Abstract
Background
Orogastric tube feeding is indicated in neonates with an impaired ability to ingest food normally and can be administered with an intermittent bolus or continuous feeding schedule.
Objectives
The objectives were to 1) compare the long-term effect of continuous with intermittent feeding on growth using the newborn pig as a model, 2) determine whether feeding frequency alters lean tissue and fat mass gain, and 3) identify the signaling mechanisms by which protein deposition is controlled in skeletal muscle in response to feeding frequency.
Design
Neonatal pigs were fed the same amount of a balanced formula by orogastric tube either as an intermittent bolus meal every 4 h (INT) or as a continuous infusion (CON). Body composition was assessed at the start and end of the study by dual-energy X-ray absorptiometry, and hormone and substrate profiles, muscle mass, protein synthesis, and indexes of nutrient and insulin signaling were measured after 21 d.
Results
Body weight, lean mass, spine length, and skeletal muscle mass were greater in the INT group than in the CON group. Skeletal muscle fractional protein synthesis rates were greater in the INT group after a meal than in the CON group and were associated with higher circulating branched-chain amino acid and insulin concentrations. Skeletal muscle protein kinase B (PKB) and ribosomal protein S6 kinase phosphorylation and eukaryotic initiation factor (eIF) 4E–eIF4G complex formation were higher, whereas eIF2α phosphorylation was lower in the INT group than in the CON group, indicating enhanced activation of insulin and amino acid signaling to translation initiation.
Conclusions
These results suggest that when neonates are fed the same amounts of nutrients as intermittent meals rather than continuously there is greater lean growth. This response can be ascribed, in part, to the pulsatile pattern of amino acids, insulin, or both induced by INT, which enables the responsiveness of anabolic pathways to feeding to be sustained chronically in skeletal muscle.
Keywords: infant, body composition, skeletal muscle, growth, protein synthesis
INTRODUCTION
It is widely recognized that appropriate nutrition is a major factor that influences the survival and subsequent growth and development of infants (1). When neonates lack the ability to coordinate normal food ingestion, orogastric tube feeding is prescribed and can be accomplished by using either an intermittent or continuous schedule (1, 2). However, a Cochrane analysis concluded that the clinical benefits of feeding frequency cannot be reliably recognized from published studies so far due to methodologic limitations and difficulties in controlling variables that can affect the outcome of these studies (3). Despite the incomplete evidence, intermittent feeding is considered by some to be superior to continuous feeding (4) on the basis of the occurrence of more natural feed-fast hormonal profiles (5, 6). In addition, intermittent feeding has beneficial effects for intestinal growth and development in newborn piglets by promoting greater mucosal and intestinal protein accretion than continuous feeding (7). Nonetheless, continuous feeding is indicated when neonates are unable to tolerate intermittent feeding (8).
Studies in humans (9) and animal models (10–14) have shown that neonates can efficiently deposit ingested amino acids into body proteins, and that this efficiency (which decreases with age) contributes significantly to their high rates of growth and protein turnover (9, 15–17). In skeletal muscle, the high anabolic capacity of the neonate is enabled by a high ribosomal abundance and the ability to accelerate protein synthesis after a meal. This response to feeding in muscle is regulated independently by the postprandial increase in insulin and amino acids (18). However, whether over the long-term differences in the patterns of circulating concentrations of both insulin and nutrients can affect overall growth is unknown.
Ethical considerations preclude the conduct of controlled studies required to ascertain the efficacy of potentially beneficial interventions in infants. To study the effect of feeding modality on muscle growth in early postnatal life, we used the neonatal piglet, which is considered to be an excellent surrogate for the human neonate (19). We have shown that short-term (24-h) intermittent feeding enhances protein synthesis more than continuous feeding but has no effect on protein degradation in muscles (20, 21) and results in a higher rate of protein deposition (21). Nonetheless, we do not know if this enhanced stimulation of anabolic pathways persists over the long term and promotes greater lean tissue mass gain than continuous feedings. On the basis of our previous data, therefore, we hypothesized that if the same amount of feed is administered to a neonate in intermittent meals rather than as a continuous infusion there would be greater accretion of lean tissue. The objectives of this study were as follows: 1) to compare the effects over a 3-wk period of continuous with intermittent feeding on the growth of the neonatal pig; 2) to determine whether feeding frequency alters lean tissue, fat mass gain, or both; and 3) to identify the signaling mechanisms by which protein deposition is controlled in skeletal muscle in response to feeding frequency.
METHODS
Animals and surgeries
The Animal Care and Use Committee of the Baylor College of Medicine approved all experimental procedures. This study was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. Eighteen female crossbred (Yorkshire × Landrace × Hampshire × Duroc) pigs (Agricultural Headquarters of the Texas Department of Criminal Justice) (n = 6/group) at 2 d of age and weighing ∼2 kg were placed under general anesthesia and, using sterile technique, were fitted with indwelling catheters in the carotid artery and jugular vein and a gastrostomy tube was also implanted for diet delivery as previously described (21). Pigs were placed in protective jackets and housed individually in cages equipped with swivel and tether systems to administer the diet in an environmentally controlled room maintained at 30°C with a 12-h light-dark cycle. They were provided with enrichment toys and supplemented with heating lamps during the first week of life. Pigs were allowed to recover and were fed a commercial sow-milk replacer (Soweena Litter Life; Merrik's) for 3 d postsurgery before they were randomly assigned to treatment groups at the initiation of the experimental feeding protocol.
Diet
All of the pigs received the same amount of sow-milk replacer [240 mL • kg body weight (BW)−1 • d−1; 12.8 g crude protein • kg BW−1 • d−1 and 155 kcal • kg BW−1 • d−1] via a gastrostomy tube either continuously (CON; n = 6; 10 mL • kg BW−1 • h−1) or intermittently (INT; n = 12; 40 mL • kg BW−1 • bolus−1 delivered over 15 min every 4 h) for 21 d (Table 1). Pigs that were intermittently fed were either killed immediately before a meal (INT-0) or 60 min after a meal (INT-60). Data for INT-0 and INT-60 groups were pooled for body composition, plasma amino acid and insulin concentrations, and muscle weight because these groups were treated identically from the start of the study until the time these data were obtained.
TABLE 1.
Ingredients and nutrient composition of the experimental diet
| Per kilogram of diet as fed | |
|---|---|
| Ingredient, g | |
| Whey protein concentrate (80% crude protein)1 | 73 |
| Lactose | 9 |
| FatPak 802 | 6 |
| Corn oil | 31 |
| Water | 868 |
| Xanthan gum | 2 |
| Vitamin premix3 | 2 |
| Mineral premix3 | 9 |
| Calculated analysis | |
| Crude protein, g | 58 |
| Crude fat, g | 40 |
| Carbohydrates, g | 13 |
| Metabolizable energy, kcal | 644 |
Hilmar Ingredients.
