Abstract
In this paper, we aim at discovering genetic factors of psoriasis through searching for statistically significant SNP-SNP interactions exhaustively from two real psoriasis genome-wide association study datasets (phs000019.v1.p1 and phs000982.v1.p1) downloaded from the database of Genotypes and Phenotypes. To deal with the enormous search space, our search algorithm is accelerated with eight biological plausible interaction patterns and a pre-computed look-up table. After our search, we have discovered several SNPs having a stronger association to psoriasis when they are in combination with another SNP and these combinations may be non-linear interactions. Among the top 20 SNP-SNP interactions being found in terms of pairwise p-value and improvement metric value, we have discovered 27 novel potential psoriasis-associated SNPs where most of them are reported to be eQTLs of a number of known psoriasis-associated genes. On the other hand, we have inferred a gene network after selecting the top 10000 SNP-SNP interactions in terms of improvement metric value and we have discovered a novel long distance interaction between XXbac-BPG154L12.4 and RNU6-283P which is not a long distance haplotype and may be a new discovery. Finally, our experiments with the synthetic datasets have shown that our pre-computed look-up table technique can significantly speed up the search process.
Introduction
Psoriasis is a common polygenic chronic inflammatory skin disease affecting up to 3% of population1. Recently, genome-wide association study (GWAS) provided the first opportunity to have a comprehensive screen for susceptibility genes and up to 50 loci had been reported1,2. The discovery of novel susceptibility genes by GWAS included genes coding for key cytokines involved in Th17 activation (like IL-12B, IL23A and IL23R) and NF-κB pathway also contributed to the aetiology (TNFAIP3 and TNIP1)3. In addition, macrophage and dendritic cells are also involved. HLA-C locus had been a known susceptibility gene before the GWAS era4. Exaggerated expression of keratinocyte antigens (like LCE3D) was also identified as susceptibility genes5.
Although many predisposition genes for psoriasis have been identified, individually they accounted for very small effect size, for example their odds ratios were typically less than 1.26. While heritability of psoriasis had been estimated to be as high as 60–90%, the genetic risk of all variants added up could only account for one-fourth of susceptibility due to genetics. This phenomenon is called missing heritability7. One possible source of unaccounted risk is interactions (including gene-gene and gene-environment interaction), which is the risk of certain genotype may be altered to a large extent in the presence of another risk factor, which is also known as non-additive effects6,8,9. Various algorithms have been proposed to detect the interactions between SNPs or genes in GWAS data10,11. They all faced the difficulties of large search space, exponential increase in SNP combination with increasing level of interaction, and limited statistical basis of the proposed methods. On the other hand, examples of epistasis were found in model organisms and human diseases9,12,13. We had proposed a biological framework of gene-gene interaction and suggested that typical samples size (thousands of cases and controls) should have sufficient power to detect such gene-gene interaction with simulation data14. There were also some suggestions of epistasis in psoriasis but replication in subsequent studies were lacking3,8.
Biological pathways are regulated by the interactions among bio-molecules constructed according to the genetic instructions stored in deoxyribonucleic acid (DNA). A single nucleotide polymorphism (SNP) is a variation at a specific DNA position among a population of organisms which may affect the structure of these bio-molecules. In a genome-wise association study (GWAS), DNA sequences of a large population of patient samples (cases) of a particular genetic disease and healthy samples (controls) are collected and researchers can discover disease-associated SNPs through comparing the DNA sequences between cases and controls15. These DNA sequences can be arranged into a matrix A where each column (except the last column) corresponds to a SNP and each row corresponds to a sample as shown in Table 1. Each entry Ai,j corresponds to the genotype of ith sample at jth SNP under the encoding scheme shown in Table 2. Each sample is either labelled as ‘case’ or ‘control’ through the value of the last column of the matrix. A traditional approach for finding statistically significant SNPs which has been widely adapted in many GWAS researches is to perform statistical tests after building a contingency table for each column of matrix A.
Table 1.
This table shows an example of a GWAS dataset.
| SNP1 | SNP2 | SNPm | Status | ||
|---|---|---|---|---|---|
| Sample1 | 1 | 1 | 1 | T | |
| Sample2 | 2 | 1 | 2 | F | |
| Samplen-1 | 3 | 2 | 2 | F | |
| Samplen | 3 | 2 | 2 | F |
Table 2.
This table shows the encoding scheme for SNP genotype.
| Original Genotype | Encode Value |
|---|---|
| Missing Data | 0 |
| Major Allele, Major Allele | 1 |
| Major Allele, Minor Allele | 2 |
| Minor Allele, Minor Allele | 3 |
As previously discussed, the cause of many genetic diseases can be better explained through certain combinations of SNPs (i.e. SNP-SNP interactions) rather than a number of independent SNPs alone16,17. Although many SNPs are weakly associated to the genetic diseases when they are analysed independently, some of them may show a stronger association only when they are analysed in combination with other SNPs. A typical example of this phenomenon is shown in Figs 1–3 and Supplementary Fig. S1. In Figs 1 and 2, rs3132486 and rs3130048 from dataset phs000019.v1.p1 are both weakly associated to psoriasis with a 1 degree of freedom (d.f.) chi-square p-value less significant than 1 × 10−6 and an odds ratio smaller than 1.7 if they are analysed independently. Meanwhile in Fig. 3, the combination of rs3132486 and rs3130048 are significantly associated to psoriasis with a 1 d.f. chi-square p-value of 2.52 × 10−14 and an odds ratio of 2.2719. Therefore, rs3132486 and rs3130048 may have a potential non-linear interaction associated to psoriasis. Similar but much weaker phenomena have also been observed between SNPs among two known psoriasis associated gene-gene interactions (HLA-C, IL12B)8 and (HLA-C, TNFAIP3)3 and are shown in Supplementary Figs S2 and S3 respectively.
Figure 1.
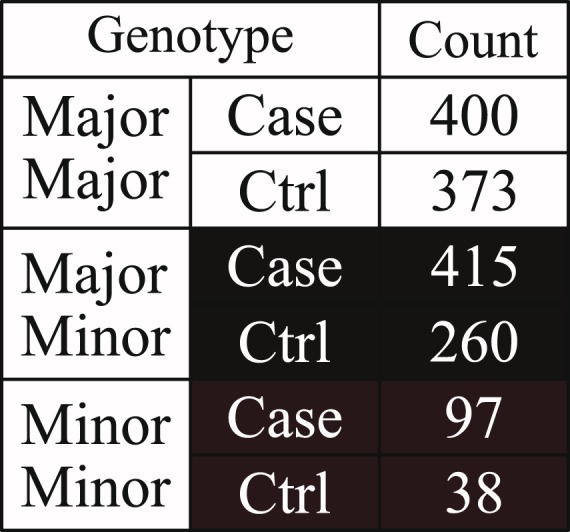
In this figure, the table shows the distribution of case and control under different genotypes of SNP rs3130048 in dataset phs000019.v1.p1. The 1 d.f. chi-square p-value and odds ratio of rs3130048 are 3.96 × 10−6 and 1.6021 respectively.
Figure 3.
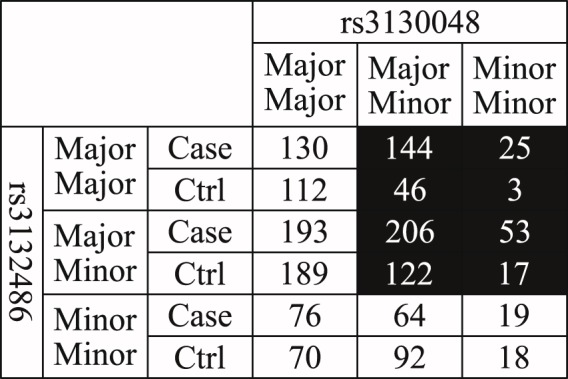
In this figure, the table shows the distribution of case and control under genotype of the combination of SNP rs3132486 and rs3130048 in dataset phs000019.v1.p1. The 1 d.f. chi-square p-value and odds ratio of the combination of rs3132486 and rs3130048 are 2.52 × 10−14 and 2.2719 respectively.
Figure 2.
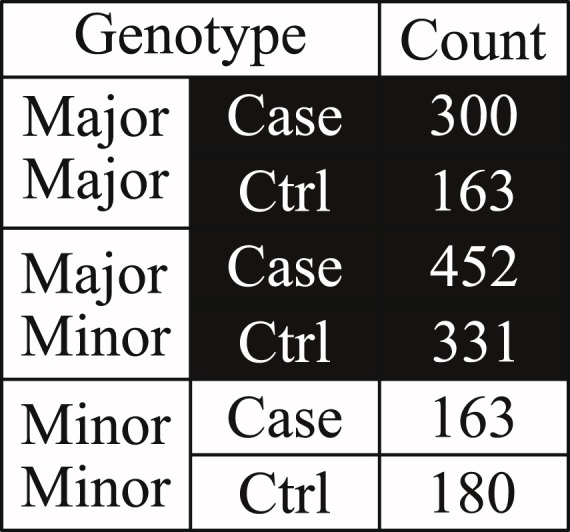
In this figure, the table shows the distribution of case and control under different genotypes of SNP rs3132486 in dataset phs000019.v1.p1. The 1 d.f. chi-square p-value and odds ratio of rs3132486 are 2.06 × 10−5 and 1.6810 respectively.
