Abstract
Background
The aim of this study was to assess the clinical and radiological outcomes of surgical treatment for primary spinal ependymoma in children.
Material/Methods
Medical records of 46 primary spinal ependymoma patients who underwent surgery in BRSHH hospital during a 12-year period from 2004 to 2015 were retrospectively reviewed. All pediatric patients (patient age <18 years) were selected as the core sample used for this study.
Results
This series included 1 female and 2 male patients between the ages of 9 and 17 years with mean age 13.3±3.9 years. The mean preoperative course was 9.1±10.5 months. The most common location was the lumbar spinal cord (n=2). The most common presenting symptoms was lower-limb weakness and numbness. Two tumors were located intradural-intramedullary and 1 was located intradural-extramedullary. Gross-total resection (GTR) was achieved in 2 patients, and a near-total resection was performed in 1 patient. No adjuvant treatment was received. The mean follow-up duration was 51.3±37.6 (17–98) months. No complications were recorded. Functional assessment of all patients by the latest follow-up evaluation showed good progress even though the patient is not fully recovered. At 6.3 years after the first operation, 1 patient presented with drop-seeding metastasis. No patients had neurofibromatosis type 2.
Conclusions
Laminoplasty and intraoperative neurophysiological monitorization are essential in surgical treatment of pediatric spinal ependymomas. GTR and recovery in pediatric spinal ependymoma are more likely than in adults. Despite the GTR, the risk of drop metastasis remains. Therefore, close clinical and radiological follow-up is recommended.
MeSH Keywords: Ependymoma, Glioma, Laminectomy
Background
Primary spinal ependymoma are very rare in the pediatric population. Population-based data indicate that the overall incidence rate per 100 000 person-years was significantly higher in male than in female patients (males 0.227±0.029, females 0.166±0.03) [1,2]. Recently, a population-based study found that only 31 spinal pediatric ependymomas (SPEs) were reported in the USA during a 31-year period from 1973 to 2003 [1]. In children, ependymomas are the most common spinal cord tumors [1,2]. Spinal ependymomas are usually well-circumscribed. Histopathological classification includes myxopapillary ependymoma (MPE) and subependymoma, which are classified as WHO grade I, classic (typical), and papillary ependymoma classified as WHO grade II, and anaplastic ependymoma referred to as WHO grade III. Low-grade (grade I and II) lesions are more common than anaplastic ependymoma.
MPEs occur most commonly in the lumbosacral region and originate from within the terminal filum or the conus medullaris. Such lesions account for 13% of all spinal cord ependymomas between all age groups. These lesions appear to be common in patients who present in their third or fourth decade of life [3]. There is a limited number of pediatric cases have been reported with details. In the pediatric population, there has been ongoing debate about the characteristics that distinguish WHO grade II and grade III ependymomas [4].
In adults, spinal ependymomas vary greatly in size and typically have a long prodrome. They frequently cause nonspecific symptoms such as numbness, nighttime back, and limb pain, which may be misinterpreted as radicular pain caused by degenerative disease [5]. This is not true for children. Thanks to their small-diameter spinal canal compared to adults, pediatric SPEs have a short prodrome (<12 months). Intramedullary ependymomas in the cervical or thoracic spine can coexist with syrinx cavities, which may lead to motor, sensory, urinary, and gait abnormalities.
Primary PSEs may be associated with neural axis dissemination, although this complication occurs with low-grade lesions rather than anaplastic ependymomas. Anaplastic ependymomas are more likely to be reported with metastasis to lung, skin, and kidney rather compared to low-grade ependymomas [6]. Spinal ependymomas have an unusual propensity to spread extraneurally. This is particularly true for subcutaneous myxopapillary tumors that arise over the sacrococcygeal region [6,7]. It has been reported that pediatric patients are more likely to experience leptomeningeal dissemination and extraneural metastasis, and their disease course may be more aggressive than in adult patients [5].
Cases appear to be rare; most are reported as case reports or retrospective studies, and different histological grades of SPEs are often reported together, making the analysis of data on these tumors in pediatric patients difficult and complicating efforts to establish a standard protocol for management of SPEs. Gross-total resection (GTR) is the main treatment for SPEs, but several studies pointed out the impact of adjuvant treatment on progression of these tumors and survival rate; however, this is still debatable [4]. Here, we present the surgical treatment outcomes of 3 consecutive pediatric spinal ependymoma cases.
Material and Methods
Patiant population
This retrospective study was approved by the Medical Ethics Committee of our hospital under decision number 507/2015. Written informed consent was obtained from both parents of all patients for publication of their cases and accompanying images.
Medical records were retrospectively reviewed and we selected 46 patients with spinal ependymoma who were diagnosed and surgically treated at the Department of Neurosurgery of our hospital between the years 2004 and 2015. All the spinal ependymoma cases of children (patients age of under 18 years) are included in the current study (n=3). The patient characteristics, clinical presentation, surgical findings, and pre- and post-operative neurological functional assessments were evaluated using a modified neurological scoring system (Table 1) [8], and long-term outcome are discussed. The relevant literature concerning ependymomas in children is also reviewed.
Table 1.
Neurological scoring system [8].
| Score | Pain intensity | Sensory disturbance, dysesthesias | Motor weakness | Gait ataxia | Sphincter function |
|---|---|---|---|---|---|
| 5 | None | Normal | Full power | Normal | Normal |
| 4 | Slight, no medication | Present, not significant | Movement against resistance | Unsteady, no aid | Slight disturbance, no catheter |
| 3 | Tolerable w/ medication | Significant, function not restricted | Movement against gravity | Mobile w/ aid | Residual in urodynamic studies, no catheter* |
| 2 | Insufficient control w/ medication | Some restriction of function | Movement w/o gravity | Few steps w/ aid | Rarely incontinent |
| 1 | Severe despite medication | Severe restriction of function | Contraction w/o movement | Standing w/ aid | Frequent catheter |
| 0 | Incapacitating | Incapacitated function | Paralysis | Paralysis | Permanent catheter |
Modified. w/ – with; w/o – without.
Statistical analysis
Statistics presented hereafter are expressed as the mean ± standard deviation values together with the range in parentheses. Differences among groups were assessed with the one-way analysis of variance (ANOVA) using the SPSS 21.0 statistical package. Significance in the multivariate model was determined using a p value <0.05. All p values are given with odds ratios (OR). OR are presented with the 95% confidential interval (CI). All tests were 2-tailed.
Surgery
Under general anesthesia and using intraoperative neurophysiological monitoring (IONM), the patients were positioned prone using a supporting roll on each side. A paramedian vertical midline incision was made. After dissecting the paraspinal muscles, laminectomy or laminotomy was completed. Bilateral lamiotomy was done using high-speed drills or kerrison rongeurs. Then, ligamentum flavum and the adipose tissue were removed. The laminectomy or laminotomy were performed up to the tumor spanned levels. The operative microscope was brought in over operation field. The thecal sac was opened in the midline and tacked up bilaterally using strong sutures. After the dura was opened, a dorsal midline myelotomy was performed on any intramedullary ependymomas to reach the intramedullary region. After exposuring all nerve roots, spinal cord/filum terminale and arachnoid bands, the neurosurgeons distinguished neural tissues from tumoral tissue using the microscope and probe of IONM. In the intramedullary region, a cavitron ultrasonic surgical aspirator was used. The tumoral tissue was completely resected in 3 cases (2 primary and 1 drop seeding metastasis lesions). Although is thought to be easy to separate neural tissues from tumoral tissue in the syrinx cavity, it seems that in the syrinx cases it is difficult to achieve GTR. Tumor tissue was removed and, to preserve neurological functions, we were careful to avoid injuring the spinal cord. After hemostasis using serum physiologic water, duraplasty was performed using 5.0 absorbable sutures (particularly, in the last reported 10 cases in this series, to avoid CSF fistula after tight closure of dura, the surgeons used fibrin sealant products). The operation field was kept clean and the CSF circulation between the neural elements was preserved. In the laminotomy cases, the laminae were placed using strong non-absorbable suture to fix them again. All layers were closed appropriately with their anatomy.
Case reports (Table 2)
Table 2.
Baseline clinical characteristics, clinical presentation and findings, surgical approaches, and outcomes of treatment in three operated pediatric patients.
| No | Clinical presentation | Age/sex | Location | Clinical findings | Surgery | Postoperative course; pathology | Survival after surgery |
|---|---|---|---|---|---|---|---|
| 1 | Unsteady gait, left leg numbness of 7 wks | 11/M | Intradural-Intrame-dullary; Lumbar; L1–L3 | Global left leg hypoesthesia | GTR using laminectomy and IONM | PO 7th day; recovered fully; MXE WHO grade I; PO 75th month presented with seeding metastasis (see below); No complication or local recurrence was recorded | Alive; 98 mns |
| Low back pain, bilateral lower extremities numbness and heavy walking of 2 mns | 17/M | Intradural-Extrame-dullary; Sacral; S1–S2 | Bilateral straight leg test at 60 degrees | GTR using laminectomy and IONM | PO 3th day; recovered fully; MXE WHO grade I (drop seeding metastasis); No complication, local recurrence or neuroaxis dissemination was recorded | Alive; 25 mns | |
| 2 | Low back and left leg pain of 2 yrs | 16/M | Intradural-Extrame-dullary; Lumbar; L1–L3 | Bilateral straight leg test at 60 degrees, local tenderness on L1–L3 | GTR using laminoplasty and IONM | PO 3th day; recovered fully; MXE WHO grade I; No complication, local recurrence or neuroaxis dissemination was recorded | Alive; 63 mns |
| 3 | Difficulty of urination and breathing of 3 dys; heavy walking, low back and left leg numbness and weakness of 35 dys | 9/F | Intradural-Intrame-dullary; Thoracic; T8–T10; (+Syrinx) | Urinary retention, 4/5 strength and global hypoesthia on the left leg, left Babinski reflex was no response and DTR of left leg were hyperactive | NTR using laminoplasty and IONM | PO 7th day; improved; Classic Ependymoma WHO grade II; She recovered with transient glob, and was discharged after rehabilitation she was doing well; No complication, local recurrence or neuroaxis dissemination was recorded | Alive; 17 mns |
GTR – gross total resection; NTR – near total resection; dys – days; wks – weeks; mns – months; yrs – years; F – Female; M – Male; PO – postoperative; DTR – deep tendon reflexes; MXE – myxopapillary ependymoma; IONM – intraoperative neurophysiological monitorization.
