Abstract
With increasing survival rates across all cancers, survivors represent a growing population that is frequently affected by persistent or permanent hair growth disorders as a result of systemic therapies, radiotherapy, surgical procedures, and therapeutic transplants. These hair disorders include persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, endocrine therapy-induced alopecia and hirsutism, post-surgery alopecia and localized hypertrichosis, alopecia attributed to therapeutic transplants, and to novel anticancer therapies. The information contained in this continuing medical education article should facilitate a better understanding on hair disorders in cancer survivors, so that adequate support and therapies may be provided to cancer survivors.
Keywords: Alopecia, cancer therapy, cancer survivors, endocrine therapy, persistent alopecia, persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, quality of life, hypertrichosis, hirsutism, therapeutic transplants
INTRODUCTION
The National Cancer Institute defines survivorship as the focus on “the health and life of a person with cancer post treatment until the end of life.”1 It covers the physical, psychosocial, and economic issues of cancer, beyond the diagnosis and treatment phases. Survivorship also includes late effects of treatment, and quality of life (QoL).1 Cancer survivors represent a growing population with a prevalence projected to approach 18 million by 2022 in the US,2 and over 32 million worldwide.3 Over the past decades, advances in cancer treatment have increased the overall 5-year survival to approximately 70% for childhood and adult cancers.4 Currently, around one in 530 young adults is a survivor of childhood cancer,5, 6 and approximately 1 in 30 adults have been diagnosed with cancer.7 Moreover, these improvements in survival rates have resulted in increased attention to treatment sequelae, in areas including cardiac, endocrine, neurologic, cutaneous and psychosocial domains.8 Approximately 1 in 4 cancer survivors reports a decreased QoL due to physical problems.7
Whereas the acute dermatologic adverse events (AEs) of anticancer therapies have received considerable attention, long-term dermatologic AEs such as hair growth disorders, dyspigmentation and scarring remain relatively unknown in the dermatologic community, limiting care and potential therapeutic efforts in this patient population. Indeed, the incidence of persistent or permanent alopecia after cancer was reported in 14% of 14,358 childhood cancer survivors and in 30% of adult breast cancer survivors.9 The occurrence of alopecia and scarring in cancer survivors is notably associated with psychological disorders such as depression, anxiety and low self-esteem, eventually leading to lowering of health-related QoL.10 The information in this article will allow clinicians to better understand hair disorders in cancer survivors so that adequate support and potential therapies could be offered, thus improving the QoL of cancer survivors.
ALOPECIA IN CANCER SURVIVORS: OVERVIEW AND CLINICAL FEATURES
Key points
There are an estimated 15.5 million cancer survivors in the United States, equivalent to 4.8% of the population. The majority had undergone a surgical procedure as part of their diagnosis or treatment, approximately 50% have been treated with radiotherapy, and more than 60% have received systemic anticancer therapies, all of which may result in persistent or permanent hair disorders
Breast cancer survivors treated with taxanes (paclitaxel, docetaxel) will develop persistent alopecia in 30%
Endocrine therapies are associated with pattern alopecia similar to androgenetic type in 15-25% of cases
Head and neck radiotherapy leads to persistent alopecia in 60% of survivors
In childhood cancer survivors, alopecia has been associated with anxiety and depression, and adult survivors with persistent alopecia report a negative impact on their emotions
Persistent chemotherapy-induced alopecia
The total or incomplete hair regrowth 6 months following therapy completion in patients who received cytotoxic chemotherapy is defined as persistent chemotherapy-induced alopecia (pCIA).11, 12 Also described as permanent chemotherapy-induced alopecia,13-20 or as chemotherapy irreversible alopecia.21-23 Whether most cancer survivors have been evaluated and treated for this type of alopecia or if pCIA is in fact permanent or irreversible has yet to be determined. pCIA has been mostly reported in breast cancer survivors treated with taxane-based chemotherapy11, 15, 19, 24, 25 (paclitaxel and docetaxel), and cyclophosphamide-based chemotherapy,15, 26, 27 with an incidence of 30% 36 months after completion of chemotherapy.28 In addition, pCIA have been reported in children who have undergone a conditioning therapy with busulfan29-35 (with a cumulative incidence of 19%),36 and with other chemotherapies used for stem cell transplantation (e.g. thiotepa and carboplatin)37 (Table I).
Table I.
Incidence, case reports, and clinical features of alopecia attributed to anticancer therapies in cancer survivors.
| Anticancer therapies | Predominant cancer type | Reported cases and incidence | Clinical Features |
|---|---|---|---|
|
| |||
| Cytotoxic chemotherapy (pCIA) | Reported cases of pCIA with described clinical features n=382 (%) | Non-scaring alopecia, with diffuse hair thinning and lightening is reported in 53% of cases. A similar pattern to androgenetic alopecia is also described (46.2% of cases) | |
|
|
|||
| Taxane based chemotherapy (including combinations with cyclophosphamide, epirubicin and FEC-100) | Breast | 259 (67.8%) (Incidence of 30%) | Persistent changes in texture could be observed Scarring features has been reported in 2 cases |
|
|
|||
| Cyclophosphamide based chemotherapy (including combinations with doxorubicin and CTC) | Leukemias, lymphomas, solid tumors | 67 (17.5%) | Eyelash, eyebrow, axillary and pubic hair could be involved |
|
|
|||
| Busulphan based chemotherapy (including combinations with cyclophosphamide) | Hematologic malignancies | 35 (9.2%) | |
|
|
|||
| Other chemotherapies (including cisplatin, methotrexate, vincristine) | Solid tumors, hematologic malignancies | 21 (5.5%) | |
|
| |||
| Radiotherapy (pRIA) | Scarring and non-scaring features may be present | ||
|
|
|||
| Photon radiation (traditional radiotherapy) | Primary CNS tumors, metastasis | Up to 50% risk with high fractionated follicular dose of 43 Gy | Geometric shapes of atrophic skin with scarce hairs could be seen in severe cases |
|
|
|||
| Proton radiation | Medulloblastoma, ependymoma | 13 children reported | Diffuse hair thinning in total cranial irradiation and in combination with cytotoxic chemotherapy Commonly: occipital, parietal and temporal areas |
|
| |||
| Endocrine therapies (EIA) | |||
|
|
|||
| Selective estrogen receptor modulators (e.g. tamoxifen, toremifene, raloxifene) | Breast, renal cell carcinoma | ~ 15% | Non-scaring features |
|
|
|||
| Aromatase inhibitors (e.g. anastrozole, letrozole, exemestane) | Breast | ~ 25% | Predominantly women with a similar pattern to androgenetic alopecia |
|
|
|||
| Estrogen receptor down-regulator (fulvestrant) | Breast, ovarian | 2.2- 7.9% | Diffuse hair thinning and lightening over the entire scalp is also reported |
|
|
|||
| Luteinizing hormone-releasing hormone agonist (leuprolide) | Breast, prostate | 9.5% | |
|
|
|||
| Somatostatin analog (octreotide) | Growth hormone producing tumor (pituitary) | 6.7% | |
|
| |||
| Cancer surgery | Linear scar on the scalp Hypertrophic scars may be observed | ||
|
|
|||
| Neurosurgical procedures (e.g. CNS tumor excisions and biopsies, scalp biopsies, catheters) | Primary CNS tumors, tumors in hair-bearing areas | Scarring/disfigurement and hair loss on the head/neck area in 3.557 (25.1%) of 14.358 cancer survivors | Could be associated with persistent radiotherapy- induced alopecia |
|
|
|
||
| Flaps (e.g. radial forearm flap) | Head and neck tumors | Terminal hairs in undesirable areas, such as the oral cavity or face | |
|
| |||
| Immunotherapies: CTLA-4 inhibitors (e.g. ipilimumab), PD- 1 receptor inhibitors (e.g. nivolumab and pembrolizumab), PD-L1 inhibitors (e.g. atezolizumab, avelumab) | Melanoma, lung, bladder, prostate cancer, head and neck squamous cell carcinoma | 2 cases with CTLA-4 inhibitors | |
| 1 case with PD-L1 inhibitor | Non-scaring alopecia with diffuse hair thinning and alopecia areata, evident 3-6 months after therapy completion | ||
| 1 case with CTLA-4 and PD-1 receptor inhibitors | Skin involvement may be present, including vitiligo, lichenoid reaction | ||
|
| |||
| Vismodegib | Basal cell carcinoma | 4 cases | Persistent diffuse severe alopecia (CTCAEv4.0 grade 2) |
|
| |||
| SCT (acute or chronic GvHD, conditioning therapy for SCT with busulfan, and total body irradiation) | Leukemias | ~ 20% of alopecia areata in chronic GvHD | More likely to develop alopecia areata. Skin involvement may be present (vitiligo, scleroderma, eczema and lichenoid reaction) |
| Diffuse alopecia with scarring features could be observed | |||
|
| |||
| Conditioning therapy for SCT: scalp alopecia with busulfan in 56% and 10% with total body irradiation | Diffuse hair loss and hair thinning | ||
CTC, cyclophosphamide/thiotepa/carboplatin; CNS, central nervous system; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CTCAEv4.0, Common Terminology Criteria for Adverse Events Version 4.0; EGFR, epidermal growth factor receptor; FEC-100, fluorouracil/epirubicin/cyclophosphamide; GvHD, graft versus host disease; pCIA, persistent chemotherapy-induced alopecia; PD-1, programmed cell death protein 1; PDGF, platelet derived growth factor; PD-L1, programmed death-ligand 1; SCT, Stem cell transplant; TCH, docetaxel/carboplatin/trastuzumab.
