracked PrEP use over time in the ACS and explored differences in characteristics between PrEP users and non-PrEP users.
Materials and methods
Study participants and data collection
HIV-negative MSM participating in the ACS provided data on PrEP through self-administered questionnaires in four 6-month waves from mid-2015 to mid-2017. At each ACS visit, we collected data on socio-demographic characteristics, sexual risk behavior, recreational drug use, chemsex, recent (i.e. in preceding 6 months) and lifetime PrEP use (S1 File); we also tested for HIV and STI (chlamydia, gonorrhea, syphilis) [10]. We distinguished use of study-provided PrEP (via AMPrEP/DISCOVER studies [4,12]) from use of self-obtained PrEP. Chemsex was defined as the use of γ-hydroxybutyric acid(GHB)/γ-butyrolactone (GBL), mephedrone and/or methamphetamine [13]. during sex with a casual partner.
PrEP eligibility for every participant was determined at each wave, using national guidelines provided by the Dutch Association of HIV-treating physicians [14]. PrEP eligibility criteria were 1) having had condomless anal sex (CAS) with a partner with unknown or seropositive HIV status, 2) having a rectal chlamydia or gonorrhea, and/or 3) having a post-exposure prophylaxis (PEP) prescription, all reported or diagnosed in the preceding six months.
Intention to use PrEP was measured at wave 1, separately for daily and event-driven use, by two questions on a seven-point Likert scale: “How likely are you to use PrEP once it becomes available in the Netherlands?” and “Are you planning on using PrEP once it becomes available in the Netherlands?” (S1 File). A score of >4, the median of all MSM, was defined as a high intention for either daily or event-driven PrEP use. A score of <2 was defined as low intention for PrEP use and a score of 2–4 as medium intention for PrEP use, as described previously by Bil et al. [11]. If the score differed between daily and event-driven use, we used the highest one.
Statistical analysis
To compare socio-demographic characteristics, sexual behavior, recreational drug use, chemsex, STI diagnosis, and PrEP eligibility in the six months prior to PrEP initiation between PrEP users (those reporting use at least once during waves 1–4) and non-PrEP users (those never reporting PrEP use), we employed the unpaired t-test for normally distributed numerical data, the Mann-Whitney U test for non-normally distributed numerical data, and the Chi-square or Fisher’s exact test for categorical data. MSM who reported PrEP use only before 2015 were excluded for this analysis. Information on characteristics and behaviors of PrEP users was taken from the wave prior to the one at which first-time PrEP was reported; information on non-PrEP users was taken from the wave at which most participants reported first-time PrEP use (wave 2). We performed three sensitivity analyses in which we compared PrEP users to non-PrEP users, taking information of non-PrEP users from wave 1, 3 and 4. Time trends in PrEP use and PrEP eligibility were examined in logistic regression models using generalized estimating equations to account for clustering within individuals. The effect of missing data on the PrEP eligibility estimate was explored in sensitivity analyses, assuming extreme values for missing data.
Results were considered significant at a p-value ≤0.05. Analyses were performed using STATA Intercooled 13.1 (STATA Corporation, College Station, Texas, USA).
Ethical approval and informed consent
The ACS was approved by the Medical Ethical Committee of the Academic Medical Center of Amsterdam, the Netherlands. Written informed consent was obtained from all participants at enrolment.
Results
Our study included 687 HIV-negative MSM, of whom the majority was educated at least to college degree (n = 529, 77%) and born in the Netherlands (n = 545, 79%). Median age was 40 (IQR 33–47) years at wave 1 of the study period. Median number of questionnaire waves per participant was 4 (IQR 3–4).
