Abstract
Aim
The epidemiology of human respiratory syncytial virus (HRSV) infection has not yet been systematically investigated in Africa. This systematic review and meta‐analysis are to estimate the prevalence of HRSV infections in people with acute respiratory tract infections (ARTI) in Africa.
Method
We searched PubMed, EMBASE, Africa Journal Online, and Global Index Medicus to identify observational studies published from January 1, 2000, to August 1, 2017. We used a random‐effects model to estimate the prevalence across studies. Heterogeneity (I 2) was assessed via the chi‐square test on Cochran's Q statistic. Review registration: PROSPERO CRD42017076352.
Results
A total of 67 studies (154 000 participants) were included. Sixty (90%), seven (10%), and no studies had low, moderate, and high risk of bias, respectively. The prevalence of HRSV infection varied widely (range 0.4%‐60.4%). The pooled prevalence was 14.6% (95% CI 13.0‐16.4, I 2 = 98.8%). The prevalence was higher in children (18.5%; 95% CI 15.8‐21.5) compared to adults (4.0%; 95% CI 2.2‐6.1) and in people with severe respiratory tract infections (17.9%; 95% CI 15.8‐20.1) compared to those with benign forms (9.4%; 95% CI 7.4‐11.5); P‐values <0.0001. The HRSV prevalence was not associated with sex, subregion in Africa, setting, altitude, latitude, longitude, and seasonality.
Conclusion
This study suggests a high prevalence of HRSV in people with ARTI in Africa, particularly among children and people with severe clinical form. All innovative strategies to curb the burden should first focus on children which present the highest HRSV‐related burden.
Keywords: Africa, epidemiology, meta‐analysis, respiratory syncytial virus, respiratory tract infections
1. INTRODUCTION
Worldwide, acute respiratory tract infections (ARTI) are one of the leading causes of hospitalization, morbidity, and mortality among children under five.1 Globally, human respiratory syncytial virus (HRSV) is the most common pathogen identified in infants and young children with bronchiolitis and pneumonia. Based on published and unpublished studies, a global systematic and meta‐analysis by Shi and colleagues showed that an incidence of 33.1 (95% CI, 21.6‐50.3) million of cases of ARTI was associated with HRSV each year in children aged less than 5 years. A total of 3.2 (95% CI, 2.7‐3.8) million of these infections were severe cases requiring hospitalization. Approximately 40‐74.5 thousand deaths were estimated in children <5 with ARTI in this meta‐analysis.2 Another meta‐analysis had previously showed that the incidence of HRSV infection in developing countries was more than twice greater than that of developed countries and that 91% of hospitalizations and nearly all deaths (99%) were registered in developing countries where access to health care is limited.3 Preterm infants, those under 2 years, those over 65 years, and immunocompromised patients, are at higher risk of hospitalization for HRSV infections.4 Patients with a history of atopy, congenital heart disease, congestive heart failure, chronic obstructive pulmonary disease, neuronal and muscular disorders, and cancer are also at high risk for the development of severe HRSV infections.5 To date, there are more than 60 HRSV vaccine development programs at various stages. Some of them could be used in the next decade.
Africa, a continent in which most countries are developing with limited resources for health, the ecology in this continent can give it a particular epidemiology regarding HRSV infection. To the best of our knowledge, to date, no systematic review and meta‐analysis have been conducted in Africa on the epidemiology and drivers of HRSV infection. Therefore, we conducted a systematic review and meta‐analysis to estimate the prevalence and drivers of HRSV infection among people with ARTI in Africa. We have done this work to provide accurate data to guide health policymakers and to identify information gaps to guide future research.
2. MATERIAL AND METHODS
2.1. Search strategy and selection criteria
We performed a comprehensive and exhaustive search of MEDLINE through PubMed, Excerpta Medica Database (EMBASE), Africa Journals Online, and Global Index Medicus to identify all relevant articles published on HRSV in Africa from January 1, 2000, until August 31, 2017, regardless of language of publication. Both text words and medical subject heading terms were used. The following terms and their variants were used for HRSV: “HRSV,” “human respiratory syncytial virus,” and “respiratory syncytial virus.” Individual country names for the 54 African countries and African subregion names such as “Northern Africa” or “Southern Africa” were also used as additional key search terms for more abstracts on the subject. African country names were introduced both in English and in languages relevant to each country, for example, “Ivory Coast” and “Côte d'Ivoire”. Where country names have changed over time, old and new names were included, such as “Zaire” and “Democratic Republic of Congo”. Titles and abstracts of all eligible papers were reviewed, and full texts of articles were accessed. Search strategy conducted in PubMed is shown in Table S1. This search strategy was adapted to fit with other databases. A manual search which consists in scanning the reference lists of eligible papers and other relevant review articles was conducted. The search in electronic databases was conducted on September 6, 2017.
We considered observational studies (cross‐sectional, case‐control, and cohort). In the case of duplicate reports, the most comprehensive/complete and up‐to‐date version was considered. We considered studies including patients with clinical diagnosis of acute respiratory tract infection as defined in each study. Studies among populations with underlying medical conditions, studies conducted during an outbreak period, case series or studies in which HRSV was imported cases were excluded. The search for HRSV had to be conducted systematically or by sampling of the population in the presence of defined inclusion criteria (respiratory signs) and HRSV detection by polymerase chain reaction (PCR) technique on respiratory samples. Studies lacking or with not extractable primary data and/or explicit method description were excluded. In the case of missing data, we contacted authors of the paper. We planned to use Google Translate in the case of other languages than French, English, or Spanish.
Two investigators independently screened records based on titles and abstracts for eligibility. Full texts of articles deemed potentially eligible were retrieved. Further, these investigators independently assessed the full text of each study for eligibility and consensually retained studies to be included. Disagreements when existing were solved through a discussion.
2.2. Data extraction and management
Data were extracted using a preconceived and tested data abstraction form. In the cases of missing data, authors were directly contacted to provide missing information. Two investigators independently extracted data including name of the first author, publication year, study design, setting, sampling method, respiratory samples collection period, timing of data analysis, number of viruses screened, site of recruitment location (country, city, latitude, longitude, and altitude), clinical presentation, number of patients screened, number of patients infected with HRSV, diagnostic techniques used, and proportion of male participants. We assigned a United Nations Statistics Division (UNSD) African region (central, eastern, northern, southern, and western) to each study regarding the country of recruitment.6 We considered two groups of clinical presentation; severe respiratory tract infection (SRTI) including severe acute respiratory infection, acute lower respiratory infections, bronchitis, bronchiolitis, pneumonia, severe or very severe pneumonia, and benign respiratory tract infection (BRTI) including upper respiratory tract infection, and influenza‐like illness. Using Google Global Positioning System, we assigned altitude, latitude, and longitude according to the cities and country of recruitment.7 In the case of multicities, we considered the median. Disagreements between investigators were reconciled through discussion and consensus or an arbitration of a third investigator.
