Abstract
Peripheral blood is the prefered source for hematopoietic stem cells for hematopoietic stem cell transplantation. The efficiency of peripheral blood stem cell (PBSC) collection can vary among devices. In this study we aimed to compare feasibility and effectivity of apheresis procedures of the different systems. Two apheresis systems [Com.Tec (Fresenius Healthcare) and Spectra Optia (Caridian BCT)] were used in our center for the collection of PBSCs for autologous and allogeneic transplantation. We retrospectively analysed 190 apheresis procedures performed in healthy donors and patients between June 2012 and November 2014 in Department of Hematology, Dokuz Eylul University. PBSCS were collected by Fresenius cell separator (64 procedure) or Spectra Optia cell separator (126 procedure). Mobilization treatments were G-CSF (26.8%), cyclophosphamide plus G-CSF (48.4%), prelixafor plus G-CSF (14.7%), ESHAP (10%) and others. Patient and donor characteristics (age, weight, volume processed, disease, mobilization regimes) were similar in Fresenius and Spectra Optia apheresis groups. Altough both collected PBSCs efficiently, the amount of CD34+ cell in product collected by Spectra Optia device was significantly higher (p < 0.05) and product volume was lower than Fresenius Com.Tec significantly (p < 0.05). “CD34+ collection efficiency” with Spectra Optia was significantly higher than Fresenius Com.Tec (CE2: 87%, 70%, p = 0.033) regarding all procedures. High collection efficiency and low product volume may be a significant characteristic of Spectra Optia device (mean 187 mL, product CD34+ cell: 1576 µL).
Keywords: Apheresis, Peripheral blood stem cell, Autologous transplantation, Allogeneic transplantation, Blood cell seperators
Introduction
Autologous and allogeneic peripheral blood stem cell (PBSC) transplantation are used frequently in hematological malignancies. In recent years, peripheral blood became the preferred source for hematopoietic stem cells for HSCT [1–4]. Collection of stem cells from peripheral blood is a cheaper, painless procedure and hematological recovery is faster [5]. It can be performed using automated or semi automated devices [6]. Patients are generally mobilized after granulocyte-colony stimulating factor (G-CSF) alone or with plerixafor, or chemotherapy plus G-CSF [7, 8]. For successful mobilization, age, diagnosis, stage of disease, type of chemotherapy and radiotherapy, the amount of peripheral CD34+ cells are important.
However, the performance of the devices can also affect the success of mobilization. Therefore, it is good to know the collection efficiency (CE) of the machines being used. Simply, the CE is the number of cells that is collected from the total number of cells processed by the apheresis machine and can be calculated by various formulae. If we know the platelet count prior and after the apheresis procedure and also the number of platelets in the collection bag, CE1 formula can be used. When the post apheresis platelet count is not known as in our study, CE2 formula can be applied [9]. Knowing mean CE of a machine also enables us to calculate required volume of blood needed to collect a specific number of stem cells.
In this study, we examined the efficiency of stem cell apheresis with two different seperators (Fresenius Com.Tec cell and Spectra Optia) and presented the factors affecting results for contribution to the literature.
Materials and Methods
We retrospectively evaluated a total of 190 apheresis data from 97 patients and healthy donors whose hematopoietic stem cell apheresis procedures were performed in apheresis unit of Dokuz Eylul University between June 2012 and November 2014 with Fresenius Com.Tec and Spectra Optia. Apheresis was performed according to pre-apheresis CD34+ cell counts by flow cytometric analysis of peripheral blood sample of patients and donors. If peripheral blood CD34+ cell count was higher than 10 µL, apheresis was performed for the patient. The demographic features, diagnosis, peripheral blood CD34+ cell values, post-apheresis product CD34+ cell counts and product volume, pre-apheresis complete blood counts of patients and healthy donors, number of apheresis procedures and device type were recorded. CE2 of CD34+ cells was calculated as the number of CD34+ cells collected in the product bag divided by the number of CD34+ cells presented to the collection nozzle, asuming stable pre-apheresis CD34+ cell concentrations over the time of apheresis by using the following formula:
Statistical Analysis
Normally distributed variables were expressed as mean ± SD, and non- normally distributed variables were expressed as median (range). Categorical variables were expressed as frequency (percentage). Independent samples t test and Mann–Whitney U tests were used to compare normally and non-normally distributed variables among independent groups. Categorical variables were compared by the Chi square test. Correlation analyses were performed by using either Pearson’s or Spearman’s coefficients accordingly. Statistical analysis was performed by using the Statistical Package of Social Science (SPSS Inc, Chicago, IL, USA), version 15.0 for Windows. A two-tailed p value less than 0.05 was accepted as statistically significant.
