Abstract
Acute Respiratory Distress Syndrome (ARDS) is a condition of varied etiology characterized by the acute onset (within 1 week of the inciting event) of hypoxemia, reduced lung compliance, diffuse lung inflammation and bilateral opacities on chest imaging attributable to noncardiogenic (increased permeability) pulmonary edema. Although multi-organ failure is the most common cause of death in ARDS, an estimated 10–15% of the deaths in ARDS are caused due to refractory hypoxemia, i.e.- hypoxemia despite lung protective conventional ventilator modes. In these cases, clinicians may resort to other measures with less robust evidence –referred to as “salvage therapies”. These include proning, 48 h of paralysis early in the course of ARDS, various recruitment maneuvers, unconventional ventilator modes, inhaled pulmonary vasodilators, and Extracorporeal membrane oxygenation (ECMO). All the salvage therapies described have been associated with improved oxygenation, but with the exception of proning and 48 h of paralysis early in the course of ARDS, none of them have a proven mortality benefit. Based on the current evidence, no salvage therapy has been shown to be superior to the others and each of them is associated with its own risks and benefits. Hence, the order of application of these therapies varies in different institutions and should be applied following a risk-benefit analysis specific to the patient and local experience. This review explores the rationale, evidence, advantages and risks behind each of these strategies.
Keywords: Acute respiratory distress syndrome, Refractory hypoxemia, Salvage therapies, Lung
1. Introduction
The Berlin definition categorizes severe ARDS based on the degree of hypoxemia, as a Pa02/FiO2 ratio less than 100, with a mortality rate of 45% [1]. According to the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory failure (LUNGSAFE study), the estimated incidence of ARDS is around 34 cases per 100, 000 patients per year in the United States [2,3]. 23.4% of the patients in this group had severe ARDS defined based on the Berlin criteria [2]. The in hospital mortality reported for severe ARDS was 46.1% with a median duration of mechanical ventilation among survivors of 11 days and median ICU length of stay of 14 days [2].
Supportive treatment with mechanical ventilation along with conservative fluid management strategies forms the cornerstone of management of ARDS [4,5]. The “lung protective ventilation” (LPV) with recommended parameters of tidal volume of 4–8 ml/kg ideal body weight (IBW) with a modest positive end expiratory pressure (PEEP), while maintaining a plateau pressure (P plat) of < 30 cm H20 [6] has become the paradigm for mechanical ventilation in ARDS. The goal of this approach is to minimize ventilator induced lung injury (VILI) (Table 1) [7]- a collective term used to describe the different mechanisms of lung injury caused secondary to mechanical ventilation. VILI develops as a result of four different mechanisms; a) Volutrauma-lung injury caused by alveolar overdistension secondary to increased transpulmonary pressure (alveolar pressure – pleural pressure), b) barotrauma-lung injury secondary to increased transpulmonary pressure causing alveolar rupture and air leaks, resulting in pulmonary interstitial emphysema, pneumothorax and pneumo-mediastinum, c) atelectrauma-shearing lung injury caused by repetitive opening and closing of alveoli and d) biotrauma-activation and release of pro-in-flammatory cytokines which further promotes pulmonary and extra-pulmonary injury, predisposing to multi-organ failure [7–9].
Table 1.
| Term | Definition |
|---|---|
| Atelectrauma | Lung injury caused by sheear forces from cyclic opening and collapse of atelectatic but recruitable lungs. |
| Barotrauma | Lung injury caused by high transpulmonary pressure |
| Volutrauma | Lung injury caused by alveolar overdistension |
| Biotrauma | Additional lung and extrapulmonary organ injury caused by proinjurious inflammatory response to mechanical lung injury. |
| Stress | Force applied to an area; in lung- represented by transpulmonary pressures. |
| Strain | Physical deformation or change in shape of an alveolus, caused by stress. |
Most of the mortality in ARDS is due to multi-organ failure, but an estimated 10–15% of patients die of refractory hypoxemia [4], which may be defined as persistent or worsening hypoxemia unresponsive to LPV. Although no standard definition exists for refractory hypoxemia; for the purpose of this review-we define refractory hypoxemia as PaO2/ Fio2 less than 150 while on PEEP of 5 cm H20 or greater on LPV settings, as most rescue interventions in ARDS patients have focused on this subset of patients [10]. In this group, most of which have severe ARDS, the clinicians may need to use additional and/or alternative “salvage” therapies to mitigate life-threatening hypoxemia [4] while the lung injury resolves. Salvage therapies by definition includes therapies that are given when conventional therapies fail, which in this case-refers to failure to alleviate hypoxemia by conventional modes of mechanical ventilation, while on lung protective ventilator settings.
These salvage therapies will be the focus of this review, where we will discuss the evidence, benefits, risks and disadvantages of each of the commonly used salvage therapies. Most of the studies evaluating salvage therapies have employed them within 36–48 h, a time at which potential for alveolar recruitment is highest [11]. However-we suggest that these strategies may be employed as early as after 6 h, if maximal ventilator support with LPV cannot correct hypoxemia. Indeed, in the LUNG SAFE study, one or more salvage therapies was used in 61% of patients [2] After discussing the various rescue strategies (Table 2) in more detail, we outline our algorithm for considering them in the conclusion.
Table 2.
Table showing different salvage therapies for ARDS.