Case 1
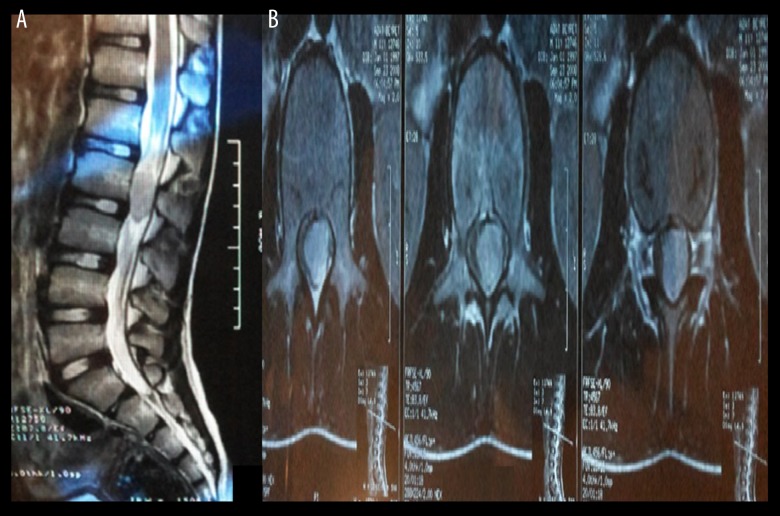
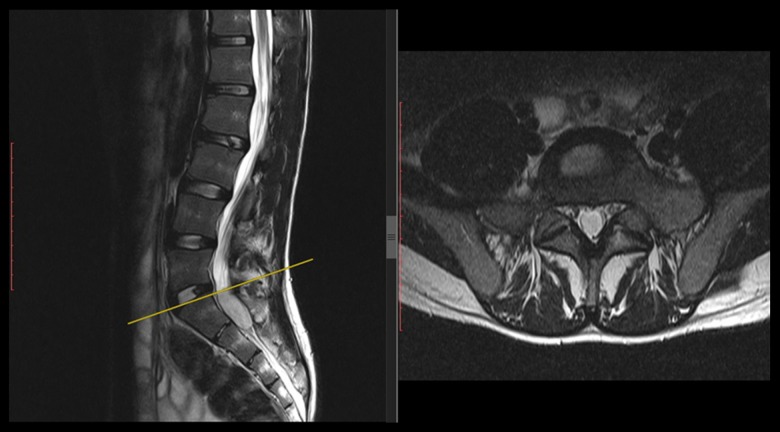
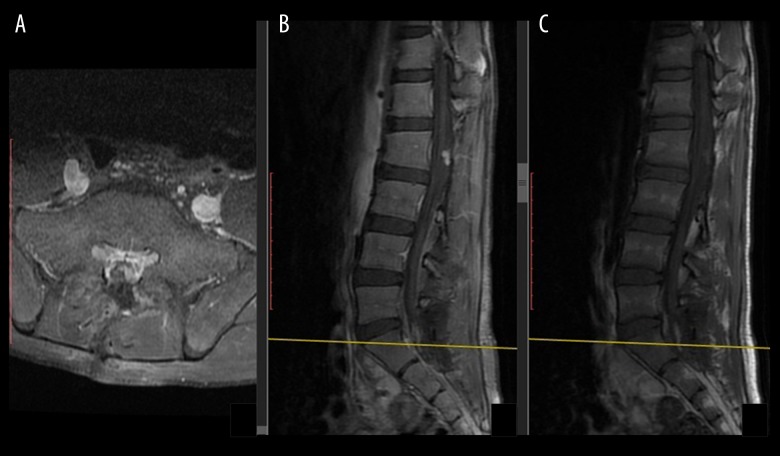
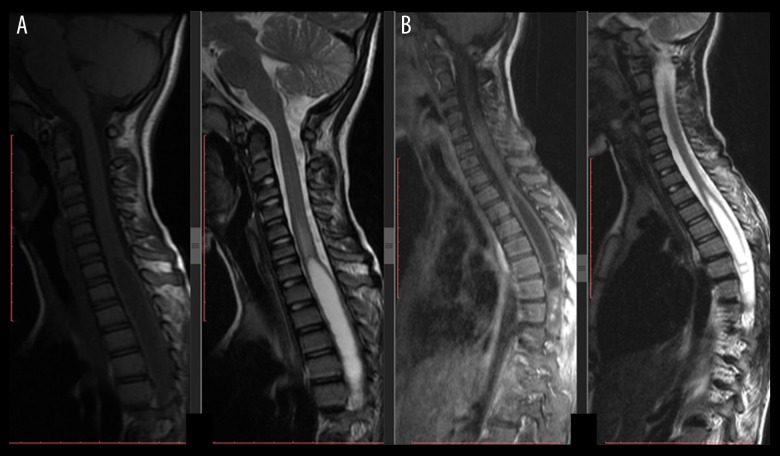
An 11-year-old male was referred to our hospital with unsteady gait and left leg numbness of 7 weeks. His complaints were more severe at nights. His neurological examination was intact, except for global hypoesthesia on left leg. T1-weighted MR images demonstrated hyperintense areas, T2-weighted MR images showed iso-hyperintense areas, and there was a well-circumscribed intradural intramedullary lumbar (filum terminale) lesion measuring 12×24×33 mm at L1–L3 level (Figure 1A, 1B). The patient underwent GTR of the lesion using total L2 laminectomy. He recovered well and was discharged after 7 days without any complication. Histopathologically, the mass lesion was confirmed to be a MPE (WHO grade I). Yearly, MRI and neurological examinations were performed. On his doctor visit at 75 months, the patient presented with low back pain, bilateral lower-extremities numbness, and heavy walking. MRI showed a well-circumscribed intradural sacral lesion measuring 14×8.2×30.1 mm at S1–S2 level (Figure 2). The patient underwent GTR of lesion using total S1-2 laminectomy (Figure 3). He recovered well and was discharged after 3 days without any complication. Histopathologically, the mass lesion was confirmed to be a MPE (drop-seeding metastasis). The patient had complete resolution of his symptoms. The patient was doing well at his postoperative 98th/25th month doctor visit.
Figure 1.
An 11-year-old male was referred to our hospital with unsteady gait and left leg numbness of 7-week duration; (A) Preoperative T2-weighted sagittal MRI demonstrated an iso-hyperintense, well-circumscribed, intradural, intramedullary, lumbar lesion measuring 12×24×33 mm at L1–L3 level; (B) T2-weighted axial MRI, note that the tumor is located intramedullary.
Figure 2.
The same patient as in Figure 1. At 75 months after surgery, he presented with low back pain, bilateral lower-extremities numbness, and heavy walking. T2-weighted MRI showed a well-circumscribed intradural sacral lesion measuring 14×8.2×30.1 mm at S1–S2 level. Note that there is no local recurrence or residue at L1–L3 level; defects of L2 laminectomy.
Figure 3.
Second operation early postoperative contrast-enhanced T1-weighted MRI showed that GTR was achieved, as well as postoperative changes and defect of S1 laminectomy; (A) Axial contrast-enhanced T1-weighted MRI demonstrated postoperative changes and S1 laminectomy; (B) Sagittal contrast-enhanced T1-weighted MRI; (C) Sagittal T1-weighted MRI.
Case 2
A 16-year-old male presented to our out-patient clinic with low back and left leg pain of 2-year duration. His neurological examination was intact, except the straight leg raise (Lasegue) test was bilaterally positive at 60 degrees, and there was local tenderness on spinous processes of L1–L3. T2-weighted MR images were iso-hyperintense and demonstrated a well-circumscribed intradural extramedullary lumbar (cauda equine) lesion measuring 15x23x54 mm at L1–L3 level. The patient underwent GTR of the lesion using total L1–L3 laminoplasty. He recovered well and was discharged after 3 days without any complication. Histopathologically, the mass lesion was confirmed to be a MPE (WHO grade I). Yearly, MRI and neurological examinations were performed. No recurrence was observed. The patient had complete resolution of his symptoms and was doing well on his postoperative 63th month doctor visit.
Case 3
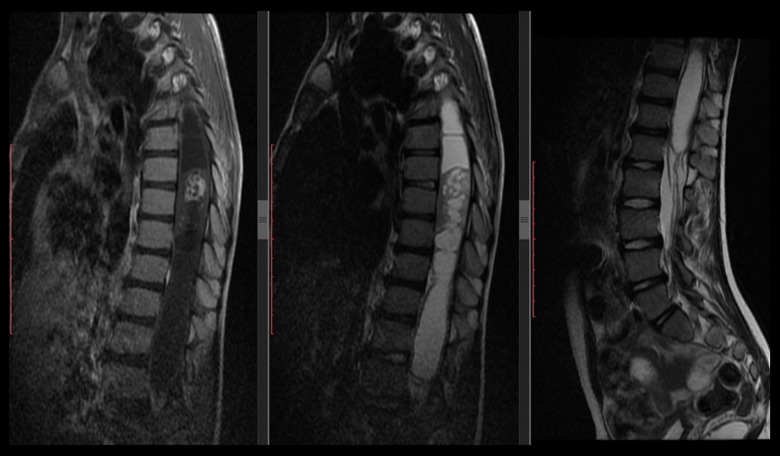
A 9-year-old female was referred to our Emergency Department with urinary retention, difficulty of breathing (3-day duration), heavy walking, low back and left leg numbness and weakness lasting 35 days. Her neurological examination revealed 4/5 strength and global hypoesthesia on the left leg, left Babinski test was no response, and deep tendon reflexes of left side lower extremity were hyperactive. T1-weighted MR images demonstrated hyperintense and T2-weighted MR images showed an iso-hyperintense, irregular, diffuse, intradural, intramedullary thoracic lesion with several septae, measuring 24×33×46 mm at T8-10 level. The lesion up and down the side of the syrinx cavity extended from C5 to L1 levels (Figure 4). The patient underwent near-total resection (NTR) of the lesion using T8–T10 laminoplasty. She recovered with transient glob, and when discharged after rehabilitation was doing well. Histopathologically, the mass lesion was confirmed to be a classic ependymoma (WHO grade II). No adjuvant treatment was carried out for syrinx. The 3rd and 6th postoperative MRI (Figure 5) showed regress of syrinx cavities, and no progression or seeding metastasis were detected. On her 3-month doctor visits, she had complete resolution of her symptoms and was doing well on her postoperative 17-month doctor visit.
