Abstract
Recent work has implicated the Lateral Habenula (LHb) in the production of anxiogenic and aversive states. It is innervated by all the major monoamine neurotransmitter systems and has projections that have been shown to modulate the activity of both dopaminergic and serotonergic brain regions. Cocaine is a stimulant drug of abuse that potentiates neurotransmission in these monoamine systems and recent research suggests that the drug’s behavioral effects may be related in part to its actions within the LHb. The present research was therefore devised to test the hypothesis that alterations in serotonin (5-HT) function within the LHb can affect the behavioral response to cocaine. Male rats were fitted with intracranial guide cannula and trained to traverse a straight alleyway once a day for a 1mg/kg i.v. injection of cocaine. Intra-LHb pretreatment with the 5-HT1B agonist CP 94,253 (0, 0.1, or 0.25μg/side) attenuated the development of approach/avoidance “retreat” behaviors known to be a consequence of cocaine’s dual rewarding (approach) and anxiogenic (avoidance) properties. This effect was reversed by co-administration of a selective 5-HT1B antagonist, NAS-181 (0.1μg/side), demonstrating drug specificity at the 5-HT1B receptor. These data suggest that 5-HT1B signaling within the LHb contributes to the anxiogenic effects of cocaine.
Keywords: Cocaine, Lateral Habenula, self-administration, anxiety, runway, serotonin
1.1 Introduction
Cocaine has long been known to produce both an initial euphoric “high” followed by an aversive “crash” characterized by dysphoria, irritability, anxiety, and cravings (Resnick et al. 1977; Gawin 1991; Williamson et al. 1997). This pattern of the rewarding and aversive effects of cocaine fits well with Solomon & Corbit’s “Opponent Process Theory” of motivated behavior (Solomon and Corbit 1974), where an initial shift in affect (either positive or negative) is followed by a delayed opponent process that serves to return the organism to affective homeostasis. The actions of cocaine closely adhere to this theory, in that while the initial effects of the drug are rewarding, this positive state is followed in time by a strong aversive/anxiogenic state (Ettenberg 2004). So, for example, animals develop preferences for distinct places paired with the immediate effects of the drug, but come to avoid places associated with the drug effects present 15-min after an i.v. injection (Ettenberg et al. 1999; Knackstedt et al. 2002; Jhou et al. 2013; Wenzel et al. 2014). Koob and others, have suggested that one of the primary features of drug addiction involves a dysregulation of these homeostatic processes whereby tolerance builds to the initial rewarding effects of the drug while the delayed negative effects become sensitized (Koob and Le Moal 1997; Kreek and Koob 1998; Koob 2003; Ben-Shahar et al. 2004; Koob and Le Moal 2008; Su et al. 2013).
The Lateral Habenula (LHb) has recently been implicated in mediating both rewarding and aversive states (Meye et al. 2013; Lecca et al. 2014; Velasquez et al. 2014). Electrophysiological studies of the LHb suggest a role for this region in preventing or blocking the behavioral impact of rewarding stimuli via an inhibition of midbrain substantia nigra dopamine (DA) “reward” neurons (Matsumoto and Hikosaka 2007; Matsumoto and Hikosaka 2009). Neuroimaging studies in humans have likewise shown that the LHb appears to be selectively activated by aversive stimuli (Ullsperger and Cramon 2003; Shepard et al. 2006). Many of the recent studies of the habenula have focused on its ability to regulate DA release (Hikosaka et al. 2008; Lecourtier et al. 2008). However, it has long been known that the LHb has prominent reciprocal projections to both the Dorsal (DRN) and Median (MRN) Raphé nuclei of the brainstem (Lidov and Molliver 1982; Luo et al. 2015; Metzger et al. 2017). More recent anatomical investigations of this region have revealed that it is the medial portions of the LHb that receive direct serotonergic innervation that in turn send projections to acetylcholine- (ACh), DA- and serotonin- (5-HT) releasing cells of the midbrain and hindbrain. The more lateral portion of the LHb directs its output towards the rostromedial tegmental nucleus (RMTg), a GABA-ergic cell group that exerts inhibition upon both the DA cells of the ventral tegmental area (VTA), as well as on serotonergic cells located in both the dorsal and median raphé nuclei (Metzger et al. 2017). Thus, the LHb is in a prime position to regulate the brain serotoninergic system through both direct and indirect pathways.
Given that 5-HT has been strongly linked to the development and expression of anxiety-like behavior (Watson and Man 2000; Sena et al. 2003; Abrams et al. 2005), coupled with the fact that cocaine is known to elevate synaptic 5-HT through inhibition of the serotonin transporter (SERT) (Cunningham et al. 1992a; Cunningham et al. 1992b; Walsh and Cunningham 1997; Filip et al. 2010) suggests a putative role for 5-HT in the aversive behavioral response to cocaine. Consistent with this hypothesis are prior studies from our own laboratory demonstrating that inactivation of 5-HT cell bodies in the DRN (Ettenberg et al. 2011) and systemic treatment with the 5-HT1A partial agonist, buspirone (Ettenberg and Bernardi 2006), reduced the development of approach/avoidance “retreat” behavior in a runway model of drug self-administration. Buspirone was also effective at selectively diminishing the delayed aversive effects of the drug, while leaving the initial positive effects intact as measured in tests of place conditioning (Ettenberg and Bernardi 2007).
The LHb expresses a variety of 5-HT receptors, including the 5-HT1, 5-HT2, 5-HT3, 5-HT5, and 5-HT7 families (Metzger et al. 2017). Of particular interest to the current study was the presence of inhibitory 5-HT1B receptors in the LHb (Tchenio et al. 2016; Wagner et al. 2016b; Wagner et al. 2016a). The 5-HT1B receptor is an inhibitory Gi-coupled receptor that is primarily located on axon terminals as an auto- or hetero-receptor (Boschert et al. 1994). Additionally, systemic treatment with 5-HT1B agonists has been shown to produce anxiolytic-like effects across multiple behavioral tests (Tatarczynska 2004; Chojnacka-Wojcik et al. 2005), which further supports the hypothesis that this receptor may modulate the anxiogenic response to cocaine. Thus, the present study was designed to investigate the role of 5-HT signaling in the LHb via manipulation of 5-HT1B receptors on the development of the anxiogenic effects of self-administered cocaine.