With the commonly used chemotherapy regimens combining taxanes with anthracyclines, the risk of severe pCIA was significantly higher than the combination of doxorubicin and cyclophosphamide alone (10.5 vs. 2.7%). In a questionnaire-based cross-sectional study of 265 breast cancer survivors, 7.2% reported severe pCIA (hair loss >50%).21
Multiple clinical features have been described for pCIA (Table I). The most common is a non-scarring, diffuse alopecia (53% of pCIA reported cases)15, 17, 25, 31 (Figure 1), and a pattern similar to androgenetic alopecia has been reported in 46.2%.16, 17 pCIA may also be associated with madarosis, axillary, and pubic alopecia, although the incidence of persistent alopecia in body sites other than the scalp is unknown.18, 35
Figure 1.
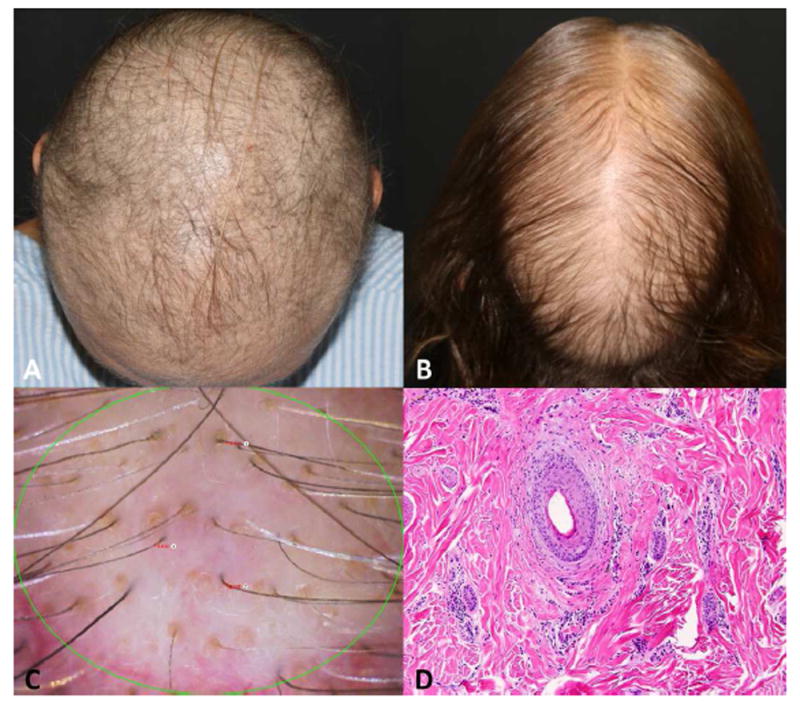
Persistent chemotherapy-induced alopecia (pCIA). A. Diffuse alopecia in a breast cancer survivor, 2 years after taxane based chemotherapy completion. B. pCIA in a breast cancer survivor, 1.6 years after taxane based chemotherapy completion with similar pattern of androgenetic alopecia, predominant hair thinning on the crown area. C. Trichoscopy of patient in figure B, featuring hair thinning, miniaturized hairs, and yellow dots. D. Histology section featuring mild perifollicular inflammation and fibrosis, (hematoxylin-eosin stain).
Persistent radiotherapy-induced alopecia
Hair regrowth generally occurs within 2–4 months after radiotherapy to the head and neck.38, 39 Persistent radiotherapy-induced alopecia (pRIA) is the total or incomplete hair regrowth 6 months following radiotherapy completion, and is commonly related to high-dose radiotherapy to the scalp.40 In 26 patients with primary brain tumors, the doses reported to cause pRIA were correlated with radiotherapy dose to the hair follicles in a particular radiotherapy field, with a 50% risk for pRIA with a fractionated follicular dose of ≥ 43 Gy.41 In 12 children with medulloblastoma treated with proton beam radiation in combination with high dose vincristine-based chemotherapy, pRIA was observed in 75%, and was correlated with a craniospinal radiotherapy dose above 21 Gy.42
Clinical presentation of pRIA includes well defined alopecic and atrophic skin confined to the area of radiotherapy, and is usually asymptomatic (Table I). Occipital, parietal, and temporal scalp are commonly focal sites of radiotherapy for brain metastases and central nervous system tumors such as glioblastoma and astrocytoma43 (Figure 2). Diffuse pRIA is also described with whole brain radiotherapy for brain metastases,44, 45 especially when combined with chemotherapy.46 In addition, pRIA could be also observed in any other hair-bearing area were radiotherapy is received, such as the face, neck,47 or the extremities in patients with other solid tumors.48
Figure 2.
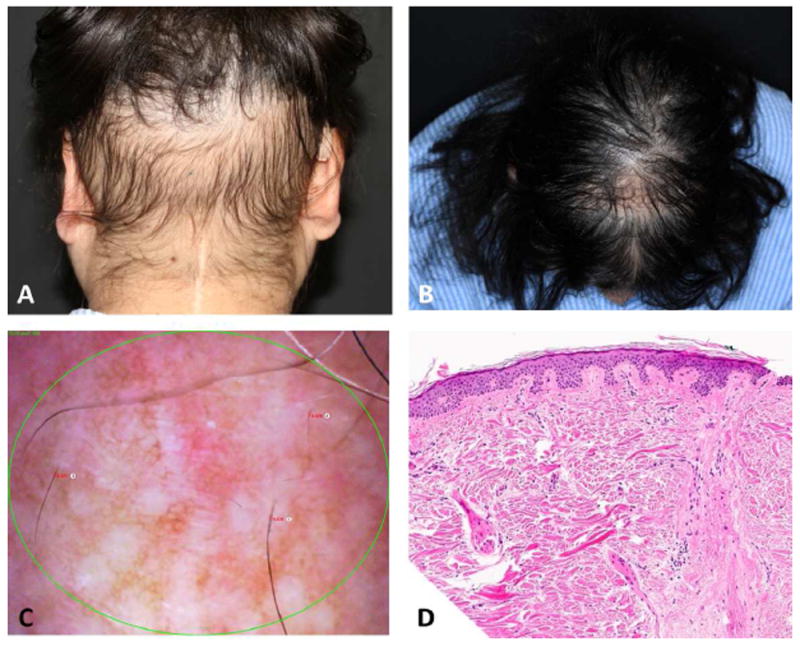
Persistent radiotherapy-induced alopecia (pRIA). A. Localized alopecia in a childhood cancer survivor with medulloblastoma treated with surgery and traditional (photon) radiotherapy. B. Diffuse hair loss with hair thinning on the crown area with a central scar after traditional radiotherapy and cytotoxic chemotherapy for a SNC tumor. C. Phototrichogram of patient in figure A, Featuring hair thinning miniaturized hairs, and white dots. D, Histology section featuring hair follicle replaced by fibrous tract. There is no significant inflammation (hematoxylin-eosin stain).