Two investigators evaluated risk of bias in included studies using an eight‐item rating scale.8 These items included (a) participation response rate more than 75% agree to participate or analysis to show whether respondents and nonrespondents were similar for the sociodemographic characteristics; (b) ARTI clearly defined; (c) method of inclusion identical for all subjects; (d) description of diagnostic technique; (e) same type of sample collected for all patients (nasopharyngeal aspirate, nasal, or throat swab); (f) standardized method for sample collection (quantity of aspirate or of liquid used for the nasal wash with any virological medium transport for swabs); (g) analysis performed according to relevant subgroups (by age classes, by center, or by symptomatology, for example); and (h) and presentation of data sources (counts are presented, not only percentages). Each item was assigned a score of 1 (Yes) or 0 (No), and each score was summed across items to generate an overall study quality score. The total score was ranged from 0 to 8 with the overall score categorized as follows: 6‐8: “low risk of bias,” 3‐5: “moderate risk,” and 0‐2: “high risk.” Disagreements were solved through discussion and consensus.
2.3. Data synthesis and analysis
Data analyses used the “meta” and “metafor” packages of the statistical software r (version 3.3.3, The R Foundation for statistical computing, Vienna, Austria). Unadjusted prevalence and standard errors of HRSV infection were recalculated based on the information of crude numerators and denominators provided by individual studies. To keep the effect of studies with extremely small or extremely large prevalence estimates on the overall estimate to a minimum, the variance of the study‐specific prevalence was stabilized with the Freeman‐Tukey double‐arcsine transformation before pooling the data within a random‐effects meta‐analysis model.9 Symmetry of funnel plot and Egger's test served to assess the presence of publication bias.10 A P‐value <0.10 on Egger test was considered indicative of statistically significant publication bias.
Heterogeneity was evaluated by the chi‐square test on Cochrane's Q statistic,11 which was quantified by H and I 2 values. The I 2 statistic estimates the percentage of total variation across studies due to true between‐study differences rather than chance. In general, I 2 values greater than 60%‐70% indicate the presence of substantial heterogeneity. The value of H close to 1 is indicative of some homogeneity between studies.12 Subgroup analyses were performed for the following subgroup: age groups (0‐5/>5 years, children/adults), sex, clinical presentation, setting, hemisphere (north/south), location according to Greenwich meridian (East/West), by median altitude, and UNSD African Regions. Univariable and multivariable meta‐regressions were used to test for an effect of study and participants’ characteristics. To be included in multivariable meta‐regression analysis, a P value <0.25 in univariable analysis was considered. For categorical variables, the global P value was considered for the inclusion in multivariable models. A P value <0.05 was considered statistically significant. We reported the explained heterogeneity (R²) for the residual heterogeneity of HRSV prevalence. Following crude overall prevalence, two sensitivity analyses were conducted: one considering only studies with low risk of bias and another one considering only studies conducted in complete season(s).
The Centre for Reviews and Dissemination guidelines was used for the methodology of this review.13 The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines served as the template for reporting the present review (Table S2).14 This review is registered in the PROSPERO International Prospective Register of Systematic Reviews, registration number CRD42017076352.
3. RESULTS
3.1. Review process
We identified 718 records; after elimination of duplicates, 683 records remained. After screening of titles and abstracts, we found 539 records to be irrelevant and excluded them. We assessed full texts of the remaining 144 papers for eligibility, of which 78 were excluded with reasons (Figure 1). The inter‐rater agreement for study selection was high (κ = 0.81). Finally, 66 full texts including 67 studies were included (one paper included two studies).15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80
Figure 1.
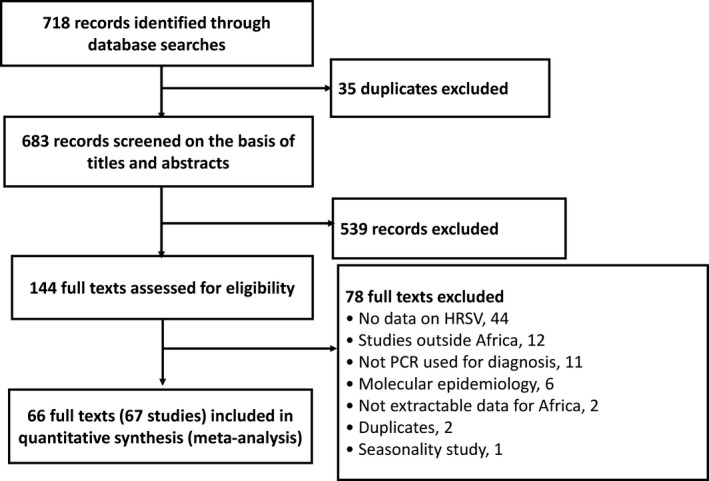
Review process
3.2. Characteristics of included studies
Sixty (90%), seven (10%), and no studies had low, moderate, and high risk of bias, respectively. Studies were published between 2010 and 2017. Data were from 20 countries. Respiratory samples were collected between January 2006 and April 2016. Most of data came from cross‐sectional studies, used consecutive sampling method, and prospective design. Most of studies included only children with proportion of males varying between 44.1% and 69.8%. Real‐time reverse transcription‐polymerase chain reaction was the most used diagnosis method. Most of studies was conducted in both urban and rural settings and in Eastern Africa region (Table 1). Individual characteristics of included studies are in the Table S3.
Table 1.
