Abstract
Early life stressors, including general anesthesia, can have adverse effects on adult neural and behavioral outcomes, such as disruptions in inhibitory signaling, stress responsivity and increased risk of psychiatric disorders. Here we used a rat model to determine the effects of combined exposure to etomidate (ET) neonatal anesthesia and maternal separation on adult amygdala expression of genes for corticotropin-releasing hormone (Crh) and the chloride co-transporters Nkcc1 and Kcc2, as well as ethanol intake. Male and female Sprague-Dawley rats were subjected to 2 h of ET anesthesia on postnatal days (P) 4, 5, or 6 followed by maternal separation for 3 h on P10 (ET + SEP). During the P91-P120 period rats had daily 2 h access to three 0.05% saccharin solutions containing 0%, 5%, or 10% ethanol, followed by gene expression analyses. The ET + SEP group had increased Crh mRNA levels and Nkcc1/Kcc2 mRNA ratios in the amygdala, with greater increases in Nkcc1/Kcc2 mRNA ratios in males. A moderate increase in 5% ethanol intake was evident in the ET + SEP males, but not females, after calculation of the ratio of alcohol intake between the last week and first week of exposure. In contrast, control males tended to decrease alcohol consumption during the same period. A brief exposure to ET combined with a subsequent episode of stress early in life induced significant alterations in expression of amygdala Crh, Nkcc1 and Kcc2 with greater changes in the Cl− transporter expression in males. The possibility of increased alcohol intake in the exposed males requires further confirmation using different alcohol intake paradigms.
Keywords: Etomidate, Stress, Developing brain, NKCC1/KCC2, Alcohol intake
Brief summary:
Neonatal rat exposure to etomidate followed by a single episode of maternal separation results in long-term increases in amygdala Nkcc1/Kcc2 mRNA ratio and Crh mRNA levels.
As many as 1.5 million children per year in the U.S. alone are exposed to general anesthesia during the first year of life [1]. Many retrospective epidemiological studies have found that such general anesthesia exposure is associated with later learning disabilities, long-term memory impairment, and attention-deficit/hyperactivity disorder (ADHD) [2–10]. Although establishing a causal role for general anesthesia in such deficits can be challenging, strong support from animal research raises serious concerns that general anesthetics administered during early life may alter subsequent neurodevelopmental trajectories. The development of translational strategies to mitigate these effects is impeded by an incomplete understanding, even in rodent models, of the full range of physiological functions altered by early life anesthetic exposure, as well as the mechanisms of such alterations [11].
In a recent series of publications, we have shown that in neonatal rats, administration of general anesthetic agents that share the ability to enhance GABA type A receptor (GABAAR) activity, such as sevoflurane, propofol and etomidate, induce not only behavioral deficiencies, but also neuroendocrine abnormalities that are reminiscent of those induced by neonatal exposure to stress [12–17]. These neuroendocrine abnormalities, which are more robust in males, include increased anxiety-like behavior and exacerbated corticosterone responses to stress. In addition, the rats have elevated corticotropin-releasing hormone (Crh) mRNA levels in the hypothalamus, as well as up- and down-regulated hypothalamic mRNA levels of the Cl− transporters Na+-K+-2Cl− (Nkcc1) and K+-2Cl− (Kcc2), respectively [13–17].
Normally, an elevated NKCC1/KCC2 ratio supports depolarizing (excitatory) GABAAR signaling in immature neurons, while developmental reduction in the NKCC1/KCC2 ratio, mainly due to increases in neuronal-specific KCC2, forms the basis for the shift toward inhibitory GABAAR signaling later on [18–20]. The GABA-initiated depolarization and resulting Ca++ influx in immature neurons regulate a wide spectrum of developmental processes from gene expression to synapse formation [18–20]. Obviously, an early-in-life anesthesia-induced delay in GABAAR signaling transition to inhibitory [13,16,17] may affect numerous GABAAR signaling-regulated functions in the body. One example may be dysregulated stress responsivity because of impaired GABAAR-mediated inhibitory control of CRH-secreting neurons [21,22]. Impaired NKCC1/KCC2 maturation and the resulting shift in GABAAR signaling toward excitatory, as well as dysregulated stress systems, have been implicated in development of psychiatric conditions, including male-predominant autism spectrum disorders (ASD) and schizophrenia, as well as increased ethanol intake in adulthood [23–27]. Of special interest, clinical studies report a high level of co-occurrence of ASD with ADHD, with the latter being significantly increased in those who had early in life exposure to general anesthesia.