2. Methods
2.1 Subjects
The subjects were 122 male Sprague–Dawley rats (Charles River Labs, Hollister, CA) weighing approximately 300 g at the time of surgery. Rats were pair-housed within a temperature-controlled (22 °C) vivarium maintained on a reverse 12-h light/dark cycle (lights on at 20:00 h) and had ad libitum access to both food (Purina rat chow) and water. Animals were handled daily for at least 7 days prior to surgery. All methods were conducted in strict adherence to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCSB Institutional Animal Care and Use Committee.
2.2 Surgery
As previously described (Klein et al., 2017), each subject was deeply anesthetized with an intramuscular injection of ketamine and xylazine (56.25 and 7.5 mg/kg, respectively; Abbott Laboratories) and fitted with an indwelling intravenous (i.v.) catheter (13 mm of microrenathane tubing, 0.014 mm inner diameter, 0.33 mm outer diameter; Braintree Scientific) inserted into the right jugular vein, secured in place by silk sutures, and subcutaneously (s.c.) passed to a threaded guide cannula (catalog #313G; Plastics One) that exited though a 2 mm hole on the animal’s back. The guide cannula was cemented to a 3cm square piece of Mersiline mesh (Bard) that was laid flat subcutaneously on the animal’s back where it was secured with surgical glue. While still anesthetized, each rat was then fitted with bilateral intracranial guide cannulae (22 gauge, 9 mm; Catalog #313GA/SPC; Plastics One) stereotaxically aimed 1 mm above the LHb using the following coordinates relative to bregma: AP −3.4, ML ±1.5, and DV −3.2 from skull surface with a lateral inclination of 11°(Paxinos and Watson 2007). During surgery, subjects received the non-opiate analgesic meloxicam, (2 mg/kg s.c. at a concentration of 5 mg/ml in saline) to control for post-surgical pain in addition to Buprenorphine HCl (0.05mg/kg, 0.3mg/ml), and saline for rehydration (3.0 ml s.c.). The catheters were flushed with the antibiotics cefazolin + gentamycin (0.25ml of 1 mg/ml & 5 mg/ml, respectively) to prevent infection and heparinized saline (6.25 IU, 0.1 ml i.v.) to retain patency.
After surgery, catheter patency was maintained via daily flushing with 0.1ml cefazolin and gentamycin (1 mg/ml & 5 mg/ml, respectively) followed by 0.1 ml of heparinized 0.9% physiological saline. Animals were allowed to recover for at least 7 days prior to behavioral testing. Catheter patency was periodically assessed through observation of the loss of the righting reflex following an i.v. injection of the fast-acting barbiturate, methohexital (Brevital, 2.0 mg/kg/0.1 ml). Rats that were unresponsive to Brevital prior to the start of behavioral testing were reimplanted with a new catheter using the left jugular vein and given additional days for recovery. Catheter patency failure during the course of behavioral testing resulted in subject removal from data analysis.
2.3 Drugs
Cocaine hydrochloride (provided by the National Institute on Drug Abuse) was dissolved in 0.9% physiological saline and sterile filtered through a 0.22 μm filter (ThermoScientific). Cocaine was diluted to a dose of 1 mg/kg delivered in a volume of 0.1 ml over a period of 4.6 s via a 10 ml syringe nested in a motorized syringe pump (Razel Scientific Instruments). The dose of 1 mg/kg i.v. cocaine was chosen based upon the results of previous runway work from our laboratory (Raven et al. 2000; Ettenberg 2004; Ettenberg and Bernardi 2006; Wenzel et al. 2011; Wenzel et al. 2014).
The 5-HT1B agonist CP 94,253 dihydrochloride (Sigma- Aldrich) was prepared in a vehicle solution of aCSF (l-ascorbic acid 0.35 g/L, NaCl 8.47 g/L, KCl .20 g/L, MgCl2 .20 g/L, CaCl2 .18 g/L, NaH2PO4 .276 g/L, Na2HPO4 .5362 g/L) for intracranial infusion at the concentrations of 0.1 or 0.25 μg/0.5 μl. In Experiment II, the 5-HT1B antagonist NAS-181 was prepared in the same vehicle as CP 94,253 and co-infused at a dose of 0.1/0.5 μl/side. The drug CP 94,253 was selected because it shows the greatest affinity for 5-HT1B over other receptors in the 5-HT1 family (Koe et al. 1992), and has produced behavioral effects in prior studies using intracranial administration at comparable doses (De Almeida et al. 2006; Veiga and Miczek 2007). The drug NAS-181 was selected due to its high affinity for the 5-HT1B receptor and ability to block agonist binding (Stenfors et al. 2000; De Groote et al. 2002; De Groote et al. 2003). Previous work from our laboratory has shown that 0.1μg of NAS-181 is sufficient to reverse the behavioral effects of an even higher dose of CP 94,253 than was used in the present study (Klein et al. 2017).
2.4 Apparatus
Experimental testing was conducted in two identical wooden straight-arm runways. Each apparatus measured 155 cm (L) × 15 cm (W) × 40 cm (H). On opposite ends of the straight alley were identically sized start and goal boxes (each measuring 24 cm × 25 cm × 40 cm) each separated from the middle runway section of the apparatus by retractable doors. Along the interior length of the alley were 13 infrared photodetector- emitter pairs positioned in the walls 16 cm apart from one another. Input from these photocells was fed through an Any-Maze interface (Stoetling) to a laptop computer running Any-Maze software, which recorded the subjects’ locations in the runway in real time throughout each trial. For a more detailed description of the runway apparatus and drug delivery system see Geist and Ettenberg (1990).