Results
A hundred and ninety collections of 97 patients and healthy donors were investigated, 38.9% of the patients were female and 61.1% of the patients were male. The percentage of patients under 18 years of age was 3.2%; between 18 and 60 age was 70%, > 60 age was 26.8%, median age was 49 (6–71), median height was 167.5 cm, median weight was 74 kg. At apheresis time, mean laboratory values were as follows: hemoglobin (hb) 11.1 g/dL (7.1–16 g/dL), white blood cell count (wbc) 24.8 × 103/µL (1.7 × 103/µL–82.3 × 103/µL), platelet (plt) 116 × 103/µL (16 × 103/µL–316 × 103/µL).
Diagnosis of patients were as follows: 42.1% multiple myeloma, 23.7% non hodgkin lymphoma, 13.7% hodgkin lymphoma, 9.5% acute myeloid leukemia (AML), 8.4% acute lymphocytic leukemia (ALL) and 2.6% others. Whereas stem cell apheresis for autologous transplantation was performed in 80% of all collections, for allogeneic stem cell transplantation in 20% (Table 1). Mobilization regimens were G-CSF (n: 51, 26.8%), cyclophosphamide plus G-CSF (n: 92, 48.4%), prelixafor plus G-CSF(n: 28, 14.8%) and ESHAP (Etoposide, Methylprednisolone, Cytarabine, Cisplatine) and others (n: 19, 10%).
Table 1.
Demographic and clinical characteristics of patients and donors
| Patients (autologous) n = 151 (%) | Donors (for allogeneic transplantation) n = 39 (%) | |
|---|---|---|
| Age (median) | 52 ± 12 | 40 ± 14 |
| Disease | ||
| Multiple myeloma | 80 (53) | 0 (0) |
| Non-hodgkin lymphoma | 46 (30.5) | 1 (2.6) |
| Hodgkin lymphoma | 19 (12.6) | 2 (5.2) |
| Acute myeloid leukemia | 0 (0) | 18 (46.1) |
| Acute lymphoblastic leukemia | 0 (0) | 17 (43.6) |
| Medullablastoma | 4 (2.6) | 0 (0) |
| Myelodysplastic syndrome | 0 (0) | 1 (2.6) |
| Neuroblastoma | 1 (1.3) | 0 (0) |
| Mobilization regimens | ||
| G-CSF alone | 13 (8.6) | 39 (100) |
| G-CSF+ chemotherapy | 95 (62.9) | 0 (0) |
| G-CSF+ plerixafor | 24 (5.9) | 0 (0) |
| ESHAP and other | 19 (12.6) | 0 (0) |
The devices used in apheresis were Fresenius Com.Tec (64 process) (33.7%) and Spectra Optia (126 process) (66.3%). Apheresis processes were performed in 53.8% of patients from jugulary vein catheter, in 46.2% of patients from peripheral venous access. All patients received an intravenous infusion of 10% calcium gluconate during the procedure. All apheresis procedures were well tolerated. No side effects were observed. No positive microbial cultures were detected in all the samples analyzed.
We found a statistically significant relation between WBC at the beginning of the apheresis and CD34+ cell value (p = 0.037). In patients whose WBC were high, high product CD34+ cells were collected (p = 0.001). There was also a significant relation between initial platelet and product CD34+ cell count (p = 0.009). Age and sex of patients didn’t affect CD34+ cell count. Pre-apheresis donor CD+ 34 cell counts and product CD34+ cell counts were correlated (p < 0.05). There wasn’t a statistically significant difference regarding the number of CD34+ cell/kg in the product between the Fresenius Com.Tec and the Spectra Optia (3.2 × 106 vs. 3.75 × 106 p = 0.12) (Table 2). However, the mean volume of ACD used in collections in the Fresenius Com.Tec was less than Spectra Optia (p = 0.001).