Endocrine therapy-induced alopecia and hirsutism
Endocrine therapies (ET) are standard of care in survivors of hormone receptor-positive breast cancer (around 70% of all breast cancers).49 ET, including selective estrogen receptor modulators (e.g., tamoxifen, toremifene), aromatase inhibitors (e.g., anastrozole, letrozole, exemestane) and gonadotropin-releasing hormone agonist (e.g., leuprolide) are usually administered for 5-10 years in the adjuvant setting to reduce the risk of recurrence.50, 51 Breast cancer survivors are known to have substantial AE attributed to estrogen deprivation from ET,52 and up to 8% of survivors will discontinue therapy due to alopecia related to adjuvant therapy with aromatase inhibitors.52
In a hospital registry-based survey study of 851 female breast cancer survivors, 22 % of those who received aromatase inhibitors reported hair loss, and 32% reported hair thinning.53 Additionally, a meta-analysis of 13,415 patients in 35 clinical trials including different ETs reported an overall incidence of all-grade alopecia of 4.4%, with the highest incidence (25%) in patients treated with aromatase inhibitors.54
In a retrospective study of 112 breast cancer patients with ET-induced alopecia (EIA), causal agents included aromatase inhibitors in 67% and tamoxifen in 33% of patients. The mean time to alopecia development was 16.8 months (range 1-91 months).55 These patients usually present with frontoparietal hairline recession (Table I), mimicking the pattern of androgenetic alopecia (Figure 3).56 The trichoscopic features observed in patients with EIA include the concomitant presence of vellus and terminal hairs, also a hallmark of androgenetic alopecia.55, 57 Iatrogenic hirsutism has been reported in less than 10% of survivors receiving ET for breast cancer58 (Figure 3).
Figure 3.
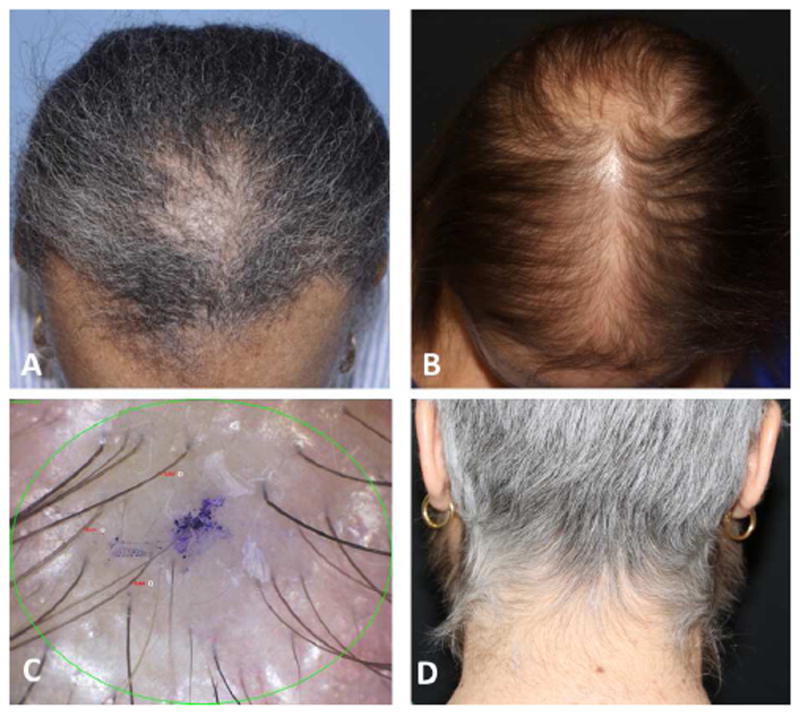
Endocrine therapy-induced alopecia (EIA). A. EIA with similar pattern of androgenetic alopecia, predominant on the frontoparietal hair line. B. EIA with similar pattern of androgenetic alopecia, predominant on the crown area. C. Trichoscopy featuring vellus hairs, and intermediate and thick terminal hairs. D. Hirsutism in a patient receiving endocrine therapy.
In patients treated with cytotoxic chemotherapy followed by ET (the majority of hormone-receptor positive breast cancers), a complete medical history must be obtained to define whether alopecia is attributed to the actual ET (EIA) or to the previous chemotherapy (pCIA), or a combination of both (pCIA+EIA).
Permanent surgery-induced alopecia and localized hypertrichosis
Alopecia and scarring in cancer survivors usually arise secondary to tissue biopsies, placement of catheters, and surgeries to resect scalp primary or metastatic tumors.59-61 In brain cancer patients, surgery is preferred for tumor removal.62 However, complete resection is generally limited.63 Therefore, combination therapy (e.g., radiotherapy and cytotoxic chemotherapy) is usually required;64 these therapies combined with surgery, enhance the risk of permanent alopecia and scarring. The clinical presentation of permanent surgery-induced alopecia includes the linear shape of the scar on the scalp. When additional radiotherapy is combined with surgery, a geometric alopecic patched confined to the area of radiotherapy is also observed (Figure 4). Hypertrophic scars may also be associated with disfigurement, pain, or pruritus.65 Conversely, undesirable hair growth in recipient areas after reconstruction of cancer defects may be observed66-68 (Figure 5).
Figure 4.
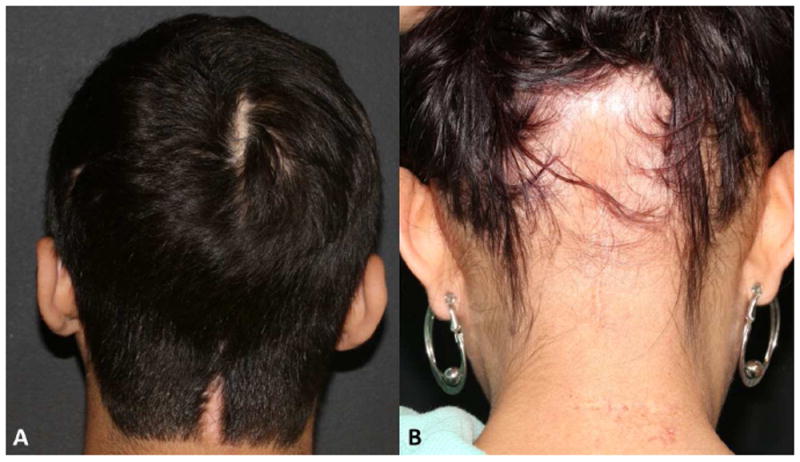
A. Linear scar on the scalp after surgery of a skull base tumor. B. Post-surgery and persistent radiotherapy-induced alopecia.
Figure 5.
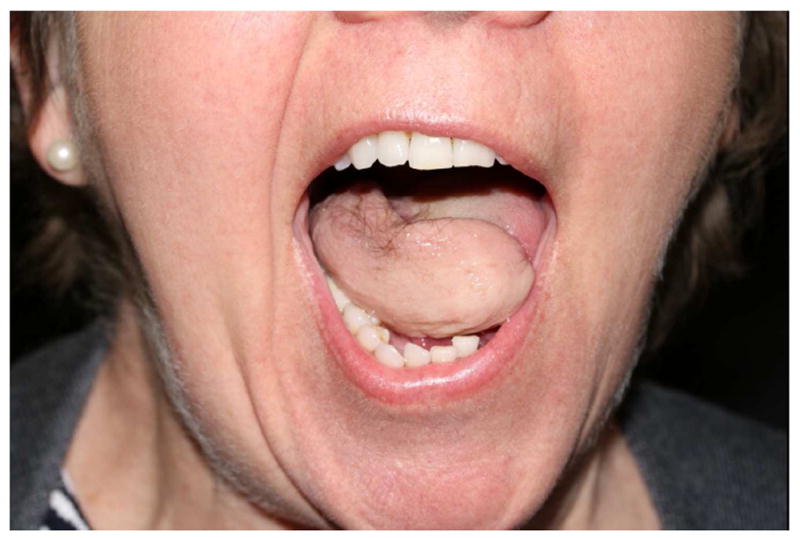
Post-surgery hair disorders. Terminal hairs on the tongue after reconstruction using a hairy donor site.
Persistent hair changes induced by other anticancer therapies
Anticancer therapies such as vismodegib,69 and immunotherapies,70 have been reported to cause persistent alopecia after drug interruption or discontinuation. Chronic graft versus host diseases after stem cell transplants may induce both diffuse alopecia (15.6%),31 and alopecia areata (in around 20%)71 (Table I).