Given that amygdala dysfunction may play a role in ADHD and ASD etiology [28–30], and that CRH and GABAergic neurotransmission in the amygdala are involved in regulation of alcohol consumption in rodents [31,32], in the present study we used rats to test whether relatively brief neonatal exposure to ET combined with a single episode of maternal separation affects the expression of Nkcc1, Kcc2 and Crh in the amygdala of adult rats, as well as their alcohol intake. This anesthesia regimen, which was also used in our recently published study [13], may be of significant translational value, as many infants who require exposure to general anesthesia are also exposed to stressors, such as surgery, diseases, pain, and/or psychosocial stress.
Materials and methods
Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee (Gainesville, FL). Breeding pairs of Sprague-Dawley rats were purchased from Charles River and bred at the University of Florida animal care facility. Prior to the alcohol consumption studies (see below) rats were housed under controlled illumination (12-h light/dark, lights on at 0700) and temperature (23–24 °C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched groups of two for the rest of the study. At the beginning of anesthesia with etomidate, the pups were well nourished, as judged by their stomachs being full of milk (detectable through the transparent abdominal wall). To control for litter variability, pups from the same litter were used for different experimental conditions. Multiple sets of rats were used in the experiments. The data reported in this study were collected from 94 male and 87 female rats, obtained from a total of 27 litters.
Experimental groups
The experimental groups and number of animals per group are described in Table 1. During etomidate anesthesia and maternal separation, rat pups were kept in a temperature-controlled chamber (+37 °C) with a continuous supply of 30% oxygen in air. Gas monitoring was performed using a calibrated Datex side stream analyzer (Datex-Ohmeda, Helsinki, Finland), which sampled from the interior of the chamber. On either P4, P5, or P6, the etomidate group (ET) received 8 mg/kg of etomidate intraperitoneally for induction of anesthesia followed by second injection of etomidate (4 mg/kg, intraperitoneally) 50 min after the first administration. At these doses of ET, the pups did not exhibit a righting reflex for 2 h. We have shown previously that this ET administration regimen induces a depth of anesthesia that is in a similar range to that induced by 2.1% sevoflurane [13,15]. As a control for the period of maternal separation during ET anesthesia, rats in the Sep condition were separated from the dams for 120 min in a temperature-controlled chamber with a continuous supply of 30% oxygen in air without exposure to anesthesia. To simulate postanesthesia stress, half of the rats in the ET and Sep groups were subjected to maternal separation for 3 h at P10 (the etomidate plus maternal separation group (ET + SEP) and the maternal separation at P4, 5 or 6 plus maternal separation at P10 (Sep + SEP) group, respectively). To assess the effects of maternal separation alone, a separate group of rats was subjected to maternal separation for 3 h at P10 only (the SEP group). Finally, a group of control rats was subjected to animal facility rearing only (the Con group; i.e., neither ET nor separation).
Table 1.
| Group Number | Treatment | Number of animals per group | |
|---|---|---|---|
| male | female | ||
| 1 | Facility rearing only (the Con group) | 16 | 16 |
| 2 | Maternal separation for 120 min at P4, 5 or 6 (the Sep group) | 15 | 15 |
| 3 | Anesthesia with Etomidate for 120 min at P4, 5 or 6 (the ET group) | 16 | 14 |
| 4 | Maternal separation for 180 min at P10 only (the SEP group) | 16 | 15 |
| 5 | Maternal separation for 120 min at P4, 5 or 6 plus maternal separation for 180 min at P10 (the Sep + SEP group) | 16 | 14 |
| 6 | Anesthesia with Etomidate for 120 min at P4, 5 or 6 plus maternal separation for 180 min at P10 (the ET + SEP group) | 15 | 13 |
Alcohol consumption studies
Ethanol intake was assessed between P91 and P120 using a modified drinking-in-the-dark model with three bottles containing 0.05% saccharine, 5% ethanol in 0.05% saccharine, and 10% ethanol in 0.05% saccharine [33]. For this experiment, the rats were individually housed in a designated room with a reverse light-dark cycle (lights off at 0800 and on at 2000). This ensured the rats would be in their active (dark) cycle when the alcohol was available. The rats underwent a one week habituation period to this new environment. During this time they did not receive any alcohol and had access to water and food ad libitum. Following the habituation period, the rats were given 2 consecutive days of 24-h access to the three bottles, for a total of 48 h. Ethanol access always began 3 h after the start of the dark cycle and fresh bottles were exchanged after 24 h. This was termed the acquisition period. After the acquisition period the rats had a 24 h “off day” on which no alcohol was provided, before beginning the experimental period. For the experimental period the rats were given 2-h access to the three solutions for 30 consecutive days, again beginning 3 h after the start of the dark cycle. The bottles were placed on the rats’ cages each day for 2 h, with the position of the 3 bottles randomized across days. Bottles were weighed before and after each drinking period and weights were recorded. Rats were weighed weekly in order to account for growth when calculating grams of ethanol consumed/kilograms of body weight. After observing the normal drinking habits of the rats, we determined that amounts greater than 35 grams were not plausible and could therefore be deemed a “leak day”. Less than 1 percent of the consumption data met this criterion, and they were not included in the data analysis. The rats had water ad lib outside the 2h/day when the 3 bottles were on the cage.