| Salvage therapies | Physiology/mechanism | Pros | Cons | Proposed benefits | Adverse effects |
|---|---|---|---|---|---|
| Proning [12–14] | - Improvement of V/Q, - Facilitates more homogeneous aeration of lung - Decreases VILI - Improves mobilization of secretions |
- No equipment Requirement - Low cost and widely available |
- Risk of local complications (pressure sores, facial edema) - Need for training personnel and specialized equipment such as proning bed or special foams - Unfamiliarity - Difficulty with regular nursing care - Need for increased sedation and paralytics |
- Mortality benefit - Possible decrease in infection risks |
- Risk of arrhythmias, - Accidental dislodgement of ET tube, invasive lines - Increased abdominal pressure with hepatic/renal dysfunction |
| NMBA [31,34,35] | - Improvement of patient ventilator asynchrony. - Improvement of V/Q - Decreases VILI |
- Low cost, widely available | - Risk of delirium due to concomitant sedation - Prolonged weakness |
- Mortality benefit - Increases ventilator free days. - Decreases risk of barotrauma and pneumothorax. |
- Possible increased risk of neuromyopathy, weakness |
| Recruitment maneuvers [39,40,50,51] |
- Recruitment of alveoli by elevated transpulmonary pressure, followed by high PEEP keeps alveoli open at end-expiration, minimizing atelectrauma. |
- Easily applied through any standard mechanical ventilator by ancillary staff |
- Difficult to determine “optimal” PEEP - Unlikely that “one size fits all” - Need for increased sedation and paralytics |
- Decreases need for other salvage therapies, especially in patients with recruitable lungs. |
- Risk of overdistension, volutrauma and barotrauma - Risk of hemodynamic compromise - May worsen mortality |
| APRV [53,56] | - Improvement in shunt, dead space, V/Q - Spontaneous ventilation can improve cardiac output - Decreases atelectrauma |
- Available on most ventilators |
- Unfamiliarity - Lack of RCT - No mortality benefit |
- Improvement in patient ventilator synchrony, - Decreased need for sedation and paralysis |
- Risk of barotrauma - AutoPEEP in obstructive lung diseases - Risk of hemodynamic instability due to high mean airway pressures |
| HFOV [53,66,69,71,73] | - Smaller tidal volumes at high frequencies and high mean airway pressure maintains lung recruitment. - Decreases risk of VILI/atelectrauma. |
- Possible mortality benefit with very low Pa02/Fi02 ratio of < 60 | - Need for increased sedation and/ or NMBAs. - Barrier to transportation. - Unfamiliarity with use |
- Helps mobilization of secretions. | - Increased mortality. - Higher risk of hemodynamic instability due to high mean airway pressures. - Risk of worsening RV afterload |
| Inhaled pulmonary vasodilators [76,77,80] |
- Local delivery allows selective vasodilation of ventilated lung units, thus increasing V/ Q matching |
- Ease of delivery (except for NO) and no systemic effects |
- Lack of data that supports its use - NO requires special equipment for delivery - No mortality benefit |
- Reduction in pulmonary vascular pressures and hence RV afterload. |
NO may cause methemoglobinemia and/or renal failure |
| ECMO [85–87] | - Ensures adequate tissue oxygenation while maintaining LPV settings. - Negates risk of VILI |
- Allows for protective lung settings. - “Buys time” for lungs to heal |
- Requires additional equipment and personnel - Cannot be used when there is a major contraindication to systemic anticoagulation - Not widely available. Often requires referral to specialized centers |
- Survival benefit reported in young patients with severe ARDS in the setting of influenza in the range of 63–77%. - Early institution in severe ARDS within 7 days may result in mortality benefits and decreased incidence of renal failure. |
- Risk of local complications with cannulation (vascular injury), thrombus formation in circuit. - Risk of hemorrhage, intracerebral bleeds |
2. Prone positioning
The physiological benefits of prone positioning are believed to primarily involve improvement of ventilation-perfusion (V/Q) by reducing ventral-dorsal transpulmonary pressure difference. This effect results in more homogeneous lung inflation and reduced VILI compared to the supine posture [12,13]. Moreover, the now open dorsal lung regions remain well perfused despite their non-dependent orientation, which minimizes shunt. The other added speculated benefits include better drainage assisted by gravity yielding decreased infection rates [12–17].
More than 30 years ago, observational studies reported that prone positioning improved oxygenation in many patients with acute respiratory failure [18,19]. However, several trials performed with prone positioning in patients with ARDS [20–23] showed mixed results with consistent improvement in oxygenation, but no major impact on mortality. A meta-analysis of studies prior to 2013 suggested a survival benefit of patients with PaO2/FiO2 less than 140 mm Hg at admission [24]. Based on this background, Guerin et al. conducted a multicenter study, termed the PROSEVA study (Prone positioning in Severe ARDS patients), which was designed with longer proning sessions (> 16 h/ day), applied only in severe and early ARDS, used protocols of lung protective ventilation and neuromuscular blockade and was conducted by staff experienced in the techniques of prone positioning and ARDS management [14].
This trial compared prone positioning of patients with severe ARDS within 36 h of onset with standard care in the supine position only. Enrolled patients had a PaO2/FiO2 ratio less than 150 mm Hg with a FiO2 of 0.6 and PEEP of at least 5 mm Hg. All were managed with low tidal volume ventilation. Patients randomized to the intervention group spent atleast 16 consecutive hours in the prone position daily until prespecified oxygenation improvements were achieved or safety issues arose. Patients in the prone group underwent an average of 4.4 sessions. 28-day mortality in the prone positioning group was 16% versus 32.8 in the supine group [16]. Of note, a recent meta-analysis suggested prone positioning works best in the context of open lung protective ventilation whereas studies which have evaluated prone positioning without concomitant use of lung protective ventilation have not shown important benefits [25].
Complications that have been described with proning include development of pressure ulcers [26] airway obstruction, vomiting, increased abdominal pressure with resultant hepatic and renal dysfunction, loss of venous access and dislodgement of endotracheal tubes [23]. However, many of these complications develop during the initial turning of the patient, and can be prevented by using a careful and regimented prone positioning protocol followed by an experienced team [27]. Contraindications to prone positioning include severe facial, neck trauma, elevated intracranial pressure, pelvic/spinal instability, hemoptysis or a high probability of patient requiring cardiopulmonary resuscitation [14].
With these caveats in mind, prone positioning should be considered for any patient with moderate-severe ARDS and refractory hypoxemia [27], applied for extended periods of time, early in the course of the disease and executed by well-trained personnel to minimize complications. The authors of recently published evidence-based guidelines recommend that patients with severe ARDS receive prone positioning for more than 12 h per day (strong recommendation, moderate to high confidence in effect estimates) [28].
3. Neuromuscular blocking agents
Neuromuscular blocking agents (NMBA) are frequently used to abolish the inspiratory and expiratory efforts of patients, in order to improve patient-ventilator synchrony and to minimize the muscle oxygen consumption. In addition, paralytics can reduce the stress/ strain generated in the lung by attenuating the negative pleural pressure during spontaneous efforts [29], thus avoiding the generation of harmful increases in regional trans-pulmonary pressures [30]. Hence, they modify thoraco-pulmonary mechanics and the V/Q ratio, with associated increase in functional residual capacity and decrease in intra-pulmonary shunt. The positive effects of NMBA could also be related to a decrease in VILI [31].