Characteristics of included studies
| Characteristics | Data |
|---|---|
| Design, n (%) | |
| Cross‐sectional | 63 (94) |
| Case‐control | 4 (6) |
| Setting, n (%) | |
| Rural | 12 (18) |
| Urban | 10 (15) |
| Urban and rural | 29 (43) |
| Not described | 16 (24) |
| Sampling method, n (%) | |
| Consecutive | 57 (85) |
| Systematic | 3 (4) |
| Random | 6 (9) |
| Timing, n (%) | |
| Prospective | 64 (96) |
| Retrospective | 3 (4) |
| Clinical presentation | |
| BRTI | 9 (13) |
| SRTI | 38 (57) |
| BRTI and SRTI | 20 (30) |
| Population, n (%) | |
| Children | 39 (58) |
| Adults | 2 (3) |
| Children and adults | 26 (39) |
| Region, n (%) | |
| Eastern (Kenya = 17; Madagascar = 2; Malawi = 1; Mozambique = 2; Reunion = 1) | 22 (33) |
| Southern (Botswana = 1; Malawi = 1; Mozambique = 1; South Africa = 12; Zambia = 1) | 15 (22) |
| Northern (Egypt = 10; Morocco = 3; Sudan = 1) | 14 (21) |
| Western (Burkina Faso = 2; Côte d'Ivoire = 1; Ghana = 2; Niger = 1; Nigeria = 1; Senegal = 5) | 12 (18) |
| Central (Cameroon = 2; Gabon = 1; Central Africa Republic = 1) | 4 (6) |
| Diagnostic technique, n (%) | |
| Real‐time RT‐PCR | 64 (96) |
| Conventional RT‐PCR | 3 (4) |
| Latitude, median (1st; 3rd quartiles), in decimal degrees | 3.5 (−9.4; 15.0) |
| Longitude, median (1st; 3rd quartiles), in decimal degrees | 30.5 (11.8‐34.4) |
| Altitude, median (1st; 3rd quartiles), in meters | 252 (28.5‐1012.5) |
3.3. Prevalence of HRSV infection among ARI in Africa
Table 2 summarizes results from meta‐analyses. The prevalence varied widely from 0.4% to 60.4% across countries. The distribution of the prevalence was not uniform in the continent (Figure 2). The overall prevalence was 14.6% (95% CI 13.0‐16.4) in a pooled sample of 154 000 participants with ARTI (Figure 3). The funnel plot did not suggest any publication bias (Figure S1), and this result is confirmed by the Egger test (Table 2). The prevalence in studies with low risk of bias was not different to the overall prevalence (Table 2). Substantial heterogeneity was present for overall and within all subgroups (Table 2). Publication bias was found for the following subgroup analyses: SRTI, North Hemisphere, and Northern Region of Africa (Table 2).
Table 2.
Summary and comparison statistics of human respiratory syncytial virus prevalence in Africa
| Groups | N studies | N participants | Prevalence, % (95% confidence interval) | I 2 (95% confidence interval | H (95% confidence interval) | P heterogeneity | P Egger test | P difference |
|---|---|---|---|---|---|---|---|---|
| Overall | 67 | 154 000 | 14.6 (13.0‐16.4) | 98.8 (98.7‐98.9) | 9.1 (8.7‐9.5) | <0.0001 | 0.298 | ‐ |
| Complete season(s) studies | 40 | 82 048 | 13.6 (11.2‐16.2) | 99.0 (98.9‐99.1) | 10.0 (9.4‐10.6) | <0.0001 | 0.148 | ‐ |
| Low risk of bias studies | 60 | 151 017 | 14.3 (12.6‐16.1) | 98.9 (98.7‐99.0) | 9.3 (8.9‐9.8) | <0.0001 | 0.404 | ‐ |
| Subgroup analyses | ||||||||
| Age group | ||||||||
| Children (0‐15 y) | 34 | 39 984 | 18.5 (15.8‐21.5) | 98.0 (97.6‐98.3) | 7.0 (6.5‐7.6) | <0.0001 | 0.512 | <0.0001 |
| Adults | 5 | 11 976 | 4.0 (2.2‐6.1) | 94.6 (90.2‐97.0) | 4.3 (3.2‐5.8) | <0.0001 | 0.904 | |
| Age group | ||||||||
| 0‐5 y | 19 | 29 843 | 22.6 (18.8‐26.6) | 98.2 (97.8‐98.6) | 7.5 (6.8‐8.3) | <0.0001 | 0.747 | <0.0001 |
| >5 y | 8 | 15 171 | 6.0 (3.8‐8.6) | 96.0 (94.0‐97.4) | 5.0 (4.1‐6.2) | <0.0001 | 0.134 | |
| Clinical presentation | ||||||||
| SRTI | 45 | 118 083 | 17.9 (15.8‐20.1) | 98.8 (98.6‐98.9) | 9.1 (8.6‐9.6) | <0.0001 | 0.033 | <0.0001 |
| BRTI | 20 | 30 472 | 9.4 (7.4‐11.5) | 97.0 (96.2‐97.6) | 5.7 (5.1‐6.5) | <0.0001 | 0.367 | |
| Settings | ||||||||
| Rural | 10 | 16 973 | 14.1 (10.4‐18.2) | 97.8 (96.9‐98.3) | 6.7 (5.7‐7.8) | <0.0001 | 0.308 | 0.402 |
| Urban | 10 | 7150 | 16.6 (12.5‐21.1) | 95.6 (93.6‐97.0) | 4.8 (3.0‐5.8) | <0.0001 | 0.462 | |
| Sex | ||||||||
| Male | 2 | 755 | 31.1 (9.8‐57.8) | 94.2 (81.8‐98.2) | 4.2 | <0.0001 | NA | 0.948 |
| Female | 2 | 817 | 32.6 (5.3‐68.8) | 96.8 (91.6‐98.8) | 5.6 | <0.0001 | NA | |
| Hemisphere | ||||||||
| North | 38 | 75 069 | 14.6 (12.5‐16.9) | 98.5 (98.2‐98.7) | 8.1 (7.5‐8.6) | <0.0001 | 0.078 | 0.960 |
| South | 29 | 78 931 | 14.7 (12.2‐17.4) | 99.0 (98.9‐99.1) | 10.0 (9.4‐10.7) | <0.0001 | 0.991 | |
| Meridian | ||||||||
| East | 56 | 137 481 | 14.6 (12.9‐16.5) | 98.8 (98.7‐98.9) | 9.1 (8.6‐9.5) | <0.0001 | 0.474 | 0.886 |
| West | 11 | 16 519 | 14.9 (10.3‐20.3) | 98.4 (97.9‐98.8) | 7.9 (7.0‐9.1) | <0.0001 | 0.096 | |
| Regions | ||||||||
| Central | 4 | 2278 | 8.0 (4.0‐13.1) | 93.4 (86.4‐96.8) | 3.9 (2.7‐5.6) | <0.0001 | 0.488 | 0.087 |
| Eastern | 22 | 38 950 | 14.3 (11.8‐17.1) | 98.1 (97.6‐98.4) | 7.2 (6.5‐7.9) | <0.0001 | 0.545 | |
| Northern | 14 | 34 884 | 16.1 (13.2‐19.2) | 97.5 (96.7‐98.1) | 6.3 (5.5‐7.2) | <0.0001 | 0.053 | |
| Southern | 15 | 62 968 | 16.7 (12.7‐21.0) | 99.5 (99.4‐99.5) | 13.5 (12.5‐14.6) | <0.0001 | 0.693 | |
| Western | 12 | 14 920 | 13.8 (9.2‐19.0) | 98.1 (97.5‐98.5) | 7.3 (6.4‐8.3) | <0.0001 | 0.162 | |
BRTI, benign respiratory tract infection; NA, not applicable; RT‐PCR, reverse transcription‐polymerase chain reaction; SRTI, severe respiratory tract infection.
