Abstract
Background
Evidence in humans is equivocal in regards to whether resting energy expenditure (REE) decreases to a greater extent than predicted for the loss of body mass with weight loss, and whether this disproportionate decrease in REE persists with weight-loss maintenance.
Objectives
We aimed to1) determine if a lower-than-predicted REE is present in a sample of successful weight-loss maintainers (WLMs) and 2) determine if amount of weight loss or duration of weight-loss maintenance are correlated with a lower-than-predicted REE in WLMs.
Design
Participants (18–65 y old) were recruited in 3 groups: WLMs (maintaining ≥13.6 kg weight loss for ≥1 y, n = 34), normal-weight controls [NCs, body mass index (BMI; in kg/m2) similar to current BMI of WLMs, n = 35], and controls with overweight/obesity (OCs, BMI similar to pre–weight-loss maximum BMI of WLMs, n = 33). REE was measured (REEm) with indirect calorimetry. Predicted REE (REEp) was determined via 1) a best-fit linear regression developed with the use of REEm, age, sex, fat-free mass, and fat mass from our control groups and 2) three standard predictive equations.
Results
REEm in WLMs was accurately predicted by equations developed from NCs and OCs (±1%) and by 3 standard predictive equations (±3%). In WLMs, individual differences between REEm and REEp ranged from −257 to +163 kcal/d. A lower REEm compared with REEp was correlated with amount of weight lost (r = 0.36, P < 0.05) but was not correlated with duration of weight-loss maintenance (r = 0.04, P = 0.81).
Conclusions
We found no consistent evidence of a significantly lower REE than predicted in a sample of long-term WLMs based on predictive equations developed from NCs and OCs as well as 3 standard predictive equations. Results suggest that sustained weight loss may not always result in a substantial, disproportionately low REE.
This trial was registered at clinicaltrials.gov as NCT03422380.
Keywords: adaptive thermogenesis, energy metabolism, metabolic adaptation, weight-loss maintenance, weight regain, obesity
INTRODUCTION
Metabolic adaptations occur during weight loss that result in both an increase in hunger and a decrease in energy requirements (1). The resulting “energy gap” (discrepancy between energy desired and energy required) is thought to contribute to the propensity for weight regain (1–6), and subsequent poor long-term weight-loss maintenance (7). Adaptive thermogenesis (AT), defined as a decrease in resting energy expenditure (REE) beyond what is predicted from changes in body mass and composition (5, 8, 9), is one such metabolic adaptation. Studies in rodents have provided consistent evidence that weight reduction is accompanied by suppression in REE [adjusted for fat-free mass (FFM)], that this suppression in REE is sustained during weight-loss maintenance, and that it has a significant impact on the risk of weight regain (10–14).
However, evidence for AT in humans is equivocal. Several studies have suggested that REE after weight loss is reduced to a greater extent than predicted (9, 15–19) and that this disproportionate reduction in REE persists long-term (1–6 y) (9, 20, 21). For example, a recently published study (9) evaluated 14 contestants from “The Biggest Loser,” a televised weight-loss competition. Using contestants’ baseline data to develop predictive equations for REE, Fothergill et al. (9) reported that mean REE after 6-y follow-up was ∼500 kcal/d lower than predicted. These data have been interpreted by some to suggest that weight-loss maintenance is a futile undertaking given this substantial metabolic penalty imposed by a lower-than-predicted REE (22, 23). However, other studies in weight-reduced humans have found no evidence of AT or suggest that it resolves during sustained weight-loss maintenance (24–29). Furthermore, there may be individual-specific factors (such as amount of weight loss or duration of weight-loss maintenance) that determine the extent to which metabolic adaptation occurs during weight reduction (5, 9, 20). It is possible that individuals for whom this adaptive response is minimal, absent, or resolves with time, may be more likely to successfully maintain weight loss.
We conducted a case-control study (NCT03422380) to 1) evaluate whether evidence of a lower-than-predicted REE exists in a unique group of successful weight-loss maintainers (WLMs, maintaining weight loss of ≥13.6 kg for ≥1 y) and 2) explore whether amount of weight loss or weight-loss maintenance duration are correlated with the degree of AT, if present. The magnitude of AT was determined by comparing measured REE (REEm) with predicted REE (REEp) via 4 different predictive equations. We hypothesized that the magnitude of adaptation would be positively correlated with the amount of weight loss and inversely correlated with the duration of weight-loss maintenance (i.e., resolution of the adaptive response over time), and that these parameters could explain the wide range of individual variability.
METHODS
This case-control study was conducted between October 2009 and August 2012 at the University of Colorado Anschutz Medical Campus and was approved by the Colorado Multiple Institutional Review Board.
Subject recruitment
Subjects were recruited through e-mail announcements and campus-wide flyers. To enhance recruitment of WLMs, we sent a letter to members of the National Weight Control Registry (NWCR) living in the Denver Metro area, inviting them to participate in the study. The NWCR was established in 1994 as a prospective cohort study to better understand successful long-term weight-loss maintenance. NWCR entry criteria include maintenance of ≥13.6 kg weight loss for ≥1 y (30). Interested individuals were screened by telephone to determine if they met preliminary eligibility criteria for 1 of 3 study groups: 1) WLMs [maintaining ≥13.6 kg (30 lb) weight loss for ≥1 y]; 2) normal-weight controls (NCs, BMI similar to the current BMI of the WLMs), and 3) controls with overweight/obesity (OCs, BMI similar to the maximum BMI of the WLMs). The NC and OC groups self-reported not maintaining a >13.6 kg weight loss. A nested subject selection procedure was used to obtain similar distributions for age (categories <36, 36–49, and ≥50 y) and sex (male compared with female) across all 3 groups. It was also designed to ensure similar distribution for BMI between NCs' and WLMs' current BMI (kg/m2; categories <22, 23–25, and 26–30) and similar distribution for BMI between OCs' and WLMs' pre–weight-loss maximum BMI (categories 26–30, 31–35, 36–40, and ≥41).
