Abstract
Cytotoxic chemotherapies, molecularly targeted therapies, immunotherapies, radiotherapy, stem cell transplants, and endocrine therapies may lead to hair disorders (including alopecia, hirsutism, hypertrichosis, pigmentary and textural hair changes). The mechanisms underlying these changes are varied and remain incompletely understood, hampering the development of preventive or therapeutic guidelines. The psychosocial impact of chemotherapy -induced alopecia has been well-documented mainly in the oncology literature, however the effect of other alterations such as radiation-induced alopecia, hirsutism, changes in hair color or texture on quality of life have not been described. This article reviews clinically significant therapy-related hair disorders in cancer patients, underlying pathophysiological mechanisms, severity grading scales, patient reported quality of life instruments, management strategies, and future translational research opportunities.
Keywords: chemotherapy-induced alopecia, anagen effluvium, catagen effluvium, brittleness, curling, depigmentation, hirsutism, hyperpigmentation, hypertrichosis, hypopigmentation, eyelash alopecia, eyebrow alopecia, hair repigmentation, straightening, trichomegaly, cancer patients
Introduction
Cancer is a major public health problem worldwide. In 2016, more than 1.6 million new cancer cases were projected in the United States and 32 million worldwide, and of these, approximately 60% received systemic therapies, and 50% underwent radiotherapy.1 The most commonly encountered hair disorder is cytotoxic chemotherapy-induced alopecia (CIA).2-4 However, several other anticancer therapies may also be related to alopecia, such as radiation,5 targeted therapies,6 immunotherapies,7 stem cell transplants,8 and endocrine agents.9 In addition, alterations in hair pigmentation, texture, and growth may also be encountered,10 although they have not been systematically documented in this patient population. The impact of these disorders, namely alopecia, on cancer patients' quality of life (QoL) cannot be disregarded.11 Indeed, 17% of patients with gynecologic cancers reported that alopecia was the most traumatic adverse event (AE) during their treatment, 30% were severely limited, and up to 14% would consider rejecting curative cancer therapies if they are associated with alopecia.12-14
The information contained in this continuing medical education article intends to contribute to greater knowledge on these untoward events in order to optimize the assessment and management of hair disorders in cancer patients.
Epidemiology
Key point
Hair changes attributed to anticancer therapies are expected to occur in at least 65% of patients receiving cytotoxic therapies, 15% with targeted therapies, <2% on immunotherapies, and around 100% in areas of the head treated with radiotherapy
The widespread use of systemic anticancer therapies, their numerous combinations, and underreporting of hair disorders yield mixed incidence reports (Table 1), but these events at varying degrees of severity are frequent across almost all types of interventions. The estimated incidence of CIA is approximately 65% and tends to vary by the specific drugs and different regimens.15 Alopecia is typically observed in almost every patient undergoing radiotherapy for central nervous system malignancies treated with photon radiotherapy (traditional radiotherapy) or proton radiotherapy, and intensity and rate would increase as the dose exceeds the threshold.16, 17 With targeted therapies, the calculated overall incidence of all-grade alopecia was reported at 15%.6, 18 An overall incidence of all grades of alopecia were reported at 1-2% with immune checkpoint inhibitors, including cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), and the programmed cell death protein-1 (PD-1) receptors and its ligand (PD-L1).7, 19 In nearly 100% of patients status post hematopoietic stem cell transplantation, complete alopecia is attributed to the conditioning cytotoxic chemotherapies (i.e. busulfan, melphalan, fludarabine).20 Endocrine therapies (i.e. anastrozole, letrozole, exemestane) have been related with alopecia in up to 25%.9 Whereas alopecia receives the most attention among hair disorders in the oncology realm, changes in hair structure (becoming curly or straight) and color (hyper or hypopigmentation) have been reported in approximately 65% of patients during and after cytotoxic chemotherapies,21 in 30% of those receiving targeted therapies,22 and 2% with immunotherapies19 (Table 1).
Table 1. Selected anticancer therapies (representative) commonly causing hair changes.
| Hair disorders and cancer therapy | Tumor types indicated or under investigation | Incidence (%) or case reports |
|---|---|---|
| Alopecia/hair loss | ||
| Chemotherapies | ||
| Cyclophosphamide23-27 | Breast cancer, leukemia, lymphoma, multiple myeloma, neuroblastoma, retinoblastoma, ovarian cancer | 25 (low dose) ∼ 100 (high dose) |
| Daunorubicin 28, 29 | AML, ALL | ∼ 100 |
| Docetaxel30-34 | Breast cancer, gastric cancer, head and neck cancer, lung cancer, prostate cancer | ∼ 100 |
| Doxorubicin35-37 | ALL, AML, thyroid cancer, breast cancer, gastric cancer, lung cancer, bladder cancer, lymphomas, neuroblastoma, sarcomas, Wilms tumor | 80 - 100 |
| Etoposide38-40 | Small cell lung cancer, testicular cancer | ∼ 55 |
| Idarubicin41-43 | AML | ∼ 50 |
| Irinotecan44-48 | Colorectal cancer | ∼ 58 |
| Paclitaxel49-55 | Breast cancer, lung cancer, gynecologic malignancies, Kaposi's sarcoma | ∼ 100 |
| Topotecan56-59 | Small cell lung cancer, ovarian cancer, cervical cancer | 20 (oral); 49 (i.v) |
| Radiotherapies | ||
| Photon radiotherapy (traditional radiotherapy)16 | Primary CNS tumors, brain metastasis, head and neck cancer | ∼ 100 |
| Proton radiotherapy60, 61 | Medulloblastoma, ependymoma, other primary CNS | 75 - 100 |
| Targeted therapies6, 18 | ||
| Vismodegib62 | Basal cell carcinoma (locally advanced or unresectable) | 62 |
| Sorafenib22 | Hepatocellular carcinoma, renal cell carcinoma, thyroid cancer | 26 |
| Sunitinib22 | Metastatic renal cell carcinoma, gastrointestinal stromal tumor | 6 |
| Regorafenib | Colorectal cancer | 4 |
| Vemurafenib | Melanoma (stage IV) | 24 |
| Dabrafenib | Melanoma (stage IV) | 19 |
| Targeted therapies in pediatric population (including imatinib, dasatinib, erlotinib, vandetanib, sorafenib, cabozantinib, pazopanib)63 | Hematologic and CNS tumors | 3 |
| Immunotherapies7, 19 | ||
| CTLA-4 | Melanoma (stage III-IV) | 1-2 |
| PD-1 and PD-L1 | Melanoma (stage IV), lung, Hodgkin lymphoma, urothelial carcinoma | 2 |
| Stem cell transplants8, 64 | ||
| Conditioning chemotherapy (busulfan, topotecan, thiotepa, tacrolimus, melphalan, methotrexate, etoposide, cyclophosphamide) | Leukemia | ∼ 100 |
| Acute graft vs host disease | 20 | |
| Neoadjuvant endocrine therapies9 | ||
| Leuprolide | Breast cancer, hepatocellular carcinoma, neuroendocrine | 2 |
| Octreotide | tumors | 6.7 |
| Aromatase inhibitors (anastrozole, letrozole, exemestane) | Metastatic estrogen–receptor-positive (ER+) breast cancer | ∼ 25 |
| Pigmentary hair changes | ||
| Targeted therapies (cabozantinib, pazopanib, sorafenib, sunitinib, imatinib)65-67 | Renal cell carcinoma, thyroid cancer, hepatocarcinoma, desmoid tumors, gastrointestinal stromal tumor, pancreatic neuroendocrine tumors | ∼ 30 |
| PD-1 and PD-L168 | Melanoma (stage IV), lung, Hodgkin lymphoma, urothelial carcinoma, head and neck squamous cell carcinoma | 27 |
| Textural hair changes | ||
| Targeted therapies (erlotinib, cetuximab, gefitinib, lapatinib, panitumumab), BRAF inhibitors69, 70 | Tumors with EGFR mutation, including non-small-cell lung cancer, squamous cell carcinoma of the head and neck | ∼ 30 |
| Cytotoxic chemotherapies (cyclophosphamide, taxanes)21 | Breast cancer, AML, ALL, lymphoma, multiple myeloma, neuroblastoma, ovarian cancer | 65 |
| Hirsutism and hypertrichosis | ||
| EGFR/MEK inhibitors (erlotinib, cetuximab, gefitinib, afatinib, lapatinib, panitumumab)70, 71 | Tumors with EGFR mutation, including non-small-cell lung cancer, squamous cell carcinoma of the head and neck | ∼ 50 |
| Targeted therapies in pediatric population (EGFR/MEK inhibitors, and MKIs) 63 | CNS tumors, squamous cell carcinoma, sarcomas | 7 |
| Eyelash trichomegaly | ||
| Targeted therapies: EGFR inhibitors 72-74 | Tumors with EGFR mutations, including non-small-cell lung cancer, squamous cell carcinoma of the head and neck | 8 (EGFR inhibitors) and case reports for BRAF and MKIs |
| Targeted therapies in pediatric population (including imatinib, dasatinib, erlotinib, vandetanib, sorafenib, cabozantinib, pazopanib) 63 | Leukemia, CNS tumors | 17 |
AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia; BRAF, proto-oncogene B-Raf; CNS, central nervous system; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFR, epidermal growth factor receptor inhibitor; MKIs, Multikinase inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; ∼, approximately.
