Abstract
Diabetic retinopathy (DR) is the leading cause of vision-loss among working-age adults. The interplay between hyperglycemia and endothelial activation in inducing endoplasmic reticulum (ER) stress pathways and visual deficits in DR is not fully understood. To address this, we employed a mouse model of chronic vascular activation using endothelial-specific TNF-α-expressing (tie2-TNF) mice to induce diabetes with Streptozotocin (STZ). At 4 weeks post STZ; a significant 2–10 fold increase in retinal neurovascular inflammatory gene transcript response in tie2-TNF mice was further increased in diabetic tie2-TNF mice. A decrease in visual acuity and scotopic b-wave amplitude in tie2-TNF mice was further accentuated in diabetic tie2-TNF mice and these changes correlated with a multi-fold increase in retinal ER stress markers and a reduction in adherens junctions. Cultured retinal endothelial cells showed a significant decrease in trans-endothelial resistance as well as VE-cadherin expression under TNF-α and high glucose stress. These changes were partly rescued by tauroursodeoxycholic acid (TUDCA), a potent ER stress inhibitor. Taken together, constant endothelial activation induced by TNF-α further exacerbated by hyperglycemia results in activation of ER stress and chronic pro-inflammation in a feed forward loop ultimately resulting in endothelial junction protein alterations leading to visual deficits in the retina. Inhibition of ER stress and endothelial activation may prove to be a novel therapeutic target in DR.
Keywords: Inflammation, TNF, ER stress, UPR, Hyperglycemia, TUDCA, ERG, OKN, DR
INTRODUCTION
Diabetic retinopathy (DR) is the most common vascular complication in patients with long-standing diabetes, and is the leading cause of blindness in working-age adults. [Duh et al., 2017]. Clinically, DR can be classified into non-proliferative DR (NPDR) and proliferative DR (PDR), with macular edema occurring at any stage of DR. The vascular endothelium is the main target of hyperglycemic damage and the common denominator of diabetic complications [Abcouwer and Gardner, 2014; Yang et al., 2016]. The current therapeutics of DR, including laser photocoagulation, steroid treatment and anti-vascular endothelial growth factor antibodies, are inherently destructive or partially successful in preserving the vision loss in PDR and are not useful in the treatment of early stages of DR [Cheung et al., 2010]. Thus, new treatment strategies that are preventative and/or can provide interventions earlier in diabetes to delay or prevent the progression of early NPDR are useful [Robinson et al., 2012]. Vascular dysfunction, a hallmark for both macular edema and DR, is propagated by risk factors such as hyperglycemia, oxidative stress and inflammation[Brownlee, 2005]. Within the context of inflammation, tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, induces inflammatory genes, facilitates leukocyte recruitment, and activates endothelial cells, thereby potentially contributing to DR pathology [Behl et al., 2008; Huang et al., 2011]. Clinically, elevated levels of pro-inflammatory cytokines interleukin-1β (IL-1β) and TNF-α have been detected in the vitreous humor as well as serum of diabetic patients with PDR [Demircan et al., 2006].
Emerging data suggest that one of the central elements in the activation of inflammatory pathways is endoplasmic reticulum (ER) stress[Schroder and Kaufman, 2005] in various cell types including in endothelial cells[Li et al., 2011]. Under physiological conditions, cells evoke the unfolded protein response (UPR) as an adaptive mechanism. The UPR includes at least the following three signaling pathways initiated by ER stress transducers:the inositol-requiring enzyme 1α (IRE1α), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6), which are bound to glucose regulated protein 78 (GRP78), a molecular chaperone. During ER stress, GRP78 gets dissociated from these transducers to activate the cellular response to unfolded proteins, which includes: (a) downregulation of protein translation, (b) enhanced expression of ER chaperone proteins that promote protein refolding, and (c) activation of proteases involved in the degradation of misfolded proteins[Schroder and Kaufman, 2005]. Under pathological conditions, deregulation of these pathways has been shown to increase ER stress, specifically in diabetes and its complications [Li et al., 2009].
To further investigate the relationship between endothelial activation and ER stress in diabetes, we used a mouse model that is transgenic for the transmembrane form of TNF-α (tmTNF) expression under tie2 promoter exclusively in endothelial cells. The constant presence of TNF-α in the endothelial cells leads to continuous activation of those cells [Rajashekhar et al., 2006] and chronic inflammation [Rajashekhar et al., 2007]. We tested the hypothesis that constant endothelial activation by TNF-α exacerbates hyperglycemia-induced activation of ER stress pathways in vivo, leading to neural (visual) deficits observed in NPDR. To test and postulate that ER stress plays an important role in the endothelial activation induced disruption of endothelial junction proteins, we administered tauroursodeoxycholic acid (TUDCA), a known ER stress inhibitor[Amin et al., 2012] to determine if it could inhibit the drop in retinal transendothelial resistance induced by TNF-α and high glucose in vitro. Our studies using tie2-TNF transgenic mice not only shed light on the potential role of TNF-α-induced activation of endothelial cells in important aspects of DR, but also on new potential treatment strategies to inhibit early NPDR.
MATERIALS AND METHODS
STZ-induced diabetes in tie2-TNF transgenic animals
Animal studies were approved by the Institutional Animal Care and Use Committee, UTHSC, Memphis following the guidelines per the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. In tie2-TNF transgenic animals the endothelial promoter tie2-driven overexpression of transmembrane TNF, through a mutation of its TNF-α converting enzyme (TACE) cleavage site, remains bound to endothelium [Willuweit et al., 2001], leading to constant endothelial activation and chronic inflammation[Rajashekhar et al., 2007; Rajashekhar et al., 2006]. The construction of transgene and generation of tie2-TNF transgenic animals was described previously [Willuweit et al., 2001], in which the cDNA of the uncleavable murine tmTNF-α mutant [mTNF-α 1–9, K(11)E] was cloned between the endothelial-specific tie2 promoter and the tie2 first intron to localize TNF-α specific to the endothelium. Mice used for this study had been backcrossed for more than eight generations in C57BL/6 animals. Animals were housed under a constant 12-hr light–dark cycle at a temperature between 21°C and 23°C and allowed free access to food and water. Six to eight week old mice were injected intraperitoneally with 35mg/kg STZ dissolved in sodium citrate buffer (0.01 M; pH 4.5) for 5 consecutive days to induce diabetes. In the following week, animals with blood glucose >250mg/dL were considered diabetic and used in the studies. Littermate, wild type age-matched C57BL/6 animals with and without diabetes served as controls. To avoid catabolic effects, diabetic mice received insulin injections once a week (Humulin, 1 unit/mouse) via subcutaneous route. Experiments proposed in this study were performed after 4 weeks of STZ injection.
Optokinetic reflex measurement
Awake mice were placed on a platform inside the OptoMotry virtual reality optokinetic reflex system to quantify the visual acuity and contrast sensitivity thresholds (OptoMotry, CerebralMechanics, Lethbride, Alberta, Canada) as described previously [Prusky et al., 2004]. A step-wise paradigm defined by OptoMotry software was used with the screens of contrasting bars of light not visible to the investigator and the investigator was blinded to the groups. Vision testing was carried out at 4 weeks after diabetes. Acuity testing was performed at 100% contrast with varying spatial frequency threshold (i.e. white versus black stripes), while contrast sensitivity testing was performed at fixed spatial frequency threshold (0.042 c/d).
