Abstract
Background
Adverse birth outcomes, including preterm birth and stunting at birth, have long-term health implications. The relation between adverse birth outcomes and chronic, asymptomatic gastrointestinal inflammation (environmental enteric dysfunction—EED) is poorly understood.
Objective
We aimed to examine the relation between maternal EED and adverse birth outcomes in a sample of pregnant Ugandan women and their newborn infants.
Design
We conducted a prospective cohort study in Mukono, Uganda. A total of 258 pregnant women were enrolled at their first prenatal visit (∼18 weeks of gestation). EED was measured by urinary lactulose:mannitol (L:M) ratio and serum concentrations of antibodies to the bacterial components flagellin and LPS. Covariates were obtained from survey data collected at 2 time points. Associations were assessed through the use of unadjusted and adjusted simple linear regression models.
Results
Complete birth outcome data were recorded for 220 infants within 48 h of delivery. Mean ± SD gestational age was 39.7 ± 2.1 wk, and 7% were born preterm. Mean ± SD length and length-for-age z score (LAZ) at birth were 48.1 ± 3.2 cm and −0.44 ± 1.07, respectively. L:M ratio was not associated with any birth outcome. In adjusted models, higher concentrations of natural log-transformed anti-flagellin immunoglobin G (IgG) and anti-LPS IgG were significantly associated with shorter length of gestation (β: −0.89 wk; 95% CI: −1.77, −0.01 wk, and β: −1.01 wk; 95% CI: −1.87, −0.17 wk, respectively) and with reduced length (β: −0.80 cm; 95% CI: −1.55, −0.05 cm, and β: −0.79 cm; 95% CI: −1.54, −0.04 cm, respectively) and LAZ at birth (β −0.44 z score; 95% CI: −0.83, −0.05, and β: −0.40 z score; 95% CI: −0.79, −0.01, respectively).
Conclusion
Maternal anti-flagellin and anti-LPS IgG concentrations in pregnancy, but not L:M ratio, were associated with shorter gestation and reduced infant length at birth. Further research on the relation between maternal EED and birth outcomes is warranted.
Keywords: environmental enteric dysfunction, inflammation, intestinal permeability, intestinal biomarkers, dual sugar absorption test, L:M test, anti-flagellin antibodies, anti-LPS antibodies, birth outcomes, Uganda
INTRODUCTION
Stunting, defined as a length (LAZ)/height-for-age z score >2 SDs below the WHO Child Growth Standards median (1), remains a pervasive form of undernutrition globally, affecting ∼155 million children <5 y of age (2). Stunted children are not only short for their age but are at an increased risk of developing the “stunting syndrome” (3), characterized by numerous health and economic consequences including increased morbidity and mortality (4, 5), impaired cognitive development (6, 7), and lower economic productivity later in life (8). An estimated 20% of stunting has in utero origins driven largely by intrauterine growth restriction, premature births, or both (9).
Environmental enteric dysfunction (EED) is a subclinical inflammatory disorder of the small intestine characterized by altered gut morphology, reduced absorptive capacity, and impaired barrier function (10, 11). The condition is thought to develop from chronic exposure to enteropathogens as the result of living in a contaminated environment with poor water, sanitation, and hygiene conditions (12, 13). EED is an alarming global health concern, especially given its high prevalence and demonstrated association with poor growth outcomes in young children living in low- and middle-income countries (14–16).
EED can be diagnosed via small bowel biopsy but the invasive nature of this approach makes it ill-suited for population-based research studies. Numerous biomarkers for EED have been proposed which measure different domains of EED, including intestinal permeability, absorption, and inflammation (17). One common marker is the lactulose:mannitol (L:M) dual sugar absorption test. Although the L:M test is a widely used proxy for EED, it has several limitations. Most notably, in addition to being time-consuming and expensive to implement, the test lacks formal evaluation studies, and its correlation with EED symptoms and growth outcomes is debated (18).
Drawbacks of the L:M test have underpinned a search for new EED biomarkers. Anti-flagellin and anti-LPS Igs have been recently proposed, supported by their elevated presence in a range of diseases associated with and/or promoted by inflammation such as short bowel syndrome (SBS) (19), Crohn's disease (20, 21), and irritable bowel syndrome (IBS) (22). Because large bacterial-derived molecules like flagellin and LPS do not readily cross the epithelium, elevated concentrations of anti-flagellin and anti-LPS antibodies are thought to signify a state of increased intestinal permeability, microbial translocation, and systemic immune activation.
Although we are unaware of any study that has examined the role of maternal EED as a risk factor for adverse birth outcomes in low- and middle-income countries, women with idiopathic inflammatory bowel disease have pregnancies marked by higher rates of preterm birth, small-for-gestational-age (SGA) infants, and other complications (23–26). The primary objective of the study was to examine the association between maternal EED biomarkers, namely L:M ratio and serum concentrations of antibodies to the bacterial components flagellin and LPS, and adverse birth outcomes.
METHODS
Study design
We performed a prospective cohort study between February and November 2017 in Mukono District, Central Region, Uganda. Mukono is a semiurban district situated 20 km east of the capital city, Kampala. The study was based at Mukono Health Center IV (MHC IV), a public outpatient health facility located in the center of Mukono Town.