Analyses of mRNA levels for Nkcci, Kcc2 and Crh
Three weeks after finishing the alcohol intake study, rats were deeply anesthetized with sevoflurane and decapitated. Whole brains were removed and immediately put in a prefrozen rat brain matrix. The amygdala regions were dissected from the coronal slices, according to the atlas of Paxinos and Watson [34]. All the tissues were immediately submerged in RNAlater solution (Invitrogen, Carlsbad, CA) and kept overnight at 4°C, followed by long-term storage at −20 °C. Crh, Nkcci, and Kcc2 mRNA in the amygdala were analyzed via real-time reverse transcription-PCR (qRT-PCR) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). RNA was extracted from the samples using an RNeasy Plus Kit (Qiagen, Valencia, CA, USA), reverse transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Laboratories, Hercules, CA, USA), and analyzed via qRT-PCR using an Applied Biosystems StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). Oligonucleotide primers and Taqman probes specific for the above genes were obtained from Applied Biosystems (Carlsbad, CA, USA): Crh (Rn01462137_m1), Nkcc1 (Rn00582505_m1), Kcc2 (Rn00592624_m1). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA (Rn01775763_g1). Gene expression was calculated using the ΔΔCT method and data presented as relative fold change from that of control animals.
Statistical analysis
For each condition, 0%, 5%, and 10% w/v ethanol, intake was indexed by three measures. For the first and last week of exposure, intake (grams) on each day was normalized to body weight (kilograms) then averaged for each animal (i.e. mean daily intake on the first or last 7 days, g/kg bodyweight). Additionally, the ratio of each of these measures was taken (mean daily intake last 7 days/mean daily intake first 7 days, referred to as the intake ratio). All measures were summarized as mean ± SEM. Outliers (intake > 35 g in one day) were recorded as missing before measure construction.
ANOVA or Welch ANOVA (one-way) was used to assess mean differences for each intake measure across combined treatment and separation groups (Con, Sep, ET, SEP, Sep + SEP, ET + SEP), separately by sex. Welch ANOVA and the separate analyses by sex were conducted due to violations of the homogeneity of variance assumption for comparisons between sexes and across treatment conditions for some outcomes. ANOVA (one-way) was used to analyze gene expression data. Bonferroni or Tamhane (for unequal variances) tests were used for post-hoc multiple comparisons. P < 0.05 was considered statistically significant. All analyses were conducted using SPSS v. 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
Results
mRNA levels of Nkcc1, Kcc2 and Crh in the amygdala
In male rats, there were statistically significant group differences in amygdala Nkcc1 mRNA levels (F(5,21) =5.971, P = 0.001; Fig. 1A), and Bonferroni post-hoc comparisons showed that the ET + SEP group had elevated Nkcc1 mRNA levels (P = 0.002 vs Con; P = 0.005 vs Sep; and P = 0.015 vs SEP). There were also statistically significant group differences in amygdala Kcc2 mRNA levels (F(5,21) = 11.283, P < 0.001; Fig. 1B). The Bonferroni post-hoc tests showed that the ET + SEP group had reduced levels of Kcc2 mRNA (P = 0.002 vs Con; P < 0.001 vs Sep; and P = 0.023 vs SEP). In addition, the Sep + SEP group had decreased levels of Kcc2 mRNA in the amygdala compared to the Sep group (P < 0.001) and the Con group (P = 0.015). Moreover, the ET group had decreased the amygdala levels of Kcc2 mRNA when compared to the Sep group (P = 0.007). Finally there were statistically significant group differences in the resulting Nkcc1/Kcc2 mRNA ratio (F(5,21) = 4.611, P = 0.005; Fig. 1C). Post-hoc tests showed that the Nkcc1/Kcc2 ratio was higher in the ET + SEP group (P = 0.021 vs Con; P = 0.019 vs Sep; P = 0.02 vs SEP).
Figure 1.