Electroretinogram measurement
Following 4 weeks of diabetes, control and diabetic mice were dark adapted overnight and prepared for ERG recording under dim red light. Scotopic threshold ERG recordings were obtained using the Espion E2 ERG system (Diagnosys LLC, Lowell, MA). Animals were anesthetized with ketamine/ dexmedetomidine hydrochloride (0.5 mg/kg & 0.025 mg/kg SC respectively). Body temperatures of the mice were regulated with a heating pad at 37°C during recordings. Tropicamide (0.3%) was used to dilate eyes and 2.5% sterile hypromellose ophthalmic demulcent solution (AKORN) was applied. Electrodes were placed on the cornea of both eyes; the reference electrode was positioned in between the eyes and a ground electrode in the tail. Mice were presented with a different flash of increasing intensity (0.0025, 0.025, 0.25, 2.5, 25 cd.s.m2), each repeated five times, with an inter-stimulus interval ranging from 20 s for dim flashes to 1 min for the brightest flashes. Three to five ERG traces at each flash luminance were averaged to measure b-wave amplitude.
Gene expression analysis
Whole mouse retinal tissue was used to isolate RNA using NucleoSpin® RNA Plus kit (Macherey-Nagel GmbH, Takara Bio USA), following the manufacturers protocol. Subsequently, about 250 ng of total RNA from each tissue was converted to cDNA using SuperScript III first-strand synthesis supermix (ThermoFisher Scientific). The resulting cDNA sample served as a template for real time qPCR using TaqMan probes (Table 1) and accompanying Master Mix (Applied Biosystems, Foster City, CA). PCR amplification was carried out using Quantstudio3 (Applied Biosystems) with cycle conditions (initial cycle: 50°C for 2 min, initial denaturation 95°C for 15 sec, 40 cycles of denaturation 95°C for 15 sec, and annealing/extension of 60°C for 1 min). The expression levels of gene transcripts were determined using 2−DDCt and normalized to β-actin as described by us previously [Rajashekhar et al., 2005].
Table 1.
Gene transcript TaqMan Gene Expression Primer\Probes
| Genes | Assay ID | Reference Sequence |
Amplicon length |
|---|---|---|---|
| 18S ribosomal RNA (18s) | Mm04277571 | NR_003278 | 115 |
| heat shock protein 5 (GRP78) | Mm00517691 | NM_001163434 | 75 |
| eukaryotic translation initiation factor 2 alpha kinase 3 (PERK) | Mm00438700 | NM_010121 | 62 |
| endoplasmic reticulum (ER) to nucleus signaling 1 (IRE1α) | Mm00470233 | NM_023913 | 95 |
| activating transcription factor 6 (ATF6) | Mm01295319 | NM_001081304 | 74 |
| X-box binding protein 1 (XBP1) | Mm00457357 | NM_001271730 | 56 |
| DNA-damage inducible transcript 3 (CHOP) | Mm01135937 | NM_001290183 | 92 |
| intercellular adhesion molecule 1 (ICAM-1) | Mm01175876_g1 | NM_010493.2 | 94 |
| vascular cell adhesion molecule 1 (VCAM1) | Mm01320973_m1 | NM_011693.3 | 126 |
| integrin alpha M (Itgam) / CD11b | Mm01271262_m1 | NM_001082960.1 | 107 |
| chemokine (C-C motif) ligand 2 (CCL2) | Mm00441242_m1 | NM_011333.3 | 74 |
| laminin, alpha 5 (LAMA5) | Mm01222029_m1 | NM_001081171.2 | 64 |
| endothelin 2 (EDN2) | Mm00432983_m1 | NM_007902.2 | 74 |
| guanylate binding protein 2 (GBP2) | Mm00494576_g1 | NM_010260.1 | 77 |
| lectin, galactose binding, soluble 9 (LGALS9) | Mm00495295_m1 | NM_001159301.1 | 68 |
| tissue inhibitor of metalloproteinase 1 (TIMP1) | Mm01341361_m1 | NM_001044384.1 | 100 |
| cholesterol 25-hydroxylase (CH25H) | Mm00515486_s1 | NM_009890.1 | 126 |
| tumor necrosis factor alpha (TNF-α) | Mm00443258_m1 | NM_001278601.1 | 81 |
| tumor necrosis factor alpha receptor 1 (TNFR 1) | Mm00441883_g1 | NM_011609.4 | 82 |
| tumor necrosis factor alpha receptor 2 (TNFR 2)/Tnfrsf1b | Mm00441889_m1 | NM_011610.3 | 64 |
Protein expression analysis
Whole mouse retinal tissues were lysed using RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 0.2% sodium azide, 1% Triton X-100, 0.25% sodium deoxycholate, and 1× protease inhibitor]. Homogenate was further sonicated and incubated for 1 hr on ice and centrifuged at 16,000 g for 5 min at 4°C. The clear supernatant was collected, and quantified for total protein by the Bradford method. About 50µg of protein was resolved on a NuPAGE Bis-Tris pre-cast gels (ThermoScientific) and transferred to a nitrocellulose membrane. After blocking the membrane for an hour in 5% bovine serum albumin (BSA) in TBST and probed with appropriate primary antibodies (Table 2), this was followed by incubation with HRP-conjugated secondary antibodies. Detection was performed using an enhanced chemiluminescence kit (GE Healthcare). Targeted proteins were probed in separate blots. Mean densitometry data from independent experiments were calculated using Image-J (NIH, Bethesda, MD) software and represented as the ratio of target protein to β-tubulin or total protein of interest, where applicable.
Table 2.