HISTOPATHOLOGY AND PATHOBIOLOGY
Key point
Destruction of epithelial hair follicle stem cells by anticancer therapies prevents hair follicle cycling
Histopathologic features of permanent or persistent alopecia attributed to anticancer therapies are not specific. However, a non-scarring pattern is usually described in pCIA, with an increased number of miniaturized and telogen hair follicles.30 Other reported histopathologic features of pCIA include scarring alopecia, with concentric fibrosis and a discrete perifollicular lymphoid cell infiltrate24, 30 (Figure 2). In pRIA the predominant features are compatible with a scarring alopecia,72 and likely similar histopathologic features are present in permanent surgery-induced alopecia (including fibrosis along with decreased numbers or absence of hair follicles). In EIA, histopathologic features similar to androgenetic alopecia have been reported.24
Although the cause of permanent or persistent alopecia in cancer survivors has not been identified, irreversible damage to epithelial hair follicle stem cells (eHFSC) in the bulge region of the hair follicle are thought to play a crucial role.73 Compared with their differentiated progeny in the hair matrix, eHFSC in the bulge have a low proliferation rate and are generally less sensitive to chemotherapy,74 but highly sensitive to ionizing radiation.75, 76 Additionally, anticancer therapies associated with pCIA overcome the relative chemoresistance of eHFSC (for as yet not understood mechanism), and thus deplete the eHFSC pool that is vitally required for hair follicle regeneration during the next hair cycle.73, 77
A 50% reduction in mitotic indices of hair matrix cells was found in experimentally irradiated mice, suggesting that there is a persistent or permanent decrease in the number or growth fraction of eHFSC.78 The effects of chemotherapy and radiotherapy on hair regrowth are related to the interval between chemotherapy sessions, dose administered, and radiotherapy exposure.78, 79 This enhancing effect may also depend on the phase of hair follicle cycle in which the activity was arrested.46
Estrogens and androgens act as potent hair growth modulators.80, 81 ET block the function and signaling of endocrine receptors, and estrogens are unable to modify the androgen metabolism in the hair follicle, increasing the amount of 5-dihydrotestosterone.82 Indeed, androgenetic alopecia in women likely results not only from the undesired effects of androgen stimulation of androgen-sensitive hair follicles, but also from a relative lack of hair follicle stimulation by estrogens,83 which may explain the clinical similarities between EIA and androgenetic alopecia.24 Permanent surgery-induced alopecia results from a hair follicle ablation during the inflammatory, proliferative, and remodeling phases of scarring, which may extend beyond the field of surgical intervention, partially by pressure atrophy of the surrounding skin appendages, and by infiltration of the fibrotic-associated tissue into neighboring skin appendages.84, 85
QUALITY OF LIFE IN CANCER SURVIVORS WITH HAIR DISORDERS
Key point
Persistent or permanent alopecia after cancer therapies has been associated with depression, anxiety, and increased somatization
Alopecia is often considered by health care professionals as a ‘temporary’ and ‘cosmetic’ issue in cancer survivors, even though the actual distress associated with hair loss is complex, and can be overwhelming.86 Moreover, permanent or persistent alopecia is rarely included as an AE during clinical trials and usually underrecognized by healthcare providers.87 Some patients also accept alopecia as a trade-off for a cure and therefore do not present with complaints of hair-related QoL issues.88 Yet, permanent alopecia related to anticancer therapies was associated with depression, anxiety, and increased somatization.9 In addition, head and neck scarring and permanent or persistent alopecia (after chemotherapy, surgery and or cranial radiotherapy) has been reported as the strongest predictors of distress, suggesting that outward physical appearance played a prominent role in emotional adjustment of survivors.9, 89
Distressing psychological consequences were common and severe among breast cancer survivors with pCIA, as reflected by the wearing hairpieces or scarves in 14 of 20 patients.26 Additionally, in 18 breast cancer survivors with pCIA, 33% were worried about their alopecia, and it interfered with functioning in 28%.90 Also, the satisfaction score regarding the state of their hair in breast cancer survivors with pCIA was significantly lower when compared to breast cancer patients without pCIA.21
The clinical severity of alopecia may not correlate with the negative impact on a patients’ QoL.26, 91 In 112 patients diagnosed with EIA, 93% had mild alopecia (grade 1, <50% of hair loss) based on the Common Terminology Criteria for Adverse Events Version 4.0 (CTCAEv4.0). However, these patients reported a negative emotional impact when compared to the other psychological domains.55 Therefore, it is important to consider the distress that any grade of alopecia may have on cancer survivors’ QoL. Focus groups interviews including 25 breast cancer patients treated with taxane-based chemotherapy revealed that madarosis has a significant emotional impact.92 Conversely, the impact of hirsutism attributed to anticancer therapies on QoL has not been defined.
MANAGEMENT
Key point
Management of hair disorders in cancer survivors is supported by anecdotal reports and case series that fail to meet strict evidence-based medicine standards
Management of permanent or persistent alopecia in cancer survivors is mostly based on case series, case reports, and expert opinion (level of evidence, IV). Despite these limitations, we have reviewed the available information (Table II). Our pCIA experience is concordant with recent case series,24 in which the alopecia remains stable, and few may improve with topical or systemic therapies. Therefore, prevention of persistent or permanent hair growth disorders is key in order to mitigate this untoward consequence in cancer survivors. Scalp cooling has become the most widely utilized standard for the prevention of chemotherapy-induced alopecia, showing prevention of grade 2 (>50% alopecia) in 51-67% of patients.93, 94 However, there is no long-term follow-up data available on the efficacy of scalp cooling to prevent pCIA.
Table II.
Hair disorders in cancer survivors: management and recommendations.
| Hair disorder | Intervention | Level of evidence |
|---|---|---|
|
| ||
| Persistent chemotherapy-induced alopecia (pCIA) | CTCAEv4.0 grade 1: | Level IV |
| Topical minoxidil foam 5% twice daily | ||
| CTCAEv4.0 grade 2: | ||
| Spironolactone (escalating dose up to 150mg daily) in addition to therapy recommended in alopecia grade 1 (caution due to the theoretical risk of hormonal stimulation of endocrine receptor-positive tumors) | ||
| Oral minoxidil (potential adverse events should be considered) | ||
|
| ||
| Persistent radiotherapy-induced alopecia (pRIA) | CTCAEv4.0 grade 1: | |
| Topical minoxidil foam 5% twice daily | ||
| Botulinum toxin type A: 5 U per 0.1 ml saline every 3 months for 12 months | ||
| CTCAEv4.0 grade 2: | ||
| Scalp reconstruction (e.g. simple excision or flaps, tissue expansion) | ||
| Hair transplant (if not severe skin damage) | ||
|
| ||
| Endocrine therapy-induced alopecia (EIA) | CTCAEv4.0 grade 1: | |
| Topical minoxidil foam 5% twice daily | ||
| CTCAEv4.0 grade 2: | ||
| Spironolactone (escalating dose up to 150mg daily) in addition to therapy recommended in alopecia grade 1 (caution due to the theoretical risk of hormonal stimulation of endocrine receptor-positive tumors) | ||
|
| ||
| Hirsutism and hypertrichosis | CTCAEv4.0 grade 1 (mild hair growth): | |
| Local therapy such as epilation, waxing, depilation, bleaching | ||
| CTCAEv4.0 grade 2 (prominent thick hairs, associated with psychosocial impact): | ||
| Laser or intense pulsed light | ||
| Spironolactone appeared to be as effective as flutamide and finasteride (avoid in hormonal-sensitive tumors) | ||
| Other physiologic causes of hirsutism may be ruled out | ||
| Laser (Nd:YAG) for hair in unwanted areas (e.g., oral cavity) | ||
|
| ||
| Permanent surgery-induced alopecia | First line: | |
| Management of scar symptoms if present (topical or intralesional steroid, laser and light-based treatment) | ||
| Second line: | ||
| Hair transplant | ||
| Scalp reconstruction (e.g. simple excision or flaps, tissue expansion) | ||
|
| ||
| Eyebrow and eyelashes alopecia | Topical bimatoprost gel 0.03% | Level IB |
|
| ||
| SCT (Chronic GvHD, conditioning therapy for SCT with chemotherapy and/or total body irradiation) | In GvHD depends upon the organs involved and severity of symptoms Topical and intralesional steroid for alopecia areata (Level II-III), and in steroid resistant; janus kinase (JAK) inhibitors (Level IV) | Level II-IV |
|
| ||
| Conditioning chemotherapy and/or total body irradiation; follow the interventions of pCIA and pRIA respectively | Level IV | |
|
| ||
| Immunotherapies: CTLA-4 inhibitors (e.g. ipilimumab), PD-1 receptor inhibitors (e.g. nivolumab and pembrolizumab), PD-L1 inhibitors (e.g. atezolizumab, avelumab) | Potent topical steroid, and orthosilicic acid | Level IV |
|
| ||
| Vismodegib | Not reported | No evidence |
|
| ||
| General recommendations | Therapy should be discussed to have realistic expectations of therapy outcome | |
| Follow-up at least 3 months after alopecia therapy started | ||
| Laboratory analysis including, ferritin, Vitamine D, Zinc levels, and thyroid function may be requested if other causes of alopecia (e.g., androgenetic, telogen effluvium, thyroid-related) are suspected | ||
| Camouflages techniques should be provided (e.g. crayons, powder, volumizers, hair weaves/hair extension, scalp micropigmentation/tattoo and hairpieces) | ||
| If emotionally affected; psychological counseling is recommended | ||
| Involve nurses and other health care providers in the cancer survivors care | ||
CTCAEv4.0, Common Terminology Criteria for Adverse Events Version 4.0; CTCAEv4.0 grade 1 for alopecia, Hair loss of <50% of normal for that individual that is not obvious from a distance but only on close inspection; a different hair style may be required to cover the hair loss but it does not require a wig or hair piece to camouflage; CTCAEv4.0 grade 2 for alopecia, Hair loss of >=50% normal for that individual that is readily apparent to others; a wig or hair piece is necessary if the patient desires to completely camouflage the hair loss; associated with psychosocial impact; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; SCT, Stem cell transplant; Nd:YAG, neodymium-doped yttrium aluminium garnet; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
The main objectives of reactive alopecia therapy in cancer survivors are to stop or reduce the hair loss and stimulate growth. Duration of therapy should be guided by clinical response, and a laboratory analysis may help to exclude other causes of alopecia, such as thyroid disease, and vitamin or mineral deficiency.