Use of PrEP and characteristics of PrEP users
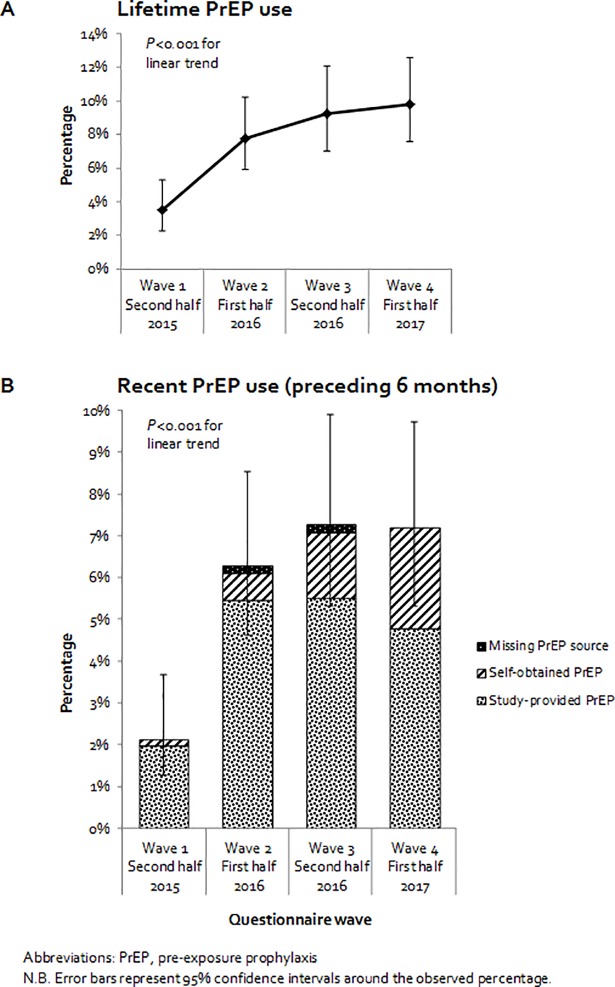
Recent PrEP use was reported by 57 (8%; 95% confidence interval [CI] 6–11%), whereas 621 (90%) reported no PrEP use, and 9 (1%) only reported use before 2015. Of the 57 users, 38 (67%) used study-provided PrEP, and 19 (33%) used self-obtained PrEP at first-time initiation. As for regimen, 20 (35%) reported daily use, 22 (39%) reported event-driven use, and 15 (26%) had missing information on regimen at first-time initiation. Median age at first-time initiation was 40 (IQR 36–48) years. PrEP use increased from 2015 to 2017, with both recent and lifetime use showing linear trends (P<0.001, Fig 1).
Fig 1. Reported lifetime and recent PrEP use among MSM participating in the Amsterdam Cohort Studies between 2015–2017 (four 6-montly waves of questionnaires).
Behavioral data were available for 52/57 PrEP users and 541/621 non-users. Users and non-users did not differ in age, country of birth, or educational level (Table 1). PrEP users had a significantly higher median number of casual partners compared to non-PrEP users (21 [IQR 12–40] vs. 4 [IQR 0–11], P<0.001), and were more likely to report chemsex (43% vs. 7%, P<0.001) and CAS (85% vs. 26%, p<0.001) with casual partners. STIs were more often diagnosed among PrEP users than non-PrEP users (27% vs. 11%, P = 0.001), mainly rectal chlamydia (13% vs. 4%, p = 0.005) and syphilis (8% vs. 1%, P = 0.008). No differences were found in having a steady partner or having CAS with a steady partner (all P>0.05). Of 48 PrEP users with available eligibility information, 34 (71%) were eligible for PrEP, compared to 118/493 (24%) non-PrEP users (P<0.001). Of 16 users of self-obtained PrEP, 10 (63%) were eligible for PrEP prior to initiation. These results were comparable to the results of the sensitivity analyses using information on non-PrEP users of wave 1, 3, and 4.
Table 1. Characteristics of MSM participating in the Amsterdam Cohort Studies between 2015 and 2017: PrEP users before first-time PrEP initiation versus non-PrEP-users.