Figure 4.
A 9-year-old female was referred to our Emergency Department with urinary retention, difficulty breathing, low back and left leg pain, numbness, and weakness. Preoperative T1-weighted MRI demonstrated hyperintense areas, T2-weighted MRI showed an iso-hyperintense irregular diffuse intradural intramedullary thoracic lesion with several septae, measuring 24×33×6 mm at T8–T10 level. The lesion up and down side syrinx cavities extends from C5 to L1 levels.
Figure 5.
Postoperative 3- and 6-month MRIs of the same patient as in Figure 4. She underwent NTR of the lesion using T8-10 laminoplasty. No adjuvant treatment was carried out for the syrinx. Both MRIs showed regress of syrinx cavities, and no progression or seeding metastasis was detected; (A) Postoperative 3-month MRI, T1-weighted MRI in left side and T2-weighted MRI in right side; (B) Postoperative 6-month MRI, T1-weighted MRI in left side and T2-weighted MRI in right side. Note that the syrinx cavities were regressed without additional therapy.
Results
This series included 1 female (33.3%) and 2 male patients (66.7%) between the ages of 9 and 17 years, with mean age 13.3±3.9 years. The basline clinical characteristics, location, treatment, outcomes, and complications of treatment for all cases are given in Table 2. The mean preoperative course (prodrome) was 9.1±10.5 months (range, 35 days to 24 months). The most common location was the lumbar spinal cord (n=2). The most common presenting symptoms were lower-limb numbness and ataxic gaits, which were seen in 3 of 4 cases per each (including the drop-seeding metastasis case). Two tumors were located intradural-intramedullary and 1 was located intradural-extramedullary. GTR was achieved in 2 patients, and NTR was performed in 1 patient. No adjuvant treatment was received. The mean follow-up duration was 51.3±37.6 months, with a range of 17 to 98 months. No complications were recorded. According to the modified neurological scoring system, functional assessment of all patients at the latest follow-up evaluation showed that were good (Table 3). Two MPEs and 1 classic ependymoma were histopathologically diagnosed. At 6.3 years after the first operation, 1 patient presented with drop-seeding metastasis. The 45- and 51-month progression-free survival rates were 100% and 75%, respectively, and the 4-year survival rate was 100%. The mean length of hospital stay was 5.3±3.2 (range, 3–10) days. No recurrence, morbidity, or mortality was recorded. No patient had neurofibromatosis type 2. All our pediatric patients attended a normal schooling system.
Table 3.
Clinical outcomes according to neurological scoring system.
| No* | Leg ± back pain intensity Preop/postop |
Sensory disturbance, dysesthesias Preop/postop |
Motor weakness Preop/postop |
Gait ataxia Preop/postop |
Sphincter function Preop/postop |
Surgical outcome |
|---|---|---|---|---|---|---|
| 1 | 5/5 | 3/4 | 5/5 | 3/5 | 5/5 | Recovered good |
| 2/5 | 4/5 | 5/5 | 4/5 | 5/5 | Recovered good | |
| 2 | 3/5 | 5/5 | 5/5 | 5/5 | 5/5 | Recovered good |
| 3 | 5/5 | 3/4 | 4/5 | 4/5 | 3/4 | Improved** |
Case No in this table referes to the same case No in Table 2.
Improved: if the patient’s complaints decreased but did not mean full recovery.
Table 4 shows a comprasion between our spinal primary ependymoma adult and child patients who were treated surgically in the same institute and during the same time period. Two symptoms were significantly different between children and adults who had spinal ependymomas; ‘gait impairment’ symptom was more likely to appear in children than in adults (OR 15.4, P=0.026), while ‘radicular (extremity) pain’ symptom was more likely to appear in adults than in children (OR 0.01 P=0.026). The mean prodrome period was shorter in child than in adult patients (9.1±10.5 and 25.0±43 months, respectively) [P=0.006].
Table 4.
Comparison between our spinal primary ependymoma adult and child patients who were treated surgically in same institute and during the same period.
| Children | Adults | Comments | |
|---|---|---|---|
| Numbar of Patients | 3 (1F; 2M) | 43 (20F; 23M) | OR 1.74, CI=0.15–20.65; P=0.57 |
| Age | 13.3±3.9 (9–17) yrs | 39.2±12.0 (22–67) yrs | – |
| F: | 9 yrs | 40.3±13.2 (22–67) yrs | – |
| M: | 14.67±3.2 (11–17) yrs | 38.3±11.1 (22–55) yrs | – |
| Complaints | |||
| – Radicular (extremity) pain | 25% (1/4*) | 83.7% (36/43) | OR 0.01, CI=0.01–0.72; P=0.026 |
| – Local pain | 50% (2/4) | 79% (34/43) | OR 0.25, CI=0.03–2.02; P=0.21 |
| – Loss of sensation | 75% (3/4) | 41.9% (18/43) | OR 4.17, CI=0.4–43.4; P=0.28 |
| – Motor deficit | 25% (1/4) | 27.9% (12/43) | OR 0.86, CI=0.1–9.1; P=0.7 |
| – Gait impairment | 75% (3/4) | 16.3% (7/43) | OR 15.4, CI=1.4–170.7; P=0.026 |
| – Urination disturbance | 25% (1/4) | 4.7% (2/43) | OR 6.8, CI=0.47–98.8; P=0.24 |
| – Difficulty of breathing | 25% (1/4) | 0 | P=0.085 |
| – Neuropathic pain | 0 | 4.7% (2/43) | P=0.84 |
| – Headache | 0 | 2.3% (1/43) | P=0.91 |
| – Neck pain | 0 | 2.3% (1/43) | P=0.91 |
| Mean prodrome | 9.1±10.5 mn (35 dy–24 mn) | 25.0±43 mn (10 dy–20 yr) | P=0.006 |
| Location | |||
| – Cervical (all Int) | 0 | 10 (7F; 3M) [23.3%] | – |
| – Thoracic [Int (4); Ext (2)] | 1 (F) [33.3%] | 5 (3F; 2M) [11.6%] | – |
| – Lumbar [Int (3); Ext (25)]: | 2 (M) [66.7%] | 26 (10F; 16M) [60.5%] | – |
| – Sacral | 0 | 0 | – |
| – Multiple (all Ext) | 0 | 2 (M) [4.6%] | – |
| Treatment choice | |||
| – GTR (n=25) | 2 [Int (1); Ext (1)] 66.7% | 23 [Int (5); Ext (18)] 53.5% | OR 1.58, CI=0.13–18.8; P=0.6 |
| – NTR (n=12) | 1 [Int (1)] 33.4% | 11 [Int (4); Ext (7)] 25.6% | |
| – STR (n=5) | 0 | 5 [Int (4); Ext (1)] 11.6% | |
| – GTR+RT (n=1) | 0 | 1 [Int (1)] 2.3% | |
| – STR+RT (n=3) | 0 | 3 [Int (2); Ext (1)] 7.0% | |
| Surgical outcome | |||
| – Recovered | 2 (GTR) [66.7%] | 27 (17: GTR; 6: NTR; 4: STR) [62.8%] | OR 1.1; P=0.72 |
| – Improved | 1 (NTR) [33.3%] | 8 (3: GTR; 4: NTR; 1: STR) [18.6%] | |
| – Unchanged | 0 | 4 (2: GTR; 2: STR) [9.3%] | |
| – Worsened | 0 | 3 (2: GTR; 1: STR) [7.0%] | |
| – Dead | 0 | 1 (NTR) [2.3%] | |
| WHO grading | |||
| – Grade I | 2 MPE(2M) [66.7%] | 18 MPE (7F; 11M) [41.9%] | – |
| – Grade II | 1 (F) [33.3%] | 23 (11F; 12M) [53.5%] | |
| – Grade III | 0 | 2 (F) [4.6%] | |
| Recurrence | 0 (1F; 1M) [5.1%] | ||
| Neuroaxis dissemination | (n=1) 33.3% | 0 | |
| Mean LOS (dys) | 5.3±3.2 (3–10) | 8.7±9.6 (2–64) | P=0.36 |
| Mean follow-up (mns) | 51.3±37.6 (17–98) | 90.2±50.9 (14–156) | – |
| 4-year PFS rate | 66.7% (1 Dis case) | 90.5% (4 recurrent cases) | – |
| 4-year OS rate | 100% | 97.7% (related surgery) | – |
Cases in children included drop seeding metastasis case.
F – Female; M – Male; Int – intradural-intramedullary; Ext – intradural-extramedullary; dy – days; mn – months; yr – years; GTR – gross total resection; NTR – near total resection; STR – subtotal resection; RT – postoperative radiotherapy; LOS – length of hospital stay; PFS – progression-free survival; OS – overall survival.
Although most studies report both children and adults, small numbers of children were included, and these numbers are insufficient to perform a meaningful review or statistical analysis, even if data are pooled. All distinguished SPE cases and series are given in Table 5.
Table 5.