In previous GWAS researches, filtering out a large proportion of statistically insignificant SNPs or performing search with greedy and stochastic search algorithms are two popular techniques for shrinking the search space of SNP-SNP interactions18,19. Any researcher who performs his/her analysis with a search space confined to statistically significant single SNPs only will prematurely filtered out some disease-associated SNPs which are statistically insignificant on their own similar to the ones shown in Figs 1 and 2. Meanwhile, greedy and stochastic search selectively explore the search space under the guidance of heuristic functions thus they may also fail to detect some disease-associated but statistically insignificant SNPs as well. However, performing exhaustive search for interactions among k different SNPs (i.e. kth order SNP-SNP interaction) would take a time complexity of O(mkn) where m is the number of SNPs and n is the number of samples. Given the fact that there are around 38 million SNPs with one million tag SNPs across the whole human genome20 and a GWAS dataset often contains thousands of samples and hundreds of thousands of SNPs, this problem is difficult to be solved without any advanced computational technique. Driven by the continuous growth of computational power, it is now becoming possible to perform exhaustive search for 2nd order SNP-SNP interactions in a GWAS dataset within a reasonable amount of time21–28. However, existing exhaustive search algorithms are not driven by any biological knowledge and they evaluate the disease association of each SNP combination solely based on heuristic or statistical parameters. Therefore, they may found statistical significant SNP-SNP interactions which may not be biologically interpretable.
We have developed an exhaustive search algorithm driven by eight biological plausible SNP-SNP interaction and applied it on two psoriasis GWAS datasets (phs000019.v1.p1 and phs000982.v1.p1). We have first discovered a number of statistically significant SNP-SNP interactions which may have a stronger association to psoriasis then their component SNPs measured independently similar to the ones shown in Fig. 3 where the SNPs involved in these interactions are far less significant when they are considered individually. After that, we have discovered 27 novel potential psoriasis-associated SNPs among the top 20 statistically significant SNP-SNP interactions in terms of p-value and improvement metric value. Most of these novel potential psoriasis-associated SNPs are reported to be Expression quantitative trait loci (eQTLs) of known psoriasis-associated genes like HLA-B and HLA-C in GTEx Portal29. After mapping the nearest gene to each SNP involved in top 10000 SNP-SNP interactions in terms of improvement metric value from both dataset, we have constructed a disease-associated gene network. In our network, almost half of the gene-gene interactions inferred are consistent with existing literature. Meanwhile, some of the remaining gene-gene interactions are potentially due to the long-distance haplotype interactions presence in the HLA region of Chromosome 6. Furthermore, we have discovered an interaction between 2 SNPs located in gene XXbac-BPG154L12.4 and RNU6-283P of the HLA loci, which doesn’t correspond to any short-distance or even long-distance haplotype interactions and hasn’t been reported in existing literature as well. Therefore, the interaction between XXbac-BPG154L12.4 and RNU6-283P is a potential new discovery. In addition, we have shown that counting contingency table through a pre-computed look-up table is effective in speeding up the process of exhaustive search.
Results
GWAS datasets
We have downloaded two psoriasis GWAS datasets namely phs000019.v1.p1 and phs000982.v1.p1 from the database of Genotypes and Phenotypes (dbGaP). Data pre-processing has been performed on these two datasets to remove low quality SNPs and samples with Plink30. The parameters of data cleansing can be found as Supplementary Tables S1 and S2 which followed the common recommendations from NCBI31.
After data cleansing, there are 352945 SNPs and 1593 samples (cases: 917, controls: 676) in dataset phs000019.v1.p1 and there are 790527 SNPs and 2689 samples (cases: 1363, controls: 1326) in dataset phs000982.v1.p1. The genotypes of every SNP in these two datasets are encoded as 0, 1, 2, 3 according to the encoding scheme shown in Table 2.
Measurement metric for ranking and filtering SNP-SNP interaction
In this paper, SNP-SNP interactions are prioritised with the following two measurement metrics. The pairwise p-value of a 2nd order SNP combination under a particular genotype interaction pattern is referring to the 1 d.f. chi-square p-value of its 2 × 2 contingency table founded from a real psoriasis dataset with the procedure illustrated in the Methods section. Additionally, we have defined another measurement metric called improvement metric value to prioritise statistically significant SNP-SNP interactions where its two components SNPs are far less associated to psoriasis when they are analysed independently. It compares the pairwise p-value of a 2nd order SNP combination under a particular genotype interaction pattern against the standalone 1 d.f. chi-square p-value of both of its component SNPs and can be calculated by equation 1. If a 2nd order SNP combination has a higher improvement metric value, its component SNPs are far more statistically significant when they are considered as a SNP combination rather than separately considered as two independent SNPs.
| 1 |
Exhaustive search on psoriasis datasets phs000019.v1.p1 and phs000982.v1.p1
We have performed exhaustive search on the two cleansed psoriasis GWAS datasets to discover biologically plausible and statistically significant 2nd order SNP-SNP interactions. There are 62284910040 and 312466073601 unique 2nd order SNP combinations in datasets phs000019.v1.p1 and phs000982.v1.p1 respectively. Each SNP combination is subjected to eight 1 d.f. chi-square statistic tests corresponding to eight genotype interaction patterns and eight pairwise p-value is thus calculated. Therefore, there are 498279280320 and 2499728588808 statistical tests performed on phs000019.v1.p1 and phs000982.v1.p1 datasets respectively. Among these tests, there are 3058119 and 59810682 statistically significant pairwise SNP-SNP interactions found in datasets phs000019.v1.p1 and phs000982.v1.p1 which have a pairwise p-value smaller than 1 × 10−13 and 1 × 10−14 respectively. We have sorted these statistically significant SNP-SNP interactions by their pairwise p-value and improvement metric value separately for further analysis.
Analysis on top 20 most statistical significant SNP-SNP interaction
After sorting the interactions found in datasets phs000019.v1.p1 and phs000982.v1.p1 by their pairwise p-value, we have selected the top 20 statistical significant SNP-SNP interactions in terms of pairwise p-value which are listed in Supplementary Tables S3 and S4 respectively.
Among the interactions shown in Supplementary Table S3, there are six SNPs which are already reported to be associated to psoriasis in existing literature: rs121918773,32–35, rs126507832,33,36, rs289420732, rs313046732, rs313051732 and rs313057333. According to GTEx Portal29, there are nine SNPs acting as eQTLs of the following four known psoriasis associated genes, HLA-C37,38, HCP539,40, PSORS1C141,42 and MICB43. First, rs2244027 and rs2894176 are found to be eQTLs of HLA-C. After that, rs2516417, rs2516510, rs2523708 and rs2844502 are found to be eQTLs of HCP5. Then, rs9262492 and rs9262498 are found to be eQTLs of PSORS1C1. Finally, rs2534666 is found to be eQTLs of MICB. Therefore, these nine SNPs have a high potential to be associated to psoriasis and may be new discoveries.
On the other hand among the interactions we have selected in dataset phs000982.v1.p1, there are 3 psoriasis associated SNPs which are already reported in existing literature: rs1320389544, rs1048455439,45–47 and rs1772833847–50. Meanwhile, rs4349859 and rs4418214 have already been found to be strongly associated to HLA-b27 allele51 and HIV infection52 respectively in other studies. According to GTEx Portal29, rs45533135 is an eQTLs of MICA. Since HIV53, HLA-B37 and MICA54–56 are strongly associated to psoriasis, these three SNPs have a high potential to be associated to psoriasis and may be new discoveries.
Analysis on SNP-SNP interactions with the top 20 improvement metric value
After sorting the statistically significant SNP-SNP interactions found in both datasets by their improvement metric value, we have selected top 20 statistically significant SNP-SNP interactions in terms of improvement metric value and are listed in Supplementary Tables S5 and S6. We can observe that these SNP-SNP interactions have a pairwise p-value much smaller than the standalone p-value of their component SNPs. Therefore, these interactions may be non-linear and further verification through wet-lab experiments should be performed in the future.
In Supplementary Table S5, there are nine SNPs which are present in Supplementary Table S3. Meanwhile, there are six SNPs rs9380237, rs7756521, rs2853950, rs2844645, rs7773175 and rs8365 which are not found in Supplementary Table S3. Among these six SNPs, rs777317532, rs938023732 and rs285395057 are literature reported psoriasis associated SNPs. According to GTEx Portal29, there are three SNPs which are eQTLs of the following two known psoriasis associated genes, PSORS1C141,42 and HLA-DQB158,59. First, rs7756521 and rs2844645 are found to be eQTLs of PSORS1C1. After that, rs8365 is found to be an eQTL of HLA-DQB1. The association between these three SNPs and psoriasis may be new discoveries.
Meanwhile there are no common SNPs between Supplementary Tables S6 and S4. Among the SNPs found in Supplementary Table S6, we observed that there is a SNP rs1576 which is reported to be associated to psoriasis in existing literature60,61. Meanwhile, SNPs rs1265112 and rs746647 are reported to be in complete linkage disequilibrium (r2 = 1.00) with the SNP rs1576 in an existing literature62. Therefore, SNPs rs1265112 and rs746647 can both be considered as a proxy SNP of a literature-reported psoriasis associated SNP and they are not new discoveries. According to GTEx Portal29, there are ten SNPs which are eQTLs of the following two known psoriasis associated genes, HLA-C37,38 and MICA54–56. Four of them are found to be reported as eQTLs of HLA-C namely rs2517985, rs1265079, rs1265114 and rs1265067. Meanwhile, six of them are found to be reported as eQTLs of MICA namely rs4358666, rs2395491, rs4624908, rs7754026, rs13194571 and rs7775117. The association between these ten eQTL SNPs and psoriasis may be new discoveries.