Pregnant women were recruited during their first prenatal visit at MHC IV, which occurred at ∼18 weeks of gestation. Eligible women were 18–45 y of age, residing within 10 km of Mukono Town, and carrying a singleton pregnancy. Women were excluded from the study if they were <18 or >45 y old, HIV positive (verified via routine rapid HIV test conducted at the first prenatal visit), severely malnourished [defined as BMI (kg/m2) <16.0], severely anemic (defined as hemoglobin <7 g/dL), or planning to move away from Mukono District before delivery.
The study was approved by the Tufts Health Sciences Institutional Review Board in Boston, MA; the Mengo Hospital Research Ethics Committee in Kampala, Uganda; and the Uganda National Council for Science and Technology in Kampala, Uganda. Before enrollment, written consent in either Luganda or English was obtained from each participant.
Participation
Participation in the study involved 4 visits over a 4–6-mo period: enrollment visit, immediately after the first prenatal visit (MHC IV); L:M test visit, within 1 wk of the first visit (participant's residence); follow-up visit, 3 wk before the expected date of delivery (also at the participant's residence); and postdelivery visit, within 48 h of delivery (either at the participant's residence, MHC IV, or another health facility).
Enrollment visit
An ultrasound scan was performed by a trained professional at MHC IV to both confirm a singleton pregnancy and determine participants’ estimated date of delivery. Hemoglobin was measured with a portable hemoglobinometer (HemoCue Hb 301; HemoCue, Inc., Brea, CA). A venous blood draw was performed by the phlebotomist at MHC IV (BD Vacutainer, Becton Dickinson, Durham, NC). Systolic and diastolic blood pressure (DBP) measurements were taken with a digital upper arm blood pressure monitor (Omron 10 Series, Omron Healthcare, Kyoto, Japan).
All anthropometry measurements were performed in triplicate and the mean was used for analysis. Weight was measured to the nearest 0.1 kg with the use of a digital weight scale (Seca 874, Hanover, MD). Height was measured to the nearest 0.1 cm with the use of a portable, rigid height board (Infant/Child/Adult ShorrBoard, Shorr Production, Olney, MD). These measurements were used to calculate BMI. Midupper arm circumference (MUAC) was measured to the nearest 0.1 cm with the use of a standard tricolored, nonstretch adult MUAC tape.
Finally, a questionnaire was administered by the study nurse that included questions related to demographics, prior pregnancies, health status, diet, food security [using the Household Food Insecurity Access Scale (HFIAS)] (27), and water, sanitation, and hygiene practices (see Supplemental File 1 for the complete questionnaire).
L:M test visit
Within 1 wk of the enrollment visit, a household visit was conducted to perform a L:M dual sugar absorption test. Participants with diarrhea (≥3 loose stools/d) in the last 2 wk had their test rescheduled for a different day.
After urination to void the bladder and an observed 1-h fast, participants consumed a 50-mL solution containing 5 g of lactulose (Lactulose Solution; Mckesson, San Francisco, CA) and 2 g of mannitol (D-mannitol powder; Sigma-Aldrich, St. Louis, MO) completely dissolved in sterile water. Urine was collected for a period of 4 h in a 2-L plastic collection bottle containing 0.05 mL of 50% thimerosal (Sigma-Aldrich, St. Louis, MO) as a preservative. Water intake was permitted ad libitum 1 h after ingestion of the solution, and women were encouraged to drink a minimum of 500 mL of water during the test to ensure sufficient urine output. A final urine sample was collected at the 4-h time-point and total urine volume was measured to the nearest 1.0 mL with the use of a graduated cylinder in the field. Samples were frozen at −20°C at the MHC IV laboratory before being transferred to a −80°C freezer in Kampala.
Follow-up visit
A second household visit was conducted 3 wk before participants’ estimated delivery date, which consisted of a follow-up survey with questions related to pregnancy risk factors. In addition, weight and MUAC measurements were taken following identical procedures to those used at the enrollment visit. Finally, participants were asked to provide a sample of water from their drinking water storage container for the purposes of a water quality test.
Post-delivery visit
Infant characteristics (live birth, date and time of delivery, sex, weight, length, and head circumference) were collected within 48 h of delivery. Birth weight was measured to the nearest 0.1 kg with the use of a digital weigh scale (Seca 874, Hanover, MD), and birth length was measured to the nearest 0.1 cm with the use of a portable, rigid height board (ShorrBoard, Shorr Production, Olney, MD). Head circumference was measured to the nearest 0.1 cm with a flexible measuring tape. All anthropometry measurements were taken in triplicate and averaged. In the case of a stillbirth or neonatal death, only birth date, time, and infant sex were recorded.
Laboratory analyses
Urine samples were analyzed for concentrations of lactulose and mannitol with the use of previously described HPLC methods at the Shulman Laboratory at Baylor College of Medicine (28). The L:M ratio was calculated by dividing the urinary lactulose concentration by the urinary mannitol concentration. Lactulose (%LE) and mannitol excretion ratios were calculated from the measured amount of each in urine (concentration × total urine volume) relative to the initial dose of each sugar.
Blood samples were centrifuged at 1900 × g for 5 min at room temperature, and serum was divided into aliquots in 2.0-mL clear plastic cryovials. Anti-flagellin and anti-LPS Ig concentrations (IgA and IgG) were measured at the Gewirtz Laboratory at Georgia State University via previously described ELISA methods (19). Concentrations of serum biomarkers are reported as optical density units throughout.