Clinical Features
Key Point
The spectrum of hair disorders in cancer patients encompasses all hair changes including alopecia, pigmentary changes, textural changes, and cycle alterations
Hair disorders in cancer patients occur due to disturbances in hair follicle cycling and functioning, and hair shaft synthesis, which result in effluvium during anagen or telogen.3, 4, 75 Clinical features of hair disorders induced by anticancer therapies vary depending on the anticancer therapy given, its half-life, dose, schedule, route and rate of administration, whether it is administered alone or in combination with other anticancer therapies, as well as patient factors (Table 2).76
Table 2. Clinical features of hair disorders attributed to anticancer therapies.
| Cancer therapy | Clinical features by predominant hair disorders |
|---|---|
| Cytotoxic chemotherapies |
1. Alopecia: non-scarring, patchy or diffuse dystrophic anagen or catagen effluvium with predominance on areas of increased friction (crown and temporo-occipital areas). Usually recover in 2-6 months after chemotherapy cessation. Eyelash, eyebrow, axillary and pubic hair could be involved (recovery is generally more rapid than scalp alopecia). Trichoscopy: black dots, yellow dots, exclamation mark hairs, and color and thickness changes along the hair may exist 2. Pigmentary and textural hair changes: Slight changes from dark to graying, and from graying to dark. Upon regrowth, straight hair may become curly or wavy, and finer 3. Hirsutism: hirsutism may appear |
| Alkylating agents (e.g. busulfan, cyclophosphamide, etoposide) | |
| Topoisomerase-interacting agents (e.g. teniposide, daunorubicin, doxorubicin, idarubicin, irinotecan, topotecan) | |
| Antimicrotubule agents (e.g. paclitaxel, docetaxel, vincristine, vinblastine) | |
| Radiotherapy |
1. Alopecia: non-scaring, geometric shapes or diffuse anagen effluvium is usually seen,in relation with the irradiated area. May coexist with different grades of radiationdermatitis. Trichoscopy: yellow and black dots, short vellus hair, peripilar sign andbroken hair 2. Pigmentary and textural hair changes: hair hypopigmentation and decreased shaftdiameter may be seen |
| Targeted therapies |
1. Hirsutism, hypertrichosis and trichomegaly: hirsutism and periocular hypertrichosis could be marked. Eyelashes and eyebrows hypertrichosis are frequent, with long and curly eyelashes that may affect vision (predominantly with EGFR inhibitors) 2. Pigmentary and textural hair changes: hair discoloration, includinghypopigmentation (VEGFR/PDGFR) and hyperpigmentation (EGFR inhibitors) 3. Alopecia: diffuse alopecia with non-scarring (EGFR inhibitors, VEGFR/PDGFR)and scarring features (EGFR inhibitors) after severe inflammatory follicular reactions (e.g. erosive pustular dermatosis of the scalp, and tufted hair folliculitis) |
| EGFR inhibitor (e.g. cetuximab, panitumumab,gefitinib, erlotinib, afatinib, lapatinib) | |
| VEGFR/PDGFR inhibitor (e.g. sorafenib, regorafenib, imatinib, dasatinib, sunitinib, nilotinib, ponatinib,axitinib, pazopanib) | |
| BRAF inhibitor (e.g. vemurafenib, dabrafenib) | |
| Immunotherapies |
1. Alopecia: non-scaring with diffuse hair thinning and alopecia areata. Skininvolvement may coexist, including vitiligo and lichenoid reactions 2. Pigmentary hair changes: diffuse hyperpigmentation and hypopigmentation |
| Ipilimumab (CTLA-4) | |
| Programmed cell death protein (PD-1) receptors and its ligand (PD-L1) (e.g. pembrolizumab, nivolumab, avelumab, atezolizumab) | |
| Stem cell transplantation |
1. Alopecia: non-scarring, patchy or diffuse anagen effluvium (similar as described forCIA –related to conditioning chemotherapies-). Scarring alopecia, and alopecia areatawith acute or chronic graft versus host disease 2. Pigmentary and textural hair changes: hypopigmentation and decreased diameterof hair shafts |
| Vismodegib | 1. Alopecia: non-scarring, patchy or diffuse anagen effluvium (similar as described for CIA) |
| Endocrine therapies (e.g. leuprolide, tamoxifen, raloxifene, anastrozole, exemestane, letrozole, octreotide) |
1. Alopecia: pattern alopecia, similar to androgenetic type 2. Pigmentary and textural hair changes: finer hairs 3. Hirsutism: hirsutism may be observed |
CIA, chemotherapy-induced alopecia; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFRI, epidermal growth factor inhibitor; PDGFR, platelet-derived growth factor receptors; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; VEGF, vascular endothelial growth factor.
Alopecia
CIA usually begins within weeks after the first dose of cytotoxic chemotherapy as a patchy or diffuse anagen effluvium with predominance on areas of increased friction, such as crown and temporo-occipital areas, that may progress to complete alopecia in 2-3 months.77 In addition, eyelash and eyebrow alopecia, and alopecia of other body areas could be observed in association with scalp alopecia in up to 33% of patients receiving taxanes.78 Although usually asymptomatic, CIA could also be related with trichodynia and pruritus (11%).77 CIA is typically reversible within 2-6 months after chemotherapy is discontinued.64, 79 Trichoscopic findings in CIA include black dots, yellow dots, exclamation mark hairs, and color and thickness changes along the hair shaft (Figure 1).80, 81
Figure 1.
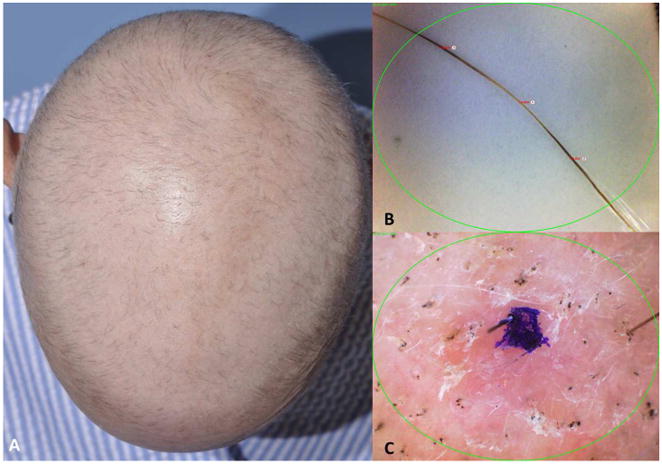
Chemotherapy-induced alopecia (CIA). A. Clinical image of scalp alopecia one month after first taxane-based chemotherapy cycle. B. Pohl-Pinkus constrictions observed in monilethrix-like hairs following weekly cyclophosphamide-based chemotherapy. C. Trichoscopy of CIA shows black dots, fractured hair shafts and vellus hairs.
Radiotherapy-induced alopecia (RIA) is characterized by an anagen effluvium due to acute damage of the hair follicle.82 An alopecia patch confined to the area of radiotherapy is usually observed 1-3 weeks after the first irradiation,83 and hair regrowth usually occurs 2-6 months after radiotherapy.60, 84 Radiation dermatitis and cutaneous injury may also accompany the alopecia (Figure 2).85 Yellow and black dots have been described as the predominant trichoscopic findings in 60% of RIA, followed by short vellus hair (50%), peripilar sign (20%), and broken hairs (10%), trichoscopic findings also described in alopecia areata and androgenetic alopecia.86,87
Figure 2.
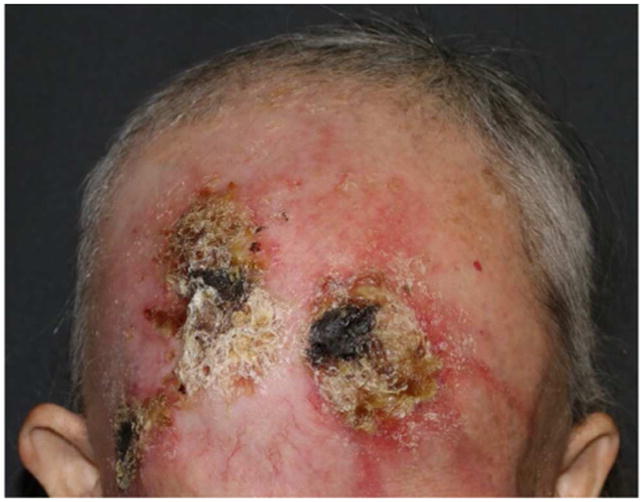
Radiotherapy-induced alopecia (RIA). Alopecia, erythema and ulceration related to photon radiotherapy used for treating scalp metastasis. Ulceration resulted in permanent/cicatricial alopecia.
Whereas mild diffuse alopecia is frequent with EGFR inhibitors (e.g. erlotinib, afatinib, cetuximab, panitumumab), scarring alopecia have been reported in 5% of patients treated with cetuximab, which may be a consequence of a secondary scalp bacterial infection (Figure 3), manifested as erosive pustular dermatosis,69, 88-90 or tufted hair folliculitis.91 Alopecia areata and universalis are considered immune related AE occurring in 2% of patients receiving ipilimumab.92
Figure 3.
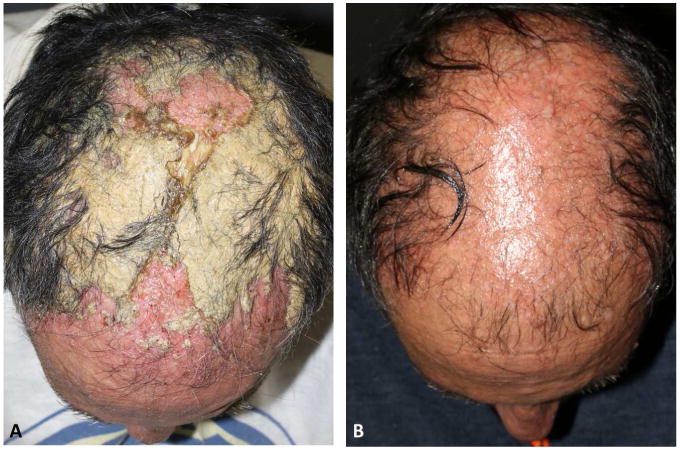
Scalp folliculitis (Skin infection grade 3 (CTCAEv4)) in a patient receiving cetuximab. A. Before therapy with oral doxycycline and topical high potency corticosteroids. B. Two weeks after dermatologic therapy.
Among patients undergoing stem cell transplantation, diffuse alopecia is reported in association with conditioning chemotherapy in around 100%.64 Hair changes may occur with acute graft-versus-host disease, including features of nonscaring alopecia with diffuse hair thinning, patchy hair loss, and premature graying. Alopecia areata and other autoimmune skin conditions such as vitiligo have been reported in the graft-versus-host disease setting.93, 94 Endocrine therapies have been associated with alopecia, usually mild in severity with a pattern similar to androgenetic alopecia.95 Despite the fact that most cases of alopecia in cancer patients are transitory, 14% of childhood cancer survivors,96 and 30% of breast cancer survivors 97 will develop persistent or even permanent alopecia.
Pigmentary and textural hair changes
Textural and pigmentary hair changes are frequent with anticancer therapies; however these are reversible upon drug discontinuation. Pigmentary hair changes include; depigmentation (Figure 4) and hyperpigmentation, most apparent on the scalp, although the eyebrows, eyelashes and body hair may be affected as well. Hair hypopigmentation has been reported with the MKIs (pazopanib, sunitinib, regorafenib), 98-104 whereas EGFR inhibitors result in hyperpigmentation of scalp and facial hair (∼ 50%).105-107 Hair repigmentation has been described as a possible marker of tumor response in 14 patients receiving anti-PD1/anti–PD-L1 therapy for lung cancer.68
Figure 4.
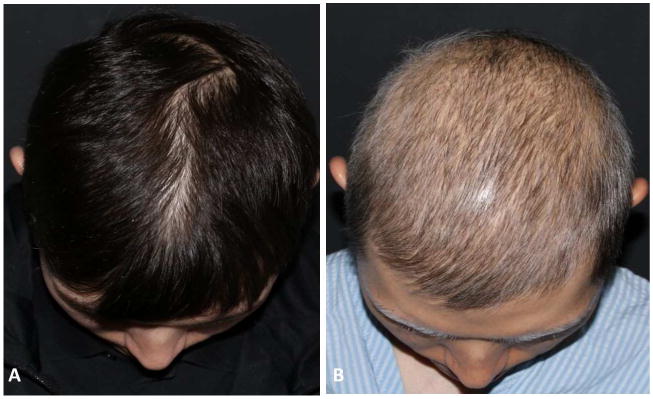
Depigmentation and hair thinning in a patient receiving tremelimumab. A. Before therapy. B. 3 months after first dose of treatment.