Antibodies used in the study
| GRP-78 | Thermo Fisher Scientific Cat# PA1-014A, RRID:AB_559 |
| PERK | Thermo Fisher Scientific Cat# PA5-38811, RRID:AB_2555404 |
| pPERK | Thermo Fisher Scientific Cat# PA5-40294, RRID:AB_2576881 |
| IRE | Thermo Fisher Scientific Cat# PA5-20189, RRID:AB_11152827 |
| pIRE | Thermo Fisher Scientific Cat# PA1-16927, RRID:AB_2262241 |
| ATF6 | Thermo Fisher Scientific Cat# PA5-20215, RRID:AB_11156621 |
| sXBP | Thermo Fisher Scientific Cat# PA5-18940, RRID:AB_10982665 |
| CHOP | Thermo Fisher Scientific Cat# MA1-250, RRID:AB_2292611 |
| btubulin | Thermo Fisher Scientific Cat# MA5-16308, RRID:AB_2537819 |
| pVE-cadherin | Abcam Cat# ab119785, RRID:AB_10971838 |
| VE-cadherin | Santa Cruz Biotechnology Cat# sc-9989, RRID:AB_2077957 |
Retinal trans-endothelial electrical resistance in vitro
Measurements of trans-endothelial electrical resistance (TER) were performed using an electric cell-substrate impedance-sensing (ECIS) device (ECIS 1600R; Applied Biophysics, Troy, NY), as described by us previously [Rajashekhar et al., 2014b]. Human retinal endothelial cells (HREC; Cell Systems, Inc.) were seeded at a density of 5 × 105 cells/mL on gold electrodes (8W10E+; Applied Biophysics, Inc.) and grown for 16 hr until maximum resistance was attained (~1200 Ω). Cells were treated with high glucose media (HG; 30mM) and TNF-α (20ng/mL; Sino Biological Inc.) with and without the ER stress inhibitor TUDCA (20µM; Sigma), and changes in resistance were monitored for up to 18 hr. Tunicamycin (Sigma) was used at 2µM concentration to induce ER stress served as positive control. For neutralization experiments, TNF-α (20ng/ml) was incubated with TNF-α neutralizing antibody (5 µg/ml, clone MP6-XT3, BD Biosciences) for 1 hr and used in the experiments. Resistance values for multiple wells, at 4000 Hz, were normalized to an identical starting resistance value and averaged and presented as normalized resistance over time.
Immunocytochemistry
Immunocytochemistry for VE-cadherin in HREC was performed by a method as described by us previously with slight modifications [Shivanna et al., 2010]. Endothelial cells were grown on 10mm round coverslips placed in a 24-well plate until a confluent monolayer was formed. Cells were then treated with desired drug treatments, fixed with 4% paraformaldehyde for 15 min, and blocked with 10% BSA, 5% Normal goat serum, 0.5% triton ×100 for 1 hr. This was followed by staining for VE-cadherin (F-8 mouse monoclonal, Santa Cruz Biotech, 1: 200 dilution) for overnight at 4°C. This was followed by washing and incubation with secondary antibody (goat anti-mouse IgG Alexa Fluor 546, ThermoFisher Scientific, 1:500). For nuclear localization, cells were incubated with DAPI for 5 min. Cells without exposure to the primary antibody were used as controls for immunostaining. Stained cells on coverslips were mounted using aqueous mounting medium (Lab Vision PermaFlour, ThermoFisher Scientific) and were visualized using a Zeiss LSM 710 laser scanning confocal microscope with a 40× oil immersion objective.
Statistical Analysis
Results are expressed as mean ± SEM. For all quantitative experiments, statistical analyses were performed with a two way ANOVA with Newmann-Keuls posthoc test for multiple group comparisons (Prism 4 software; GraphPad Software, La Jolla, CA, USA) and a p-value < 0.05 was considered statistically significant.
RESULTS
Streptozotocin-induced diabetes in tie2-TNF transgenic mice
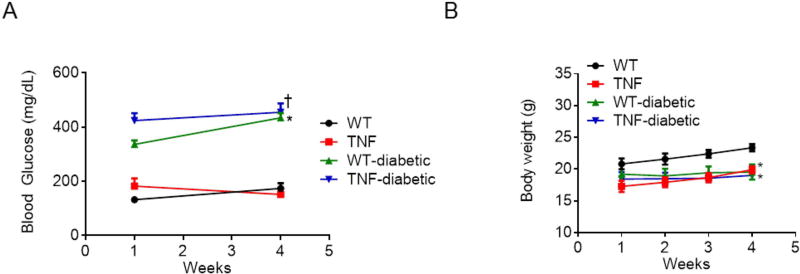
All mice treated with STZ developed sustained hyperglycemia when compared to nondiabetic WT mice. While blood glucose levels in tie2-TNF animals were 152 mg/dL, diabetic tie2-TNF and diabetic WT animals had significantly elevated levels (TNF-diabetic, 455±32 mg/dL; WT-diabetic, 434±18 mg/dL, p<0.05) as early as three days to a week after STZ, and remained >250 mg/dL until after four months of STZ injections (Fig. 1A). Diabetic mice in this study failed to gain weight at a normal rate compared to age matched non-diabetic controls (Fig. 1B).
Fig 1. Mice from diabetic groups had markedly higher blood glucose levels and lower body weights.
(A) Blood glucose concentration (mg/dL) in the study groups. (B) Animal body weights of the study groups. Data represent Mean ± SEM from n=3–5 animals. *p<0.05 compared to WT mice and † p<0.05 compared to tie2-TNF (TNF) mice.
tie2-TNF transgenic mice exhibit markers of retinal inflammation
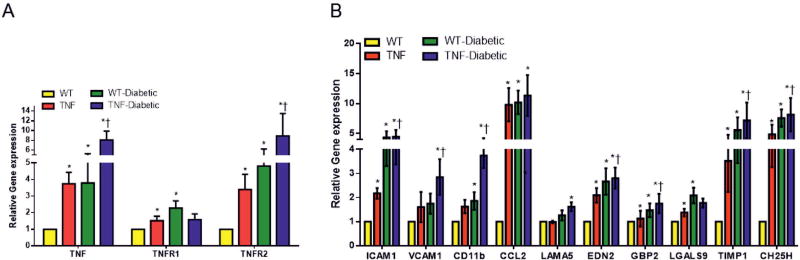
To establish endothelial TNF exhibit retinal inflammation as observed in early stage DR, we assessed retinal mRNA gene transcripts by quantitative real-time qPCR assay in all study groups. Since diabetes alone is known to upregulate TNF-α in mouse retina [Jiang et al., 2014], we first checked for TNF-α expression in tie2-TNF and diabetic tie2-TNF mice 4 weeks after STZ injection. As predicted, the TNF-α levels were significantly higher in tie2-TNF mice compared to WT mice; with a further two fold increase observed in diabetic tie2-TNF mice (Fig. 2A). As we have shown previously, the transmembrane form of TNF-α (tmTNF) elicits chronic pro-inflammatory signals via both TNF receptor 1 and receptor 2 (TNFR1 and TNFR2) [Rajashekhar et al., 2007]. Here we demonstrate a significant increase in both receptors in tie2-TNF mice with TNFR2 being further increased in diabetic tie2-TNF mice (Fig. 2A). Complementing this increase in TNF-α and TNF receptors, a significant increase in adhesion molecules including ICAM-1 as well as pro-inflammatory cytokines and biomarker panel genes implicated in DR research[Freeman et al., 2010; Freeman et al., 2009] including chemokine (C-C motif) ligand 2 (CCL2), laminin subunit alpha 5 (LAMA5), endothelin 2 (EDN2), guanylate binding protein 2 (GBP2), lectin galactose binding soluble 9 (LGALS9), tissue inhibitor of metalloproteinase 1 (TIMP1) and cholesterol 25-hydroxylase (CH25H) were significantly upregulated (>2 fold, p<0.05) in tie2-TNF retina compared to WT retina. Interestingly, vascular cell adhesion molecule-1 (VCAM1), integrin alpha M (CD11b), EDN2, GBP2, TIMP1, and CH25H were further increased in diabetic tie2-TNF mice (>2 fold, p<0.05) compared with tie2-TNF mice (Fig. 2B).