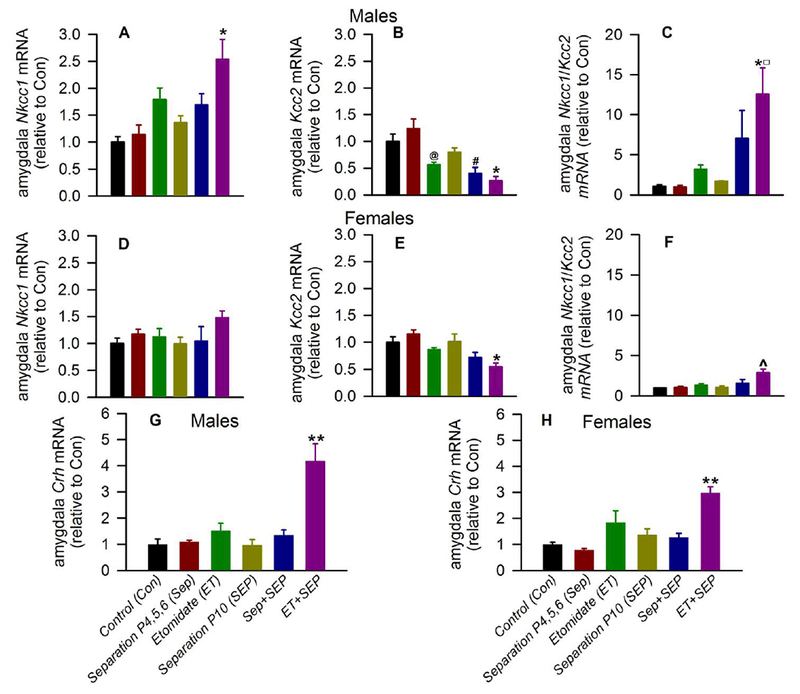
Anesthesia with etomidate (ET) for 2 h on postnatal days (P) 4, 5 or 6 followed by maternal separation for 3 h at P10 induced alterations in amygdala levels of Nkcc1, Kcc2 and Crh mRNA in adult rats. Amygdala tissue samples were collected at P>120 for qRT-PCR analyses. Shown are levels of Nkcc1 mRNA (A) and Kcc2 mRNA (B), and the resulting Nkcc1/Kcc2 mRNA ratios (C) in male rats. The respective data for female rats are shown in (D) Nkcc1 mRNA levels, (E) Kcc2 mRNA levels and (F) the resulting Nkcc1/Kcc2 mRNA ratios. Data normalized against control are means ± SEM from 4-5 rats per treatment group. *P < 0.05 vs. Con, Sep, and SEP; #P < 0.05 vs. Con and Sep; @P < 0.05 vs. Sep; ^P < 0.05 vs Con, Sep, SEP, and ET; ◻P < 0.05 vs ET + SEP females). (G,H) Shown are levels of Crh mRNA in males (G) and females (H). Data normalized against control are means ± SEM from 4-5 rats per treatment group. **P < 0.05 vs. all experimental groups; Color coding in Fig. 1G, H is applicable to the entire figure.
In female rats, there were no statistically significant group differences in amygdala Nkcc1 mRNA levels (F(5,22) = 1.562, P = 0.212; Fig. 1D). There were statistically significant group differences in amygdala Kcc2 mRNA levels (F(5,22) = 5.837, P = 0.001; Fig. 1E), with Bonferroni post-hoc tests showing that the ET + SEP group had decreased Kcc2 mRNA levels (P = 0.043 vs Con; P = 0.001 vs Sep; and P = 0.022 vs SEP). There were also statistically significant group differences in amygdala Nkcc1/Kcc2 mRNA ratios (F(5,22) = 6.073, P = 0.001; Fig. 1F). Post-hoc tests showed that the ET + SEP group had higher Nkcc1/Kcc2 mRNA ratios (P = 0.004 vs Con; P = 0.003 vs Sep; P = 0.003 vs SEP; and P = 0.014 vs ET). Finally, the ET + SEP-induced increase in Nkcc1/Kcc2 mRNA ratio was greater in males than in females (t(8) = 2.955, P = 0.0183, Fig. 1C,F).
In male rats, there were statistically significant group differences in amygdala Crh mRNA levels (F(5,24) = 14.157, P < 0.001; Fig. 1G), with Bonferroni post-hoc tests showing that male rats in the ET + SEP group had increased amygdala Crh mRNA levels (P < 0.001 vs each other experimental group). There were also statistically significant group differences in amygdala Crh mRNA levels in female rats (F(5,24) = 10.569, P < 0.001; Fig. 1H). Post-hoc tests showed that rats in the ET + SEP group had elevated Crh mRNA levels (P < 0.001 vs Con and Sep; P = 0.001 vs SEP and Sep + SEP; and P = 0.045 vs ET). The ET + SEP-induced increases in Crh mRNA levels in the amygdala were similar in male and female rats (t(8) = 1.716, P = 0.125, Fig. 1G,H).
