Abstract
Aim of the study
This meta-analysis evaluated serum interleukin-6 (IL-6) levels in hepatocellular carcinoma (HCC) patients compared with healthy controls and hepatitis and cirrhotic patients.
Material and methods
The three databases PubMed, Scopus, and Web of Science were searched for assessment of IL-6 levels in HCC patients (without cirrhosis and hepatitis) compared with healthy controls (without HCC, cirrhosis and hepatitis) and the studies were selected based on inclusion and exclusion criteria. A random-effect meta-analysis was performed with RevMan 5.3 software, using mean difference (MD) and 95% confidence intervals (CIs).
Results
Out of 503 studies searched in databases, 18 studies were included in the meta-analysis. The pooled analysis with continuous data demonstrated that the IL-6 level in HCC patients was significantly higher than that in healthy controls (MD = 12.44; 95% CI: 9.02-15.85; p < 0.00001). Also, the pooled analysis demonstrated that the IL-6 levels in cirrhotic patients (MD = –6.98; 95% CI: –12.91-1.05; p < 0.02) and patients with hepatitis (MD = –8.43; 95% CI: –11.91-4.95; p < 0.00001) were significantly lower than the level in HCC patients, and the subgroup analyses had high heterogeneity.
Conclusions
The elevated IL-6 levels in HCC patients compared with hepatitis and cirrhosis patients and healthy controls may show a significant association of this cytokine with increased risk of HCC and its potential as a diagnostic marker for HCC in future diagnostic and therapeutic strategies.
Keywords: hepatocellular carcinoma, interleukin-6, serum
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world and is highly prevalent in sub-Saharan Africa and Southeast Asia [1]. Most cases have chronic inflammatory liver diseases in the background [1, 2]. Inflammatory cytokines may have a positive or negative effect on development and progression of HCC [3]. Interleukin-6 (IL-6) as an important pro-inflammatory mediator is a potent mitogen for cholangiocytes and cholangiocarcinoma cells [4, 5]. Meanwhile, IL-6 is also known as an autocrine growth factor in cancer [6]. IL-6 appears to increase cholangiocarcinoma cells’ growth by stimulating a mitogen-activated protein kinase signaling pathway [5, 7]. Important risk factors of recurrence in successfully treated early-stage HCC patients are higher Child-Pugh score, larger tumor size, low platelet count, liver cirrhosis, elevated a-fetoprotein (AFP) level, and high serum hepatitis B virus (HBV) load [8, 9]. AFP is the major tumor marker for HCC [10, 11] with a sensitivity of 41-65% [11]. Using AFP as a screening test in high risk patients can detect more early tumors and prolong the survival of patients [10]. Also, recognizing interleukins can help immunotherapy treatments in HCC patients [12]. This meta-analysis aimed to evaluate serum IL-6 levels in HCC patients in comparison with healthy controls as well as hepatitis and cirrhotic patients.
Material and methods
Search strategies
We used the keywords ‘IL-6’, ‘interleukin-6’, ‘liver cancer’, ‘HCC’ and ‘hepatocellular carcinoma’ in the three databases PubMed, Scopus and Web of Science from January 1980 to March 2017 for publications with an English abstract.
Study selection
One author (M.S.) searched the articles, followed by the second author (M.R.), blinded to the first reviewer. If there was any disagreement between two reviewers, the third reviewer (E.S.) resolved the problem. All studies were searched for evaluation of IL-6 levels in the HCC patients compared with healthy controls, cirrhotic patients or patients with hepatitis. The inclusion criteria for the studies included: i) studies reporting the mean or median IL-6 level in HCC patients and healthy controls; ii) HCC patients or healthy controls with liver diseases could be included in the study; iii) studies reporting healthy control, hepatitis or cirrhosis groups compared with a HCC group; iv) only studies with an English-language abstract could be included; v) human studies; vi) HCC was confirmed histologically or based on biopsy or cytologic findings; vii) blood sample of IL-6 was taken after overnight fasting. The exclusion criteria were: 1) the studies did not report mean or median IL-6 level; 2) data from incomplete reports (no sufficient information) and review studies were not eligible for this study; 3) studies reporting the healthy control with liver diseases.