Fig 2. tie2-TNF transgenic mice and the diabetic tie2-TNF mice demonstrate an increased inflammatory gene expression.
(A) Assessment of TNFα and its receptor gene expression (B) markers of retinal inflammation by Taqman qPCR expressed as fold change normalized to internal control in the study groups. Data represent Mean ± SEM from n=5 animals. *p<0.05 compared to WT mice and † p<0.05 compared to tie2-TNF (TNF) mice.
Characterization of visual deficits in TNF transgenic mice
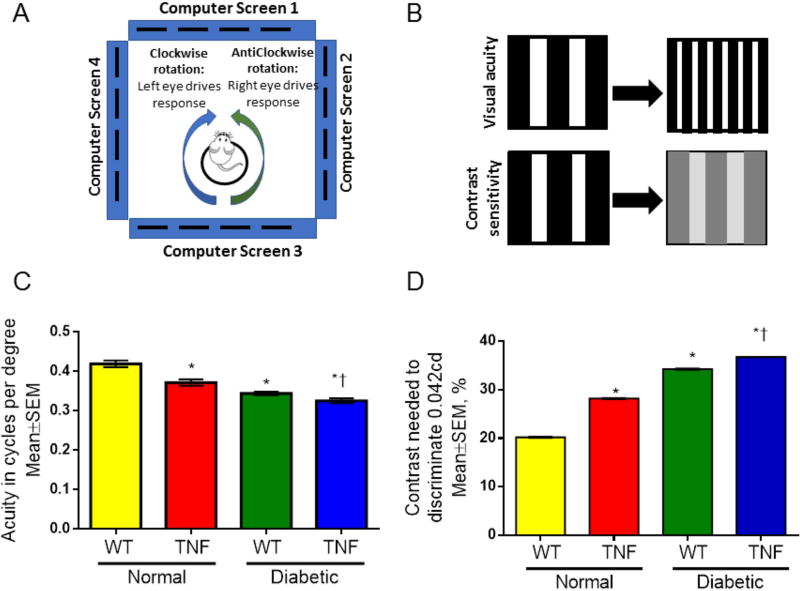
To better understand the correlation of endothelial activation to visual deficits, optokinetic measurements (OKN) were performed after 4 weeks of diabetes in all study groups. We assessed visual acuity (spatial frequency threshold) at high contrast (100% contrast) and contrast sensitivity at low spatial frequency threshold [0.042 cycles per degree (c/d), i.e., wide stripes] (Fig. 3). WT mice had a visual acuity of 0.42±0.008 c/d (both eyes combined). This is within the reported normal visual acuity range for a C57Bl/6 mouse using the same system[Prusky et al., 2004]. On the other hand, the visual acuity in tie2-TNF mice was significantly decreased when compared with age-matched WT mice (tie2-TNF, 0.37± 0.008; WT, 0.42±0.008 c/d, p<0.05). Interestingly, diabetic tie2-TNF mice demonstrated a further decrease in visual acuity when compared with the tie2-TNF group (tie2-TNF-diabetic, 0.33±0.006; tie2-TNF, 0.37± 0.008 c/d, p<0.05). This decrease in visual acuity is also seen in WT-diabetic animals (WT-diabetic, 0.34± 0.004; WT, 0.42±0.008 c/d, p<0.05). In corroboration with a decrease in visual acuity, contrast sensitivity was significantly increased in tie2-TNF mice compared to WT mice (TNF, 28.2± 0.016; WT, 20.2±0.014 %, p<0.05) with a further increase in both tie2-TNF and WT diabetic mice (tie2-TNF-diabetic, 36.7±0.004; WT-diabetic, 34.2±0.03 %, p<0.05).
Fig 3. tie2-TNF transgenic mice and the diabetic tie2-TNF mice show significant loss of visual acuity and increase in requirement for contrast sensitivity.
(A). A schematic representation of visual acuity and contrast sensitivity measurements by observing the optomotor responses of mice to rotating sinusoidal gratings (OptoMotry). Mice reflexively respond to rotating vertical gratings by moving their head in the direction of grating rotation. The protocol used yields independent measures of the acuities of right and left eyes based on the unequal sensitivities of the two eyes to pattern rotation: right and left eyes are most sensitive to counter-clockwise and clockwise rotations, respectively. (B). Visual acuity was measured by presenting black and white bars of varying spatial frequencies at 100% contrast and the contrast sensitivity was measured by changing the gradient that generates tracking at a fixed spatial frequency of 0.042 c/d (C). Visual acuity in mice expressed as cycles per degree (D). Contrast sensitivity in mice expressed as percentage. Data represent Mean ± SEM from n=5 animals. *p<0.05 compared to WT mice and † p<0.05 compared to tie2-TNF (TNF) mice.
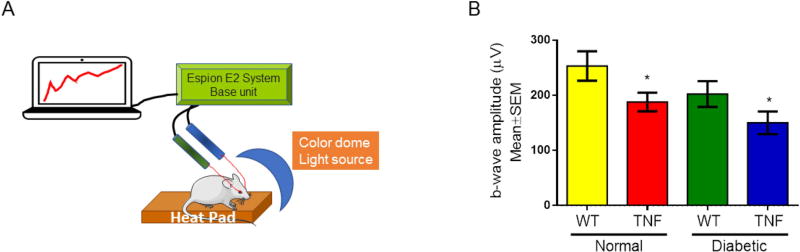
Next we examined the electrophysiological changes generated by neuronal and non-neuronal cells in the mouse retina as a result of chronic inflammation and STZ-induced diabetes. Dark-adapted scotopic ERG responses were recorded from both eyes of WT and tie2-TNF transgenic mice with and without 4 weeks of diabetes (Fig. 4). When compared with age-matched WT mice, tie2-TNF mice demonstrated a substantial decrease in the b-wave amplitude in the scotopic range with the data being significant at the 25cd.s.m2 flash intensity (tie2-TNF, 150±21; WT, 253±27 µV, p<0.05). This decrease was further exacerbated when the mice were made diabetic with diabetic tie2-TNF mice demonstrating the worst amplitudes (tie2-TNF-diabetic, 188±17; tie2-TNF, 150±21 µV, p>0.05). However, diabetic tie2-TNF mice did not show a statistically significant decrease in ERG response compared with normal tie2-TNF mice, despite the reduction in b-wave amplitudes in these mice in comparison to age-matched WT mice with diabetes.
Fig 4. tie2-TNF transgenic mice and the diabetic tie2-TNF mice show altered b-wave amplitudes.
(A) Schematic diagram of ERG in anesthetized mice. A color dome with Xenon lamp delivers the flash intensity, while the b-wave amplitudes are recorded through a differential amplifier connected to both eyes via platinum ring corneal electrodes, a reference electrode inserted subcutaneously into the forehead and a ground electrode into the tail. A computer driven software records the data. (B) b-wave amplitude measurement in mice expressed as µV. Data represent Mean ± SEM from n=5 animals. *p<0.05 compared to WT mice.