Figure 2.
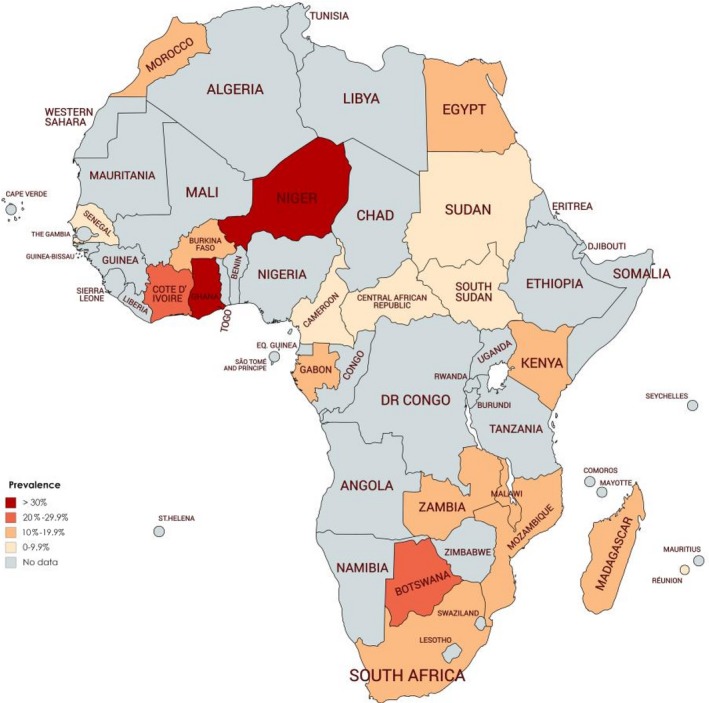
Distribution of prevalence of Human Respiratory Syncytial virus infection among patients with ARI in Africa continent
Figure 3.
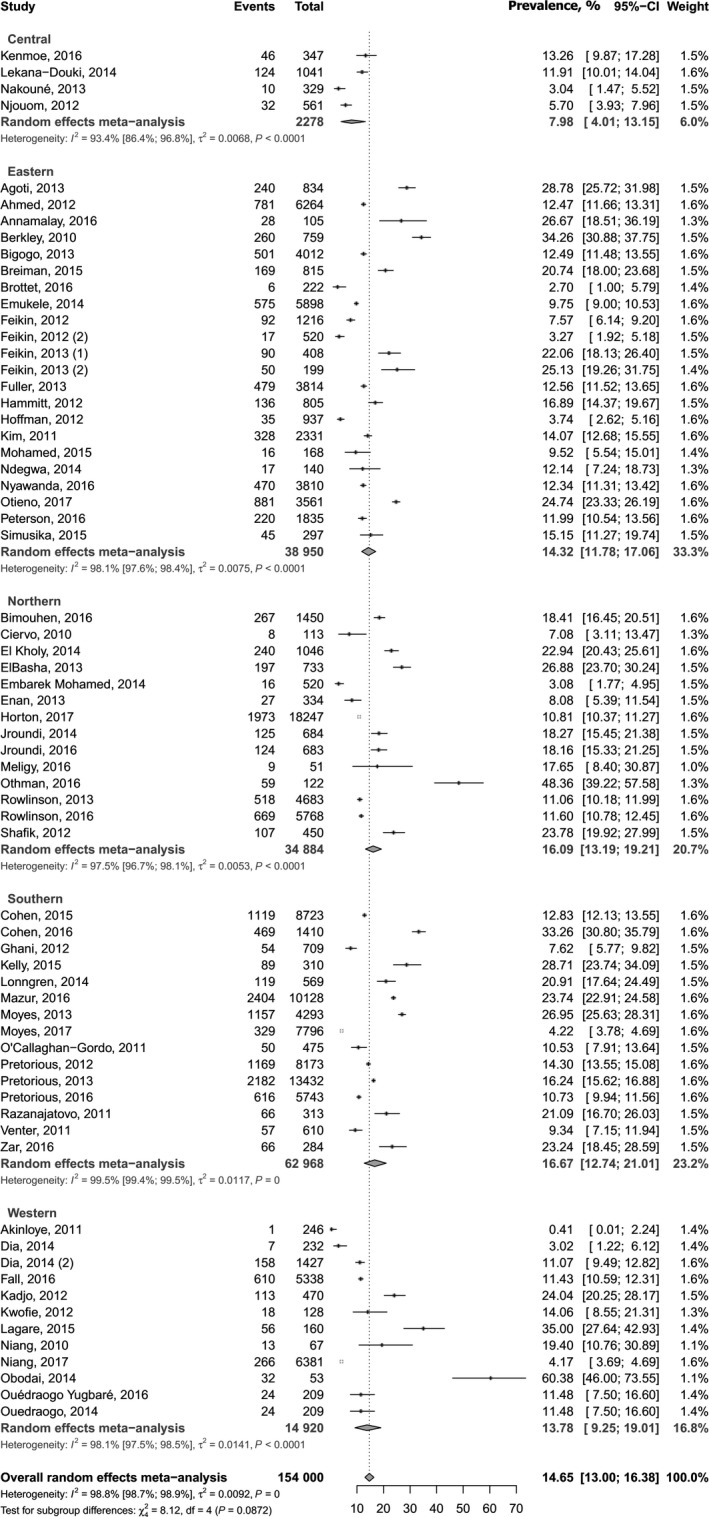
Meta‐analysis results for the prevalence of human respiratory syncytial virus prevalence in Africa
3.4. Subgroup analyses
The prevalence was higher among children (18.5%, 95% CI 15.8‐21.5) compared to adults (4.0%, 95% CI 2.2‐6.1; P < 0.0001), among people aged <5 years (22.6%, 95% CI 18.8‐26.6) compared to those aged >5 years (6.0%, 95% CI 3.8‐8.6; P < 0.0001), and among people with SRTI (17.9%, 95% CI 15.8‐20.1) compared to those with BRTI (9.4%, 95% CI 7.4‐11.5; P < 0.0001) (Table 2). There was no difference for UNSD subregions of Africa, sex, setting, hemisphere, and distribution by Greenwich meridian (Table 2).