Topical therapy with minoxidil 2 or 5% has been shown to stabilize or improve alopecia in case reports of pCIA.24, 26 However, in 14 of 20 breast cancer survivors it was unsuccessful after >3 months of therapy.26 On the other hand, treatment with topical minoxidil 5% daily in 46 breast cancer survivors with EIA, moderate or significant improvement was observed in 80%.55 A case report showed that oral minoxidil improved pCIA in a breast cancer survivor.13 In contrast, pCIA treated with spironolactone (150 mg/day, 3 months) in one breast cancer survivor showed no efficacy.26 However, in our experience with breast cancer survivors using spironolactone alone or in combination with minoxidil 5% for pCIA and EIA, 60% of patients tend to have a moderate or significant clinical improvement as confirmed with baseline clinical and trichoscopy standardized pictures (Figure 6). These positive clinical outcomes have been mostly observed in patients with alopecia grade 1 (CTCAEv4.0). Other options should be discussed in patients with alopecia grade 2 (CTCAEv4.0), so that the expectations of alopecia improvement are realistic. There is a putative risk of hormonal stimulation of endocrine receptor-positive tumors with the use of systemic therapies for androgenic alopecia (e.g., spironolactone, finasteride), so these agents must be used with caution. Support groups may be helpful, and patients with pCIA can be directed to http://aheadofourtime.org/.
Figure 6.
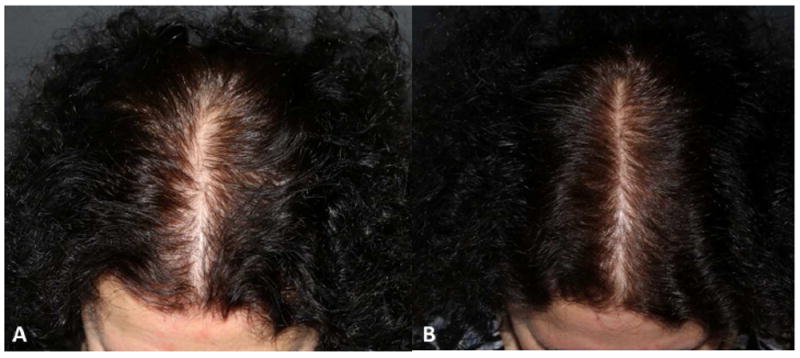
Endocrine therapy-induced alopecia (EIA). A. Before and B. Six months after therapy with topical minoxidil foam 5% twice a day.
For hypertrichosis and hirsutism in cancer survivors, ruling out common causes of hyperandrogenism is important.95 In our experience, hirsutism attributed to anticancer therapies is predominantly mild (grade 1, CTCAEv4.0) in cancer survivors, especially in postmenopausal once they have completed cytotoxic chemotherapy or in those receiving ET, and requires only reassurance and local therapies such as epilation, laser therapies, or bleaching if needed (Table II). Nd:YAG laser therapy appears to have a hair clearance of >90% after 1-4 sessions in 5/9 patients suffering from growth of terminal hair in the oral cavity after reconstruction using a hairy donor site.96
Multiple scalp reconstructive options have been described to improve the appearance of the localized pRIA, including tissue expansion, and hair transplantation.40 However, the success of these techniques will rely on the skin viability and severity of hair follicle damaged.97 There is no reported experience using platelet-rich plasma to treat alopecias in cancer survivors, and costs may be elevated. Hair follicle neogenesis with autologous cell populations may become a future therapeutic option for pCIA and pRIA.98, 99
A randomized controlled trial including 20 patients treated with bimatoprost 0.03% gel for chemotherapy-induced eyelash alopecia, improvement in length (1.50 vs 0.46 mm), and thickness (3 vs 2, in a scale of 1-5) of treated eyelashes was observed, with an increase of patient satisfaction (16 vs 26) after 3 moths of therapy100(Figure 7).
Figure 7.

Topical bimatoprost 0.03% gel for persistent chemotherapy-induced eyelashes alopecia. A. Before therapy and B. Six months after therapy.
Interventions with skin camouflage (including powders, scalp micropigmentation/tattoo, hair color and hairstyle changes) can modify the concerning body image to mask skin discoloration and alopecia. This effect acts to improve the visible impact of deformity.101, 102 When camouflage may be needed, options should be provided. Initially, most patients are reluctant to use any camouflage, but they can enhance self-confidence and QoL.103 The market for home-use cosmetic devices is rapidly expanding, however there are no reports measuring their efficacy for hair growth disorders in cancer survivors.
The ongoing CHANCE Study (A Study of Chemotherapy-Induced Hair Changes and Alopecia, Skin Aging and Nail Changes in Women With Non-Metastatic Breast Cancer; ClinicalTrials.gov dentifier: NCT02530177) examining the incidence, risk factors, psychosocial impact, and clinical features of pCIA and EIA in 500 cancer survivors will yield additional information, critical towards the identification of preventive and treatment strategies, and similar studies in other patient populations are needed
CHALLENGES AND FUTURE PERSPECTIVES
Further efforts should be made to understand the mechanism of hair follicle alteration and to identify effective strategies for the prevention and treatment of permanent or persistent alopecia in cancer survivors. Patient counseling regarding the possibility of developing persistent or permanent hair disorders with anticancer therapies will also be important so that anticipatory coping can take place. Prospective studies evaluating patients during and after their treatments are needed to identify the real incidence and severity of these conditions. Therapeutic options remain limited in number and efficacy, and additional research is needed in order to determine optimal preventive and therapeutic approaches for the various hair disorders observed in the growing population of cancer survivors.
Acknowledgments
Funding Support: This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. M.E.L. was supported by the RJR Oncodermatology Fund. A.F.M was partially supported by Beca Excelencia, Academia Española de Dermatología y Venereología (AEDV)-Fundación Piel Sana. R.P. is funded by the NIHR Manchester Biomedical Research Centre. Funders/sponsors were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
ABBREVIATIONS USED
- AE
Adverse event
- CTCAEv4.0
Common Terminology Criteria for Adverse Events Version 4.0
- eHFSC
Epithelial hair follicle stem cells
- ET
Endocrine therapy
- EIA
Endocrine therapy-induced alopecia
- pCIA
Persistent chemotherapy-induced alopecia
- pRIA
Persistent radiotherapy-induced alopecia
- QoL
Quality of life
Footnotes
Learning objectives:
To diagnose and manage hair disorders in cancer survivors.