2.5 Procedures
Prior to the first cocaine-reinforced trial, subjects were individually placed into the start box and permitted to freely explore the runway for 10 min to allow for acclimation to the apparatus (the goal door remained closed to prevent entry into the goal box). The first of 16 single daily runway trials began the following day.
The subjects were administered bilateral intra-LHb infusions (0.5 μl/side) of one of two doses of CP 94,253 (0.1 or 0.25 μg/side (Experiment I), or the combination of 0.25μg CP 94,253 with 0.1μg NAS-181 per side (Experiment II), or vehicle prior to each runway trial. The infusions were administered slowly over 120 s using a 25 μl Hamilton syringe that was seated in a motorized syringe pump (KD Scientific). The syringe was connected via PE20 tubing to 28-gauge internal cannula (catalog #313LI/SPC Plastics One) that, when inserted into the implanted intracranial guide cannula, projected 2 mm beyond the tip of the guide cannula. The internal cannula were left in place for 60 s following each infusion to permit diffusion of the drug away from the injection tip. After 10 min, each subject was moved to the runway apparatus, connected to the i.v. drug delivery system, and placed into the start box where, after 5 s, the start door was opened and the trial initiated. Animals were free to traverse the runway until they entered the goal box at which point the goal door automatically closed behind them (to prevent retracing) and an i.v. infusion of 1.0 mg/kg cocaine (in 0.1 ml) was administered over 4.6 s. After 5 min, the subjects were removed from the goal box, disconnected from the drug delivery system, and returned to their home cages. On the rare occasion that an animal did not enter the goal box within 10 min, it was gently encouraged (pushed from behind) to enter the goal box, where it then received an i.v. injection of cocaine. All trials for a given subject were conducted in the same apparatus. To maintain catheter patency, animals were flushed with 0.1 ml of cefazolin/gentamycin followed by 0.1 ml heparinized saline after removal from the apparatus.
2.6 Spontaneous locomotor activity
To ensure that central application of the intra-LHb infusions did not produce nonspecific alterations in the response capacity of the subjects, they were examined in a test of spontaneous locomotor activity following completion of runway testing. Locomotor behavior (total distance traveled) was measured in 12 identical Plexiglas chambers each measuring 20 cm (L) × 40 cm (W) × 20 cm (H) (Kinder Scientific). Each test chamber was equipped with an array of fifteen infrared photodetector-emitter pairs evenly spaced along its long axis and seven along its narrow axis, all 8 cm above the floor surface. Movement within the chamber produced photobeam interruptions that were recorded by a desktop computer running Motor Monitor software (Kinder Scientific). At the start of testing, all animals were allowed to acclimate to the locomotor chambers for 60 min. Rats were then removed from the test chambers, administered the same bilateral microinjections that they had received previously during runway testing, and immediately returned to the locomotor chambers for an additional 15-min test session.
2.7 Histology
After completion of behavioral testing, animals were euthanized with an overdose of sodium pentobarbital and phenytoin sodium solution (Euthasol; Virbac) and perfused through the heart with 120 mL phosphate-buffered saline (PBS) followed by 120 mL 4% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed in 4% PFA, after which cannula placements were determined from Nissl-stained 40 μm frozen coronal sections.
3. Results
3.1 Histology
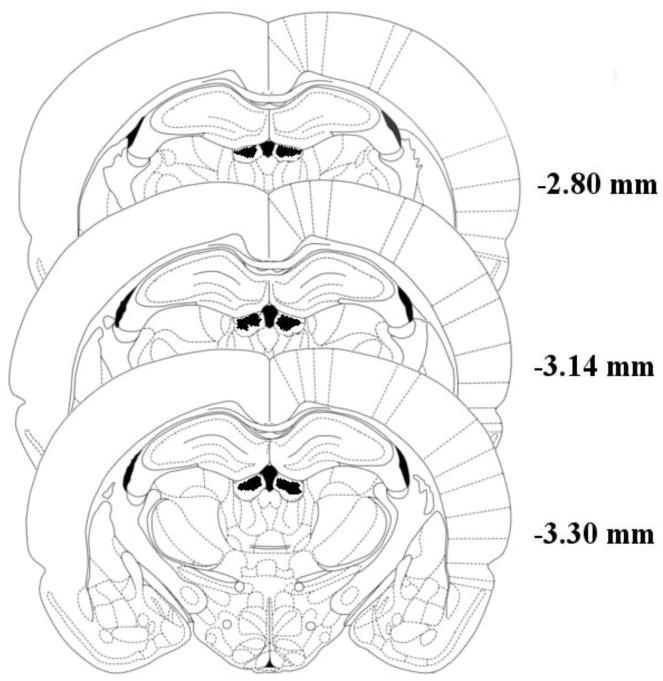
Figure 1 shows the placement of bilateral cannula tips located in the targeted region (figure adapted from the brain atlas of Paxinos & Watson, 2005). Inclusion of subjects in the experiment required strict histological confirmation that both cannula tips were accurately localized to the LHb. Any animal with microinjector tips located outside the target region or displaying significant necrosis around injector tips was removed from the study (n=23). The final histological determination of a subject’s inclusion in the study was made by an individual blind to that animal’s treatment group (AE). An additional 30 animals failed to maintain catheter patency throughout the duration of the study and were similarly removed from the data analysis resulting in a final total sample size of 69 subjects for analysis.
Figure 1.
Shaded areas indicate regions where successful cannula tip placements could be verified. Numbers represent distance of coronal slices posterior to bregma. Due to the volume of drug injected (0.5 μl/side) and the target region’s close proximity to the midline, the area affected likely includes a broad region of both the Lateral Habenula (LHb) and Medial Habenula (MHb). Figure adapted from Paxinos & Watson, 2005.