Milk Specialties Global Animal Nutrition.
Dyets, Inc. The vitamin premix provided the following (in grams per kilogram): thiamin HCl (0.1), riboflavin (0.375), pyridoxine HCl (0.1), niacin (1), calcium pantothenate (1.2), folic acid (0.13), biotin (0.02), cyanocobalamin (1.5), retinyl palmitate (0.8), cholecalciferol (0.05), tocopheryl acetate (8.8), and menadione sodium bisulfite (0.08). The trace mineral premix provided (in g/kg): calcium phosphate, dibasic (187); calcium carbonate (279); sodium chloride (85); potassium phosphate, monobasic (155); magnesium sulfate, anhydrous (44); manganous carbonate (0.93); ferric citrate (10); zinc carbonate (1.84); cupric carbonate (0.193); potassium iodate (0.005); and sodium selenite (0.007).
Body-composition and anthropometric measurements
On day −3 and 18 d after starting the feeding trial, body composition was determined on anesthetized pigs by dual-energy X-ray absorptiometry (DXA) with the use of a fan-beam densitometer (Hologic QDR4500A) in the infant whole-body scan mode. Previously published calibration equations specific to young piglets were used to estimate fat and lean mass (22). Spine length was determined from the DXA images and defined as the distance between the first cervical to the sixth lumbar vertebrae. Spine length and body composition at the start (day 0) and end (day 21) of the feeding period were inferred from DXA measurements at days −3 and 18, respectively. Spine length was calculated by using the daily increment in length determined from the measurement at −3 and 18 d and assuming a linear rate of change. The percentage of fat and total lean mass were calculated from the product of BW at days 0 and 21 and percentage fat or percentage lean at −3 and 18 d assuming no change in composition between −3 and 0 d and 18 and 21 d (−3-d and 18-d values are shown in Supplemental Table 1). Total fat is the product of percentage fat and body weight. After being killed, the longissimus dorsi, gastrocnemius, and soleus muscles were quantitatively dissected and weighed. The efficiency of food conversion to BW gain was calculated with the use of the following formula:
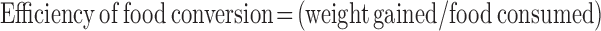 |
(1) |
where weight gained is the change in BW in kilograms from day 0 to 21 of the feeding protocol and food consumed in kilograms over the same 21 d. Total body protein was calculated from DXA lean values with the use of the following equation (23):
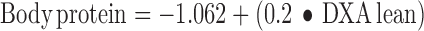 |
(2) |
Efficiency of energy retention was calculated from total fat and protein deposition calculated from the DXA measurements. Protein retention efficiency was calculated as follows:
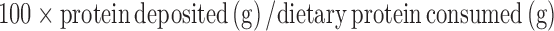 |
(3) |
The efficiency of energy deposition was calculated as follows:
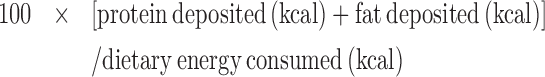 |
(4) |
assuming energy values for protein and fat of 5680 and 9460 kcal/kg, respectively.
Blood sampling
On the 21st day of feeding, blood samples were taken over a 4-h period, every 15 min from 0 to 90 min after feeding and every 30 min thereafter. Plasma samples were stored at −20°C until analysis. Samples were analyzed for glucose using a glucose oxidase method (model 2300; Yellow Springs Instrument Co.), branched-chain amino acids using a leucine oxidase method (24), and insulin using a porcine insulin radioimmunoassay (Millipore).
Fractional protein synthesis
Tissue fractional protein synthesis rates were measured by using a flooding dose of l-[4-3H]phenylalanine (12). Piglets received l-[4-3H]phenylalanine (1.5 mmol/kg BW, 0.5 mCi/kg BW; American Radiolabeled Chemicals, Inc.). Pigs in the INT-0 group were injected 30 min before a feeding, and killed 30 min later before being fed. Those in the INT-60 group were given the tracer 30 min after a feeding and killed 30 min later. For the CON group, the isotope was injected without interrupting the feeding and the piglets killed 30 min later. After pigs were killed with an overdose of pentobarbital, samples were immediately frozen in liquid nitrogen and stored at −70°C until analysis (12). The specific radioactivities of phenylalanine incorporated into tissue proteins and the tissue-free pools were analyzed as previously described (15). Fractional rates of protein synthesis (KS; %/d) were calculated by using the following formula:
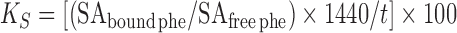 |
(5) |
where SAbound phe and SAfree phe (dpm/nmol) are the specific radioactivity of protein-bound and tissue-free phenylalanine, respectively, t (minutes) is the time after tracer administration, and 1440 is a conversion factor (minutes to days).
Protein synthetic capacity and efficiency
The protein concentration in muscle homogenates was determined by using the bicinchoninic acid assay (Thermo Scientific) and total RNA by the method of Munro and Fleck (25). Protein synthetic capacity (CS; µg RNA/mg protein) was calculated as the RNA-to-protein ratio and protein synthetic efficiency (KRNA; g protein • d−1 • g RNA−1) as the total protein synthesized in a day per total RNA.
Reverse transcriptase and real-time quantitative polymerase chain reaction
Polysomal fractions were prepared with the use of sucrose density gradients. Isolated RNA was reverse transcribed to complementary DNA and quantitated by quantitative reverse transcriptase–polymerase chain reaction. The proportion of ribosomal protein (rp) S4 (rpS4), rpS8, and ornithine decarboxylase (ODC) mRNAs in the polysomal fraction was determined in longissimus muscle as described previously (26).
Protein Western blot analysis
Proteins from longissimus dorsi muscle homogenates were separated by SDS-PAGE as described previously (21). Immunoblotting was performed using the following primary antibodies: eukaryotic elongation factor 2 (eEF2; total and phosphorylated Thr56; Cell Signaling Technology), eukaryotic initiation factor (eIF) 2α (eIF2α; total and phosphory-lated Ser51; Cell Signaling Technology), eIF4E (gift of Leonard Jefferson, Penn State University College of Medicine), eIF4G (EMD Millipore), F-box protein atrogin-1/MAFbx (Atrogin-1; ECM Biosciences), forkhead transcription factor 3 (Fox03; total and phosphorylated Ser253; Cell Signaling Technology), insulin receptor (IR; Santa Cruz Biotechnology), insulin receptor substrate 1 (IRS-1; Santa Cruz Biotechnology), microtubule-associated protein 1 light-chain 3 (LC3; Cell Signaling Technology), muscle ring finger 1 (MuRF1; R&D Systems), lysosome-associated membrane protein 2 (LAMP2; Epitomics), l-type amino acid transporter 1 (LAT1; Cell Signaling Technology), phospho-tyrosine (p-Tyr; Cell Signaling Technology), phospho-threonine (p-Thr; Santa Cruz Biotechnology), protein kinase B (PKB; total; Santa Cruz Biotechnology; phosphorylated Thr308; Cell Signaling Technology), ribosomal protein S6 kinase 1 (S6K1; total; Santa Cruz Biotechnology; phosphorylated Thr389; EMD Millipore), sodium-coupled neutral amino acid transporter 2 (SNAT2; Santa Cruz Biotechnology), and α-tubulin (Santa Cruz Biotechnology). Protein loading was normalized against α-tubulin abundance, and immunoblots that were probed with antiphospho-specific antibodies were normalized to the corresponding non–phospho-specific antibodies. Blots were developed by using an enhanced chemiluminescence kit (GE Health Sciences) and visualized and analyzed using a ChemiDoc-It Imaging System (UVP).