ER stress activation in TNF transgenic mice
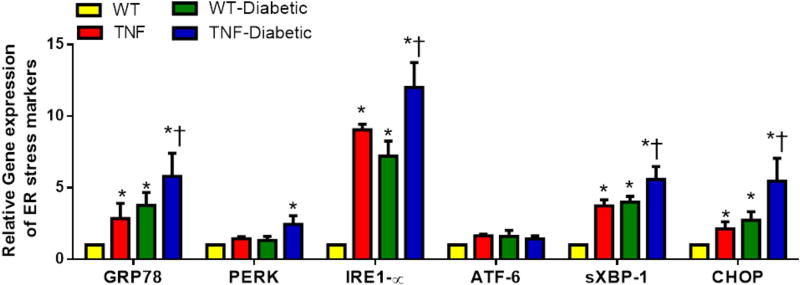
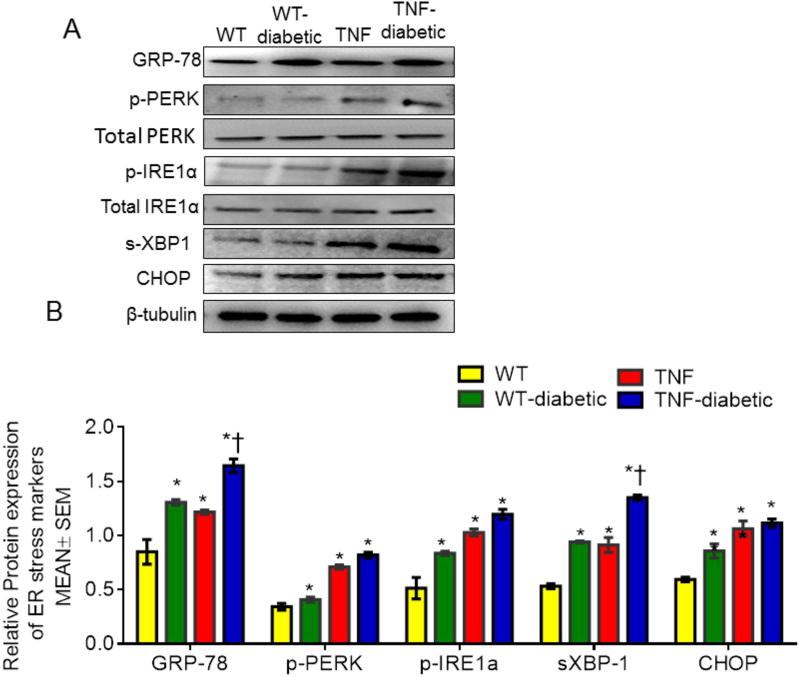
To investigate whether ER stress is involved in the early-stages of DR in these mice, we checked mRNA and protein levels of ER stress markers including GRP78, PERK, IRE1α, ATF6, spliced X box binding protein (sXBP-1) and C/EBP homologous protein (CHOP) in the retinal tissues at 4 weeks of diabetes (Fig. 5). While mRNA expression of GRP78, IRE1α, sXBP-1 and CHOP were significantly elevated in retinal tissues of tie2-TNF mice compared to WT mice (>2 fold, p<0.05), others tended to increase, without achieving statistical significance. Interestingly, the expression of these genes further increased significantly in diabetic tie2-TNF mice compared to tie2-TNF mice. While PERK displayed no significant alterations in the mRNA expression in tie2-TNF mice, protein levels were elevated in tie2-TNF mice compared to WT mice (Fig. 6). Consistent with the gene transcript data, protein expression of other ER stress markers, GRP78, p-PERK, p-IRE1α, sXBP1 and CHOP, were also increased in tie2-TNF mice compared to WT mice. On the other hand, GRP78 and sXBP-1 protein levels further increased in diabetic tie2-TNF mice compared to tie2-TNF mice, while others showed an increase without achieving statistical significance.
Fig 5. tie2-TNF transgenic mice and the diabetic tie2-TNF mice demonstrate an increased gene expression of ER stress markers.
Assessment of gene expression by Taqman qPCR and expressed as fold change normalized to internal control in the study groups. Data represent Mean ± SEM from n=3–5 animals. *p<0.05 compared to WT mice and † p<0.05 compared to tie2-TNF (TNF) mice.
Fig 6. tie2-TNF transgenic mice and the diabetic tie2-TNF mice demonstrate an increased protein expression of ER stress markers.
(A) Representative immunoblots of ER stress markers in the retina collected from the study groups. (B) Densitometric analysis of target to internal control or total protein of interest. Data represent Mean ± SEM from n=3–5 animals. *p<0.05 compared to WT mice and † p<0.05 compared to tie2-TNF (TNF) mice.
TNFα induced decrease in trans-endothelial electrical resistance protected by an ER stress inhibitor in vitro
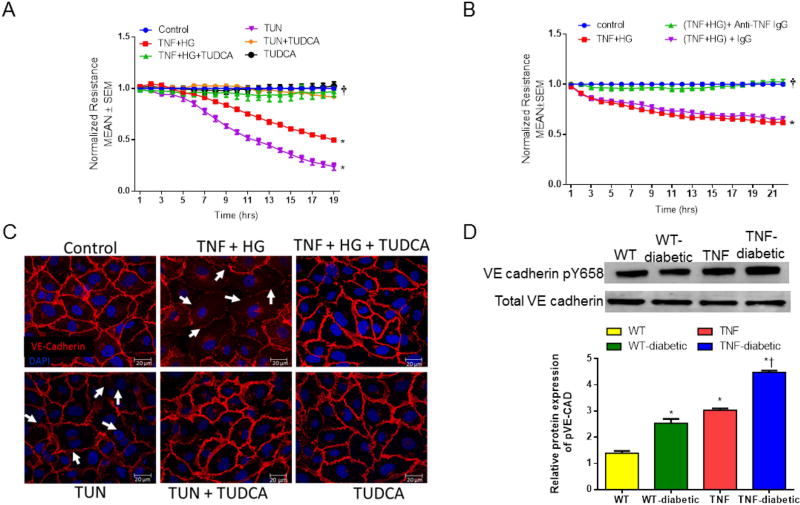
To further evaluate the influence of ER stress in endothelial activation, we measured trans-endothelial electrical resistance (TER) in vitro (Fig. 7A). Human retinal endothelial cells (HREC) exposed to high glucose (HG) and TNF-α induced a sustained reduction in barrier integrity as evidenced by decreased TER (TNF+HG, 0.49±0.01; control, 1.0±0.0 AU, p<0.05). On the other hand, these effects were rescued by treatment with an ER stress inhibitor, TUDCA (TNF+HG+TUDCA, 0.96±0.04 A.U., p<0.05). Tunicamycin, a known ER stress inducer, which acts through inhibition of N-linked glycosylation served as a positive control[Lenin et al., 2012] in the assay system. Much similar to TUDCA, cells pre-treated with TNF-α neutralizing antibody abolished the decrease in TER when challenged with TNF-α and HG (Fig. 7B). Mannitol used as an osmolality control did not influence the TER measurements (data not shown).