COI Disclosure Statement: AFM, CvdH and JJ have nothing to disclose. JS: Consultant for Aclaris, Samumed, Incyte, Replicel Life Sciences, Shook, Hardy, Bacon LLP who represent Sanofi Aventis US LLC. AR: consultant for Cutera lasers and Vivscal. RP: has a speaking, consultant or advisory role with Giuliani/Italy, Unilever/UK, Reckitt Benkiser/UK and Shiseido/Japan. SG: has a speaking, consultant or advisory role with Adgero Biopharmaceuticals, AMAG pharmaceuticals, Procter and Gamble and Valeant women’s health pharmaceuticals. MEL: has a speaking, consultant or advisory role with Abbvie, Quintiles, Boehringer Ingelheim, AstraZeneca pharmaceuticals, Legacy Healthcare, Foamix, Adgero Bio Pharmaceuticals, Janssen R&D, Novartis, Paxman and Novocure, and also receives research grants from Berg and Bristol-Myers Squibb.
The Contents of the manuscript have not been previously published and are not currently submitted elsewhere. The authors accept responsibility for the scientific integrity of the work described in this manuscript. All listed authors have seen and approved of the manuscript and will sign off on any subsequent manuscript revisions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [4 Jan 2017]; https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=445089.
- 2.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 5.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:2108–17. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. The New England journal of medicine. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 9.Kinahan KE, Sharp LK, Seidel K, Leisenring W, Didwania A, Lacouture ME, et al. Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2466–74. doi: 10.1200/JCO.2011.39.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi M, Oishi K, Zubal B, Lacouture ME. Unanticipated toxicities from anticancer therapies: survivors’ perspectives. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1461–8. doi: 10.1007/s00520-009-0769-1. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois H, D F, Kerbrat P, Combe M, Lamy R, Egreteau J, Delecroix V, Deguiral P, Van Hulst S, Hadjarab Y, Simon H, Berton-Rigault D, Priou F, Hardy-Bessard A, Porneuf M, Raoul Y, Desclos H, Abadie-Lacourtoisie S, El Khouri C, Metges J, Riche C, Grude F. Long Term Persistent Alopecia and Suboptimal Hair Regrowth after Adjuvant Chemotherapy for Breast Cancer: Alert for an Emerging Side Effect: ALOPERS Observatory. Cancer Res. 2009;69 Abstractnr 3174. [Google Scholar]
- 12.Bourgeois HP, Kerbrat P, Combe M, Tuchais C, Delecroix V, Egreteau J, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: Alert for an emerging side effect: French alopers observatory. Annals of Oncology. 2010;21:viii83–viii4. [Google Scholar]
- 13.Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. The Australasian journal of dermatology. 2015 doi: 10.1111/ajd.12350. [DOI] [PubMed] [Google Scholar]
- 14.Tosti A, Piraccini BM, Vincenzi C, Misciali C. Permanent alopecia after busulfan chemotherapy. The British journal of dermatology. 2005;152:1056–8. doi: 10.1111/j.1365-2133.2005.06469.x. [DOI] [PubMed] [Google Scholar]
- 15.Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clinical journal of oncology nursing. 2009;13:13–4. doi: 10.1188/09.CJON.13-14. [DOI] [PubMed] [Google Scholar]
- 16.Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. Journal of the American Academy of Dermatology. 2010;63:333–6. doi: 10.1016/j.jaad.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Miteva M, Misciali C, Fanti PA, Vincenzi C, Romanelli P, Tosti A. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. The American Journal of dermatopathology. 2011;33:345–50. doi: 10.1097/DAD.0b013e3181fcfc25. [DOI] [PubMed] [Google Scholar]
- 18.Palamaras I, Misciali C, Vincenzi C, Robles WS, Tosti A. Permanent chemotherapy-induced alopecia: a review. Journal of the American Academy of Dermatology. 2011;64:604–6. doi: 10.1016/j.jaad.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand M, Mailliez A, Vercambre S, Kotecki N, Mortier L, Bonneterre J. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: Long term follow up. Cancer Research. 2013;73 [Google Scholar]
- 20.Champagne C, Taylor M, Farrant P. Permanent chemotherapy-induced nonscarring alopecia and premature ovarian failure. Clinical and experimental dermatology. 2015;40:589–90. doi: 10.1111/ced.12596. [DOI] [PubMed] [Google Scholar]
- 21.Kim GM, Kim S, Park HS, Kim JY, Nam S, Park S, et al. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast cancer research and treatment. 2017;163:527–33. doi: 10.1007/s10549-017-4204-x. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Crespo M, Betlloch I, Ballester I, Lucas A, Mataix J, Niveiro M. Irreversible alopecia due to busulphan in a 7-year-old girl. European journal of dermatology : EJD. 2009;19:192–3. doi: 10.1684/ejd.2008.0617. [DOI] [PubMed] [Google Scholar]
- 23.Prevezas C, Matard B, Pinquier L, Reygagne P. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. The British journal of dermatology. 2009;160:883–5. doi: 10.1111/j.1365-2133.2009.09043.x. [DOI] [PubMed] [Google Scholar]
- 24.Fonia A, Cota C, Setterfield JF, Goldberg LJ, Fenton DA, Stefanato CM. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: Clinicopathologic findings in a cohort of 10 patients. Journal of the American Academy of Dermatology. 2017;76:948–57. doi: 10.1016/j.jaad.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Park HS, Kim JY, Nam S, Kim GM, Sohn JH, et al. Irriversible chemotherapy-induced alopecia in breast cancer patient. Cancer Research. 2016;76 [Google Scholar]
- 26.Kluger N, Jacot W, Frouin E, Rigau V, Poujol S, Dereure O, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2879–84. doi: 10.1093/annonc/mds095. [DOI] [PubMed] [Google Scholar]
- 27.Thorp NJ, Swift F, Arundell D, Wong H. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Research. 2015;75 [Google Scholar]
- 28.Kang D, Kim IR, Lee DY, Ahn JS, Park JH, Guallar E, et al. 80PIncidence of permanent chemotherapy-induced alopecia among breast cancer patients: A five-year prospective cohort study. Annals of Oncology. 2017;28:mdx655.022–mdx655.022. [Google Scholar]
- 29.Baker BW, Wilson CL, Davis AL, Spearing RL, Hart DN, Heaton DC, et al. Busulphan/cyclophosphamide conditioning for bone marrow transplantation may lead to failure of hair regrowth. Bone marrow transplantation. 1991;7:43–7. [PubMed] [Google Scholar]
- 30.Basilio FM, Brenner FM, Werner B, Rastelli GJ. Clinical and histological study of permanent alopecia after bone marrow transplantation. Anais brasileiros de dermatologia. 2015;90:814–21. doi: 10.1590/abd1806-4841.20154013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bresters D, Wanders DC, Louwerens M, Ball LM, Fiocco M, van Doorn R. Permanent diffuse alopecia after haematopoietic stem cell transplantation in childhood. Bone marrow transplantation. 2017 doi: 10.1038/bmt.2017.15. [DOI] [PubMed] [Google Scholar]
- 32.Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G. Busulfan concentration in relation to permanent alopecia in recipients of bone marrow transplants. Bone marrow transplantation. 1995;15:869–71. [PubMed] [Google Scholar]
- 33.Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone marrow transplantation. 2007;40:979–82. doi: 10.1038/sj.bmt.1705817. [DOI] [PubMed] [Google Scholar]
- 34.Socié G, Clift RA, Biaise D, Devergie A, Ringden O, Martin PJ, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: Long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–74. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- 35.Tran D, Sinclair RD, Schwarer AP, Chow CW. Permanent alopecia following chemotherapy and bone marrow transplantation. The Australasian journal of dermatology. 2000;41:106–8. doi: 10.1046/j.1440-0960.2000.00405.x. [DOI] [PubMed] [Google Scholar]
- 36.Bresters D, Wanders D, Louwerens M, Van Doorn R, Ball L. Permanent alopecia is a common late effect of hematopoietic stem cell transplantation, especially in younger children and after busulfan conditioning. Bone marrow transplantation. 2016;51:S219. [Google Scholar]