Water quality was assessed in the field through the use of a compartment bag test (Aquagenx, Chapel Hill, NC). One hundred mL of drinking water from each household was mixed with an Escherichia coli chromogenic growth medium and incubated for a period of 48 h inside a sealed plastic bag containing 5 compartments of varying volumes. Risk categories were determined by noting which, if any, compartments changed from yellow to green/blue during the incubation period and matching that to a most probable number table based on the WHO guidelines (29). Because there is no safe level of E. coli contamination, a dichotomous (safe/unsafe) water variable was created, corresponding to no E. coli detected and any E. coli detected, respectively.
Statistical analyses
Based on studies of maternal idiopathic inflammatory bowel diseases and adverse birth outcomes, the RR of maternal EED and subsequent preterm birth was assumed to be 2.0 (30, 31). Assuming 80% power, 0.05 significance, a 5% frequency of preterm birth, and 15% loss to follow-up, a sample size of 258 allowed for the detection of an RR of 2.0 within 50% of the true risk parameters.
All analyses were carried out with the use of STATA 15 software (Stata Corps, College Station, TX). Weight and length measurements were converted to z scores for weight-for-age (WAZ), LAZ, and weight-for-length (WLZ) with the use of the WHO standards (1). Outliers were defined as WAZ <−6 or >+5, WLZ <−5 or >+5, and LAZ <−6 or >+6. On this basis, 7 observations were excluded from WLZ analyses; none of the other measures had any outliers.
Stillbirth was defined as fetal death after 20 weeks of gestation, and preterm birth was defined as a live birth before 37 weeks of gestation. Low birth weight (LBW) was defined as weighing <2500 g at birth, and SGA was defined as birth weight for gestational age <10th percentile via sex-specific INTERGROWTH-21st standards (32). Stunted and wasted at birth were defined as LAZ <−2 and WHZ <−2, respectively.
Before analysis, distributions of biomarker values were assessed for outliers and normality. Because of their skewed distribution, L:M ratios, %LE, and serum biomarkers were natural log transformed (ln) in all regression models. Associations between EED biomarkers (continuous, independent variables) and birth outcomes [i.e., gestational age, length, and LAZ (continuous, primary outcomes) and weight, WAZ, WLZ, and head circumference (continuous, secondary outcomes)] were assessed via simple unadjusted and adjusted linear regression models. For adjusted models, covariates were selected via bivariate analyses, with gestational age at birth and birth length as dependent variables, and a P value <0.25. Before inclusion in the model, correlation among covariates was assessed through the use of Pearson correlation coefficients, and the absence of multicollinearity was verified with the use of the variance inflation factor.
t Tests were used to assess differences in maternal biomarker means for infants born stillborn, preterm, LBW, SGA, stunted, and wasted compared with not. Pearson correlations were calculated to evaluate agreement between ln L:M ratio and ln serum biomarkers. In all cases, statistical significance was determined by a P value <0.05.
RESULTS
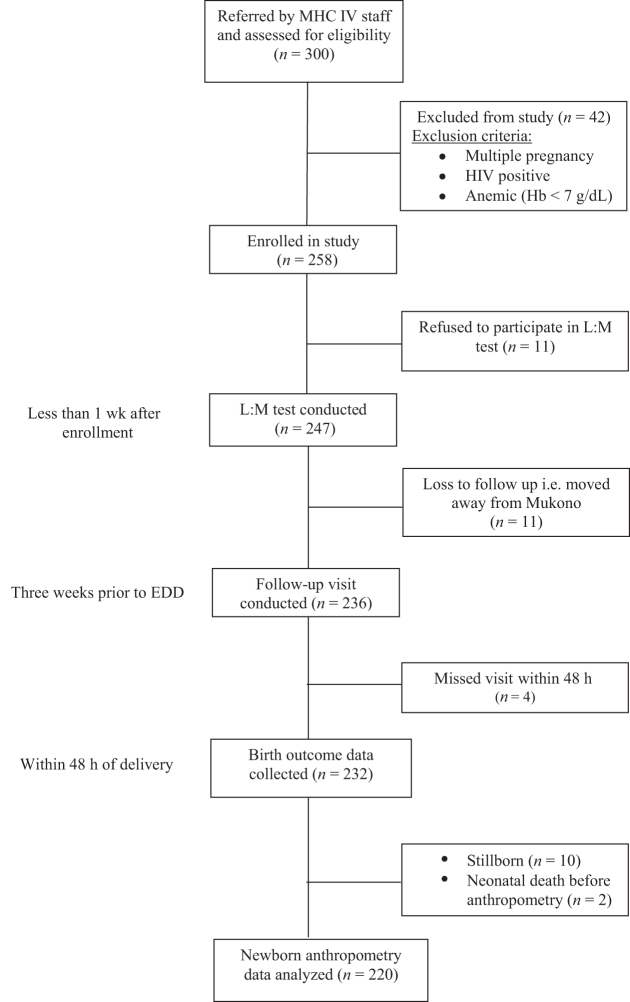
A flow diagram for the prospective cohort study is presented in Figure 1. Of the 300 pregnant women screened, 258 met the inclusion criteria and were enrolled in the study. Of these 258 participants, 247 had an L:M test conducted within 1 wk of enrollment, and 11 refused the test and dropped out of the study. Of these, 236 participants had a follow-up visit conducted before delivery, and 11 were considered lost to follow-up because they moved away from Mukono District. Birth outcome data were collected within 48 h for 232 infants. Ten infants were stillborn, and 2 infants died before anthropometry measurements could be taken. Descriptive characteristics, maternal EED biomarkers, and birth outcome data were therefore analyzed for 220 participants and their infants.
FIGURE 1.