Quantification of eIF4E–eIF4G complex
The complex was immunoprecipitated with an anti-eIF4E monoclonal antibody from aliquots of fresh tissue homogenates as previously described (21).
Statistical analysis
Treatments were assigned to experimental units with the use of a complete randomized block design. A sample of 6 pigs/treatment was expected to provide 80% power (α = 0.05; 2-tailed) to detect a difference between treatments of 30% with the assumption of 15% between animal variation. To investigate temporal effects, data were analyzed as repeated measures with the use of the MIXED procedure of SAS (version 9.4; SAS Institute). Various autocorrelation structures were investigated on the basis of the Bayesian information criterion for model selection. First-order autoregressive covariance structure gave the best fit (smallest Bayesian information criterion) and this structure was then used for all repeated-measures analyses. All other data were analyzed by ANOVA using the MIXED procedure of SAS. When a significant effect was detected, means were compared by using the Tukey-Kramer post hoc test. Data are presented as least-square means ± SEMs, and differences were considered significant at P ≤ 0.05.
RESULTS
Growth and body composition
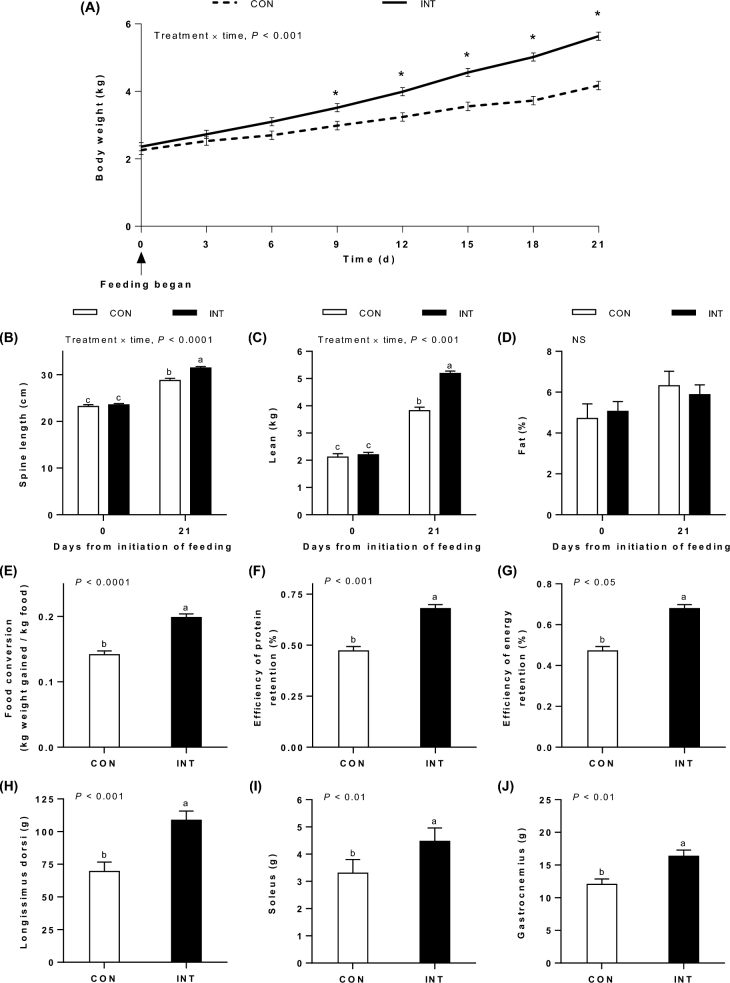
BW was greater for INT than for CON pigs on day 9 and at each day measured thereafter (P < 0.001; Figure 1A). Spine length (Figure 1B), lean tissue mass (Figure 1C), and fat mass (data not shown) determined by DXA were greater for INT than for CON pigs after 21 d of feeding (P < 0.0001, P < 0.001, and P < 0.05, respectively). However, mean values for percentage of fat (Figure 1D) were not affected by feeding modality. Intermittent feeding resulted in improved food conversion efficiency (Figure 1E) compared with continuous feeding (0.20 compared with 0.14 kg BW gain/kg milk; P < 0.0001). In addition, the efficiencies of protein and energy retentions (Figure 1F, G) were improved by 30% and 44%, respectively, with intermittent compared with continuous feeding (P < 0.001 and P < 0.05, respectively). Longissimus dorsi (Figure 1H), soleus (Figure 1I), and gastrocnemius (Figure 1J) muscles were 56%, 35%, and 36% heavier (P < 0.001, P < 0.01, and P < 0.01, respectively) in the INT group than in the CON group.
FIGURE 1.
Growth and efficiency of nutrient deposition. Body weight (A) over the 21-d feeding period; spine length (B), lean mass (C), and percentage of fat (D) by dual X-ray absorptiometry at 0 and 21 d; food conversion (E); efficiency of protein retention (F) and efficiency of energy retention (G) integrated over the 21-d feeding period; and longissimus dorsi muscle mass (H), soleus muscle mass (I), and gastrocnemius muscle mass (J) after 21 d of feeding neonatal pigs either continuously or intermittently. Values are means ± SEMs; n = 6 (CON) and 12 (INT). “INT” represents INT-0 and INT-60 data that were pooled because these groups were treated identically from the start of the study until the time these data were obtained. Spine length and body composition at the start (day 0) and end (day 21) of the feeding period were inferred from dual X-ray absorptiometry measurements at days –3 and 18, respectively, as described in Methods. Statistical analyses were conducted by using mixed-model repeated-measures ANOVA. When a significant effect was detected, all means were compared using a Tukey-Kramer post hoc test. *Body weight of INT pigs differed from that of CON pigs, P ≤ 0.001. Means without a common letter differ significantly. CON, continuously fed; INT, intermittently fed; INT-0, intermittently fed just before a meal; INT-60, intermittently fed 60 min after a meal.