- 37.de Jonge ME, Mathot RA, Dalesio O, Huitema AD, Rodenhuis S, Beijnen JH. Relationship between irreversible alopecia and exposure to cyclophosphamide, thiotepa and carboplatin (CTC) in high-dose chemotherapy. Bone marrow transplantation. 2002;30:593–7. doi: 10.1038/sj.bmt.1703695. [DOI] [PubMed] [Google Scholar]
- 38.Cox MC, Kusters JM, Gidding CE, Schieving JH, van Lindert EJ, Kaanders JH, et al. Acute toxicity profile of craniospinal irradiation with intensity-modulated radiation therapy in children with medulloblastoma: A prospective analysis. Radiation oncology (London, England) 2015;10:241. doi: 10.1186/s13014-015-0547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suneja G, Poorvu PD, Hill-Kayser C, Lustig RA. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatric blood & cancer. 2013;60:1431–6. doi: 10.1002/pbc.24554. [DOI] [PubMed] [Google Scholar]
- 40.Rannan-Eliya YF, Rannan-Eliya S, Graham K, Pizer B, McDowell HP. Surgical interventions for the treatment of radiation-induced alopecia in pediatric practice. Pediatric blood & cancer. 2007;49:731–6. doi: 10.1002/pbc.20689. [DOI] [PubMed] [Google Scholar]
- 41.Lawenda BD, Gagne HM, Gierga DP, Niemierko A, Wong WM, Tarbell NJ, et al. Permanent alopecia after cranial irradiation: dose-response relationship. International journal of radiation oncology, biology, physics. 2004;60:879–87. doi: 10.1016/j.ijrobp.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Min CH, Paganetti H, Winey BA, Adams J, MacDonald SM, Tarbell NJ, et al. Evaluation of permanent alopecia in pediatric medulloblastoma patients treated with proton radiation. Radiation oncology (London, England) 2014;9:220. doi: 10.1186/s13014-014-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ounsakul V, Iamsumang W, Suchonwanit P. Radiation-Induced Alopecia after Endovascular Embolization under Fluoroscopy. Case reports in dermatological medicine. 2016;2016:8202469. doi: 10.1155/2016/8202469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siqueira M, de L, Trope BM, Cavalcante RB, Campos-do-Carmo G, Ramos ESM. Dermoscopy of multiple radiation-induced basal cell carcinomas in a patient treated previously for pinealoma. Journal of dermatological case reports. 2014;8:115–7. doi: 10.3315/jdcr.2014.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinmann D, Paelecke-Habermann Y, Geinitz H, Aschoff R, Bayerl A, Bolling T, et al. Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC cancer. 2012;12:283. doi: 10.1186/1471-2407-12-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haider M, Hamadah I, Almutawa A. Radiation- and chemotherapy-induced permanent alopecia: case series. Journal of cutaneous medicine and surgery. 2013;17:55–61. doi: 10.2310/7750.2012.12033. [DOI] [PubMed] [Google Scholar]
- 47.Sanada T, Nakayama H, Irisawa R, Okubo M, Tsuboi R, Tokuuye K. Clinical outcome and dose volume evaluation in patients who undergo brachytherapy for angiosarcoma of the scalp and face. Molecular and clinical oncology. 2017;6:334–40. doi: 10.3892/mco.2017.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolek TW, Marcus RB, Jr, Mendenhall NP, Scarborough MT, Graham-Pole J. Local control and functional results after twice-daily radiotherapy for Ewing’s sarcoma of the extremities. International journal of radiation oncology, biology, physics. 1996;35:687–92. doi: 10.1016/0360-3016(96)00145-9. [DOI] [PubMed] [Google Scholar]
- 49.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. Journal of the National Cancer Institute. 2003;95:142–53. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 50.Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2255–69. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sousa MS, Peate M, Jarvis S, Hickey M, Friedlander M. A clinical guide to the management of genitourinary symptoms in breast cancer survivors on endocrine therapy. Therapeutic advances in medical oncology. 2017;9:269–85. doi: 10.1177/1758834016687260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moscetti L, Agnese Fabbri M, Sperduti I, Fabrizio N, Frittelli P, Massari A, et al. Adjuvant aromatase inhibitor therapy in early breast cancer: what factors lead patients to discontinue treatment? Tumori. 2015;101:469–73. doi: 10.5301/tj.5000376. [DOI] [PubMed] [Google Scholar]
- 53.Gallicchio L, Calhoun C, Helzlsouer KJ. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast cancer research and treatment. 2013;142:435–43. doi: 10.1007/s10549-013-2744-2. [DOI] [PubMed] [Google Scholar]
- 54.Saggar V, Wu S, Dickler MN, Lacouture ME. Alopecia with endocrine therapies in patients with cancer. The oncologist. 2013;18:1126–34. doi: 10.1634/theoncologist.2013-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freites-Martinez Azael, S J, Donald Chan, Fornier Monica, Modi Shanu, Gajria Devika, Dusza Stephen, Goldfarb Shari, Lacouture Mario E. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA dermatology. 2018 doi: 10.1001/jamadermatol.2018.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossi A, Iorio A, Scali E, Fortuna MC, Mari E, Maxia C, et al. Aromatase inhibitors induce ’male pattern hair loss’ in women? Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:1710–1. doi: 10.1093/annonc/mdt170. [DOI] [PubMed] [Google Scholar]
- 57.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. International Journal of Trichology. 2009;1:123–30. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Niaimi F, Lyon C. Tamoxifen-induced hirsutism. Journal of drugs in dermatology : JDD. 2011;10:799–801. [PubMed] [Google Scholar]
- 59.Gosain AK, Zochowski CG, Cortes W. Refinements of tissue expansion for pediatric forehead reconstruction: a 13-year experience. Plastic and reconstructive surgery. 2009;124:1559–70. doi: 10.1097/PRS.0b013e3181babc49. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Lei B, Zheng M, Li Z, Huang S, Deng Y. Prognostic factors in patients treated with surgery for brain metastases: A single-center retrospective analysis of 125 patients. International journal of surgery (London, England) 2017 doi: 10.1016/j.ijsu.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 61.Yoo TK, Chae BJ, Kim SJ, Lee J, Yoon TI, Lee SJ, et al. Identifying long-term survivors among metastatic breast cancer patients undergoing primary tumor surgery. Breast cancer research and treatment. 2017 doi: 10.1007/s10549-017-4309-2. [DOI] [PubMed] [Google Scholar]
- 62.Antuna AR, Vega MA, Sanchez CR, Fernandez VM. Brain Metastases of Non-Small Cell Lung Cancer: Prognostic Factors in Patients with Surgical Resection. Journal of neurological surgery Part A, Central European neurosurgery. 2017 doi: 10.1055/s-0037-1601874. [DOI] [PubMed] [Google Scholar]
- 63.Albright AL, Sposto R, Holmes E, Zeltzer PM, Finlay JL, Wisoff JH, et al. Correlation of neurosurgical subspecialization with outcomes in children with malignant brain tumors. Neurosurgery. 2000;47:879–85. doi: 10.1097/00006123-200010000-00018. discussion 85-7. [DOI] [PubMed] [Google Scholar]
- 64.Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. The Lancet Oncology. 2016;17:484–95. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.English RS, Shenefelt PD. Keloids and hypertrophic scars. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 1999;25:631–8. doi: 10.1046/j.1524-4725.1999.98257.x. [DOI] [PubMed] [Google Scholar]
- 66.Endo T, Nakayama Y, Kikuchi M. Oral-cavity hair growth after free-flap transfer: case report. Journal of reconstructive microsurgery. 2001;17:37–8. doi: 10.1055/s-2001-12686. [DOI] [PubMed] [Google Scholar]
- 67.Liang J, Yu T, Wang X, Zhao Y, Fang F, Zeng W, et al. Free tissue flaps in head and neck reconstruction: clinical application and analysis of 93 patients of a single institution. Brazilian journal of otorhinolaryngology. 2017 doi: 10.1016/j.bjorl.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan BY, Hibler BP, Connolly KL, Rossi AM. Intraoral Laser Hair Removal of a Palate Free Flap: Tips and Technique. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2017 doi: 10.1097/DSS.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 69.Alkeraye S, Maire C, Desmedt E, Templier C, Mortier L. Persistent alopecia induced by vismodegib. The British journal of dermatology. 2015;172:1671–2. doi: 10.1111/bjd.13630. [DOI] [PubMed] [Google Scholar]
