Abstract
Setting
Tuberculosis (TB) drug resistance survey was conducted in 2016–2017 to estimate the burden of drug-resistant TB in Côte d'Ivoire.
Design
A cross-sectional cluster-based survey was conducted. All eligible smear positive patients were interviewed using a structured questionnaire to collect clinical and sociodemographic information and tested by the Xpert Mycobacterium tuberculosis/rifampicin (MTB/RIF) assay. If resistant to rifampicin, solid and liquid cultures were performed. Phenotypic drug susceptibility testing (DST) was conducted in liquid medium for rifampicin, isoniazid, ethambutol, streptomycin, ofloxacin, and amikacin.
Results
Of the 1105 sputum smear positive patients enrolled, 995 new and 100 previously treated patients were positive for Mycobacterium tuberculosis complex by Xpert. Proportion of patients with rifampicin resistance was 4.6% (95% CI: 2.4–6.7) and 22% (95% CI: 13.7–30.3), respectively, for new and previously treated patients. Second-line DST results were available for most rifampicin-resistant patients. None were resistant to amikacin, only two were ofloxacin-resistant. Apart from the antecedent of previously treatment for TB, no other risk factors for rifampicin resistance were detected.
Conclusion
Prevalence of rifampicin resistance among TB patients in Côte d'Ivoire is higher than that in other countries in the region. Surveillance of drug resistance, through an expanded GeneXpert network, and programmatic management of drug-resistant TB (PMDT) must be strengthened in Côte d'Ivoire.
Keywords: Tuberculosis, survey, prevalence, drug resistance, Xpert MTB/RIF
Introduction
Antimicrobial resistance threatens global health and security. In 2014, the World Health Assembly called for the international community to take action against antimicrobial resistance [1]. Development of drug-resistant Mycobacterium tuberculosis strains, especially multidrug-resistant tuberculosis (MDR-TB) due to resistance to the two most powerful first-line anti-tuberculosis (TB) drugs, isoniazid and rifampicin, poses a major challenge to the control of TB worldwide [2, 3].
Guidelines recommended by the World Health Organization (WHO) to diagnose, treat, and prevent TB have been implemented in Côte d'Ivoire since 1995 [4–6]. There were an estimated 36,000 new cases of TB in 2016 [7]. The main method used for diagnosis of TB is sputum smear microscopy (SSM) based on Ziehl–Neelsen or Auramin staining of two sputum samples (spot and morning) on consecutive days.
Surveillance is a critical component of detecting, planning, and responding to the public health challenge of drug-resistant TB. As Côte d'Ivoire does not yet have the capacity to routinely test all TB patients for drug susceptibility, two national drug resistance surveys were conducted in 1995 and 2006. The proportions of new TB cases with MDR-TB in these surveys were 5.3% (95% CI: 3.1–8.4%) and 2.5% (95% CI: 1.1–4.9%), respectively [8, 9]. The third national survey was conducted in 2016 under routine programmatic conditions to provide updated data on the burden of drug-resistant TB.
Methods
Study Design
This cross-sectional survey targeted all consecutively notified new and previously treated sputum smear-positive pulmonary TB diagnosed in the country, including both adults and children. The survey was implemented using a cluster design, with 35 clusters selected from a list of all of the 230 TB diagnostic centres in the country using a probability-proportional-to-size approach. These 35 clusters were distributed across 28 diagnostic centres. The target sample size was 971 new patients, with previously treated patients being enrolled opportunistically until the sample size for new patients was reached. The enrolment period was 12 months from 1 March 2016 to 28 February 2017. A questionnaire was administered by a trained interviewer at the time of enrolment, which included questions relating to demographics, TB treatment history (new or previously treated), social factors (education level, previous incarceration), and other health indicators (history of smoking, human immunodeficiency virus [HIV] status). Ethical clearance for the survey was obtained from the National Ethics Committee of Côte d'Ivoire.
Laboratory Methods
Two sputum specimens (spot and morning samples) were collected from all eligible patients.