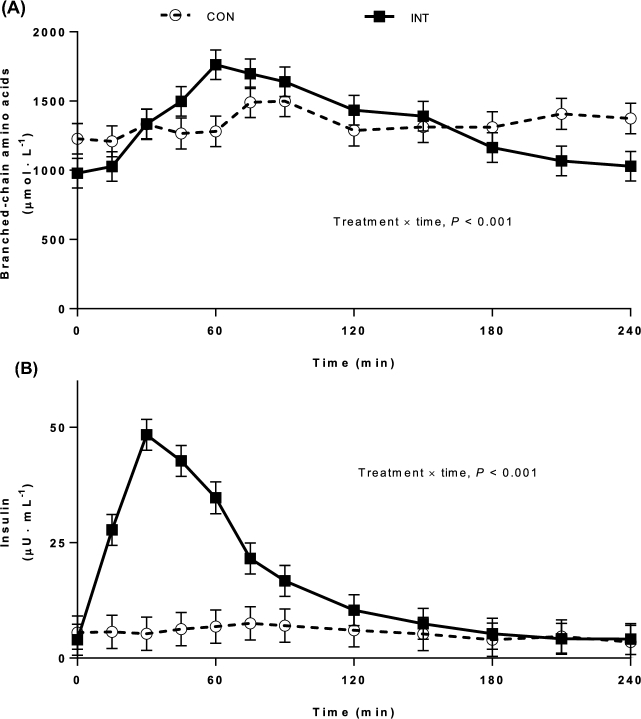
Branched-chain amino acids and insulin
Arterial branched-chain amino acid (Figure 2A) and insulin (Figure 2B) concentrations, measured on the last day of feeding, were greater in INT pigs after the meal than in CON pigs (P < 0.001). The mean arterial branched-chain amino acid concentration was 1300 µmol/L for the CON group, and for INT pigs concentrations reached a maximum of 1760 µmol/L at 60 min before returning to baseline (1100 µmol/L) by 180 min postfeeding. In the INT pigs, maximum insulin concentrations were reached at 30 min after the meal and returned to prefeeding levels by 3 h, whereas circulating insulin concentrations remained low and constant in the CON group.
FIGURE 2.
Arterial plasma branched-chain amino acid (A) and insulin (B) concentrations on day 21 of feeding neonatal pigs either continuously or intermittently for 21 d. Values are means ± SEMs; n = 6 (CON) and 12 (INT). “INT” represents INT-0 and INT-60 data that were pooled because these groups were treated identically from the start of the study until the time these data were obtained. Statistical analyses were conducted by using mixed-model repeated-measures ANOVA. When a significant effect was detected, all means were compared using a Tukey-Kramer post hoc test. CON, continuously fed; INT, intermittently fed; INT-0, intermittently fed just before a meal; INT-60, intermittently fed 60 min after a meal.
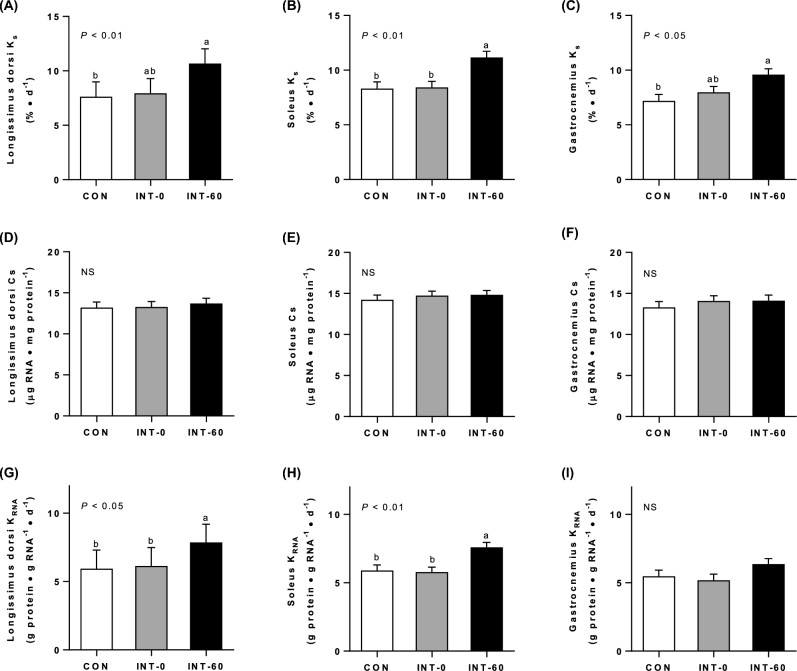
Fractional protein synthesis rates
Fractional protein synthesis rates (KS) in the INT-60 group increased by 40% in longissimus dorsi (Figure 3A; P < 0.01) and by 34% in the soleus and gastrocnemius (Figure 3B, C; P < 0.01 and P < 0.05, respectively) muscles compared with those in the CON group. There were no differences in Ks between the CON group and the INT-0 group.
FIGURE 3.
Fractional protein synthesis rates (Ks) of longissimus dorsi (A), soleus (B), and gastrocnemius (C) muscles; RNA synthetic capacity (Cs) of longissimus dorsi (D), soleus (E), and gastrocnemius (F) muscles; and RNA synthetic efficiency (KRNA) of longissimus dorsi (G), soleus (H), and gastrocnemius (I) muscles of neonatal pigs fed for 21 d either continuously or intermittently with measurements made just before (INT-0) or 60 min after (INT-60) a meal. Values are means ± SEMs; n = 6. Statistical analyses were conducted by mixed-model ANOVA. When a significant effect was detected, all means were compared using a Tukey-Kramer post hoc test. Means without a common letter differ significantly. “NS” denotes no significant differences between treatments. CON, continuously fed; INT, intermittently fed.
Protein synthetic capacity and efficiency
There was no effect of feeding modality on total RNA concentration, a measure of ribosomal abundance and protein synthetic capacity (Cs) in the longissimus, soleus, and gastrocnemius muscles (Figure 3D–F) (27). However, the efficiency with which ribosomes translate mRNA into protein (KRNA) was significantly higher in the longissimus (P < 0.05) and soleus (P < 0.01) muscles of INT-60 pigs compared with INT-0 and CON pigs (Figure 3G, H), but was not different for the gastrocnemius muscle (Figure 3I).
Ribosomal proteins
The expression of ODC was used as a reference. There were no differences in the proportion of longissimus dorsi muscle rpS4 and rpS8 mRNA in polysomes in response to feeding frequency (Supplemental Figure 1A, B).