Flow diagram for prospective birth cohort study of pregnant women in Mukono, Uganda. EED, environmental enteric dysfunction; Hb, hemoglobin; L:M, lactulose:mannitol; MHC IV, Mukono Health Center IV.
Descriptive characteristics for 220 participants (Table 1) and their infants (Table 2) are shown. Descriptive characteristics are presented as mean ± SD or n (%) for continuous and categorical outcomes, respectively. At enrollment, participants were ∼24 y old and ∼18 weeks of gestation, and ∼40% were pregnant for the first time. In all cases, characteristics were not significantly different between participants who dropped out of the study or were lost to follow-up and those that remained in the study.
TABLE 1.
Descriptive characteristics for 220 pregnant women and their households in Mukono, Uganda1
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Enrollment characteristics | |
| Age, y | 23.9 ± 4.3 |
| Gestation at enrollment, wk | 17.8 ± 3.5 |
| Weight, kg | 60.7 ± 9.8 |
| Height, cm | 158.5 ± 6.1 |
| BMI, kg/m2 | 24.1 ± 3.5 |
| MUAC, cm | 27.1 ± 3.4 |
| Hb, g/dL | 11.9 ± 1.4 |
| Anemic (Hb < 12 g/dL) | 104 (47.3) |
| Systolic blood pressure, mm Hg | 109.8 ± 11.3 |
| Diastolic blood pressure, mm Hg | 72.7 ± 8.3 |
| First pregnancy, yes | 87 (39.6) |
| Household head, yes | 9 (4.1) |
| Household members | 3.5 ± 2.1 |
| Marital status | |
| Married/cohabitating, monogamous | 191 (86.8) |
| Married/cohabitating, polygamous | 18 (8.2) |
| Single | 11 (5.0) |
| Employed, yes | 100 (45.5) |
| Education, y | 9.9 ± 2.9 |
| HFIAS | |
| Food secure | 56 (25.5) |
| Mildly food insecure | 62 (28.2) |
| Moderately food insecure | 86 (39.1) |
| Severely food insecure | 16 (7.3) |
| Pregnancy characteristics | |
| Antenatal visits | 3.5 (0.8) |
| Weight change, g/wk | 292.7 ± 187.2 |
| Safe water, yes | 94 (42.7) |
| Water source | |
| Piped water | 12 (5.5) |
| Protected well/spring | 40 (18.2) |
| Tube well/borehole | 156 (70.9) |
| Rainwater | 4 (1.8) |
| Unprotected well/spring | 6 (2.7) |
| Surface water | 2 (0.9) |
| Toilet type | |
| Flush toilet | 8 (3.6) |
| Improved pit latrine | 115 (52.3) |
| Unimproved pit latrine | 97 (44.1) |
| Iron supplementation, d | 47.9 (26.5) |
| IPT, courses | 2.3 ± 1.1 |
| Deworming medication, yes | 139 (63.2) |
| Second-hand tobacco exposure, yes | 20 (9.1) |
| EED biomarkers | |
| L:M test | |
| Urine volume, mL | 250.60 ± 234.13 |
| L:M ratio | 0.09 ± 0.10 |
| Urinary lactulose, % dose excreted | 0.52 ± 0.77 |
| Urinary mannitol, % dose excreted | 18.23 ± 16.40 |
| Serum markers (OD) | |
| Anti-flagellin IgA | 1.61 ± 0.68 |
| Anti-LPS IgA | 1.48 ± 0.55 |
| Anti-flagellin IgG | 1.15 ± 0.30 |
| Anti-LPS IgG | 1.81 ± 0.60 |
EED, environmental enteric dysfunction; Hb, hemoglobin; HFIAS, Household Food Insecurity Access Scale; IPT, intermittent preventive treatment (i.e., sulfadoxine pyrimethamine) for malaria; L:M, lactulose:mannitol; MUAC, midupper arm circumference; OD, optical density.
TABLE 2.
Birth outcome characteristics for 220 newborn infants in Mukono, Uganda1
| Birth characteristic | Mean ± SD or n (%) |
|---|---|
| Female | 115 (52.3) |
| Gestational age, wk | 39.7 ± 2.1 |
| Preterm, <37 wk | 15 (6.8) |
| Birth weight, kg | 3.3 ± 0.5 |
| Birth length, cm | 48.1 ± 3.2 |
| Head circumference, cm | 35.2 ± 1.5 |
| LBW, <2500 g | 8 (3.6) |
| SGA, <10th percentile | 24 (11.3) |
| Weight-for-length z score | 0.47 ± 1.54 |
| Weight-for-age z score | −0.10 ± 1.01 |
| Length-for-age z score | −0.44 ± 1.07 |
| Wasted, <−2 SD | 13 (6.5) |
| Stunted, <−2 SD | 15 (7.2) |
LBW, low birth weight; SGA, small for gestational age.