Data extraction
The name of author, year of publication, country, number/age/male (%) of HCC, cirrhosis, hepatitis and healthy control groups were the relevant data extracted from every study.
Statistical analysis
A random-effect meta-analysis was performed with Review Manager 5.3 (RevMan 5.3, The Cochrane Collaboration, Oxford, United Kingdom) using mean difference (MD) and 95% confidence intervals (CIs). Heterogeneity between estimates was assessed by the Q and I2 statistic that for the Q statistic. Heterogeneity was considered if p < 0.1 and also 95% CI and p-value (2-sided) < 0.05 was considered statistically significant in this meta-analysis. Publication bias was assessed through funnel plot analysis with Begg’s and Egger’s tests. If the data were presented using the standard error (SE), then the formula was used to calculate standard deviation (SD). If the data were presented using several SDs, the mean SD was calculated by the formula (n = sample size) [3]. We used the formula for estimation of mean and SD if the study reported median plus range [13, 14] or median plus interquartile range [15]. The unit of measurement of IL-6 in this meta-analysis is pg/ml.
Results
Study selection
Out of 503 studies searched in databases, 62 studies were assessed for eligibility. Of these studies, 44 studies were excluded with reasons (Figure 1). Therefore, 18 studies reported the mean or median IL-6 level in HCC and healthy control groups and therefore were included in this meta-analysis.
Fig. 1.
Study flowchart
Study characteristics
The characteristics of 18 studies included in the meta-analysis are shown in Table 1. All studies were published in the period from 2000 to 2014. Three studies were reported in Italy [16-18], three in Egypt [19-21], three in Taiwan [22-24], two in Korea [25, 26], three in China [27-29], two in Turkey [30, 31], one in Thailand [32] and one in Japan [33]. In the meta-analysis, 1187 HCC patients and 1414 healthy controls were included. Five studies reported the median IL-6 level [16-18, 22, 23], five studies reported SE [20-23, 25] and one study reported two means and SDs for HCC patients [19].
Table 1.
Characteristics of studies included in meta-analysis (n = 18)
| Study (year) | Country | HCC patients (n/Mean age, yrs/male %) | Controls (n/Mean age, yrs/male %) | Cirrhosis (n/Mean age, yrs/male %) | Hepatitis (n/Mean age, yrs/male %) |
|---|---|---|---|---|---|
| Chau (2000) [22] | Taiwan | 67/63.4/89.6 | 27/–/– | – | – |
| Giannitrapani (2002) [16] | Italy | 87/62.2/68.9 | 28/55.4/67.8 | – | – |
| Coskun (2004) [30] | Turkey | 11/–/– | 12/–/– | – | – |