Sputum smear microscopy was performed after Auramin staining, as per national guidelines for routine TB diagnosis. One sputum sample from each sputum smear-positive patient was tested by Xpert Mycobacterium tuberculosis/rifampicin (MTB/RIF) (Cepheid, CA, USA) for the identification of the M. tuberculosis complex (MTBC) and the determination of rifampicin susceptibility status, either at the peripheral diagnostic centre or in the capital, Abidjan, at the laboratories of the Institut Pasteur or the Centre de Diagnostic et de Recherche sur le SIDA. All samples with resistance to rifampicin were transported on ice to the central laboratories in Abidjan for culture and phenotypic drug susceptibility testing (DST), as well as a randomly selected 10% of rifampicin-susceptible samples as a control.
Handling of sputum for culture was performed in a Class II biosafety cabinet in a biosafety level 3 (BSL3) laboratory. Sputum samples were decontaminated by the standard N-acetyl-l-cysteine–4% NaOH procedure. The supernatant was discarded, and the pellet was resuspended with sterile phosphate buffer to a final volume of 2 mL. This was inoculated into one BACTEC Mycobacteria Growth Indicator Tube (MGIT) 960 tube (0.5 mL) and two Lowenstein–Jensen slants (0.2 mL each). Prior to inoculation, the BACTEC MGIT 960 media were supplemented with an antibiotic mixture containing polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA) and growth supplement (Becton Dickinson). BACTEC MGIT 960 tubes were incubated at 37 °C in the BACTEC MGIT 960 instrument, in which they were automatically monitored each hour for fluorescence development for 42 days or until a positive signal developed. Solid media were incubated at 37 °C for 8 weeks and were inspected weekly or until mycobacterial colonies were seen. Cultures were considered positive only after detection of MTBC by the immunochromatographic assay, SD BIOLINE TB Ag MPT64 (Standard Diagnostics, Seoul, South Korea). This assay is rapid, simple, and reliable with a demonstrated high sensitivity and specific on clinical isolates [10, 11].
Phenotypic DST with the automated MGIT system was performed on MTBC positive cultures according to the manufacturer's instructions using the BD BACTEC MGIT SIRE drug kit (Becton, Dickinson & Company, Sparks, MD, USA). The DST assays were performed at the following concentrations: 0.1 mg/L for isoniazid, 1.0 mg/L for rifampicin, 1.0 mg/L for streptomycin, and 5.0 mg/L for ethambutol. For rifampicin-resistant isolates, phenotypic DST was also performed for amikacin and ofloxacin at concentrations of 1.0 mg/L and 2.0 mg/L, respectively. Phenotypic DST tubes were inoculated with 0.5 mL of MGIT culture. A drug-free control was also inoculated with 0.5 mL of a 1:100 dilution of positive culture broth in sterile saline. The tubes were incubated in the BACTEC MGIT instrument. The H37rv strain was used for internal quality control of phenotypic DST.
Statistical Analysis
Data were entered into a pre-designed EpiData Entry 3.1 software (EpiData Association, Odense, Denmark) and analysed using Stata version 14.0 (StatCorp, Texas, USA). Proportions of new and previously treated cases with rifampicin resistance and MDR-TB with 95% confidence intervals (95% CI) were computed using logistic regression with robust standard errors to account for clustering. The analysis was performed both with and without sample weights (target cluster size divided by the actual number enrolled in cluster) to account for under- and over-enrolment, and the results were compared. For samples with a rifampicin result from both Xpert MTB/RIF and phenotypic DST, the level of interrater agreement was assessed by the Kappa test.
Results
Patient Inclusion
A total of 1002 new sputum smear positive pulmonary TB patients were enrolled, corresponding to an enrolment rate of 103%. Additionally, 103 previously treated patients were enrolled across the 35 clusters (Figure 1). The median age of patients was 33 years (range 15–80). Males represented 65.5% of the patients, and 12% were HIV-positive (Table 1). An Xpert MTB/RIF assay result was available for 1104 cases. Of these, 1095 were positive for MTBC, including 995 new and 100 previously treated cases. The concordance between smear microscopy and GeneXpert for bacteriological confirmation was 99.2%.