5% Alcohol, 10% alcohol and saccharine intake
In male rats, there were no statistically significant group differences in mean intake of 5% alcohol in the first 7 days (F(5,88) = 2.174, P = 0.064; Fig. 2A) or last 7 days (F(5,88) = 1.627, P = 0.161; Fig. 2B). To determine whether early life exposure to etomidate and the stress of maternal separation changed alcohol consumption across the 30 day exposure to alcohol, we compared the ratio of alcohol consumption between the last and first week (the intake ratio). There were statistically significant group differences for the intake ratio (Welch F(5,40) = 7.484, P < 0.001; Fig. 2C). The Tamhane post-hoc tests showed that compared to the Con group, only the ET+SEP group (P < 0.001) had a higher intake ratio.
Figure 2.
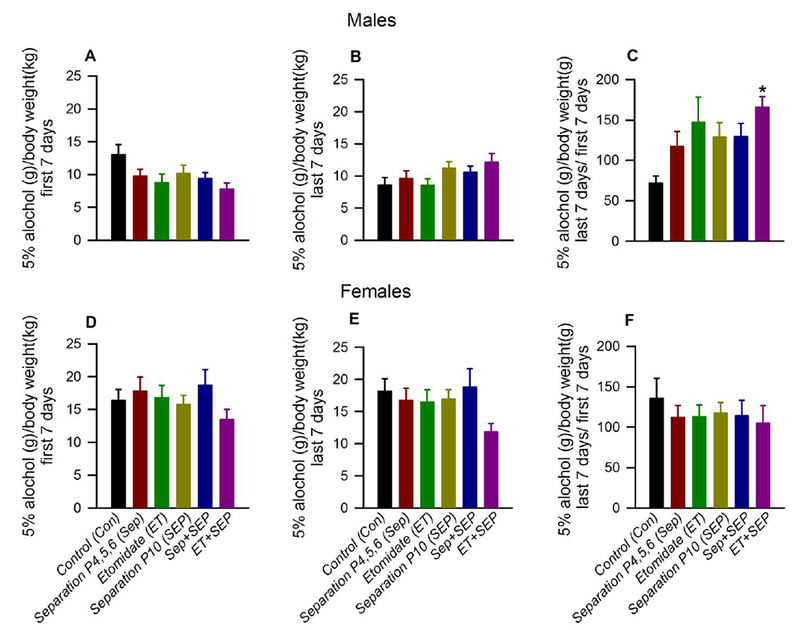
Anesthesia with etomidate (ET) for 2 h at postnatal days (P) 4, 5 or 6 followed by maternal separation for 3 h at P10 led to an increase in 5% ethanol intake in male, but not female, adult rats. Shown are: (A) mean daily 5% ethanol intake first 7 days [ethanol intake (grams) on each day was normalized to body weight (kilograms) then averaged for each animal]; (B) mean daily intake last 7 days; and (C) the intake ratio (mean daily intake last 7 days / mean daily intake first 7 days, in g ethanol / kg body weight) for male rats. (D-F) show respective data for female rats: (D) mean daily intake first 7 days; (E) mean daily intake last 7 days; and (F) the intake ratio. Data are means ± SEM from 13-16 rats per experimental group. *P < 0.05 vs. the Con group. Color coding in Fig. 2D-F is applicable to the entire figure.
In female rats, there were no statistically significant group differences in mean intake during first 7 days (F(5,81) = 0.867, P = 0.507; Fig. 2D), last 7 days (F(5,81) = 1.426, P = 0.224; Fig. 2E) or in intake ratio (F(5,81) = 0.315, P = 0.903; Fig. 2F).
There were no statistically significant group differences in mean intake of 10% alcohol or saccharine during the first 7 days, last 7 days or in intake ratio in male and female rats. Also, there were no statistically significant group differences in mean body weight of male rats during the first 7 days (F(5,88) = 0.515, P = 0.764), last 7 days (F(5,88) = 0.353, P = 0.879) or in their the body weight ratio (mean body weight last 7 days / mean body weight first 7 days) (F(5,88) = 0.880, P = 0.498). Similarly, there were no statistically significant group differences in mean body weight of female rats during the first 7 days (F(5,81) = 0.285, P = 0.92), last 7 days (F(5,81) = 0.695, P = 0.629) or in their body weight ratio (F(5,81) = 0.185, P = 0.968).
Discussion
Neonatal exposure to general anesthesia can cause a range of adverse neural and behavioral outcomes in adulthood. Here we report the novel findings that early life exposure to a brief ET anesthesia combined with a single episode of maternal separation induces adult upregulation of Nkcc1 and downregulation of Kcc2 Cl− co-transporter expressions in the amygdala in a sex-dependent manner, suggesting a fundamental change in functioning of the main inhibitory neurotransmitter GABA. Amygdala expression of Crh, one of principal hormones of the stress response system, was also significantly enhanced in adult rats neonatally exposed to ET and maternal separation. In addition to profound differences in gene expression, adult male (but not female) rats exposed to ET plus maternal separation exhibited opposite changes in consumption of 5% alcohol compared to their unexposed counterparts (an increase and decrease, respectively, across 30 days of access).