Three randomized trials evaluating the use of NMBA in ARDS have been performed. The first trial by Gainnier et al., in 2004, randomized 56 patients with severe ARDS, defined as PA02/Fi02 less than 150 while on PEEP of 5 cm H20 or greater to two groups with one group receiving NMBA while both groups remained on conventional ventilation with low tidal volumes. They observed consistently better oxygenation and a decrease in PEEP levels at 48, 96 and 120 h in the NMBA group [32]. The same group performed another randomized control trial involving 36 patients with ARDS within 48 h of onset, with Pa02/ Fio2 less than 200 and PEEP of 5 cm H20 and greater with NMBA infusion compared to placebo while on conventional lung protective ventilation and confirmed the same findings: the NMBA group again had better oxygenation and also had significantly lower pulmonary and systemic inflammatory response as measured by decreased IL-6 and IL-8 compared to the non NMBA group [33]. The third and the largest trial-the ARDS et curarisation systematique (ACURASYS) randomized 340 patients with severe ARDS (defined as PaO2/Fio2 less than 150 while on PEEP of 5 cm H20 and higher) to receive a 48-h continuous infusion of NMBAs or placebo while on conventional low tidal volume ventilation. Compared to placebo, the NMBA group had lower mortality (31% vs. 40%) at 90 days, increased number of ventilator free days (53% vs. 44%) and lower incidence of pneumothorax (4% vs. 11%) with no increase in myopathy [34]. A meta-analysis considering all the above-mentioned three randomized controlled trials showed that use of NMBA in the early phase of ARDS improves outcomes with a trend towards lower mortality, more ventilator-free days at day 28, higher Pa02- FiO2 ratios and less barotrauma [35]. A much larger randomized multicenter trial- Reevaluation of Systemic Early Neuromuscular blockade (ROSE) (NCT 02509078) is currently underway to assess the mortality benefit of NMBA in moderate to severe ARDS.
Thus, NMBAs should be considered in patients with moderate-severe ARDS within the first 48 h, particularly in the subset of patients with PaO2/Fio2 less than 150. However, risks of ICU- acquired weakness should be considered especially patients with hyperglycemia, patients receiving steroids and care should be taken to minimize use of NMBA beyond 48 h due to increased risk of weakness [31].
4. Recruitment maneuvers and high PEEP
A recruitment maneuver (RM) is a transient sustained increase in transpulmonary pressure in an attempt to open previously collapsed alveoli and thus increase lung compliance and improve gas exchange [36]. ARDS is associated with dependent atelectasis, which is compounded by increased lung weight from interstitial and alveolar edema as well as low tidal volume ventilation [4,37]. Atelectasis exacerbates lung injury by reducing the size of the lung available for mechanical ventilation as well as increasing stress at the interface between atelectatic and aerated lungs and worsening atelectrauma [28,38]. RMs applied to reverse atelectasis followed by high PEEP to keep the recruited alveoli open forms the basis of the “ open lung approach” which aims to improve gas exchange and minimize VILI by reducing stress and atelectrauma [39].
Several methods for performing recruitment maneuvers have been studied. The most common one used is sustained inflation with continuous positive airway pressure (CPAP) mode with applied pressure of 30–40cmH2O applied for 30–40 s period [40]. Still other methods use ‘sighs’ [41,42] stepwise recruitment maneuvers with incremental PEEP with constant driving pressure (eg- 15 cm H20) or fixed tidal volumes (4–8 ml/kg IBW) [10,43]. Stepwise recruitment maneuvers have been suggested as more effective approaches with less risk of hemodynamic compromise [43]. Once the lungs are recruited, they must be kept open by maintaining extrinsic PEEP at a pressure above the point of derecruitment, as determined at the bedside by changes in compliance and/ or oxygen desaturation [43]. Sedation along with paralytics is commonly used, which maximize the effectiveness of recruitment.
Most of the meta-analyses on RMs in patients with moderate to severe ARDS have been confounded by the co-intervention with higher PEEP, poor sample size in trials and a high risk of bias [28,43,44]. A single multicenter randomized controlled trial which did not involve the co-intervention of PEEP in patients with moderate or severe ARDS compared recruitment maneuvers along with conventional ventilation to conventional ventilation alone and reported mortality benefit (32.7% vs 52.7% in ICU and 41.8 vs 56.4% in hospital) with the use of RMs, however this trial had methodological limitations due to limited sample size, imprecision and risk of bias [44,45].
Three large randomized controlled trials of higher PEEP with or without RMs in patients with ARDS and a PaO2/FiO2 of 300 or less did not demonstrate any significant mortality benefit compared to lower PEEP settings; with all groups maintained on low tidal volume ventilation [46–48]. An individual patient data meta-analysis of these three trials suggested that higher PEEP reduced mortality in patients with more severe hypoxemia, i.e PaO2/FiO2 of 200 or less [49]. However, a recent well conducted multicenter trial (Alveolar Recruitment for ARDS trial- or ART) randomized 1010 patients with moderate to severe ARDS to receive lung recruitment maneuvers and high PEEP titrated to respiratory system compliance against conventional low PEEP settings, and showed increased 28- day (55.3% vs. 49.3%) and 6-month mortality in the group receiving recruitment maneuvers and high PEEP (65.3 vs. 59.9%) with increased incidence of barotrauma (5.6% vs. 1.2%) and pneumothorax requiring drainage (3.2 vs. 1.2%) [50]. Both groups were maintained on low tidal volume ventilation. Thus, high PEEP may not be “patient-protective” in spite of the physiological benefits [51]. Other potential risks of PEEP include increase in right atrial pressure affecting venous return and consequently cardiac output as well as increase in pulmonary vascular resistance leading to cor pulmonale [28,51].
The open lung approach has consistently shown to improve oxygenation in moderate-severe ARDS [51]. RMs along with high PEEP may still therefore have a role in selected patients with refractory hypoxemia, particularly following an episode of extensive alveolar derecruitment, such as endotracheal suctioning, disconnection from the ventilator or bronchoscopy and in patients with a substantial amount of recruitable nonaerated lung tissue [40,52], which can be ascertained based on the response to RMs with improvement in oxygenation and lung compliance (usually better seen in early ARDS between 4 and 5 days of onset and ARDS secondary to non pulmonary causes) [43,44]. Caution must be exercised in the application of RMs however in hypovolemia or in shock [28]. The authors of recently published evidence-based clinical practice guidelines suggest that patients with ARDS receive recruitment maneuvers (conditional recommendation, low to moderate confidence in the effect estimates) [28]. However, these guidelines were published prior to the ART trial and future guidelines may change based on the findings of this study.
5. Unconventional ventilator modes
5.1. Airway Pressure Release Ventilation
Airway Pressure Release Ventilation (APRV) is a time cycled, pressure targeted mode of ventilation which consists of continuous positive airway pressure (CPAP) that is intermittently released to allow a brief expiratory interval. APRV provides increased airway pressure as a potential recruitment mechanism [53].