Figure 1.
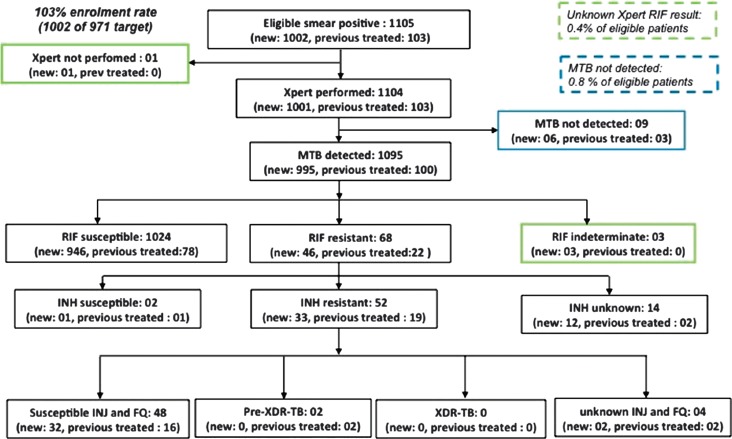
Flowchart of patients enrolled (FQ = fluoroquinolones; INH = isoniazid; INJ = second-line injectable agents; RIF = rifampicin; MTB = Mycobacterium tubercolosis complex; XDR-TB: Extensively drug-resistant tuberculosis)
Table 1.
Characteristics of GeneXpert MTB-positive patients included in the national anti-TB drug resistance survey
| Characteristic | Number of patients (n = 1095) |
|---|---|
| History of treatment | |
| New TB cases | 995 (90.9%) |
| Previously treated | 100 (9.1%) |
| Sex | |
| Male | 715 (65.3%) |
| Female | 380 (34.7%) |
| Age | |
| Range | 15–80 years |
| Median | 33 years |
| HIV status | |
| Negative | 958 (87.5%) |
| Positive | 130 (12%) |
| Unknown | 7 (0.6%) |
Drugs Susceptibility Testing Results
Rifampicin resistance was detected by Xpert MTB/RIF in 68 patients, representing 46 new patients and 22 previously treated patients. The proportion of new TB cases with rifampicin resistance was 4.6% (95% CI: 2.4–6.7), with 22% (95% CI: 13.7–30.3) among previously treated cases. Due to most clusters reaching their target sample size, sample weights had minimal impact on the results and were not used in final analysis.
Of the 68 cases with rifampicin resistance detected by Xpert MTB/RIF, cultures were performed for only 58, due to challenges in sample transport. Of these, two cultures were contaminated, and two were negative. Phenotypic DST was therefore successfully performed for 54 Xpert MTB/RIF rifampicin-resistant patients (Table 2). Discordant results between Xpert MTB/RIF and phenotypic drug susceptibility testing in liquid medium were observed for 8 of the 54 isolates (14.8%) (all were rifampicin-resistant by Xpert MTB/RIF but rifampicin-susceptible by phenotypic testing). DST was performed for 114 Xpert MTB/RIF rifampicin-susceptible samples of which 112 phenotypic DST results were available.
Table 2.