3.2 Experiment I: Intra-LHb Infusion of CP 94,253
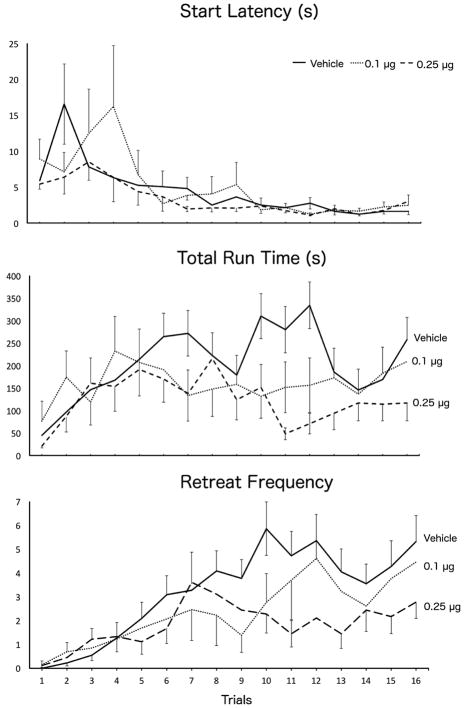
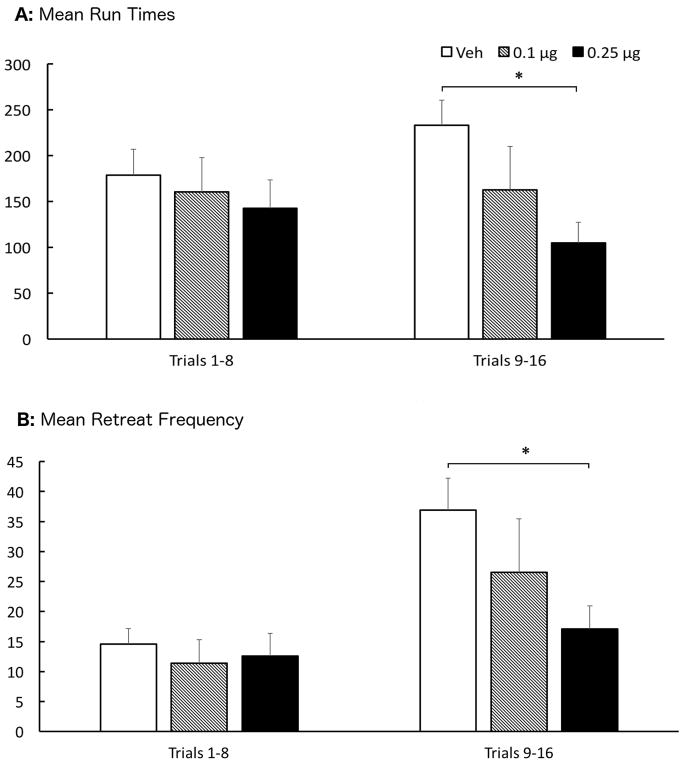
This experiment tested the effects of bilateral intra-LHb microinjections of CP 94,253 (0, 0.1, or 0.25 μg/0.5 μl/side) on the runway behavior of animals approaching and entering a goal box associated with the administration of 1.0mg/kg i.v. cocaine. Group sizes were N = 22, 13, 18, respectively. Figure 2 depicts the mean (+SEM) runway performance of the 3 groups of animals during the 16 days of runway testing. A two-factor (Group x Trials) Analysis of Variance (ANOVA) conducted on the start latency data revealed a significant main effect of Trial F(15,750)=6.822, p < .001, but no main effect of Group and no Group x Trial interaction (p’s > .05). Thus, all animals, irrespective of group, learned the association between the goal box and drug delivery and displayed an increasing motivation to seek the drug (faster start times) as testing progressed. However, despite the fast start latencies, animals took longer and longer to enter the goal box. Another two-factor (Group x Trial) ANOVA computed on the run time data confirmed a significant main effect of Trial (F(15,750)=2.876, p < .001) but in the opposite direction of start times (see middle panel of figure 2). While there was no main effect of Group (F(2,50)=2.298, p=.111), there was a statistically significant Group x Trial interaction (F(30,750)=1.641, p = .017), reflecting the fact that on the early trials of testing the Groups performed comparably while by the end of testing the run times of the three groups diverged. A series of pairwise comparisons computed on these data revealed a significant group difference between animals treated with vehicle versus the 0.25μg dose (p=.038). To confirm this assessment, the mean Run Times for the three groups were recomputed by averaging performance during the first 8 trials to that observed during the final 8 trials. Those results are depicted in Figure 3A. A two-factor (Group x Trials) ANOVA computed on these data produced results consistent with the ANOVA computed on all trials. There was no main effect of Trials (F(1,50)=0.185, p=.669), or Group (F(2,50)=2.298, p=.111), however there was a significant Group X Trials interaction (F(2,50)=3.982, p=.025). To elucidate the source of this interaction, a one-way ANOVA was computed on the data for each set of trials, which revealed no group differences during trials 1–8 (F(2,52)=0.373, p=.691), but a significant effect of Group during trials 9–16 (F(2,52)=4.778, p=.013). Post hoc analysis by LSD testing showed that the 0.25μg dose was significantly different than vehicle-treated animals (p=.003).
Figure 2.
Group Mean (±SEM) start latencies (top panel), run times (middle panel), and approach-avoidance retreat behavior (bottom panel) of animals running a straight alley once daily for an infusion of 1 mg/kg i.v. cocaine after pretreatment with either 0.1 (dotted lines) or 0.25 μg (dashed lines) CP 94,253, or vehicle (solid lines) into the LHb.
Figure 3.