Table 2.
Leukapheresis collection and product data
| Fresenius Com.Tec | Spectra Optia | p value | |
|---|---|---|---|
| Number of collection (n) | 64 | 126 | p = 0.11 |
| Age at collection | 50.1 ± 14.6 | 49.6 ± 13.5 | p = 0.08 |
| Weight at collection (kg) | 74.9 ± 12 | 73.7 ± 16 | p = 0.94 |
| Number of apheresis per case | 2 ± 1 | 2 ± 1 | p = 0.06 |
| ACD used (mL)* | 564 ± 118 | 802 ± 207 | p = 0.001 |
| Total blood volume (mL)* | 4.1 ± 0.9 | 2.1 ± 0.4 | p = 0.001 |
| Processed blood volume (L)* | 7.6 ± 1.4 | 10.5 ± 1.1 | p = 0.04 |
| Plasma volume (mL) | 103 ± 28 | 98 ± 28 | p = 0.22 |
| Product volume (mL)* | 231 ± 58 | 185 ± 61 | p = 0.039 |
| Collection time (min)* | 171 ± 30 | 226 ± 37 | p = 0.01 |
| Product WBC × 103 (µL)* | 215 (60–500) | 232 (60–570) | p = 0.027 |
| Product Hct (%)* | 0.61% (0.3–3.6%) | 1.24% (0.3–3.1%) | p = 0.001 |
| Peripheral CD34+ cell count (µL) | 52 ± 36 | 48 ± 53 | p = 0.13 |
| CD34+ cell count in Product (µL)* | 1105 ± 1164 | 1576 ± 1475 | p = 0.01 |
| CD34+ cells infused (106/kg) | 3.2 ± 3.7 | 3.75 ± 3.7 | p = 0.41 |
| CD34+ collection efficiency 2 (CE2) %* | 70% (3–200%) | 87% (6–390%) | p = 0.033 |
*p < 0.05 is significant
When relationship between product CD34+ cell collected and type of separator was analysed, product CD34+ cell collected via Spectra Optia device was found significantly high (Table 2, p = 0.011). Processed blood volumes were greater by Fresenius Com.Tec compared to Spectra Optia (Table 2, p = 0.001). Spectra Optia enabled more CD34+ cell collection by less volume. Product quality data of autologous apheresis collections are summarized in Table 2. The small product volume with significantly greater CD34+ cell product was achieved with Spectra Optia comparing to Fresenius Com.Tec (p = 0.039). However; we found statistically significant relationship between initial wbc, plt count and product CD34+ cell value in all collections. Processed blood volume was higher with Spectra Optia.
Product quality data of autologous apheresis collections and allogeneic collections are summarized in Tables 3 and 4. The numbers of apheresis procedures with donor collections were less than autologous collections. Altough product CD34+ cell count was similar for the two devices, product volume was lower and used ACD volume was higher with Spectra Optia device (p = 0.037). Product Hct values were higher by Fresenius Com.Tec (p = 0.001). Product wbc values were similar between Spectra Optia and Fresenius Com.Tec.
Table 3.
