Abstract
Although mesenchymal stem cells (MSCs) transplantation has been shown to promote the lung respiration in acute lung injury (ALI) in vivo, its overall restorative capacity appears to be restricted mainly because of low retention in the injured lung. Angiotensin II (Ang II) are upregulated in the injured lung. Our previous study showed that Ang II increased MSCs migration via Ang II type 2 receptor (AT2R). To determine the effect of AT2R in MSCs on their cell migration after systemic injection in ALI mice, a human AT2R expressing lentiviral vector and a lentivirus vector carrying AT2R shRNA were constructed and introduced into human bone marrow MSCs. A mouse model of lipopolysaccharide‐induced ALI was used to investigate the migration of AT2R‐regulated MSCs and the therapeutic potential in vivo. Overexpression of AT2R dramatically increased Ang II‐enhanced human bone marrow MSC migration in vitro. Moreover, MSC‐AT2R accumulated in the damaged lung tissue at significantly higher levels than control MSCs 24 and 72 hours after systematic MSC transplantation in ALI mice. Furthermore, MSC‐AT2R‐injected ALI mice exhibited a significant reduction of pulmonary vascular permeability and improved the lung histopathology and had additional anti‐inflammatory effects. In contrast, there were less lung retention in MSC‐ShAT2R‐injected ALI mice compared with MSC‐Shcontrol after transplantation. Thus, MSC‐ShAT2R‐injected group exhibited a significant increase of pulmonary vascular permeability and resulted in a deteriorative lung inflammation. Our results demonstrate that overexpression of AT2R enhance the migration of MSCs in ALI mice and may provide a new therapeutic strategy for ALI. Stem Cells Translational Medicine 2018;7:721–730
Keywords: Acute lung injury, Mesenchymal stem cells, AT2R, Migration, Lentiviral vector
Significance Statement.
Patients suffering from acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) experience a high rate of mortality. Mesenchymal stem cells (MSCs) exerted therapeutic effects in preclinical models of ARDS. However, the therapeutic effect of MSC‐based therapeutics relies on its ability to reach the sites of injured lung. Results of this study showed that AT2R overexpression could increase MSC migration to the injured lung in ALI mice. Meanwhile, the presence of more MSCs at the site of injury improve the therapeutic effect of MSCs on ARDS. This finding provided a new target to improve stem cell therapy in clinical settings.
INTRODUCTION
Over the past few years mesenchymal stem cells (MSCs) have been extensively investigated for their potential in developing cell base therapies for the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) 1, 2. Recently, it is encouraging that MSCs have been reported to be clinical safe for patients with ARDS 3. However, the therapeutic effect of MSC‐based therapeutics to clinical practice has been limited by questions including the mechanisms of MSC homing and ultimate function 4, 5, 6.
The utility of the MSCs in the treatment of ALI is mainly dependent on its ability to reach the sites of injured lung. In the pathogenesis of ALI, inflammation and the development of lung tissue damage results in large part from the mobilization of inflammatory cells by cytokines. Evidence has demonstrated that the renin‐angiotensin system involved in the pathophysiology of ARDS 7 and ALI results in increased production of angiotensin II (Ang II) 8, 9. The mediators that promote inflammation might also be the same molecules that attract MSCs to the site of tissue injury. Furthermore, we have found that Ang II promote the migration of MSCs in vitro mainly by interaction with AT2R in our previous study 10. However, the literature showed that chemokine receptors expression on MSCs decrease as cells are expanded in vitro 11, ultimately limiting their benefit in vivo.
To improve MSCs migration to the lung and the therapeutic potential of MSCs in ALI, a construct containing AT2R was developed for high and stable long‐term expression in MSCs via lentiviral‐mediated gene transfer. Migration of the AT2R expressing MSCs (AT2R‐MSCs) were examined in vitro. The potential therapeutic efficiency of AT2R‐MSCs was subsequently investigated in the in vivo model of ALI induced by lipopolysaccharide (LPS) and assessed on the migration of MSCs to the damage lung tissue. In contrast, MSCs with AT2R gene knockdown (MSC‐ShAT2R) were also developed to determine the role of AT2R in the migration ability and the therapeutic effects of MSCs on ARDS in vivo.
Materials and Methods
Human MSC Culture
Human bone marrow MSCs were purchased from Cyagen Biosciences Inc. (Guangzhou, China). The cells were identified by detecting cell surface phenotypes and their multipotent potential for differentiation along the adipogenic, osteogenic, and chondrogenic lineages and maintained as previously described 10, 12. Cells were passaged every 3–4 days by 0.25% trypsin‐ethylenediaminetetraacetic acid (Gibco, Carlsbad, CA, USA) when they reached about 80% confluence and were used for the experimental protocols between passages 5 and 10 13.