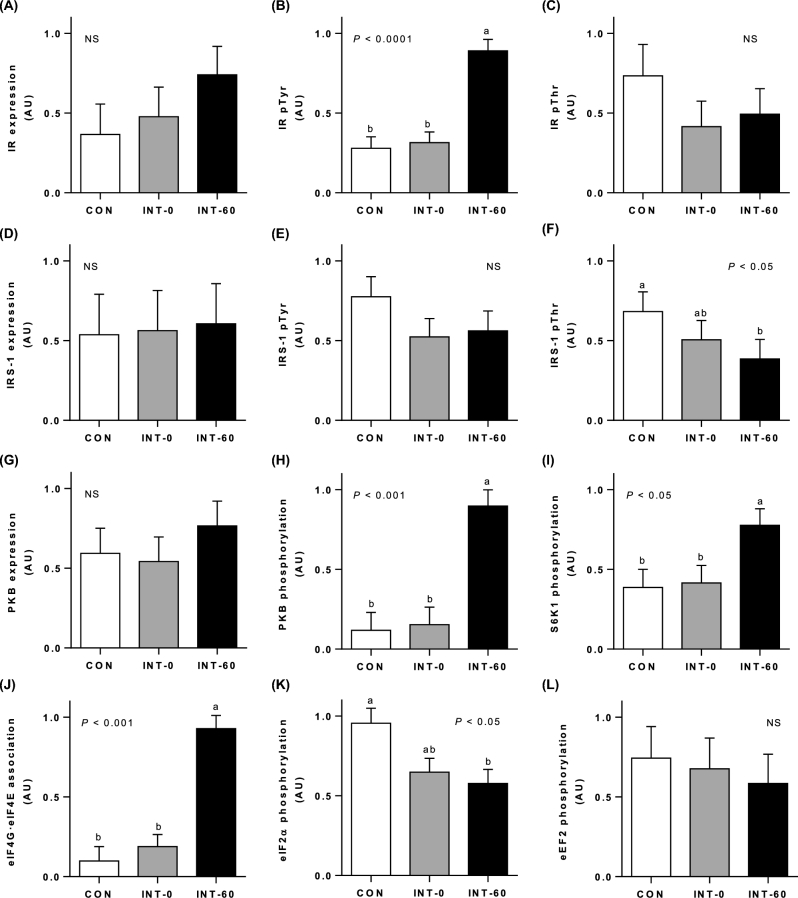
Translation initiation and intracellular signaling
In the longissimus dorsi muscle, relative IR abundance was unaffected by feeding modality (Figure 4A). Tyrosine phosphorylation of IR (Figure 4B), corrected for abundance, increased in INT-60 pigs compared with CON and INT-0 pigs (P < 0.0001); however, IR threonine phosphorylation (Figure 4C) was not affected by treatment. IRS-1 abundance (Figure 4D) and IRS-1 tyrosine phosphorylation (Figure 4E) were similar between all 3 treatments, but IRS-1 threonine phosphorylation (Figure 4F) was lower (P < 0.05) for the INT-60 group than for the CON group, and the INT-0 group was intermediate in value. PKB abundance (Figure 4G) was not affected by feeding frequency. However, PKB phosphorylation (Figure 4H) was greater in the INT-60 than in the INT-0 and CON muscles (P < 0.001).
FIGURE 4.
Insulin pathway and translation initiation signaling. IR expression (A), IR pTyr (B), IR pThr (C), IRS-1 expression (D), IRS-1 pTyr (E), IRS-1 pThr (F), PKB expression (G), PKB phosphorylation (H), S6K1 phosphorylation (I), association of the eIF4G–eIF4E complex (J), eIF2α phosphorylation (K), and eEF2 phosphorylation (L) in the longissimus dorsi muscle of neonatal pigs fed for 21 d either continuously or intermittently with measurements made just before (INT-0) or 60 min after (INT-60) a meal. Values are means ± SEMs; n = 6. Statistical analyses were conducted by using mixed-model ANOVA. When a significant effect was detected, all means were compared using a Tukey-Kramer post hoc test. “NS” denotes no significant differences between treatments. Means without a common letter differ significantly. AU, arbitrary units; CON, continuously fed; eEF2, eukaryotic elongation factor 2; eIF, eukaryotic initiation factor; INT, intermittently fed; IR, insulin receptor; IRS-1, insulin receptor substrate 1; PKB, protein kinase B; pThr, threonine phosphorylation; pTyr, tyrosine phosphorylation; S6K1, ribosomal protein S6 kinase 1.
Phosphorylation of S6K1 (Figure 4I) and formation of the active eIF4E–eIF4G complex (Figure 4J) were enhanced in longissimus dorsi muscle in response to meal feeding in the INT-60 pigs compared with CON or INT-0 pigs (P < 0.05 and P < 0.001, respectively). Phosphorylation of eIF2α (Figure 4K), which inhibits protein synthesis, was lower in INT-60 pigs than in CON pigs (P < 0.05), and INT-0 pigs were intermediate between the 2. There was no effect of treatment on eEF2 phosphorylation (Figure 4L). Expression of the amino acid transporters LAT1 and SNAT2 was not different between the 3 treatments (Supplemental Figure 2A, B). Furthermore, the abundances of eIF2α, eEF2, S6K1, eIF4E, and eIF4G were not affected by feeding modalities (data not shown).
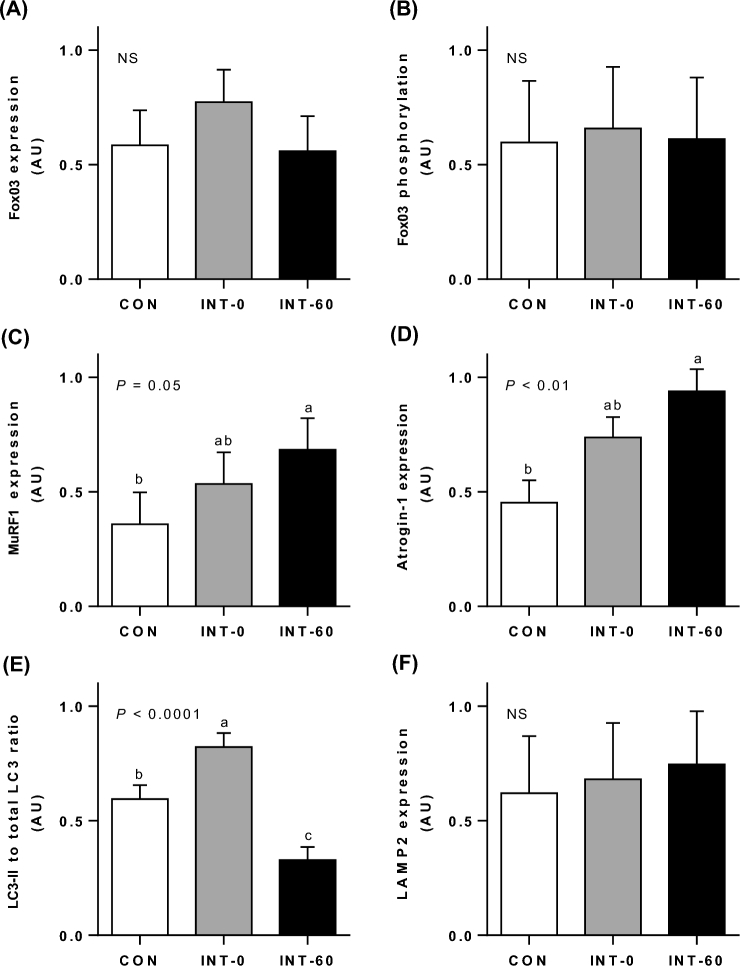
Fox03 abundance and phosphorylation (Figure 5A, B) were not affected by treatment; however, the abundance of the ubiquitin ligases MuRF1 and Atrogin-1 (Figure 5C, D) was highest for INT-60 pigs compared with CON pigs (P = 0.05 and P < 0.01, respectively), with INT-0 pigs being intermediate between the 2. The LC3-II to total LC3 ratio (Figure 5E) was greater in INT-0 pigs than in CON pigs (P < 0.001), but after feeding (INT-60), the ratio decreased below levels in CON pigs (P < 0.0001). There was no effect of treatment on LAMP2 expression (Figure 5F).