| PrEP users | Non-PrEP-users | ||||
|---|---|---|---|---|---|
| (n = 52) | (n = 541) | ||||
| Socio-demographic characteristics | N | n (%) | N | n (%) | P-value |
| Age in years (median, IQR) | 52 | 40 [36–48] | 541 | 41 [34–48] | 0.459 |
| Born in the Netherlands | 49 | 43 (88) | 512 | 437 (85) | 0.647 |
| High education level (college degree or higher) | 52 | 41 (79) | 541 | 422 (78) | 0.888 |
| Sexual behavior | |||||
| Steady partner(s) in preceding 6 months | |||||
| ≥1 Steady partner | 48 | 29 (60) | 519 | 327 (63) | 0.723 |
| Anal sex with steady partner | 48 | 21 (44) | 519 | 238 (46) | 0.966 |
| CAS with steady partner(s) | 48 | 20 (42) | 519 | 200 (39) | 0.670 |
| CAS with HIV+ steady partner or partner with unknown HIV status | 48 | 3 (6) | 508 | 2(5) | 0.687 |
| Casual partner(s) in preceding 6 months | |||||
| ≥1 Casual partner | 48 | 48 (100) | 514 | 357 (69) | <0.001 |
| Number of casual partner(s) (median, IQR) | 48 | 21 [12–40] | 514 | 4 [0–11] | <0.001 |
| Anal sex with casual partner | 48 | 48 (100) | 512 | 297 (58) | <0.001 |
| CAS with casual partner(s) | 48 | 41 (85) | 511 | 134 (26) | <0.001 |
| CAS with HIV+ casual partner(s) or partner with unknown HIV status | 48 | 31 (64) | 511 | 76 (15) | <0.001 |
| Recreational drug use | |||||
| Any illicit drug use* in preceding 6 months | 47 | 27 (57) | 517 | 197 (38) | 0.009 |
| Chemsex** with casual partners in preceding 6 months | 47 | 20 (43) | 517 | 37 (7) | <0.001 |
| Injecting drug use in preceding 6 months | 47 | 0 | 518 | 0 | - |
| STI diagnosis in preceding 6 months | |||||
| Any bacterial STI | 52 | 14 (27) | 527 | 60 (11) | 0.001 |
| Any rectal STI (chlamydia/gonorrhea) | 52 | 8 (15) | 527 | 35 (7) | 0.022 |
| Chlamydia, urethral | 52 | 2 (4) | 541 | 10 (2) | 0.347 |
| Chlamydia, rectal | 52 | 7 (13) | 527 | 23 (4) | 0.005 |
| Gonorrhea, urethral | 52 | 2 (4) | 527 | 7 (1) | 0.190 |
| Gonorrhea, rectal | 52 | 4 (8) | 527 | 16 (3) | 0.079 |
| Syphilis | 52 | 4 (8) | 527 | 6 (1) | 0.008 |
| PrEP eligibility and criteria | |||||
| Eligible for PrEP in the preceding 6 months | 48 | 34 (71) | 493 | 118 (24) | <0.001 |
| CAS with partner with unknown or seropositive HIV status | 48 | 31 (60) | 501 | 96 (19) | <0.001 |
| Rectal chlamydia or gonorrhea | 52 | 8 (15) | 527 | 35 (7) | 0.022 |
| PEP prescription | 49 | 4 (8) | 539 | 7 (1) | 0.001 |
| Intention to use PrEP | |||||
| High intention to use PrEP in 2015 (vs. low/medium) | 45 | 36 (80) | 455 | 119 (26) | <0.001 |
| High intention for daily use (vs. low/medium) | 45 | 25 (56) | 455 | 65 (14) | <0.001 |
| High intention for event-driven use (vs. low/medium) | 45 | 25 (56) | 455 | 84 (18) | <0.001 |
Abbreviations: CAS, condomless anal sex; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; STI, sexually transmitted infection; PrEP, pre-exposure prophylaxis; PEP, post-exposure prophylaxis.
*Defined as the use of amphetamines, benzodiazepines, cocaine, 2,5-dimethoxy-4-bromophenethylamine (2-CB), 4-Fluoroamphetamine (4-FA), γ-hydroxybutyric acid(GHB)/γ-butyrolactone (GBL), heroin, ketamin, mephedrone, methamphetamin, opioids, 3,4-methylenedioxymethamphetamine (XTC/MDMA), 3-mmc, methoxetamin (MXE), 4-methylethcathinone (4-mec).