Benefits of APRV are unproven and speculated to be linked to spontaneous breathing and include: 1. Better patient-ventilator synchrony 2. Improvement in ventilation/perfusion matching by promoting a more physiological gas distribution to non-dependent lung regions. 3. Decreased need for sedation and paralysis 4. Improvement in cardiac performance-due to reduced sedation and decreased intrathoracic and right atrial pressures [53] 5. Decrease in VILI associated with cyclic recruitment and atelectrauma [54,55]. However, the mode does not have a mechanism for limiting tidal volume, and patients can potentially receive very large transpulmonary pressures, which increase the risk of overdistension. Given that most of the benefits of APRV are related to spontaneous breathing, this method is not usually applied for patients who require deep sedation and neuromuscular blockade [53]. Also, given the very low time allotted for the expiratory phase, it is relatively contraindicated in patients with obstructive lung diseases due to potential of developing auto-PEEP [56].
The few published randomized controlled trials (RCTs) evaluating APRV have had small sample sizes and did not compare APRV with best practices in conventional mechanical ventilation for ARDS. Moreover, they used surrogate endpoints such as sedation levels and yielded conflicting results [53,57–59]. The only RCT that compared APRV with the conventional low tidal volume ventilation was in a series of adult trauma patients in acute respiratory failure, which did not show any mortality benefit-rather a trend towards increased duration of mechanical ventilation and ICU days was noted in the APRV group [60]. While some studies show improvement in oxygenation [61], improvement in respiratory mechanics [61,62] others have shown increases in ventilator days [63] or no difference in clinically significant outcomes [64]. In short, most studies show physiological benefits and improvement in some short-term clinical outcomes such as oxygenation and respiratory mechanics, but no mortality benefit [53].
In the absence of mortality benefit and lack of universality from the available evidence so far, a definite recommendation for the use of APRV cannot be made and controversy regarding its use will continue to exist until a well-designed multi-center RCT assessing patient related outcomes is completed [10]. However, it continues to be used in several centers and may be considered in refractory hypoxemia, especially in patients with recruitable lung in ARDS.
5.2. High frequency oscillatory ventilation
High frequency oscillatory ventilation (HFOV), delivers a small tidal volume (1–4 ml/kg) at a frequency range of 3–15 Hz while maintaining a high mean airway pressure [65]. In HFOV, the lungs are held inflated with high pressures to maintain oxygenation while carbon dioxide is cleared by small volumes of gas moved in and out of the respiratory system at high frequencies, which aim to facilitate alveolar recruitment and minimize atelectrauma [66]. Observational Studies and trials [67,68] showed improvement in oxygenation in patients with ARDS with HFOV but were limited by the use of outdated conventional ventilator strategies and small sample sizes.
Two large randomized controlled trials of HFOV in ARDS published in 2013 showed no mortality benefit in ARDS compared to lung protective ventilation strategy in conventional ventilation modes, and one of the two trials showed harm [66,69]. The High-frequency oscillation in early acute respiratory distress syndrome (OSCILLATE) trial [69] randomized 548 patients with early moderate to severe ARDS, with PaO2/FiO2 less than 200 in a 1: 1 ratio to either HFOV or conventional low volume ventilation and high PEEP and showed an absolute increase in hospital mortality (47% in HFOV vs. 35% in conventional lung protective ventilation modes). The mechanism of poor outcomes with HFOV was unclear but may relate to hemodynamic influences of high airway pressure, worsening right ventricular failure secondary to increased afterload and/or substantial sedation requirements of this approach [70] [71]. A Cochrane review of 10 randomized controlled trials showed no mortality benefit in the 1779 patients with moderate or severe ARDS on HFOV, despite improved oxygenation in 18–26% [72]. A recent patient level meta-analysis confirmed increased mortality with HFOV in mild-moderate ARDS, but suggested a survival benefit in very severe ARDS with Pa02/FiO2 less than 60 [73].
The authors of recently published evidence-based clinical practice guidelines recommend that HFOV not be used routinely in patients with moderate or severe ARDS (strong recommendation, moderate to high confidence in effect estimates) [28]. However it continues to be used in many centers in the setting of severe refractory ARDS, perhaps because HFOV is less well studied in the rescue setting.
6. Pulmonary vasodilator therapy
Inhaled pulmonary vasodilators will theoretically dilate the blood vessels preferentially in the well-ventilated lung units. Redirection of blood flow from the poorly ventilated lung zones will thus improve the V/Q mismatch [74]. They may also reduce hypoxia mediated vasoconstriction and pulmonary hypertension [75]. The localized delivery and short half-life of these medications substantially reduces their systemic effects. However, despite the physiological benefits of inhaled vasodilators, studies performed in ARDS patients have not established any mortality benefit [76,77]. Of note, clinical trials in some cases were designed to reduce PEEP by protocol in the context of iNO induced oxygenation improvement; arguably, this strategy may have offset any potential benefits of iNO if the PEEP were indeed lung protective.
Nitric oxide:
Inhaled nitric oxide (iNO) when delivered at concentrations of 5–80 ppm, can diffuse across alveolar walls and dilate the capillaries in those lung units that are well-ventilated. Once initiated, parameters of improvement usually monitored are PaO2 and pulmonary vascular pressures. Use of iNO is considered when the patient is not responding to conventional management and requires high FiO2 and PEEP. Inhaled NO is also not routine therapy for adults with ARDS, since studies have not proven a mortality benefit [76,78,79]. Additionally, iNO is associated with more complications, particularly methemoglobinemia and increased renal impairment [80]. Methemoglobin levels should be checked before and monitored during therapy. iNO also requires a specialized delivery system, resulting in iNO being largely replaced by inhaled prostacyclins.
Prostacyclins:
Inhaled prostacyclins (PGI2 or epoprostenol) may be especially useful for patients with refractory hypoxemia accompanied by pulmonary hypertension and right ventricular dysfunction [77]. It is usually initiated at doses of 50 ng/kg/min followed by monitoring of PaO2 and pulmonary artery pressures (if available), and titrated down based on improvement of these parameters.