3.5. Factors associated with prevalence of HRSV infections
In univariable meta‐regression analysis, the prevalence was associated with clinical presentation (R 2 = 8.99%) and age groups (R 2 = 38.84%). In multivariable meta‐regression analysis, the prevalence was higher in children compared to adults (adjusted odds ratio: 1.28, 95% CI: 1.09‐1.52, P = 0.0041). Variables included in the multivariable model (clinical presentation, age groups, diagnostic method) explained 42.8% of the 97.6% residual heterogeneity of HRSV prevalence (Table S4).
4. DISCUSSION
This systematic review and meta‐analysis of data from 67 studies involving 154 000 individuals found a pooled prevalence of HRSV infection of 14.6% in people with ARTI in Africa, with substantial heterogeneity. Sensitivity analysis including only studies with low risk of bias and another one including only studies conducted in complete seasons gave similar results. The drivers of high prevalence of HRSV infection included being children and having SRTI.
The prevalence of HRSV infection in people with ARTI from Africa found in this review is close to that found in other systematic reviews from another place. A systematic review with meta‐analysis in China found a prevalence of 18.7% (95% CI 17.1‐20.5, 135 studies from 2010 to 2015) attributable to HRSV.81 Another systematic review with meta‐analysis of 21 studies (1996‐2013) in Iran yielded to a prevalence of 18.7%.82 Another meta‐analysis including participants with lower ARTI from Latin America found a prevalence varying from 40.9% among children aged less than 1 year to 12.6% adults aged more than 65 years.83 One should note that these three systematic reviews included studies using less sensitive and less specific immunofluorescence and immune‐chromatographic techniques. Our review included studies using only reverse transcriptase PCR. The interpretation of the prevalence found in our study should consider the involvement of other infectious agents in the occurrence of ARTI. Indeed, the presence of coinfection with other viruses should be considered. These coinfections may have a synergistic effect in the occurrence of ARTI. Similarly, bacterial superinfection that may favor clinical presentation in the form of SRTI should also be considered. Thus, this prevalence of 14.6% in our study could not represent the proportion of ARTI cases attributable to HRSV alone, but its implication on the occurrence of ARTI in people from Africa. However, this hypothesis, involvement of bacterial superinfection or viral coinfection on the occurrence of STRI in the presence of HRSV infection, needs to be investigated in futures studies.
We found in this review that the prevalence of HRSV infection was higher in children compared to adults. This is consistent with other reviews.4, 5, 81, 82, 83 Similar data were found in developed countries.84 This means that the etiologies of ARTI including viruses and other agents such as bacteria are not the same between adults and children. Indeed, in the published literature, HRSV infections appear as one of the major cause of mortality and morbidity including causes of hospitalizations in children and not among adults.85, 86, 87 Children may have a naturally weak immune system compared to adults when facing HRSV infection.88 Innovative strategies to curb the burden should first focus on children which present the highest HRSV‐related burden.2 Effective and efficient maternal HRSV vaccine and monoclonal antibody use may help to curb the disease burden in this vulnerable population. In fact, an effective maternal immunization or if newborn antibody immunization is successful,89, 90 it is possible to decrease by 80% the involvement of HRSV in the occurrence of ARTI in children.2, 91
We found a higher prevalence of HRSV infection in SRTI compared to BRTI patients. A review in China reported a higher prevalence in inpatients compared outpatients.81 SRTI clinical presentation may mainly due to the coinfection with bacteria; therefore, this clinical presentation could reflect the consequence of a superinfection of HRSV infection. We did not find any association between prevalence and altitude, latitude, and longitude suggesting that these parameters do not influence the distribution of HRSV throughout the Africa continent. In addition, there was no difference in distribution across Africa subregions. One would have expected that the increasing distance from the equator or in case of the increasing altitude, one can have an increase of the prevalence.
Results from this study should be interpreted with caution in the context of its limitations. First, we found substantial heterogeneity in estimation of the prevalence of HRSV infection in people with ARI across studies. Although we identified some sources of heterogeneity, there may still be other sources of heterogeneity not investigated. These other sources of heterogeneity could include environmental temperature, humidity, pluviometry, exposure to smoking, and air pollution. However, we were unable to assess these factors as source of heterogeneity because they were not reported in primary included studies. Second, countries and UNSD African regions were not uniformly represented, partly owing to difficult retrieval of African medical literature, especially for older articles and those published in local journals. This can limit the generalizability of findings to the entire African continent. Thirdly, even though we aimed at including all age groups, most of studies were conducted in pediatric populations. Our findings therefore mainly reflect the prevalence of HRSV infection in children with ARI.
To the best of our knowledge, this article is among the first systematic reviews that use meta‐analysis to summarize data on prevalence of HRSV infection in people with ARI in Africa. Strengths of this systematic review and meta‐analysis include the use of predefined protocol, a comprehensive search strategy, and involvement of two independent investigators in all stages of the review process. We included only studies that identified HRSV using a reference standard method for diagnosis, the reverse transcriptase PCR. Although we found publication bias in some subgroup analyses, no publication bias was found in the main analyses suggesting that we were unlikely to have missed studies that could alter the findings. Nine‐tenths of the studies were assessed as having low risk of bias in their methodological quality, suggesting that we can be confident to the quality of the findings in this review. In addition, the sensitivity analyses including only studies with low risk and studies with complete season(s) yielded to very close prevalence to that in the crude analysis. A multivariable meta‐regression analysis was conducted helping to control potential confounders for source of variation in HRSV prevalence.
This study suggests a high prevalence of HRSV in people with ARTI in Africa, particularly among children and people with severe clinical form presentation. As such, HRSV infections in people with ARTI in Africa should deserve more attention from healthcare providers, researchers, policymakers, and stakeholders from the health sector for improved detection, overall proper management, and efficient control. Actual influenza surveillance systems implemented in Africa could add routine screening of HRSV in collected samples. Efforts to address this burden could mainly focus on primary prevention including development and implementation of vaccines against HRSV. Strategies and funding to expand the use of vaccines and other primary prevention methods in this resource‐constrained continent are needed. All innovative strategies to curb the burden should first focus on children which present the highest HRSV‐related burden.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
DATA SHARING AND DATA ACCESSIBILITY
All data generated or analyzed during this study are included in this published article and its supplementary information files.