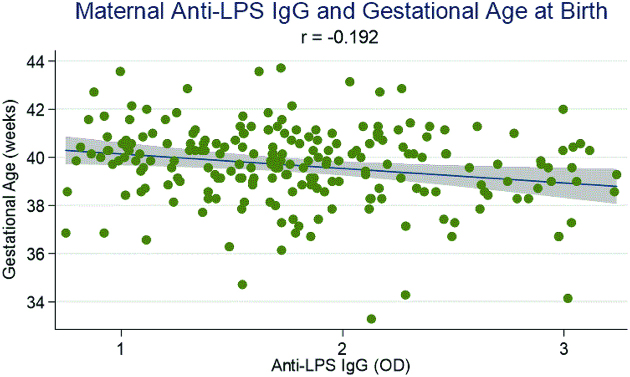
As shown in Figure 2, concentrations of maternal anti-LPS IgG were significantly negatively associated with infant gestational age at birth (ρ = −0.192, P < 0.05). Table 3 shows the association between maternal EED biomarkers and infant gestational age, length, and LAZ at birth in adjusted and unadjusted linear regression models. In adjusted analyses, controlling for maternal age, height, DBP, years of education, first pregnancy (yes/no), HFIAS, safe water (yes/no), and infant birth weight, higher concentrations of ln anti-flagellin IgG and ln anti-LPS IgG were significantly associated with shorter gestation (β: −0.89 wk, 95% CI: −1.77, −0.01 wk; and β: −1.01 wk, 95% CI: −1.87, −0.17 wk, respectively).
FIGURE 2.
Maternal anti-LPS IgG concentration and its correlation with infant gestational age at birth (weeks) for 220 mother-infant pairs. Graph shows the best-fit trend line with 95% CI (gray area). Correlation significant at P < 0.05. OD, optical density.
TABLE 3.
Biomarkers of maternal EED as predictors of infant gestational age (weeks), length (centimeters), and LAZ at birth (n = 220) in unadjusted and adjusted linear regression models1
| Gestational age, wk | Length, cm | LAZ | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| L:M | 0.04 (−0.22, 0.30) P = 0.761 | 0.02 (−0.24, 0.29) P = 0.858 | 0.04 (−0.21, 0.30) P = 0.746 | 0.01 (−0.22, 0.24) P = 0.901 | 0.03 (−0.11, 0.16) P = 0.712 | 0.01 (−0.11, 0.13) P = 0.842 |
| %LE | 0.02 (−0.25, 0.29) P = 0.897 | 0.006 (−0.27, 0.28) P = 0.968 | −0.03 (−0.29, 0.24) P = 0.850 | −0.03 (−0.27, 0.20) P = 0.776 | −0.008 (−0.15, 0.13) P = 0.915 | −0.009 (−0.13, 0.11) P = 0.881 |
| Anti-flagellin IgA | −0.26 (−0.96, 0.44) P = 0.463 | −0.37 (−1.10, 0.36) P = 0.322 | 0.11 (−0.57, 0.79) P = 0.743 | −0.15 (−0.79, 0.49) P = 0.643 | 0.05 (−0.31, 0.41) P = 0.785 | −0.11 (−0.44, 0.23) P = 0.533 |
| Anti-LPS IgA | −0.24 (−1.06, 0.58) P = 0.566 | −0.25 (−1.10, 0.60) P = 0.564 | −0.36 (−1.15, 0.43) P = 0.372 | −0.48 (−1.22, 0.25) P = 0.195 | −0.21 (−0.63, 0.21) P = 0.323 | −0.28 (−0.67, 0.10) P = 0.152 |
| Anti-flagellin IgG | −0.79 (−1.66, 0.08) P = 0.075 | −0.89 (−1.77, −0.01) P = 0.047* | −0.68 (−1.52, 0.16) P = 0.110 | −0.80 (−1.55, −0.05) P = 0.036* | −0.38 (−0.83, 0.06) P = 0.089 | −0.44 (−0.83, −0.05) P = 0.029* |
| Anti-LPS IgG | −0.98 (−1.82, −0.15) P = 0.021* | −1.01 (−1.87, −0.17) P = 0.019* | −0.50 (−1.32, 0.32) P = 0.234 | −0.79 (−1.54, −0.04) P = 0.039* | −0.29 (−0.72, 0.15) P = 0.197 | −0.40 (−0.79, −0.01) P = 0.043* |
Values are β-coefficients (95% CIs) and P values; all EED biomarkers were natural log transformed before analysis. Adjusted model controls for maternal age, height, diastolic blood pressure, years of education, first pregnancy (yes/no), *P < 0.05. Household Food Insecurity Access Scale score, safe water (yes/no), and infant birth weight. EED; environmental enteric dysfunction; LAZ, length-for-age z score; L:M, lactulose:mannitol; %LE, percentage lactulose excretion.
Furthermore, in adjusted analyses with the same controls, higher concentrations of ln anti-flagellin IgG and ln anti-LPS IgG were significantly associated with reduced infant length (β: −0.80 cm; 95% CI: −1.55, −0.05 cm, and β: −0.79 cm; 95% CI: −1.54, −0.04 cm, respectively) and LAZ at birth (β −0.44 z score; 95% CI: −0.83, −0.05, and β: −0.40 z score; 95% CI: −0.79, −0.01, respectively). In contrast, no association was observed between ln L:M ratio or ln %LE and infant gestational age, length, or LAZ at birth.
In analyses of secondary birth outcomes (Supplemental Table 1), we found no significant association between maternal EED biomarkers and infant weight, WAZ, or head circumference at birth in either unadjusted or adjusted linear regression models. However, in unadjusted analyses, higher concentrations of ln anti-flagellin IgG and ln anti-LPS IgG were significantly associated with higher infant WLZ at birth (β: 0.85 z score; 95% CI: 0.21, 1.49, and β: 0.65 z score; 95% CI: 0.10, 1.29, respectively). For ln anti-flagellin IgG, the relation remained significant in the adjusted model controlling for maternal age, height, DBP, years of education, first pregnancy (yes/no), HFIAS, and safe water (yes/no) (0.79 z score; 95% CI: 0.14, 1.44).