FIGURE 5.
Protein degradation signaling. Fox03 expression (A), Fox03 phosphorylation (B), MuRF1 expression (C), Atrogin-1 expression (D), LC3-II to total LC3 ratio (E), and LAMP2 expression (F) in the longissimus dorsi muscle of neonatal pigs fed for 21 d either continuously or intermittently with measurements made just before (INT-0) or 60 min after (INT-60) a meal. Values are means ± SEMs; n = 6. Statistical analyses were conducted by using mixed-model ANOVA. When a significant effect was detected, all means were compared using a Tukey-Kramer post hoc test. “NS” denotes no significant differences between treatments. Means without a common letter differ significantly. Atrogin-1, F-box protein atrogin 1/MAFbx; AU, arbitrary units; CON, continuously fed; Fox03, forkhead transcription factor 3; INT, intermittently fed; LC3, microtubule-associated protein 1 light chain 3; LAMP2, lysosome-associated membrane protein 2; MuRF1, muscle ring finger 1.
DISCUSSION
We showed that the surge in insulin and amino acids after an intermittent bolus meal activates the signaling components that regulate translation initiation and stimulate protein synthesis, whereas the low and constant hormone-substrate pattern incurred with continuous feeding attenuates protein synthesis in the neonatal piglet model (20, 21, 26). Whether these differences are maintained over the long term is uncertain due to the limited published data from either human or animal model studies. Herein, our findings show that the protein synthetic response to feeding modality underlies the differences observed between groups in muscle and whole-body lean growth. The results of the present study also suggest that intermittent feeding enhances lean tissue accretion more than does continuous feeding in full-term neonatal pigs by promoting a pulsatile pattern of amino acid– or insulin-induced translation initiation or both.
It has been shown that intermittent feeding results in equal (28–30) or better (31) weight gains than continuous feeding. Previously, we reported that protein synthesis in muscle increases after a meal in a neonatal piglet model (26). We also established that intermittent feeding enhances protein accretion by upregulating protein synthesis with no changes to degradation (21). In these studies, however, the feeding protocols were administered only for 24 h, and it was uncertain if the responses would be maintained over a more chronic and clinically relevant length of time. In the current study, after 21 d of intermittent feeding, BW gain was enhanced compared with continuous feeding.
Our results showed that after 21 d fractional protein synthesis was similar in pigs fed intermittently right before a meal and those fed continuously but was higher 60 min after a meal in INT pigs than in CON pigs, which is in agreement with our previous findings (20). The rate of protein synthesis depends on the capacity and efficiency of the translation process (15). Our data indicate that in the longissimus dorsi muscle, the synthesis of rpS4 and rpS8 was not affected by feeding modality. However, the increase in the fractional rate of protein synthesis in the longissimus dorsi and soleus muscles in INT pigs 60 min after a meal was driven by the enhanced protein synthetic efficiency.
The regulation of protein synthetic efficiency by feeding is mediated through the control of translation initiation or elongation mechanisms or both in response to feeding-induced changes in the activity of insulin and amino acid–signaling pathways (14, 18). The abundances of IR and IRS-1 were not affected by feeding frequency; however, tyrosine phosphorylation of the IR and phosphorylation of PKB increased in INT pigs 60 min after a meal. This is in accordance with our previous findings (20, 21, 32) that the rapid increase in insulin that occurs with feeding (33) stimulates the IR and activates the signal transduction pathway leading to PKB phosphorylation on Thr308 (34). The activation of PKB after a meal occurs through insulin-mediated phosphorylation but independently of the changes in amino acid concentrations (34).
Prolonged hyperphosphorylation of S6K1 (35) serves as a negative feedback process controlling IRS-1 activity (36) through serine/threonine phosphorylation (37), which leads to its degradation (38, 39). Conversely, our data suggest that, although threonine phosphorylation of IRS-1 was reduced in INT compared with CON piglets, the abundance of IRS-1 was similar between the 2 feeding modalities. Thus, it seems likely that the pulsatile changes in circulating insulin contributed to the enhanced rate of protein synthesis and lean deposition in the INT pigs via activation of insulin-induced signaling leading to protein synthesis.
There are 2 mechanisms that control translation initiation. The first is via eukaryotic initiation factor 4E binding protein 1 (4EBP1) and S6K1, leading to the formation of the eIF4G–eIF4E complex (40, 41). In the current study, S6K1 phosphorylation and eIF4G–eIF4E complex formation increased 60 min after the meal in the INT pigs but was not different in the CON or INT pigs before the meal. The postprandial surges in both insulin and amino acids are likely to have been responsible for these changes.
The second mechanism that regulates translation initiation is via the eIF2-dependent process (40, 41). In our previous studies, phosphorylation of the α-subunit of eIF2, which negatively regulates translation initiation, did not differ after 1 d of continuous or intermittent bolus feeding (20, 21). In contrast, in this study, phosphorylation of the α-subunit of eIF2 decreased in INT compared with CON pigs, suggesting that eIF2-dependent regulation of protein synthesis is sensitive to the chronic meal frequency pattern. Thus, by stimulating both key steps that regulate translation initiation, intermittent feeding resulted in a greater increase in protein synthesis and ultimately improved gain in muscle mass.
Skeletal muscle degradation through the autophagy-lysosomal (42) and the ubiquitin-proteasome (43) systems becomes an important source of amino acids when dietary nutrient intake is insufficient (43). Although activation of the ubiquitin proteasome system occurs in response to severe cachectic and catabolic states (44–49), high rates of degradation also occur during muscle hypertrophy (16, 50) and allow cellular protein remodeling to occur (51). This is critically important for skeletal muscle when undergoing maturation.