**Defined as the use of GHB/GBL, mephedrone and/or methamphetamine [13] during sex with a casual partner.’
PrEP eligibility and intention to use PrEP
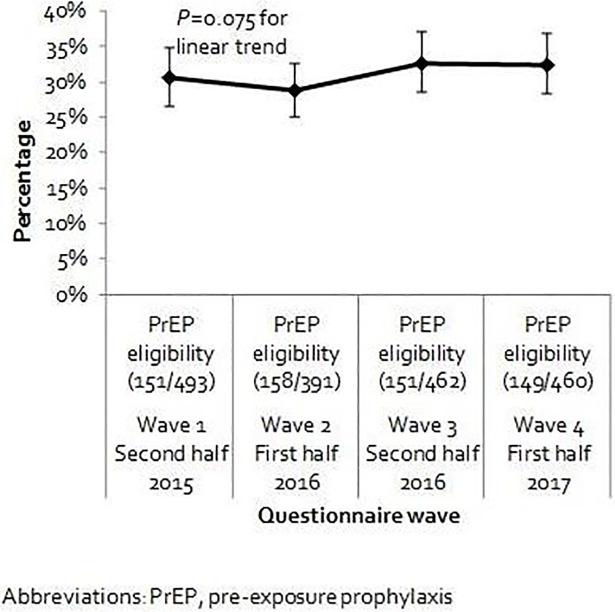
We observed a small increase in PrEP eligibility from 2015 to 2017, but the effect was not statistically significant (P = 0.075, Fig 2). Of 460 MSM with eligibility information available at wave 4, 149 (32%, 95% CI 28–37%) met ≥1 PrEP eligibility criteria; of these, 31/149 (21%) reported lifetime PrEP use. Among eligible MSM, the majority (n = 127/149, 85%) reported CAS with a partner with positive (n = 29) and/or unknown HIV serostatus (n = 103). In total, 21/149 (14%) MSM were considered eligible for PrEP based only on reported CAS with an HIV-positive partner. Thirty-nine of 149 (26%) MSM had a rectal chlamydia or gonorrhea diagnosis in the preceding 6 months. PEP, the third PrEP eligibility criterion, had been prescribed to 6/149 (4%) MSM. In our sensitivity analysis on missing eligibility data, PrEP eligibility at wave 4 was 27% (n = 149/541, 95% CI 24–31%) when MSM with missing data (81/541, 15%) were considered not eligible, whereas it was 43% (230/541, 95% CI 38–47%) when all were considered eligible.
Fig 2. PrEP eligibility among MSM participating in the Amsterdam Cohort Studies between 2015–2017.
Of 548 MSM with data on intention to use PrEP, 165 (30% [95% CI 26–34%]) reported a high intention, 277 (51% [95% CI 46–55%]) a medium intention and 106 (19% [95% CI 16–23%]) a low intention. The proportion with a high intention was greater among eligible compared to non-eligible MSM (51% [95% CI 43–59%] vs. 24% [95% CI 20–29%], p<0.001), and also greater among MSM who initiated PrEP between 2015 and 2017 compared to MSM who did not (80% vs. 26%, P<0.001). A high intention for daily use was reported by 96 (17%) MSM, and for event-driven use by 114 (21%) MSM.
Discussion
Approximately 10% of HIV-negative participants of a prospective MSM cohort in Amsterdam, the Netherlands, reported lifetime PrEP use through the first half of 2017. The number of PrEP users had increased since 2015 and most obtained PrEP through participation in studies. PrEP users did not differ from non-PrEP users in terms of socio-demographic characteristics. According to national guidelines, about 30% were eligible for PrEP at the most recent wave, of whom about half had a high intention to use PrEP and one-fifth had ever used PrEP.
This study is subject to some limitations. First, ACS participants were predominantly highly educated native Dutch MSM from the Amsterdam area who may not represent nationwide MSM. They are likely to be more informed about PrEP, given the close proximity of ongoing PrEP studies at the Public Health Service of Amsterdam. Second, not all MSM with a study visit at wave 4 had complete eligibility information available. Sensitivity analysis showed that in extreme-value scenarios, eligibility ranged between 27% and 43%. Third, our eligibility criterion concerning CAS with a partner with positive or unknown HIV status did not include the undetectable viral load criterion specified in the national guidelines, as data was not available. We may therefore have misclassified some of the 21/149 MSM who were eligible based only on reported CAS with an HIV-positive partner, as eligible. Finally, the differences in sexual risk behavior and STI between PrEP users and non-users likely reflect the eligibility criteria of the studies through which most users in this cohort obtained PrEP. The low number of PrEP users did not permit subgroup analyses to compare MSM using PrEP from various sources or those following different dosing regimens.