Fig 7. Trans-endothelial resistance is protected by ER stress inhibitor in vitro.
Representative ECIS tracings plotted as normalized resistance expressed as Mean ± SEM from TNF+HG with TUDCA (A) and TNF neutralizing antibody (B). Data represented from two independent experiments performed in duplicates. *p<0.05 compared to control and † p<0.05 compared to TNF+HG. (C) Representative confocal immunofluorescence images of VE-cadherin in HREC. White arrows indicate loss of cell-cell contacts. (D) Immunoblots of phospho-VE-cadherin and VE-Cadherin in the retina collected from the study groups. Data represent Mean ± SEM from n=3–5 animals, *p<0.05 compared to WT mice, † p<0.05 compared to tie2-TNF (TNF) mice.
We next examined whether alterations in TER observed in TNF-α and HG treated endothelial cells coincided with a change in VE-cadherin distribution (Fig. 7C). While control untreated cells displayed a continuous pattern of VE-cadherin staining associated with the lateral cell borders, treatment with TNF-α and HG or the ER stress inducer, Tunicamycin caused a disruption in the pattern of VE-cadherin with punctate staining on the cell surface. On the other hand, HREC treated simultaneously with TUDCA and challenged with TNF-α and HG demonstrated restoration of VE-cadherin staining similar to control cells. Interestingly, the tyrosine phosphorylated VE-Cadherin levels in retinal tissues of tie2-TNF mice were increased 3-fold compared to control mice with a further significant increase in diabetic tie2-TNF mice (Fig. 7D). Taken together these findings suggest that endothelial activation in response to high glucose and TNF-α may be occurring via loss of adheren junction proteins, mediated by ER stress pathways in retinal endothelium.
DISCUSSION
In this study, using an animal model of tie2-TNF transgenic mice that display endothelial activation and pro-inflammatory retinal gene expression, we show a significant increase in visual deficits using ERG and OKN measurements. Interestingly, these deficits are further exacerbated in diabetic tie2-TNF transgenic mice. In addition, the retinal tissues collected from these mice exhibited an enhanced expression of multiple ER stress markers including GRP78, PERK, IRE1α, ATF6, XBP-1 and CHOP, both at the mRNA transcript and protein levels. Furthermore, our results point towards ER stress as one of the causal agents in the loss of trans-endothelial resistance, which could be ameliorated by blocking ER stress.
TNF-α, a well-known pro-inflammatory cytokine, has been shown to be detrimental in DR [Behl et al., 2008]. TNF-α exists in two forms, with differing mechanisms of action for each. The transmembrane-spanning TNF (tmTNF) mostly acts through TNFR2 and elicits proliferation and survival signaling, while the soluble TNF-α only acts through TNFR1, and elicits apoptotic signaling [Rajashekhar et al., 2007]. We and others have demonstrated a variety of roles for tmTNF signaling including: protection from con-A induced acute hepatitis[Willuweit et al., 2001]; resistance to apoptosis and drug resistance in breast cancer[Yu et al., 2013]; proliferation of endothelial colony forming cells[Green et al., 2016] and neuroprotection[Novrup et al., 2014]. However, the role of tmTNF in retinal diseases remains to be studied.
It has been shown earlier that the tie2-TNF mouse model used in this study develops nephropathy characterized by inflammatory exudates in kidney with tubular damage and fibrosis within 6 months after birth [Rajashekhar et al., 2012]. Based on this and because similar risk factors induce the “microvascular disease” associated with nephropathy as well as retinopathy [Viswanathan et al., 2012], the present study explored whether the tie2-TNF inflammatory mouse model exhibits hallmarks of human retinopathy when rendered diabetic. Retinal gene expression changes observed in this model are consistent with other DR animal models [Brucklacher et al., 2008; Freeman et al., 2010; Rajashekhar et al., 2014a], TNF-expressing endothelial cells[Rajashekhar et al., 2007], and the kidneys of tie2-TNF mice[Rajashekhar et al., 2012]. Specifically, genes coding proteins involved in the infection and immune response (GBP2; LGALS9; CH25H) and in intracellular signal transduction pathways activated by cytokines and chemokines, such as adhesion and tissue structure (VCAM-1; ICAM-1; LAMA5), inflammation (CCL2; EDN2; CD11b) and protein degradation (TIMP1), were strongly represented and have been shown to be associated with endothelial activation. Although more detailed temporal expression and gain or loss of function studies of these genes/proteins is necessary, our current results endorse the endothelial activation induced retinopathy features in this model. Finally, it is compelling to note that several genes mentioned above significantly changed in tie2-TNF mice after 4 weeks of STZ injection, while other STZ model studies did not observe significant gene expression changes until after about 3 months [Brucklacher et al., 2008; Freeman et al., 2009], perhaps suggesting a remarkable phenotype of DR that is worth pursuing in future studies.
Neural alterations of DR can be monitored in real-time via visual function tests such as ERG and OKN. The decrease in visual acuity and an increase in contrast sensitivity in our tie2-TNF and diabetic tie2-TNF mice are consistent with the defects observed in other diabetic models including the Ins2Akita mouse[Barber et al., 2005] and Long Evans rats[Aung et al., 2013]. The b-wave in ERG represents the corneal-positive deflection originating from the inner layers of the retina, specifically the Müller and bipolar cells. Our results in this study demonstrate significantly lower b-wave amplitudes in tie2-TNF mice at 3 months of age, suggesting retinal damage in this model. On the other hand, diabetic tie2-TNF mice demonstrated a further decrease in ERG amplitudes, which is in agreement with previously reported data in diabetic rodent models [Ramsey et al., 2006; Yeh et al., 2016]. A number of reasons could be considered for the observed visual deficits in our mice. Retinal inflammation has been shown to be both associated [Yeh et al., 2016] and independent[Samuels et al., 2017] of ERG defects in diabetic rodents. Diabetic animals experience global metabolic changes and any change post-synaptic to the photoreceptors could affect b-wave [Ramsey et al., 2006]. Similarly, any disturbances in post retinal connections downstream of accessory optic system[Aung et al., 2013] have been linked to OKN defects. Since the neural retina is nourished by retinal microcirculation and the outer retina by retinal choroidal circulation, any change in the blood flow and changes in neurovascular coupling may adversely affect the ERG in mice [Barber, 2015; Muir et al., 2012]. We need more elegant experiments of pharmacological agonists or antagonists to tease out the exact cellular origins of these defects in our model.