Further analysis on the component SNPs of the SNP-SNP interactions being discovered with CADD SNP annotation
By referring to the genome assembly GRCh37 published by Genome Reference Consortium, the genomic position of every SNP can be retrieved. After knowing the genomic position of every SNP, we have annotated every component SNP of the top 20 SNP-SNP interactions in terms of improvement metric value through CADD version 1.363. The genomic position of these SNPs and their nearest genes (if available) are shown in Supplementary Table S7. Among these SNPs, rs13191519 is located at an intron region of a RNA gene XXbac-BPG248L24.13 (also known as LOC105375015). Since LOC105375015 is reported to be associated to HIV and AIDS progression64 and HIV is associated to psoriasis, rs13191519 may be associated to psoriasis as well. Meanwhile, rs3094205 is located at the upstream region of CDSN and CDSN is reported to be associated to psoriasis in an existing literature65,66. Therefore these two SNPs are likely to be a associated to psoriasis and may be a new discovery.
Predicting gene-gene interactions with CADD SNP annotation
By making an assumption that if SNPi and SNPj have an SNP-SNP interaction, Genei and Genej will have a gene-gene interaction where Genei and Genej are the closest genes to SNPi and SNPj respectively, we can predict gene-gene interactions based on the SNP-SNP interactions we have found after annotated with CADD.
Analysis on common gene-gene interactions predicted by statistically significant SNP-SNP interactions with top 10000th ranking in improvement metric value
We have selected top 10000 statistically significant SNP-SNP interactions in terms of improvement metric value from both datasets. Then we have predicted a number of gene-gene interactions based on these SNP-SNP interactions. As shown in Supplementary Fig. S4, we have predicted 3501 unique gene-gene interactions from dataset phs000019.v1.p1 and 430 unique gene-gene interactions from dataset phs000982.v1.p1. There are 62 common gene-gene interactions between these two datasets. After excluding 2 self-looping interactions found on gene XXbac-BPG248L24.13 and CCHCR1, there are 60 interactions left and are listed in Supplementary Table S8.
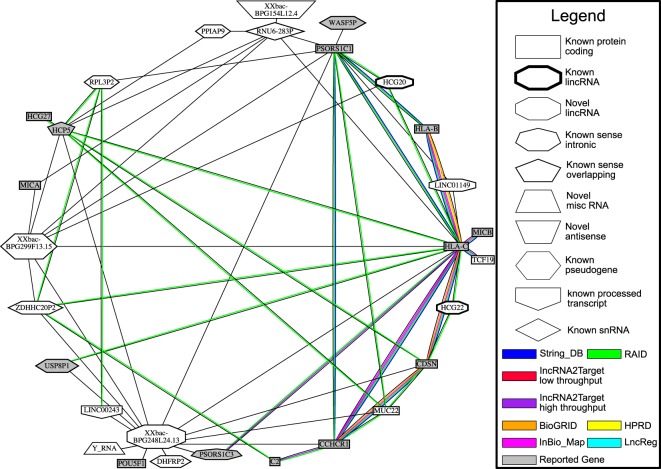
Among these 60 common gene-gene interactions, there are 29 unique genes. Through referencing Ensembl release 75, the positions of these 29 genes under GRCh37 can be found (see Supplementary Table S9). Among these 29 genes, there are 14 genes which are reported by existing literature to be associated to psoriasis: HLA-B37, HLA-C37,38, PSORS1C1(which was previous named as SEEK1)41,42, CCHCR167,68, HCP539,40, CDSN65,66, USP8P144, MICA54,69, PSORS1C365,70, HCG2771, POU5F172, WASF5P44, MICB43, C273. On the other hand, these 60 common gene-gene interactions can be arranged into a network with a circular layout as shown in Fig. 4. Each gene is represented by a node and each common gene-gene interaction is represented by a black edge. If a gene is reported in an existing literature, its corresponding node will be highlighted in grey colour. If a predicted gene-gene interaction is reported as a direct interaction or an indirect interaction through an intermediate gene in one of the following seven biomolecule interaction databases: String74, RAID75, lncRNA2target76, LncReg77, InBio_Map78, HPRD79 and BioGRID80, a new edge with a colour corresponding to the reporting database will be added to the network. Furthermore, the gene network can also be arranged with a linear layout (see Supplementary Fig. S5), where the genes are laid down according to their genomic position on Chromosome 6. The recombination rate and SNP pairs with significant linkage disequilibrium score (r ≥ 0.9) along Chromosome 6 from position 30734602 to 32233615 are shown in Supplementary Fig. S5a,b respectively. The linkage disequilibrium score is calculated with the samples from phs000982.p1.v1 using Plink30.
Figure 4.
This figure shows a gene network constructed from the 60 common gene-gene interactions predicted from the top 10000 SNP-SNP interactions in terms of improvement metric value found from datasets phs000019.v1.p1 and phs000982.v1.p1 with a circular layout. Genes which are already reported to be associated to psoriasis in existing literatures are coloured in grey colour. Meanwhile, if a gene-gene interaction is supported by a database, a thickened edge with a specific colour will be added to the network.
As seen in Fig. 4, 28 out of 60 common gene-gene interactions predicted can be verified by one or more than one existing databases as direct or indirect gene-gene interactions. Since almost half of the gene-gene interactions are supported by existing databases, our gene network is generally supported. Furthermore, there is a long non-coding RNA (lincRNA) gene XXbac-BPG248L24.13 acting as a hub and is interacting with eight literature reported psoriasis-associated genes namely PSORS1C3, CDSN, POU5F1, PSORS1C1, CCHCR1, HLA-C, USP8P1 and HCP5. Therefore, XXbac-BPG248L24.13 may be a new discovery. However, since XXbac-BPG248L24.13 and two other literature reported psoriasis-associated genes HLA-C and USP8P1 are located in the same LD region as shown in Supplementary Fig. S5b. Therefore, XXbac-BPG248L24.13 may be a false discovery and the SNPs being mapped to XXbac-BPG248L24.13 by CADD may be proxy SNPs of the disease-associated SNPs located in HLA-C or USP8P1. Further verification through wet-lab experiments should be performed. Meanwhile, there is a pseudogene XXbac-BPG299F13.15 which is located in the same LD region as HLA-C and is interacting with 4 other literature reported psoriasis-associated genes (PSORS1C1, MICA, HCP5, HLA-C). Similar to XXbac-BPG248L24.13, XXbac-BPG299F13.15 may be a discovery and its effect can only be verified through wet-lab experiments. In Supplementary Fig. S5b, there is long-distance gene-gene interactions between HCP5 and MUC22. Since there is a long-distance haplotype between HCP5 and MUC22, the gene-gene interaction predicted has a high potential to be representing the effect of a long-distance haplotype. Similarly, LINC00243 and PSORS1C1 have a predicted gene-gene interaction which corresponds to a long-distance haplotype. Clearly, long-distance disease-associated haplotype can be found through our exhaustive search algorithm as well. Finally, the gene-gene interaction predicted between XXbac-BPG154L12.4 and RNU6-283P may be a novel discovery. Although RNU6-283P and a known psoriasis-associated gene HLA-B are both located at the same LD region, XXbac-BPG154L12.4 is not in any LD region which contains any known psoriasis associated gene. Therefore, XXbac-BPG154L12.4 may be interacting with a proxy gene of a known psoriasis-associated gene HLA-B. Furthermore, there is a strong recombination site in between XXbac-BPG154L12.4 and any other known psoriasis associated gene. Therefore, the interaction between XXbac-BPG154L12.4 and RNU6-283P cannot be simply explained as a long-distance haplotype and it may be a new discovery.
Simulations on speeding up counting of contingency table with a pre-computed look-up table
We have performed simulations to compare the time of counting contingency table of each SNP-SNP interaction under every pattern with pre-computed look-up table instead of naively counting the number of cases and controls under black and white genotype under every pattern. In this simulation, we have executed our program under the synthesis datasets generated by us. The average run-time of our program with or without pre-computed look-up table under datasets with different numbers of SNPs and heritabilities are shown in Supplementary Table S10. By observing Supplementary Table S10, we can observe that our program executed with the pre-computed look-up table is at least 8 times faster under datasets with 1000 SNPs and at least 10 times faster under datasets with 5000 and 10000 SNPs. This shows that the pre-computed look-up table is a effective mean to accelerate the counting of contingency table. Since the loading time of the pre-computed look-up table is constant under any dataset inputted, the impact of loading pre-computed look-up table on run-time is far more significant under a smaller dataset. Therefore, the speed-up under datasets with 1000 SNPs is less significant than the speed-up under dataset with 1000 and 10000 SNPs.
Discussion
This project studies the genetic risk factors of psoriasis. Psoriasis is a chronic inflammatory dermatitis characterised by hyperproliferation of the epidermis. It is a common dermatitis affecting up to 3% of the general population1. A strong role of genetic predisposition in its etiology has been confirmed by recent family-based linkage studies and twin studies. Recent GWAS also confirmed a number of predisposition SNPs, particularly in Chromosome 6 HLA loci region1,2. In this paper, we aim at demonstrating SNP-SNP interactions also play an important role in causing psoriasis. Our results have shown that interactions between SNPs are present in psoriasis patients. Although these interacting SNPs are mostly found in the extended region of HLA in Chromosome 6, there are SNP-SNP interactions spanned across recombination hotspots which excluded the possibility that the epistasis is due to long-distance haplotype effect. Existing gene/protein interaction database also confirmed that the bio-molecule products of these genes in the extended region of HLA in Chromosome 6 are indeed interacting with each other.