Characteristics of patient’s apheresis
| Spectra Optia | Fresenius Com.Tec | p value | |
|---|---|---|---|
| WBC × 103/µL | 18.85 (1.7–82.3) | 21.2 (2.1–63.0) | p = 0.37 |
| PLT × 103/µL | 90.5 (16–229) | 107 (33–300) | p = 0.08 |
| Procedure time (min) | 227 (136–358) | 174 (112–259) | p = 0.001 |
| Number of apheresis per case | 2.3 (1–5) | 2.3 (1–4) | p = 0.63 |
| ACD used (mL) | 806 (336–1630) | 580 (319–925) | p = 0.001 |
| Total blood volume (mL) | 2.1 (1.3–4.8) | 4.2 (2–5.2) | p = 0.001 |
| Product volume (mL) | 177 (28–360) | 220 (40–330) | p = 0.001 |
| Processed blood volume (L) | 11,002 (3697–101,269) | 7717 (4715–12,015) | p = 0.07 |
| Peripheral CD34+ cell count (µL) | 46.68 (1.2–356) | 49 (4.2–142) | p = 0.77 |
| CD34+ cell count in Product (µL) | 1627 (32–8207) | 1155 (0.2–6474) | p = 0.018 |
| CD34+ cells infused (106/kg) | 3.69 (0.1–26.1) | 3.3 (0.2–19.4) | p = 0.58 |
| Product WBC × 103/µL | 280 (60–500) | 212 (60–570) | p = 0.02 |
| Product Hct % | 0.58% (0.1–2%) | 1.17% (0.1–3%) | p = 0.001 |
| CD34+ collection efficiency 2 (CE2) % | 89% (6–390%) | 71% (3–200%) | p = 0.07 |
Table 4.
Characteristics of donor apheresis
| Spectra Optia | Fresenius Com.Tec | p value | |
|---|---|---|---|
| WBC × 103/µL | 39.0 (7.6–64.6) | 36.7 (8.2–62.7) | p = 0.7 |
| PLT × 103/µL | 172 (85–282) | 199 (103–336) | p = 0.14 |
| Procedure time (min) | 220 (157–306) | 165 (126–228) | p = 0.001 |
| Number of apheresis per case | 2.1 (1–3) | 2.7 (1–5) | p = 0.11 |
| ACD used (mL) | 784 (579–1077) | 518 (337–807) | p = 0.001 |
| Total blood volume (mL) | 2 (1.7–2.5) | 3.9 (1.6–5) | p = 0.01 |
| Product volume (mL) | 224 (100–360) | 265 (210–360) | p = 0.026 |
| Processed blood volume (L) | 8724 (6302–11,946) | 7391 (5620–11,360) | p = 0.016 |
| Peripheral CD34+ cell count (µL) | 53.7 (14.3–120) | 62.9 (11–120) | p = 0.11 |
| CD34+ cell count in Product (µL) | 1347.5 (267–2675) | 958 (51.7–2643) | p = 0.001 |
| CD34+ cells infused (106/kg) | 4 (0.76–8.6) | 3.2 (0.1–7.9) | p = 0.19 |
| Product WBC × 103/µL | 275 (96–537) | 255 (140–350) | p = 0.43 |
| Product Hct % | 1.36% (0.4–3.6%) | 0.7% (0.4–3.6%) | p = 0.024 |
| CD34+ collection efficiency 2 (CE2) % | 66% (3–250%) | 79% (2–200%) | p = 0.34 |
CD34+ CE2 value with Spectra Optia was significantly higher than Fresenius Com.Tec in all total apheresis procedures (CE2 optia × Com.Tec: 87% × 70%, p = 0.033). However, there was no significant difference among the two devices regarding CE2 of autologous and allogeneic apheresis procedures independently (p = 0.078, p = 0.34). CD34+ CE2 value with Spectra Optia was significantly higher than Fresenius Com.Tec in mobilization by G-CSF+ chemotherapy (p = 0.026) and CE2 were similar with another mobilization regimens (p = 0.2).
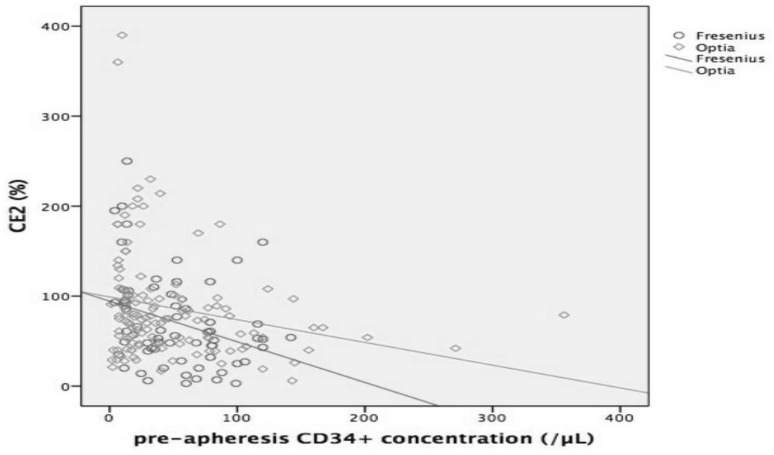
CD34+ CE2 value with Spectra Optia was significantly higher than Fresenius Com.Tec when pre-apheresis CD34+ cell value was > 30 µL (p = 0.003). CE2 was similar between two devices when pre-apheresis CD34+ cell value was < 30 µL. (Figure 1). Likewise when pre-apheresis CD34+ cell value was < 20 µL or between 20 and 30 µL there was no significant difference between two devices in terms of CE2.