Top Panel (A): Group Mean (±SEM) Total Run Times averaged over the first half (left) of trials and the last half (right) for animals treated with bilateral infusions of vehicle (Veh), 0.1μg or 0.25μg of CP 94,253 into the LHb. Bottom Panel (B): Group Mean (±SEM) retreat frequency summed over the first half and the last half of runway trials. *(p<.05) when compared to vehicle
The increase in Run Times observed as testing progressed was mirrored by the frequency of approach-avoidance retreat behaviors (see figures 2 & 3B). Not surprisingly, an animal that stops and retreats back toward the start box will necessarily delay its entry into the goal box. This was confirmed via Pearson correlational analyses, whereby each animal’s run time was correlated with the frequency of approach-avoidance retreats during each of the 16 days of testing. Unsurprisingly, the correlation between run times and retreats was statistically significant on all 16 days of testing (Table 1).
Table 1.
Correlations between Total Run Time and Retreat Frequency across trials
| Trial | Pearson R= | P - Value |
|---|---|---|
| 1 | .442 | .001 |
| 2 | .392 | .004 |
| 3 | .614 | <.001 |
| 4 | .716 | <.001 |
| 5 | .608 | <.001 |
| 6 | .728 | <.001 |
| 7 | .620 | <.001 |
| 8 | .558 | <.001 |
| 9 | .638 | <.001 |
| 10 | .725 | <.001 |
| 11 | .732 | <.001 |
| 12 | .770 | <.001 |
| 13 | .775 | <.001 |
| 14 | .788 | <.001 |
| 15 | .694 | <.001 |
| 16 | .750 | <.001 |
A two-factor (Group x Trials) ANOVA was computed on the approach-avoidance retreat data (Figure 2, bottom panel), which revealed a significant main effect of Trials (F(15,750)=8.865, p<.001 ), but no effect of Group (F(2,50)=1.909, p=.159), and only a marginal Group x Trials interaction (F(30,750)=1.404, p=.075. However, as was done for the run time analysis, the data was analyzed by comparing performance over the first half of trials versus the last half of trials. The results were comparable to those observed for run times. A two-factor (Group X Trials) ANOVA was computed on the data collapsed across trials. This analysis revealed a significant main effect of Trials (F(1,50)=30.742, p<.001), no main effect of Group (F(2,50)=1.909, p=.159), and a significant Group x Trials interaction (F(2,50)=4.891, p=.011). As was the case for Run Times, a one-way ANOVA comparing group behavior over the first half of trials produced no reliable differences (F(2,52)=.233, p=.793), while a significant group effect was identified during the second half of runway trials (F(2,52)=3.260, p=.047). Post hoc analysis via LSD testing showed a significant difference between the 0.25μg dose group and vehicle (p=.014). Indeed, as Fig 3B illustrates, while retreats increased during the second half of the experiment in the vehicle group (as we have seen previously; e.g. see review by Ettenberg, 2004), intra-LHb pretreatment with the 5-HT1B agonist CP 94,253 dose-dependently reversed this effect.
3.3 Experiment I: Locomotor Activity
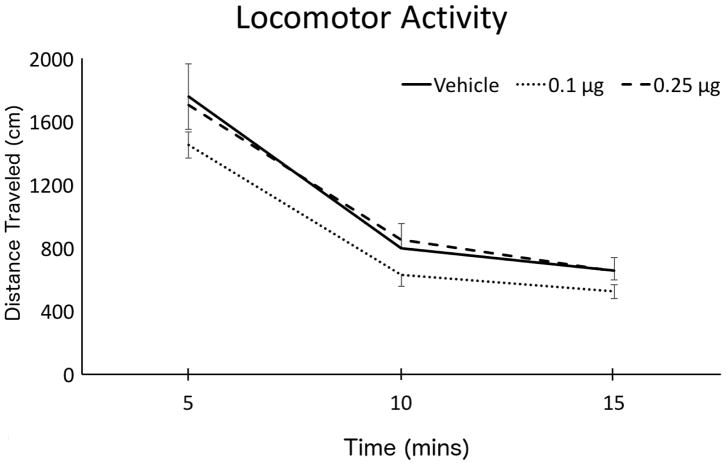
The effects of CP 94,253 on spontaneous locomotor activity was assessed by a two-factor (Group x Time) ANOVA computed on data acquired for 15 mins following drug infusion (depicted in Figure 4). Although there was an expected reduction in locomotor activity over time as animals habituated to the apparatus (significant main effect of Time: F(2,86)=165.85, p<.001), there was no main effect of Group(F(2,43)=1.315, p=.279) and no Group x Time interaction (F(4,86)=.442, p=.778). Thus, manipulation of the 5-HT1B receptor in the LHb produced no perceptible alterations in the spontaneous ambulatory behavior of subjects relative to vehicle control.
Figure 4.
Group Mean (±SEM) spontaneous locomotor activity immediately after infusion of CP 94,253. The total distance traveled (cm) was recorded over 5-min intervals for a 15-min test session.