Dysfunction of the ER, or ER stress, is implicated in Muller cell-derived retinal inflammation [Zhong et al., 2012]. However, the role of endothelial activation in DR is less clear. Conditions that disrupt endothelial junctions might not only increase inflammation by opening intercellular gaps but also might change the endothelial cell responses to their environment and to surrounding cells. Inflammation and the accompanying cytokines have been shown to induce UPR through the damaging effects of oxidative stress on protein folding in the ER [Xue et al., 2005] and may be propagated through the signaling molecules such as c-Jun N-terminal kinase (JNK) [Urano et al., 2000] and nuclear factor-kappaB (NF-κB)[Balakumar et al., 2016]. The causative role for ER stress in retinal inflammation was shown with periocular injection of tunicamycin [Li et al., 2009]. It is interesting to note that mice that received tunicamycin demonstrated increased TNF-α and VEGF levels, which are also elevated in the retina of other diabetic models [Li et al., 2009; Yang et al., 2011]. This could be of relevance as TNF-α induced ER stress may lead to further amplification of pro-inflammatory signals, possibly leading to exacerbation of DR. One possible mechanism of pro-inflammatory stress may result in tyrosine phosphorylation of VE-cadherin that is linked to loss of junctional integrity in retinal endothelial cells [Rangasamy et al., 2011]. In accordance with this, diabetic tie2-TNF mice downstream of TNF signaling demonstrated elevation of VCAM-1 and ICAM-1 coupled with increased phosphorylation of VE-cadherin proteins and a complimentary remarkable decrease in VE-cadherin expression in retinal endothelial cells exposed to HG and TNF-α. It is interesting to note that Tunicamycin, a known ER stress inducer also reduced VE-cadherin expression and demonstrated similar reduction in TER in vitro, suggesting that ER stress pathways are downstream of HG/TNF-α signaling and can be rescued with TUDCA. These findings suggest that ER stress is a critical mediator of endothelial activation induced retinal inflammation. Whether endothelial activation with increased TNF-α levels observed in the diabetic model is part of a feed forward loop of ER stress leading to chronic retinal inflammation is worth pursuing further with specific inhibitors to design therapies that address both ER stress and endothelial activation.
There exists a significant health burden attributable to diabetic complications because of a continuing increase in the prevalence of diabetes, along with poor glycemic control in the early stages of disease. Our study presents a comprehensive analysis of the involvement of chronic inflammation and hyperglycemia in the activation of ER stress in activated endothelial cells, which eventually leads to visual deficits in the context of DR. A close look into the mechanisms by which endothelial activation leads to ER stress and/or identification of key protective genes against ER stress may provide new insight into the pathogenesis of the disease and pave the way for new treatment in DR. Nevertheless, early intervention that reduces ER stress and enhances the adaptive mechanism to restore ER homeostasis and promotes cell survival may be important for protecting retinal vascular cells and neurons from diabetic damage. As seen from our administration of TUDCA, which is under clinical trials for treatment of amyotrophic lateral sclerosis (NCT03127514; AMX0035 in Patients With Amyotrophic Lateral Sclerosis) and diabetes (NCT02218619; Tauroursodeoxycholic Acid (TUDCA) in New-Onset Type 1 Diabetes), it is plausible that ER stress inhibitors could be of therapeutic value in DR and other diabetic complications. In support of this, other ER stress inhibitors, 4-phenylbutyrate and ursodeoxycholic acid have shown successful alleviation of ER stress-induced pathology in retinal inflammation [Chung et al., 2017; Zhong et al., 2012]. Future studies, beyond the scope of this study will explore the administration of TUDCA in our diabetic tie2-TNF mice to evaluate this supposition.
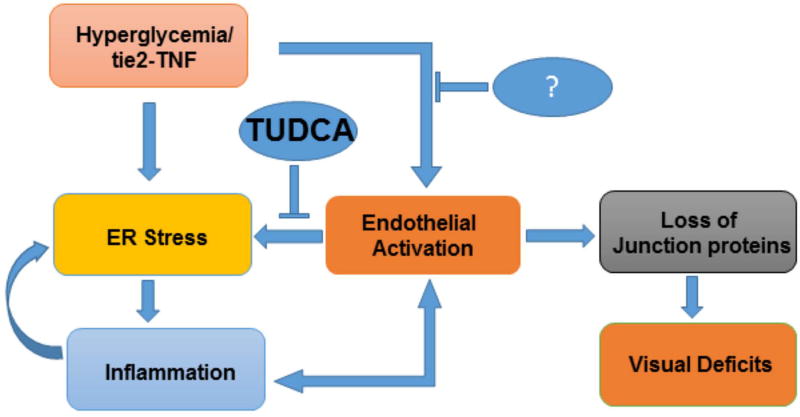
In conclusion, using a constant endothelial activation animal model of diabetes, we show that chronic inflammatory activation of vascular endothelial cells and hyperglycemia promotes visual deficits downstream of ER stress signaling in a feed forward loop of pro-inflammation and loss of endothelial junction proteins. Based on these and our in vitro analysis, future studies aiming at controlling ER stress pathways and endothelial activation may help protect against disruption of junctional proteins in retinal endothelial cells and possibly aid in the recovery of visual deficits observed in our DR model (Fig. 8).
Fig 8. Schematic representation of critical role of ER stress in the visual deficits under chronic endothelial activation.
Endothelial tie2-TNF and hyperglycemia induces ER stress and chronic pro-inflammation in a feed forward loop ultimately resulting in endothelial junction protein alterations leading to visual deficits in the retina. While inhibition of ER stress by taurosodeoxycholic acid (TUDCA) enhances the adaptive mechanisms to restore ER homeostasis, inhibition of endothelial activation in parallel may be necessary as shown by us previously with soluble thrombomodulin[Rajashekhar et al., 2012].
Acknowledgments
Authors wish to acknowledge Abby Marin, Indiana University, for technical support and Timothy S Kern, Case Western Reserve University, for helpful discussions. This study was supported by grants from the National Eye Institute (EY023427), gifts from the Hamilton Eye Institute, and an unrestricted grant from Research to Prevent Blindness. RL is a recipient of postdoc fellowship awards from Neuroscience Institute, UTHSC and International Retinal Research Foundation. The funders played no role in the conduct of the study, collection of data, management of the study, analysis of data, interpretation of data, or preparation of the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS:
Conceived and designed the experiments: RL, RG. Performed the experiments: RL, PGN, SA, VRR. Analyzed the data: RL, VRR, MAC, UBK, RG. Contributed reagents/materials/analysis tools: MAC, UBK, RG. Wrote and reviewed the paper: All authors.
COMPETING FINANCIAL INTERESTS:
None of the other authors declare any financial interests relevant to the studies presented here.