The major advantage of inhaled prostacyclin compared to inhaled NO is its ease of delivery. Aerosolized prostacyclin can be delivered via nebulizer connected to the mechanical ventilation circuit. Additionally, inhaled epoprostenol is considerably less expensive than inhaled NO [81]. Adverse events with the use of prostacyclin are infrequent. There were concerns about prostacyclins’ inhibitory effects on platelet aggregation [82]. However, studies that have utilized prostacyclins in ARDS have not reported major bleeding events [83]. Thus, inhaled pulmonary vasodilators though not supported for routine use based on existing evidence, may be considered as adjunctive therapy in severe ARDS especially if associated with pre-existing pulmonary hypertension [4,79].
7. Extra- corporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) has been used successfully in patients with severe acute respiratory distress syndrome (ARDS) since 1972 [84]. There has been a marked increase in the number of patients receiving extracorporeal support for respiratory failure especially after the H1N1 influenza pandemic [85]. In patients with moderate-severe ARDS, and refractory hypoxemia on LPV, when part of the gas exchange can be taken over by veno-venous ECMO (VVECMO), tidal volumes and plateau pressures can be substantially reduced on the ventilator to provide time for recovery of the injured lungs.
In 2009, a UK based multicenter trial Conventional Ventilation or ECMO for severe adult respiratory failure (CESAR) [86] randomized 180 adult patients in 1:1 ratio with severe but reversible respiratory failure to receive ECMO (n = 90) or conventional management (n = 90). Patients randomized to ECMO were transferred to a single specialized ECMO center, while patients randomized to conventional mechanical ventilation remained at their local hospital. Only 68 of the 90 (75%) patients randomized to the ECMO group, actually received ECMO. Patients were maintained on lung protective ventilation while on VV ECMO which was continued until lung recovery, or until apparently irreversible multi-organ failure. The study found an absolute mortality reduction by 16% without severe disability in the ECMO group (63% survivors in ECMO group vs. 47% in non ECMO group). But the CESAR study had several weaknesses -which include a) Absence of standardized ventilation protocol in conventional ventilation arm b) Among those referred to ECMO centers, only 75% received ECMO and c) Significantly higher percentage of patients in the ECMO group received LPV, as compared to conventional management arm. In fact, if one only considers the mortality of those patients who actually received ECMO and compare it to those who were managed with conventional ventilation regardless of location, there is no significant difference in survival (51.5% ECMO vs. 56.9% conventional ventilation) Thus, it is unclear whether the improvement in survival was attributable to ECMO or to better care and adherence to LPV in specialized centers [86].
The 2009 H1N1 pandemic stimulated the use of ECMO for severe ARDS in various parts of the world- and demonstrated survival rates between 68 and 77%. However, all of the reported case series were uncontrolled and shared the same shortcomings of the CESAR trial and whether improvement in survival could be attributed to ECMO or better care with lung protective ventilation/rescue strategies in specialized hospitals is unclear [87–89].
The authors of a recently published evidence based clinical practice guidelines state that additional evidence is necessary to make a definitive recommendation for or against the use of ECMO in severe ARDS [28]. To date, there are no well-defined clinical criteria to determine the specific patient population that would benefit from the use of ECMO over conventional therapy. In a recently concluded randomized controlled trial- the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA trial)- early use of ECMO within 7 days for severe ARDS (defined as Pao2:Fio2 ratio < 50 for more than 3 h; or PaO2/FiO2 ratio < 80 for 6 h) was compared with conventional treatment group (low tidal volume mechanical ventilation and on LPV settings). At 60 days, the ECMO group did not have a statistically significant mortality benefit compared with the control group. Notably though, 28% of patients in the control group crossed over to the ECMO group, possibly diluting the true effect of ECMO initiation in these patients. Hence the data is still inconclusive for clinical benefit from ECMO, although a trend towards decreased mortality and renal failure was observed in the arm with early institution of ECMO (within 7 days) [90].
8. Conclusions
Refractory hypoxemia in ARDS continues to pose a major treatment challenge and is associated with considerable mortality. Salvage therapies, with the exception of extra-corporeal membrane oxygenation and pulmonary vasodilators work on the physiological principles of opening up collapsed alveoli, with resultant reduction in shunt fractions and dead space, thus improving lung compliance and alveolar recruitment and consequently ventilation –perfusion ratios [91–94].
In light of the present evidence suggesting a mortality benefit, we suggest proning and paralysis as first line therapy. There currently are no data to recommend which other salvage therapies should be initiated and the use of such measures is likely going to be dependent upon clinician familiarity, resource availability, patient risk and cost considerations [91]. Future research to identify mechanical or biological markers predicting response to these therapies may provide more targeted patient selection than simply hypoxemia [92–94]. A proposed flow chart is outlined below (Fig. 1).
Fig. 1.
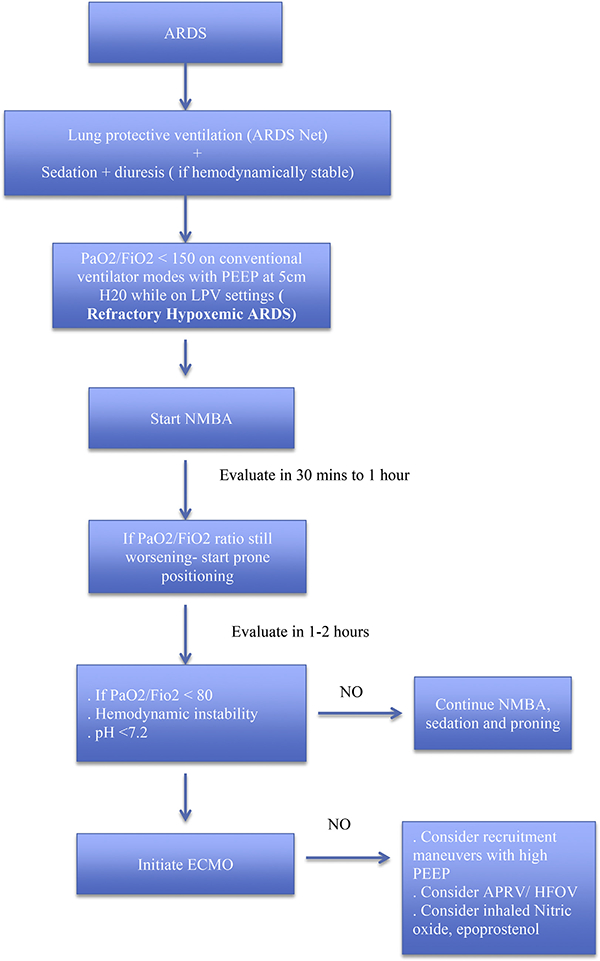
Proposed flow chart of management of refractory hypoxemia in moderate-to severe ARDS.