Performing GWAS through analysing the independent effect of every SNP did not provide an adequate explanation for the hereditability of psoriasis. Previous studies have shown that monozygotic twins were more likely to be affected together than dizygotic twins81. Its heritability is as high as 90% which is one of the highest among common diseases and it can equally affect both male and female82. On the other hand, the best predisposition SNPs found in HLA-C could only increase disease risk by around 4 folds4,39,83. This level of odds ratio is not sufficient to account for the high heritability of psoriasis. Other mechanisms must be involved. Gene-gene interactions or gene-environmental interactions are among the most likely explanations for this phenomenon of missing heritability.
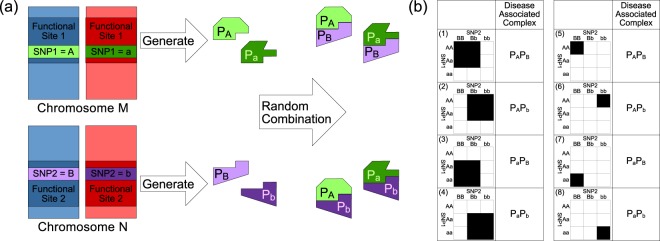
Unlike other existing GWAS researches, we mainly focus on searching SNP-SNP interactions which can be explained as interactions between two bio-molecules. Although there are many potential mechanisms which can explain the interaction between two loci, interaction between 2 bio-molecules is the most valid and feasible hypothesis. With this idea in mind, we can introduce new constraints to the genotype interaction patterns to shrink the search space. Given any 3 × 3 genotype table of any 2 SNPs, there are total 29 possible genotype interaction patterns available. Such exhaustive enumeration of interaction patterns is unnecessary as most of these patterns are not biologically interpretable under our assumption. Based on our previous analysis on the distribution of bio-molecule complexes between two different bio-molecules, we have shown that the search space can be restricted to only 8 genotype interaction patterns as shown in Fig. 5b. After we have applied our algorithm to two psoriasis GWAS datasets, we have identified interaction between bio-molecules generated from the extended HLA region in Chromosome 6.
Figure 5.
In this figure, part (a) shows the bio-molecule interaction mechanism behind a 2nd order SNP-SNP interaction14 where SNP1 and SNP2 are both having genotype (major, minor). Meanwhile, part (b) shows the eight biologically plausible 2nd order genotype interaction patterns and their corresponding disease-associated complexes. Major alleles are represented by upper-case letters (i.e. A, B) and minor alleles are represented by lower-case letters (i.e. a,b).
Among the gene-gene interactions we have found, some are obviously associated to psoriasis. For example, HLA-C and HLA-B, these 2 genes are probably expressed together and their protein products can interact with antigens on cell surface. It is possible some alleles of the HLA lead to a more intense inflammatory response. For example, HLA-b27 has been known to be a genetic factor of psoriasis. Similar HLA alleles have already been found in other diseases like HIV infection and Diabetes.
Knowledge of interaction between other bio-molecules in the extended HLA loci is still less understood. However, genome-wide protein-protein interactions were evident in numerous wet experiment. In fact, it is not uncommon to have interacting gene partners (like ligands and receptors) located in nearby genomic region, with the benefit that they could be regulated simultaneous during organism development. This genomic arrangement leads to various example of gene clusters, like those of cytokines, chemokines and their receptor etc.
Methods
Restricting search space of SNP-SNP interaction with biologically plausible genotype interaction pattern
The number of cases and controls under each genotype of any 2nd order SNP combination (SNPi, SNPj) can be arranged into a 3 × 3 genotype table similar to the table shown in Fig. 3, where each cell in the 3 × 3 table corresponds to a genotype of (SNPi, SNPj). A genotype in a 3 × 3 table can be labelled as a high-risk or low-risk genotype through applying statistical or heuristic algorithms like multi-factor dimensionality reduction (MDR) algorithm and its derivatives84. However, the patterns of the genotype label generated by these algorithms may not be biologically interpretable. In this paper, we have applied eight 2nd order biological plausible SNP-SNP interaction patterns14 for labelling genotypes into high-risk and low risk genotypes. The principles and assumptions in deriving these eight SNP-SNP interaction patterns are shown in Fig. 5a and are explained below.
SNP1 and SNP2 are located within two different functional sites Site1 and Site2 respectively. Major alleles are represented by upper-case letters (i.e. A, B) and minor alleles are represented by lower-case letters (i.e. a, b).
SNP1 and SNP2 can affect their respective functional sites and cause each site to produce at most two different subtypes of bio-molecules. For example, bio-molecules pA is generated from Site1 with SNP1 having an major allele.
The bio-molecules generated from Site1 and Site2 can randomly dock with each other to form at most four different bio-molecule complexes For example, complex pApB is composed by bio-molecules pA and pB generated from Site1 and Site2 respectively.
A bio-molecule complex is associated to a genetic disease if (1) Only its solo presence (i.e. no other bio-molecules are present) or (2) Its presence can either promote or inhibit a disease.
The eight biologically plausible SNP-SNP interaction patterns are shown in Fig. 5b. Considering pattern 1 in Fig. 5b, if pApB is the only disease-associated bio-molecule complex and its presence can either promote or inhibit a disease (i.e. condition 4b), samples carrying genotype {“AA”, “BB”}, {“AA”, “Bb”}, {“Aa”, “BB”} and {“Aa”, “Bb”} obviously would have a different level of disease risk comparing to samples carrying other genotypes. After labelling these two groups of genotypes with two different colours, pattern 1 can hence be defined. On the other hand considering pattern 5 in Fig. 5b, if pApB is the only disease-associated bio-molecule complex and only its solo presence can either promote or inhibit a disease (i.e. condition 4a), samples carrying genotype AA, BB would have a different level of disease risk comparing to samples with other genotypes. Similarly, other patterns shown in Fig. 5b can be also defined through a similar argument shown above.
Since other genotype interaction patterns are not biologically plausible, we can reduce our search space on genotype interaction patterns from 29 to the eight patterns shown in Fig. 5b and thus significantly reduce the size of the search space.
Finding statistically significant SNP-SNP interactions with exhaustive search
In Supplementary Fig. S6, the process of converting the 3 × 3 table of a 2nd order SNP combination (SNPi, SNPj) into a 2 × 2 contingency table is being demonstrated. Considering a 2nd order SNP combination (SNPi, SNPj) after its genotypes being labelled according to genotype interaction pattern 1 in Fig. 5b, the number of cases and controls having black genotypes are summed up as ND,B and NH,B respectively. Meanwhile the number of cases and controls of white genotypes are summed up as ND,W and NH,W respectively. This summation process is shown in Supplementary Fig. S6a. After that, the counts of cases and controls ND,B, NH,B and ND,W and NH,W can then be arranged into a 2 × 2 contingency table as shown in Supplementary Fig. S6b. Finally, the 2 × 2 contingency table of every SNP-SNP interaction is subjected to statistical tests and the SNP-SNP interaction found to be statistically significant are analysed. Imputation of SNPs on selected chromosomes were carried out on Michigan Imputation Server (https://imputationserver.sph.umich.edu) which is based on Minimac3 imputation algorithm85.
Accelerating counting of contingency table with a pre-computed look-up table
We propose to accelerate the exhaustive search process through a pre-computed look-up table. For each SNPi, it corresponds to a vector (i.e. the ith column of matrix A in Table 1). Each vector can be spliced into two different vectors and , where case vector only has genotypes of SNPi from cases and control vector has genotypes of from controls. Each genotype can be considered as a 2 bit integer and thus every p genotypes in a vector can be combined into a 2p bit integer g. Given any possible pair of integer g, the distribution of black and white genotypes in cases and controls under every genotype interaction pattern can be pre-computed and stored in a look-up table located at the main memory. Therefore, the 2 × 2 contingency table of any pairs of SNP can be found without direct counting. Instead, the distribution of black and white genotypes in cases under any pair of SNP SNPi and SNPj can be found by retrieving and summing the distribution of black and white genotypes of every corresponding pair of integer g between case vectors and from the look-up table in the main memory. Meanwhile, the distribution of black and white genotypes in controls can be obtained in a similar fashion. This significantly accelerates the time needed to build the contingency table of each SNP-SNP interaction.
Electronic supplementary material
Acknowledgements
Datasets phs000019.v1.p1. and phs000982.v1.p1. used for the analyses described in this paper were obtained from the database of Genotypes and Phenotypes (dbGaP). Dataset phs000019.v1.p1 was collected by Dr. James T. Elder (University of Michigan, Ann Arbor, MI), Gerald G. Krueger (University of Utah, Salt Lake City, UT), Anne Bowcock (Washington University, St. Louis, MO) and Gonçalo R. Abecasis (University of Michigan, Ann Arbor, MI). Data collection was funded by the National Institutes of Health, the Foundation for the National Institutes of Health, and the National Psoriasis Foundation. Support for genotyping of samples was provided through the Genetic Association Information Network (GAIN). For a description of the dataset, phenotypes, genotype data and quality control procedures see Nair et al. (2009) Nature Genetics 41:200-204. Dataset phs000982.v1.p1 was collected by James T. Elder, University of Michigan, with collaborators Dr. Dafna Gladman, University of Toronto and Dr. Proton Rahman, Memorial University of Newfoundland, providing samples. Data collection was supported by grants from the National Institutes of Health, the Canadian Institute for Health Research, and the Krembil Foundation. Additional support was provided by the Babcock Memorial Trust and by the Barbara and Neal Henschel Charitable Foundation. JTE is supported by the Ann Arbor Veterans Affairs Hospital.