Additionally, straight hair may become curly or wavy in 65% of cancer patients after treatment with cytotoxic chemotherapy.21 With targeted therapies, the hair growth on scalp can slow down, become finer, curlier and brittler.69, 108
Hirsutism, hypertrichosis and trichomegaly
Excessive hair growth over the periocular area,109, 110 hirsutism,69 and trichomegaly (Figure 5) have been mostly reported as an AE of EGFR inhibitors.72-74 Eyelash trichomegaly also has been reported after pan-FGF receptor inhibitor therapy.111 These alterations typically resolve after discontinuation of treatment, although in some cases, they can persist for several months.112 Hirsutism could be seen in cancer patients who receive endocrine therapies (anti-estrogen agents), and the low incidence is likely due to underreporting.
Figure 5.
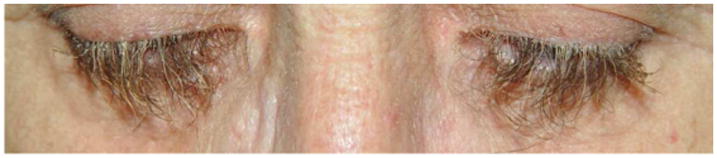
Trichomegaly in a patient on erlotinib during a year.
Etiology and Pathogenic Mechanisms
Key points
The pathogenic mechanisms of anticancer therapy-induced alopecia and other hair disorders will vary depending on causal therapy
The hair matrix keratinocytes of anagen hair follicles have a high mitotic activity, which makes them especially vulnerable to anticancer therapies
Paradoxically, some anticancer therapies can promote hair growth, textural and hair color changes
Although the clinical manifestation of CIA from different therapies may be similar (albeit with subtle variations), accumulating evidence suggests that the molecular underpinnings are varied and share several molecular damage-response pathways.75, 113 In particular, massive p53-dependent apoptotic cell death of hair follicle matrix keratinocytes and of hair follicle melanocytes plays a pivotal role.114-116 In the rodent and human CIA models, rapidly proliferating anagen hair follicles and their pigmentary system, which are both very sensitive to toxins, are the main targets of cytotoxic chemotherapy-induced hair follicle damage, reducing the proliferation rate and promoting the apoptosis of matrix keratinocytes and melanogenesis in the hair bulb.75, 116 Telogen hair follicles are less sensitive than anagen hair follicles to chemotherapy, presumably because of low-level proliferation and arrested pigmentary activity.75 RIA is a dose-dependent treatment related acute damage to actively dividing matrix cells of anagen follicles, followed by telogen shedding due to premature catagen entry of follicles in late anagen.82 Doses as low as 2 Gy in a single fraction have been shown to cause temporary alopecia.117
Targeted therapies work by blocking oncogenic pathways needed for cell growth and survival. In mice, dysregulation of the EGFR-Ras-Raf pathway can result in abnormal hair follicle morphogenesis. However, it is not known why paradoxically, EGFR inhibitors result in scalp alopecia, but result in hirsutism and eyebrow and lash trichomegaly.
Immunotherapies (anti-CTLA-4, PD-1, PDL1) and stem cell transplants may also result in alopecia, with a clinical and histologic pattern consistent with alopecia areata, likely due to the activation of inflammatory responses against hair follicle antigens, and an imbalance of the immune tolerance in the hair follicle environment. 7. These findings are concordant with other immune-related AEs reported in immunotherapy-treated patients, in which autoimmune T and B cell-driven mechanisms underlie toxicities.7, 94
The mechanisms by which endocrine therapies result in alopecia is via counteracting the anagen-prolonging effects of 17-β-estradiol 118-120 and result in increased hair growth-inhibitory actions of androgens, which is probably reflected by the development of hair thinning with a predominant androgenetic pattern.121, 122
The pigmentary changes in regrowing hairs could be explained by an impaired transfer of melanin from hair follicle keratinocytes to the hair shaft, and the generation of an oxidative damage, 123 with induction of apoptosis of hair follicle melanocytes and melanocyte stem cells.116 Changes in hair structure depend on multiple interacting parameters, including hair shaft keratins, and hair follicle asymmetric cell proliferation.124, 125
Hair Disorder Severity Grading
Key point
Adverse events in oncology clinical trials are graded using the Common Terminology Criteria for Adverse Events
The documentation of AEs is critical for patient safety and for the development of a toxicity profile for each anticancer drug/regimen. In the oncology literature, alopecia is graded by the following AE grading instruments; the World Health Organization (WHO),126 Dean scale,127 the Eastern Cooperative Oncology Group (ECOG),128 Sredni et al,129 the National Cancer Institute (NCI),130 the EGFR inhibitors Skin Toxicity Tool (MESTT),131 and the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0) (Table 3).132 Of all these grading instruments, the CTCAE v4.0 132 is the standard for the description and exchange of drug safety information in cancer treatments. Its use is mandatory in oncology trials, therefore data on alopecia incidence and severity is reported according to the CTCAE. There are some limitations in the alopecia grading (CTACE grade 1 and 2) that may not capture subtle changes in severity or the pattern, therefore more granular severity grading tools are recommended for studies investigating anticancer therapy-induced alopecia.
Table 3. Grading scales used for anticancer therapy-induced hair changes.
| Alopecia grading | |||
|---|---|---|---|
| CTCAE V4.0 | |||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Hair loss of <50% of normal for that individual that is not obvious from a distance but only on close inspection; a different hairstyle may be required to cover the hair loss but it does not require a wig or hairpiece to camouflage | Hair loss of >=50% normal for that individual that is readily apparent to others; a wig or hair piece is necessary if the patient desires to completely camouflage the hair loss; associated with psychosocial impact | - | - |
| Dean's grading scale of hair loss protection from anticancer therapies | |||
| >0 to 25% hair loss | 25 to 50% hair loss | 50 to 75% hair loss | 75 to 100% hair loss |
| WHO handbook for reporting results of cancer treatment | |||
| Minimal hair loss | Moderate, patchy hair loss | Complete alopecia, but reversible | Non-reversible alopecia |
| EGFR Inhibitor Skin Toxicity Tool (MESTT) | |||
| Hair loss <50% of normal for that individual that may or may not be noticeable to others but is associated with increased shedding and overall feeling of less volume. May require different hairstyle to cover but does not require hairpiece to camouflage | 2A. Hair loss associated with marked increase shedding and 50-74% loss compared to normal for that individual Hair loss is apparent to others, may be difficult to camouflage with change in hairstyle and may require a hairpiece 2B. Marked loss of at least 75% hair compared to normal for that individual with inability to camouflage except with a full wig OR new cicatricial hair loss documented by biopsy that covers at least 5%scalp surface area. May impact on functioning in social, personal or professional situations |
- | - |
| Hypertrichosis grading | |||
| CTCAE V4.0 | |||
| Increase in length, thickness or density of hair that the patient is either able to camouflage by periodic shaving or removal of hairs or is not concerned enough about the overgrowth to use any form of hair removal | Increase in length, thickness or density of hair at least on the usual exposed areas of the body [face (not limited to beard/moustache area) plus/minus arms] that requires frequent shaving or use of destructive means of hair removal to camouflage; associated with psychosocial impact | - | - |
| Hirsutism grading | |||
| CTCAE V4.0 | |||
| In women, increase in length, thickness or density of hair in a male distribution that the patient is able to camouflage by periodic shaving, bleaching, or removal of hair | In women, increase in length, thickness or density of hair in a male distribution that requires daily shaving or consistent destructive means of hair removal to camouflage; associated with psychosocial impact | - | - |
For eyelash and eyebrow alopecia, there are no validated grading scales, whereas hypertrichosis and trichomegaly are graded with the CTCAE v4.0. 132 The modified Ferryman–Gallwey scoring system may also be used to grade hyperthrichosis.133
Quality of Life In Cancer Patients With Hair Disorders
Key point
Chemotherapy-induced scalp, eyelash, and eyebrow alopecia lead to a negative psychosocial impact. The impact of other hair disorders in oncology has not been reported, but is expected to be significant
Hair-related AEs have a profound impact on cancer patients' QoL. CIA is one of the most clinically visible and distressing AE,2 and has been cited as the most disturbing anticipated AE by 58% of breast cancer patients prior to chemotherapy.134 In a multicenter study, 55% of 168 breast cancer patients reported high psychological distress from CIA.135 The impact can be so immense that coping with hair loss was felt more difficult than with loss of a breast,136, 137 and can even lead patients to refuse treatment (8%).136, 138 Additionally, eyelash and eyebrow alopecia resulted in psychological distress in breast cancer patients treated with taxane-based chemotherapy.139
The impact on QoL of changes of hair color and texture, hirsutism and hypertrichosis in cancer patients has not been reported. Treatment-related growth of unwanted facial hair can also significantly affect QoL, as shown in a previous survey-based study of several thousand women, where 62% had concerns regarding unwanted hair on the upper lip.140, 141 Therefore, it would be important to query and measure the impact on QoL of hair disorders attributed to anticancer therapies, given the longer times patients are on therapy and the increasing number of medications with these events.
Specific Quality Of Life Instruments
Assessing the patient's own perception of their symptoms using patient reported outcome measures such as PRO-CTCAE, may complement our understanding of drug-induced hair disorders.142 Specific instruments to assess the impact of hair disorders on cancer patients' QoL, include the Chemotherapy-induced Alopecia Distress Scale (CADS)143 (validated in Korean patients and translated into English), which comprises 17 questions in 4 domains. In addition, the Eyelash Satisfaction Questionnaire (ESQ) was validated in a cohort of 595 cancer patients, and it includes 23 questions in 3 domains. 144, 145
Management
Key point
Most preventive or reactive strategies are based on uncontrolled studies, however the FDA has cleared two dynamic scalp cooling devices for the prevention of CIA in patients treated with cytotoxic therapies for solid tumors
Anticancer Therapy-Induced Alopecia
Given the varied alopecia-inducing mechanisms of action of anticancer therapies, and the individual's inherent susceptibility, no one strategy might work for alopecia induced by different therapies.75 Management for anticancer-therapy induced alopecia can be broadly divided into preventive and reactive strategies, as summarized in Table 4.