Eligible individuals were invited to attend an in-person screening visit. After providing informed written consent, participants completed a health history, weight-loss history, and a physical exam with the study physician. Participants were excluded if they had any physical or medical condition that restricted physical activity (including diabetes, cardiovascular disease, cancer, and/or significant musculoskeletal, neurologic, or psychiatric disorders), if they had bariatric surgery, were currently taking weight-loss medications or other medications known to affect appetite or metabolism, were smokers or had quit smoking within the past 6 mo, were not weight stable (>5 kg fluctuation in body weight over the past 6 mo), or were pregnant or lactating. Eligible participants were invited to participate in the study and scheduled for a 1-wk free-living monitoring period.
Body weight and composition
Weight was measured with a calibrated digital scale (to the nearest 0.2 lb; Tanita, BWB-800) and height with a wall-mounted stadiometer to the nearest 0.1 cm. Weight was collected at screening, and on days 1 and 8 during the free-living monitoring period. Waist circumference was measured just over the iliac crests at screening with the use of a tape measure. Body composition was measured by dual-energy X-ray absorptiometry (Delphi-W version 13.1.0, Hologic Inc., Bedford, MA) at screening. One OC participant's supine body width exceeded the scan window dimensions, so fat mass (FM) and FFM were determined from bioelectrical impedance analysis (Tanita, TBF-105).
REEm
REE was measured through the use of standard indirect calorimetry (Truemax 2400, ParvoMedics, Salt Lake City, UT) with the ventilated hood technique. Before each test was performed, the metabolic cart gas analyzers and flow meter were calibrated per the manufacturer's recommendations. Participants were instructed to fast for 12 h overnight, which was confirmed by study staff upon arrival in the clinic. Upon arrival (∼0700), participants rested supine, awake, and lightly clothed in a thermoneutral (20–23°C), dimly lit, quiet room for 30 min. Respiratory gas exchange was measured for 15 min, using the last 10 min to average REE. Criteria used to determine if the REE measurement was acceptable included stability (CV of the final 10 min <5%) and average metabolic equivalents (<1.10). REE measurements that did not meet these criteria were considered invalid and excluded from the analysis. REE was measured on days 1 and 8 of the free-living monitoring period, and averaged to produce a single value for REEm (intraclass correlation coefficient = 0.96).
REEp
Data from 1) NCs only, 2) OCs only, and 3) NCs and OCs combined were used to generate least squares best-fit linear regression equations for REE as a function of FFM (kg), FM (kg), age (years), and sex. These are the same predictor variables used by Fothergill et al. (9) to develop a predictive equation for REE in “The Biggest Loser” contestants. We then applied the predictive equations to WLMs, using their corresponding FFM, FM, age, and sex to calculate REEp. The resulting predictive equations (REEp) were as follows:
- NCs (n = 35):
- REE = 561.5 + 19.4 (FFM) + 1.4 (FM) − 2.4 (age) + 100 (sex*) (R2 = 0.93)
- OCs (n = 33):
- REE = 749.8 + 15.0 (FFM) + 9.4 (FM) − 5.8 (age) + 134 (sex*) (R2 = 0.89)
- NCs + OCs (n = 68):
- REE = 650.8 + 16.9 (FFM) + 7.6 (FM) − 4.5 (age) + 132 (sex*) (R2 = 0.92)
*F = 0, M = 1.
To compare REEm with REEp within all 3 study groups, REEp was also determined through the use of 4 previously published predictive equations: 1) Harris and Benedict (31), based on a sample of “normal” men (n = 136) and women (n = 247) “of widely different ages” studied in 1918; 2) Mifflin et al. (32), derived from 498 healthy adults (251 men, 247 women), aged 19–78 y, including individuals of normal weight (n = 264) and individuals with obesity (n = 234); 3) a predictive equation based on tissue-specific metabolic rates (brain, skeletal muscle, adipose tissue, bone, and residual mass) that was validated in 154 healthy adults (74 men, 80 women; mean ± SD age: 33.8 ± 8.6 y, BMI: 24.4 ± 4.1), through the use of methods described previously (33, 34); and 4) a least squares best-fit linear regression equation for REE as a function of baseline FFM, FM, age, and sex from 16 contestants in “The Biggest Loser”, a televised weight-loss competition (mean ± SD age: 34.9 ± 10.3 y, BMI: 49.5 ± 10.1), published by Fothergill et al. (9).
Statistics
Statistical analyses were performed with the use of SAS version 9.4 (SAS System for Microsoft, SAS Institute Inc., Cary, NC), with the type I error rate fixed at 0.05 (2-tailed). Fisher's exact tests compared categoric demographic characteristics (sex, ethnicity, and race) across the 3 groups. Normality of outcome measures was checked with the Shapiro-Wilk test. For variables where Shapiro-Wilk P < 0.05, data transformations were used. A natural log transformation was used for weight, minimum weight, maximum BMI, maximum weight ever lost, FFM, and REEm. A square root transformation was used for maximum weight. Untransformed means ± SDs are presented in the tables and figures.
Differences between REEm and REEp were determined and compared via a paired-samples t test. ANOVA (PROC GLM, SAS) and 1-factor ANCOVA were used to test the null hypothesis that 1) REEm, 2) REEm adjusted for FFM only, 3) REEm adjusted for FFM and FM, 4) REEp, and 5) the difference between REEm and REEp, in the 3 groups are drawn from populations with the same mean values. A significant difference between REEm and REEp that was <0 would provide evidence of AT. Omnibus F test P values are reported, followed by between-group pairwise comparisons. Pearson correlations were used to determine whether maximum weight, maximum BMI, maximum weight lost, and duration of weight-loss maintenance were correlated with the difference between REEm and REEp, predicted from NCs, in WLMs. Results are presented as mean ± SD unless otherwise stated. Results were not corrected for multiple comparisons. There was no a priori power analysis for the outcome variables in this secondary analysis.