AUTHOR'S CONTRIBUTIONS
SK, JJB, and RN involved in conception and design. SK and JJB involved in literature search.SK and JJB involved in study selection. SK, JJB, FBNS, and EAW extracted the data. JJB and SK involved in data synthesis and analysis.SK and JJB made the first draft. SK, JJB, EAW, FBNS, VBP, AV, and RN involved in critical revision of successive drafts of the paper. RN is guarantor of the review. All authors approved the final version.
Supporting information
ACKNOWLEDGEMENTS
None.
Kenmoe S, Bigna JJ, Well EA, et al. Prevalence of human respiratory syncytial virus infection in people with acute respiratory tract infections in Africa: A systematic review and meta‐analysis. Influenza Other Respi Viruses. 2018;12:793–803. 10.1111/irv.12584
Funding information
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post‐2015 priorities: an updated systematic analysis. Lancet (London, England). 2015;385(9966):430‐440. [DOI] [PubMed] [Google Scholar]
- 2. Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet (London, England). 2017;390(10098):946‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet (London, England). 2010;375(9725):1545‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi T, Balsells E, Wastnedge E, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta‐analysis. J Glob Health. 2015;5(2):020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welliver RC Sr, Checchia PA, Bauman JH, Fernandes AW, Mahadevia PJ, Hall CB. Fatality rates in published reports of RSV hospitalizations among high‐risk and otherwise healthy children. Curr Med Res Opin. 2010;26(9):2175‐2181. [DOI] [PubMed] [Google Scholar]
- 6. Statistics Division United Nations . Geographic Regions. https://unstats.un.org/unsd/methodology/m49/. Accessed September 1, 2017.
- 7. Google . Coordonnées GPS et Google Map. https://www.coordonnees-gps.fr/. Accessed September 1, 2017.
- 8. Lefebvre A, Manoha C, Bour JB, et al. Human metapneumovirus in patients hospitalized with acute respiratory infections: a meta‐analysis. J Clin Virol. 2016;81:68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974‐978. [DOI] [PubMed] [Google Scholar]
- 10. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cochran GW. The combination of estimates from different experiments. Biometrics. 1954;10(1):101‐129. [Google Scholar]
- 12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Reviews and Dissemination . CRD's Guidance for Undertaking Reviews in Healthcare. Centers for Reviews and Dissemination; 2009. http://www.york.ac.uk/crd/SysRev/!SSL!/WebHelp/SysRev3.htm. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ (Clinical research ed). 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agoti CN, Otieno JR, Gitahi CW, Cane PA, Nokes DJ. Rapid spread and diversification of respiratory syncytial virus genotype ON1, Kenya. Emerging Infect Dis. 2014;20(6):950‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed JA, Katz MA, Auko E, et al. Epidemiology of respiratory viral infections in two long‐term refugee camps in Kenya, 2007–2010. BMC Infect Dis. 2012;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akinloye OM, Rönkkö E, Savolainen‐Kopra C, et al. Specific viruses detected in Nigerian children in association with acute respiratory disease. J Trop Med. 2011;2011:690286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Annamalay A, Lanaspa M, Bizzintino J, Khoo S, Le Souef P, Bassat Q. Viral aetiology of acute lower respiratory infections in children in rural mozambique. Respirology. 2014;19:127. [Google Scholar]
- 19. Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303(20):2051‐2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bimouhen A, El Falaki F, Ihazmad H, Regragui Z, Benkerroum S, Barakat A. Circulation of Respiratory Syncytial Virus in Morocco during 2014–2016: findings from a sentinel‐based virological surveillance system for influenza. East Mediterr Health J. 2016;22(7):483‐490. [PubMed] [Google Scholar]
- 21. Breiman RF, Cosmas L, Njenga M, et al. Severe acute respiratory infection in children in a densely populated urban slum in Kenya, 2007–2011. BMC Infect Dis. 2015;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ciervo A, Mancini F, Puzelli S, et al. Detection and correlates of Chlamydophila pneumoniae among children with acute respiratory infections. J Pediatric Infect Dis. 2010;5(3):249‐254. [Google Scholar]
- 23. Cohen C, Walaza S, Moyes J, et al. Epidemiology of viral‐associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009–2012. Pediatr Infect Dis J. 2015;34(1):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ElBasha N, El Rifai N, Draz I, El Kholy A. Contribution of viruses to severe pneumonia in children. Egypt Pediatr Assoc Gaz. 2013;61(2):73‐77. [Google Scholar]
- 25. El Kholy AA, Mostafa NA, Ali AA, et al. Risk factors of prolonged hospital stay in children with viral severe acute respiratory infections. J Infect Dev Count. 2014;8(10):1285‐1293. [DOI] [PubMed] [Google Scholar]
- 26. Emukule GO, Khagayi S, McMorrow ML, et al. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009–2012. PLoS One. 2014;9(8):e105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Enan KA, Nabeshima T, Kubo T, et al. Survey of causative agents for acute respiratory infections among patients in Khartoum‐State, Sudan, 2010–2011. Virol J. 2013;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feikin DR, Njenga MK, Bigogo G, et al. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007–2010. Pediatr Infect Dis J. 2013;32(1):e14‐e19. [DOI] [PubMed] [Google Scholar]
- 29. Feikin DR, Njenga MK, Bigogo G, et al. Etiology and incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS One. 2012;7(8):e43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuller JA, Njenga MK, Bigogo G, et al. Association of the CT values of real‐time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J Med Virol. 2013;85(5):924‐932. [DOI] [PubMed] [Google Scholar]
- 31. Ghani AS, Morrow BM, Hardie DR, Argent AC. An investigation into the prevalence and outcome of patients admitted to a pediatric intensive care unit with viral respiratory tract infections in Cape Town, South Africa. Pediatr Crit Care Med. 2012;13(5):e275‐e281. [DOI] [PubMed] [Google Scholar]
- 32. Hammitt LL, Kazungu S, Morpeth SC, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54(suppl 2):S190‐S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann J, Rabezanahary H, Randriamarotia M, et al. Viral and atypical bacterial etiology of acute respiratory infections in children under 5 years old living in a rural tropical area of Madagascar. PLoS One. 2012;7(8):e43666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horton KC, Dueger EL, Kandeel A, et al. Viral etiology, seasonality and severity of hospitalized patients with severe acute respiratory infections in the Eastern Mediterranean Region, 2007–2014. PLoS One. 2017;12(7):e0180954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jroundi I, Mahraoui C, Benmessaoud R, et al. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J Trop Pediatr. 2014;60(4):270‐278. [DOI] [PubMed] [Google Scholar]