| Ataseven (2006) [31] | Turkey | 22/59.8/68.0 | 25/37.1/44 | 23/45.5/47 | – |
| Soresi (2006) [17] | Italy | 93/62.2/65.6 | 42/54.9/73.8 | 72/56.5/66.7 | – |
| Cheon (2007) [25] | Korea | 26/62.8/65.3 | 23/53.5/65.2 | – | – |
| Tangkijvanich (2007) [32] | Thailand | 70/55.0/85.7 | 10/–/– | – | – |
| Porta (2008) [18] | Italy | 30/Median: 62.3/70.0 | 30/matched/matched | 30/matched/matched | – |
| Wong (2009) [27] | China | 37/Median: 55.0/89.0 | – | – | 37/Median: 55.0/89.0 |
| El-Folly (2010) [19] | Egypt | 50/54.9/68.0 | 25/34.0/56.0 | – | – |
| Pang (2011) [28] | China | 32/–/– | 18/–/– | – | – |
| Chao (2012) [23] | Taiwan | 73/matched/matched | 30/matched/matched | – | – |
| Jang (2012) [26] | Korea | 110/58.6/71.8 | 24/–/– | – | – |
| Kao (2012) [24] | Taiwan | 47/60.1/80.9 | 139/43.9/56.8 | 57/52.9/82.5 | 291/43.8/63.2 |
| Metwaly (2012) [20] | Egypt | 40/57.6/77.5 | 15/50.7/73.3 | 31/53.7/83.9 | – |
| Othman (2013) [21] | Egypt | 20/–/60.0 | 20/–/50 | 20/–/75.0 | 20/–/65.0 |
| Tang (2013) [29] | China | 148/60.2/74.3 | 265/41.6/72.8 | 153/51.6/72.5 | 292/43.9/72.7 |
| Ohishi (2014) [33] | Japan | 224/66.4/60.7 | 644/63.7/60.1 | – | – |
HCC – hepatocellular carcinoma, n – number
Meta-analysis
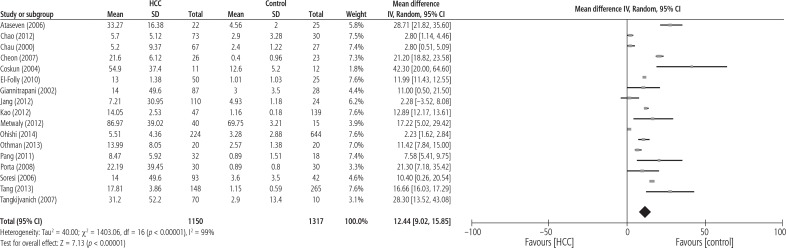
Figure 2 shows MD of serum IL-6 levels in 1150 HCC patients compared with 1377 healthy controls. The pooled analysis with continuous data demonstrated that the IL-6 level in HCC patients was significantly higher than that in healthy controls (MD = 12.44; 95% CI: 9.02-15.85; p < 0.00001) and I2 = 99% (p < 0.00001).
Fig. 2.
Forest plot of random-effect of serum IL-6 level in hepatocellular carcinoma patients compared with healthy controls (n = 17)
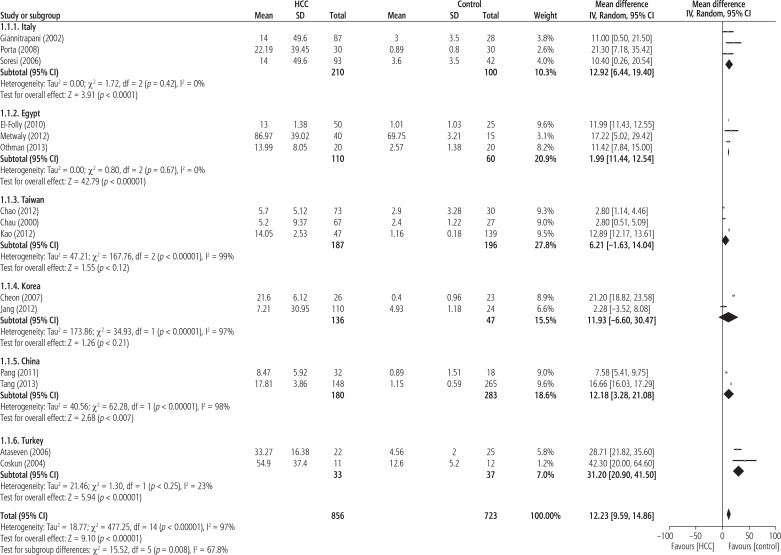
Figure 3 shows MD of serum IL-6 levels in HCC patients compared with healthy controls in different countries. The pooled subgroup analysis with continuous data demonstrated that the IL-6 levels in HCC patients were significantly higher than in healthy controls in Italy (MD = 12.92; 95% CI: 6.44-19.40; p < 0.0001), Egypt (MD = 11.99; 95% CI: 11.44-12.54; p < 0.00001), China (MD = 12.18; 95% CI: 3.28-21.08; p < 0.007) and Turkey (MD = 31.20; 95% CI: 20.90-41.50; p < 0.00001) with I2 = 0% (p < 0.42), I2 = 0% (p < 0.67), I2 = 98% (p < 0.00001) and I2 = 23% (p < 0.25), respectively. This analysis did not show significantly higher levels of IL-6 in HCC patients than in healthy controls in Taiwan (MD = 6.21; 95% CI: –1.63-14.04; p < 0.12) or Korea (MD = 11.93; 95% CI: –6.60-30.47; p < 0.21) with I2 = 99% and I2 = 97%, respectively.