RESULTS
Study enrollment and subject characteristics
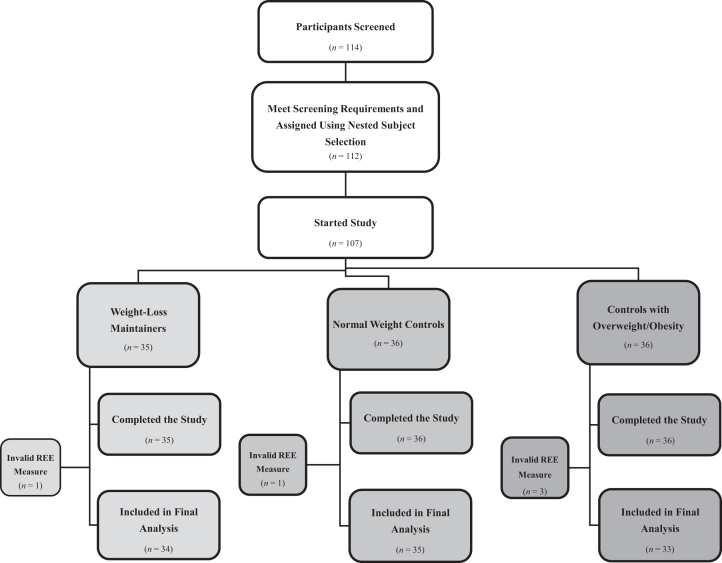
A total of 114 participants were screened, and 107 completed the study. Subjects with ≥1 valid REEm (day 1 or day 8) were included in the final analysis. Five subjects (WLMs: n = 1, NCs: n = 1, OCs: n = 3) did not have a valid day 1 or day 8 REEm and were excluded. Thus, 102 subjects were included in the final analysis (34 WLMs, 35 NCs, 33 OCs; Figure 1). Of these, 14/102 (14%) had only 1 valid REEm (WLMs: n = 2, NCs: n = 7, OCs: n = 5). Change in weight (day 1 – day 8) was not significantly different from 0 in any subject group (WLMs: 0.09 ± 0.73, NCs: 0.07 ± 0.74, OCs: 0.29 ± 1.06 kg; P > 0.05). In addition, day 1 and 8 weights had low variation, with a within-individual CV of 0.63% ± 0.50%.
FIGURE 1.
Study CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials; REE, resting energy expenditure.
The nested subject selection procedures successfully achieved similar group means for age, sex, and BMI (i.e., BMI of NCs similar to WLMs' current BMI, and BMI of OCs similar to WLMs' pre–weight-loss maximum BMI) (Table 1). Current BMI of WLMs (24.0 ± 2.2) was not different from current BMI of NCs (22.8 ± 1.9). Current BMI of OCs (33.1 ± 4.7) was not different from the pre–weight-loss maximum BMI of WLMs (33.0 ± 4.6, P = 0.90). WLMs reported maintaining a weight loss of 26.7 ± 10.5 kg for a median of 6.0 y (IQR 3–12 y).
TABLE 1.
Baseline characteristics of study participants1
| Characteristic | WLMs (n = 34) | NCs (n = 35) | OCs (n = 33) | P value, omnibus F test | P value, WLMs:NCs | P value, WLMs:OCs | P value, NCs:OCs |
|---|---|---|---|---|---|---|---|
| Age, y | 43.8 ± 11.3 | 45.6 ± 13.5 | 47.2 ± 10.7 | 0.50 | 0.52 | 0.24 | 0.59 |
| Anthropometric measures | |||||||
| Weight,2 kg | 68.3 ± 10.0 | 64.6 ± 11.5 | 93.2 ± 18.8 | <0.01* | 0.14 | <0.01* | <0.01* |
| Height, cm | 168.3 ± 9.32 | 167.6 ± 10.3 | 167.3 ± 9.7 | 0.90 | 0.77 | 0.66 | 0.88 |
| BMI, kg/m2 | 24.0 ± 2.2 | 22.8 ± 1.9 | 33.1 ± 4.7 | <0.01* | 0.11 | <0.01* | <0.01* |
| Waist circumference,3 cm | 83.0 ± 6.9 | 82.4 ± 7.7 | 104.5 ± 12.8 | <0.01* | 0.80 | <0.01* | <0.01* |
| Maximum weight,4 kg | 93.5 ± 14.8 | 69.0 ± 12.0 | 99.5 ± 21.3 | <0.01* | <0.01* | 0.17 | <0.01* |
| Minimum weight,2,5 kg | 63.1 ± 10.4 | 55.5 ± 1.2 | 65.4 ± 15.4 | 0.02* | 0.04* | 0.61 | 0.01* |
| Maximum BMI,2 kg/m2 | 33.0 ± 4.6 | 24.4 ± 2.0 | 35.3 ± 5.6 | <0.01* | <0.01* | 0.03* | <0.01* |
| Maximum weight ever lost,2 kg | 26.7 ± 10.5 | 6.1 ± 5.0 | 12.3 ± 7.8 | <0.01* | <0.01* | <0.01* | <0.01* |
| Weight-loss maintenance duration,6 y | 9.1 ± 9.2 | n/a | n/a | n/a | |||
| Sex, male, n (%) | 8 (24) | 10 (29) | 6 (18) | 0.60 | |||
| Ethnicity, n (%) | 0.43 | ||||||
| Hispanic/Latino | 1 (3) | 4 (11) | 3 (9) | ||||
| Not Hispanic/Latino | 33 (97) | 31 (89) | 30 (91) | ||||
| Race, n (%) | 0.02* | ||||||
| Caucasian | 34 (100) | 29 (83) | 29 (88) | ||||
| Black/African American | 0 (0) | 2 (6) | 4 (12) | ||||
| Asian | 0 (0) | 3 (8) | 0 (0) | ||||
| Not reported | 0 (0) | 1 (3) | 0 (0) | ||||
| DXA scan measures | |||||||
| Fat mass, kg | 17.9 ± 4.7 | 17.7 ± 4.4 | 37.1 ± 9.7 | <0.01* | 0.93 | <0.01* | <0.01* |
| Fat mass, % | 26.9 ± 6.8 | 28.3 ± 6.8 | 40.2 ± 5.3 | <0.01* | 0.36 | <0.01* | <0.01* |
| Fat-free mass,2 kg | 49.1 ± 9.5 | 45.8 ± 10.7 | 54.7 ± 11.3 | <0.01* | 0.12 | 0.03* | <0.01* |
| Fat-free mass, % | 73.1 ± 6.8 | 71.7 ± 6.8 | 59.9 ± 5.4 | <0.01* | 0.36 | <0.01* | <0.01* |
Values are means ± SDs unless otherwise indicated. Chi-square or Fisher's exact test was used for categoric variables; continuous variables were analyzed via 1-factor ANOVA. *Significant P values (α < 0.05). DXA, dual energy X-ray absorptiometry; NC, normal-weight control; n/a, not applicable; OC, control with overweight/obesity; WLM, weight-loss maintainer.