The developmental down-regulation of NKCC1 and up-regulation of KCC2 determines the ontogenetic shift in GABAAR signaling from depolarizing/stimulatory to inhibitory. This shift occurs at older ages in male compared to female pups [35–37], suggesting that this may be one of reasons for the sex-specific (and more prominent in males) developmental effects of GABAergic anesthetics. Our current findings, together with previously published data [13,17], demonstrate that GABAergic anesthetics administered to neonatal rats may not only exacerbate GABAAR-mediated depolarization/stimulation at the time of exposure of neonatal rats to anesthesia, but may also induce long-term impairment in the transition of GABAAR signaling to inhibitory that persists into adulthood. Considering that similar molecular mechanisms, such as an increased NKCC1/KCC2 ratio and impaired GABAAR-mediated inhibition, may be involved in early life environmental stressor-induced dysregulation of stress response systems and neurobehavioral abnormalities [38–40], it is plausible that developmental effects of early life anesthesia and environmental stressors may be additive or synergistic. The concept of the cumulative effects of early life anesthesia and subsequent environmental stressor(s) is supported by our current findings in the amygdala and our recently published data on the effects of etomidate or sevoflurane in combination with a subsequent single episode of maternal separation in the hypothalamus and hippocampus, respectively [13,16]. These findings may offer a mechanistic explanation of the observation that human patients may develop anesthesia-related abnormalities even though the typical anesthesia duration in humans, when normalized to the life span, is much shorter than that shown to induce developmental deficiencies in rodents [41]. The environmental stressors, such as diseases or psychosocial stress, may contribute to developmental outcomes of early life anesthetic exposure in humans. In future studies, it will be important to determine whether significant developmental effects can be detected in animals exposed to even shorter anesthetic durations when post-anesthesia stress regimens more closely model the stressful conditions human subjects may experience.
Although it was not robust, a significant increase in 5% alcohol intake across 30 days of access in male, but not female, rats exposed to ET plus maternal separation could be detected when compared to their control counterparts that exhibited an opposite trend in alcohol consumption. Several factors, including type of anesthetic, duration of anesthesia, and strength of stressor, may contribute to this specific effect. Etomidate is a general anesthetic that shares with propofol and sevoflurane the property of enhancing GABAAR activity. Unlike propofol and sevoflurane, however, ET is known to disrupt adrenal synthesis of corticosterone by inhibiting 11-β-hydroxylase [42]. Hence, ET may activate the HPA axis via reduced negative feedback by corticosterone. In addition, ET-induced 11-β-hydroxylase inhibition increases substrate availability for neuroactive steroid synthesis, which, by enhancing GABAAR depolarization/stimulation, may further increase CRH production [42, 43]. Both increased CRH and corticosterone levels have been linked to greater intake of drugs of abuse, particularly alcohol [44]. Because of expected lower corticosterone levels with ET, due to the anesthetic-caused inhibition of the corticosterone producing enzyme, 11-β-hydroxylase, the effect on alcohol intake might not be as robust as it might have been with other anesthetics. A comparison of the alcohol intake-inducing effects of ET and other GABAergic anesthetics may provide a more complete understanding of the role of neonatal anesthesia in predisposition to alcohol consumption later in life.
Expression and function of CRH in the amygdala has been linked to alcohol intake, particularly in the presence of stressors [45, 46]. Hence, it is possible that the increase in amygdala Crh expression in rats exposed to ET plus maternal separation was causally linked to the increase in alcohol intake. Notably, however, the increased alcohol intake was only evident in male rats, whereas the increase in Crh expression was evident in both sexes. In contrast, the increase in amygdala Nkcc1/Kcc2 ratio was evident only in males, suggesting that it might be more closely related to the change in alcohol intake. Indeed, increased amygdala excitability (the expected outcome of a greater Nkcc1/Kcc2 ratio) would be expected to be anxiogenic, which should promote alcohol drinking [46]. In future studies it will be important to determine whether the changes in expression of these genes and in levels of their respective proteins are localized to specific subregions of the amygdala, as well as to other distinct brain regions. Of direct relevance to this latter point, recent studies have linked downregulated KCC2 and the consequent shift toward more excitatory GABAAR signaling in the ventral tegmental area (VTA) to increased alcohol self-administration, while a KCC2 agonist administered into the VTA reduced alcohol consumption [26, 27].