3.4 Experiment II: Co-administration of 5-HT1B agonist and antagonist
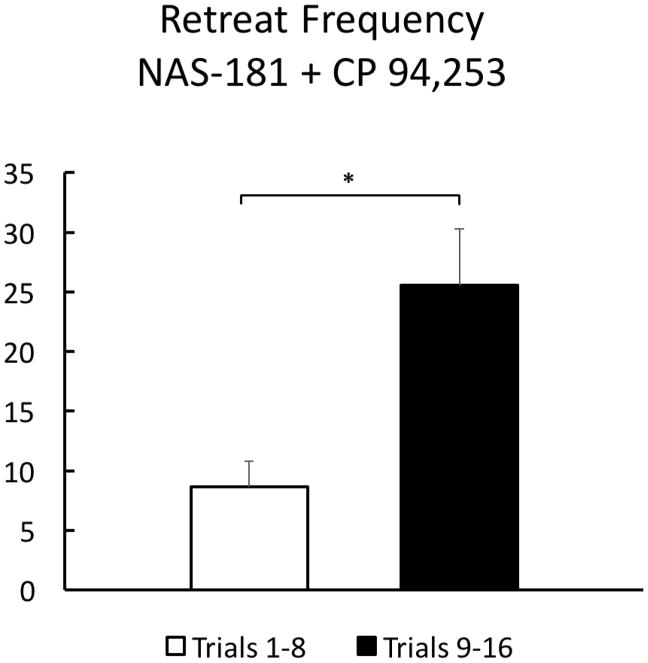
To demonstrate specificity to the 5-HT1B receptor subtype, the effective dose of 0.25μg CP 94,253 was challenged by co-administration of the highly selective 5-HT1B receptor antagonist, NAS-181 (0.1μg/side; N=16). NAS-181 was effective at reversing the effect of the agonist on approach-avoidance retreat behavior when co-administered with 0.25μg of CP 94,253 (Figure 5). As in the previous experiment, data for this group was collapsed into the first half and second half of runway trials, and then analyzed by a paired sample two-tailed t-test, which revealed a significant difference in retreat behavior over the course of trials (t(15)=−2.503, p=.024). Indeed, these animals behaved much like the vehicle-treated subjects in Experiment 1 (Fig 3B) and increased the number of retreats as testing progressed. A direct comparison between the retreat behavior of Vehicle-treated animals from Experiment I on Trials 9–16 and the animals in Experiment II from the same time period showed no differences between the groups (t(36)=1.207, p=.235). The antagonist, therefore, reversed the retreat-suppressing effects of the 5-HT1B agonist alone. Additionally, in a subsequent locomotor test, treatment with CP 94,253 + NAS-181 produced no reliable changes (compared to the agonist alone) in locomotor behavior (data not shown).
Figure 5.
Group Mean (±SEM) retreat frequency was summed over the first half (left) of trials and the last half (right) for the N=16 animals who received the combined infusion of 0.25μg CP 94,253 and 0.1μg NAS-181 prior to runway testing. * p<.05
4.1 Discussion
The present study examined the impact of 5-HT1B receptor stimulation in the LHb on the behavior of animals running a straight alley for i.v. cocaine reinforcement. This work builds upon an extensive body of work in our laboratory which shows that cocaine-reinforced animals develop a characteristic pattern of approach-avoidance retreat behavior that reflects the conflict of entering a location in which subjects had formed mixed positive and negative associations (Ettenberg et al. 1999; Raven et al. 2000; Ettenberg 2004), and which has been replicated by others (Jhou et al. 2013). Treatment with a selective 5-HT1B agonist into the LHb produced a dose-dependent decrease in the frequency of retreat behaviors, an effect that was reversed by co-administration with a selective 5-HT1B antagonist.
These results cannot be easily accounted for by some form of nonspecific motoric effects of the treatment, since neither treatment protocol produced alterations in the spontaneous locomotor activity of subjects. Alternatively, one might argue that the observed reductions in retreat behavior produced by the 5-HT1B agonist do not stem from a treatment-induced decrease in the drug’s aversive effects, but rather an increase in the rewarding properties of cocaine. For example, Parsons et al. (1998) reported that systemic or intra-ventricular administration of 5-HT1B agonists produced changes in cocaine self-administration that mirrored what is seen when the unit dose of cocaine is increased (Parsons et al. 1998). It has also been suggested that 5-HT1B receptors present on the GABAergic interneurons of the VTA could lead to a disinhibition of dopamine release, which would be consistent with an enhancement of cocaine reward (Castanon et al. 2000; Filip et al. 2003). It is of course entirely possible that the same drug, when applied to different brain regions, produces region-specific alterations in the activity of different neurotransmitter systems. To turn the argument on its head, the putative enhancement in cocaine reward observed by Parsons et al. (1998) could have been due to either a potentiation of the dopaminergic reward signal from the VTA, or alternatively from a reduction in the drug’s aversive effects via the LHb, leading to a net increase in reward. Indeed, when a 5-HT1B agonist is administered systemically, it would be difficult to tease apart potentially opposing actions stemming from drug effects in different brain regions. Additionally, in the current study, we note that CP 94,253 had no effect on subjects’ start latencies, a measure reflective of the positive incentive properties of goal box stimuli (such as cocaine) (Ettenberg, 2004, 2009). When taken together, the current findings are therefore consistent with the hypothesis that the observed reductions in approach-avoidance retreat behaviors were due, not to an elevation in the rewarding properties of cocaine, but rather to a reduction in the drug’s negative/anxiogenic effects.
There is ample evidence demonstrating an intimate inverse relationship between the VTA and LHb. Early studies investigating the relationship between these two regions found that stimulation of the LHb produces an inhibition of VTA DA cell firing (Christoph et al. 1986). It was later shown by Matsumoto & Hikosaka (2007) that this circuit is responsible for encoding an “anti-reward” signal. Their experiments elegantly showed how the LHb responds to both negative outcomes, as well as the lack of an expected reward, by an increase in its own activity while simultaneously inhibiting the VTA. Likewise, the rewarding conditions that produced strong activations of VTA DA cells also inhibited the activity of LHb neurons (Matsumoto and Hikosaka 2007). In later research, these projections from the LHb were found to be glutamatergic and inhibitory to the VTA via activation of GABAergic cells of the RMTg (Jhou et al. 2009; Stamatakis and Stuber 2012). Additionally, the reverse pathway has also been identified using optogenetic methods—a population of presumptively GABAergic VTA neurons that inhibit the LHb and promote reward (Stamatakis et al. 2013). Jhou et al. (2013) have also reported that the initial rewarding effects of cocaine are associated with an inhibition of LHb neurons and that the onset of the delayed anxiogenic response to the drug is directly associated with an increase in LHb activity and a resulting suppression of VTA DA neurons. When viewed in this context, the current findings suggest a role for 5-HT release within the LHb as a contributing factor to the LHb’s modulation of the affective response to cocaine.