Table 4. Management of hair disorders in patients receiving anticancer therapies.
| Hair disorder | Interventions | Level of evidence |
|---|---|---|
| Chemotherapy-induced alopecia (CIA) |
Preventive strategies: Scalp cooling: caps or cooling systems, only for cancer patients with solid tumors Contraindications to scalp cooling include hematological malignancies, and the following: cold sensitivity and cold trigged diseases, central nervous system malignancies, small cell carcinoma of the lung, cancers of the head and neck, skin cancer, and in pediatric patients Topical minoxidil 2% daily, over the entire scalp throughout chemotherapy and up to 4 months post-chemotherapy |
Level IB |
| Level IB | ||
| Radiotherapy-induced alopecia (RIA) |
Reactive strategies: Topical minoxidil 5% daily (radiation dermatitis should be managed first with topical corticosteroid, if present) |
Level IV |
| Alopecia attributed to targeted therapies (e.g. EGFR inhibitors, VEGFR/PDGFR/BRAF inhibitors) |
Reactive strategies: Non-inflammatory alopecia (VEGFR/PDGFR): topical minoxidil 5% daily continued until 6 months after therapy ends If scalp inflammation (EGFR inhibitors): topical corticosteroids; if secondary infection present treat with culture/sensitivity-driven oral antibiotics |
|
| Alopecia attributed to Immunotherapies (e.g. CTCLA-4, PD1, PDL-1) | High potency topical corticosteroid if alopecia areata; rule out thyroid dysfunction (immune related adverse event) | |
| Alopecia attributed to stem cell transplant | If alopecia areata, topical corticosteroids or Janus kinase inhibitors If diffuse or pattern alopecia (similar pattern to CIA); topical minoxidil 5% daily | |
| Alopecia attributed to vismodegib |
CTCAEv4.0 grade 1 and grade 2: Topical minoxidil 5% daily continued until 6 months post-therapy |
|
| Endocrine therapy-induced alopecia (EIA) |
CTCAEv4.0 grade 1 and grade 2: Topical minoxidil 5% daily |
|
| Eyebrow and eyelashes alopecia | Chemotherapy-induced alopecia: topical bimatoprost solution 0.03% | Level IB |
| Pigmentary and textural hair changes | If needed, options such as hair coloring and changes in hairstyle should be recommended (e.g. hair straightener, hair permanent) | Level IB |
| Hirsutism and hypertrichosis |
Reactive strategies: TCAEv4.0 grade 1 (mild hair growth): Local therapy such as epilation, depilation, shaving, eflornithine, laser treatment CTCAEv4.0 grade 2 (prominent thick hairs, associated with psychosocial impact): Laser or intense pulsed light Trimming for eyelash trichomegaly, referral to an ophthalmologist when irritation or discomfort is present Patients can be reassured that these hair changes are temporary; normal growth should begin within 1 month after cessation of medication |
Level III |
| General recommendations | As a prevention of patient distress, we recommend patient education and support Most of the hair changes are temporary. However, if therapy is requested, this should be discussed to have realistic expectations of therapy outcome Camouflaging techniques (e.g. crayons, powder, volumizers, hair weaves/hair extension, scalp micropigmentation/tattoo and hairpieces) could be recommended If emotionally affected; psychological counseling is recommended Involve nurses and other health care providers in the cancer patients hair care |
|
CTCAEv4.0, Common Terminology Criteria for Adverse Events Version 4.0; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; EGFRI, epidermal growth factor inhibitor; Nd:YAG, neodymium-doped yttrium aluminium garnet; PDGF-R, Platelet-derived growth factor receptors; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; VEGF, vascular endothelial growth factor.
Preventive strategies
There are no preventive pharmacologic strategies that have demonstrated satisfactory efficacy to justify their general use. For example, topical minoxidil 2% twice a day, showed no benefit to prevent CIA in a prospective trial of 10 patients.146 However, in a randomized trial including 22 breast cancer patients who were treated with cytotoxic chemotherapy, topical minoxidil 2% solution reduced the duration of alopecia by 50 days when compared to placebo (87 vs. 137 days).147 Trials evaluating this potential preventative strategy are needed. The topical calcitriol (BPM31543) showed benefit in a phase 1 trial of 31 patients and is currently under development.148
Scalp cooling has become the most widely utilized method for the prevention of CIA.149 Scalp cooling systems include static devices (e.g. glycerin-based, Chemocoldcaps™, Penguin™),150-153 and the recently FDA-cleared dynamic scalp cooling systems (DigniCap™, 2015; Orbis Paxman™, 2017).154-156 The plausible mechanisms for conferring protection to the hair follicle include the reduced availability of cytotoxic drug to the hair follicle (vasoconstriction induces a decrease of 20% of scalp blood flow),127, 156, 157 the relative reduced follicular uptake of cytotoxic therapies,158 and decreased follicular metabolic activity.159
The success of scalp cooling appears to depend on the type of the chemotherapy regimen. In a prospective study in 124 women with breast cancer receiving taxane-based chemotherapy, Dignicap scalp cooling conferred protection against hair loss (Dean score of 0-2) in 66% compared to 0% in the uncooled group.160 In a multicenter, randomized study using the Orbis Paxman scalp cooling system,161 hair preservation was observed in 50% (cooling group) vs. 0% (controls) after the 4th chemotherapy cycle (taxane/ anthracycline/ or both). Another prospective randomized study of 79 patients (41 receiving Dignicap scalp cooling) reported lower hair preservation rates (39%) among patients undergoing scalp cooling versus 0% in the no scalp cooling arm.162, 163 Differences between devices are likely related to operator experience and types of chemotherapy regimens of patients enrolled, with patients on taxane-based regimens showing a higher benefit from scalp cooling. Regarding safety, the most common adverse device events include headache (11%), nausea (4%), and dizziness (3%).161 A meta-analysis of 10 trials that included 1,959 patients demonstrated no differences in the incidence of scalp metastases between cooled (0.61%) vs non-cooled patients (0.41%).164 Scalp cooling has been reported effective in 3 breast cancer patients treated with taxane-based chemotherapy and with CIA grade 2 (CTCAE) in order to prevent alopecia in future chemotherapy sessions, and consequently allow sooner reestablishment of scalp hair density, however, the risk of frostbite should be considered.165 In general, pre-cooled caps remain very popular because of their widespread availability. Although four cases of thermal injury has been reported due to improper caps applications.166
Although the 30% incidence of persistent CIA 97 augments the interest in preventing CIA, there is no data on the efficacy of scalp cooling devices on persistent alopecia, hence their use cannot be recommended for this purpose. Despite the relative success of the scalp cooling system in preventing CIA, this system has been reported as not effective to prevent RIA.167 Additionally, scalp cooling system use is not reimbursable by insurance companies, and costs may be elevated. Financial assistance may be obtained through hairtostay.org and coldcapitalfund.org.
There is no data on preventive strategies for non-cytotoxic agent-induced alopecia. Therefore, we recommend pre-therapy counseling and on-therapy evaluations by the oncology team for atypical patterns or severity of alopecia, so that any comorbidities or exacerbating factors may be addressed and patient is referred to a dermatologist if indicated
Reactive strategies
The reactive strategies are mostly based on case series, case reports, and expert opinion. Immunotherapies and stem cell transplant have been related with alopecia areata.7, 93 Therefore, topical and intralesional therapy with corticosteroids have shown efficacy in anecdotal reports of 2 patients.7 Cancer patients may have hormone-sensitive tumors that could react with systemic therapies used for androgenetic alopecia (e.g. spironolactone, finasteride, cyproterone acetate); we have therefore held with caution these strategies for cases of persistent alopecia in cancer survivors. Camouflage and supportive care should be provided to patients with CIA (Table 4).
Eyelash and Eyebrow Alopecia
Relatively inexpensive cosmetics and camouflage products could be used as long as eyelashes are still present. The effect of bimatoprost solution 0.03% on chemotherapy-induced eyelash hypotrichosis was examined in a controlled study of 130 breast cancer patients receiving cytotoxic chemotherapy showing that treatment with bimatoprost resulted in increased length and thickness compared to the vehicle control group (eyelash length 38% vs 16%; eyelash thickness 245% vs 33%).168
Pigmentary and Textural Hair Changes
Hair repigmentation and textural hair shaft changes have been reported with targeted and immunotherapies.68 Initially, most patients are satisfied with their new hair characteristics. Nevertheless, if management is requested, options such as hair dyeing and changes in hairstyle (i.e. straightening or curling) should be recommended as they pose no additional risk in cancer patients. The use of hair dyes and their association with cancer development is conflicting with non-Hodgkin lymphoma, none to insignificant for leukemias, and non-existent for breast cancer.169
Hirsutism, Hipertrichosis And Trichomegaly
Topical or cosmetic interventions are recommended (e.g. waxing or bleaching).170 Laser and photoepilation treatments are the most effective, especially in patients with lighter skin and dark-colored hairs.171 For eyelash and eyebrow trichomegaly related with targeted therapies, eyelash clipping and referral to an ophthalmologist is indicated for patients with ocular symptoms.172
Experimental Therapies
Several pre-clinical approaches have been tried for CIA and RIA, although only a few have shown some clinical benefit. Moreover, there are no public trials for the prevention or management of other anticancer therapy-induced hair disorders.
AS101 (ammonium trichloro (dioxoethylene-O,O-) tellurate) is an immunomodulatory tellurium compound based on a derivative of cisplatin. The drug has been shown to protect against CIA by reducing the severity but it does not prevent hair loss.129 A topical botanical blend solution for the treatment of androgenetic alopecia 173 is currently being investigated for the prevention of permanent CIA in a double-blind, randomized controlled trial in breast cancer survivors.174 It is hypothesized that this herbal medication may normalize apoptotic process in the hair follicle cells, and reduces chemotherapy-induced inflammation in the scalp.
Tempol is a nitric oxide radioprotector and its topical formulation has been found to be protective against RIA in both animal models and humans.175 In a phase Ib study, the application of tempol gel 15 minutes before radiotherapy followed by wash-off, led to full scalp retention in 3/5 evaluable patients.176
Challenges and Future Perspectives
Despite the prevalence and psychosocial impact of the anticancer therapy-induced hair disorders, research into their clinical presentation, pathophysiology, and management strategies has not received the attention it justifies. A comprehensive knowledge of the impact of hair disorders on QoL would be critical in order to optimize the shared decision-making process between doctors and patients regarding cancer therapies. As patients live longer on cancer therapies, there is a need to identify risk factors of clinically significant events and to develop improved and widely available preventive strategies, all of which would contribute to the optimal and comprehensive care of cancer patients.
Acknowledgments
Funding Support: This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. M.E.L. is supported by the RJR Oncodermatology Fund. A.F.M is partially supported by Beca Excelencia, Academia Española de Dermatología y Venereología (AEDV)-Fundación Piel Sana. R.P. is funded by the NIHR Manchester Biomedical Research Centre. Funders/sponsors were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Abbreviations
- AE
Adverse event
- CIA
Chemotherapy-induced alopecia
- CTCAEv4.0
Common Terminology Criteria for Adverse Events Version 4.0
- EGFR
Epidermal growth factor receptor
- MKIs
Multikinase inhibitor
- QoL
Quality of Life
- RIA
Radiotherapy-induced alopecia
Footnotes
COI Disclosure Statement: AFM and JJ have nothing to disclose. JS: Consultant for Aclaris, Samumed, Incyte, Replicel Life Sciences, Shook, Hardy, Bacon LLP who represent Sanofi Aventis US LLC. SG: has a speaking, consultant or advisory role with Adgero Biopharmaceuticals, AMAG pharmaceuticals, Procter and Gamble and Valeant women's health pharmaceuticals. JN: clinical trial funding from Paxman to Baylor College of Medicine for conduct of the SCALP trial. RP: has a consultant role with or receives research funding from Giuliani/Italy and Unilever/UK, and is founder/owner of Monasterium Laboratory/Germany. MEL: has a speaking, consultant or advisory role with Abbvie, Quintiles, Boehringer Ingelheim, AstraZeneca pharmaceuticals, Legacy Healthcare, Foamix, Adgero Bio Pharmaceuticals, Janssen R & D, Novartis, Paxman and Novocure, and also receives research grants from Berg and Bristol-Myers Squibb.