Analyzed with the use of log (natural base) transformation, but untransformed means ± SDs are presented.
n = 34 for NCs and n = 32 for OCs.
Excluding pregnancy. Analyzed via square root transformation, but untransformed means ± SDs are presented.
After age 18 y and excluding illness.
Calculated as the difference between baseline visit year and the year the participant lost ≥13.6 kg.
REEm
Day 1 and 8 REEm had low variation (within-individual CV = 3.09% ± 1.99%). Unadjusted REEm was lower in WLMs and NCs compared with OCs (Table 2). After adjusting for FFM, both WLMs and NCs had significantly lower REEm compared with OCs (Table 2). However, after adjusting for differences in FFM and FM, there were no significant differences between groups (Table 2).
TABLE 2.
Comparison of resting energy expenditure across subject group1
| Outcome, n | WLMs (n = 34) | NCs (n = 35) | OCs (n = 33) | P value, omnibus F test | P value, WLMs:NCs | P value, WLMs:OCs | P value, NCs:OCs |
|---|---|---|---|---|---|---|---|
| REEm,2 kcal/d | 1442.7 (1359.1, 1526.4) | 1390.6 (1308.2, 1473.1) | 1668.2 (1583.3, 1753.1) | <0.01* | 0.24 | <0.01* | <0.01* |
| REEm,2 kcal/d, adjusted for FFM | 1457.5 (1421.0, 1494.0) | 1474.0 (1437.2, 1510.9) | 1564.6 (1526.2, 1602.9) | <0.01* | 0.96 | <0.01* | <0.01* |
| REEm,2 kcal/d, adjusted for FFM and FM | 1485.4 (1445.3, 1525.5) | 1500.7 (1460.7, 1540.6) | 1507.5 (1453.7, 1561.3) | 0.49 | 0.93 | 0.27 | 0.25 |
Values are least square means (95% CIs) unless otherwise indicated. Results from 1-factor ANOVA or 1-factor ANCOVA. *Significant P values (α < 0.05). FFM, fat-free mass; FM, fat mass; NC, normal-weight control; OC, control with overweight/obesity; REEm, measured resting energy expenditure; REEp, predicted resting energy expenditure; WLM, weight-loss maintainer.
Analyzed with the use of log (natural base) transformation, but untransformed means and 95% CIs are presented.
REEm compared with REEp
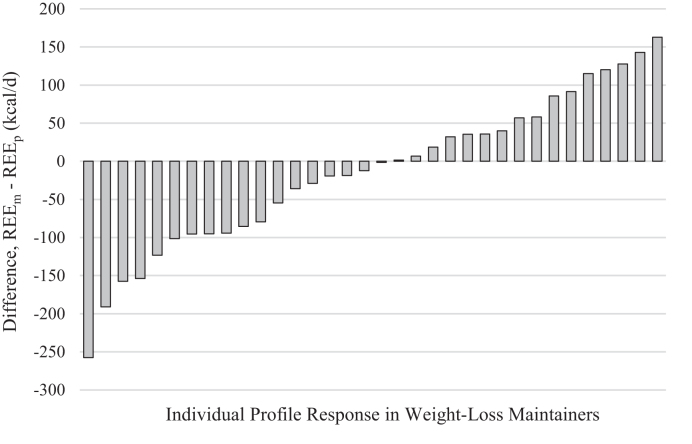
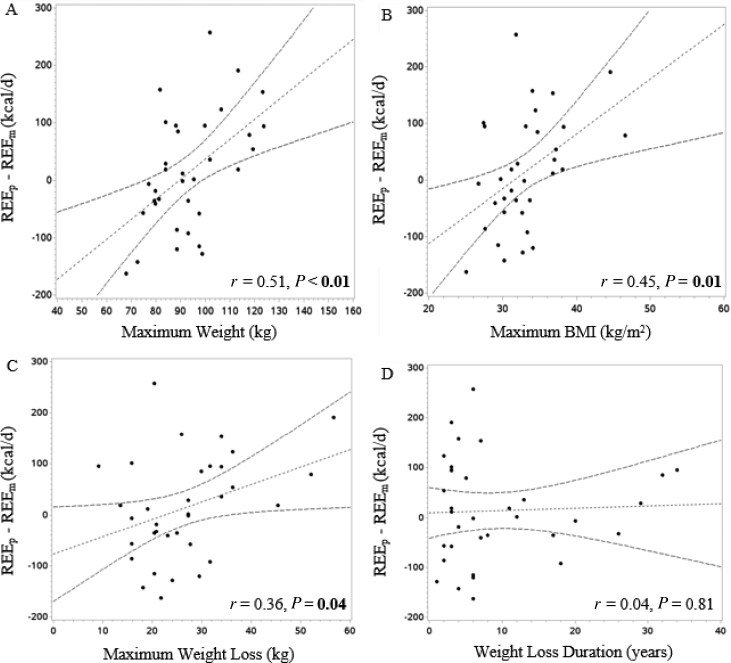
We compared REEm with REEp in WLMs based on the predictive equations developed from our control groups. There were no significant differences between REEm and REEp (Table 3). For additional analyses, we chose to rely on the NCs-only equation because this equation 1) is based on a sample of individuals that are most similar to our WLM sample and 2) had the highest R2 of the 3 predictive equations. Individual differences between REEm and REEp, predicted from NCs, ranged from −257 to +163 kcal/d (Figure 2). In WLMs, a lower-than-predicted REE (predicted from NCs) was positively correlated with maximum weight, maximum BMI, and maximum weight lost (r = 0.51, r = 0.45, r = 0.36, respectively; P < 0.05), but was not correlated with duration of weight-loss maintenance (Figure 3A−D).