In addition to its role in regulating the VTA, the LHb is also one of the major inputs to the dorsal and median raphé 5-HT systems (see Metzger, Bueno, & Lima, 2017 for a recent review). While the LHb has been shown to receive reciprocal projections back from the DRN (Zhao et al. 2015) we note that the bulk of 5-HT innervation targets the medial habenula (MHb) (Morin and Meyer-Bernstein 1999; Tchenio et al. 2016; Wagner et al. 2016b; Wagner et al. 2016a; Metzger et al. 2017). Analysis of mRNA transcripts in this region suggests that the 5-HT1B receptor has an elevated expression in the LHb relative to the MHb (Wagner et al. 2016b; Wagner et al. 2016a; Metzger et al. 2017), which does support the hypothesis that the observed behavioral effects in this study were due primarily to modulation of the LHb, rather than MHb. However, due to the relatively small size and immediate adjacency of the LHb and MHb, it cannot be conclusively determined with any anatomical precision whether our manipulations produced their effects via modulation of the LHb, MHb, or both structures. Although most of the research in this area has focused on the LHb, the MHb has also been implicated in modulating the same affective states as the LHb, including drug withdrawal, depression, stress, and anxiety (Viswanath et al. 2014). The MHb has also received a renewed interest for its role in nicotine addiction, due to the discovery that it contains a high concentration of nicotinic ACh receptors and has outputs to areas that regulate ACh release throughout the brain (Viswanath et al. 2014). Clearly there is a need for more research on the MHb and its role in the behavioral effects of drugs of abuse.
5-HT itself appears to have mixed effects in the LHb. On post-synaptic LHb neurons, 5-HT produces primarily stimulatory effects via activation of 5-HT2/3 receptors which enhance cellular depolarization (Han et al. 2015; Zuo et al. 2016). 5-HT can also act as an inhibitory signal through activation of inhibitory 5-HT1B heteroreceptors located presynaptically on the glutamatergic inputs to the LHb (Hwang and Chung 2014; Xie et al. 2016). Additionally, local application of a 5-HT1B agonist into the LHb has been shown to reduce 5-HT release as measured through microdialysis (Adell et al. 2001). Thus, there are two possible mechanisms that could explain the results observed in this study. First, it could be that the 5-HT1B agonist is reducing LHb activity indirectly via inhibition of glutamatergic inputs to the LHb; alternatively, these results could be explained by an inhibition of 5-HT release in the LHb via activation of the 5-HT1B autoreceptors localized on the serotonergic fibers innervating this region. Preliminary studies in our lab are currently underway to address these two possibilities.
Highlights.
Rats running an alley for 1mg/kg cocaine develop a characteristic pattern of retreats
5-HT1B receptors in the LHb mediate the development of retreat behavior
Infusion of a 5-HT1B antagonist reversed the agonist induced reduction of retreats
No changes were observed in spontaneous locomotor activity
Acknowledgments
The authors wish to thank Dr. Skirmantas Janusonis for his help in understanding the anatomy of 5-HT innervation of the habenula. This work was supported by NIDA grant DA-033370 awarded to AE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hay-Schmidt a, et al. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Artigas F. The role of 5-HT 1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Boschert U, Aït Amara D, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, et al. Modulation of the effects of cocaine by 5-HT1B receptors: A comparison of knockouts and antagonists. Pharmacol Biochem Behav. 2000;67:559–566. doi: 10.1016/S0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Chojnacka-Wojcik E, Klodzinska A, Tatarczynska E. The anxiolytic-like effect of 5-HT1B receptor ligands in rats: a possible mechanism of action. J Pharm Pharmacol. 2005;57:253–257. doi: 10.1211/0022357055399. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham Ka, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992a;11:112–23. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- Cunningham Ka, Paris JM, Goeders NE. Serotonin neurotransmission in cocaine sensitization. Ann N Y Acad Sci. 1992b;654:117–27. doi: 10.1111/j.1749-6632.1992.tb25960.x. [DOI] [PubMed] [Google Scholar]
- De Almeida RMM, Rosa MM, Santos DM, et al. 5-HT(1B) receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology (Berl) 2006;185:441–50. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- De Groote L, Klompmakers AA, Olivier B, Westenberg HGM. An evaluation of the effect of NAS-181, a new selective 5-HT1B receptor antagonist, on extracellular 5-HT levels in rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:89–94. doi: 10.1007/s00210-002-0685-0. [DOI] [PubMed] [Google Scholar]
- De Groote L, Olivier B, Westenberg HG. Extracellular serotonin in the prefrontal cortex is limited through terminal 5-HT1B autoreceptors: A microdialysis study in knockout mice. Psychopharmacology (Berl) 2002;162:419–424. doi: 10.1007/s00213-002-1117-z. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–8. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi R. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006;85:393–399. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi R. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Ofer O, Mueller C. Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol …. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Raven Ma, Danluck Da, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–12. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegaliński E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol. 2010;15:227–49. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Papla I, Nowak E, et al. Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on cocaine discrimination in rats. Eur J Pharmacol. 2003;459:239–245. doi: 10.1016/S0014-2999(02)02873-X. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine Psychology and Addiction: Neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Geist T, Ettenberg A. A Simple Method for Studying Intravenous Drug Reinforcement in a Runway. Pharmacol Biochem Behav. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Han LN, Zhang L, Li LB, et al. Activation of serotonin2C receptors in the lateral habenular nucleus increases the expression of depression-related behaviors in the hemiparkinsonian rat. Neuropharmacology. 2015;93:68–79. doi: 10.1016/j.neuropharm.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: Crossroad between the Basal Ganglia and the Limbic System. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EK, Chung JM. 5HT1B receptor-mediated pre-synaptic depression of excitatory inputs to the rat lateral habenula. Neuropharmacology. 