Fig. 3.
Forest plot of random-effect of serum IL-6 level in hepatocellular carcinoma patients compared with healthy controls based on country reported
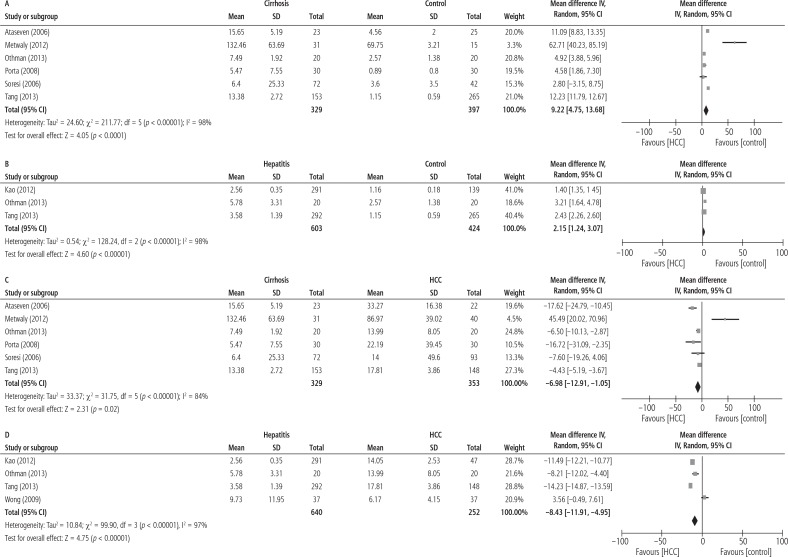
The MDs of serum IL-6 levels in 329 cirrhotic patients versus 397 controls, 603 patients with hepatitis versus 424 controls, 329 cirrhotic patients versus 353 HCC patients and 640 patients with hepatitis versus 252 HCC patients are shown in Figure 4. The pooled analysis with continuous data demonstrated that the IL-6 levels in cirrhotic patients (MD = 9.22; 95% CI: 4.75-13.68; p < 0.0001) and patients with hepatitis (MD = 2.15; 95% CI: 1.24-3.07; p < 0.00001) were significantly higher than in healthy controls with I2 = 99% and I2 = 98%, respectively. Also, the pooled analysis demonstrated that the IL-6 levels in cirrhotic patients (MD = –6.98; 95% CI: –12.91- –1.05; p < 0.02) and patients with hepatitis (MD = –8.43; 95% CI: –11.91-4.95; p < 0.00001) were significantly lower than in HCC patients with I2 = 84% and I2 = 97%, respectively.
Fig. 4.