Acknowledgments
Financial disclosures
The authors have no financial disclosures to declare and no conflicts of interest to report.
Glossary
- PEEP
Positive End Expiratory Pressure
- V/Q ratio
Ventilation-perfusion ratio
- TT
Endotracheal tube
- NMBA
Neuromuscular Blocking Agents
- NO
Nitric Oxide
References
- [1].Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. , Acute respiratory distress syndrome: the Berlin Definition, J. Am. Med. Assoc 307 (23) (2012) 2526–33. [DOI] [PubMed] [Google Scholar]
- [2].Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. , Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries, J. Am. Med. Assoc 315 (8) (2016) 788–800. [DOI] [PubMed] [Google Scholar]
- [3].Cannon JW, Gutsche JT, Brodie D, Optimal strategies for severe acute respiratory distress syndrome, Crit. Care Clin 33 (2) (2017) 259–75. [DOI] [PubMed] [Google Scholar]
- [4].Pipeling MR, Fan E, Therapies for refractory hypoxemia in acute respiratory distress syndrome, J. Am. Med. Assoc 304 (22) (2010) 2521–7. [DOI] [PubMed] [Google Scholar]
- [5].Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. , Comparison of two fluid-management strategies in acute lung injury, N. Engl. J. Med 354 (24) (2006) 2564–75. [DOI] [PubMed] [Google Scholar]
- [6].Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome, N. Engl. J. Med 342 (18) (2000) 1301–1308. [DOI] [PubMed] [Google Scholar]
- [7].Slutsky AS, Ranieri VM, Ventilator-induced lung injury, N. Engl. J. Med 369 (22) (2013) 2126–2136. [DOI] [PubMed] [Google Scholar]
- [8].Beitler JR, Malhotra A, Thompson BT, Ventilator-induced lung injury, Clin. Chest Med 37 (4) (2016) 633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sweeney RM, McAuley DF, Acute respiratory distress syndrome, Lancet 388 (10058) (2016) 2416–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Narendra DK, Hess DR, Sessler CN, Belete HM, Guntupalli KK, Khusid F, et al. , Update in management of severe hypoxemic respiratory failure, Chest 152 (4) (2017) 867–879. [DOI] [PubMed] [Google Scholar]
- [11].Esan A, Hess DR, Raoof S, George L, Sessler CN, Severe hypoxemic respiratory failure: part 1–ventilatory strategies, Chest 137 (5) (2010) 1203–1216. [DOI] [PubMed] [Google Scholar]
- [12].Pelosi P, Brazzi L, Gattinoni L, Prone position in acute respiratory distress syndrome, Eur. Respir. J 20 (4) (2002) 1017–1028. [DOI] [PubMed] [Google Scholar]
- [13].Gattinoni L, Taccone P, Carlesso E, Marini JJ, Prone position in acute respiratory distress syndrome. Rationale, indications, and limits, Am. J. Respir. Crit. Care Med 188 (11) (2013) 1286–1293. [DOI] [PubMed] [Google Scholar]
- [14].Scholten EL, Beitler JR, Prisk GK, Malhotra A, Treatment of ARDS with prone positioning, Chest 151 (1) (2017) 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Richter T, Bellani G, Scott Harris R, Vidal Melo MF, Winkler T, Venegas JG, et al. , Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury, Am. J. Respir. Crit. Care Med 172 (4) (2005) 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. , Prone positioning in severe acute respiratory distress syndrome, N. Engl. J. Med 368 (23) (2013) 2159–2168. [DOI] [PubMed] [Google Scholar]
- [17].Pelosi P, D’Andrea L, Vitale G, Pesenti A, Gattinoni L, Vertical gradient of regional lung inflation in adult respiratory distress syndrome, Am. J. Respir. Crit. Care Med 149 (1) (1994) 8–13. [DOI] [PubMed] [Google Scholar]
- [18].Piehl MA, Brown RS, Use of extreme position changes in acute respiratory failure, Crit. Care Med 4 (1) (1976) 13–14. [DOI] [PubMed] [Google Scholar]
- [19].Langer M, Mascheroni D, Marcolin R, Gattinoni L, The prone position in ARDS patients. A clinical study, Chest 94 (1) (1988) 103–107. [DOI] [PubMed] [Google Scholar]
- [20].Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. , Effect of prone positioning on the survival of patients with acute respiratory failure, N. Engl. J. Med 345 (8) (2001) 568–573. [DOI] [PubMed] [Google Scholar]
- [21].Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, et al. , Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial, J. Am. Med. Assoc 292 (19) (2004) 2379–2387. [DOI] [PubMed] [Google Scholar]
- [22].Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, et al. , A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome, Am. J. Respir. Crit. Care Med 173 (11) (2006) 1233–1239. [DOI] [PubMed] [Google Scholar]
- [23].Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. , Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial, J. Am. Med. Assoc 302 (18) (2009) 1977–1984. [DOI] [PubMed] [Google Scholar]
- [24].Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. , Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis, Intensive Care Med 36 (4) (2010) 585–599. [DOI] [PubMed] [Google Scholar]
- [25].Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, et al. , Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis, Intensive Care Med 40 (3) (2014) 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Girard R, Baboi L, Ayzac L, Richard JC, Guerin C, The impact of patient positioning on pressure ulcers in patients with severe ARDS: results from a multicentre randomised controlled trial on prone positioning, Intensive Care Med 40 (3) (2014) 397–403. [DOI] [PubMed] [Google Scholar]
- [27].Marini JJ, Josephs SA, Mechlin M, Hurford WE, Should early prone positioning Be a standard of care in ARDS with refractory hypoxemia? Respir. Care 61 (6) (2016) 818–829. [DOI] [PubMed] [Google Scholar]
- [28].Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. , An official American thoracic society/european society of intensive care medicine/ society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome, Am. J. Respir. Crit. Care Med 195 (9) (2017) 1253–1263. [DOI] [PubMed] [Google Scholar]
- [29].Hraiech S, Yoshida T, Papazian L, Balancing neuromuscular blockade versus preserved muscle activity, Curr. Opin. Crit. Care 21 (1) (2015) 26–33. [DOI] [PubMed] [Google Scholar]
- [30].Chiumello D, Brioni M, Severe hypoxemia: which strategy to choose, Crit. Care 20 (1) (2016) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hraiech S, Forel JM, Papazian L, The role of neuromuscular blockers in ARDS: benefits and risks, Curr. Opin. Crit. Care 18 (5) (2012) 495–502. [DOI] [PubMed] [Google Scholar]
- [32].Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. , Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome, Crit. Care Med 32 (1) (2004) 113–119. [DOI] [PubMed] [Google Scholar]