Polyubiquitination of target myofibrillar proteins in skeletal muscles (52–54) by muscle-specific E3 ubiquitin-ligases Atrogin-1 and MuRF1 (43, 55–57) is the rate-limiting step of the ubiquitin-proteasome system (42). In the current study, expression of Atrogin-1 and MuRF1 was increased in muscle of the intermittently fed pigs despite no changes in Fox03 phosphorylation. Although expression of MuRF1 and Atrogin-1 decreased after phosphorylation of Fox03 (47) by PKB (57), it appears that, contrary to atrophy states, Fox03 signaling is not correlated with transcription of MuRF1 and Atrogin-1 in response to muscle loading (58) or feeding frequency (current study). It is also possible that transcription may have been regulated through different post-translational modifications, signaling pathways, or both (59).
A reduction in PKB phosphorylation induces autophagy (42) through activation of LC3 (LC3-II) and the formation of autophagosomes (42, 60). The autophagy-lysosomal system appeared to be more sensitive to acute dietary changes than the ubiquitin-proteasome system (20, 61). However, despite the sensitive nature of this system, protein degradation was not different between continuous and intermittent feeding protocols of shorter (1 d) duration (21). An alternative explanation is that an increase in LC3-II is caused by accumulation of autophagosomes, a process that precedes autolysosome maturation and muscle protein loss (42). This possibility, however, is unlikely because LAMP2, a protein involved in the fusion of autophagosomes and lysosomes (62), was not different between the groups. Our results further show that the autophagy-lysosomal system is sensitive to feeding frequency because activation of this pathway was reduced in pigs fed intermittently after a meal.
In conclusion, our data suggest that intermittent bolus compared with continuous feeding induces a pulsatile pattern of circulating insulin and amino acids, which activates the insulin and amino acid signaling pathways, leading to translation initiation and stimulation of protein synthesis in muscle of the neonate. This rapid increase in insulin and amino acids likely contributes to the enhanced protein synthetic efficiency and the consequent increase in lean mass with prolonged intermittent feeding. The downregulation of the autophagy-lysosomal pathway, but upregulation of the ubiquitin-proteasome pathway, with intermittent bolus feeding may imply fine-tuning of protein degradation systems to amino acid supply during a period of rapid growth when tissue remodeling is critical. Our results provide direct evidence that the intermittent bolus pattern of feeding is more advantageous than continuous feeding in improving lean body mass and growth of neonates.
Supplementary Material
Acknowledgements
We thank DG Burrin and B Stoll for helpful discussions, RD Almonaci for technical assistance, JC Stubblefield for animal care, and E O'Brian Smith for statistical assistance.
The authors’ responsibilities were as follows—SWE-K, MLF, and TAD: designed the research and wrote the manuscript; SWE-K, CB, MCG, AS, RAO, NS, HVN, and SRK: conducted the research; SWE-K, AS, MLF, and TAD: analyzed the data; TAD: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors declared that no conflicts of interest exist.
Notes
Supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grants AR044474 (TAD) and AR46308 (MLF), National Institute of Child Health and Human Development grants HD072891 (TAD) and HD085573 (TAD), USDA National Institute of Agriculture grant 2013-67015-20438 (TAD), and by the USDA–Agricultural Research Service under cooperative agreement 6250-510000-055 (TAD).
This work is a publication of the USDA–Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Supplemental Table 1 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- Atrogin-1
F-box protein atrogin 1/MAFbx
- BW
body weight
- CON
continuously fed
- DXA
dual-energy X-ray absorptiometry
- eEF2
eukaryotic elongation factor 2
- eIF
eukaryotic initiation factor
- Fox03
forkhead transcription factor 3
- INT
intermittently fed
- IR
insulin receptor
- IRS-1
insulin receptor substrate 1
- LAMP2
lysosome-associated membrane protein 2
- LC3
microtubule-associated protein 1 light-chain 3
- MuRF1
muscle ring finger 1
- PKB
protein kinase B
- rp
ribosomal protein
- S6K1
ribosomal protein S6 kinase 1
REFERENCES
- 1. American Academy of Pediatrics Nutritional needs of the preterm infant. In: Kleinman RE, editor. Pediatric nutrition handbook. Elk Grove Village (IL): American Academy of Pediatrics; 2003. p. 23–54. [Google Scholar]
- 2. Marchand V. Enteral nutrition tube feedings. In: Baker S, Baker RD, Davis A, editors. Pediatric nutrition support. Sudbury (MA): Jones and Bartlett Publishers; 2007. p. 249–60. [Google Scholar]
- 3. Premji SS, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev 2011;11:CD001819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103(6 Part 1):1150–7. [DOI] [PubMed] [Google Scholar]
- 5. Aynsley-Green A. The endocrinology of feeding in the newborn. Baillieres Clin Endocrinol Metab 1989;3(3):837–68. [DOI] [PubMed] [Google Scholar]
- 6. Mashako MN, Bernard C, Cezard JP, Chayvialle JA, Navarro J. Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr Gastroenterol Nutr 1987;6(6):948–52. [DOI] [PubMed] [Google Scholar]
- 7. Shulman RJ, Redel CA, Stathos TH. Bolus versus continuous feedings stimulate small-intestinal growth and development in the newborn pig. J Pediatr Gastroenterol Nutr 1994;18(3):350–4. [DOI] [PubMed] [Google Scholar]
- 8. Dollberg S, Kuint J, Mazkereth R, Mimouni FB. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am Coll Nutr 2000;19(6):797–800. [DOI] [PubMed] [Google Scholar]
- 9. Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res 1991;30(1):23–7. [DOI] [PubMed] [Google Scholar]
- 10. Burrin DG, Davis TA, Fiorotto ML, Reeds PJ. Stage of development and fasting affect protein synthetic activity in the gastrointestinal tissues of suckling rats. J Nutr 1991;121(7):1099–108. [DOI] [PubMed] [Google Scholar]
- 11. Burrin DG, Davis TA, Fiorotto ML, Reeds PJ. Hepatic protein synthesis in suckling rats: effects of stage of sevelopment and sasting. Pediatr Res 1992;31(3):247–52. [DOI] [PubMed] [Google Scholar]
- 12. Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 1996;270(5):E802–9. [DOI] [PubMed] [Google Scholar]
- 13. Davis TA, Fiorotto ML, Nguyen HV, Burrin DG, Reeds PJ. Response of muscle protein synthesis to fasting in suckling and weaned rats. Am J Physiol Regul Integr Comp Physiol 1991;261(6 Part 2):R1373–80. [DOI] [PubMed] [Google Scholar]
- 14. Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol Regul Integr Comp Physiol 1993;265(2):R334–40. [DOI] [PubMed] [Google Scholar]
- 15. Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol 1989;257:R1141–6. [DOI] [PubMed] [Google Scholar]
- 16. Goldspink DF, Kelly FJ. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J 1984;217(2):507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis SE, Kelly FJ, Goldspink DF. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J 1984;217(2):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 2003;285(1):E40–53. [DOI] [PubMed] [Google Scholar]
- 19. Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care 2008;11(5):601–6. [DOI] [PubMed] [Google Scholar]