TABLE 3.
Difference between REEm and REEp in weight-loss maintainers predicted with the use of control group data1
| Predictive equation | REEm, kcal/d | REEp, kcal/d | Difference, kcal/d (95% CI) | P value, difference |
|---|---|---|---|---|
| NCs + OCs | 1442.7 ± 192.7 | 1449.7 ± 202.6 | −6.9 ± 87.6 (−37.5, 23.6) | 0.65 |
| NCs only | 1442.7 ± 192.7 | 1456.7 ± 217.9 | −13.9 ± 100.9 (−49.1, 21.2) | 0.43 |
| OCs only | 1442.7 ± 192.7 | 1431.1 ± 187.3 | 11.6 ± 86.3 (−18.5, 41.7) | 0.44 |
Value are means ± SDs unless otherwise indicated. Results from paired-samples t test; total n = 34. NC, normal-weight control; OC, control with overweight/obesity; REEm, measured resting energy expenditure; REEp, predicted resting energy expenditure.
FIGURE 2.
Individual variability in difference between REEm and REEp in WLMs. Bar chart of individual data for difference between REEm and REEp, predicted via the NCs-only equation in WLMs (n = 34). NC, normal-weight control; REEm, measured resting energy expenditure; REEp, predicted resting energy expenditure; WLM, weight-loss maintainer.
FIGURE 3.
Correlation between adaptive thermogenesis and other factors in WLMs: (A) maximum weight, (B) maximum BMI, (C) maximum weight loss, (D) weight-loss duration. Scatter plot displaying results determined through the use of Pearson correlation to examine which factors were correlated with AT (calculated as REEp – REEm) in WLMs (n = 34), with REEp predicted from NCs-only equation. A positive value on the x axis is indicative of AT. (A) Positive correlation between AT and maximum weight (i.e., those with a higher maximum weight exhibited a greater degree of AT). (B) Positive correlation between AT and maximum BMI (i.e., those with a higher maximum BMI exhibited a greater degree of AT). (C) Positive correlation between AT and maximum weight lost (i.e., those with greater weight loss exhibited a greater degree of AT). (D) No correlation between AT and duration of weight-loss maintenance. AT, adaptive thermogenesis; NC, normal-weight control; REEm, measured resting energy expenditure; REEp, predicted resting energy expenditure; WLM, weight-loss maintainer.
We also compared REEm with REEp in all 3 study groups through the use of 3 standard predictive equations (31, 32, 34) as well as the equation published by Fothergill et al. (9), developed from “The Biggest Loser” contestants’ baseline data (Table 4). When using the Harris and Benedict equation (31), REEm was significantly lower than REEp in both WLMs and NCs, but not in OCs, with no between-group differences in REEm− REEp. When using the Mifflin equation (32), REEm was significantly higher than REEp in all 3 groups, with no between-group differences in REEm − REEp. Using the Mifflin equation (32), the ranges of the difference between REEm and REEp were as follows: WLMs, −124 to 214 kcal/d; NCs, −117 to 205 kcal/d; and OCs, −156 to 264 kcal/d. When using the organ-size equation (34), REEm was not significantly different from REEp in WLMs and NCs but was significantly greater than REEp in OCs; the difference between REEm and REEp in OCs was significantly greater than the difference between REEm and REEp in both WLMs and NCs. When using “The Biggest Loser” equation (9), REEm was significantly lower than REEp in all 3 groups with OCs having a significantly smaller difference between REEm and REEp compared with both WLMs and NCs. The accuracy with which REEm was predicted from REEp, calculated via the 4 equations, within each study group is shown in Table 4.
TABLE 4.