- 70.Zarbo A, Belum VR, Sibaud V, Oudard S, Postow MA, Hsieh JJ, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. The British journal of dermatology. 2017;176:1649–52. doi: 10.1111/bjd.15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ceovic R, Desnica L, Pulanic D, Serventi Seiwerth R, Ilic I, Grce M, et al. High frequency of cutaneous manifestations including vitiligo and alopecia areata in a prospective cohort of patients with chronic graft-vs-host disease. Croatian medical journal. 2016;57:229–38. doi: 10.3325/cmj.2016.57.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Severs GA, Griffin T, Werner-Wasik M. Cicatricial alopecia secondary to radiation therapy: case report and review of the literature. Cutis. 2008;81:147–53. [PubMed] [Google Scholar]
- 73.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. The Lancet Oncology. 2013;14:e50–9. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- 74.Purba TS, Haslam IS, Poblet E, Jimenez F, Gandarillas A, Izeta A, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. BioEssays : news and reviews in molecular, cellular and developmental biology. 2014;36:513–25. doi: 10.1002/bies.201300166. [DOI] [PubMed] [Google Scholar]
- 75.Aoki H, Hara A, Motohashi T, Kunisada T. Keratinocyte stem cells but not melanocyte stem cells are the primary target for radiation-induced hair graying. The Journal of investigative dermatology. 2013;133:2143–51. doi: 10.1038/jid.2013.155. [DOI] [PubMed] [Google Scholar]
- 76.Nanashima N, Ito K, Ishikawa T, Nakano M, Nakamura T. Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia. International journal of molecular medicine. 2012;30:579–84. doi: 10.3892/ijmm.2012.1018. [DOI] [PubMed] [Google Scholar]
- 77.Haslam IS, Pitre A, Schuetz JD, Paus R. Protection against chemotherapy-induced alopecia: targeting ATP-binding cassette transporters in the hair follicle? Trends in pharmacological sciences. 2013;34:599–604. doi: 10.1016/j.tips.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Malkinson FD, Keane JT. Radiobiology of the skin: review of some effects on epidermis and hair. The Journal of investigative dermatology. 1981;77:133–8. doi: 10.1111/1523-1747.ep12479347. [DOI] [PubMed] [Google Scholar]
- 79.Malkinson FD, Griem ML, Marianovic R. Persistent impairment of hair growth afterssingle large doses of x-rays. Radiation research. 1970;43:83–91. [PubMed] [Google Scholar]
- 80.Alonso LC, Rosenfield RL. Molecular genetic and endocrine mechanisms of hair growth. Hormone research. 2003;60:1–13. doi: 10.1159/000070821. [DOI] [PubMed] [Google Scholar]
- 81.Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocrine reviews. 2006;27:677–706. doi: 10.1210/er.2006-0020. [DOI] [PubMed] [Google Scholar]
- 82.Niiyama S, Happle R, Hoffmann R. Influence of estrogens on the androgen metabolism in different subunits of human hair follicles. European journal of dermatology : EJD. 2001;11:195–8. [PubMed] [Google Scholar]
- 83.Langan EA, Paus R. Female pattern hair loss: beyond an androgenic aetiology? The British journal of dermatology. 2010;163:1140–1. doi: 10.1111/j.1365-2133.2010.09955.x. [DOI] [PubMed] [Google Scholar]
- 84.Stenn KS, Sundberg JP, Sperling LC. Hair follicle biology, the sebaceous gland, and scarring alopecias. Archives of dermatology. 1999;135:973–4. doi: 10.1001/archderm.135.8.973. [DOI] [PubMed] [Google Scholar]
- 85.Harries MJ, Paus R. Scarring alopecia and the PPAR-gamma connection. The Journal of investigative dermatology. 2009;129:1066–70. doi: 10.1038/jid.2008.425. [DOI] [PubMed] [Google Scholar]
- 86.Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. The Journal of investigative dermatology. 2004;123:455–7. doi: 10.1111/j.0022-202X.2004.23237.x. [DOI] [PubMed] [Google Scholar]
- 87.van den Hurk CJ, Winstanley J, Young A, Boyle F. Measurement of chemotherapy-induced alopecia-time to change. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015;23:1197–9. doi: 10.1007/s00520-015-2647-3. [DOI] [PubMed] [Google Scholar]
- 88.Peerbooms M, van den Hurk CJ, Breed WP. Familiarity, opinions, experiences and knowledge about scalp cooling: a Dutch survey among breast cancer patients and oncological professionals. Asia-Pacific journal of oncology nursing. 2015;2:35–41. doi: 10.4103/2347-5625.152404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harrison S, Sinclair R. Optimal management of hair loss (alopecia) in children. Am J Clin Dermatol. 2003;4:757–70. doi: 10.2165/00128071-200304110-00004. [DOI] [PubMed] [Google Scholar]
- 90.Beisecker A, Cook MR, Ashworth J, Hayes J, Brecheisen M, Helmig L, et al. Side effects of adjuvant chemotherapy: perceptions of node-negative breast cancer patients. Psycho-oncology. 1997;6:85–93. doi: 10.1002/(SICI)1099-1611(199706)6:2<85::AID-PON247>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 91.Reid EE, Haley AC, Borovicka JH, Rademaker A, West DP, Colavincenzo M, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. Journal of the American Academy of Dermatology. 2012;66:e97–e102. doi: 10.1016/j.jaad.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 92.Smith K, Winstanley J, Boyle F, O’Reilly A, White M, Antill Y. Madarosis: Assessing perceptions and experience of patients with early breast cancer treated with taxane-based chemotherapy. 2017 doi: 10.1007/s00520-017-3852-z. [DOI] [PubMed] [Google Scholar]
- 93.Nangia J, Wang T, Osborne C, Niravath P, Otte K, Papish S, et al. Effect of a Scalp Cooling Device on Alopecia in Women Undergoing Chemotherapy for Breast Cancer: The SCALP Randomized Clinical Trial. Jama. 2017;317:596–605. doi: 10.1001/jama.2016.20939. [DOI] [PubMed] [Google Scholar]
- 94.Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast cancer research and treatment. 2017;163:199–205. doi: 10.1007/s10549-017-4185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heidelbaugh JJ. Endocrinology Update: Hirsutism. FP essentials. 2016;451:17–24. [PubMed] [Google Scholar]
- 96.Kaune KM, Haas E, Jantke M, Kramer FJ, Gruber R, Thoms KM, et al. Successful Nd:YAG laser therapy for hair removal in the oral cavity after plastic reconstruction using hairy donor sites. Dermatology (Basel, Switzerland) 2013;226:324–8. doi: 10.1159/000350685. [DOI] [PubMed] [Google Scholar]
- 97.Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326–41. doi: 10.1148/radiol.2542082312. [DOI] [PubMed] [Google Scholar]
- 98.Balana ME, Charreau HE, Leiros GJ. Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World journal of stem cells. 2015;7:711–27. doi: 10.4252/wjsc.v7.i4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chueh SC, Lin SJ, Chen CC, Lei M, Wang LM, Widelitz R, et al. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert opinion on biological therapy. 2013;13:377–91. doi: 10.1517/14712598.2013.739601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morris CL, Stinnett S, Woodward J. The role of bimatoprost eyelash gel in chemotherapy-induced madarosis: an analysis of efficacy and safety. Int J Trichology. 2011;3:84–91. doi: 10.4103/0974-7753.90809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen SC, Huang BS, Lin CY, Fan KH, Chang JT, Wu SC, et al. Psychosocial effects of a skin camouflage program in female survivors with head and neck cancer: A randomized controlled trial. Psycho-oncology. 2016 doi: 10.1002/pon.4308. [DOI] [PubMed] [Google Scholar]
- 102.Huang S, Liu HE. Effectiveness of cosmetic rehabilitation on the body image of oral cancer patients in Taiwan. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16:981–6. doi: 10.1007/s00520-008-0417-1. [DOI] [PubMed] [Google Scholar]
- 103.Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia: Lymphocytic primary cicatricial alopecias, including chronic cutaneous lupus erythematosus, lichen planopilaris, frontal fibrosing alopecia, and Graham-Little syndrome. Journal of the American Academy of Dermatology. 2016;75:1081–99. doi: 10.1016/j.jaad.2014.09.058. [DOI] [PubMed] [Google Scholar]