Biomarker means for dichotomous birth outcomes, including stillbirth, preterm birth, LBW, SGA, stunting, and wasting, are presented in Supplemental Table 2. We found significantly higher L:M ratio (0.16 ± 0.26 compared with 0.08 ± 0.12, P < 0.05) and anti-flagellin IgA concentrations (1.93 ± 0.75 compared with 1.58 ± 0.67, P < 0.05) for mothers who delivered preterm (n = 15) compared with term infants. In addition, we found significantly higher maternal %LE (0.92 ± 1.16 compared with 0.49 ± 0.71, P < 0.05) and lower anti-flagellin IgG (0.99 ± 0.35 compared with 1.16 ± 0.29, P < 0.05) for infants born wasted (n = 13) compared with those not.
Pearson correlations between maternal EED biomarkers are presented in Supplemental Table 3. Overall, we observed modest negative correlations between ln L:M ratio and ln serum biomarkers, with significant associations observed between ln L:M ratio and ln anti-LPS IgA and ln anti-LPS IgG (r = −0.15 and −0.19, respectively, P < 0.05 for both). As expected, because both measure exposure to bacterial components, pairs of ln anti-flagellin and ln anti-LPS Ig concentrations had significant positive associations (P < 0.001 in all cases).
DISCUSSION
In this prospective cohort study of pregnant women in Mukono, Uganda, we tested the hypothesis that maternal EED biomarkers, specifically the L:M ratio and serum concentrations of anti-flagellin and anti-LPS Igs measured at ∼18 weeks of gestation, are associated with adverse birth outcomes, primarily shorter gestation and reduced infant length at birth. To our knowledge, this is one of the first studies to measure EED biomarkers in a sample of pregnant women as well as the first to examine the relation between EED biomarkers and adverse birth outcomes.
We found that higher maternal ln anti-flagellin and ln anti-LPS IgG, but not ln IgA, concentrations were significantly associated with shorter gestation and reduced infant length and LAZ at birth in adjusted linear regression models. Because these markers were not associated with birth weight, maternal ln anti-flagellin and ln anti-LPS IgG were also significantly associated with higher infant WLZ at birth. In other words, elevations in maternal ln anti-flagellin and ln anti-LPS IgG were associated with being infants born both shorter and heavier. We did not find this or any relation between maternal ln L:M ratio or ln %LE and any primary or secondary birth outcome of interest.
In addition, we found modest negative correlations between serum biomarker concentrations and the L:M ratio, which is consistent with results from several recent studies. In 539 young Bangladeshi children aged 18 mo, Campbell et al. (33) found low to modest agreement among a panel of EED biomarkers. In addition, in 375 Brazilian 6- to 26-mo-old children, Guerrant et al. (34) found similarly weak correlations among 18 proposed EED biomarkers, including between anti-flagellin and anti-LPS Igs and the L:M ratio. One reasonable explanation for this disparity is that the L:M test, particularly %LE, reflects intestinal permeability whereas anti-flagellin and anti-LPS Ig concentrations capture the immune/inflammatory response to increased bacterial translocation (35). However, it is worth noting that our results differ from findings by Campbell et al. (36) which reported a significant association between plasma concentrations of both endotoxin and immunoglobulin (Ig)G-endotoxin core antibody (EndoCAb) and raised lactulose recovery (r = 0.36, P < 0.02 and r = 0.35, P < 0.005, respectively) as well as between plasma concentrations of both endotoxin and EndoCAb and poor growth (r = −0.30, P < 0.02 and r = −0.64, P < 0.0001, respectively) in Gambian infants.
To date, elevations in anti-flagellin and anti-LPS Ig concentrations have been observed in cases of other chronic enteric conditions such as SBS and IBS. Ziegler et al. (19) compared serum from parenteral nutrition–dependent patients with SBS (n = 23) with non-SBS control subjects (n = 48 healthy adults and n = 37 adults requiring parenteral nutrition during critical illness) and found flagellin, LPS, or both in 61% of SBS patients compared with 0% in control subjects. Patients with SBS had significantly elevated anti-flagellin Igs, including IgA, IgG, and IgM, compared with control subjects (P < 0.001). Similarly, Dlugosz et al. (22) compared serum from patients with 3 different subtypes of IBS (total n = 88) with healthy control subjects (n = 106) and found significantly elevated concentrations of LPS in patients with diarrhea-predominant IBS compared with control subjects (P = 0.0155). They found significantly elevated concentrations of antibodies to flagellin in all patients with IBS compared with control subjects (P = 0.0018).
Elevations in anti-flagellin and anti-LPS Ig concentrations have also been linked to poor growth outcomes in a limited number of studies. Most notably, in 590 Tanzanian children, McDonald et al. (37) found that infants at 6 wk of age that fell in the highest quartile of anti-flagellin IgA, anti-LPS IgA, anti-flagellin IgG, and anti-LPS IgG concentrations were 2.02 (95% CI: 1.11, 3.67), 1.84 (95% CI: 1.03, 3.27), 1.94 (95% CI: 1.04, 3.62), and 2.31 (95% CI: 1.25, 4.27) times, respectively, more likely to become underweight over 18 mo of follow-up than were children with Ig concentrations in the lowest quartile (P-trend <0.05).