DST pattern of clinical isolates
| GeneXpert MTB/RIF assay results | Drug resistance pattern | New cases (n = 130) |
Previous treated patients (n = 32) |
|---|---|---|---|
| MTB detected and rifampicin susceptible | RIFS + INHS+ STRS + EMBS + AMKS + OFXS | 85 | 7 |
| RIFS + INHS+ STRR + EMBS + AMKS + OFXS | 1 | 4 | |
| RIFS + INHR+ STRS + EMBS + AMKS + OFXS | 8 | 2 | |
| RIFS + INHR+ STRR + EMBS + AMKS + OFXS | 4 | 1 | |
| MTB detected and rifampcin resistant | RIFS + INHR+ STRR + EMBS + AMKS + OFXS | 1 | 2 |
| RIFS + INHR + STRR + EMBR + AMKS + OFXS | 5 | – | |
| RIFR + INHR + STRS + EMBS + AMKS + OFXS | 5 | 1 | |
| RIFR + INHR + STRR + EMBS + AMKS + OFXS | 5 | 5 | |
| RIFR + INHR + STRR + EMBR + AMKS + OFXS | 14 | 7 | |
| RIFR + INHR + STRS + EMBR + AMKS + OFXS | 2 | 1 | |
| RIFR + INHR + STRS + EMBR + AMKS + OFXR (pre-XDR-TB) | – | 1 | |
| RIFR + INHR + STRR + EMBS + AMKS + OFXR (pre-XDR-TB) | – | 1 |
RIFS: rifampin susceptible; RIFR: rifampin resistant; INHS: isoniazid susceptible; INHR: isoniazid resistant; STRS : streptomycin susceptible; STRR: streptomycin resistant; EMBS: ethambutol susceptible; EMBR : ethambutol resistant; AMKS: amikacin susceptible; AMKR: amikacin resistant; OFXS: ofloxacin susceptible; OFXR: ofloxacin resistant; XDR-TB: extensively drug-resistant TB.
Six DST results were not interpretable, 2 for rifampicin (Xpert rifampicin-susceptible), 1 in new and previous traited patients (Table 1). The 4 others were for second line drug (Figure 1 and Table 2)
Overall, the level of agreement between Xpert MTB/RIF and phenotypic DST for the 168 samples for which results were available by both tests was 94% (kappa = 0.859).
Among 68 samples with rifampicin resistance on Xpert MTB/RIF, an isoniazid result was available for 54 (79%). Most of these (96%) were MDR-TB. Fifty of these 54 patients also had phenotypic DST results available for second-line drug susceptibility testing. No resistance was detected to second-line injectable drugs and resistance to ofloxacin was only detected in two patients.
Given that isoniazid susceptibility testing was only conducted among rifampcin-resistant cases, for which results were missing for 20%, as well as a subset of rifampicin-susceptible cases, statistical multiple imputations of missing values could not be performed. Minimum and maximum estimations of MDR-TB were calculated by assuming that all of the rifampicin-resistant cases missing an isoniazid result were either isoniazid-susceptible or isoniazid-resistant, respectively. Minimum estimations were 3.3% (95% CI: 1.9–4.8%) in new and 19.2 (95%CI: 10.8–27.6%) in previously treated cases. Maximum estimations were 4.5% (95% CI: 2.4–6.7%) in new and 21.2% (95% CI: 13.0–29.4) in previously treated cases (Table 3).
Table 3.
Prevalence of MDR-TB and rifampicin resistance from 1995 to 2016
| Prevalences | 1995 [8] | 2006 [9] | 2016 | |
|---|---|---|---|---|
| New cases (n = 320) | New cases (n = 320) | New cases (n = 995) | Previously treated (n = 100) | |
| Rifampicine resistance | Not applicable | Not applicable | 46 (4.6%) (95% CI: 2.4–6.8%) | 22 (22%) (95% CI: 13.7–30.3%) |
| MDR-TB | 17 (5.3%) | 8 (2.5%) | mE*: 3.3% (95% CI: 1.9–4.8%) | mE*: 19.2% (95% CI: 10.8–27.6%) |
| (95% CI: 3.1–8.4%) | (95% CI: 1.1–4.9%), | ME: 4.5% (95% CI: 2.4–6.7%) | ME: 21.2% (95% CI: 13.0–29.4%) | |
CI: confidence interval; mE : minimum estimation of MDR-TB prevalence; ME: maximum estimation MDR-TB prevalence.
Risks Factors For Rifampicin Resistance
No associations were found between rifampicin resistance and age, sex, alcohol consumption, history of incarceration, or smoking. Patients who had been previously treated for TB had odds of rifampicin-resistant TB that was 6.0 (95%CI: 3.4–10.7) times higher than new cases (p < 0.001).