Lentiviral Vector Construction, Packaging, and Transduction of MSCs
AT2R Overexpression
The full‐length coding sequence of AT2R (NM_000686.4; 1,192 bp) was amplified from human MSC cRNA by polymerase chain reaction (PCR), gel purified, and ligated with T4 DNA ligase into the GV358 vector (Ubi‐MCS‐3FLAG‐SV40‐GFP‐IRES‐puromycin; GeneChem Co., Ltd., Shanghai, China), which carries the green fluorescent protein (GFP) gene, to develop a construct coexpressing human AT2R and GFP. The ligation was transformed into competent Escherichia coli, and the transformation was plated onto an ampicillin plate. Colonies were picked 16 hours later and inoculated onto Luria‐Bertani medium. Bacteria were grown in shaking culture at 37°C for 16 hours. Plasmid was extracted by the alkaline lysis method, confirmed by PCR and sequenced.
The following primers were used: AT2R (1,133 bp), sense 5′‐GAGGATCCCCGGGTACCGGTCGCCACCATGAAGGGCAACTCCACCCTTG‐3′ and antisense 5′‐TCCTTGTAGTCCATACCAGACACAAAGGTCTCCATTTC‐3′.
AT2R Downregulation
Three different sequences targeted to human AT2R mRNA were designed and provided by GeneChem Co. Ltd. (http://www.genechem.com.cn; Supporting Information Table S1). The sense and antisense strands of single stranded DNA oligo (short‐hairpin RNAs [shRNAs]) are shown in Supporting Information Table S2. Briefly, the human AT2R knockdown constructs expressing shRNA targeting endogenous AT2R were encoded into a lentivirus‐based ShRNA vector, GV248 vector (hU6‐MCS‐Ubiquitin‐EGFP‐IRES‐puromycin; GeneChem Co., Ltd.), which carries the enhanced GFP (EGFP) gene and puromycin.
All the constructs were transfected into 293 T packaging cells (GeneChem Co., Ltd.) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) to produce lentivirus. MSCs were transduced with viral supernatant (multiplicity of infection = 50), and mRNA expression of AT2R was detected by using reverse transcription‐polymerase chain reaction (RT‐PCR) 10 3 days after transduction to detect the effect of overexpression and screen for the optimal shRNA (ShRNA‐AT2R; TTCCTCTATGGGCAACCTA). The transduction efficiency was evaluated by detecting the expression of GFP with an Olympus IX51 fluorescence microscope (Olympus Co., Tokyo, Japan). MSCs carrying either GFP (MSC‐GFP, MSC‐Shcontrol) alone or both AT2R/ShRNA‐AT2R and GFP (MSC‐AT2R, MSC‐ShAT2R) were harvested after selection using puromycin at the minimal lethal concentration (1.5 μg/ml) as previously described 14. The puromycin‐resistant cells were then collected for further use.
In Vitro Transwell Migration Assay
Gene‐modified MSCs and control groups were added to the upper chambers of 0.8 μm cell‐culture inserts (Corning Inc., Corning, NY) at a density of 500,000 per milliliter cells per insert well. Dulbecco's modified Eagle's medium/F12 (1:1) supplemented with 2% fetal bovine serum containing concentration of 100 nM Ang II were used in the bottom chambers of the Transwell and cultured for 12 hours at 37°C. Cells from the upper chambers of the Transwells were removed. The migrated cells on the undersides of the membranes were stained with crystal violet (Beyotime, Haimen, China). Migratory cells were imaged and counted under a light microscope (Olympus).
ALI Model and Cell Transplantation Procedures
All experiments were performed in accordance with Chinese legislation regarding experiment animals and approved by the Committee of Animal Care and Use of Southeast University. Male C57BL/6 mice aged 8–10 weeks were purchased from the Laboratory Animal Center (Shanghai, China). Mice were housed in individual microisolator cages under specific pathogen‐free conditions, with free access to water and chow.
After they were anesthetized with an intraperitoneal injection of 5.0% (w/v) pentobarbital sodium at 4.0 ml/kg, mice were subjected to intratracheal administration of LPS (E. coli 0111:B4; Sigma‐Aldrich, St. Louis, MO) as previously described 9. Sham operation was performed in a similar manner with same volume of normal saline.
Gene‐modified MSCs and control groups (1 × 105 cells suspended in 150 μl phosphate buffered solution [PBS]) 15 were injected into the tail vein 4 hours after LPS challenge. Mice without LPS instillation were injected with PBS as a control. The mice were sacrificed at 24 and 72 hours after MSC injection and samples were collected for further analysis.