Fig. 1.
High pre-apheresis CD34+ cell values correlate with collection efficiency (CE2). CD34+ cell values are plotted on the X-axis and CE2 are on the Y-axis, Spectra Optia: green rings, Fresenius: blue rings
Discussion
In this study, we compare the performance characteristics between Fresenius Com.Tec and Spectra Optia cell separators for peripheral stem cell collections.
In the study of Altuntas et al., the effectiveness of Amicus and Fresenius cell separators for peripheral blood progenitor cell collection was investigated. The median product volume was found higher with the Fresenius Com.Tec and there wasn’t a significant difference between the two for the number of CD34+ cell/kg in the product. The product volume was lower with Amicus [4]. In our study we compared the Fresenius and Spectra Optia cell separators for peripheral blood progenitor cell collection. There wasn’t a significant difference for the number of CD34+ cell/kg in the product and the median product volume was lower with Optia.
Dihenescikova VR et al. evaluated 169 collection procedures with 2 types of apheresis devices (Amicus and Cobe Spectra). Cobe Spectra processed larger blood volumes with higher CD34+ cell collection efficiency compared to Amicus [5]. Kim et al. compared the Fresenius Com.Tec and COBE Spectra cell separators for PBSC in pediatric patients. PBSC collections by the Com.Tec separator had avantages like high processing rate, low product volume and low contamination by platelets of product. The median CE was less than 60% in both groups (50 and 48.4%) [10].
Bambi et al. evaluated three apheresis machines in children: MCS 3P (Haemonetics-10 procedures), Spectra (Cobe-8 procedures) and AS104 (Fresenius-24 procedures). All apheresis machines seem to have comparable PBSC extraction with no significant differences in CD34+ cell extraction [11].
In the study of Flommersfeld et al., they compared 186 apheresis procedures and effectiveness of the three different apheresis systems for autologous and allogeneic stem cell collections (Fresenius, COBE Spectra and Spectra optia). 131 autologous and 56 allogeneic apheresis procedures were evaluated. Spectra Optia had high collection efficiency and low product volume. The best collection efficiency was seen with spectra Optia regarding the autologous collections. There was a negative relationship between the CD34+ cell/kg and total processed volume in all devices. There was no significant correlation between initial pre-apheresis wbc counts and collection efficiency. However, in our study we observed a correlation between initial pre-apheresis wbc counts and collection efficiency. Similarly, high collection efficiency and low product volume favoured Spectra Optia [12].
In the study of Brauninger et al., two systems for the allogeneic donor peripheral blood “stem cell” apheresis were compared prospectively. They reported that, the mean CD34+ cell collection efficiency was 7.9% greater with the Spectra Optia compared with COBE Spectra [13].
In another study, usability and function of automatic interface control of Spectra Optia were compared to COBE Spectra [14]. Most collection parameters, including collection efficiency (Spectra Optia or COBE Spectra, slightly greater than % 40) were similar. Platelet loss with Spectra Optia was 25% less than with COBE Spectra MNC. Products contained fewer erythrocytes, but more granulocytes.
It is well reported that the circulating CD34+ cell/µL is correlated with the product CD34+ cell/µL [15, 16]. Our study showed that there is a correlation between circulating and product CD34+ cell counts for the two devices.