- [33].Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. , Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome, Crit. Care Med 34 (11) (2006) 2749–2757. [DOI] [PubMed] [Google Scholar]
- [34].Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. , Neuromuscular blockers in early acute respiratory distress syndrome, N. Engl. J. Med 363 (12) (2010) 1107–1116. [DOI] [PubMed] [Google Scholar]
- [35].Neto AS, Pereira VG, Esposito DC, Damasceno MC, Schultz MJ, Neuromuscular blocking agents in patients with acute respiratory distress syndrome: a summary of the current evidence from three randomized controlled trials, Ann. Intensive Care 2 (1) (2012) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, et al. , Recruitment and derecruitment during acute respiratory failure: a clinical study, Am. J. Respir. Crit. Care Med 164 (1) (2001) 131–140. [DOI] [PubMed] [Google Scholar]
- [37].Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L, Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver, Am. J. Respir. Crit. Care Med 163 (7) (2001) 1609–1613. [DOI] [PubMed] [Google Scholar]
- [38].Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A, A high positive endexpiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial, Crit. Care Med 34 (5) (2006) 1311–1318. [DOI] [PubMed] [Google Scholar]
- [39].Lachmann B, Open up the lung and keep the lung open, Intensive Care Med 18 (6) (1992) 319–321. [DOI] [PubMed] [Google Scholar]
- [40].Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, et al. , Recruitment maneuvers for acute lung injury: a systematic review, Am. J. Respir. Crit. Care Med 178 (11) (2008) 1156–1163. [DOI] [PubMed] [Google Scholar]
- [41].Lim CM, Koh Y, Park W, Chin JY, Shim TS, Lee SD, et al. , Mechanistic scheme and effect of “extended sigh” as a recruitment maneuver in patients with acute respiratory distress syndrome: a preliminary study, Crit. Care Med. 29 (6) (2001) 1255–1260. [DOI] [PubMed] [Google Scholar]
- [42].Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, et al. , Sigh in acute respiratory distress syndrome, Am. J. Respir. Crit. Care Med 159 (3) (1999) 872–880. [DOI] [PubMed] [Google Scholar]
- [43].Hess DR, Recruitment maneuvers and PEEP titration, Respir. Care 60 (11) (2015) 1688–1704. [DOI] [PubMed] [Google Scholar]
- [44].Goligher EC, Hodgson CL, Adhikari NKJ, Meade MO, Wunsch H, Uleryk E, et al. , Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis, Ann. Am. Thorac. Soc 14 (Supplement_4) (2017) S304–s11. [DOI] [PubMed] [Google Scholar]
- [45].Xi XM, Jiang L, Zhu B, Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial, Chinese Med J 123 (21) (2010) 3100–3105. [PubMed] [Google Scholar]
- [46].Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. , Positive endexpiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial, J. Am. Med. Assoc 299 (6) (2008) 646–655. [DOI] [PubMed] [Google Scholar]
- [47].Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. , Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial, J. Am. Med. Assoc 299 (6) (2008) 637–645. [DOI] [PubMed] [Google Scholar]
- [48].Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. , Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome, N. Engl. J. Med 351 (4) (2004) 327–336. [DOI] [PubMed] [Google Scholar]
- [49].Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. , Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis, J. Am. Med. Assoc 303 (9) (2010) 865–873. [DOI] [PubMed] [Google Scholar]
- [50].Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, et al. , Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial, J. Am. Med. Assoc 318 (14) (2017) 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sahetya SK, Brower RG, Lung recruitment and titrated PEEP in moderate to severe ARDS: is the door closing on the open lung? J. Am. Med. Assoc 318 (14) (2017) 1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, et al. , Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury, Am. J. Respir. Crit. Care Med 167 (9) (2003) 1215–1224. [DOI] [PubMed] [Google Scholar]
- [53].Facchin F, Fan E, Airway pressure release ventilation and high-frequency oscillatory ventilation: potential strategies to treat severe hypoxemia and prevent ventilator-induced lung injury, Respir. Care 60 (10) (2015) 1509–1521. [DOI] [PubMed] [Google Scholar]
- [54].Frawley PM, Habashi NM, Airway pressure release ventilation: theory and practice, AACN Clin Issues 12 (2) (2001) 234–46; quiz 328–329. [DOI] [PubMed] [Google Scholar]
- [55].Stawicki SP, Goyal M, Sarani B, High-frequency oscillatory ventilation (HFOV) and airway pressure release ventilation (APRV): a practical guide, J. Intensive Care Med 24 (4) (2009) 215–229. [DOI] [PubMed] [Google Scholar]
- [56].Modrykamien A, Chatburn RL, Ashton RW, Airway pressure release ventilation: an alternative mode of mechanical ventilation in acute respiratory distress syndrome, Cleve. Clin. J. Med 78 (2) (2011) 101–110. [DOI] [PubMed] [Google Scholar]
- [57].Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, et al. , Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury, Am. J. Respir. Crit. Care Med 164 (1) (2001) 43–49. [DOI] [PubMed] [Google Scholar]
- [58].Varpula T, Jousela I, Niemi R, Takkunen O, Pettila V, Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury, Acta Anaesthesiol. Scand 47 (5) (2003) 516–524. [DOI] [PubMed] [Google Scholar]
- [59].Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettila VV, Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome, Acta Anaesthesiol. Scand 48 (6) (2004) 722–731. [DOI] [PubMed] [Google Scholar]
- [60].Maxwell RA, Green JM, Waldrop J, Dart BW, Smith PW, Brooks D, et al. , A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure, J. Trauma 69 (3) (2010) 501–510 discussion 11. [DOI] [PubMed] [Google Scholar]
- [61].Lim J, Litton E, Robinson H, Das Gupta M, Characteristics and outcomes of patients treated with airway pressure release ventilation for acute respiratory distress syndrome: a retrospective observational study, J. Crit. Care 34 (2016) 154–159. [DOI] [PubMed] [Google Scholar]
- [62].Dart BWt, Maxwell RA, Richart CM, Brooks DK, Ciraulo DL, Barker DE, et al. , Preliminary experience with airway pressure release ventilation in a trauma/surgical intensive care unit, J. Trauma 59 (1) (2005) 71–76. [DOI] [PubMed] [Google Scholar]
- [63].Maung AA, Schuster KM, Kaplan LJ, Ditillo MF, Piper GL, Maerz LL, et al. , Compared to conventional ventilation, airway pressure release ventilation may increase ventilator days in trauma patients, J. Trauma Acute Care Surg 73 (2) (2012) 507–510. [DOI] [PubMed] [Google Scholar]