Ex Vivo Optical Imaging
MSC‐GFP, AT2R‐MSC, MSC‐Shcontrol, and MSC‐ShAT2R (1 × 105 cells) labeled with CellVue NIR815 dye (eBioscience Inc., San Diego, CA) were transplanted into ALI mice through tail vein. Three mice at each time point (24 and 72 hours post transplantation) were imaged for ex vivo lung using a Maestro In Vivo Optical Imaging System 16. Images were obtained and analyzed using Maestro 2.10.0 software (CRi) as previously described 17
Fluorescence Microscopy
Lung tissues sections were fixed in 4% paraformaldehyde (30 minutes), recovered with antigen retrieval buffer (20 minutes), permeabilized with 0.3% Triton X‐100 (10 minutes), and blocked with 5% bovine serum albumin (30 minutes at room temperature). Primary antibody GFP (1:50; Santa Cruz Biotechnology, Paso Robles, CA) was added and incubated overnight at 4°C. Then Alexa Fluor 488‐labeled secondary antibodies (1:500; Abcam Incorporated, Cambridge, UK) were added and incubated 50 minutes in dark. After washed 3 times, the sections were stained with DAPI for 10 minutes and visualized using a fluorescence microscope (Olympus).
DNA Preparation and RT‐PCR Analysis
Real‐time PCR assays for human specific Alu sequences 18 were detected according to protocol described by Lee et al. 6. Briefly, total DNA of lung tissue was extracted using DNA extraction kit (Takara Bio, Inc., Kyoto, Japan) according to the manufacturer's protocol. The assays were performed in a volume of 50 μl that contained 25ul AceQ qPCR Probe Master Mix (Vazyme, Piscataway, NJ), 900 nM each of the forward and reverse primers, 250 nM TaqMan probe, and 200 ng DNA produced above. Real‐time PCR assays for human and mouse genes for glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) were performed in a volume of 50 μl that contained 25 μl AceQ qPCR SYBR Green Master Mix (Vazyme), 200 nM each of the forward and reverse primers, and 200 ng DNA produced above. Reactions were incubated at 50°C for 2 minutes and at 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute by StepOne Plus Real Time PCR System (Life Technologies). The final value for total DNA in the sample was corrected by parallel real‐time PCR assays with primers that amplified both the human and the mouse genes for GAPDH and calculated using the 2(−△△Ct) method.
The following primers were used: Alu Forward Primer 5′‐CATGGTGAAACCCCGTCTCTA‐3′; Alu Reverse Primer 5′‐GCCTCAGCCTCCCGAGTAG‐3′; Alu Probe 5′‐FAM‐ATTAGCCGGGCGTGGTGGCGTAMRA‐3′; h/mGAPDH Forward Primer 5′‐CAGCGACAC CCACTCCTCCACCTT‐3′; h/mGAPDH Reverse Primer 5′‐CATGAG GTCCACCACCCTGTTGCT‐3′.
Hematoxylin and Eosin Staining and Lung Injury Scoring
Lung tissues were fixed in 4.0% paraformaldehyde, embedded in paraffin, and cut into 5 μm sections, which were stained with H&E and viewed by light microscopy (Olympus). The degree of lung damage was quantified and scored blindly by a pathologist, as what we have did previously 9.
Pulmonary Capillary Permeability Measurement
The lung wet weight/body weight ratio (LWW/BW) was measured to reflect the microvascular endothelial permeability of the lung. In separate experiments, Evans blue dye (20 mg/kg in 80 μl saline) was administered intravenously. After 30 minutes, the intravascular dye in the lung was flushed by right ventricular perfusion with 10 ml of heparinized saline. The lungs were harvested and immersed into 2 ml formamide (Merck, Kenilworth, NJ) for extraction for 24 hours at 60°C. The optical density value of the extracts was read at 630 nm wavelength as previously described 19.
Total Cell Counts, Neutrophil Counts, and Cytokine Measurements in Bronchoalveolar Lavage Fluid
Bronchoalveolar lavage fluid (BALF) was collected as we previously described 20. Total cell counts were determined using hemocytometer (Thermo Fisher Scientific, Inc.). And a differential of the white blood cell was obtained using Wright's dye staining (Jiancheng Bioengineering Co., Nanjing, China) 9. Enzyme‐linked immunosorbent assay (ELISA) was used to detect cytokines IL‐1β, IL‐6, and IL‐10 in BALF strictly according to manufacturer's protocols (ExcellBio, Shanghai, China).
Statistical Analysis
Data are expressed as mean ± SD. Statistics analysis was performed by the one‐way ANOVA followed by Bonferroni's post hoc test using SPSS 20.0 software (Chicago, IL, USA). Statistical significant level was defined as p < .05.