Although neonatal anesthesia with ET and subsequent maternal separation induced greater changes in the alcohol intake ratio in males, females in general, independent of treatment condition, exhibited greater mean alcohol intake compared to males (Fig. 2). Greater alcohol consumption by adult female rats is consistent with data in the literature [47, 48]. Vetter-O’Hagen et al. [49] suggested that greater alcohol intake by adult female rats can be explained by the suppressive role of the male sex hormone testosterone on ethanol intake in adult male rats, as castration increases ethanol intake and testosterone replacement is sufficient to return ethanol intake to pre-castration levels. As GABAAR signaling plays a crucial role in regulating the function of steroid hormones in general, and sex steroids in particular [50], investigation of effects of developmental exposure to GABAergic anesthetics on adult sex hormones is an important topic of future work.
In summary, a combination of relatively brief ET anesthesia and a single episode of maternal separation during early postnatal development induced significant alterations in expression of amygdala Nkcc1, Kcc2 and Crh, as well as moderate increase in alcohol intake in adulthood, with greater changes in males.
Highlights.
Subsequent stress potentiated developmental effects of neonatal etomidate in rats
The effects included altered expressions of Ch transporters and Crh in the amygdala
Exposed and unexposed rats exhibited opposite changes in alcohol consumption
The effects were sex-specific with greater changes in males
Acknowledgments
Sources of financial support: Supported by the National Institutes of Health (R01GM93036, R01NS091542 and R01NS091542-S to A.E.M.), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (to N.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Servick K, Biomedical Research Researchers struggle to gauge risks of childhood anesthesia, Science 346 (2014) 1161–1162. [DOI] [PubMed] [Google Scholar]
- [2].Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM, Are anesthesia and surgery during infancy associated with altered academic performance during childhood, Anesthesiology 117 (2012) 494–503. [DOI] [PubMed] [Google Scholar]
- [3].Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson AC, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Warner DO, Association between Exposure of Young Children to Procedures Requiring General Anesthesia and Learning and Behavioral Outcomes in a Population-based Birth Cohort, Anesthesiology 127 (2017) 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dimaggio C, Sun LS, Li G, Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort, Anesth. Analg. 113 (2011) 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G, A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children, J. Neurosurg. Anesthesiol. 21 (2009) 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR, Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years: a retrospective matched cohort study, Anesthesiology 125 (2016) 667–677. [DOI] [PubMed] [Google Scholar]
- [7].Ing C, Sun M, Olfson M, DiMaggio CJ, Sun LS, Wall MM, Li G, Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis, Anesth. Analg 125 (2017) 1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP, Behavior and development in children and age at the time of first anesthetic exposure, Anesthesiology 110 (2009) 805–812. [DOI] [PubMed] [Google Scholar]
- [9].Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanić K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO, Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia, Mayo Clin. Proc. 87 (2012) 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO, Early exposure to anesthesia and learning disabilities in a population-based birth cohort, Anesthesiology 110 (2009) 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jevtovic-Todorovic V, Exposure of Developing Brain to General Anesthesia: What Is the Animal Evidence, Anesthesiology 128 (2018) 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE, Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats, Anesthesiology 117 (2012) 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ju LS, Yang JJ, Gravenstein N, Seubert CN, Morey TE, Sumners C, Vasilopoulos T, Yang JJ, Martynyuk AE, Role of environmental stressors in determining the developmental outcome of neonatal anesthesia, Psychoneuroendocrinology 81 (2017) 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tan S, Xu C, Zhu W, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE, Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats, Anesthesiology 121 (2014) 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu C, Tan S, Zhang J, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE, Neonatal anesthesia with sevoflurane: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl- importer antagonists, Psychoneuroendocrinology 60 (2015) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang JJ, Ju LS, Jia M, Zhang H, Sun XR, Ji MH, Yang JJ, Martynyuk AE, Subsequent maternal separation exacerbates neurobehavioral abnormalities in rats neonatally exposed to sevoflurane anesthesia, Neurosci. Lett. 661 (2017) 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE, Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane to the next generation of male, but not female, rats. Br. J. Anaesth. 2018, doi: 10.1016/j.bja.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E, The GABA excitatory/inhibitory shift in brain maturation and neurological disorders, Neuroscientist 18 (2012) 467–486. [DOI] [PubMed] [Google Scholar]
- [19].Ben-Ari Y, The GABA excitatory/inhibitory developmental sequence: a personal journey, Neuroscience 279 (2014) 187–219. [DOI] [PubMed] [Google Scholar]