- 20. El-Kadi SW, Gazzaneo MC, Suryawan A, Orellana RA, Torrazza RM, Srivastava N, Kimball SR, Nguyen HV, Fiorotto ML, Davis TA. Viscera and muscle protein synthesis in neonatal pigs is increased more by intermittent bolus than by continuous feeding. Pediatr Res 2013;4(10):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 2012;302(6):E674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koo WWK, Hammami M, Shypailo RJ, Ellis KJ. Bone and body composition measurements of small subjects: discrepancies from software for fan-beam dual energy x-ray absorptiometry. J Am Coll of Nutr 2004;23(6):647–50. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell A, Scholz A. Efficiency of energy and protein deposition in swine measured by dual energy X-ray absorptiometry (DXA). Arch Tierzucht 2008;51(2):160–72. [Google Scholar]
- 24. Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem 1996;240(1):48–53. [DOI] [PubMed] [Google Scholar]
- 25. Munro HN, Fleck A. Analysis of tissues and body fluids for nitrogenous constituents. In: Munro HN, editor. Mammalian protein metabolism 3. New York: Academic Press; 1969. p. 465–83. [Google Scholar]
- 26. Gazzaneo MC, Orellana RA, Suryawan A, Tuckow AP, Kimball SR, Wilson FA, Nguyen HV, Torrazza RM, Fiorotto ML, Davis TA. Differential regulation of protein synthesis and mTOR signaling in skeletal muscle and visceral tissues of neonatal pigs after a meal. Pediatr Res 2011;70(3):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fiorotto ML, Davis TA, Reeds PJ, Burrin DG. Nonnutritive factors in colostrum enhance myofibrillar protein synthesis in the newborn pig. Pediatr Res 2000;48(4):511–7. [DOI] [PubMed] [Google Scholar]
- 28. Macdonald PD, Skeoch CH, Carse H, Dryburgh F, Alroomi LG, Galea P, Gettinby G. Randomised trial of continuous nasogastric, bolus nasogastric, and transpyloric feeding in infants of birth weight under 1400 g. Arch Dis Child 1992;67(4 Spec No):429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silvestre MA, Morbach CA, Brans YW, Shankaran S. A prospective randomized trial comparing continuous versus intermittent feeding methods in very low birth weight neonates. J Pediatr 1996;128(6):748–52. [DOI] [PubMed] [Google Scholar]
- 30. Akintorin S, Kamat M, Pildes R, Kling P, Andes S, Hill J, Pyati S. A prospective randomized trial of feeding methods in very low birth weight infants. Pediatrics 1997;100(4):E4. [DOI] [PubMed] [Google Scholar]
- 31. Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 1999;103(2):434–9. [DOI] [PubMed] [Google Scholar]
- 32. Gazzaneo MC, Suryawan A, Orellana RA, Torrazza RM, El-Kadi SW, Wilson FA, Kimball SR, Srivastava N, Nguyen HV, Fiorotto ML et al.. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr 2011;141(12):2152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 2001;281(5):E908–15. [DOI] [PubMed] [Google Scholar]
- 34. Suryawan A, O'Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 2004;134(1):24–30. [DOI] [PubMed] [Google Scholar]
- 35. Hinault H, Mothe-Satney I, Gautier N, Lawrence JC Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J 2004;18(15):1894–6. [DOI] [PubMed] [Google Scholar]
- 36. Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE 2005;268:pe4. [DOI] [PubMed] [Google Scholar]
- 37. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J et al.. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004;431(7005):200–5. [DOI] [PubMed] [Google Scholar]
- 38. Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol 2000;14(6):783–94. [DOI] [PubMed] [Google Scholar]
- 39. Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes 2001;50(1):24–31. [DOI] [PubMed] [Google Scholar]
- 40. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009;10(5):307–18. [DOI] [PubMed] [Google Scholar]
- 41. Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009;136(4):731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandri M. Autophagy in health and disease: 3. Autophagy involvement in muscle atrophy. Am J Physiol Cell Physiol 2010;298:C1291–7. [DOI] [PubMed] [Google Scholar]
- 43. Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 2006;18(6):631–5. [DOI] [PubMed] [Google Scholar]
- 44. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E et al.. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 2007;6(5):376–85. [DOI] [PubMed] [Google Scholar]
- 45. Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol 2007;292(1):R328–36. [DOI] [PubMed] [Google Scholar]
- 46. Larsen AE, Tunstall RJ, Carey KA, Nicholas G, Kambadur R, Crowe TC, Cameron-Smith D. Actions of short-term fasting on human skeletal muscle myogenic and atrogenic gene expression. Ann Nutr Metab 2006;50(5):476–81. [DOI] [PubMed] [Google Scholar]
- 47. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117(3):399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. Endocrinology 2008;197(1):1–10. [DOI] [PubMed] [Google Scholar]
- 49. Sishi BJ, Engelbrecht AM. Tumor necrosis factor alpha (TNF-alpha) inactivates the PI3-kinase/PKB pathway and induces atrophy and apoptosis in L6 myotubes. Cytokine 2011;54(2):173–84. [DOI] [PubMed] [Google Scholar]
- 50. Millward DJ, Garlick PJ, Stewart RJ, Nnanyelugo DO, Waterlow JC. Skeletal-muscle growth and protein turnover. Biochem J 1975;150(2):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hesselink MK, Minnaard R, Schrauwen P. Eat the meat or feed the meat: protein turnover in remodeling muscle. Curr Opin Clin Nutr Metab Care 2006;9(6):672–6. [DOI] [PubMed] [Google Scholar]
- 52. Polge C, Heng A-E, Jarzaguet M, Ventadour S, Claustre A, Combaret L, Béchet D, Matondo M, Uttenweiler-Joseph S, Monsarrat B et al.. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J 2011;25(11):3790–802. [DOI] [PubMed] [Google Scholar]
- 53. Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 2004;287(4):E591–601. [DOI] [PubMed] [Google Scholar]
- 54. Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol 2005;350(4):713–22. [DOI] [PubMed] [Google Scholar]
- 55. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 2014;307(6):E469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bodine SC, Latres E, Baumhueter S, Lai VKM, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K et al.. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001;294(5547):1704–8. [DOI] [PubMed] [Google Scholar]
- 57. Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin–proteasome pathway in normal and disease states. J Am Soc Nephrol 2006;17(7):1807–19. [DOI] [PubMed] [Google Scholar]
- 58. Baehr L, Tunzi M, Bodine S. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol 2014;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Myatt SS, Lam EWF. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007;7:847. [DOI] [PubMed] [Google Scholar]
- 60. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A et al.. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008;4(2):151–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab 2013;305(5):E620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hubert V, Peschel A, Langer B, Groger M, Rees A, Kain R. LAMP-2 is required for incorporating syntaxin-17 into autophagosomes and for their fusion with lysosomes. Biol Open 2016;5(10):1516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.