Discussion
In this manuscript, we report the results of the third national anti-TB drug resistance survey conducted in Côte d'Ivoire. High quality clinical, laboratory, and sociodemographic data collected from TB patients allowed a national representative analysis of patient characteristics, drug resistance patterns, and risk factors for resistance. With 4.6% of new and 22% of previously treated TB cases being infected by rifampicin-resistant strains, the burden of drug-resistant TB in Côte d'Ivoire is notably higher than in other countries in West Africa, such as Burkina Faso (2.1% rifampicin resistance in new and 14.3% in previously treated cases in 2017), Ghana (1.5% rifampicin resistance in new and 7% in previously treated cases in 2017), or Senegal (0.5% MDR-TB in new and 17.5% in previously treated cases in 2014). However, at the regional level, the prevalence of drug resistance among TB patients in Africa is lower than in other parts of the world (regional estimate of 2.7% rifampicin resistance among new patients) [2].
Although the standard first-line anti-TB treatment regimen recommended by WHO (2RHZE/4RH) has been implemented since 2002, Côte d'Ivoire suffered a civil war from 2002 to 2011. It is possible that disruption to health services during this period may have negatively impacted on the control of TB and facilitated the emergence of resistant strains. Prior to 2012, there were no national guidelines for programmatic management of drug-resistant TB (PMDT), including DST among presumptive MDR-TB cases or provision of an appropriate treatment regimen for drug-resistant patients. This may have allowed ongoing transmission of drug-resistant strains in Côte d'Ivoire. Encouragingly, resistance to second-line drugs among rifampicin-resistant strains is very low which means that most of these patients would be eligible for the shorter MDR-TB treatment regimen of 9–12 months [12]. There is ongoing investment in strengthening PMDT, with 27 MDR-TB treatment sites currently across the country.
Not unsurprisingly, a history of previous treatments for TB was significantly associated with rifampicin resistance. In this survey, 11 of the 22 rifampicin-resistant previously treated patients were the cases who had failed a treatment course. According to national guidelines, treatment failures are identified only 5 months after treatment initiation. This poses a risk for the transmission of drug-resistant strains in the community. The WHO's End TB Strategy calls for universal DST for all TB patients at the time of treatment initiation [13]. The ongoing expansion of the GeneXpert network in Côte d'Ivoire is allowing more patients to access rapid testing. There are currently 13 instruments across the country, and there is a plan to equip each of the 29 health districts in the country with an instrument by 2020. This will facilitate prompter diagnosis of drug resistance and initiation of an appropriate treatment regimen. However, sputum transport networks, laboratory capacity and data management systems must also be strengthened in order to achieve the ultimate aim of establishing a continuous surveillance system for anti-TB drug resistance in Côte d'Ivoire.
Among the 54 rifampicin-resistant samples detected by Xpert MTB/RIF for which results were also available by phenotypic DST, discordance was noted in 14.8%. This discordance may have been due to administrative errors such as sample mislabeling, or technical errors in conducting phenotypic DST. Furthermore, the BACTEC MGIT 960 system has been shown to miss some mutations in the rpoB gene that are known to confer resistance to rifampicin [14]. Overall, among the 168 samples for which results were available by both genotypic and phenotypic tests, the level of agreement was high, reflecting the reliable performance of Xpert MTB/RIF compared to conventional methods.
There are some limitations to this survey. Firstly, the survey enrolled sputum smear positive patients only. However, there is no evidence that the prevalence of resistance differs between smear positive and smear negative patients [15]. Secondly, phenotypic DST for first and second-line drugs was only performed on sputum samples that were resistant to rifampicin by Xpert MTB/RIF. This means that other resistance patterns in the absence of rifampicin resistance could not be investigated. However, the use of this assay greatly simplified logistics. A rifampicin result was available for almost all of the enrolled patients, allowing an accurate estimation of the prevalence of rifampicin resistance among new and previously treated patients.
Conclusion
The third national anti-TB drug resistance survey in Côte d'Ivoire detected a higher prevalence of rifampicin resistance among TB patients than in neighbouring countries. Drug-resistant TB poses a major threat to reducing the burden of TB in Côte d'Ivoire. The surveillance of resistance must be strengthened through expansion of the GeneXpert network, coupled with improved access to appropriate care through PMDT.