Forest plot of random-effect of comparison of serum IL-6 levels in (A) cirrhosis and healthy control group, (B) hepatitis and healthy control groups, (C) cirrhosis and HCC groups, (D) hepatitis and HCC groups
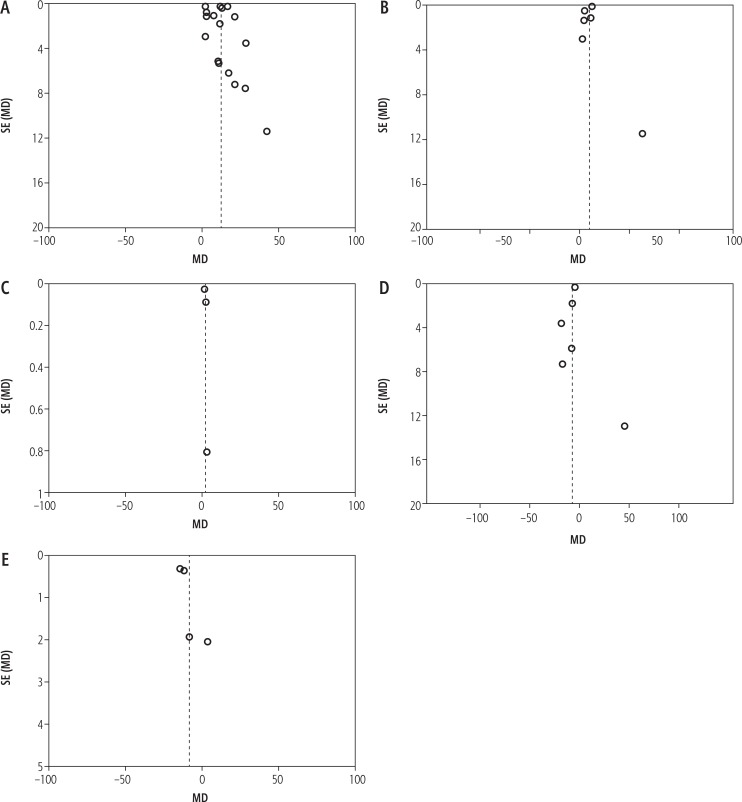
Publication bias
A funnel plot for every analysis is shown in Figure 4. The results of Begg’s and Egger’s tests revealed that no publication biases existed in terms of the comparison of serum IL-6 levels in HCC and healthy control groups (Figure 5A), the comparison of serum IL-6 levels in cirrhosis and healthy control groups (Figure 5B), the comparison of serum IL-6 levels in hepatitis and healthy control groups (Figure 5C), the comparison of serum IL-6 levels in cirrhosis and HCC groups (Figure 5D) and the comparison of serum IL-6 levels in hepatitis and healthy control groups (Figure 5E).
Fig. 5.
Funnel plot of random effect of comparison of serum IL-6 levels in (A) hepatocellular carcinoma (HCC) and healthy control groups, (B) cirrhosis and healthy control group, (C) hepatitis and healthy control groups, (D) cirrhosis and HCC groups, (E) hepatitis and HCC groups
Discussion
This meta-analysis showed that serum IL-6 level in HCC patients was significantly higher than in healthy controls and patients with hepatitis and cirrhosis. Also, IL-6 levels in patients with hepatitis and cirrhosis were significantly higher than in healthy controls. IL-6 is the most important cytokine in predicting survival of HCC [26]. A high serum IL-6 level is an indicator of the tumor burden, i.e. tumor size, stage, and aggressiveness such as portal vein invasion and metastasis. IL-6 is a pleiotropic cytokine that has a central role in hematopoiesis and acute phase responses [18, 34]. A number of studies [4, 17, 20, 21, 35, 36] have reported that IL-6 concentration was significantly higher in the HCC group than healthy controls and liver cirrhosis patients. Porta et al. [18] reported that IL-6 titers were 4-fold higher in HCC vs. cirrhotic patients and 25-fold higher than in healthy controls.
Among patients with a low (< 20 ng/ml) AFP level, IL-6 expression was significantly associated with the presence of HCC [36]. IL-6 may play an extremely important role in determining liver disease progression. Statistically significant differences were demonstrated in the level of IL-6 in patients of advanced liver disease compared with those of mild or moderate to severe liver disease [24]. In patients with advanced HCC, elevated levels of IL-6 were found, correlating with the tumor burden [37]. Soresi et al. [17] concluded that IL-6 values in HCC stage III were higher than in stages I and II of the disease. Also, El-Folly et al. [19] found that the mean IL-6 levels in patients with Child classification C was higher than in those with Child A and B. One study showed that serum IL-6 levels were also elevated in patients with relatively early and resectable disease and were also correlated with tumor size [22]. Two studies [4, 22] found that there was no significant correlation between IL-6 and AFP levels, but other studies reported an association between serum IL-6 level and AFP levels [19-21, 27, 36].