2014;81:153–165. doi: 10.1016/j.neuropharm.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, et al. Article The Rostromedial Tegmental Nucleus (RMTg), a GABAergic Afferent to Midbrain Dopamine Neurons, Encodes Aversive Stimuli and Inhibits Motor Responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–12. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AK, Brito MA, Akhavan S, et al. Attenuation of the anxiogenic effects of cocaine by 5-HT1B autoreceptor stimulation in the bed nucleus of the stria terminalis of rats. Psychopharmacology (Berl) 2017;234:485–495. doi: 10.1007/s00213-016-4479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav. 2002;72:931–936. doi: 10.1016/S0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- Koe BK, Nielsen Ja, Macor JE, Heym J. Biochemical and behavioral studies of the 5-HT{-1B} receptor agonist, CP-94, 253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- Koob G. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: An anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, DeFrancesco A, Moghaddam B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov HGW, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: Ascending pathways and terminal fields. Brain Res Bull. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Luo XF, Zhang BL, Li JC, et al. Lateral habenula as a link between dopaminergic and serotonergic systems contributes to depressive symptoms in Parkinson’s disease. Brain Res Bull. 2015;110:40–46. doi: 10.1016/j.brainresbull.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M, Bueno D, Lima LB. The lateral habenula and the serotonergic system. Pharmacol Biochem Behav. 2017 doi: 10.1016/j.pbb.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Lecca S, Valentinova K, Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Front Hum Neurosci. 2013;7:860. doi: 10.3389/fnhum.2013.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Meyer-Bernstein EL. The ascending serotonergic system in the hamster: Comparison with projections of the dorsal and median raphe nuclei. Neuroscience. 1999;91:81–105. doi: 10.1016/S0306-4522(98)00585-5. [DOI] [PubMed] [Google Scholar]
- Parsons L, Weiss F, Koob G. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CR. The Rat Brain in Stereotaxic Coordinates. 2007. [DOI] [PubMed] [Google Scholar]
- Raven Ma, Necessary BD, Danluck Da, Ettenberg a. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8:117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195:696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RLH, et al. The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav Brain Res. 2003;142:125–133. doi: 10.1016/S0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Holcomb HH, Gold JM. The Presence of Absence: Habenular Regulation of Dopamine Neurons and the Encoding of Negative Outcomes. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–53. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors C, Yu H, Ross SB. Enhanced 5-HT metabolism and synthesis rate by the new selective r5-HT1B receptor antagonist, NAS-181 in the rat brain. Neuropharmacology. 2000;39:553–560. doi: 10.1016/S0028-3908(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Su Z-I, Wenzel J, Ettenberg A, Ben-Shahar O. Prior extended daily access to cocaine elevates the reward threshold in a conditioned place preference test. Addict Biol. 2013 doi: 10.1111/adb.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZI, Wenzel J, Baird R, Ettenberg A. Comparison of self-administration behavior and responsiveness to drug-paired cues in rats running an alley for intravenous heroin and cocaine. Psychopharmacology (Berl) 2011;214:769–778. doi: 10.1007/s00213-010-2088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarczynska E. Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav …. 2004:523–534. doi: 10.1097/00008877-200412000-00001. [DOI] [PubMed] [Google Scholar]
- Tchenio A, Valentinova K, Mameli M. Can the Lateral Habenula Crack the Serotonin Code? Front Synaptic Neurosci. 2016;8:1–7. doi: 10.3389/fnsyn.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Von Cramon DY. Error Monitoring Using External Feedback: Specific Roles of the Habenular Complex, the Reward System, and the Cingulate Motor Area Revealed by Functional Magnetic Resonance Imaging. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga C, Miczek K. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Brazilian J. 2007;40:825–830. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Front Hum Neurosci. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath H, Carter AQ, Baldwin PR, et al. The medial habenula: still neglected. Front Hum Neurosci. 2014;7:1–6. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F, Bernard R, Derst C, et al. Microarray analysis of transcripts with elevated expressions in the rat medial or lateral habenula suggest fast GABAergic excitation in the medial habenula and habenular involvement in the regulation of feeding and energy balance. Brain Struct Funct. 2016a;221:4663–4689. doi: 10.1007/s00429-016-1195-z. [DOI] [PubMed] [Google Scholar]
- Wagner F, French L, Veh RW. Transcriptomic-anatomic analysis of the mouse habenula uncovers a high molecular heterogeneity among neurons in the lateral complex, while gene expression in the medial complex largely obeys subnuclear boundaries. Brain Struct Funct. 2016b;221:39–58. doi: 10.1007/s00429-014-0891-9. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Cunningham Ka. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Watson S, Man MS. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14:419–421. doi: 10.1177/026988110001400415. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cotten SW, Dominguez HM, et al. Noradrenergic β-receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci. 2014;34:3467–74. doi: 10.1523/JNEUROSCI.3861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Waldroup SA, Haber ZM, et al. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology (Berl) 2011;217:221–30. doi: 10.1007/s00213-011-2267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, et al. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/S0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Xie G, Zuo W, Wu L, et al. Serotonin modulates glutamatergic transmission to neurons in the lateral habenula. Sci Rep. 2016;6:23798. doi: 10.1038/srep23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhang BL, Yang SJ, Rusak B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav Brain Res. 2015;277:89–98. doi: 10.1016/j.bbr.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Zuo W, Zhang Y, Xie G, et al. Serotonin stimulates lateral habenula via activation of the post-synaptic serotonin 2/3 receptors and transient receptor potential channels. Neuropharmacology. 2016;101:449–459. doi: 10.1016/j.neuropharm.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]