- [20].Khazipov R, Valeeva G, Khalilov I, Depolarizing GABA and developmental epilepsies CNS, Neurosci. Ther 21 (2015) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mody I, Maguire J, The reciprocal regulation of stress hormones and GABA(A) receptors, Front. Cell. Neurosci. 6 (2012) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kakizawa K, Watanabe M, Mutoh H, Okawa Y, Yamashita M, Yanagawa Y, Itoi K, Suda T, Oki Y, Fukuda A, A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence, Sci. Adv. 2 (2016) e1501723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ben-Ari Y, Damier P, Lemonnier E, Failure of the Nemo Trial: Bumetanide is a promising agent to treat many brain disorders but not newborn seizures, Front. Cell. Neurosci 10 (2016) 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cellot G, Cherubini E, GABAergic signaling as therapeutic target for autism spectrum disorders. Front. Pediatr. 2 (2014) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y, Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring, Science 343 (2014) 675–679. [DOI] [PubMed] [Google Scholar]
- [26].Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA, Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area, Neuron 92 (2016) 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA, Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration, Cell Rep 23 (2018) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rausch A, Zhang W, Beckmann CF, Buitelaar JK, Groen WB, Haak KV, Connectivity-Based Parcellation of the Amygdala Predicts Social Skills in Adolescents with Autism Spectrum Disorder, J. Autism Dev. Disord 48 (2018) 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tajima-Pozo K, Yus M, Ruiz-Manrique G, Lewczuk A, Arrazola J, Montañes-Rada F, Amygdala abnormalities in adults with ADHD, J. Atten. Disord. 22 (2018) 671–678. [DOI] [PubMed] [Google Scholar]
- [30].Sharp BM, Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction, Transl. Psychiatry 7 (2017) e1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roberto M, Gilpin NW, Siggins GR, The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides, Cold Spring Harb Perspect Med 2 (2012) a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Silberman Y, Winder DG, Ethanol and corticotropin releasing factor receptor modulation of central amygdala neurocircuitry: An update and future directions, Alcohol 49 (2015) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J, Peripheral oxytocin administration reduces ethanol consumption in rats, Pharmacol. Biochem. Behav. 140 (2016) 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Paxinos G, Watson C, The rat brain in stereotaxic coordinates, Sixth ed., Academic Press, San Diego, 2007 [Google Scholar]
- [35].Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM, Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus, J. Neuroendocrinol. 19 (2007) 302–308. [DOI] [PubMed] [Google Scholar]
- [36].Galanopoulou AS, Moshé SL, Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra, Exp. Neurol. 184 (2003) 1003–1009. [DOI] [PubMed] [Google Scholar]
- [37].Nuñez JL, McCarthy MM, Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus, Neuroscience 158 (2009) 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Furukawa M, Tsukahara T, Tomita K, Iwai H, Sonomura T, Miyawaki S, Sato T, Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression, Biochem. Biophys. Res. Commun. 493 (2017) 1243–1249. [DOI] [PubMed] [Google Scholar]
- [39].O’Malley D, Dinan TG, Cryan JF, Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 214 (2011) 221–229. [DOI] [PubMed] [Google Scholar]
- [40].Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP, Chronic Unpredictable Mild Stress Induces Loss of GABA Inhibition in Corticotrophin-Releasing Hormone-Expressing Neurons through NKCC1 Upregulation, Neuroendocrinology 104 (2017) 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A, Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats, Anesthesiology 110 (2009) 849–861. [DOI] [PubMed] [Google Scholar]
- [42].Kaminski RM, Rogawski MA, 11β-Hydroxylase inhibitors protect against seizures in mice by increasing endogenous neurosteroid synthesis, Neuropharmacology 61 (2011) 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mody I, Maguire J, The reciprocal regulation of stress hormones and GABA(A) receptors, Front. Cell. Neurosci. 6 (2012) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Phillips TJ. Reed C, Pastor R, Preclinical evidence implicating corticotropin-releasing factor signaling in ethanol consumption and neuroadaptation, Genes Brain Behav. 14 (2015) 98–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bruijnzeel AW, Gold MS, The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence, Brain Res. Brain Res. Rev. 49 (2005) 505–528. [DOI] [PubMed] [Google Scholar]
- [46].Spanagel R, Noori HR, Heilig M, Stress and alcohol interactions: animal studies and clinical significance, Trends Neurosci. 37 (2014) 219–227. [DOI] [PubMed] [Google Scholar]
- [47].Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA, “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood, Horm. Behav. 58 (2009) 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vetter-O’Hagen CS, Varlinskaya EI, Spear LP, Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood, Alcohol Alcohol 44 (2009) 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vetter-O’Hagen CS, Sanders KW, Spear LP, Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats, Behav. Brain. Res. 224 (2011) 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Handa RJ, Weiser MJ, Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis, Front. Neuroendocrinol. 35 (2014) 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]