Author Contributions
Kwan-Yeung Lee conducted the project and developed the the algorithms used in this study under the supervision of Man-Hon Wong and Kwong-Sak Leung and interpreted the biological meaning of the experimental results under the guidance of Nelson L.S. Tang who pioneered the interaction models. Kwan-Yeung Lee also ran the experiments and wrote the main manuscript text. All authors discussed the results and reviewed the manuscript thoroughly.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kwan-Yeung Lee, Email: kylee@cse.cuhk.edu.hk.
Nelson L. S. Tang, Email: nelsontang@cuhk.edu.hk
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33493-w.
References
- 1.Harden Jamie L., Krueger James G., Bowcock Anne M. The immunogenetics of Psoriasis: A comprehensive review. Journal of Autoimmunity. 2015;64:66–73. doi: 10.1016/j.jaut.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anbunathan Hima, Bowcock Anne M. The Molecular Revolution in Cutaneous Biology: The Era of Genome-Wide Association Studies and Statistical, Big Data, and Computational Topics. Journal of Investigative Dermatology. 2017;137(5):e113–e118. doi: 10.1016/j.jid.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair Rajan P, Duffin Kristina Callis, Helms Cynthia, Ding Jun, Stuart Philip E, Goldgar David, Gudjonsson Johann E, Li Yun, Tejasvi Trilokraj, Feng Bing-Jian, Ruether Andreas, Schreiber Stefan, Weichenthal Michael, Gladman Dafna, Rahman Proton, Schrodi Steven J, Prahalad Sampath, Guthery Stephen L, Fischer Judith, Liao Wilson, Kwok Pui-Yan, Menter Alan, Lathrop G Mark, Wise Carol A, Begovich Ann B, Voorhees John J, Elder James T, Krueger Gerald G, Bowcock Anne M, Abecasis Gonçalo R, Nair Rajan P, Duffin Kristina Callis, Helms Cynthia, Ding Jun, Stuart Philip E, Goldgar David, Gudjonsson Johann E, Li Yun, Tejasvi Trilokraj, Paschall Justin, Malloy Mary J, Pullinger Clive R, Kane John P, Gardner Jennifer, Perlmutter Amy, Miner Andrew, Feng Bing-Jian, Hiremagalore Ravi, Ike Robert W, Lim Henry W, Christophers Enno, Henseler Tilo, Schreiber Stefan, Franke Andre, Ruether Andreas, Weichenthal Michael, Gladman Dafna, Rahman Proton, Schrodi Steven J, Prahalad Sampath, Guthery Stephen L, Fischer Judith, Liao Wilson, Kwok Pui-Yan, Menter Alan, Lathrop G Mark, Wise C, Begovich Ann B, Voorhees John J, Elder James T, Krueger Gerald G, Bowcock Anne M, Abecasis Gonçalo R. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nature Genetics. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strange Amy, Capon Francesca, Spencer Chris C A, Knight Jo, Weale Michael E, Allen Michael H, Barton Anne, Band Gavin, Bellenguez Céline, Bergboer Judith G M, Blackwell Jenefer M, Bramon Elvira, Bumpstead Suzannah J, Casas Juan P, Cork Michael J, Corvin Aiden, Deloukas Panos, Dilthey Alexander, Duncanson Audrey, Edkins Sarah, Estivill Xavier, Fitzgerald Oliver, Freeman Colin, Giardina Emiliano, Gray Emma, Hofer Angelika, Hüffmeier Ulrike, Hunt Sarah E, Irvine Alan D, Jankowski Janusz, Kirby Brian, Langford Cordelia, Lascorz Jesús, Leman Joyce, Leslie Stephen, Mallbris Lotus, Markus Hugh S, Mathew Christopher G, McLean W H Irwin, McManus Ross, Mössner Rotraut, Moutsianas Loukas, Naluai Åsa T, Nestle Frank O, Novelli Giuseppe, Onoufriadis Alexandros, Palmer Colin N A, Perricone Carlo, Pirinen Matti, Plomin Robert, Potter Simon C, Pujol Ramon M, Rautanen Anna, Riveira-Munoz Eva, Ryan Anthony W, Salmhofer Wolfgang, Samuelsson Lena, Sawcer Stephen J, Schalkwijk Joost, Smith Catherine H, Ståhle Mona, Su Zhan, Tazi-Ahnini Rachid, Traupe Heiko, Viswanathan Ananth C, Warren Richard B, Weger Wolfgang, Wolk Katarina, Wood Nicholas, Worthington Jane, Young Helen S, Zeeuwen Patrick L J M, Hayday Adrian, Burden A David, Griffiths Christopher E M, Kere Juha, Reis André, McVean Gilean, Evans David M, Brown Matthew A, Barker Jonathan N, Peltonen Leena, Donnelly Peter, Trembath Richard C. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nature Genetics. 2010;42(11):985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Cid Rafael, Riveira-Munoz Eva, Zeeuwen Patrick L J M, Robarge Jason, Liao Wilson, Dannhauser Emma N, Giardina Emiliano, Stuart Philip E, Nair Rajan, Helms Cynthia, Escaramís Georgia, Ballana Ester, Martín-Ezquerra Gemma, Heijer Martin den, Kamsteeg Marijke, Joosten Irma, Eichler Evan E, Lázaro Conxi, Pujol Ramón M, Armengol Lluís, Abecasis Gonçalo, Elder James T, Novelli Giuseppe, Armour John A L, Kwok Pui-Yan, Bowcock Anne, Schalkwijk Joost, Estivill Xavier. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nature Genetics. 2009;41(2):211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray-Jones Helen, Eyre Stephen, Barton Anne, Warren Richard B. One SNP at a Time: Moving beyond GWAS in Psoriasis. Journal of Investigative Dermatology. 2016;136(3):567–573. doi: 10.1016/j.jid.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Golan David, Lander Eric S., Rosset Saharon. Measuring missing heritability: Inferring the contribution of common variants. Proceedings of the National Academy of Sciences. 2014;111(49):E5272–E5281. doi: 10.1073/pnas.1419064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Wen-Hua, Massey Jonathan, Worthington Jane, Barton Anne, Warren Richard B. Genotypic variability-based genome-wide association study identifies non-additive loci HLA-C and IL12B for psoriasis. Journal of Human Genetics. 2017;63(3):289–296. doi: 10.1038/s10038-017-0350-6. [DOI] [PubMed] [Google Scholar]
- 9.Kuzmin Elena, VanderSluis Benjamin, Wang Wen, Tan Guihong, Deshpande Raamesh, Chen Yiqun, Usaj Matej, Balint Attila, Mattiazzi Usaj Mojca, van Leeuwen Jolanda, Koch Elizabeth N., Pons Carles, Dagilis Andrius J., Pryszlak Michael, Wang Jason Zi Yang, Hanchard Julia, Riggi Margot, Xu Kaicong, Heydari Hamed, San Luis Bryan-Joseph, Shuteriqi Ermira, Zhu Hongwei, Van Dyk Nydia, Sharifpoor Sara, Costanzo Michael, Loewith Robbie, Caudy Amy, Bolnick Daniel, Brown Grant W., Andrews Brenda J., Boone Charles, Myers Chad L. Systematic analysis of complex genetic interactions. Science. 2018;360(6386):eaao1729. doi: 10.1126/science.aao1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niel, C., Sinoquet, C., Dina, C. & Rocheleau, G. A survey about methods dedicated to epistasis detection, 10.3389/fgene.2015.00285 (2015). [DOI] [PMC free article] [PubMed]
- 11.Ritchie Marylyn D. Methods in Molecular Biology. New York, NY: Springer New York; 2014. Finding the Epistasis Needles in the Genome-Wide Haystack; pp. 19–33. [DOI] [PubMed] [Google Scholar]
- 12.Chen Anlu, Liu Yang, Williams Scott M., Morris Nathan, Buchner David A. Widespread epistasis regulates glucose homeostasis and gene expression. PLOS Genetics. 2017;13(9):e1007025. doi: 10.1371/journal.pgen.1007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip Danny Kit-Sang, Chan Landon L, Pang Iris K, Jiang Wei, Tang Nelson L S, Yu Weichuan, Yip Kevin Y. A network approach to exploring the functional basis of gene–gene epistatic interactions in disease susceptibility. Bioinformatics. 2018;34(10):1741–1749. doi: 10.1093/bioinformatics/bty005. [DOI] [PubMed] [Google Scholar]
- 14.Chu, S. K., Xu, S. G., Xu, F. & Tang, N. L. S. Gene-gene Interaction Analysis by IAC (Interaction Analysis by Chi-Square) - A Novel Biological Constraint-based Interaction Analysis Framework. In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies, 10.5220/0005654601420150 (2016).