Acknowledgments
The authors would like to thank all patients who were involved in this survey. We also wish to thank all chief doctors of TB centres and Dennis Falzon (World Health Organization, Global TB Programme, Geneva, Switzerland) for their support.
Footnotes
Funding Sources
This study was funded by Global Fund to Fight against tuberculosis.
Author Contributions
K.N., T.O., G.D.A., D.C., and A.S.D. conceptualized and designed the study. A.S.D. and V.I. performed statistical analysis. K.N., J.K., A.S.D., and R.A. analyzed and interpreted the data. K.N. and R.A. supervised the study.
Conflict of Interest
The authors have no commercial associations or sources of support that might pose a conflict of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
References
- 1.Resolution WHA67/25: antimicrobial resistance. Presented at the 67th World Health Assembly. Geneva, May 24, 2014 (Resolutions and Decisions; Annexes [WHASS1/2014WHA67/2014/REC/25]). Geneva, Switzerland; 2014. [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2017. WHO/HTM/TB/2017.23. Geneva, Switzerland; 2017. [Google Scholar]
- 3.World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision. WHO/HTM/TB/2013.2. Geneva, Switzerland; 2013. [Google Scholar]
- 4.World Health Organization. What is DOTS? A Guide to Understanding the WHO-recommended TB Control Strategy Known as DOTS. WHO/CDS/CPC/TB/99.270. World Health Organization, Geneva, Switzerland; 1999. [Google Scholar]
- 5.World Health Organization. THE STOP TB STRATEGY: Building on and enhancing DOTS to meet the TB-related Millennium Development Goals. WHO/HTM/TB/2006.368. World Health Organization, Geneva, Switzerland; 2006. [Google Scholar]
- 6.World Health Organization. The End TB Strategy. WHO/HTM/TB/2015.19. World Health Organization, Geneva, Switzerland; 2015. [Google Scholar]
- 7.World Health Organization. Global Tuberculosis Report 2016. WHO/HTM/TB/2016. 13. World Health Organization, Geneva, Switzerland; 2016. [Google Scholar]
- 8.Dosso M, Bonnard D, Msellati P, Doulhourou C, Bamba A, Peyre M, et al. Surveillance des résistances secondaires aux anti-tuberculeux en Côte d'Ivoire 1995–1996. Int J Tuberc Lung Dis. 1999;3:805–9. [PubMed] [Google Scholar]
- 9.N'guessan K, Nahoua I, San Koffi M, Kouakou J, Dosso M. Primary resistance to antituberculosis drugs: trends in Côte d'Ivoire from 1996 to 2006. Med Mal Infect. 2008;38:231–2. [DOI] [PubMed] [Google Scholar]
- 10.Hopprich R, Shephard L, Taing B, Kralj S, Smith A, Lumb R. Evaluation of (SD) MPT64 antigen rapid test, for fast and accurate identification of Mycobacterium tuberculosis complex. Pathology 2012;44:642–3. [DOI] [PubMed] [Google Scholar]
- 11.Fabre M, Vong R, Gaillard T, Merens A, Gérome P, Saint-Blancard P, et al. Evaluation of the SD BIOLINE TB Ag MPT64 Rapid® for the diagnosis of tuberculosis. Pathol Biol. 2011;59:26–8. [DOI] [PubMed] [Google Scholar]
- 12.Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49. DOI: 10.1183/13993003.02308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al. WHO's new End TB Strategy. Lancet 2015;385:1799–801. [DOI] [PubMed] [Google Scholar]
- 14.Rigouts L, Gumusboga M, Bram de Rijk W, Nduwamahoro E, Uwizeye C, De J, et al. Mutations tuberculosis isolates with specific rpoB liquid culture system for Mycobacterium rifampin resistance missed in automated. J Clin Microbiol. 2013;51:2641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zignol M, Dean AS, Falzon D, Van Gemert W, Wright A, Van Deun A, et al. Twenty years of Global Surveillance of Antituberculosis-Drug Resistance. N Engl J Med. 2016;375:1081–9. [DOI] [PubMed] [Google Scholar]