Estimated numbers of PrEP users and PrEP-eligible persons in Europe are scarce and mainly based on personal communications [2]. Estimations range from 500–1500 eligible persons in Belgium to 20,000–100,000 in England [2]. To our knowledge, our study provides the first estimates of PrEP eligibility and intention to use among MSM in the Netherlands. These findings can be used to provide crude estimates of the number of indicated and expected PrEP users, and associated costs, in the absence of more nationally representative data. Based on our findings, the estimated total number of MSM in Amsterdam [15], and the HIV prevalence among MSM [16], we roughly estimate that between 2,903–5,370 eligible MSM in Amsterdam can be expected to use PrEP (S2 File). This is higher than a previous estimate using STI clinic data and the ACS data from 2012 (n = 936–2,358) [11,17]. Additionally, between 2,986–5,118 non-eligible MSM may also use PrEP (S2 File). These estimates may be slightly overestimated as PrEP use among ACS participants might be somewhat ahead of PrEP use in the general MSM population due to having been informed about PrEP earlier by study participation. Moreover, we used intention as a marker of uptake, but intention does not fully predict use, and uptake depends on multiple factors [18].
The percentage of MSM with a high intention to use PrEP has more than doubled since a 2012 study in the same cohort [11], possibly due to increased PrEP awareness, new results on PrEP effectiveness [19,20], and the added option of event-driven PrEP use. The increased PrEP awareness might have been influenced by long-term study participation, but also by an increased information provision on PrEP to MSM in the Netherlands which has been going on since 2015. The proportion with a high intention to use daily PrEP was smaller (17%) but was still increased compared to 2012. Since about half of eligible MSM did not report a high intention, future work is warranted on how to motivate MSM at risk of HIV to use PrEP. As observed in the 2012 study, this includes increasing PrEP knowledge; addressing psychosocial determinants such as feelings of shame and perception of self-efficacy; and encouraging the use of condoms or other risk reduction strategies alongside increasing PrEP accessibility [11, 21]. We furthermore expect PrEP intention and use to increase with increasing awareness and decreasing PrEP costs [10,20–22]. This is supported by the observed increasing proportion of recent self-obtaining PrEP users.
In conclusion, this study provides important descriptive data for decision-making on PrEP implementation in the Netherlands. The majority of eligible MSM are currently not using PrEP, suggesting that a large proportion of MSM at risk for HIV could benefit from it once accessibility improves. As accessibility improves, there should be a focus on improving intention to use PrEP among eligible MSM who do not have a high intention to use PrEP. Research on motives for and barriers to using PrEP can be helpful to increase PrEP uptake among eligible MSM.
Supporting information
(PDF)
(PDF)
Acknowledgments
The authors gratefully acknowledge the Amsterdam Cohort Studies (ACS) on HIV infection and AIDS, which is a collaboration between the Public Health Service of Amsterdam, the Academic Medical Center of the University of Amsterdam, Sanquin Blood Supply Foundation, Medical Center Jan van Goyen, and the HIV Focus Center of the DC-Clinics. ACS is part of the Netherlands HIV Monitoring Foundation and is financially supported by the Center for Infectious Disease Control of the Netherlands National Institute for Public Health and the Environment. The authors thank all ACS participants, research nurses and data managers for their contribution. They also explicitly thank Lucy Phillips for editing this manuscript and Anders Boyd for his input on the statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Coleman R, Prins M. Options for affordable pre-exposure prophylaxis (PrEP) in national HIV prevention programmes in Europe. Euro Surveill. 2017;22(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoornenborg E, Krakower DS, Prins M, Mayer KH. Pre-exposure prophylaxis for MSM and transgender persons in early adopting countries. AIDS. 2017;31(16):2179–91. 10.1097/QAD.0000000000001627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoornenborg E, Achterbergh RC, van der Loeff MFS, Davidovich U, van der Helm JJ, Hogewoning A, et al. Men who have sex with men more often chose daily than event-driven use of pre-exposure prophylaxis: baseline analysis of a demonstration study in Amsterdam. J Int AIDS Soc. 2018;21(3):e25105 10.1002/jia2.25105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DISCOVER Trial Register [Internet]. 2016 [cited 15 January 2018]. Available from: https://www.avac.org/trial/discover
- 5.European Medicines Agency. First medicine for HIV pre-exposure prophylaxis recommended for approval in the EU [Internet]. 2016 July 22 [cited 10 July 2018]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/07/news_detail_002578.jsp&mid=WC0b01ac058004d5c1
- 6.Aidsfonds. PrEP nu voor 50 euro beschikbaar bij alle apotheken [Internet]. 2018 Jan 12 [cited 15 Jan 2018]. Available from: https://aidsfonds.nl/nieuws/prep-nu-voor-50-euro-beschikbaar-bij-alle-apotheken. Dutch.