- 15.Hirschhorn Joel N., Daly Mark J. Genome-wide association studies for common diseases and complex traits. Nature Reviews Genetics. 2005;6(2):95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 16.Gerke J., Lorenz K., Cohen B. Genetic Interactions Between Transcription Factors Cause Natural Variation in Yeast. Science. 2009;323(5913):498–501. doi: 10.1126/science.1166426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore J. H., Asselbergs F. W., Williams S. M. Bioinformatics challenges for genome-wide association studies. Bioinformatics. 2010;26(4):445–455. doi: 10.1093/bioinformatics/btp713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Wen-Hua, Hemani Gibran, Haley Chris S. Detecting epistasis in human complex traits. Nature Reviews Genetics. 2014;15(11):722–733. doi: 10.1038/nrg3747. [DOI] [PubMed] [Google Scholar]
- 19.Li P., Guo M., Wang C., Liu X., Zou Q. An overview of SNP interactions in genome-wide association studies. Briefings in Functional Genomics. 2014;14(2):143–155. doi: 10.1093/bfgp/elu036. [DOI] [PubMed] [Google Scholar]
- 20. Altshuler, D. M. et al. An integrated map of genetic variation from 1,092 human genomes. Nat., 10.1038/nature11632 (2012). [DOI] [PMC free article] [PubMed]
- 21.Wienbrandt Lars, Kässens Jan Christian, González-Domínguez Jorge, Schmidt Bertil, Ellinghaus David, Schimmler Manfred. FPGA-based Acceleration of Detecting Statistical Epistasis in GWAS. Procedia Computer Science. 2014;29:220–230. doi: 10.1016/j.procs.2014.05.020. [DOI] [Google Scholar]
- 22.Zhu Zhixiang, Tong Xiaoran, Zhu Zhihong, Liang Meimei, Cui Wenyan, Su Kunkai, Li Ming D., Zhu Jun. Development of GMDR-GPU for Gene-Gene Interaction Analysis and Its Application to WTCCC GWAS Data for Type 2 Diabetes. PLoS ONE. 2013;8(4):e61943. doi: 10.1371/journal.pone.0061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudey Benjamin, Rawlinson David, Wang Qiao, Shi Fan, Ferra Herman, Campbell Richard M, Stern Linda, Inouye Michael T, Ong Cheng Soon, Kowalczyk Adam. GWIS - model-free, fast and exhaustive search for epistatic interactions in case-control GWAS. BMC Genomics. 2013;14(Suppl 3):S10. doi: 10.1186/1471-2164-14-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yung Ling Sing, Yang Can, Wan Xiang, Yu Weichuan. GBOOST: a GPU-based tool for detecting gene–gene interactions in genome–wide case control studies. Bioinformatics. 2011;27(9):1309–1310. doi: 10.1093/bioinformatics/btr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Domínguez Jorge, Schmidt Bertil, Kässens Jan Christian, Wienbrandt Lars. Lecture Notes in Computer Science. Cham: Springer International Publishing; 2014. Hybrid CPU/GPU Acceleration of Detection of 2-SNP Epistatic Interactions in GWAS; pp. 680–691. [Google Scholar]
- 26.Hu Xiaohan, Liu Qiang, Zhang Zhao, Li Zhiqiang, Wang Shilin, He Lin, Shi Yongyong. SHEsisEpi, a GPU-enhanced genome-wide SNP-SNP interaction scanning algorithm, efficiently reveals the risk genetic epistasis in bipolar disorder. Cell Research. 2010;20(7):854–857. doi: 10.1038/cr.2010.68. [DOI] [PubMed] [Google Scholar]
- 27.González-Domínguez Jorge, Schmidt Bertil. GPU-accelerated exhaustive search for third-order epistatic interactions in case–control studies. Journal of Computational Science. 2015;8:93–100. doi: 10.1016/j.jocs.2015.04.001. [DOI] [Google Scholar]
- 28.Hemani G., Theocharidis A., Wei W., Haley C. EpiGPU: exhaustive pairwise epistasis scans parallelized on consumer level graphics cards. Bioinformatics. 2011;27(11):1462–1465. doi: 10.1093/bioinformatics/btr172. [DOI] [PubMed] [Google Scholar]
- 29.Lonsdale John, Thomas Jeffrey, Salvatore Mike, Phillips Rebecca, Lo Edmund, Shad Saboor, Hasz Richard, Walters Gary, Garcia Fernando, Young Nancy, Foster Barbara, Moser Mike, Karasik Ellen, Gillard Bryan, Ramsey Kimberley, Sullivan Susan, Bridge Jason, Magazine Harold, Syron John, Fleming Johnelle, Siminoff Laura, Traino Heather, Mosavel Maghboeba, Barker Laura, Jewell Scott, Rohrer Dan, Maxim Dan, Filkins Dana, Harbach Philip, Cortadillo Eddie, Berghuis Bree, Turner Lisa, Hudson Eric, Feenstra Kristin, Sobin Leslie, Robb James, Branton Phillip, Korzeniewski Greg, Shive Charles, Tabor David, Qi Liqun, Groch Kevin, Nampally Sreenath, Buia Steve, Zimmerman Angela, Smith Anna, Burges Robin, Robinson Karna, Valentino Kim, Bradbury Deborah, Cosentino Mark, Diaz-Mayoral Norma, Kennedy Mary, Engel Theresa, Williams Penelope, Erickson Kenyon, Ardlie Kristin, Winckler Wendy, Getz Gad, DeLuca David, MacArthur Daniel, Kellis Manolis, Thomson Alexander, Young Taylor, Gelfand Ellen, Donovan Molly, Meng Yan, Grant George, Mash Deborah, Marcus Yvonne, Basile Margaret, Liu Jun, Zhu Jun, Tu Zhidong, Cox Nancy J, Nicolae Dan L, Gamazon Eric R, Im Hae Kyung, Konkashbaev Anuar, Pritchard Jonathan, Stevens Matthew, Flutre Timothèe, Wen Xiaoquan, Dermitzakis Emmanouil T, Lappalainen Tuuli, Guigo Roderic, Monlong Jean, Sammeth Michael, Koller Daphne, Battle Alexis, Mostafavi Sara, McCarthy Mark, Rivas Manual, Maller Julian, Rusyn Ivan, Nobel Andrew, Wright Fred, Shabalin Andrey, Feolo Mike, Sharopova Nataliya, Sturcke Anne, Paschal Justin, Anderson James M, Wilder Elizabeth L, Derr Leslie K, Green Eric D, Struewing Jeffery P, Temple Gary, Volpi Simona, Boyer Joy T, Thomson Elizabeth J, Guyer Mark S, Ng Cathy, Abdallah Assya, Colantuoni Deborah, Insel Thomas R, Koester Susan E, Little A Roger, Bender Patrick K, Lehner Thomas, Yao Yin, Compton Carolyn C, Vaught Jimmie B, Sawyer Sherilyn, Lockhart Nicole C, Demchok Joanne, Moore Helen F. The Genotype-Tissue Expression (GTEx) project. Nature Genetics. 2013;45(6):580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purcell Shaun, Neale Benjamin, Todd-Brown Kathe, Thomas Lori, Ferreira Manuel A.R., Bender David, Maller Julian, Sklar Pamela, de Bakker Paul I.W., Daly Mark J., Sham Pak C. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. The American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson Carl A, Pettersson Fredrik H, Clarke Geraldine M, Cardon Lon R, Morris Andrew P, Zondervan Krina T. Data quality control in genetic case-control association studies. Nature Protocols. 2010;5(9):1564–1573. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fang, S., Fang, X. & Xiong, M. Psoriasis prediction from genome-wide SNP profiles. BMC Dermatol., 10.1186/1471-5945-11-1 (2011). [DOI] [PMC free article] [PubMed]
- 33.Climer Sharlee, Templeton Alan R., Zhang Weixiong. Allele-Specific Network Reveals Combinatorial Interaction That Transcends Small Effects in Psoriasis GWAS. PLoS Computational Biology. 2014;10(9):e1003766. doi: 10.1371/journal.pcbi.1003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Bing-Jian, Sun Liang-Dan, Soltani-Arabshahi Razieh, Bowcock Anne M., Nair Rajan P., Stuart Philip, Elder James T., Schrodi Steven J., Begovich Ann B., Abecasis Gonçalo R., Zhang Xue-Jun, Callis-Duffin Kristina P., Krueger Gerald G., Goldgar David E. Multiple Loci within the Major Histocompatibility Complex Confer Risk of Psoriasis. PLoS Genetics. 2009;5(8):e1000606. doi: 10.1371/journal.pgen.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elder J T. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes & Immunity. 2009;10(3):201–209. doi: 10.1038/gene.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Miaozhu, Wu Yumei, Chen Guoliang, Yang Yifeng, Zhou Daizhan, Zhang Zhou, Zhang Di, Chen Yinwei, Lu Zhiyong, He Lin, Zheng Jie, Liu Yun. Deletion of the Late Cornified Envelope Genes LCE3C and LCE3B Is Associated with Psoriasis in a Chinese Population. Journal of Investigative Dermatology. 2011;131(8):1639–1643. doi: 10.1038/jid.2011.86. [DOI] [PubMed] [Google Scholar]
- 37. Gladman, D. D., Anhorn, K. A., Schachter, R. K. & Mervart, H. HLA antigens in psoriatic arthritis. The J. rheumatology (1986). [PubMed]