- 36. Kelly MS, Smieja M, Luinstra K, et al. Association of respiratory viruses with outcomes of severe childhood pneumonia in Botswana. PLoS One. 2015;10(5):e0126593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kenmoe S, Tchendjou P, Vernet MA, et al. Viral etiology of severe acute respiratory infections in hospitalized children in Cameroon, 2011–2013. Influenza Other Respir Viruses. 2016;10(5):386‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim C, Ahmed JA, Eidex RB, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real‐time reverse transcription‐PCR assays. PLoS One. 2011;6(6):e21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwofie TB, Anane YA, Nkrumah B, Annan A, Nguah SB, Owusu M. Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol J. 2012;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lagare A, Mainassara HB, Issaka B, Sidiki A, Tempia S. Viral and bacterial etiology of severe acute respiratory illness among children < 5 years of age without influenza in Niger. BMC Infect Dis. 2015;15:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lonngren C, Morrow BM, Haynes S, Yusri T, Vyas H, Argent AC. North‐South divide: distribution and outcome of respiratory viral infections in paediatric intensive care units in Cape Town (South Africa) and Nottingham (United Kingdom). J Paediatr Child Health. 2014;50(3):208‐215. [DOI] [PubMed] [Google Scholar]
- 42. Mazur NI, Bont L, Cohen AL, et al. Severity of respiratory syncytial virus lower respiratory tract infection with viral coinfection in HIV‐uninfected children. Clin Infect Dis. 2017;64(4):443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meligy B, Sayed A, Ismail DK, Kamal D, Abdel‐Latif W, Erfan DM. Detection of viral acute lower respiratory tract infection in hospitalized infants using real‐time PCR. Egypt Pediatr Assoc Gaz. 2016;64(1):13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niang MN, Diop OM, Sarr FD, et al. Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal: the EVIRA project. J Med Virol. 2010;82(5):866‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Obodai E, Asmah R, Boamah I, Goka B, Odoom JK, Adiku T. Respiratory syncytial virus genotypes circulating in urban Ghana: February to November 2006. Pan Afr Med J. 2014;19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Callaghan‐Gordo C, Bassat Q, Morais L, et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J. 2011;30(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 47. Othman HT, Abu Elhamed WA, Hassan DM, Soliman MS, Abdel Baset RW. Respiratory syncytial virus and human metapneumovirus in severe lower respiratory tract infections in children under two. J Infect Dev Count. 2016;10(3):283‐289. [DOI] [PubMed] [Google Scholar]
- 48. Otieno JR, Kamau EM, Agoti CN, et al. Spread and evolution of respiratory syncytial virus a genotype ON1, Coastal Kenya, 2010–2015. Emerg Infect Dis. 2017;23(2):264‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ouedraogo S, Traore B, Nene Bi ZA, et al. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso). PLoS One. 2014;9(10):e110435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peterson I, Bar‐Zeev N, Kennedy N, et al. Respiratory virus‐associated severe acute respiratory illness and viral clustering in Malawian children in a setting with a high prevalence of HIV infection, malaria, and malnutrition. J Infect Dis. 2016;214(11):1700‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pretorius MA, Madhi SA, Cohen C, et al. Respiratory viral coinfections identified by a 10‐plex real‐time reverse‐transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness–South Africa, 2009–2010. J Infect Dis. 2012;206(suppl 1):S159‐S165. [DOI] [PubMed] [Google Scholar]
- 52. Pretorius MA, van Niekerk S, Tempia S, et al. Replacement and positive evolution of subtype A and B respiratory syncytial virus G‐protein genotypes from 1997–2012 in South Africa. J Infect Dis. 2013;208(suppl 3):S227‐S237. [DOI] [PubMed] [Google Scholar]
- 53. Pretorius MA, Tempia S, Walaza S, et al. The role of influenza, RSV and other common respiratory viruses in severe acute respiratory infections and influenza‐like illness in a population with a high HIV sero‐prevalence, South Africa 2012–2015. J Clin Virol. 2016;75:21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rowlinson E, Dueger E, Taylor T, et al. Incidence and clinical features of respiratory syncytial virus infections in a population‐based surveillance site in the Nile Delta Region. J Infect Dis. 2013;208(suppl 3):S189‐S196. [DOI] [PubMed] [Google Scholar]
- 55. Rowlinson E, Dueger E, Mansour A, et al. Incidence and etiology of hospitalized acute respiratory infections in the Egyptian Delta. Influenza Other Respir Viruses. 2017;11(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shafik CF, Mohareb EW, Yassin AS, et al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect Dis. 2012;12:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Venter M, Lassauniere R, Kresfelder TL, Westerberg Y, Visser A. Contribution of common and recently described respiratory viruses to annual hospitalizations in children in South Africa. J Med Virol. 2011;83(8):1458‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zar HJ, Barnett W, Stadler A, Gardner‐Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case‐control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4(6):463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cohen C, Moyes J, Tempia S, et al. Epidemiology of acute lower respiratory tract infection in HIV exposed uninfected infants. Pediatrics. 2016;137(4):e20153272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dia N, Richard V, Kiori D, et al. Respiratory viruses associated with patients older than 50 years presenting with ILI in Senegal, 2009 to 2011. BMC Infect Dis. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fall A, Dia N, Cisse el HA, et al. Epidemiology and molecular characterization of human respiratory syncytial virus in Senegal after four consecutive years of surveillance, 2012–2015. PLoS One. 2016;11(6):e0157163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kadjo HA, Ekaza E, Coulibaly D, et al. Sentinel surveillance for influenza and other respiratory viruses in Cote d'Ivoire, 2003–2010. Influenza Other Respir Viruses. 2013;7(3):296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lekana‐Douki SE, Nkoghe D, Drosten C, Ngoungou EB, Drexler JF, Leroy EM. Viral etiology and seasonality of influenza‐like illness in Gabon, March 2010 to June 2011. BMC Infect Dis. 2014;14:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moyes J, Cohen C, Pretorius M, et al. Epidemiology of respiratory syncytial virus‐associated acute lower respiratory tract infection hospitalizations among HIV‐infected and HIV‐uninfected South African children, 2010–2011. J Infect Dis. 2013;208(suppl 3):S217‐S226. [DOI] [PubMed] [Google Scholar]