References
- Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci. 2014;1311:174–90. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A, Choi SK, Galan M, Kassan M, Partyka M, Kadowitz P, Henrion D, Trebak M, Belmadani S, Matrougui K. Chronic inhibition of endoplasmic reticulum stress and inflammation prevents ischaemia-induced vascular pathology in type II diabetic mice. J Pathol. 2012;227:165–74. doi: 10.1002/path.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MH, Kim MK, Olson DE, Thule PM, Pardue MT. Early visual deficits in streptozotocin-induced diabetic long evans rats. Invest Ophthalmol Vis Sci. 2013;54:1370–7. doi: 10.1167/iovs.12-10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar M, Raji L, Prabhu D, Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol Cell Biochem. 2016;423:93–104. doi: 10.1007/s11010-016-2828-5. [DOI] [PubMed] [Google Scholar]
- Barber AJ. Diabetic retinopathy: recent advances towards understanding neurodegeneration and vision loss. Sci China Life Sci. 2015;58:541–9. doi: 10.1007/s11427-015-4856-x. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–8. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–8. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Brucklacher RM, Patel KM, VanGuilder HD, Bixler GV, Barber AJ, Antonetti DA, Lin C-M, LaNoue KF, Gardner TW, Bronson SK, Freeman WM. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Medical Genomics. 2008;1:26–26. doi: 10.1186/1755-8794-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Chung YR, Choi JA, Koh JY, Yoon YH. Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice. J Diabetes Res. 2017;2017:1763292. doi: 10.1155/2017/1763292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 2006;20:1366–9. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Bixler GV, Brucklacher RM, Lin CM, Patel KM, VanGuilder HD, LaNoue KF, Kimball SR, Barber AJ, Antonetti DA, Gardner TW, Bronson SK. A multistep validation process of biomarkers for preclinical drug development. Pharmacogenomics J. 2010;10:385–95. doi: 10.1038/tpj.2009.60. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Bixler GV, Brucklacher RM, Walsh E, Kimball SR, Jefferson LS, Bronson SK. Transcriptomic comparison of the retina in two mouse models of diabetes. J Ocul Biol Dis Infor. 2009;2:202–213. doi: 10.1007/s12177-009-9045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LA, Njoku V, Mund J, Case J, Yoder M, Murphy MP, Clauss M. Endogenous Transmembrane TNF-Alpha Protects Against Premature Senescence in Endothelial Colony Forming Cells. Circ Res. 2016;118:1512–24. doi: 10.1161/CIRCRESAHA.116.308332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–44. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Thakran S, Bheemreddy R, Ye EA, He H, Walker RJ, Steinle JJ. Pioglitazone normalizes insulin signaling in the diabetic rat retina through reduction in tumor necrosis factor alpha and suppressor of cytokine signaling 3. J Biol Chem. 2014;289:26395–405. doi: 10.1074/jbc.M114.583880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenin R, Maria MS, Agrawal M, Balasubramanyam J, Mohan V, Balasubramanyam M. Amelioration of glucolipotoxicity-induced endoplasmic reticulum stress by a “chemical chaperone” in human THP-1 monocytes. Exp Diabetes Res. 2012;2012:356487. doi: 10.1155/2012/356487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583:1521–7. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JJ, Zhang SX. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J Biol Chem. 2011;286:4912–21. doi: 10.1074/jbc.M110.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir ER, Renteria RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012;53:6488–94. doi: 10.1167/iovs.12-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novrup HG, Bracchi-Ricard V, Ellman DG, Ricard J, Jain A, Runko E, Lyck L, Yli-Karjanmaa M, Szymkowski DE, Pearse DD, Lambertsen KL, Bethea JR. Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. J Neuroinflammation. 2014;11:159. doi: 10.1186/s12974-014-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–6. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Rajashekhar G, Grow M, Willuweit A, Patterson CE, Clauss M. Divergent and convergent effects on gene expression and function in acute versus chronic endothelial activation. Physiol Genomics. 2007;31:104–13. doi: 10.1152/physiolgenomics.00157.2006. [DOI] [PubMed] [Google Scholar]
- Rajashekhar G, Gupta A, Marin A, Friedrich J, Willuweit A, Berg DT, Cramer MS, Sandusky GE, Sutton TA, Basile DP, Grinnell BW, Clauss M. Soluble thrombomodulin reduces inflammation and prevents microalbuminuria induced by chronic endothelial activation in transgenic mice. Am J Physiol Renal Physiol. 2012;302:F703–12. doi: 10.1152/ajprenal.00558.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekhar G, Loganath A, Roy AC, Chong SS, Wong YC. Extracellular matrix-dependent regulation of angiogenin expression in human placenta. J Cell Biochem. 2005;96:36–46. doi: 10.1002/jcb.20507. [DOI] [PubMed] [Google Scholar]
- Rajashekhar G, Ramadan A, Abburi C, Callaghan B, Traktuev DO, Evans-Molina C, Maturi R, Harris A, Kern TS, March KL. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One. 2014a;9:e84671. doi: 10.1371/journal.pone.0084671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekhar G, Shivanna M, Kompella UB, Wang Y, Srinivas SP. Role of MMP-9 in the breakdown of barrier integrity of the corneal endothelium in response to TNF-alpha. Exp Eye Res. 2014b;122:77–85. doi: 10.1016/j.exer.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, Helisch A, Clauss M. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vasc Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- Ramsey DJ, Ripps H, Qian H. An electrophysiological study of retinal function in the diabetic female rat. Invest Ophthalmol Vis Sci. 2006;47:5116–24. doi: 10.1167/iovs.06-0364. [DOI] [PubMed] [Google Scholar]
- Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3784–91. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech. 2012;5:444–56. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Portillo JC, Miao Y, Kern TS, Subauste CS. Loss of CD40 attenuates experimental diabetes-induced retinal inflammation but does not protect mice from electroretinogram defects. Vis Neurosci. 2017;34:E009. doi: 10.1017/S0952523817000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Shivanna M, Rajashekhar G, Srinivas SP. Barrier dysfunction of the corneal endothelium in response to TNF-alpha: role of p38 MAP kinase. Invest Ophthalmol Vis Sci. 2010;51:1575–82. doi: 10.1167/iovs.09-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Viswanathan V, Tilak P, Kumpatla S. Risk factors associated with the development of overt nephropathy in type 2 diabetes patients: a 12 years observational study. Indian J Med Res. 2012;136:46–53. [PMC free article] [PubMed] [Google Scholar]
- Willuweit A, Sass G, Schoneberg A, Eisel U, Tiegs G, Clauss M. Chronic inflammation and protection from acute hepatitis in transgenic mice expressing TNF in endothelial cells. J Immunol. 2001;167:3944–52. doi: 10.4049/jimmunol.167.7.3944. [DOI] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917–25. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu R, Cui Z, Chen ZQ, Yan S, Pei H, Li B. Functional characterization of 58-kilodalton inhibitor of protein kinase in protecting against diabetic retinopathy via the endoplasmic reticulum stress pathway. Mol Vis. 2011;17:78–84. [PMC free article] [PubMed] [Google Scholar]
- Yang X, Scott HA, Monickaraj F, Xu J, Ardekani S, Nitta CF, Cabrera A, McGuire PG, Mohideen U, Das A, Ghosh K. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J. 2016;30:601–11. doi: 10.1096/fj.15-277962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P-T, Huang H-W, Yang C-M, Yang W-S, Yang C-H. Astaxanthin Inhibits Expression of Retinal Oxidative Stress and Inflammatory Mediators in Streptozotocin-Induced Diabetic Rats. PLoS ONE. 2016;11:e0146438. doi: 10.1371/journal.pone.0146438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou W, Gan H, Wang J, Jiang X, Yin B, Li Z. Targeting transmembrane TNF-alpha suppresses breast cancer growth. Cancer Res. 2013;73:4061–74. doi: 10.1158/0008-5472.CAN-12-3946. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]