- 38.Nair Rajan P., Stuart Philip E., Nistor Ioana, Hiremagalore Ravi, Chia Nicholas V.C., Jenisch Stefan, Weichenthal Michael, Abecasis Gonçalo R., Lim Henry W., Christophers Enno, Voorhees John J., Elder James T. Sequence and Haplotype Analysis Supports HLA-C as the Psoriasis Susceptibility 1 Gene. The American Journal of Human Genetics. 2006;78(5):827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Ying, Helms Cynthia, Liao Wilson, Zaba Lisa C., Duan Shenghui, Gardner Jennifer, Wise Carol, Miner Andrew, Malloy M. J., Pullinger Clive R., Kane John P., Saccone Scott, Worthington Jane, Bruce Ian, Kwok Pui–Yan, Menter Alan, Krueger James, Barton Anne, Saccone Nancy L., Bowcock Anne M. A Genome-Wide Association Study of Psoriasis and Psoriatic Arthritis Identifies New Disease Loci. PLoS Genetics. 2008;4(4):e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X.-L., Yu H., Wu G.-S. Investigating the genetic association ofHCP5,SPATA2,TNIP1,TNFAIP3andCOG6with psoriasis in Chinese population. International Journal of Immunogenetics. 2014;41(6):503–507. doi: 10.1111/iji.12150. [DOI] [PubMed] [Google Scholar]
- 41.Holm Sofia J., Carlen Lina M., Mallbris Lotus, Stahle-Backdahl Mona, O'Brien Kevin P. Polymorphisms in the SEEK1 and SPR1 genes on 6p21.3 associate with psoriasis in the Swedish population. Experimental Dermatology. 2003;12(4):435–444. doi: 10.1034/j.1600-0625.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 42.Rahman P. Association of SEEK1 and psoriatic arthritis in two distinct Canadian populations. Annals of the Rheumatic Diseases. 2005;64(9):1370–1372. doi: 10.1136/ard.2004.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight Jo, Spain Sarah L., Capon Francesca, Hayday Adrian, Nestle Frank O., Clop Alex, Barker Jonathan N., Weale Michael E., Trembath Richard C. Conditional analysis identifies three novel major histocompatibility complex loci associated with psoriasis. Human Molecular Genetics. 2012;21(23):5185–5192. doi: 10.1093/hmg/dds344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou, F. et al. Epigenome-wide association data implicates DNA methylation-mediated genetic risk in psoriasis. Clin. Epigenetics, 10.1186/s13148-016-0297-z (2016). [DOI] [PMC free article] [PubMed]
- 45.Denny Joshua C, Bastarache Lisa, Ritchie Marylyn D, Carroll Robert J, Zink Raquel, Mosley Jonathan D, Field Julie R, Pulley Jill M, Ramirez Andrea H, Bowton Erica, Basford Melissa A, Carrell David S, Peissig Peggy L, Kho Abel N, Pacheco Jennifer A, Rasmussen Luke V, Crosslin David R, Crane Paul K, Pathak Jyotishman, Bielinski Suzette J, Pendergrass Sarah A, Xu Hua, Hindorff Lucia A, Li Rongling, Manolio Teri A, Chute Christopher G, Chisholm Rex L, Larson Eric B, Jarvik Gail P, Brilliant Murray H, McCarty Catherine A, Kullo Iftikhar J, Haines Jonathan L, Crawford Dana C, Masys Daniel R, Roden Dan M. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nature Biotechnology. 2013;31(12):1102–1111. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lysell Josefin, Padyukov Leonid, Kockum Ingrid, Nikamo Pernilla, Ståhle Mona. Genetic Association with ERAP1 in Psoriasis Is Confined to Disease Onset after Puberty and Not Dependent on HLA-C*06. Journal of Investigative Dermatology. 2013;133(2):411–417. doi: 10.1038/jid.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VILLARREAL-MARTÍNEZ ALEJANDRA, GALLARDO-BLANCO HUGO, CERDA-FLORES RICARDO, TORRES-MUÑOZ IRIS, GÓMEZ-FLORES MINERVA, SALAS-ALANÍS JULIO, OCAMPO-CANDIANI JORGE, MARTÍNEZ-GARZA LAURA. Candidate gene polymorphisms and risk of psoriasis: A pilot study. Experimental and Therapeutic Medicine. 2016;11(4):1217–1222. doi: 10.3892/etm.2016.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowes J., Orozco G., Flynn E., Ho P., Brier R., Marzo-Ortega H., Coates L., McManus R., Ryan A. W., Kane D., Korendowych E., McHugh N., FitzGerald O., Packham J., Morgan A. W., Bruce I. N., Barton A. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Annals of the Rheumatic Diseases. 2011;70(9):1641–1644. doi: 10.1136/ard.2011.150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Q., Liu H., Qu L., Fu X., Yu Y., Yu G., Tian H., Yu Y., Sun D., Peng J., Bao F., Yuan C., Lu N., Li J., Zhang Y., Zhang F. Investigation of 20 non-HLA (human leucocyte antigen) psoriasis susceptibility loci in Chinese patients with psoriatic arthritis and psoriasis vulgaris. British Journal of Dermatology. 2013;168(5):1060–1065. doi: 10.1111/bjd.12142. [DOI] [PubMed] [Google Scholar]
- 50.Das Sayantan, Stuart Philip E, Ding Jun, Tejasvi Trilokraj, Li Yanming, Tsoi Lam C, Chandran Vinod, Fischer Judith, Helms Cynthia, Duffin Kristina Callis, Voorhees John J, Bowcock Anne M, Krueger Gerald G, Lathrop G Mark, Nair Rajan P, Rahman Proton, Abecasis Goncalo R, Gladman Dafna, Elder James T. Fine mapping of eight psoriasis susceptibility loci. European Journal of Human Genetics. 2014;23(6):844–853. doi: 10.1038/ejhg.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans David M, Spencer Chris C A, Pointon Jennifer J, Su Zhan, Harvey David, Kochan Grazyna, Oppermann Udo, Dilthey Alexander, Pirinen Matti, Stone Millicent A, Appleton Louise, Moutsianas Loukas, Leslie Stephen, Wordsworth Tom, Kenna Tony J, Karaderi Tugce, Thomas Gethin P, Ward Michael M, Weisman Michael H, Farrar Claire, Bradbury Linda A, Danoy Patrick, Inman Robert D, Maksymowych Walter, Gladman Dafna, Rahman Proton, Morgan Ann, Marzo-Ortega Helena, Bowness Paul, Gaffney Karl, Gaston J S Hill, Smith Malcolm, Bruges-Armas Jacome, Couto Ana-Rita, Sorrentino Rosa, Paladini Fabiana, Ferreira Manuel A, Xu Huji, Liu Yu, Jiang Lei, Lopez-Larrea Carlos, Díaz-Peña Roberto, López-Vázquez Antonio, Zayats Tetyana, Band Gavin, Bellenguez Céline, Blackburn Hannah, Blackwell Jenefer M, Bramon Elvira, Bumpstead Suzannah J, Casas Juan P, Corvin Aiden, Craddock Nicholas, Deloukas Panos, Dronov Serge, Duncanson Audrey, Edkins Sarah, Freeman Colin, Gillman Matthew, Gray Emma, Gwilliam Rhian, Hammond Naomi, Hunt Sarah E, Jankowski Janusz, Jayakumar Alagurevathi, Langford Cordelia, Liddle Jennifer, Markus Hugh S, Mathew Christopher G, McCann Owen T, McCarthy Mark I, Palmer Colin N A, Peltonen Leena, Plomin Robert, Potter Simon C, Rautanen Anna, Ravindrarajah Radhi, Ricketts Michelle, Samani Nilesh, Sawcer Stephen J, Strange Amy, Trembath Richard C, Viswanathan Ananth C, Waller Matthew, Weston Paul, Whittaker Pamela, Widaa Sara, Wood Nicholas W, McVean Gilean, Reveille John D, Wordsworth B Paul, Brown Matthew A, Donnelly Peter. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nature Genetics. 2011;43(8):761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.International HIV Controllers Study, T. I. H. C. et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science (New York, N.Y.), 10.1126/science.1195271 (2010). [DOI] [PMC free article] [PubMed]
- 53.Arnett, F. C., Reveille, J. D. & Duvic, M. Psoriasis and psoriatic arthritis associated with human immunodeficiency virus infection. Rheum. diseases clinics North Am (1991). [PubMed]
- 54.Choi H.B., Han H., Youn J.I., Kim T.Y., Kim T.G. MICA 5.1 allele is a susceptibility marker for psoriasis in the Korean population. Tissue Antigens. 2000;56(6):548–550. doi: 10.1034/j.1399-0039.2000.560609.x. [DOI] [PubMed] [Google Scholar]
- 55.González Segundo, Brautbar Chaim, Martínez-Borra J, López-Vazquez A, Segal Rafael, Blanco-Gelaz M.A, Enk Claes D, Safriman Cilly, López-Larrea C. Polymorphism in MICA rather than HLA-B/C genes is associated with psoriatic arthritis in the Jewish population. Human Immunology. 2001;62(6):632–638. doi: 10.1016/S0198-8859(01)00242-7. [DOI] [PubMed] [Google Scholar]
- 56.Chang Y.T., Tsai S.F., Lee D.D., Shiao Y.M., Huang C.Y., Liu H.N., Wang W.J., Wong C.K. A study of candidate genes for psoriasis near HLA-C in Chinese patients with psoriasis. British Journal of Dermatology. 2003;148(3):418–423. doi: 10.1046/j.1365-2133.2003.05166.x. [DOI] [PubMed] [Google Scholar]
- 57.Wu Xuesen, Dong Hua, Luo Li, Zhu Yun, Peng Gang, Reveille John D., Xiong Momiao. A Novel Statistic for Genome-Wide Interaction Analysis. PLoS Genetics. 2010;6(9):e1001131. doi: 10.1371/journal.pgen.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang, X. et al. HLA-DQA1 and DQB1 alleles are associated with genetic susceptibility to psoriasis vulgaris in Chinese Han. Int J Dermatol (2004). [DOI] [PubMed]