Learning objectives: To prevent, diagnose and treat hair disorders developing during anticancer therapies.
The Contents of the manuscript have not been previously published and are not currently submitted elsewhere. The authors accept responsibility for the scientific integrity of the work described in this manuscript. All listed authors have seen and approved of the manuscript and will sign off on any subsequent manuscript revisions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psycho-oncology. 2008;17:317–28. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 3.Chon SY, Champion RW, Geddes ER, Rashid RM. Chemotherapy-induced alopecia. Journal of the American Academy of Dermatology. 2012;67:e37–47. doi: 10.1016/j.jaad.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Rossi A, Fortuna MC, Caro G, Pranteda G, Garelli V, Pompili U, et al. Chemotherapy-induced alopecia management: Clinical experience and practical advice. Journal of cosmetic dermatology. 2017 doi: 10.1111/jocd.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali SY, Singh G. Radiation-induced Alopecia. Int J Trichology. 2010;2:118–9. doi: 10.4103/0974-7753.77528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belum VR, Marulanda K, Ensslin C, Gorcey L, Parikh T, Wu S, et al. Alopecia in patients treated with molecularly targeted anticancer therapies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26:2496–502. doi: 10.1093/annonc/mdv390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarbo A, Belum VR, Sibaud V, Oudard S, Postow MA, Hsieh JJ, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649–52. doi: 10.1111/bjd.15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bresters D, Wanders DC, Louwerens M, Ball LM, Fiocco M, van Doorn R. Permanent diffuse alopecia after haematopoietic stem cell transplantation in childhoods. Bone marrow transplantation. 2017 doi: 10.1038/bmt.2017.15. [DOI] [PubMed] [Google Scholar]
- 9.Saggar V, Wu S, Dickler MN, Lacouture ME. Alopecia with endocrine therapies in patients with cancer. The oncologist. 2013;18:1126–34. doi: 10.1634/theoncologist.2013-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosti A, Misciali C, Piraccini BM, Peluso AM, Bardazzi F. Drug-induced hair loss and hair growth. Drug Safety. 1994;10:310–7. doi: 10.2165/00002018-199410040-00005. [DOI] [PubMed] [Google Scholar]
- 11.Munstedt K, Manthey N, Sachsse S, Vahrson H. Changes in self-concept and body image during alopecia induced cancer chemotherapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 1997;5:139–43. doi: 10.1007/BF01262572. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi M, Oishi K, Zubal B, Lacouture ME. Unanticipated toxicities from anticancer therapies: survivors' perspectives. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1461–8. doi: 10.1007/s00520-009-0769-1. [DOI] [PubMed] [Google Scholar]
- 13.Bezjak A, Tu D, Bacon M, Osoba D, Zee B, Stuart G, et al. Quality of Life in Ovarian Cancer Patients: Comparison of Paclitaxel Plus Cisplatin, With Cyclophosphamide Plus Cisplatin in a Randomized Study. Journal of Clinical Oncology. 2004;22:4595–603. doi: 10.1200/JCO.2004.08.080. [DOI] [PubMed] [Google Scholar]
- 14.Hackbarth M, Haas N, Fotopoulou C, Lichtenegger W, Sehouli J. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women's cancers. Results of a prospective study Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16:267–73. doi: 10.1007/s00520-007-0318-8. [DOI] [PubMed] [Google Scholar]
- 15.Trueb RM. Chemotherapy-induced alopecia. Seminars in cutaneous medicine and surgery. 2009;28:11–4. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Gerrard GE, Prestwich RJ, Edwards A, Russon LJ, Richards F, Johnston CF, et al. Investigating the palliative efficacy of whole-brain radiotherapy for patients with multiple-brain metastases and poor prognostic features. Clin Oncol (R Coll Radiol) 2003;15:422–8. doi: 10.1016/s0936-6555(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 17.Mooney RB, McKinstry CS, Kamel HA. Absorbed dose and deterministic effects to patients from interventional neuroradiology. The British journal of radiology. 2000;73:745–51. doi: 10.1259/bjr.73.871.11089467. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy Journal of the American Academy of Dermatology. 2014;71:217.e1–e11. doi: 10.1016/j.jaad.2014.04.013. quiz 27-8. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. European journal of cancer (Oxford, England : 1990) 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Beelen DW, Quabeck K, Graeven U, Sayer HG, Mahmoud HK, Schaefer UW. Acute toxicity and first clinical results of intensive postinduction therapy using a modified busulfan and cyclophosphamide regimen with autologous bone marrow rescue in first remission of acute myeloid leukemia. Blood. 1989;74:1507–16. [PubMed] [Google Scholar]
- 21.Fairlamb DJ. Hair changes following cytotoxic drug induced alopecia. Postgrad Med J. 1988;64:907. doi: 10.1136/pgmj.64.757.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WJ, Lee JL, Chang SE, Lee MW, Kang YK, Choi JH, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. The British journal of dermatology. 2009;161:1045–51. doi: 10.1111/j.1365-2133.2009.09290.x. [DOI] [PubMed] [Google Scholar]
- 23.Mendelson D, Block JB, Serpick AA. Effect of large intermittent intravenous doses of cyclophosphamide in lymphoma. Cancer. 1970;25:715–20. doi: 10.1002/1097-0142(197003)25:3<715::aid-cncr2820250332>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Coggins PR, Ravdin RG, Eisman SH. Clinical evaluation of a new alkylating agent: cytoxan (cyclophosphamide) Cancer. 1960;13:1254–60. doi: 10.1002/1097-0142(196011/12)13:6<1254::aid-cncr2820130614>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney MJ, Tuttle AH, Etteldorf JN, Whittington GL. Cyclophosphamide in the treatment of common neoplastic diseases of childhood. The Journal of pediatrics. 1962;61:702–8. doi: 10.1016/s0022-3476(62)80341-2. [DOI] [PubMed] [Google Scholar]
- 26.Brincker H, Mouridsen HT, Andersen KW. Adjuvant chemotherapy with cyclophosphamide or CMF in premenopausal women with stage II breast cancer. Breast cancer research and treatment. 1983;3:91–5. doi: 10.1007/BF01806239. [DOI] [PubMed] [Google Scholar]
- 27.McLean RD. Cyclophosphamide in the management of advanced bronchial carcinoma. Thorax. 1965;20:555–61. doi: 10.1136/thx.20.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiernik PH, Serpick AA. A randomized clinical trial of daunorubicin and a combination of prednisone, vincristine, 6-mercaptopurine, and methotrexate in adult acute nonlymphocytic leukemia. Cancer Res. 1972;32:2023–6. [PubMed] [Google Scholar]
- 29.Weil M, Glidewell OJ, Jacquillat C, Levy R, Serpick AA, Wiernik PH, et al. Daunorubicin in the therapy of acute granulocytic leukemia. Cancer Res. 1973;33:921–8. [PubMed] [Google Scholar]
- 30.Taxotere [package insert] Bridgewater, NJ: Sanofi-Aventis; 2010. accessed from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf, on Jun 26, 2017. [Google Scholar]
- 31.Cortes JE, Pazdur R. Docetaxel. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13:2643–55. doi: 10.1200/JCO.1995.13.10.2643. [DOI] [PubMed] [Google Scholar]
- 32.Sjostrom J, Blomqvist C, Mouridsen H, Pluzanska A, Ottosson-Lonn S, Bengtsson NO, et al. Docetaxel compared with sequential methotrexate and 5-fluorouracil in patients with advanced breast cancer after anthracycline failure: a randomised phase III study with crossover on progression by the Scandinavian Breast Group. European journal of cancer (Oxford, England : 1990) 1999;35:1194–201. doi: 10.1016/s0959-8049(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 33.Bonneterre J, Roche H, Monnier A, Guastalla JP, Namer M, Fargeot P, et al. Docetaxel vs 5-fluorouracil plus vinorelbine in metastatic breast cancer after anthracycline therapy failure. British journal of cancer. 2002;87:1210–5. doi: 10.1038/sj.bjc.6600645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 35.Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clinical journal of oncology nursing. 2009;13:13–4. doi: 10.1188/09.CJON.13-14. [DOI] [PubMed] [Google Scholar]
- 36.Henderson IC, Allegra JC, Woodcock T, Wolff S, Bryan S, Cartwright K, et al. Randomized clinical trial comparing mitoxantrone with doxorubicin in previously treated patients with metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7:560–71. doi: 10.1200/JCO.1989.7.5.560. [DOI] [PubMed] [Google Scholar]
- 37.Lawton PA, Spittle MF, Ostrowski MJ, Young T, Madden F, Folkes A, et al. A comparison of doxorubicin, epirubicin and mitozantrone as single agents in advanced breast carcinoma. Clin Oncol (R Coll Radiol) 1993;5:80–4. doi: 10.1016/s0936-6555(05)80851-9. [DOI] [PubMed] [Google Scholar]
- 38.Saxman S, Loehrer PJ, Sr, Logie K, Stephens D, Workman F, Scullin D, et al. Phase II trial of daily oral etoposide in patients with advanced non-small cell lung cancer. Investigational new drugs. 1991;9:253–6. doi: 10.1007/BF00176978. [DOI] [PubMed] [Google Scholar]
- 39.Sahmoud T, Postmus PE, van Pottelsberghe C, Mattson K, Tammilehto L, Splinter TA, et al. Etoposide in malignant pleural mesothelioma: two phase II trials of the EORTC Lung Cancer Cooperative Group. European journal of cancer (Oxford, England : 1990) 1997;33:2211–5. doi: 10.1016/s0959-8049(97)00183-4. [DOI] [PubMed] [Google Scholar]
- 40.Fulton D, Urtasun R, Forsyth P. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma. Journal of neuro-oncology. 1996;27:149–55. doi: 10.1007/BF00177478. [DOI] [PubMed] [Google Scholar]
- 41.Idarubicin [package insert] New York, NY: Pharmacia & Upjohn Co., Division of Pfizer Inc.; 2006. accessed from: https://www.pfizer.com/files/products/uspi_idamycin.pdf, on Jun 29, 2017. [Google Scholar]
- 42.Vogler WR, Velez-Garcia E, Weiner RS, Flaum MA, Bartolucci AA, Omura GA, et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: a Southeastern Cancer Study Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10:1103–11. doi: 10.1200/JCO.1992.10.7.1103. [DOI] [PubMed] [Google Scholar]
- 43.Lopez M, Contegiacomo A, Vici P, Dello Ioio C, Di Lauro L, Pagliarulo C, et al. A prospective randomized trial of doxorubicin versus idarubicin in the treatment of advanced breast cancer. Cancer. 1989;64:2431–6. doi: 10.1002/1097-0142(19891215)64:12<2431::aid-cncr2820641206>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Armand JP, Ducreux M, Mahjoubi M, Abigerges D, Bugat R, Chabot G, et al. CPT-11 (irinotecan) in the treatment of colorectal cancer. European journal of cancer (Oxford, England : 1990) 1995;31a:1283–7. doi: 10.1016/0959-8049(95)00212-2. [DOI] [PubMed] [Google Scholar]