Several previous studies showed that a value greater than 20/µL for circulating CD34+ cell count was a predictor of progenitor cell yield [17]. In our study, minimum required CD34 count is 10/µL for the effective collection. CE2 was % 92 in the pre-apheresis when CD34+ cell value < 20 µL and there was no significant difference among the two devices. Additionally, CE2 was similar when pre-apheresis CD34+ cell value was between 20 and 30 µL regarding the two devices. However, CE2 with Spectra Optia was significantly higher than Fresenius Com.Tec regarding all total apheresis procedures.
Some studies showed a correlation between the absolute number of circulating CD34+ cells with the total number of CD34 cells in the product [18–20]. Also in our study; pre-apheresis CD34+ cell count and product CD34+ cell values was well correlated. The number of procedures was less when pre-apheresis CD34 cell count was high.
Conclusion
Successful collection of a product depends on diagnosis of the patient, pre-treatment CD34 level, pretreatment wbc, platelet levels, device types used for apheresis. High collection efficiency and low product volume are favorable characteristics of Spectra Optia.
Acknowledgements
Professional medical writing support and editor assistance were not supported by the company.
Compliance with Ethical Standards
Conflict of interest
The author did not receive financial compensation for authoring the manuscript.
Contributor Information
Serife Solmaz, Phone: (90) 232 2505050, Email: solmazserife@yahoo.com.
Selda Kahraman, Email: sel_ad@yahoo.com.
Omur Gokmen Sevindik, Email: omurgok17@hotmail.com.
Celal Acar, Email: celalacar@yahoo.com.
Munire Turkyilmaz, Email: munire_turk@hotmail.com.
Inci Alacacioglu, Email: inci074@yahoo.com.
Ozden Piskin, Email: ozden.piskin@deu.edu.tr.
Mehmet Ali Ozcan, Email: mehmet.ozcan@deu.edu.tr.
Hayri Guner Ozsan, Email: hayri.ozsan@deu.edu.tr.
Bulent Undar, Email: bulent.undar@deu.edu.tr.
Fatih Demirkan, Email: fatih.demirkan@deu.edu.tr.
References
- 1.Grathwohl A, Baldomero H, Schmid O, Horisberger B, Bargetzi M, Urbano-Ispizua A. Change in stem cell source for hematopoietic stem cell transplantation (HSCT) in Europe: a report of the EBMT activity survey 2003. Bone Marrow Transpl. 2005;36:575–590. doi: 10.1038/sj.bmt.1705104. [DOI] [PubMed] [Google Scholar]
- 2.Stem Cell Trialists Collaborative Group Allogeneic peripheral blood stem cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn SK, Kim JG, Baek JH, Lee KB. Diverse clinical application using advantages of allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2004;79:457–461. doi: 10.1532/IJH97.A10313.4. [DOI] [PubMed] [Google Scholar]
- 4.Altuntaş F, Koçyigit I, Ozturk A, Kaynar L, Oztekin M, Solmaz M, Eser B, Cetin M, Unal A. Comparison of the Fenwal Amicus and Fresenius Com.Tec cell separators for autologous peripheral blood progenitor cell collection. Transfus Apher Sci. 2007;36:159–167. doi: 10.1016/j.transci.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Dihenescikova VR, Mistrik M, Martina J, Zwiewka M, Bizikova I, Batorova A. Collection of peripheral hematopoietic stemprogenitor cells. Bratis Lek Listy. 2015;116:9–13. doi: 10.4149/BLL.2015.002. [DOI] [PubMed] [Google Scholar]
- 6.Adorno G, Del Proposto G, Palombi F, Bruno A, Ballatore G, Postorino M, Tednas A, Del Poeta G, Isacchi G, Amadori S. Collection of peripheral progenitor cells: a comparison between Amicus and Cobe-Spectra blood cell separators. Transfus Apher Sci. 2004;30:131–136. doi: 10.1016/jtransci.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Bachier C, Potter J, Potter G, Sugay R, Shaughnessy P, Chan K, Jude V, Madden R, LeMaistre C. High white blood cell concentration in the peripheral blood stem cell product can induse seizures during infusion of autologous peripheral blood stem cells. Am Soc Blood Marrow Transpl. 2012;18:1055–1060. doi: 10.1016/j.bbmt.2011.12.500. [DOI] [PubMed] [Google Scholar]