Although the pathway by which maternal EED contributes to adverse birth outcomes is not well established, we hypothesize that gut barrier dysfunction results in systemic exposure to flagellin and LPS, which ultimately stimulates an adaptive immune/inflammatory response. Although the resulting immune/inflammatory response likely contributes an important line of defense against bacterial infection, such a response may also contribute to the pathogenesis of EED and ultimately result in poor child growth and in adverse birth outcomes in the case of maternal EED and pregnancy. This hypothesis is supported by several studies that have previously demonstrated an association between maternal biomarkers of general inflammation and adverse birth outcomes, particularly proinflammatory cytokines (38, 39) and C-reactive protein (40, 41). In a prospective cohort study of HIV-positive (n = 44) and HIV-negative (n = 70) Tanzanian mothers and their infants, Wilkinson et al. (42) found that systemic inflammation, measured by a 9-plex panel of maternal plasma cytokines, was associated with poorer birth anthropometry. In the study, higher maternal plasma TNF-α concentrations were associated with earlier delivery (−1.7 wk, P = 0.039) and lower birthweights (−287 g, P = 0.020), and higher umbilical cord TNF-α (−1.43 cm, P = 0.036) and IL-12p70 (−2.4 cm, P = 0.008) were associated with reduced infant birth length (42). Furthermore, in a longitudinal study of Filipino mothers and their infants (n = 327), Kuzawa et al. (43) found that systemic inflammation, measured by maternal C-reactive protein during pregnancy, was associated with reduced infant body weight (−0.047 ± 0.017 kg · log-mg−1 · L−1), length (−0.259 ± 0.092 cm · log-mg−1 · L−1), and sum of skinfolds (−0.520 ± 0.190 mm · log-mg−1 · L−1) (all P < 0.05).
Overall, our study has several strengths. Date of delivery was estimated more exactly through the use of an ultrasound scan rather than calculated from an estimate of the first day of the last menstrual period. Furthermore, loss to follow-up in the study was relatively low, and birth outcome measurements, including birth length, were collected within 48 h of delivery. There are, however, several limitations. This study was designed as a pilot or proof-of-concept study and our sample size of 258 enrolled women was relatively small. Therefore, we were underpowered to determine associations between maternal EED biomarkers and less common adverse birth outcomes, such as spontaneous abortion, stillbirth, SGA, and LBW. Furthermore, we obtained measures of only 2 proposed EED biomarkers at only 1 point in time, ∼18 weeks of gestation.
In conclusion, maternal anti-flagellin and anti-LPS Ig concentrations measured at ∼18 weeks of gestation were significantly associated with shorter gestation and reduced infant length and LAZ at birth in a sample of pregnant Ugandan women and their newborn infants. These results provide preliminary evidence that maternal EED may be related to adverse birth outcomes. Going forward, we recommend larger, more robust studies which examine a more extensive panel of biomarkers at different gestational time points to better determine the possible effects of maternal EED on adverse birth outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—JML: designed the research study, conducted the research, analyzed the data, and wrote the manuscript; CPD, LMA, JKG, PW, SG, NN, and EA: provided input into the study design; CPD, LMA, JKG, PW, and SG: provided input into data analysis; HQT and ATG: analyzed samples; CPD, LMA, JKG, PW, SG, NN, EA, HQT, and ATG: contributed to the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by the Feed the Future Innovation Lab for Nutrition at Tufts University in Boston, MA, supported by the United States Agency for International Development, award AID-OAA-L-10-00006. CD was supported in part by NIH grants K24DK104676 and 2P30 DK040561.
The funding sources had no role in the publication process including the analysis of data or the writing of the manuscript.
Supplemental Tables 1–3 and Supplemental File 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- DBP
diastolic blood pressure
- EED
environmental enteric dysfunction
- HFIAS
Household Food Insecurity Access Scale
- IBS
irritable bowel syndrome
- LAZ
length-for-age z score
- LBW
low birth weight
- L:M
lactulose:mannitol
- MHC IV
Mukono Health Center IV
- MUAC
midupper arm circumference
- SBS
short bowel syndrome
- SGA
small for gestational age
- WAZ
weight-for-age z score
- WLZ
weight-for-length z score
- %LE
percentage of lactulose excretion
REFERENCES
- 1. WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 2. UNICEF, WHO, The World Bank. Levels and trends in child malnutrition: key findings of the 2017 edition. Global Database on Child Growth and Malnutrition; 2017, URL:www.who.int/nutgrowthdb/jme_brochoure2017.pdf, Accessed:January 4, 2018. [Google Scholar]
- 3. Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34:250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–8. [DOI] [PubMed] [Google Scholar]
- 5. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 6. Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–71. [DOI] [PubMed] [Google Scholar]
- 7. Walker SP, Chang SM, Powell CA, Simonoff E, Grantham-McGregor SM. Early childhood stunting is associated with poor psychological functioning in late adolescence and effects are reduced by psychosocial stimulation. J Nutr. 2007;137(11):2464–9. [DOI] [PubMed] [Google Scholar]