- 65. Moyes J, Walaza S, Pretorius M, et al. Respiratory syncytial virus in adults with severe acute respiratory illness in a high HIV prevalence setting. J Infect. 2017;75:346‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakoune E, Tricou V, Manirakiza A, et al. First introduction of pandemic influenza A/H1N1 and detection of respiratory viruses in pediatric patients in Central African Republic. Virol J. 2013;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ndegwa LK, Katz MA, McCormick K, et al. Surveillance for respiratory health care‐associated infections among inpatients in 3 Kenyan hospitals, 2010–2012. Am J Infect Control. 2014;42(9):985‐990. [DOI] [PubMed] [Google Scholar]
- 68. Niang MN, Diop NS, Fall A, et al. Respiratory viruses in patients with influenza‐like illness in Senegal: focus on human respiratory adenoviruses. PLoS One. 2017;12(3):e0174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Njouom R, Yekwa EL, Cappy P, Vabret A, Boisier P, Rousset D. Viral etiology of influenza‐like illnesses in Cameroon, January‐December 2009. J Infect Dis. 2012;206(suppl 1):S29‐S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Razanajatovo NH, Richard V, Hoffmann J, et al. Viral etiology of influenza‐like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PLoS One. 2011;6(3):e17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bigogo GM, Breiman RF, Feikin DR, et al. Epidemiology of respiratory syncytial virus infection in rural and urban Kenya. J Infect Dis. 2013;208(suppl 3):S207‐S216. [DOI] [PubMed] [Google Scholar]
- 72. Brottet E, Jaffar‐Bandjee MC, Li‐Pat‐Yuen G, Filleul L. Etiology of influenza‐like illnesses from sentinel network practitioners in Reunion Island, 2011–2012. PLoS One. 2016;11(9):e0163377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dia N, Diene Sarr F, Thiam D, et al. Influenza‐like illnesses in Senegal: not only focus on influenza viruses. PLoS One. 2014;9(3):e93227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Embarek Mohamed MS, Reiche J, Jacobsen S, et al. Molecular analysis of human metapneumovirus detected in patients with lower respiratory tract infection in upper Egypt. Int J Microbiol. 2014;2014:290793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Feikin DR, Njenga MK, Bigogo G, et al. Additional diagnostic yield of adding serology to PCR in diagnosing viral acute respiratory infections in Kenyan patients 5 years of age and older. Clin Vaccine Immunol. 2013;20(1):113‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jroundi I, Mahraoui C, Benmessaoud R, et al. A comparison of human metapneumovirus and respiratory syncytial virus WHO‐defined severe pneumonia in Moroccan children. Epidemiol Infect. 2016;144(3):516‐526. [DOI] [PubMed] [Google Scholar]
- 77. Mohamed GA, Ahmed JA, Marano N, et al. Etiology and incidence of viral acute respiratory infections among refugees aged 5 years and older in Hagadera Camp, Dadaab, Kenya. Am J Trop Med Hyg. 2015;93(6):1371‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nyawanda BO, Mott JA, Njuguna HN, et al. Evaluation of case definitions to detect respiratory syncytial virus infection in hospitalized children below 5 years in Rural Western Kenya, 2009–2013. BMC Infect Dis. 2016;16:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ouedraogo YS, Ouedraogo R, Nenebi A, et al. [Respiratory syncytial virus (RSV) infections in the pediatric teaching hospital Charles de Gaulle of Ouagadougou, Burkina Faso]. Bull Soc Pathol Exot. 2016;109(1):20‐25. [DOI] [PubMed] [Google Scholar]
- 80. Simusika P, Bateman AC, Theo A, et al. Identification of viral and bacterial pathogens from hospitalized children with severe acute respiratory illness in Lusaka, Zambia, 2011–2012: a cross‐sectional study. BMC Infect Dis. 2015;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang Y, Yuan L, Zhang Y, Zhang X, Zheng M, Kyaw MH. Burden of respiratory syncytial virus infections in China: systematic review and meta‐analysis. J Glob Health. 2015;5(2):020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Salimi V, Tavakoli‐Yaraki M, Yavarian J, Bont L, Mokhtari‐Azad T. Prevalence of human respiratory syncytial virus circulating in Iran. J Infect Public Health. 2016;9(2):125‐135. [DOI] [PubMed] [Google Scholar]
- 83. Bardach A, Rey‐Ares L, Cafferata ML, et al. Systematic review and meta‐analysis of respiratory syncytial virus infection epidemiology in Latin America. Rev Med Virol. 2014;24(2):76‐89. [DOI] [PubMed] [Google Scholar]
- 84. Centers for Disease Control and Prevention . Respiratory syncytial virus – United States, July 2012–June 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1133‐1136. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6348a3.htm. Accessed October 10, 2017. [PMC free article] [PubMed] [Google Scholar]
- 85. Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114(4):e437‐e444. [DOI] [PubMed] [Google Scholar]
- 86. Djelantik IG, Gessner BD, Soewignjo S, et al. Incidence and clinical features of hospitalization because of respiratory syncytial virus lower respiratory illness among children less than two years of age in a rural Asian setting. Pediatr Infect Dis J. 2003;22(2):150‐157. [DOI] [PubMed] [Google Scholar]
- 87. Constantopoulos AG, Kafetzis DA, Syrogiannopoulos GA, et al. Burden of respiratory syncytial viral infections on paediatric hospitals: a two‐year prospective epidemiological study. Eur J Clin Microbiol Infect Dis. 2002;21(2):102‐107. [DOI] [PubMed] [Google Scholar]
- 88. Sastre P, Ruiz T, Schildgen O, Schildgen V, Vela C, Rueda P. Seroprevalence of human respiratory syncytial virus and human metapneumovirus in healthy population analyzed by recombinant fusion protein‐based enzyme linked immunosorbent assay. Virol J. 2012;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. August A . RSV F Vaccine: Phase 2 Clinical Trial to Protect Infants via Maternal Immunization. Paper presented at: XXI FIGO World Congress of Gynecology and Obstetrics 2015; Vancouver, Canada.
- 90. Griffin MP, Khan AA, Esser MT, et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F‐targeting monoclonal antibody with an extended half‐life, in healthy adults. Antimicrob Agents Chemother 2017;61(3):e01714‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS. WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine. 2016;34(2):190‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