Difference between REEm and REEp, predicted from 4 predictive equations1
| WLMs (n = 34) | NCs (n = 35) | OCs (n = 33) | P value, omnibus F test | P value, WLMs:NCs | P value, WLMs:OCs | P value, NCs:OCs | |
|---|---|---|---|---|---|---|---|
| Harris and Benedict (31) | |||||||
| REEm, kcal/d | 1442.7 ± 192.7 | 1390.6 ± 261.8 | 1668.2 ± 275.3 | <0.01* | 0.24 | <0.01* | <0.01* |
| REEp, kcal/d | 1475.3 ± 190.3 | 1437.3 ± 225.3 | 1707.0 ± 299.9 | <0.01* | 0.52 | <0.01* | <0.01* |
| Difference, kcal/d | −32.6 ± 84.4 | −46.6 ± 86.5 | −38.8 ± 116.1 | 0.83 | 0.55 | 0.79 | 0.74 |
| P value, difference | 0.03* | <0.01* | 0.06 | ||||
| Mifflin et al. (32) | |||||||
| REEm, kcal/d | 1442.7 ± 192.7 | 1390.6 ± 261.8 | 1668.2 ± 275.3 | <0.01* | 0.24 | <0.01* | <0.01* |
| REEp, kcal/d | 1397.1 ± 202.4 | 1355.1 ± 248.8 | 1613.7 ± 274.1 | <0.01* | 0.48 | <0.01* | <0.01* |
| Difference, kcal/d | 45.7 ± 84.0 | 35.5 ± 88.7 | 54.5 ± 97.0 | 0.69 | 0.65 | 0.69 | 0.39 |
| P value, difference | 0.03* | 0.02* | <0.01* | ||||
| Hayes et al. (organ size) (34) | |||||||
| REEm, kcal/d | 1442.7 ± 192.7 | 1390.6 ± 261.8 | 1647.2 ± 251.4 | <0.01* | 0.24 | <0.01* | <0.01* |
| REEp, kcal/d | 1430.7 ± 230.1 | 1363.2 ± 271.2 | 1500.0 ± 249.2 | 0.09 | 0.27 | 0.27 | 0.03* |
| Difference, kcal/d | 12.0 ± 125.5 | 27.4 ± 80.5 | 147.2 ± 137.4 | <0.01* | 0.58 | <0.01* | <0.01* |
| P value, difference | 0.58 | 0.05 | <0.01* | ||||
| Fothergill et al. (“The Biggest Loser” equation) (9) | |||||||
| REEm, kcal/d | 1442.7 ± 192.7 | 1390.6 ± 261.8 | 1668.2 ± 275.3 | <0.01* | 0.24 | <0.01* | <0.01* |
| REEp, kcal/d | 1820.1 ± 306.0 | 1751.6 ± 363.5 | 1927.2 ± 323.9 | 0.10 | 0.39 | 0.19 | 0.03* |
| Difference, kcal/d | −377.3 ± 159.4 | −360.9 ± 137.2 | −259.0 ± 143.0 | <0.01* | 0.64 | <0.01* | 0.01* |
| P value, difference | <0.01* | <0.01* | <0.01* |
Values are means ± SDs unless otherwise indicated. Results are based on paired-samples t test for examining the difference between REEm and REEp within subject groups and 1-factor ANOVA for examining differences between subject groups. *Significant P values (α < 0.05). NC, normal-weight control; OC, control with overweight/obesity; REEm, measured resting energy expenditure; REEp, predicted resting energy expenditure; WLM, weight-loss maintainer.
One participant in the OC group did not have a dual-energy X-ray absorptiometry scan completed, so n = 32 for OCs.
DISCUSSION
Although strong biological pressures toward weight regain after weight loss have been identified (35, 36), our case-control study suggests that a substantial, disproportionately lower REE than predicted may not be a universal part of the biological pressure to regain weight after weight loss in all humans. Our primary findings are: 1) after adjusting for FFM and FM, REEm in WLMs was not different than REEm in either control group; 2) on average, REEm in WLMs was accurately predicted by equations determined from our weight-stable controls (±<1%) and by 3 standard predictive equations (±2–3%); 3) in WLMs, there was substantial interindividual variability in REEm – REEp, suggesting that some WLMs exhibit a lower REE than predicted, whereas other WLMs exhibit a higher REE than predicted; and 4)WLM with greater weight loss were more likely to exhibit a lower-than-predicted REE.
We found no significant differences in REEm between WLMs and controls after adjusting for FFM and FM, indicating that body composition is a primary driving force for the between-group differences in unadjusted REEm. All 3 predictive equations developed from our controls accurately predicted mean REEm in WLMs within ±1% (7–14 kcal/d), providing no evidence of a lower-than-predicted REE. In addition, no consistent pattern emerged from the use of 3 standard predictive equations to compare REEm with REEp in WLMs. Consistent with previous studies (37), the Harris and Benedict equation overestimated REEm in WLMs by ∼2%. The Mifflin equation underestimated REEm in WLMs by ∼3%. The organ size equation most accurately estimated REEm in WLMs (within 1%). Of note, the organ size equation underestimated REEm by ∼9% in OCs, potentially because this equation was validated in a sample of relatively normal-weight individuals (BMI 24.4 ± 4.1) and may not be generalizable to individuals with overweight/obesity. When REEp is within ±10% of REEm, the predictive equation is considered accurate (37), thus, all standard predictive equations accurately predicted REEm in all subject groups.
We also considered whether a lower-than-predicted REE might be present in a subset of WLMs. Interindividual differences in REEm – REEp (predicted from NCs) in WLMs ranged from −257 to +163 kcal/d (−16% to +12%). This variability is greater than the expected error in the accuracy of standard predictive equations for REE (±10%) (37), which suggests that some WLMs indeed exhibit a lower REE than predicted, whereas other WLMs exhibit a higher REE than predicted. A lower-than-predicted REE in WLMs was correlated with higher maximum lifetime BMI and greater maximum weight loss, but not with weight-loss maintenance duration. These correlations are consistent with previous longitudinal studies (9, 20, 38, 39) and reviews of AT (5, 40). Although the NCs-only equation explained a high degree (93%) of variance in REEm, correlations should be interpreted with caution because results could be due to the limitations of predictive equations. Future large prospective studies should explore additional factors that may contribute to interindividual differences in AT such as genetics, rate and method of weight loss, sex, race/ethnicity, physical activity level, and/or hormonal/metabolic profiles.