Reported cases of pediatric (<18 years) spinal ependymoma in the literature (to the best of our knowledge).
| No | Lead Author; year | Mean age/sex | No of Pts | Location | Prodrome (mns) | Treatment | Pathology and postoperative course | Mean of FU (mns) |
|---|---|---|---|---|---|---|---|---|
| 1 | Nisenson [24]; 1945# | 12/M | 1 | N.M | N.M | STR+RT | Ependymoma (NOS); No Rec or Dis | 42 |
| 2 | Dereymaeker [24]; 1962# | 14/F | 1 | N.M | N.M | GTR | Ependymoma (NOS); No Rec or Dis | 60; Dead |
| 3 | Hendren [25]; 1963*# | 16/M | 1 | SC | 108 | Resection*+RT + ChT | Ependymoma (NOS); Rec+Dis (Extraneural met: inguinal lymph nodes, pelvis) | 72 |
| 4 | Sloof [17]; 1964 | 14/M | 1 | L (CE) | N.M | STR+RT | MPE; 3 times Rec at 2, 4 and 8 years. Dis(+) | 150; Dead |
| 5 | Anderson [6]; 1966 | 9/1M: 2F | 3 | SC (3) | N.M | GTR (2); STR+RT (1) | MPE (3); Rec (1): in STR+RT; no PO details for 1 pt. | 45 |
| 6 | Probhaker [25]; 1969*# | 0.83/M | 1 | SC | Since birth | Resection* | Ependymoma (NOS); Rec (+) | 24 |
| 7 | Rubinstein [26]; 1970 | 17/F | 1 | L (FT) | N.M | STR+RT | Ependymoma (NOS); Rec afte 3 yrs, Dis after 6 yrs; Extraneural metastasis (Lungs, pleural and lymph nodes): DOD after 29 yrs | 348; Dead |
| 8 | Wolf [27]; 1972**# | 4/M | 1 | SC | N.M | Resection* | Ependymoma (NOS); Rec+Dis (extraneural metastas: Lungs and inguinal lymph nodes) | 228 |
| 9 | Payne [28]; 1973 | 11/F | 1 | L (CE) | Acute; Posttrauma | Resection*+RT | MPE; No Rec or Dis | 36 |
| 10 | Scharrer [25]; 1974*# | 7/F | 1 | SC | N.M | Resection* | Ependymoma (NOS); Rec/Dis (N.M) | N.M |
| 11 | Scott [29]; 1974 | 17/M | 1 | L | N.M | STR+RT | MPE; Rec (after 5 yrs); Dis (No) | 276 |
| 12 | Ammerman [24]; 1975# | 16/F | 1 | N.M | N.M | GTR | MPE; No Rec or Dis | 24 |
| 13 | Cameron [30]; 1976 | 13/F | 1 | Multiple (supra-, infratentorial and spinal) | N.M | Resection* (4 operations in 4 yrs) | MPE; Rec? and Dis? Or multiple at presentation | 78 |
| 14 | Fisher [24]; 1977# | (6–14)/4M: 4F | 8 | N.M | N.M | GTR (5); STR+RT (3) | Ependymoma grade I (5); Ependymoma grade II (2); Ependymoma NOS (1); Dead at PO 36 mn | 43.5 |
| 15 | Mavroudis [31]; 1977 | 7/M | 1 | L (CE) | N.M | GTR | MPE; Rexploration after 2yrs (No lesion); Rec (after 24 yrs); Extraneural metastasis to lungs after 29 yrs) | 348 |
| 16 | Mork [10]; 1977 | 13/2M: 3F | 5 | N.M | N.M | NOS | MPE and Classic ependymoma (NOS) | N.M |
| 17 | Bale [25]; 1980*# | 4/F | 1 | SC | 1 | Resection* | Ependymoma (NOS); Rec/Dis (No) | 168 |
| 18 | Mork [32]; 1980 | 12/M | 1 | TL | 6 | STR+RT | Anaplastic ependymoma; Rec (after 2 yrs), Dis (after 6 yrs) | 78 |
| 19 | Morris [33]; 1983 | 10/F | 1 | L (CE) | 60 | NTR+RT | Ependymoma grade I; Rec and Dis (Extraneural metastasis: lungs and hip) after 6 yrs | 144 |
| 20 | Chan [34]; 1984 | 10.6/5M: 2F | 14* | L (7) [4: CE+ 3: FT] | 10.6 | GTR (5); STR+RT (2) | MPE (7); Rec in 2 GTR;Dis in 2 pts | 105.6 |
| 21 | Helwig [35]; 1984 | 17/F | 1 | SC | 4 | Resection* | MPE; Rec+Dis after 6 mns (DOD) | 60; Dead |
| 22 | Matsuo [36]; 1985 | 11/F | 1 | SC | N.M | GTR | MPE; Rec/Dis (No) | 7 |
| 23 | Sonneland [18]; 1985 | N.M/10M: 5F | 15 | L (15) | N.M | NOS | MPE (15); Rec (5 pts); 3 pts DOD | 205.1 |
| 24 | West [37]; 1985 | 7.9/4M | 4 | T (2); L (CE) (1); Extrasacral (CS) (1) | N.M | STR+RT+ChT (1); STR+RT (1); Bx+RT (2) | Ependymoma (Low grade) (4); No Rec or Dis | 102.5 |
| 25 | Ciraldo [38]; 1986 | 0.83/2M: 3F | 5 | SC (5) | Since birth | GTR (5) | MPE (5); Rec/Dis (No) | 26 |
| 26 | Chou [27]; 1987**# | 12.5/1M: 1F | 2 | SC (2) | N.M | Resection* (2) | MPE (2); Rec or Dis (N.M) | N.M |
| 27 | Murphy [27]; 1987**# | 0.09/M | 1 | SC | Since birth | N.M | MPE; Rec/Dis (No) | N.M |
| 28 | Di Marco [39]; 1988 | 17/F | 1 | L | N.M | STR+RT | Low grade ependymoma; Rec after 169 months (treated) | 182 |
| 29 | Kramer [25]; 1988 | 15/M | 1 | SC | N.M | GTR | MPE; Rec (twice)+Dis (extraneural: inguinal, lymph nodes and skin) | 240 |
| 30 | Pulitzer [40]; 1988 | 0.6/3M: 1F | 4 | SC (4) | Since birth | Resection* | MPE (4); Rec/Dis (No) | 7–40 |
| 31 | Naidu [41]; 1989 | 14/M | 1 | L (CE) | 6 | Resection*+RT | Ependymoma (NOS); Dis after one yr, Rec after two yrs | 24 |
| 32 | Fujiyama [42]; 1990 | 6/F | 1 | TL | 6 | GTR+RT | Anaplastic Ependymoma; Dis and Rec after 3 yrs | 89 |
| 33 | Le Marc’hadour [43]; 1991 | 14/F | 1 | SC | 6 | Resection* | MPE; Rec/Dis (No) | 24 |
| 34 | Wen [44]; 1991 | 12/NOS | 2 | L (2) | 18 (Median) | STR+RT (2) | MPE (2); Both had Dis in spine at 32 and 48 mns; both DOD at 209 and 110 mns. | 159; Dead |
| 35 | Gupta [45]; 1992 | 1.5/M | 1 | SC | Since birth | NOS | MPE; since birth, Dis (metastasis to inguinal lymph nodes) PO aggressive tm | 18 |
| 36 | Serour [46]; 1992 | 11/M | 1 | SC | N.M | GTR | MPE; Rec/Dis (No) | 12 |
| 37 | Clover [47]; 1993 | 16/2M: 1F | 3 | Multiple [LS+CE] (2); LS (1) | 24 (Median) | STR+RT (2); STR (1) | MPE (3); Rec (1) after 3.2 yrs in STR+RT (F-U: 6 yrs -AWD) | 52 |
| 38 | Gagliardi [24]; 1993 | 13.5/3M: 1F | 4 | L [FT] (4) | 12 | GTR (2); STR+RT (1); STR (1) | MPE (4); 1 Rec in STR | 93.3 |
| 39 | Lunardi [48]; 1993 | 10–16/NOS | 9 | Int [NOS] (9) | NOS | GTR+RT (3); GTR (4); STR+RT (2) | Low grade ependymoma (NOS) (9); Rec (2) | 48 [Median] |
| 40 | Ross [16]; 1993 | 10.7/2M: 1F | 3 | N.M | N.M | GTR+RT (3) | MPE (3); Rec/Dis (No) | 93.3 |
| 41 | Waldron [23]; 1993 | 11.5/NOS | 4 | LS (4) | N.M | Resection*+RT (4) | MPE (1), Classic Ep (1), Anaplastic (1), NOS (1); Rec (1); Dis (3): 2 of Dis were DOD; 1 Reopere+RT (30 yrs: NED); 1 Reopere (5 yrs: NED) | 114.8 |
| 42 | Botti [49]; 1994 | 10.5/N.M | 1 | SC | 4 | GTR | MPE; Rec/Dis (No); but the pt was presented with Dis. | N.M |
| 43 | Do-Dai [50]; 1995 | 12/F | 1 | CT | 5 dys | Bx+ChT | MPE; Rec: (+), Dis (+); Dis at prersentation | 6 |
| 44 | Mottl [51]; 1997 | N.M/F | 1 | L (FT) | N.M | Partial resection+RT | WHO grade I Ependymoma; Rec: No, Dis: No; alive | N.M |
| 45 | Nagib [52]; 1997 | 9.3/1M: 2F | 3 | TL (FT) [1]; L (CE) [2] | 1.7 | GTR (3) | MPE (3); Rec: No, Dis (1) | 42.7 |
| 46 | Ilhan [53]; 1998 | 8/M | 1 | SC | 3 | GTR | MPE; Rec/Dis (No) | 20 |
| 47 | Lonjon [54]; 1998 | 14/11M: 9F## | 32## | CM (2); C (4); CT (5); T (8); TL (1); 12 MPEs were located in LS | 5–84 | GTR+RT (1); GTR (13); STR+RT (5); STR (1); No details about treatment of MPEs | MPE (12), low grade ependymoma (17), high grade ependymoma (3); Rec (2) after 2 and 3 yrs (both were treated with STR+RT; Dis (4): 2 of them were anaplastic (In 1 GTR+R, 1 GTR and 2 STR+RT). 11 presented with syrinx, 3 pts had NF Type 2. 3 operations for kyphoscoliosis, 1 operation for shunting hydrocephalus | 67 [Median](25–177) |
| 48 | Graf [55]; 1999 | 15/M | 1 | TL (CE) | 3 | GTR | MPE; Rec (+): after 32 yrs; Neuroaxial Dis to Lungs and Liver (+) after 37 yrs and dead | 480; Dead |
| 49 | Johnson [56]; 1999 | 7/M | 1 | SC | 18 | GTR | Grade II ependymoma; No Rec/Dis | 96 |
| 50 | Ohata [57]; 1999 | 16/2M | 2 | CM (1); CT (1) | N.M | GTR (1); STR+RT (1) | Grade II (1) and grade III (1); No Rec/Dis. | 86.2 |
| 51 | Aktug [27]; 2000 | 5/M | 1 | SC | 6 | GTR | MPE; Rec/Dis (No) | 36 |
| 52 | Chinn [13]; 2000 | 11/2M: 1F | 3 | Multiple (2); L (1) | 11.3 | GTR+RT (1); GTR (1); STR+RT (1) | MPE (3); Rec (1) in GTR; Dis in all pts (3): 2 at presentation, one after 7 mns of GTR | 24 |