- 8.Ozkan MC, Sahin F, Saydam G. Peripheral blood stem cell mobilization from healthy donors. Transfus Apher Sci. 2015;53:13–16. doi: 10.1016/j.transci.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Neyrinck M, Virielink H. Calculations in apheresis. J Clin Apher. 2015;30:38–42. doi: 10.1002/jca.21347. [DOI] [PubMed] [Google Scholar]
- 10.Kim SR, Choung HK, Kim DW, Sung KW, Kang ES. Evaluation of a new cell separator for collection of peripheral blood CD34 + progenitor cells in pediatric patients. Transfusion. 2011;51:306–312. doi: 10.1111/j.1537.2995.2010.02864. [DOI] [PubMed] [Google Scholar]
- 11.Bambi F, Faulkner LB, Azzari C, Gelli AM, Tamburini A, Tintori V, Lippi AA, Tucci F, Bernini G, Genovese F. Pediatric peripheral blood progenitor cell collection: haemonetics MSC3P versus COBE Spectra versus Fresenius AS104. Transfusion. 1998;38:70–74. doi: 10.1046/j.1537-2995.1998.38198141501.x. [DOI] [PubMed] [Google Scholar]
- 12.Flommersfeld S, Backchoul T, Bein G, Wachtel A, Loechelt C, Sachs UJ. A single center comparison between three different apheresis systems for autologous and allogenei stem cell collection. Transfus Apher Sci. 2013;49:428–433. doi: 10.1016/jtransci.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Brauninger S, Bialleck H, Thorausch K, Felt T, Seifried E, Bonig H. Allogeneic donor peripheral blood “stem cell” apheresis: prospective comparison of two apheresis systems. Transfusion. 2012;52:1137–1145. doi: 10.1111/j.1537.2995.2011.03414. [DOI] [PubMed] [Google Scholar]
- 14.Brauninger S, Bialleck H, Thorausch K, Felt T, Seifried E, Bonig H. Mobilized allogeneic peripheral stem/progenitor cell apheresis with Spectra Optia v. 5.0, a novel, automatic interface—controlled apheresis system: results from the first feasibility trial. Vox Sang. 2011;101:237–246. doi: 10.1111/j.1423.0410.2011.01484. [DOI] [PubMed] [Google Scholar]
- 15.Wu F, Heng K, Salleh RB, Soh TG, Lee J, et al. Comparing peripheral blood stem cell collection using the COBE Spectra, Haemonetics MCS and Amicus. Trans Apher Sci. 2012;47:345–350. doi: 10.1016/j.transci.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Basquiera AL, Abichain P, Damonte JC, et al. The number of CD34 (+) cells in peripheral blood as a predictor of the CD34+ yield in patients going to autologus stem cell transplantation. J. Clin Apher. 2006;21:92–95. doi: 10.1002/jca.20062. [DOI] [PubMed] [Google Scholar]
- 17.Mohle R, Murea S, Pforsich M, et al. Estimated of the progenitor cell yield in a Leucapheresis product by previous measurement of CD34+ cell in the peripheral blood. Vox Sang. 1996;71:90–96. doi: 10.1046/j.1423-0410.1996.7120090.x. [DOI] [PubMed] [Google Scholar]
- 18.Fante CD, Perotti C, Viarengo G, Bellotti L, Parisi C, Marchesi A, et al. Clinical impact of a new automated system employed for perpheral blood stem cell collection. J. Clin Apher. 2006;21:227–232. doi: 10.1002/jca.20102. [DOI] [PubMed] [Google Scholar]
- 19.Ford C, Chan K, Reilly W, Peterson F. An evaluation of predictive factors for CD34 cell harvest yields from patients mobilized with cheomatherapy and growth factors. Transfusion. 2003;43:622–625. doi: 10.1046/j.1537.2995.2003.0037. [DOI] [PubMed] [Google Scholar]
- 20.Schots R, Van Riet I, Damiaens S, Flament J, Lacor P, Staelens Y, et al. The absolute number of circulating CD34 cells predicts the number of hematopoetic stem cells than can be predict by apheresis. Bone Marrow Transpl. 1996;17:509–515. [PubMed] [Google Scholar]