In contrast to the relative accuracy we observed for equations developed from our control groups as well as the 3 standard predictive equations, the predictive equation developed from “The Biggest Loser” contestants’ baseline data (9) substantially overestimated REEm (by 16–26%) in all 3 subject groups. Results from the current study differ from results from Fothergill et al. (9) in which “The Biggest Loser” contestants were observed to have a substantial and sustained reduction in REEm − REEp (−499 ± 207 kcal/d) at 6-y follow-up (9). This discrepancy may be due to several differences between the 2 studies including factors related to study design (case-control study of successful WLMs compared with longitudinal study of individuals participating in a televised weight-loss competition) as well as differences between study participants in regards to pre–weight loss BMI (33.0 ± 4.6 compared with 49.5 ± 10.1), amount of weight lost (26.7 ± 10.5 compared with 58.3 ± 24.9 kg), and rate of weight loss. It should also be noted that the intent of Fothergill et al. (9) was not to develop a generalizable predictive equation for REE.
Our results provide a more optimistic picture for individual obesity treatment. The resulting media coverage from “The Biggest Loser” study (22, 23) may instill a sense of futility of efforts to produce long-term weight loss. Although strong metabolic adaptations toward weight regain after weight loss likely exist (35, 36), our results suggest that a disproportionately low REE is not observed in all weight-reduced individuals. Further, despite our finding that a subset of WLMs do exhibit a lower-than-predicted REE, these individuals were still able to maintain a reduced body weight. Therefore, the existence of AT does not necessarily preclude successful achievement of long-term weight-loss maintenance. In addition, given the overwhelming evidence that suggests extremely high recidivism rates in obesity treatment (41), future research should explore additional metabolic or behavioral adaptations that occur in response to weight loss (e.g., changes in appetite/eating behavior, work efficiency, or thermic effect of food) (1), which may provide alternative drivers of weight regain.
Our study has several limitations. Because of the case-control study design, we were unable to examine within-person changes in REE longitudinally. Estimates of amount of weight loss and weight-loss maintenance duration in WLMs were based on self-reported data, which may have been overestimated. However, given that the maximum weight loss reported (∼26 kg) was twice the amount of the minimum entry criterion (13.6 kg) for WLMs, it is unlikely that our results were affected. Our sample did not include many individuals with BMI ≥ 40 and thus our findings are not generalizable to individuals with morbid obesity. Furthermore, our sample of 34 WLMs may have only included individuals who did not experience a significant degree of AT, which may in part explain their success. It is also possible that AT exists in WLMs but that these changes were masked by behavioral strategies (e.g., high levels of physical activity) that are commonly observed in successful WLMs (42). Future studies should explore factors that contribute to the success of WLMs such as whether chronically high levels of physical activity augment REE. Given our relatively homogeneous WLM sample (76% female, 100% Caucasian), our results are not generalizable to other populations. Our sample size was small and thus our study may lack power to detect a clinically meaningful difference. However, the 95% CI for REEm − REEp (predicted from NCs) in WLMs indicates that the possible value of the mean difference ranges from −49.1 to +21.2 kcal/d with 95% confidence (43). Although differences of this magnitude appear relatively small, they may be clinically important when extrapolated over time.
In conclusion, predictive equations developed from our control groups as well as 3 standard predictive equations accurately predicted REE in WLMs (within ±3%), indicating no consistent evidence of a lower-than-predicted REE in successful WLMs who have maintained a weight loss of 26.7 ± 10.5 kg over an average of 9.1 ± 9.2 y. However, there was large interindividual variability (REEm – REEp range: −257 to +163 kcal/d), suggesting that weight-reduced individuals may be a heterogeneous group, with some who experience AT and others who may not. Results from the current study suggest that sustained weight loss may not always result in a substantial, disproportionately low REE that inexorably predisposes individuals to regain weight. Future research should explore underlying reasons for individual variability in AT, additional metabolic adaptions that may drive the high recidivism rates in obesity treatment, and behavioral strategies to help counter these adaptations and prevent weight regain.
ACKNOWLEDGEMENTS
We thank Rena Wing for providing access to the NWCR to assist with recruitment of WLMs.
The authors’ contributions were as follows—VAC, ELM, HRW, and JOH: conceived of and designed the study; VAC: conducted the research; ZP and DMO: performed the statistical analysis; DMO, ELM, AEC, SAC, PSM, JOH, and VAC: wrote the manuscript; DMO: had primary responsibility for final content; and all authors: assisted with data interpretation and read and approved the final manuscript. JOH and HRW report stock options from Retrofit, and are partners in Shakabuku LLC, companies who provide weight management services, outside the submitted work; JOH and HRW have been issued a patent on the “Energy Gap”. HRW accepts personal fees as an advisory board member for Atkins, a low-carbohydrate weight-loss program. None of the other authors reported a conflict of interest related to this study.
Notes
Supported by NIH grants K23 DK078913 (to VC), P30 DK048520 (to VC) and T32 HL116276 (to AC and SC), NIH/National Center for Advancing Translational Sciences (NCATS) Colorado Clinical and Translational Science Awards (CTSA) grant UL1 TR001082, as well as American Heart Association grant 16PRE29660012 (to DO).
Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations used:
- AT
adaptive thermogenesis
- FFM
fat-free mass
- FM
fat mass
- NC
normal-weight controls
- NWCR
National Weight Control Registry
- OC
controls with overweight/obesity
- REE
resting energy expenditure
- REEm
measured resting energy expenditure
- REEp
predicted resting energy expenditure
- WLM
weight-loss maintainer
REFERENCES
- 1. Melby CL, Paris HL, Foright RM, Peth J. Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up?. Nutrients. 2017;9(5):E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacLean PS, Bergouignan A, Cornier MA, Jackman MR. Biology's response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R581–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1306–15. [DOI] [PubMed] [Google Scholar]
- 4. Dulloo AG. Suppressed thermogenesis as a cause for resistance to slimming and obesity rebound: adaptation or illusion?. Int J Obes (Lond). 2007;31(2):201–3. [DOI] [PubMed] [Google Scholar]
- 5. Muller MJ, Bosy-Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring). 2013;21(2):218–28. [DOI] [PubMed] [Google Scholar]
- 6. Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond). 2010;34(Suppl 1):S47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phelan S, Wing RR, Loria CM, Kim Y, Lewis CE. Prevalence and predictors of weight-loss maintenance in a biracial cohort: results from the Coronary Artery Risk Development in Young Adults study. Am J Prev Med. 2010;39(6):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keys A, Brozek J, Henschel A, Mickelsen O, Taylor H. The biology of human starvation. Minneapolis: The University of Minnesota Press; 1950. [Google Scholar]
- 9. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, et al.. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring). 2016;24(8):1612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dulloo AG, Calokatisa R. Adaptation to low calorie intake in obese mice: contribution of a metabolic component to diminished energy expenditures during and after weight loss. Int J Obes. 1991;15(1):7–16. [PubMed] [Google Scholar]
- 11. Dulloo AG, Girardier L. Adaptive changes in energy expenditure during refeeding following low-calorie intake: evidence for a specific metabolic component favoring fat storage. Am J Clin Nutr. 1990;52(3):415–20. [DOI] [PubMed] [Google Scholar]
- 12. Dulloo AG, Girardier L. 24 hour energy expenditure several months after weight loss in the underfed rat: evidence for a chronic increase in whole-body metabolic efficiency. Int J Obes Relat Metab Disord. 1993;17(2):115–23. [PubMed] [Google Scholar]
- 13. MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R288–97. [DOI] [PubMed] [Google Scholar]
- 14. MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1577–88. [DOI] [PubMed] [Google Scholar]
- 15. Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69(6):1117–22. [DOI] [PubMed] [Google Scholar]
- 16. Doucet E, St-Pierre S, Almeras N, Despres JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. Br J Nutr. 2001;85(6):715–23. [DOI] [PubMed] [Google Scholar]
- 17. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. [DOI] [PubMed] [Google Scholar]
- 18. Weigle DS, Sande KJ, Iverius PH, Monsen ER, Brunzell JD. Weight loss leads to a marked decrease in nonresting energy expenditure in ambulatory human subjects. Metabolism. 1988;37(10):930–6. [DOI] [PubMed] [Google Scholar]
- 19. Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97(7):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97(5):990–4. [DOI] [PubMed] [Google Scholar]
- 21. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–12. [DOI] [PubMed] [Google Scholar]
- 22. Kolata G. That lost weight? The body finds it. The New York Times; 2016 May 2. p. 1. [Google Scholar]
- 23. Smith P. ‘Biggest Loser’ study shows how bodies fight against weight loss. NBC; 2016. [cited 2017 May 29] Available from: https://www.nbclosangeles.com/news/health/Research-Shows-Your-Body-Fights-Against-You-Losing-Weight-377841901.html. [Google Scholar]
- 24. Amatruda JM, Statt MC, Welle SL. Total and resting energy expenditure in obese women reduced to ideal body weight. J Clin Invest. 1993;92(3):1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larson DE, Ferraro RT, Robertson DS, Ravussin E. Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr. 1995;62(4):735–9. [DOI] [PubMed] [Google Scholar]
- 26. Weinsier RL, Hunter GR, Zuckerman PA, Darnell BE. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women. Obes Res. 2003;11(8):937–44. [DOI] [PubMed] [Google Scholar]
- 27. Wyatt HR, Grunwald GK, Seagle HM, Klem ML, McGuire MT, Wing RR, Hill JO. Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am J Clin Nutr. 1999;69(6):1189–93. [DOI] [PubMed] [Google Scholar]
- 28. Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72(5):1088–94. [DOI] [PubMed] [Google Scholar]
- 29. Knuth ND, Johannsen DL, Tamboli RA, Marks-Shulman PA, Huizenga R, Chen KY, Abumrad NN, Ravussin E, Hall KD. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring). 2014;22(12):2563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–46. [DOI] [PubMed] [Google Scholar]
- 31. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 33. Koehler K, Williams NI, Mallinson RJ, Southmayd EA, Allaway HC, De Souza MJ. Low resting metabolic rate in exercise-associated amenorrhea is not due to a reduced proportion of highly active metabolic tissue compartments. Am J Physiol Endocrinol Metab. 2016;311(2):E480–7. [DOI] [PubMed] [Google Scholar]
- 34. Hayes M, Chustek M, Wang Z, Gallagher D, Heshka S, Spungen A, Bauman W, Heymsfield SB. DXA: potential for creating a metabolic map of organ-tissue resting energy expenditure components. Obes Res. 2002;10(10):969–77. [DOI] [PubMed] [Google Scholar]
- 35. Purcell K, Sumithran P, Prendergast LA, Bouniu CJ, Delbridge E, Proietto J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2(12):954–62. [DOI] [PubMed] [Google Scholar]
- 36. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. [DOI] [PubMed] [Google Scholar]
- 37. Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105(5):775–89. [DOI] [PubMed] [Google Scholar]
- 38. Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity?. Obes Rev. 2012;13(Suppl 2):105–21. [DOI] [PubMed] [Google Scholar]
- 39. Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Gluer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr. 2015;102(4):807–19. [DOI] [PubMed] [Google Scholar]
- 40. Muller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5(4):413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–84. [DOI] [PubMed] [Google Scholar]
- 42. Catenacci VA, Grunwald GK, Ingebrigtsen JP, Jakicic JM, McDermott MD, Phelan S, Wing RR, Hill JO, Wyatt HR. Physical activity patterns using accelerometry in the National Weight Control Registry. Obesity (Silver Spring). 2011;19(6):1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121(3):200–6. [DOI] [PubMed] [Google Scholar]