| 53 | Constantini [9]; 2000 | <21/NOS | 26 | Int (NOS)** | 11.6** | NOS** | Ependymoma (19), MPE (7); 10-yrs survival rate is 86%; 4-yr PFS rate is 75%** | 85.1** |
| 54 | Merchant [58]; 2000 | 8.6/3M: 5F | 8 | CM (1); CT (1); TL (3); L (1); LS (2) | N.M | GTR+RT+ChT (1); STR+RT+ChT (4); STR+RT (3) | MPE (4), ependymoma (4); Rec (2): in STR+RT+ChT both were ependymomas; Dis (4): 3 were MPEs which were treatd with STR+RT+ChT (2) and STR+RT (1), 1 ependymom which was treated as GTR+RT+ChT | 85.9 |
| 55 | Nishio [59]; 2000 | 15/2M | 2 | C+T (1); L (1) | 12.5 | GTR | Ependymoma grade II/III (NOS); Rec/Dis (No) | 137 |
| 56 | Gelabert-Gonzalez [60]; 2001 | 15/F | 1 | FT (2 tms: L+S) | 2 | GTR | MPE (both lesion); Rec/Dis (No) | 6 |
| 57 | Helseth [61]; 2001 | 8.76/3M: 2F | 5 | L (CE): 5 | N.M | GTR (2); STR+RT (3) | MPE (3), grade II (2); Dis (No); Rec (2): 1 GTR, 1 STR+RT | 164 |
| 58 | Hirose [62]; 2001 | 14.1/7M: 2F | 9 | C (2); L (1); CE (6) | N.M | N.M | MPE (6), Ependymoma grade II (3); Rec and Dis: N.M (Genetic study) | N.M |
| 59 | Hanbali [63]; 2002 | 17/M | 1 | T+L (Multiple) | N.M | GTR (both tms) | Grade II ependymoma; Rec/Dis (No) | 31 |
| 60 | Goto[64]; 2003 | 12.5/2M | 2 | CM (1); CT (1) | 13 | GTR (1); STR+RT (1) | Ependymoma (NOS); Rec/Dis (No) | 206.5 |
| 61 | Hallacq [15]; 2003 | 13/M | 1 | L+S (Multiple) | 5 | GTR | MPE; Rec/Dis (No) | 60 |
| 62 | Wolf [65]; 2003 | 1.5/F | 1 | TL | 0.5 | GTR | MPE; Rec/Dis (No) | 33 |
| 63 | Peker [66]; 2004 | 9/F | 1 | C (Int) | 4 | GTR | Low grade ependymoma (NOS); Rec/Dis (No) | 108 |
| 64 | Sebire [67]; 2004 | 13/F | 1 | SC | N.M | Resection* | MPE; Rec/Dis (NOS). | N.M |
| 65 | Fassett [14]; 2005 | 11.2/4M: 1F | 5 | L (5) | 18.7 | GTR+RT (1); GTR (1); STR+RT (3) | MPE (5); Rec (1): STR+RT; Dis (4). | 58.2 |
| 66 | Lin [68]; 2005 | 12/M | 1 | C | N.M | GTR | Ependymoma grade II; Rec/Dis (No) | 48 |
| 67 | Tubbs [69]; 2005 | 2/F | 1 | S | 6 | GTR | MPE; Rec/Dis (N.M) | N.M |
| 68 | Akyurek [22]; 2006 | 15/NOS | 2 | TL (1); L (1) | N.M | GTR+RT (1); GTR (1) | MPE (2); Rec (2): GTR+RT at 15 mns and GTR at 20 mns | 131 |
| 69 | Bagley [7]; 2007 | 12.6/10M: 4F | 14 | T (1); TL (4); L (1); LS (5); S (1); Multiple (2) | 23.2 | GTR (7); STR+RT (2); STR (2); Resection* (1); Bx+RT (1); Bx (1) | MPE (14); Rec (11): in 4 GTR, 2 STR+RT, 2 STR, 1 Bx+RT, 1 Bx, 1 Resection*; Dis (6) | 63.7 |
| 70 | Mridha [70]; 2007 | 13/M | 1 | TL | 18 | GTR | MPE; Rec and Dis (1) (After 36 mns and 38 mns, repectively) | 38 |
| 71 | Jatana [71]; 2008 | 11/M | 1 | N/A | N/A | GTR | MPE; Rec and Dis (1); NF-2 pt | N/A |
| 72 | Kabler [72]; 2008 | 16/M | 1 | L | N.M | GTR | MPE; No Rec or Dis | 1.5 |
| 73 | Cho [73]; 2009 | 16/M | 1 | C (Int) | N.M | Bx+RT (No response) thenGTR | Grrade III Ependymoma; No Rec or Dis. (After 6 mns follow-up had lost) | 6 |
| 74 | Al-Halabi [12]; 2010 | 15.4/5M: 2F | 7 | TL (1); L (5); LS (1) | 12 | GTR+RT (3); GTR (1); STR+RT (2); Bx then STR+RT after progression(1) | MPE (7); Rec after STR+RT (1); Rec+Dis (2): after GTR+RT; presentation with cranial and spinal ependymomas (2) | 78 |
| 75 | Benesch [4]; 2010 | 13.6 [median]/12M: 17F | 29 | CM (1); C (3); T (2); LS (6); 2 regions (14); >2 regions (3) | 2 [Median] | GTR+RT+ChT (3); GTR+RT (5); GTR (9); STR+RT+ChT (2); STR+RT (2); STR+ChT (2); STR (3); Bx+RT+ChT (2); Bx+RT (1) | MPE (6), grade II (17), grade III (6); 1 pt grade III died PO65.mn; progressive disease or relapse: 2 GTR (both are MPEs) and 5 less than GTR (2: MPEs, 2: typical and 1: anaplastic ependymomas) | 50.4 [Median] |
| 76 | Dulai [74]; 2010 | 8/F | 1 | T (Int) | N.M | STR then GTR | MPE; Rec (PO 2 yrs)+Dis (PO 5 yrs) | 108 |
| 77 | Kaner [75]; 2010 | 17/F | 1 | CT (1) | 23.2 | GTR | Classic Ependymoma; No Rec or Dis | 14 |
| 78 | Moon [76]; 2010 | 11/M | 1 | Multiple (L+S) | 4 | GTR+RT | Classic Ependymoma; No Rec or Dis | 36 |
| 79 | Boström [77], 2011 | 12/M | 1 | TL | N.M | GTR+RT | Anaplastic ependymoma; No Rec or Dis | 30 |
| 80 | Chakraborti [78], 2012 | 0.92/F | 1 | SC | 1 | GTR | MPE with anaplastic component; Rec and Dis after 6 weeks | 13.5 |
| 81 | Choi [79]; 2012 | 13.7/1M: 2F | 3 | L (1); LS (1); T2-S1 (1) | 8.5 | GTR (1); STR+RT (1); Bx+RT (1) | MPE (3); No Rec; Dis (1): Bx+RT | 13 |
| 82 | Stephen [80]; 2012 | 13/11M: 6F | 17 | C (1); C+T (1); T (3); L (9): [FT (5)+CE (1), Int (3)]; LS (2); S (1) | 9.5 | GTR+RT (5); GTR+Proton (1); GTR (9); STR+RT (2) | MPE (7), ependyomoma (9), anaplastic (1); Rec (4): all are MPEs (Rec after an average of 37.8 mns). 1 pt had NF Type 2 (Rec in primary site). | 58.8 (4–203) |
| 83 | Agbahiwe [21]; 2013 | 14.3/9M | 9 | TL (2); L (4); LS (2); S (1) | N.M | GTR+RT (1); GTR (4); STR+RT (3); STR (1) | MPE (9); Dis in GTR+RT (1), and 2 Rec in GTR and STR | 87 [Median] (9–317) |
| 84 | Becco de Souza [81]; 2013 | 13/M | 1 | L | 3 | GTR | MPE; Rec/Dis (No) | 2 |
| 85 | Liu [5]; 2013 | 11.5/3M: 1F | 4 | C (3); CT(1) | 2.7 | GTR+RT+CT (2); GTR (1); STR (1) | Anaplastic (4); Rec in 1 STR (dead after 23 mns) | 50.7 for 3 pts, 4th (N/A) |
| 86 | Pedziwiatr [82]; 2013 | 13.3/15M: 13F | 28 | L (CE) (17); Other spinal (8); Multiple (3) | 3–60 | GTR+RT (18); GTR (2); STR+RT (2); Partial resection+RT (4); Bx+RT (2).Out of them 3 recieved ChT | MPE (13), Typical ependymoma (12), Anaplastic ependymoma (3); DOD (5): Rec (2), Rec+Dis (3): Out of them 2 anaplastic; DOC (1) after GTR | 104 [Median] 36–300 |
| 87 | Cimino[83]; 2014 | 10.3/6M: 5F | 11 | Extradural (7): [SC (5)+ Lower back (1)+ Pelvis (1)]; L (2); LS (2) | N.M | GTR (11) | MPE (11); Rec in 2 Lumbar tms followed-up for 5 and 10 years; No Dis | N.M |
| 88 | Khalatbari [84]; 2014 | 15/M | 1 | TL | Acute | GTR | MPE; Rec/Dis (No) | 48 |
| 89 | Lundar [85]; 2014 | 10.9/7M: 3F | 10 | TL (1); L (7); LS (2) | N.M | GTR (6); STR+RT (4) | MPE (6), Ependymoma grade II (2), grade III (2); Rec (1): MPE/GTR; Dis (1): MPE/STR+RT; Rec+Dis (3): 1 MPE/STR +RT, 1 Ep gr II/GTR and 1 Ep gr III/GTR | 268 |
| 90 | Pencovich [86]; 2014 | 15.5/5M: 1F | 6 | C (1); T+TL (1); T+L+LS (1); L+S (3) | 10 | GTR (2); STR+RT (2); STR (2) | MPE (5); Ependymoma grade II (1); All pts presented with regional metastasis (Multiple); No Rec or Dis | 55 |
| 91 | Lin [87]; 2015 | 11.9/30M: 34F | 64 | Spinal cord (62); CE (2) | N.M | GTR+RT (4); GTR (17); STR+RT (11); STR (5); No resection [only RT] (23); Unknown (4) | Grade II ependymopma (64); No details about Rec or Diss. [The patients were reported here thought to be duplicated in previous published studies] | 110.4 [Median] |
| 92 | Current study; 2018 | 13.3/2M: 1F | 3 | T (1); L (2) | 9.1 | GTR (2); NTR (1) | MPE (2) and Classic ependymoma (1); Dis (1): GTR (MPE) | 51.3 |
AWD – alive with disease; Bx – biopsy; C – cervical; CE – Cauda equine; ChT – chemotherapy; CM – cervicomedullary; CT – cervicothoracic; Dis – neuroaxis dissemination; DOC – dead of other causes; DOD – dead of disease; dys – days; F – Female; FT – filum terminale; GTR – gross total resection; L – lumbar; LS – lumbosacral; M – male; mns – months; N/A – not available; NED – no evidence of disease; NF – neurofibromatosis; N.M – not mentioned; No – number; NOS – non otherwised specifised; PFS – progression-free survival; PO – postoperative; pts – patients; Rec – recurrence; Resection* – surgical resection borders had not been defined; RT – radiotherapy; S – sacral; SC – sacrococcygeal; STR – subtotal resection; T – thoracic; TL – thoracolumbar; wks – weeks; yrs – years.