- [64].Gonzalez M, Arroliga AC, Frutos-Vivar F, Raymondos K, Esteban A, Putensen C, et al. , Airway pressure release ventilation versus assist-control ventilation: a comparative propensity score and international cohort study, Intensive Care Med 36 (5) (2010) 817–827. [DOI] [PubMed] [Google Scholar]
- [65].Huang CT, Lin HH, Ruan SY, Lee MS, Tsai YJ, Yu CJ, Efficacy and adverse events of high-frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome: a meta-analysis, Crit. Care 18 (3) (2014) R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. , High-frequency oscillation for acute respiratory distress syndrome, N. Engl. J. Med 368 (9) (2013) 806–813. [DOI] [PubMed] [Google Scholar]
- [67].Fort P, Farmer C, Westerman J, Johannigman J, Beninati W, Dolan S, et al. , High-frequency oscillatory ventilation for adult respiratory distress syndrome–a pilot study, Crit. Care Med 25 (6) (1997) 937–947. [DOI] [PubMed] [Google Scholar]
- [68].Mehta S, Lapinsky SE, Hallett DC, Merker D, Groll RJ, Cooper AB, et al. , Prospective trial of high-frequency oscillation in adults with acute respiratory distress syndrome, Crit. Care Med 29 (7) (2001) 1360–1369. [DOI] [PubMed] [Google Scholar]
- [69].Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. , High-frequency oscillation in early acute respiratory distress syndrome, N. Engl. J. Med 368 (9) (2013) 795–805. [DOI] [PubMed] [Google Scholar]
- [70].Alessandri F, Pugliese F, Ranieri VM, The role of rescue therapies in the treatment of severe ARDS, Respir. Care 63 (1) (2018) 92–101. [DOI] [PubMed] [Google Scholar]
- [71].Malhotra A, Drazen JM, High-frequency oscillatory ventilation on shaky ground, N. Engl. J. Med 368 (9) (2013) 863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sud S, Sud M, Friedrich JO, Wunsch H, Meade MO, Ferguson ND, et al. , High-frequency oscillatory ventilation versus conventional ventilation for acute respiratory distress syndrome, Cochrane Database Syst. Rev 4 (2016) Cd004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Meade MO, Young D, Hanna S, Zhou Q, Bachman TE, Bollen C, et al. , Severity of hypoxemia and effect of high-frequency oscillatory ventilation in acute respiratory distress syndrome, Am. J. Respir. Crit. Care Med 196 (6) (2017) 727–733. [DOI] [PubMed] [Google Scholar]
- [74].Zwissler B, Kemming G, Habler O, Kleen M, Merkel M, Haller M, et al. , Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome, Am. J. Respir. Crit. Care Med 154 (6 Pt 1) (1996) 1671–1677. [DOI] [PubMed] [Google Scholar]
- [75].Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM, Inhaled nitric oxide for the adult respiratory distress syndrome, N. Engl. J. Med 328 (6) (1993) 399–405. [DOI] [PubMed] [Google Scholar]
- [76].Adhikari NK, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, et al. , Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis, Crit. Care Med 42 (2) (2014) 404–412. [DOI] [PubMed] [Google Scholar]
- [77].Fuller BM, Mohr NM, Skrupky L, Fowler S, Kollef MH, Carpenter CR, The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis, Chest 147 (6) (2015) 1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr.et al. , Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial, J. Am. Med. Assoc 291 (13) (2004) 1603–1609. [DOI] [PubMed] [Google Scholar]
- [79].Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, et al. , Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group, Crit. Care Med 26 (1) (1998) 15–23. [DOI] [PubMed] [Google Scholar]
- [80].Afshari A, Brok J, Moller AM, Wetterslev J, Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults, Cochrane Database Syst. Rev (7) (2010) Cd002787. [DOI] [PubMed] [Google Scholar]
- [81].Torbic H, Szumita PM, Anger KE, Nuccio P, LaGambina S, Weinhouse G, Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients, J. Crit. Care 28 (5) (2013) 844–848. [DOI] [PubMed] [Google Scholar]
- [82].Burghuber OC, Silberbauer K, Haber P, Sinzinger H, Elliott M, Leithner C, Pulmonary and antiaggregatory effects of prostacyclin after inhalation and intravenous infusion, Respiration 45 (4) (1984) 450–454. [DOI] [PubMed] [Google Scholar]
- [83].Haraldsson A, Kieler-Jensen N, Wadenvik H, Ricksten SE, Inhaled prostacyclin and platelet function after cardiac surgery and cardiopulmonary bypass, Intensive Care Med 26 (2) (2000) 188–194. [DOI] [PubMed] [Google Scholar]
- [84].Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. , Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung, N. Engl. J. Med 286 (12) (1972) 629–634. [DOI] [PubMed] [Google Scholar]
- [85].Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Extracorporeal life support organization registry report 2012, Am. Soc. Artif. Intern. Organs J 59 (3) (2013) 202–210. [DOI] [PubMed] [Google Scholar]
- [86].Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. , Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial, Lancet 374 (9698) (2009) 1351–1363. [DOI] [PubMed] [Google Scholar]
- [87].Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. , Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1), J. Am. Med. Assoc 306 (15) (2011) 1659–1668. [DOI] [PubMed] [Google Scholar]
- [88].Webb SA, Pettila V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, et al. , Critical care services and 2009 H1N1 influenza in Australia and New Zealand, N. Engl. J. Med 361 (20) (2009) 1925–1934. [DOI] [PubMed] [Google Scholar]
- [89].Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. , The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks, Intensive Care Med 37 (9) (2011) 1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. , Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome, N. Engl. J. Med 378 (21) (2018. May 24) 1965–1975. [DOI] [PubMed] [Google Scholar]
- [91].Collins SR, Blank RS, Approaches to refractory hypoxemia in acute respiratory distress syndrome: current understanding, evidence, and debate, Respir. Care 56 (10) (2011) 1573–1582. [DOI] [PubMed] [Google Scholar]
- [92].Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. , Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy, Am. J. Respir. Crit. Care Med 195 (3) (2017) 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials, Lancet Respir. Med 2 (8) (2014) 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Thompson BT, Chambers RC, Liu KD, Acute respiratory distress syndrome, N. Engl. J. Med 377 (6) (2017) 562–572. [DOI] [PubMed] [Google Scholar]