- 59.Ozawa Akira, Miyahara Motomi, Sugai Junichi, Iizuka Mariko, Kawakubo Yo, Matsuo Itsuro, Ohkido Muneo, Naruse Taeko, Ando Hitoshi, Inoko Hidetoshi, Kobayashi Hitoshi, Ohkawara Akira, Takahashi Hidetoshi, Iizuka Hajime, Morita Eishin, Yamamoto Shoso, Hide Michihiro, Taniguchi Yoshiki, Shimizu Masayuki. HLA Class I and II Alleles and Susceptibility to Generalized Pustular Psoriasis: Significant Associations with HLA-Cw1 and HLA-DQB1*0303. The Journal of Dermatology. 1998;25(9):573–581. doi: 10.1111/j.1346-8138.1998.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 60.Lesueur Fabienne, Oudot Tiphaine, Heath Simon, Foglio Mario, Lathrop Mark, Prud'homme Jean-François, Fischer Judith. ADAM33, a New Candidate for Psoriasis Susceptibility. PLoS ONE. 2007;2(9):e906. doi: 10.1371/journal.pone.0000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asumalahti K. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Human Molecular Genetics. 2002;11(5):589–597. doi: 10.1093/hmg/11.5.589. [DOI] [PubMed] [Google Scholar]
- 62.Chantarangsu Soranun, Mushiroda Taisei, Mahasirimongkol Surakameth, Kiertiburanakul Sasisopin, Sungkanuparph Somnuek, Manosuthi Weerawat, Tantisiriwat Woraphot, Charoenyingwattana Angkana, Sura Thanyachai, Takahashi Atsushi, Kubo Michiaki, Kamatani Naoyuki, Chantratita Wasun, Nakamura Yusuke. Genome-wide Association Study Identifies Variations in 6p21.3 Associated With Nevirapine-Induced Rash. Clinical Infectious Diseases. 2011;53(4):341–348. doi: 10.1093/cid/cir403. [DOI] [PubMed] [Google Scholar]
- 63.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014 doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matzaraki, V., Kumar, V., Wijmenga, C. & Zhernakova, A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases, 10.1186/s13059-017-1207-1 (2017). [DOI] [PMC free article] [PubMed]
- 65.Chang Y.T., Chou C.T., Shiao Y.M., Lin M.W., Yu C.W., Chen C.C., Huang C.H., Lee D.D., Liu H.N., Wang W.J., Tsai S.F. Psoriasis vulgaris in Chinese individuals is associated with PSORS1C3 and CDSN genes. British Journal of Dermatology. 2006;155(4):663–669. doi: 10.1111/j.1365-2133.2006.07420.x. [DOI] [PubMed] [Google Scholar]
- 66.Orru S., Giuressi E., Casula M., Loizedda A., Murru R., Mulargia M., Masala M.V., Cerimele D., Zucca M., Aste N., Biggio P., Carcassi C., Contu L. Psoriasis is associated with a SNP haplotype of the corneodesmosin gene (CDSN) Tissue Antigens. 2002;60(4):292–298. doi: 10.1034/j.1399-0039.2002.600403.x. [DOI] [PubMed] [Google Scholar]
- 67.Suomela S, Kainu K, Onkamo P, Tiala I, Himberg J, Koskinen L, Snellman E, Karvonen SL, Karvonen J, Uurasmaa T, Reunala T, Kivikäs K, Jansén CT, Holopainen P, Elomaa O, Kere J, Saarialho-Kere U. Clinical Associations of the Risk Alleles of HLA-Cw6 and CCHCR1*WWCC in Psoriasis. Acta Dermato-Venereologica. 2007;87(2):127–134. doi: 10.2340/00015555-0184. [DOI] [PubMed] [Google Scholar]
- 68. Gandhi, G., Buttar, B. S., Albert, L., Hasan, Q. & Aggarwal, R. K. Psoriasis-associated genetic polymorphism in North Indian population in the CCHCR1 gene and in a genomic segment flanking the HLA-C region. Dis. Markers, 10.3233/DMA-2011-0851 (2011). [DOI] [PMC free article] [PubMed]
- 69.Romphruk A.V., Romphruk A., Choonhakarn C., Puapairoj C., Inoko H., Leelayuwat C. Major histocompatibility complex class I chain-related gene A in Thai psoriasis patients: MICA association as a part of human leukocyte antigen-B-Cw haplotypes. Tissue Antigens. 2004;63(6):547–554. doi: 10.1111/j.0001-2815.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 70.Sánchez Fabio, Carlén Lina, Mallbris Lotus, Ståhle Mona, O'brien Kevin, Holm Sofia. HLA-Cw*0602 Associates More Strongly to Psoriasis in the Swedish Population than Variants of the Novel 6p21.3 Gene PSORS1C3. Acta Dermato-Venereologica. 2005;85(1):2–8. doi: 10.1080/00015550410023527. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Kun-Ju, Lv Yong-Mei, Yin Xian-Yong, Wang Zai-Xing, Sun Liang-Dan, He Su-Min, Cheng Hui, Hu Da-Yan, Zhang Zheng, Li Yang, Zuo Xian-Bo, Zhou You-Wen, Yang Sen, Fan Xing, Zhang Xue-Jun, Zhang Feng-Yu. Psoriasis Regression Analysis of MHC Loci Identifies Shared Genetic Variants with Vitiligo. PLoS ONE. 2011;6(11):e23089. doi: 10.1371/journal.pone.0023089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang Yun-Ting, Hsu Chih-Yi, Chou Chan-Te, Lin Ming-Wei, Shiao Yu-Ming, Tsai Chang-Youh, Yu Chia-Wen, Shiue Jen-Jen, Lee Yu-Fen, Huang Cheng-Hung, Chen Chih-Chiang, Lee Ding-Dar, Wang Wen-Jen, Liu Han-Nan, Tsai Shih-Feng. The genetic polymorphisms of POU5F1 gene are associated with psoriasis vulgaris in Chinese. Journal of Dermatological Science. 2007;46(2):153–156. doi: 10.1016/j.jdermsci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Dewald G., Lange C. -E., Schmeel E., Kreysel H. -W. HLA-linked complement polymorphisms (C2, BF) in psoriasis. Archives of Dermatological Research. 1983;275(5):301–304. doi: 10.1007/BF00417201. [DOI] [PubMed] [Google Scholar]
- 74.Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., von Mering C. STRING 8--a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Research. 2009;37(Database):D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, et al. RAID: A comprehensive resource for human RNA-associated (RNA-RNA/RNA-protein) interaction. RNA. 2014 doi: 10.1261/rna.044776.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Qinghua, Wang Jixuan, Wu Xiaoliang, Ma Rui, Zhang Tianjiao, Jin Shuilin, Han Zhijie, Tan Renjie, Peng Jiajie, Liu Guiyou, Li Yu, Wang Yadong. LncRNA2Target: a database for differentially expressed genes after lncRNA knockdown or overexpression. Nucleic Acids Research. 2014;43(D1):D193–D196. doi: 10.1093/nar/gku1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Zhong, Shen Yi, Khan Muhammad Riaz, Li Ao. LncReg: a reference resource for lncRNA-associated regulatory networks. Database. 2015;2015:bav083. doi: 10.1093/database/bav083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Intomics A/S. InBio_Map, https://www.intomics.com/inbio/map (2016).
- 79.Peri S. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Research. 2004;32(90001):497D–501. doi: 10.1093/nar/gkh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stark C. BioGRID: a general repository for interaction datasets. Nucleic Acids Research. 2006;34(90001):D535–D539. doi: 10.1093/nar/gkj109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lønnberg A.S., Skov L., Skytthe A., Kyvik K.O., Pedersen O.B., Thomsen S.F. Heritability of psoriasis in a large twin sample. British Journal of Dermatology. 2013;169(2):412–416. doi: 10.1111/bjd.12375. [DOI] [PubMed] [Google Scholar]
- 82.Wuepper Kirk D., Coulter Silvija N., Haberman Abigail. Psoriasis Vulgaris: A Genetic Approach. Journal of Investigative Dermatology. 1990;95(5):S2–S4. doi: 10.1111/1523-1747.ep12505638. [DOI] [PubMed] [Google Scholar]
- 83.Stuart Philip E., Nair Rajan P., Tsoi Lam C., Tejasvi Trilokraj, Das Sayantan, Kang Hyun Min, Ellinghaus Eva, Chandran Vinod, Callis-Duffin Kristina, Ike Robert, Li Yanming, Wen Xiaoquan, Enerbäck Charlotta, Gudjonsson Johann E., Kõks Sulev, Kingo Külli, Esko Tõnu, Mrowietz Ulrich, Reis Andre, Wichmann H. Erich, Gieger Christian, Hoffmann Per, Nöthen Markus M., Winkelmann Juliane, Kunz Manfred, Moreta Elvia G., Mease Philip J., Ritchlin Christopher T., Bowcock Anne M., Krueger Gerald G., Lim Henry W., Weidinger Stephan, Weichenthal Michael, Voorhees John J., Rahman Proton, Gregersen Peter K., Franke Andre, Gladman Dafna D., Abecasis Gonçalo R., Elder James T. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. The American Journal of Human Genetics. 2015;97(6):816–836. doi: 10.1016/j.ajhg.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gola Damian, Mahachie John Jestinah M., van Steen Kristel, König Inke R. A roadmap to multifactor dimensionality reduction methods. Briefings in Bioinformatics. 2015;17(2):293–308. doi: 10.1093/bib/bbv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Das Sayantan, Forer Lukas, Schönherr Sebastian, Sidore Carlo, Locke Adam E, Kwong Alan, Vrieze Scott I, Chew Emily Y, Levy Shawn, McGue Matt, Schlessinger David, Stambolian Dwight, Loh Po-Ru, Iacono William G, Swaroop Anand, Scott Laura J, Cucca Francesco, Kronenberg Florian, Boehnke Michael, Abecasis Gonçalo R, Fuchsberger Christian. Next-generation genotype imputation service and methods. Nature Genetics. 2016;48(10):1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.