Chan et al.
[34] series contain 14 pediatric ependymoma patients, while only details of 7 patients of childhood MPE were reported.
Constantini et al.
[9] reported 164 patients 21 years of age and younger in whom intradural-intramedullary tumors were resected. Their study is not specific for ependymomas.
We could not reach to the original studies, the data included in this table about the studies, which were indicated with (#), were obtained from Gagliardi et al.’s study [24].
Lonjon et al.
[54] series contain 32 pediatric ependymoma patients, while only details of 20 patients of childhood ependymomas were reported, 12 MPEs were excluded.
The data of these studies were obtained from Kramer et al. study (1988) [25].
The data of these studies were obtained from Aktug et al. study (2000) [27].
Discussion
The occurrence of ependymomas is very rare in children younger than 10 years of age [9,10]. A review of the literature reveals that the most commonly reported primary SPEs are MPEs (WHO grade I) followed by classic/typical ependymomas (WHO grade II) and anaplastic ependymomas (WHO grade III). Out of 441 SPE cases, 197 MPE were reported in the literature (included all cases with sufficient details between 1945 and 2016). Anaplastic ependymomas are extremely rare in pediatric patients (only 24 cases were reported). In 1932, Kernohan [11] described MPE as a separate histopathological subgroup of ependymoma, and since then several case and serial reports have been reported in the pediatric population (Table 5). Kernohan found that areas of mucinous degeneration within the vascular connective tissue cores of papillary tumors helped to distinguish MPE from other subgroups of ependymoma [11]. Ependymomas may occur intra- or extramedullary. In adults, intramedullary ependymomas are most commonly observed in the cervical spine but there is no such data on pediatric patients. Lumbar spine ependymomas mostly consist of those originating from the filum terminale (FT) or cauda equine (CE). MPEs are common in the lumbar region and are extramedullary tumors. However, ependymomas grade II and grade III are most common in the cervical region followed by the thoracic region. In the present study, 3 cases (included the drop-seeding metastasis) were located in the lumbosacral region and all of their histopathological examinations showed that they were WHO grade I (MPEs). Only 1 case was histopathologically diagnosed as classic ependymoma WHO grade II and was located in the thoracic spine and coexisted with the syrinx cavity.
Mork and Loken published one of the first reported series of central nervous system ependymomas, which included ependymoma cases in Norway over a 22-year period from 1953 to 1974. They found few spinal cord ependymomas in children; of the 53 spinal ependymomas, only 5 patients (2 boys and 3 females, aged 3–19 years old) were reported [10]. The male preponderance in SPEs is well-established in all previously published reports [5,6,12–19]. The male predominance is demonstrated by the fact that 66.7% (n=2) of our patients were males, in line with the previous reports.
In our series, presentation symptoms of spinal primary ependymomas differed between children and adults (Table 4). In children, the most common presenting symptoms were lower-limb numbness and ataxic gait, which were seen in 3 of 4 cases (including the drop-seeding metastasis case). However, in adult patients, radicular and local pain were the most common presenting symptoms. Al-Halabi et al. [12] reported that the most common presenting symptom was low back pain, which was reportedin up to 100% of patients, followed by urination symptoms (reported in 3 out of 7 patients) and lower-limb weakness (reported in 2 patients). The mean prodrome period was shorter in child than in adults (9.1±10.5 and 25.0±43 months, respectively) [P=0.006]. It appears that the good surgical resection outcomes in our series were related to the short prodrome in pediatric patients. Logically, symptoms in children appear early because the space in the thecal sac is limited.
Maximal resection of the lesion for GTR to preserve neurological function is the optimal treatment for all spinal tumors such as schwannomas and ependymomas. The consensus among pediatric neurosurgeons that GTR of SPEs is the choice of treatment regardless of location, age, and WHO grade of the tumor. The extent of resection depends on the preservation of the neurological functions; therefore, especially in the pediatric population, IONM will guide neurosurgeons to maximal borders up to GTR. Nowadays, the adverse effects of radio- and chemotherapy are well known. To avoid such adverse effects, the general view is that early RT should be avoided in children with WHO grade II spinal ependymomas, irrespective of the extent of resection, but is indicated in anaplastic (WHO grade III) spinal ependymomas after STR, NTR, or GTR [9]. GTR (i.e., resection of the tumor until reaching a neural structure with absence of any residual tumor on early postoperative imaging) for intradural-intramedullary spinal ependymomas is difficult, especially for tumors coexisting with syrinx cavities. In these conditions, we recommend subtotal (STR) or NTR (i.e., decompressing the neural structures next to the tumor and excising more than 75% for STR or 95% for NTR of tumor mass, leaving parts of the tumor attached to the neural tissues or their neurovascular structures). A systematic review by Feldman et al. [20] suggested that STR with RT in children may be associated with better outcomes than is STR with RT in adults. In the present series, GTR was achieved in 3 cases (included the drop-seeding metastasis case), while NTR was achieved in 1 female patient who had syrinx coexisting with thoracic spine ependymoma.
Although almost all SPEs are histopathologically benign tumors, WHO grade I and II primary spinal ependymomas have high local recurrence and neuroaxis dissemination rates, in particular MPEs, even though they are benign tumors classified as WHO grade I. Therefore, in management of SPEs, use of RT, which has as definite impact on progression of these tumors as proven in larger series [12,19,21,22], is still controversial. Al-Halabi et al. suggested GTR alone provided suboptimal disease control in MPE, while RT resulted in control of residual, metastatic, and/or recurrent pediatric MPEs [12]. Akyurek et al. suggested that MPE patients who are less than 35 years old are at higher risk of local recurrence and distant metastasis compared counterparts older than 35 years [22]. Two studies concluded there is no significant influence on the extent of resection to control local recurrence or distant metastasis [22,23]. However, both studies reported serious postoperative RT complications. In our patients, no adjuvant treatment was received. At 6.3 years after the first operation, 1 patient presented with drop-seeding metastasis. Thus, 45- and 51-month progression-free survival rates were 100% and 75%, respectively, while the 4-year survival rate was 100%. No recurrence, morbidity, or mortality were recorded. All our pediatric patients attended a normal schooling system.
Surgical morbidity is a serious complication in SPEs. Surgical morbidity was reported in most relevant studies; for example, Constantini et al. [9] reported spinal deformity is a common and serious complication associated with surgical treatment of spinal cord tumors. Although our series was relatively small and we cannot make generalization, we did not have any serious complications in our pediatric patients. Our female patient had the same transit glob (retention of urination); however, she improved, as shown in Table 3. Our review of the literature found that several studies suggested age, histology, previous surgery, tumor size, location, tumor consistency, and the extent of resection are not correlated with surgical morbidity, while removing 2 or more laminae can lead to spinal deformity, especially in children [8,9].
Our data support the evolving literature which suggests that no accepted molecular prognostic factors that can be used to predict surgical outcome for spinal ependymomas. Age, histology grade, and the MIB-1 index, a marker of cell proliferation, do not correlate with outcome in patients with primary spinal ependymomas; however, spinal ependymomas in children may have better prognosis compared to their adult counterparts. This may be related to the short prodrome in children. Distant metastasis and local recurrence have poor prognosis.