- 8. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christian P, Lee SE, Donahue Angel M, Adair LS, Arifeen SE, Ashorn P, Barros FC, Fall CH, Fawzi WW, Hao W et al.. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42:1340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petri WA, Naylor C, Haque R. Environmental enteropathy and malnutrition: do we know enough to intervene?. BMC Med. 2014;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crane RJ, Jones K, Berkeley J. Environmental enteric dysfunction: an overview. Food Nutr Bull. 2015;36:S76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374(9694):1032–5. [DOI] [PubMed] [Google Scholar]
- 13. Lin A, Arnold BF, Afreen S, Goto R, Huda TM, Haque R, Raqib R, Unicomb L, Ahmed T, Colford JM Jr. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am J Trop Med Hyg. 2013;89(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133(5):1332–8. [DOI] [PubMed] [Google Scholar]
- 15. Lunn PG. The impact of infection and nutrition on gut function and growth in childhood. Proc Nutr Soc. 2000;59:147–54. [DOI] [PubMed] [Google Scholar]
- 16. Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP et al.. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syed S, Ali A, Duggan C. Environmental enteric dysfunction in children: a review. J Pediatr Gastroenterol Nutr. 2016;63(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis. 2014;59(Suppl 4):S213–19. [DOI] [PubMed] [Google Scholar]
- 19. Ziegler TR, Luo M, Estívariz CF, Moore DA 3rd, Sitaraman SV, Hao L, Bazargan N, Klapproth JM, Tian J, Galloway JR et al.. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294:R402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020–8. [DOI] [PubMed] [Google Scholar]
- 21. Sitaraman SV, Klapproth JM, Moore DA 3rd, Landers C, Targan S, Williams IR, Gewirtz AT. Elevated flagellin-specific immunoglobulins in Crohn's disease. J Physiol Gastrointest Liver Physiol. 2005;288(2):G403–6. [DOI] [PubMed] [Google Scholar]
- 22. Dlugosz A, Nowak P, D'Amato M, Mohammadian Kermani G, Nystrom J, Abdurahman S, Lindberg G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1747–54. [DOI] [PubMed] [Google Scholar]
- 23. Kornfeld D, Cnattignuis S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease. A population-based cohort study. Am J Obstet Gynaecol. 1997;177:942–6. [DOI] [PubMed] [Google Scholar]
- 24. Fonager K, Sorensen HT, Olsen J, Dahlerup JF, Rasmussen SN. Pregnancy outcomes for women with Crohn's disease—a follow-up study based on linkage between national registers. Am J Gastroenterol. 1988;93:3426–30. [DOI] [PubMed] [Google Scholar]
- 25. Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002;97:641–8. [DOI] [PubMed] [Google Scholar]
- 26. Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis. 2014;20(6):1091–8. [DOI] [PubMed] [Google Scholar]
- 27. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide. Version 3. Washington (DC): Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 28. Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res. 1998;44:519–23. [DOI] [PubMed] [Google Scholar]
- 29. WHO. Guidelines for drinking-water quality. 4th ed Geneva: World Health Organization; 2011. [Google Scholar]
- 30. Stephansson O, Larsson H, Pedersen L, Kieler H, Granath F, Ludvigsson JF, Falconer H, Ekbom A, Sørensen HT, Nørgaard M. Crohn's disease is a risk factor for preterm birth. Clin Gastroenterol Hepatol. 2010;8:509–15. [DOI] [PubMed] [Google Scholar]
- 31. Boyd HA, Basit S, Harpsøe MC, Wohlfahrt J, Jess T. Inflammatory bowel disease and risk of adverse pregnancy outcomes. PLoS One. 2015;10(6):e0129567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA et al.. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet North Am Ed. 2014;384(9946):857–68. [DOI] [PubMed] [Google Scholar]
- 33. Campbell RK, Schulze KJ, Shaikh S, Mehra S, Ali H, Wu L, Raqib R, Baker S, Labrique A, West KP Jr, et al.. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. J Pediatr Gastroenterol Nutr. 2017;65:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guerrant RL, Leite AM, Pinkerton R, Medeiros PHQS, Cavalcante P, DeBoer M, Kosek M, Duggan C, Gewirtz A, Kagan JC et al.. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One. 2016;11(9):e0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prendergast A, Humphrey JH, Mutasa K, Majo FD, Rukobo S, Govha M, Mbuya MNN, Moulton LH, Stoltzfus RJ. Assessment of environmental enteric dysfunction in the SHINE trial: methods and challenges. Clin Infect Dis. 2015;61(Suppl 7):S726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133(5):1332–8. [DOI] [PubMed] [Google Scholar]
- 37. McDonald CM, Manji KP, Gosselin K, Tran H, Liu E, Kisenge R, Aboud S, Fawzi WW, Gewirtz AT, Duggan CP. Elevations in serum anti-flagellin and anti-LPS Igs are related to growth faltering in young Tanzanian children. Am J Clin Nutr. 2016;103:1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Makhseed M, Raghupathy R, El-Shazly S, Azizieh F, Al-Harmi JA, Al-Azemi MMK. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. 2003;49:308–18. [DOI] [PubMed] [Google Scholar]
- 39. Georgiou HM, Thio YS, Russell C, Permezel M, Heng YJ, Lee S, Tong S. Association between maternal serum cytokine profiles at 7–10 weeks’ gestation and birthweight in small for gestational age infants. Am J Obstet Gynecol. 2011;204:415.e1–12. [DOI] [PubMed] [Google Scholar]
- 40. Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162(11):1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59:29–37. [DOI] [PubMed] [Google Scholar]
- 42. Wilkinson AL, Pedersen SH, Urassa M, Michael D, Andreasen A, Todd J, Kinung'hi SM, Changalucha J, McDermid JM. Maternal systemic or cord blood inflammation is associated with birth anthropometry in a Tanzanian prospective cohort. Trop Med Int Health. 2017;22(1):52–62. [DOI] [PubMed] [Google Scholar]
- 43. Kuzawa CW, Fried RL, Borja JB, McDade TW. Maternal pregnancy C-reactive protein predicts offspring birth size and body composition in metropolitan Cebu, Philippines. J Develop Origins Health Dis. 2017;8(6):674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.