Ehab et al. [38] reported that HCC patients have significantly higher levels of AFP and serum IL-6 than cirrhotic patients without HCC. Moreover, elevated serum levels of IL-6 were significantly associated with increased risk of HCC, especially among obese patients [33]. Othman et al. [21] found that the tumor size has a high correlation with IL-6 level and measuring this cytokine may help to identify a group of HCC patients with low AFP. Also, Soresi et al. [17] concluded that IL-6 levels in patients with liver cirrhosis associated HCC are higher than in patients with liver cirrhosis alone and controls, indicating that production of this cytokine is increased by tumor cells. Combination of IL-6 and AFP in the study of Wong et al. [27] improved the sensitivity in diagnosing HCC or predicting future HCC development. High serum IL-6 level can predict the development of HCC in chronic hepatitis B patients, and has moderate accuracy in predicting future cancer, and also the diagnostic value of IL-6 increases when it is associated with AFP measurement. The combination of these two markers can create new hopes in earlier diagnosis of HCC [19].
Limitations
There was a liver disease with HCC or healthy control in several studies. There was a difference in the percentage of each liver disease in the patients and also stage of HCC. Matching of the mean age between HCC patients and healthy controls was not performed in several studies. There was a low number of patients and controls in several studies. There was high heterogeneity between the studies in each analysis, but there was no bias. There was a difference in consumption of alcohol in the studies. There were differences in the score of Child-Pugh and AFP levels for HCC patients, cirrhosis and hepatitis groups between the studies. There was hepatitis virus related to cirrhotic patients in some studies.
Conclusions
The elevated IL-6 levels in HCC patients compared with patients with hepatitis or cirrhosis and healthy controls may show a significant association of this cytokine with increased risk of HCC and its potential as a diagnostic marker for HCC. The geographical area, lifestyle-related, clinicopathological factors and AFP levels may affect IL-6 levels in HCC patients. Therefore, clinicians should consider the roles of IL-6 and AFP in future diagnostic and therapeutic strategies. The future studies on the impact of each clinicopathological factor on IL-6 level can be done in HCC patients without cirrhosis and hepatitis compared with healthy controls in a large sample size with control of lifestyle-related and clinicopathological factors and AFP levels in similar areas.
Acknowledgments
This article is part of a research project (code: 95549) supported by a grant from Kermanshah University of Medical Sciences Research Council.
Disclosure
The authors report no conflict of interest.
References
- 1.Bosch FX, Ribes J, Díaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Alison MR, Nicholson LJ, Lin WR. Chronic inflammation and hepatocellular carcinoma. Recent Results Cancer Res. 2011;185:135–148. doi: 10.1007/978-3-642-03503-6_8. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goydos JS, Brumfield AM, Frezza E, et al. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227:398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal M, LaRusso NF, Burgarr LJ, et al. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 6.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 7.Park J, Tadlock L, Gores GJ, et al. Inhibition of interleukin-6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. American association for the study of liver, diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamiyama T, Nakanishi K, Yokoo H, et al. Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 2012;10:107. doi: 10.1186/1477-7819-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez J. Recent development in the first detection of Hepatocellular Carcinoma. Clin Biochem Rev. 2005;26:70–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Solahaye-Kahnamouii S, Farhadi F, Rahkare-Farshi M, et al. The effect of interleukin 36 gene therapy in the regression of tumor. Iran J Cancer Prev. 2014;7:197–203. [PMC free article] [PubMed] [Google Scholar]