Conclusions
GTR or NTR are sufficient to treat spinal ependymomas in children without needing adjuvant radio- or chemotherapy. Close patient follow-up, neurological assessment, and yearly craniospinal MRI are necessary to prevent serious complications in children. Timely treatment for local recurrence or distant metastasis may increase patient quality of life.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Source of support: Departmental sources
References
- 1.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: A surveillance, epidemiology, and end results study. J Neurosurg. 2009;110:725–29. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- 2.Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173–79. doi: 10.1007/s11060-007-9507-z. [DOI] [PubMed] [Google Scholar]
- 3.Celli P, Cervoni L, Cantore G. Ependymoma of the filum terminale: Treatment and prognostic factors in a series of 28 cases. Acta Neurochir (Wien) 1993;124:99–103. doi: 10.1007/BF01401130. [DOI] [PubMed] [Google Scholar]
- 4.Benesch M, Weber-Mzell D, Gerber NU, et al. Ependymoma of the spinal cord in children and adolescents: A retrospective series from the HIT database. J Neurosurg Pediatr. 2010;6:137–44. doi: 10.3171/2010.5.PEDS09553. [DOI] [PubMed] [Google Scholar]
- 5.Liu XD, Sun B, Xu QW, et al. Outcomes in treatment for primary spinal anaplastic ependymomas: a retrospective series of 20 patients. J Neurosurg Spine. 2013;19:3–11. doi: 10.3171/2013.3.SPINE12183. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MS. Myxopapillary ependymomas presenting in the soft tissue over the sacrococcygeal region. Cancer. 1966;19:585–90. doi: 10.1002/1097-0142(196604)19:4<585::aid-cncr2820190418>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Bagley CA, Kothbauer KF, Wilson S, et al. Resection of myxopapillary ependymoma in children. J Neurosurg. 2007;106(4 Suppl):261–67. doi: 10.3171/ped.2007.106.4.261. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah A, Emel E, Abdallah BG, et al. Factors affecting the surgical outcomes of tethered cord syndrome in adults: a retrospective study. Neurosurg Rev. 2018;41(2):229–39. doi: 10.1007/s10143-017-0842-z. [DOI] [PubMed] [Google Scholar]
- 9.Constantini S, Miller DC, Allen JC, et al. Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;2(93):183–93. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 10.Mork SJ, Loken AC. Ependymoma: A follow-up study of 101 cases. Cancer. 1977;40:907–15. doi: 10.1002/1097-0142(197708)40:2<907::aid-cncr2820400247>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Kernohan JW. Primary tumors of the spinal cord and intradural filum terminale. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. Vol. 3. New York: Paul B. Hoeber; 1932. pp. 993–1025. [Google Scholar]
- 12.Al-Halabi H, Montes JL, Atkinson J, et al. Adjuvant radiotherapy in the treatment of pediatric myxopapillary ependymomas. Pediatr Blood Cancer. 2010;55:639–43. doi: 10.1002/pbc.22614. [DOI] [PubMed] [Google Scholar]
- 13.Chinn DM, Donaldson SS, Dahl GV, et al. Management of children with metastatic spinal myxopapillary ependymoma using craniospinal irradiation. Med Pediatr Oncol. 2000;35:443–45. doi: 10.1002/1096-911x(20001001)35:4<443::aid-mpo13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Fassett DR, Pingree J, Kestle JR. The high incidence of tumor dissemination in myxopapillary ependymoma in pediatric patients: Report of five cases and review of the literature. J Neurosurg. 2005;102(1 Suppl):59–64. doi: 10.3171/ped.2005.102.1.0059. [DOI] [PubMed] [Google Scholar]
- 15.Hallacq P, Labrousse F, Streichenberger N, et al. Bifocal myxopapillary ependymoma of the terminal filum: the end of a spectrum? Case report. J Neurosurg. 2003;98(3 Suppl):288–89. doi: 10.3171/spi.2003.98.3.0288. [DOI] [PubMed] [Google Scholar]
- 16.Ross DA, McKeever PE, Sandler HM, Muraszko KM. Myxopapillary ependymoma. Results of nucleolar organizing region staining. Cancer. 1993;71:3114–18. doi: 10.1002/1097-0142(19930515)71:10<3114::aid-cncr2820711036>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Sloof JL, Kernohan JW, MacCarthy CS. Primary intramedullary tumors of the spinal cord and filum terminale. Philadelphia, PA: Saunders; 1964. pp. 63–251. [Google Scholar]
- 18.Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56:883–93. doi: 10.1002/1097-0142(19850815)56:4<883::aid-cncr2820560431>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Weber DC, Miller R, Villa S, et al. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: A retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys. 2008;72(Suppl 1):S204. doi: 10.1016/j.ijrobp.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Feldman WB, Clark AJ, Safaee M, et al. Tumor control after surgery for spinal myxopapillary ependymomas: Distinct outcomes in adults versus children (A systematic review) J Neurosurg Spine. 2013;19:471–76. doi: 10.3171/2013.6.SPINE12927. [DOI] [PubMed] [Google Scholar]
- 21.Agbahiwe HC, Wharam M, Batra S, et al. Management of pediatric myxopapillary ependymoma: The role of adjuvant radiation. Int J Radiat Oncol Biol Phys. 2013;85(2):421–27. doi: 10.1016/j.ijrobp.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akyurek S, Chang EL, Yu TK, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006;80:177–83. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 23.Waldron JN, Laperriere NJ, Jaakkimainen L, et al. Spinal cord ependymomas: A retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys. 1993;27:223–29. doi: 10.1016/0360-3016(93)90231-j. [DOI] [PubMed] [Google Scholar]
- 24.Gagliardi FM, Cervoni L, Domenicucci M, et al. Ependymomas of the filum terminale in childhood: report of four cases and review of literature. Childs Nerv Syst. 1993;9:3–6. doi: 10.1007/BF00301925. [DOI] [PubMed] [Google Scholar]
- 25.Kramer GWPM, Rutten E, Sloof J. Subcutaneous sacrococcygeal ependymoma with inguinal lymph node metastasis. J Neurosurg. 1988;68:474–77. doi: 10.3171/jns.1988.68.3.0474. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein LJ, Logan WJ. Extraneural metastases in ependymoma of the cauda equina. J Neurol Neurosurg Psychiatry. 1970;33:763–70. doi: 10.1136/jnnp.33.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aktug T, Hakguder G, Sarioglu S, et al. Sacrococcygeal extraspinal ependymomas: The role of coccygectomy. J Pediatr Surg. 2000;35:515–18. doi: 10.1016/s0022-3468(00)90228-8. [DOI] [PubMed] [Google Scholar]
- 28.Payne NS, 2nd, McDonald JV. Rupture of spinal cord ependymoma. Case report. J Neurosurg. 1973;39:662–65. doi: 10.3171/jns.1973.39.5.0662. [DOI] [PubMed] [Google Scholar]
- 29.Scott M. Infiltrating ependymomas of the cauda equina. Treatment by conservative surgery plus radiotherapy. J Neurosurg. 1974;41:446–48. doi: 10.3171/jns.1974.41.4.0446. [DOI] [PubMed] [Google Scholar]
- 30.Cameron MM. Surgical management of multiple neuraxial ependymomas. Case report. Eur Neurol. 1976;14:365–69. doi: 10.1159/000114760. [DOI] [PubMed] [Google Scholar]
- 31.Mavroudis C, Townsend JJ, Wilson CB. A metastasizing ependymoma of the cauda equina. Case report. J Neurosurg. 1977;47:771–75. doi: 10.3171/jns.1977.47.5.0771. [DOI] [PubMed] [Google Scholar]
- 32.M rk SJ, Risberg G, Krogness K. Anaplastic ependymoma of the spinal cord. Neuropathol Appl Neurobiol. 1980;6:307–11. doi: 10.1111/j.1365-2990.1980.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 33.Morris DM, Steinert HR, Wiernik PH. Ineffectiveness of chemotherapy in patients with metastatic ependymoma of the cauda equina. J Surg Oncol. 1983;22:33–36. doi: 10.1002/jso.2930220109. [DOI] [PubMed] [Google Scholar]
- 34.Chan HS, Becker LE, Hoffman HJ, et al. Myxopapillary ependymoma of the filum terminale and cauda equina in childhood. Neurosurgery. 1984;14:204–10. doi: 10.1227/00006123-198402000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Helwig EB, Stern JB. Subcutaneous sacrococcygeal myxopapillary ependymoma. A clinicopathologic study of 32 cases. Am J Clin Pathol. 1984;81:156–61. doi: 10.1093/ajcp/81.2.156. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo K, Kumagai K, Kawai K, Tsuchiyama H. Subcutaneous sacrococcygeal myxopapillary ependymoma. A case report and a review of the literature. Acta Pathol Japan. 1985;35(4):925–31. doi: 10.1111/j.1440-1827.1985.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 37.West CR, Bruce DA, Duffner PK. Ependymomas. Factors in clinical and diagnostic staging. Cancer. 1985;56:1812–16. doi: 10.1002/1097-0142(19851001)56:7+<1812::aid-cncr2820561319>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Ciraldo AV, Platt MS, Agamanolis DP, Boeckman CR. Sacrococcygeal myxopapillary ependymomas and ependymal rests in infants and children. J Pediatr Surg. 1986;21:49–52. doi: 10.1016/s0022-3468(86)80653-4. [DOI] [PubMed] [Google Scholar]
- 39.Di Marco A, Griso C, Pradella R, et al. Postoperative management of primary spinal cord ependymomas. Acta Oncol. 1988;27:371–75. doi: 10.3109/02841868809093557. [DOI] [PubMed] [Google Scholar]
- 40.Pulitzer DR, Martin PC, Collins PC, Ralph DR. Subcutaneous sacrococcygeal (myxopapillary) ependymal rests. Am J Surg Pathol. 1988;12(9):672–77. doi: 10.1097/00000478-198809000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Naidu MR, Dinakar I. Intramedullary mass lesions of the spinal cord. Clin Neurol Neurosurg. 1989;91:135–38. doi: 10.1016/s0303-8467(89)80034-4. [DOI] [PubMed] [Google Scholar]
- 42.Fujiyama K, Kishikawa M, Fujii H, et al. Anaplastic ependymoma of the spinal cord in childhood. A case report. Acta Pathol Jpn. 1990;40:376–82. doi: 10.1111/j.1440-1827.1990.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 43.Le’Marchadour F, Pasquier B. Subcutaneous sacrococcygeal ependymoma with incidental glomus coccygeum. Histopathology. 1991;18:570–72. doi: 10.1111/j.1365-2559.1991.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 44.Wen BC, Hussey DH, Hitchon PW, et al. The role of radiation therapy in the management of ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys. 1991;20:781–86. doi: 10.1016/0360-3016(91)90023-w. [DOI] [PubMed] [Google Scholar]