- 45.Pitot HC, Wender DB, O'Connell MJ, Schroeder G, Goldberg RM, Rubin J, et al. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2910–9. doi: 10.1200/JCO.1997.15.8.2910. [DOI] [PubMed] [Google Scholar]
- 46.Andre T, Louvet C, Maindrault-Goebel F, Couteau C, Mabro M, Lotz JP, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR European journal of cancer (Oxford, England : 1990) 1999;35:1343–7. doi: 10.1016/s0959-8049(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 47.Duffour J, Gourgou S, Desseigne F, Debrigode C, Mineur L, Pinguet F, et al. Multicentre phase II study using increasing doses of irinotecan combined with a simplified LV5FU2 regimen in metastatic colorectal cancer. Cancer chemotherapy and pharmacology. 2007;60:383–9. doi: 10.1007/s00280-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 48.Ychou M, Raoul JL, Douillard JY, Gourgou-Bourgade S, Bugat R, Mineur L, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20:674–80. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 49.Shin H, Jo SJ, Kim do H, Kwon O, Myung SK. Efficacy of interventions for prevention of chemotherapy-induced alopecia: a systematic review and meta-analysis. International journal of cancer Journal international du cancer. 2015;136:E442–54. doi: 10.1002/ijc.29115. [DOI] [PubMed] [Google Scholar]
- 50.Gill PS, Tulpule A, Espina BM, Cabriales S, Bresnahan J, Ilaw M, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi's sarcoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:1876–83. doi: 10.1200/JCO.1999.17.6.1876. [DOI] [PubMed] [Google Scholar]
- 51.Ranson M, Davidson N, Nicolson M, Falk S, Carmichael J, Lopez P, et al. Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small-cell lung cancer. Journal of the National Cancer Institute. 2000;92:1074–80. doi: 10.1093/jnci/92.13.1074. [DOI] [PubMed] [Google Scholar]
- 52.Curtin JP, Blessing JA, Webster KD, Rose PG, Mayer AR, Fowler WC, Jr, et al. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a Gynecologic Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:1275–8. doi: 10.1200/JCO.2001.19.5.1275. [DOI] [PubMed] [Google Scholar]
- 53.Lombardi D, Crivellari D, Scuderi C, Magri MD, Spazzapan S, Sorio R, et al. Long-term, weekly one-hour infusion of paclitaxel in patients with metastatic breast cancer: a phase II monoinstitutional study. Tumori. 2004;90:285–8. doi: 10.1177/030089160409000304. [DOI] [PubMed] [Google Scholar]
- 54.Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, et al. Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast cancer research and treatment. 2007;101:355–65. doi: 10.1007/s10549-006-9306-9. [DOI] [PubMed] [Google Scholar]
- 55.Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5544–52. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hycamtin [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2014. accessed from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020981s006lbl.pdf, on Jun 29, 2017. [Google Scholar]
- 57.ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2183–93. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]
- 58.Ramlau R, Gervais R, Krzakowski M, von Pawel J, Kaukel E, Abratt RP, et al. Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2800–7. doi: 10.1200/JCO.2005.03.6491. [DOI] [PubMed] [Google Scholar]
- 59.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecologic oncology. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Suneja G, Poorvu PD, Hill-Kayser C, Lustig RA. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatric blood & cancer. 2013;60:1431–6. doi: 10.1002/pbc.24554. [DOI] [PubMed] [Google Scholar]
- 61.Mizumoto M, Oshiro Y, Takizawa D, Fukushima T, Fukushima H, Yamamoto T, et al. Proton beam therapy for pediatric ependymoma. Pediatrics international : official journal of the Japan Pediatric Society. 2015;57:567–71. doi: 10.1111/ped.12624. [DOI] [PubMed] [Google Scholar]
- 62.Basset-Seguin N, Hauschild A, Kunstfeld R, Grob J, Dreno B, Mortier L, et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. European journal of cancer (Oxford, England : 1990) 2017;86:334–48. doi: 10.1016/j.ejca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Belum VR, Washington C, Pratilas CA, Sibaud V, Boralevi F, Lacouture ME. Dermatologic adverse events in pediatric patients receiving targeted anticancer therapies: a pooled analysis. Pediatric blood & cancer. 2015;62:798–806. doi: 10.1002/pbc.25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker BW, Wilson CL, Davis AL, Spearing RL, Hart DN, Heaton DC, et al. Busulphan/cyclophosphamide conditioning for bone marrow transplantation may lead to failure of hair regrowth. Bone marrow transplantation. 1991;7:43–7. [PubMed] [Google Scholar]
- 65.Valeyrie L, Bastuji-Garin S, Revuz J, Bachot N, Wechsler J, Berthaud P, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. Journal of the American Academy of Dermatology. 2003;48:201–6. doi: 10.1067/mjd.2003.44. [DOI] [PubMed] [Google Scholar]
- 66.Arora B, Kumar L, Sharma A, Wadhwa J, Kochupillai V. Pigmentary changes in chronic myeloid leukemia patients treated with imatinib mesylate. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:358–9. doi: 10.1093/annonc/mdh068. [DOI] [PubMed] [Google Scholar]
- 67.Aleem A. Hypopigmentation of the skin due to imatinib mesylate in patients with chronic myeloid leukemia. Hematology/oncology and stem cell therapy. 2009;2:358–61. doi: 10.1016/s1658-3876(09)50026-x. [DOI] [PubMed] [Google Scholar]
- 68.Rivera N, Boada A, Bielsa MI, Fernandez-Figueras MT, Carcereny E, Moran MT, et al. Hair Repigmentation During Immunotherapy Treatment With an Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Agent for Lung Cancer. JAMA dermatology. 2017 doi: 10.1001/jamadermatol.2017.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16:1425–33. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 70.Lacouture ME, Basti S, Patel J, Benson A., 3rd The SERIES clinic: an interdisciplinary approach to the management of toxicities of EGFR inhibitors. The journal of supportive oncology. 2006;4:236–8. [PubMed] [Google Scholar]
- 71.Vergou T, Stratigos AJ, Karapanagiotou EM, Matekovits AE, Dilana KD, Tsimboukis S, et al. Facial hypertrichosis and trichomegaly developing in patients treated with the epidermal growth factor receptor inhibitor erlotinib. Journal of the American Academy of Dermatology. 2010;63:e56–8. doi: 10.1016/j.jaad.2009.11.589. [DOI] [PubMed] [Google Scholar]
- 72.Dueland S, Sauer T, Lund-Johansen F, Ostenstad B, Tveit KM. Epidermal growth factor receptor inhibition induces trichomegaly. Acta oncologica (Stockholm, Sweden) 2003;42:345–6. doi: 10.1080/02841860310006038. [DOI] [PubMed] [Google Scholar]
- 73.Bouche O, Brixi-Benmansour H, Bertin A, Perceau G, Lagarde S. Trichomegaly of the eyelashes following treatment with cetuximab. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16:1711–2. doi: 10.1093/annonc/mdi300. [DOI] [PubMed] [Google Scholar]
- 74.Pascual JC, Banuls J, Belinchon I, Blanes M, Massuti B. Trichomegaly following treatment with gefitinib (ZD1839) The British journal of dermatology. 2004;151:1111–2. doi: 10.1111/j.1365-2133.2004.06265.x. [DOI] [PubMed] [Google Scholar]
- 75.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. The Lancet Oncology. 2013;14:e50–9. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- 76.Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2004;12:543–9. doi: 10.1007/s00520-003-0562-5. [DOI] [PubMed] [Google Scholar]
- 77.Yun SJ, Kim SJ. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology (Basel, Switzerland) 2007;215:36–40. doi: 10.1159/000102031. [DOI] [PubMed] [Google Scholar]
- 78.Tsalic M, Gilboa M, Visel B, Miller B, Haim N. Epiphora (excessive tearing) and other ocular manifestations related to weekly docetaxel: underestimated dose-limiting toxicity. Medical oncology (Northwood, London, England) 2006;23:57–61. doi: 10.1385/MO:23:1:57. [DOI] [PubMed] [Google Scholar]
- 79.Vowels M, Chan LL, Giri N, Russell S, Lam-Po-Tang R. Factors affecting hair regrowth after bone marrow transplantation. Bone marrow transplantation. 1993;12:347–50. [PubMed] [Google Scholar]
- 80.Pirmez R, Pineiro-Maceira J, Sodre CT. Exclamation marks and other trichoscopic signs of chemotherapy-induced alopecia. The Australasian journal of dermatology. 2013;54:129–32. doi: 10.1111/j.1440-0960.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- 81.Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. Journal of the European Academy of Dermatology and Venereology : JEADV. 2013;27:1299–303. doi: 10.1111/j.1468-3083.2012.04530.x. [DOI] [PubMed] [Google Scholar]
- 82.Wen CS, Lin SM, Chen Y, Chen JC, Wang YH, Tseng SH. Radiation-induced temporary alopecia after embolization of cerebral arteriovenous malformations. Clinical neurology and neurosurgery. 2003;105:215–7. doi: 10.1016/s0303-8467(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 83.Ounsakul V, Iamsumang W, Suchonwanit P. Radiation-Induced Alopecia after Endovascular Embolization under Fluoroscopy. Case reports in dermatological medicine. 2016;2016:8202469. doi: 10.1155/2016/8202469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cox MC, Kusters JM, Gidding CE, Schieving JH, van Lindert EJ, Kaanders JH, et al. Acute toxicity profile of craniospinal irradiation with intensity-modulated radiation therapy in children with medulloblastoma: A prospective analysis. Radiation oncology (London, England) 2015;10:241. doi: 10.1186/s13014-015-0547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haruna F, Lipsett A, Marignol L. Topical Management of Acute Radiation Dermatitis in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Anticancer research. 2017;37:5343–53. doi: 10.21873/anticanres.11960. [DOI] [PubMed] [Google Scholar]
- 86.Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part I. History and clinical examination Journal of the American Academy of Dermatology. 2014;71:415.e1–e15. doi: 10.1016/j.jaad.2014.04.070. [DOI] [PubMed] [Google Scholar]