- 13.Barde MP, Barde PJ. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect Clin Res. 2012;3:113–116. doi: 10.4103/2229-3485.100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannitrapani L, Cervello M, Soresi M, et al. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:46–52. doi: 10.1111/j.1749-6632.2002.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 17.Soresi M, Giannitrapani L, D’Antona F, et al. Interleukin-6 and its soluble receptor in patients with liver cirrhosis and hepatocellular carcinoma. World J Gastroenterol. 2006;12:2563–2568. doi: 10.3748/wjg.v12.i16.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porta C, De Amici M, Quaglini S, et al. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 19.El-Folly RF, El-Kabarity RH, Arafa NA. Assessment of the role of interleukin-6 in diagnosis of hepatocellular carcinoma. Egypt J Immunol. 2010;17:11–22. [PubMed] [Google Scholar]
- 20.Metwaly HA, Al-Gayyar MM, Eletreby S, et al. Relevance of serum levels of interleukin-6 and syndecan-1 in patients with hepatocellular carcinoma. Sci Pharm. 2012;80:179–188. doi: 10.3797/scipharm.1110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Othman MS, Aref AM, Mohamed AA, et al. Serum Levels of Interleukin-6 and Interleukin-10 as Biomarkers for Hepatocellular Carcinoma in Egyptian Patients. ISRN Hepatol. 2013;2013:412317. doi: 10.1155/2013/412317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao Y, Wu CY, Kuo CY, et al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int. 2013;7:883–892. doi: 10.1007/s12072-012-9409-9. [DOI] [PubMed] [Google Scholar]
- 24.Kao JT, Lai HC, Tsai SM, et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naïve hepatitis B infection patients. Liver Int. 2012;32:928–936. doi: 10.1111/j.1478-3231.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheon YK, Cho YD, Moon JH, et al. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am J Gastroenterol. 2007;102:2164–2170. doi: 10.1111/j.1572-0241.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 26.Jang JW, Oh BS, Kwon JH, et al. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686–693. doi: 10.1016/j.cyto.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Wong VW, Yu J, Cheng AS, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- 28.Pang XH, Zhang JP, Zhang YJ, et al. Preoperative levels of serum interleukin-6 in patients with hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1687–1693. doi: 10.5754/hge10799. [DOI] [PubMed] [Google Scholar]
- 29.Tang S, Liu Z, Zhang Y, et al. Rather than Rs1800796 polymorphism, expression of interleukin-6 is associated with disease progression of chronic HBV infection in a Chinese Han population. Dis Markers. 2013;35:799–805. doi: 10.1155/2013/508023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coskun U, Bukan N, Sancak B, et al. Serum hepatocyte growth factor and interleukin-6 levels can distinguish patients with primary or metastatic liver tumors from those with benign liver lesions. Neoplasma. 2004;51:209–213. [PubMed] [Google Scholar]
- 31.Ataseven H, Bahcecioglu IH, Kuzu N, et al. The levels of ghrelin, leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediators Inflamm. 2006;2006:78380. doi: 10.1155/MI/2006/78380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tangkijvanich P, Thong-Ngam D, Mahachai V, et al. Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J Gastroenterol. 2007;13:4345–4349. doi: 10.3748/wjg.v13.i32.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohishi W, Cologne JB, Fujiwara S, et al. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014;134:154–163. doi: 10.1002/ijc.28337. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng KS, Tang HL, Chou FT, et al. Cytokine evaluation in liver cirrhosis and hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1105–1110. [PubMed] [Google Scholar]
- 36.Hsia CY, Huo TI, Chiang SY, et al. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol. 2007;33:208–212. doi: 10.1016/j.ejso.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Malaguarnera M, Di Fazio I, Laurino A, et al. Role of interleukin 6 in hepatocellular carcinoma. Bull Cancer. 1996;83:379–384. [Article in French] [PubMed] [Google Scholar]
- 38.Moustafa EF, Galal GM, Hassany SM, et al. Serum Interleukin-6 (IL-6), Vascular Endothelial Growth Factor (VEGF), and VEGF/Platelets Ratio as Markers for Hepatocelluar Carcinoma. Life Sci J. 2012;9:930–938. [Google Scholar]