- 7.Gezondheidsraad. Preventief gebruik van hiv-remmers [Internet]. 2018 March 27 [cited 10 July]. Available from: https://www.gezondheidsraad.nl/sites/default/files/grpublication/advies_prep.pdf. Dutch.
- 8.Public Health Service of Amsterdam. Decision Minister Bruins on reimbursement of PrEP [Internet]. 2018 July 18 [cited 18 July 2018]. Available from: http://www.ggd.amsterdam.nl/english/sti-hiv-sense/prep/decision-minister/
- 9.Health Council of the Netherlands. Work Programme 2018 [Internet]. 2017 September 19 [cited 15 January 2018]. Available from: https://www.gezondheidsraad.nl/sites/default/files/grpublication/advies_prep.pdf
- 10.van Griensven GJ, de Vroome EM, Goudsmit J, Coutinho RA. Changes in sexual behaviour and the fall in incidence of HIV infection among homosexual men. BMJ. 1989;298(6668):218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bil JP, Davidovich U, van der Veldt WM, Prins M, de Vries HJ, Sonder GJ, et al. What do Dutch MSM think of preexposure prophylaxis to prevent HIV-infection? A cross-sectional study. AIDS. 2015;29(8):955–64. 10.1097/QAD.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 12.Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, Davidovich U, Hogewoning A, de Vries HJC, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS. 2017;31(11):1603–10. 10.1097/QAD.0000000000001522 [DOI] [PubMed] [Google Scholar]
- 13.Stuart D. Sexualised drug use by MSM: background, current status and response. HIV Nursing. 2013. Available from: http://www.davidstuart.org/DavidStuart-HIVN13.1.pdf. [Google Scholar]
- 14.Nederlandse Vereniging voor HIV Behandelaren. HIV Pre-exposure profylaxe (PrEP) richtlijn Nederland [Internet]. 2016 Sept 8 [cited 15 January 2018]. Available from: https://nvhb.nl/wp-content/uploads/2017/01/PrEP-richtlijn-Nederland-8-september-2016-met-logos.pdf. Dutch.
- 15.Dijkshoorn H, Hazeleger F, Janssen AP, Ujcic-Voortman JK. Amsterdamse Gezondheidsmonitor 2012 [Internet]. 2018 Feb 6 [cited 10 July 2018]. Available from: https://www.ggd.amsterdam.nl/beleid-onderzoek/gezondheidsmonitors/amsterdamse/. Dutch.
- 16.Op de Coul EL, Schreuder I, Conti S, van Sighem A, Xiridou M, Van Veen MG, et al. Changing Patterns of Undiagnosed HIV Infection in the Netherlands: Who Benefits Most from Intensified HIV Test and Treat Policies? PLoS One. 2015;10(7):e0133232 10.1371/journal.pone.0133232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urbanus AT, Blom C, Zantkuijl P, David S. Pre-Expositie Profylaxe voor hiv-negatieven in Nederland [Internet]. 2017 July 26 [cited 15 January 2018]. Available from: http://www.rivm.nl/dsresource?objectid=62176937-6710-4fde-b699-da20687a8fb8&type=org&disposition=inline. doi: 10.21945/RIVM-2017-0094 Dutch.
- 18.Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull. 2006;132(2):249–68. 10.1037/0033-2909.132.2.249 [DOI] [PubMed] [Google Scholar]
- 19.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–46. 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- 20.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bil JP, van der Veldt WM, Prins M, Stolte IG, Davidovich U. Motives of Dutch men who have sex with men for daily and intermittent HIV pre-exposure prophylaxis usage and preferences for implementation: A qualitative study. Medicine (Baltimore). 2016. 95(39):e4910 10.1097/MD.0000000000004910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gredig D, Uggowitzer F, Hassler B, Weber P, Niderost S. Acceptability and willingness to use HIV pre-exposure prophylaxis among HIV-negative men who have sex with men in Switzerland. AIDS Care. 2016;28 Suppl 1:44–7. 10.1080/09540121.2016.1146212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.