- 87.Miteva M, Tosti A. Dermatoscopy of hair shaft disorders. Journal of the American Academy of Dermatology. 2013;68:473–81. doi: 10.1016/j.jaad.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 88.Pongpudpunth M, Demierre MF, Goldberg LJ. A case report of inflammatory nonscarring alopecia associated with the epidermal growth factor receptor inhibitor erlotinib. Journal of cutaneous pathology. 2009;36:1303–7. doi: 10.1111/j.1600-0560.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- 89.Piraccini BM, Patrizi A, Fanti PA, Starace M, Bruni F, Melotti B, et al. RASopathic alopecia: hair changes associated with vemurafenib therapy. Journal of the American Academy of Dermatology. 2015;72:738–41. doi: 10.1016/j.jaad.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Fukui T, Kitamura H, Harada K, Nakano H, Sawamura D. Trichoscopic Findings of Erosive Pustular Dermatosis of the Scalp Associated with Gefitinib. Case reports in dermatology. 2017;9:44–9. doi: 10.1159/000475543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ena P, Fadda GM, Ena L, Farris A, Santeufemia DA. Tufted hair folliculitis in a woman treated with lapatinib for breast cancer. Clinical and experimental dermatology. 2008;33:790–1. doi: 10.1111/j.1365-2230.2008.02882.x. [DOI] [PubMed] [Google Scholar]
- 92.Yamazaki N, Kiyohara Y, Uhara H, Fukushima S, Uchi H, Shibagaki N, et al. Phase II study of ipilimumab monotherapy in Japanese patients with advanced melanoma. Cancer chemotherapy and pharmacology. 2015;76:997–1004. doi: 10.1007/s00280-015-2873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ceovic R, Desnica L, Pulanic D, Serventi Seiwerth R, Ilic I, Grce M, et al. High frequency of cutaneous manifestations including vitiligo and alopecia areata in a prospective cohort of patients with chronic graft-vs-host disease. Croatian medical journal. 2016;57:229–38. doi: 10.3325/cmj.2016.57.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zuo RC, Naik HB, Steinberg SM, Baird K, Mitchell SA, Kuzmina Z, et al. Risk factors and characterization of vitiligo and alopecia areata in patients with chronic graft-vs-host disease. JAMA dermatology. 2015;151:23–32. doi: 10.1001/jamadermatol.2014.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freites-Martinez A, Belum VR, Geller S, Skripnik A, Ciccolini K, Shapiro J, et al. Dermatologic adverse events in breast cancer patients receiving endocrine therapies. Journal of Clinical Oncology. 2017;35:e12533. e. [Google Scholar]
- 96.Kinahan KE, Sharp LK, Seidel K, Leisenring W, Didwania A, Lacouture ME, et al. Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2466–74. doi: 10.1200/JCO.2011.39.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang D, Kim IR, Lee DY, Ahn JS, Park JH, Guallar E, et al. 80PIncidence of permanent chemotherapy-induced alopecia among breast cancer patients: A five-year prospective cohort study. Annals of Oncology. 2017;28:mdx655.022–mdx655.022. [Google Scholar]
- 98.Sideras K, Menefee ME, Burton JK, Erlichman C, Bible KC, Ivy SP. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor pazopanib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:e312–3. doi: 10.1200/JCO.2009.26.4432. [DOI] [PubMed] [Google Scholar]
- 99.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England) 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi E, Koyama T, Kobayashi K, Setsu N, Kawashima M, Kawai A. Reversible hair depigmentation in a Japanese female treated with pazopanib. The Journal of dermatology. 2014;41:1021–2. doi: 10.1111/1346-8138.12654. [DOI] [PubMed] [Google Scholar]
- 101.Hartmann JT, Kanz L. Sunitinib and periodic hair depigmentation due to temporary c-KIT inhibition. Archives of dermatology. 2008;144:1525–6. doi: 10.1001/archderm.144.11.1525. [DOI] [PubMed] [Google Scholar]
- 102.Brzezniak C, Szabo E. Images in clinical medicine. Sunitinib-associated hair depigmentation. The New England journal of medicine. 2014;370:e27. doi: 10.1056/NEJMicm1309906. [DOI] [PubMed] [Google Scholar]
- 103.Hutterer M, Nowosielski M, Haybaeck J, Embacher S, Stockhammer F, Gotwald T, et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07) Neuro-oncology. 2014;16:92–102. doi: 10.1093/neuonc/not161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sibaud V, Munsch C, Lamant L. Eruptive nevi and hair depigmentation related to regorafenib. European journal of dermatology : EJD. 2015;25:85–6. doi: 10.1684/ejd.2014.2462. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez NA, Ascaso FJ. Trichomegaly and poliosis of the eyelashes during cetuximab treatment of metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:e532–3. doi: 10.1200/JCO.2011.34.6858. [DOI] [PubMed] [Google Scholar]
- 106.Alexandrescu DT, Kauffman CL, Dasanu CA. Persistent hair growth during treatment with the EGFR inhibitor erlotinib. Dermatology online journal. 2009;15:4. [PubMed] [Google Scholar]
- 107.Carser JE, Summers YJ. Trichomegaly of the eyelashes after treatment with erlotinib in non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1:1040–1. [PubMed] [Google Scholar]
- 108.Gerber PA, Homey B. Images in clinical medicine. Erlotinib-induced hair alterations. The New England journal of medicine. 2008;358:1175. doi: 10.1056/NEJMicm073144. [DOI] [PubMed] [Google Scholar]
- 109.Kerob D, Dupuy A, Reygagne P, Levy A, Morel P, Bernard BA, et al. Facial hypertrichosis induced by Cetuximab, an anti-EGFR monoclonal antibody. Archives of dermatology. 2006;142:1656–7. doi: 10.1001/archderm.142.12.1656. [DOI] [PubMed] [Google Scholar]
- 110.Montagut C, Grau JJ, Grimalt R, Codony J, Ferrando J, Albanell J. Abnormal hair growth in a patient with head and neck cancer treated with the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5273–5. doi: 10.1200/JCO.2005.01.9570. [DOI] [PubMed] [Google Scholar]
- 111.Betrian S, Gomez-Roca C, Vigarios E, Delord JP, Sibaud V. Severe Onycholysis and Eyelash Trichomegaly Following Use of New Selective Pan-FGFR Inhibitors. JAMA dermatology. 2017;153:723–5. doi: 10.1001/jamadermatol.2017.0500. [DOI] [PubMed] [Google Scholar]
- 112.Kudo K, Fujiwara K, Tsushima M, Mizuta M, Matsuo K, Yonei T, et al. Toxicity manifesting as cosmetic hair alterations during erlotinib treatment. Acta oncologica (Stockholm, Sweden) 2011;50:146–8. doi: 10.3109/0284186X.2010.509107. [DOI] [PubMed] [Google Scholar]
- 113.Botchkarev VA, Sharov AA. Modeling Chemotherapy-Induced Hair Loss: From Experimental Propositions toward Clinical Reality. The Journal of investigative dermatology. 2016;136:557–9. doi: 10.1016/j.jid.2015.10.068. [DOI] [PubMed] [Google Scholar]
- 114.Botchkarev VA, Komarova EA, Siebenhaar F, Botchkareva NV, Komarov PG, Maurer M, et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res. 2000;60:5002–6. [PubMed] [Google Scholar]
- 115.Yoon JS, Choi M, Shin CY, Paik SH, Kim KH, Kwon O. Development of a Model for Chemotherapy-Induced Alopecia: Profiling of Histological Changes in Human Hair Follicles after Chemotherapy. The Journal of investigative dermatology. 2016;136:584–92. doi: 10.1038/JID.2015.358. [DOI] [PubMed] [Google Scholar]
- 116.Tobin DJ, Hagen E, Botchkarev VA, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? The Journal of investigative dermatology. 1998;111:941–7. doi: 10.1046/j.1523-1747.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 117.Hamilton CS, Potten CS, Denham JW, O'Brien PC, Kron T, Ostwald P, et al. Response of human hair cortical cells to fractionated radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1997;43:289–92. doi: 10.1016/s0167-8140(97)00059-5. [DOI] [PubMed] [Google Scholar]
- 118.Conrad F, Ohnemus U, Bodo E, Bettermann A, Paus R. Estrogens and human scalp hair growth-still more questions than answers. The Journal of investigative dermatology. 2004;122:840–2. doi: 10.1111/j.0022-202X.2004.22344.x. [DOI] [PubMed] [Google Scholar]
- 119.Conrad F, Ohnemus U, Bodo E, Biro T, Tychsen B, Gerstmayer B, et al. Substantial sex-dependent differences in the response of human scalp hair follicles to estrogen stimulation in vitro advocate gender-tailored management of female versus male pattern balding. The journal of investigative dermatology Symposium proceedings. 2005;10:243–6. doi: 10.1111/j.1087-0024.2005.10115.x. [DOI] [PubMed] [Google Scholar]
- 120.Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocrine reviews. 2006;27:677–706. doi: 10.1210/er.2006-0020. [DOI] [PubMed] [Google Scholar]
- 121.Gateley CA, Bundred NJ. Alopecia and breast disease. BMJ (Clinical research ed) 1997;314:481. doi: 10.1136/bmj.314.7079.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park J, Kim JI, Yun SK, Kim HU, Ihm CW. Pattern Alopecia during Hormonal Anticancer Therapy in Patients with Breast Cancer. Annals of dermatology. 2014;26:743–6. doi: 10.5021/ad.2014.26.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 124.Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Experimental dermatology. 2017;26:483–90. doi: 10.1111/exd.13347. [DOI] [PubMed] [Google Scholar]
- 125.Bernard BA. The hair follicle enigma. Experimental dermatology. 2017;26:472–7. doi: 10.1111/exd.13337. [DOI] [PubMed] [Google Scholar]
- 126.World Health Organization. World Health Organization Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization; 1979. p. 48. [Google Scholar]
- 127.Dean JC, Salmon SE, Griffith KS. Prevention of doxorubicin-induced hair loss with scalp hypothermia. The New England journal of medicine. 1979;301:1427–9. doi: 10.1056/NEJM197912273012605. [DOI] [PubMed] [Google Scholar]
- 128.Comis R. ECOG Common Toxicity Criteria. Boston: The Organization; 2015. [revised 2007 Jun 15; cited 2017 Jun 1]. ECOG-ACRIN Cancer Research Group [Internet] Available from: http://www.ecog.org/general/common_tox.html. [Google Scholar]
- 129.Sredni B, Xu RH, Albeck M, Gafter U, Gal R, Shani A, et al. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia studies on human and animal models. International journal of cancer Journal international du cancer. 1996;65:97–103. doi: 10.1002/(SICI)1097-0215(19960103)65:1<97::AID-IJC17>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 130.National Cancer Institute [Internet] Cancer Therapy Evaluation Program: Protocol Development. Bethesda: The Organization; 1995-2002. [updated 2016 Nov 14; cited 2017 Jun 1]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 131.Lacouture ME, Maitland ML, Segaert S, Setser A, Baran R, Fox LP, et al. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:509–22. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 132.Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. Journal of the American Academy of Dermatology. 2012;67:1025–39. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 133.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. American journal of obstetrics and gynecology. 1981;140:815–30. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 134.McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer practice. 2001;9:283–9. doi: 10.1046/j.1523-5394.2001.96007.x. [DOI] [PubMed] [Google Scholar]