- 45.Gupta RK, Pratap D. Metastasising congenital subcutaneous sacrococcygeal ependymoma. Indian J Cancer. 1992;29:76–81. [PubMed] [Google Scholar]
- 46.Serour F, Gorenstein A, Finci Y, Zaidel L. Subcutaneous sacrococcygeal myxopapillary ependymoma. Ped Surg Int. 1993;8:362–65. [Google Scholar]
- 47.Clover LL, Hazuka MB, Kinzie JJ. Spinal cord ependymomas treated with surgery and radiation therapy. A review of 11 cases. Am J Clin Oncol. 1993;16:350–53. doi: 10.1097/00000421-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Lunardi P, Licastro G, Missori P, et al. Management of intramedullary tumors in children. Acta Neurochir (Wien) 1993;120:59–65. doi: 10.1007/BF02001471. [DOI] [PubMed] [Google Scholar]
- 49.Botti G, Gravina A, Cremona F, et al. Subcutaneous sacrococcygeal myxopapillary ependymoma. A case report. Eur J Cancer. 1994;30A(4):570–71. doi: 10.1016/0959-8049(94)90451-0. [DOI] [PubMed] [Google Scholar]
- 50.Do-Dai DD, Rovira MJ, Ho VB, Gomez RR. Childhood onset of myxopapillary ependymomatosis: MR features. Am J Neuroradiol. 1995;16(4 Suppl):835–39. [PMC free article] [PubMed] [Google Scholar]
- 51.Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293–95. doi: 10.1002/(sici)1096-911x(199710)29:4<293::aid-mpo10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Nagib MG, O’Fallon MT. Myxopapillary ependymoma of the conus medullaris and filum terminale in the pediatric age group. Pediatr Neurosurg. 1997;26:2–7. doi: 10.1159/000121154. [DOI] [PubMed] [Google Scholar]
- 53.Ilhan J, Berberoglu S, Kutluay L, Maden HA. Subcutaneous myxopapillary ependymoma. Med Pediatr Oncol. 1998;30:81–84. doi: 10.1002/(sici)1096-911x(199802)30:2<81::aid-mpo2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 54.Lonjon M, Goh KY, Epstein FJ. Intramedullary spinal cord ependymomas in children: treatment, results and follow-up. Pediatr Neurosurg. 1998;29:178–83. doi: 10.1159/000028718. [DOI] [PubMed] [Google Scholar]
- 55.Graf M, Blaeker H, Otto HF. Extraneural metastasizing ependymoma of the spinal cord. Pathol Oncol Res. 1999;5:56–60. doi: 10.1053/paor.1999.0056. [DOI] [PubMed] [Google Scholar]
- 56.Johnson JM, Jessurun J, Leonard A. Sacrococcygeal ependymoma: Case report and review of the literature. J Pediatr Surg. 1999;34:1405–7. doi: 10.1016/s0022-3468(99)90020-9. [DOI] [PubMed] [Google Scholar]
- 57.Ohata K, Takami T, Gotou T, et al. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir (Wien) 1999;141:341–47. doi: 10.1007/s007010050309. [DOI] [PubMed] [Google Scholar]
- 58.Merchant TE, Kiehna EN, Thompson SJ, et al. Pediatric low-grade and ependymal spinal cord tumors. Pediatr Neurosurg. 2000;32:30–36. doi: 10.1159/000028894. [DOI] [PubMed] [Google Scholar]
- 59.Nishio S, Morioka T, Fujii K, Inamura T, Fukui M. Spinal cord gliomas: Management and outcome with reference to adjuvant therapy. J Clin Neurosci. 2000;7:20–23. doi: 10.1054/jocn.1999.0128. [DOI] [PubMed] [Google Scholar]
- 60.Gelabert-Gonzalez M, Prieto-Gonzalez A, Abdulkadar-Nallib I, Cutrin-Prieto J. Double ependymoma of the filum terminale. Childs Nerv Syst. 2001;17:106–8. doi: 10.1007/s003810000369. [DOI] [PubMed] [Google Scholar]
- 61.Helseth E, Due-Tonnessen B, Lote K, et al. Ependymoma in children and young adults (0–19 years): Report of 25 consecutive cases. Childs Nerv Sys. 2001;17:24–30. doi: 10.1007/s003810000400. [DOI] [PubMed] [Google Scholar]
- 62.Hirose Y, Aldape K, Bollen A, et al. Chromosomal abnormalities subdivide ependymal tumors into clinically relevant groups. Am J Pathol. 2001;158:1137–43. doi: 10.1016/S0002-9440(10)64061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: Radical surgical resection and outcome. Neurosurgery. 2002;51:1162–72. doi: 10.1097/00006123-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Goto T, Ohata K, Takami T, et al. Prevention of postoperative posterior tethering of spinal cord after resection of ependymoma. J Neurosurg. 2003;99:181–87. doi: 10.3171/spi.2003.99.2.0181. [DOI] [PubMed] [Google Scholar]
- 65.Wolf NI, Harting I, Hartmann M, et al. Combination of caudal myxopapillary ependymoma and dermal sinus: A single shared embryologic lesion? Dev Med Child Neurol. 2003;45:568–70. doi: 10.1017/s0012162203001038. [DOI] [PubMed] [Google Scholar]
- 66.Peker S, Ozgen S, Ozek MM, Pamir MN. Surgical treatment of intramedullary spinal cord ependymomas: Can outcome be predicted by tumor parameters? J Spinal Disord Tech. 2004;17:516–21. doi: 10.1097/01.bsd.0000129585.91599.5c. [DOI] [PubMed] [Google Scholar]
- 67.Sebire NJ, Fowler D, Ramsay AD. Sacrococcygeal tumors in infancy and childhood: A retrospective histopathological review of 85 cases. Fetal Pediatr Pathol. 2004;23(5–6):295–303. doi: 10.1080/15227950490952424. [DOI] [PubMed] [Google Scholar]
- 68.Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71:205–10. doi: 10.1007/s11060-004-1386-y. [DOI] [PubMed] [Google Scholar]
- 69.Tubbs RS, Kelly DR, Mroczek-Musulman EC, Braune K, et al. Dwarfism, occult spinal dysraphism, and presacral myxopapillary ependymoma with an epidermoid cyst in a child. Acta Neurochir (Wien) 2005;147:299–302. doi: 10.1007/s00701-004-0469-z. [DOI] [PubMed] [Google Scholar]
- 70.Mridha AR, Sharma MC, Sarkar C, et al. Myxopapillary ependymoma of lumbosacral region with metastasis to both cerebellopontine angles: Report of a rare case. Childs Nerv Syst. 2007;23:1209–13. doi: 10.1007/s00381-007-0423-5. [DOI] [PubMed] [Google Scholar]
- 71.Jatana KR, Jacob A, Slone HW, et al. Spinal myxopapillary ependymoma metastatic to bilateral internal auditory canals. Ann Otol Rhinol Laryngol. 2008;117:98–102. doi: 10.1177/000348940811700204. [DOI] [PubMed] [Google Scholar]
- 72.Kabler HA, Syska BE, Springer BL, Singer JI. Ependymoma as a cause of low back pain in a young healthy athlete. Pediatr Emerg Care. 2008;24:685–87. doi: 10.1097/PEC.0b013e3181887e60. [DOI] [PubMed] [Google Scholar]
- 73.Cho JC, Miller A, Kettner NW. Cervical ependymoma in a male adolescent with neck and back pain. J Manip Physiol Ther. 2009;32:695–700. doi: 10.1016/j.jmpt.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 74.Dulai MS, Caccamo DV, Briley AL, et al. Intramedullary papillary ependymoma with choroid plexus differentiation and cerebrospinal fluid dissemination to the brain. J Neurosurg Pediatr. 2010;5:511–17. doi: 10.3171/2009.12.PEDS09130. [DOI] [PubMed] [Google Scholar]
- 75.Kaner T, Sasani M, Oktenoglu T, et al. Clinical analysis of 21 cases of spinal cord ependymoma: Positive clinical results of gross total resection. J Korean Neurosurg Soc. 2010;47:102–6. doi: 10.3340/jkns.2010.47.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moon K, Filis AK, Cohen AR. Mobile spinal ependymoma. J Neurosurg Pediatr. 2010;5:85–88. doi: 10.3171/2009.7.PEDS09244. [DOI] [PubMed] [Google Scholar]
- 77.Boström A, von Lehe M, Hartmann W, et al. Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery. 2011;68:302–9. doi: 10.1227/NEU.0b013e3182004c1e. [DOI] [PubMed] [Google Scholar]
- 78.Chakraborti S, Kini H, Pai KG, Upadhyaya V. Sacrococcygeal myxopapillary ependymoma with anaplastic ependymoma component in an infant. J Pediatr Neurosci. 2012;7:218–20. doi: 10.4103/1817-1745.106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi GH, Oh JK, Kim TY, et al. The clinical features and surgical outcomes of pediatric patients with primary spinal cord tumor. Childs Nerv Syst. 2012;28:897–904. doi: 10.1007/s00381-012-1718-8. [DOI] [PubMed] [Google Scholar]
- 80.Stephen JH, Sievert AJ, Madsen PJ, et al. Spinal cord ependymomas and myxopapillary ependymomas in the first 2 decades of life: A clinicopathological and immunohistochemical characterization of 19 cases. J Neurosurg Pediatr. 2012;9:646–53. doi: 10.3171/2012.2.PEDS11285. [DOI] [PubMed] [Google Scholar]
- 81.Becco de Souza R, Brasileiro de Aguiar G, Saade N, Esteves Veiga JC. Cauda equina syndrome caused by spontaneous bleeding in the filum terminale myxopapillary ependymoma: A rare pediatric case. Pediatr Neurosurg. 2013;48:385–88. doi: 10.1159/000354216. [DOI] [PubMed] [Google Scholar]
- 82.Pedziwiatr K, Skowronska-Gardas A, Chojnacka M. Spinal cord ependymoma in children – Results of postoperative radiotherapy. Radiother Oncol. 2013;106:181–85. doi: 10.1016/j.radonc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Cimino PJ, Agarwal A, Dehner LP. Myxopapillary ependymoma in children: a study of 11 cases and a comparison with the adult experience. Pediatr Blood Cancer. 2014;61:1969–71. doi: 10.1002/pbc.25125. [DOI] [PubMed] [Google Scholar]
- 84.Khalatbari MR, Moharamzad Y. Myxopapillary ependymoma of the conus medullaris presenting with intratumoral hemorrhage during weight lifting in a teenager. Childs Nerv Syst. 2014;30:181–83. doi: 10.1007/s00381-013-2218-1. [DOI] [PubMed] [Google Scholar]
- 85.Lundar T, Due-Tonnessen BJ, Scheie D, Brandal P. Pediatric spinal ependymomas: an unpredictable and puzzling disease. Long-term follow-up of a single consecutive institutional series of ten patients. Childs Nerv Syst. 2014;30:2083–88. doi: 10.1007/s00381-014-2491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pencovich N, Bot G, Lidar Z, et al. Spinal ependymoma with regionalmetastasisat presentation. Acta Neurochir (Wien) 2014;156(6):1215–22. doi: 10.1007/s00701-014-2048-2. [DOI] [PubMed] [Google Scholar]
- 87.Lin Y, Jea A, Melkonian SC, Lam S. Treatment of pediatric grade II spinal ependymomas: A population-based study. J Neurosurg Pediatr. 2015;15:243–49. doi: 10.3171/2014.9.PEDS1473. [DOI] [PubMed] [Google Scholar]