- 135.Choi EK, Kim IR, Chang O, Kang D, Nam SJ, Lee JE, et al. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho-oncology. 2014 doi: 10.1002/pon.3531. [DOI] [PubMed] [Google Scholar]
- 136.McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer practice. 2001;9:283–89. doi: 10.1046/j.1523-5394.2001.96007.x. [DOI] [PubMed] [Google Scholar]
- 137.Freedman TG. Social and cultural dimensions of hair loss in women treated for breast cancer. Cancer nursing. 1994;17:334–41. [PubMed] [Google Scholar]
- 138.Tierney AJ, Taylor J, Closs SJ. Knowledge, expectations and experiences of patients receiving chemotherapy for breast cancer. Scandinavian Journal of Caring Sciences. 1992;6:75–80. doi: 10.1111/j.1471-6712.1992.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 139.Smith K, Winstanley J, Boyle F, O'Reilly A, White M, Antill YC. Madarosis: a qualitative study to assess perceptions and experience of Australian patients with early breast cancer treated with taxane-based chemotherapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2018;26:483–9. doi: 10.1007/s00520-017-3852-z. [DOI] [PubMed] [Google Scholar]
- 140.Blume-Peytavi U. An overview of unwanted female hair. The British journal of dermatology. 2011;165(Suppl 3):19–23. doi: 10.1111/j.1365-2133.2011.10632.x. [DOI] [PubMed] [Google Scholar]
- 141.Rosenfield RL. Clinical practice. Hirsutism. The New England journal of medicine. 2005;353:2578–88. doi: 10.1056/NEJMcp033496. [DOI] [PubMed] [Google Scholar]
- 142.Kim J, Singh H, Ayalew K, Borror K, Campbell M, Johnson LL, et al. Use of PRO measures to inform tolerability in oncology trials: Implications for clinical review, IND safety reporting and clinical site inspections. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017 doi: 10.1158/1078-0432.CCR-17-2555. [DOI] [PubMed] [Google Scholar]
- 143.Cho J, Choi EK, Kim IR, Im YH, Park YH, Lee S, et al. Development and validation of Chemotherapy-induced Alopecia Distress Scale (CADS) for breast cancer patients. Ann Oncol. 2014;25:346–51. doi: 10.1093/annonc/mdt476. [DOI] [PubMed] [Google Scholar]
- 144.Dang J, Hansen JE, Burgess SM. PSS32 Validation and Assessment of Measurement Invariance of The Eyelash Satisfaction Questionnaire (Esq) In Us Cancer Patients. Value in Health. 2009;12:A458. [Google Scholar]
- 145.Dang J, Cole JC, Burgess SM, Yang M, Daniels SR, Walt JG. Development and Validation of the Eyelash Satisfaction Questionnaire. Aesthetic surgery journal. 2016;36:221–8. doi: 10.1093/asj/sjv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Granai CO, Frederickson H, Gajewski W, Goodman A, Goldstein A, Baden H. The use of minoxidil to attempt to prevent alopecia during chemotherapy for gynecologic malignancies. European journal of gynaecological oncology. 1991;12:129–32. [PubMed] [Google Scholar]
- 147.Duvic M, Lemak NA, Valero V, Hymes SR, Farmer KL, Hortobagyi GN, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74–8. doi: 10.1016/S0190-9622(96)90500-9. [DOI] [PubMed] [Google Scholar]
- 148.Lacouture ME, Konner JA, Belum VR, Stevens J, Dion H, Ciccolini K, et al. A phase I safety study of topical calcitriol (BPM 31543) for the prevention of chemotherapy-induced alopecia (CIA) Journal of Clinical Oncology. 2017;35:e14064. e. [Google Scholar]
- 149.Shah VV, Wikramanayake TC, DelCanto GM, van den Hurk C, Wu S, Lacouture ME, et al. Scalp hypothermia as a preventative measure for chemotherapy-induced alopecia: A review of controlled clinical trials. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017 doi: 10.1111/jdv.14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teraki Y, Shibuya M, Izaki S. Stevens-Johnson syndrome and toxic epidermal necrolysis due to anticonvulsants share certain clinical and laboratory features with drug-induced hypersensitivity syndrome, despite differences in cutaneous presentations. Clinical and experimental dermatology. 2010;35:723–8. doi: 10.1111/j.1365-2230.2009.03718.x. [DOI] [PubMed] [Google Scholar]
- 151.Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013;43:1027–37. doi: 10.1111/cea.12145. [DOI] [PubMed] [Google Scholar]
- 152.Peck HJ, Mitchell H, Stewart AL. Evaluating the efficacy of scalp cooling using the Penguin cold cap system to reduce alopecia in patients undergoing chemotherapy for breast cancer. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2000;4:246–8. doi: 10.1054/ejon.2000.0094. [DOI] [PubMed] [Google Scholar]
- 153.Katsimbri P, Bamias A, Pavlidis N. Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. European journal of cancer (Oxford, England : 1990) 2000;36:766–71. doi: 10.1016/s0959-8049(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 154.Ogawa K, Morito H, Hasegawa A, Daikoku N, Miyagawa F, Okazaki A, et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS) Journal of dermatological science. 2013;69:38–43. doi: 10.1016/j.jdermsci.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 155.Ishida T, Kano Y, Mizukawa Y, Shiohara T. The dynamics of herpesvirus reactivations during and after severe drug eruptions: their relation to the clinical phenotype and therapeutic outcome. Allergy. 2014;69:798–805. doi: 10.1111/all.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luce J, Raffetto T, Crisp I, Grief G. Prevention of alopecia by scalp cooling of patients receiving adriamycin. Cancer Chemother Rep. 1973;57:108–9. [Google Scholar]
- 157.Janssen FP, Rajan V, Steenbergen W, van Leeuwen GM, van Steenhoven AA. The relationship between local scalp skin temperature and cutaneous perfusion during scalp cooling. Physiol Meas. 2007;28:829–39. doi: 10.1088/0967-3334/28/8/006. [DOI] [PubMed] [Google Scholar]
- 158.Decorti G, Peloso I, Favarin D, Klugmann FB, Candussio L, Crivellato E, et al. Handling of doxorubicin by the LLC-PK1 kidney epithelial cell line. J Pharmacol Exp Ther. 1998;286:525–30. [PubMed] [Google Scholar]
- 159.Bulow J, Friberg L, Gaardsting O, Hansen M. Frontal subcutaneous blood flow, and epi-and subcutaneous temperatures during scalp cooling in normal man. Scandinavian journal of clinical and laboratory investigation. 1985;45:505–8. doi: 10.3109/00365518509155250. [DOI] [PubMed] [Google Scholar]
- 160.Rugo HS, Klein P, Melin SA, Hurvitz SA, Melisko ME, Moore A, et al. Association Between Use of a Scalp Cooling Device and Alopecia After Chemotherapy for Breast Cancer. Jama. 2017;317:606–14. doi: 10.1001/jama.2016.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, et al. Effect of a Scalp Cooling Device on Alopecia in Women Undergoing Chemotherapy for Breast Cancer: The SCALP Randomized Clinical Trial. Jama. 2017;317:596–605. doi: 10.1001/jama.2016.20939. [DOI] [PubMed] [Google Scholar]
- 162.Smetanay K, Junio P, Feiβt M, Hassel JC, Mayer L, Matthies LM, et al. COOLHAIR: A prospective randomized trial to investigate the efficacy and tolerability of scalp cooling in patients undergoing neoadjuvant chemotherapy for early breast cancer. Journal of Clinical Oncology. 2017;35:525. doi: 10.1007/s10549-018-4983-8. [DOI] [PubMed] [Google Scholar]
- 163.Forsberg SA. Scalp cooling therapy and cytotoxic treatment. Lancet (London, England) 2001;357:1134. doi: 10.1016/s0140-6736(00)04293-8. [DOI] [PubMed] [Google Scholar]
- 164.Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast cancer research and treatment. 2017;163:199–205. doi: 10.1007/s10549-017-4185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Barros Silva G, Donati A, van den Hurk CJ. Comments regarding “Hair regrowth during chemotherapy after scalp cooling technique”. International journal of dermatology. 2017;56:e57–e9. doi: 10.1111/ijd.13526. [DOI] [PubMed] [Google Scholar]
- 166.Belum VR, de Barros Silva G, Laloni MT, Ciccolini K, Goldfarb SB, Norton L, et al. Cold thermal injury from cold caps used for the prevention of chemotherapy-induced alopecia. Breast Cancer Res Treat. 2016;157:395–400. doi: 10.1007/s10549-016-3799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van den Hurk C, de Beer F, Dries W, van de Sande I, Hermsen N, Breed W, et al. No prevention of radiotherapy-induced alopecia by scalp cooling. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;117:193–4. doi: 10.1016/j.radonc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 168.Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. The journal of investigative dermatology Symposium proceedings. 2013;16:S73–6. doi: 10.1038/jidsymp.2013.30. [DOI] [PubMed] [Google Scholar]
- 169.Takkouche B, Etminan M, Montes-Martinez A. Personal use of hair dyes and risk of cancer: a meta-analysis. Jama. 2005;293:2516–25. doi: 10.1001/jama.293.20.2516. [DOI] [PubMed] [Google Scholar]
- 170.van Zuuren EJ, Fedorowicz Z, Carter B, Pandis N. Interventions for hirsutism (excluding laser and photoepilation therapy alone) The Cochrane database of systematic reviews. 2015:Cd010334. doi: 10.1002/14651858.CD010334.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haedersdal M, Gotzsche PC. Laser and photoepilation for unwanted hair growth. The Cochrane database of systematic reviews. 2006:Cd004684. doi: 10.1002/14651858.CD004684.pub2. [DOI] [PubMed] [Google Scholar]
- 172.Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1079–95. doi: 10.1007/s00520-011-1197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cucé LC, Rodrigues CJ, Patriota RCR. Cellium® GC: evaluation of a new natural active ingredient in 210 mg/ml topical solution, through scalp biopsy. Surg Cosmet Dermatol. 2011;3:123–28. [Google Scholar]
- 174.https://clinicaltrials.gov/ct2/show/NCT02605629
- 175.Cuscela D, Coffin D, Lupton GP, Cook JA, Krishna MC, Bonner RF, et al. Protection from radiation-induced alopecia with topical application of nitroxides: fractionated studies. The cancer journal from Scientific American. 1996;2:273–8. [PubMed] [Google Scholar]
- 176.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, et al. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:6411–7. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]