Results
AT2R Expression in Genetically Modified MSCs
We used lentiviruses to transduce MSCs with genes expressing GFP and AT2R or ShRNA‐AT2R (Fig. 1A). The cells showed robust green fluorescent signal (>90%; Fig. 1B, 1C) and were separated by using puromycin thereafter used in this study. Real‐time quantitative PCR analysis, performed in parallel, confirmed an approximate threefold increase and 80% downregulation of AT2R mRNA in MSCs (Fig. 1D). We did not analyze the protein expression of AT2R because the commercially available AT2R antibodies are nonspecific 21. Thus, the determination of mRNA expression remains the only reliable approach to date for examining AT2R expression.
Figure 1.
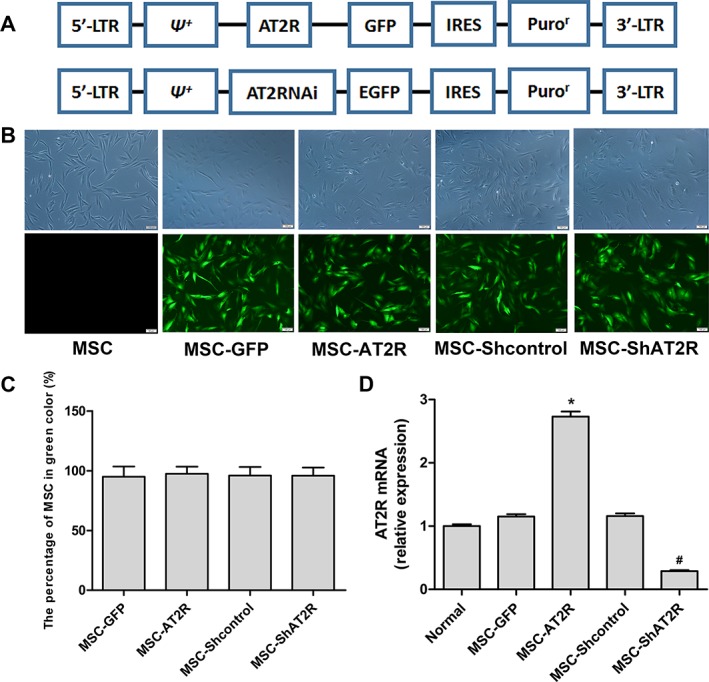
Generation of genetically modified MSCs. (A): Simplified scheme of the lentiviral vector constructs. (B): Lentiviral‐infected MSCs exhibited robust GFP signal. Phase contrast (top) and green channel (bottom) images. Scale bar = 100 μm. (C): The quantitative analysis for lentiviral transduction. (D): Evaluation of AT2R mRNA expression in lentivirally transduced MSCs. The data are shown as fold changes over normal MSCs. Means ± SD of three experiments are shown (n = 3, * p < .05 vs. the MSC‐GFP group; # p < .05 vs. the MSC‐Shcontrol group). Abbreviations: AT2R, angiotensin II type 2 receptor; EGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; IRES, internal ribosome entry site; LTR, long terminal repeat; MSC, mesenchymal stem cell; ᴪ +, extended viral packaging signal; Puror, puromycin resistance gene.
AT2R Overexpression Enhances Ang II‐Induced MSCs Migration In Vitro
To test the migration potential of engineered MSCs in vitro, all groups of MSCs were stimulated with 100 nm Ang II and assessed using a migration Transwell assay. As shown in Figure 2, overexpression of AT2R significantly increased Ang II‐induced MSC migration. In contrast, MSC‐ShAT2R demonstrated a decreased response to Ang II. These data indicate that AT2R overexpression can effectively enhance the migration of MSCs induced by Ang II in vitro.
Figure 2.
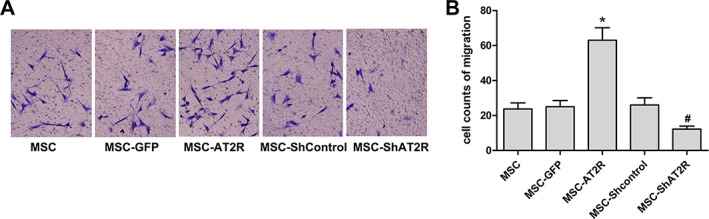
Ang II chemotaxis assay for AT2R genetically modified MSCs. After transduction, cells were plated into the upper well of a 6‐well Transwell plate, and Ang II (100 nM) were added to the lower well. Assays were incubated for 12 hours, and cells that migrated into the lower well were fixed, stained with crystal violet, and counted. (A): One representative experiment of three is shown (×200). (B): Quantitative analysis of percentage of migrated cells in total number of plated cells. Data represent the means ± SD of three wells from three independent experiments. The migration of MSC‐AT2R and MSC‐ShAT2R is compared with nontransduced cells in response to Ang II (n = 3, * p < .05 vs. the MSC‐GFP group; # p < .05 vs. the MSC‐Shcontrol group). Abbreviations: Ang II, angiotensin II; AT2R, angiotensin II type 2 receptor; GFP, green fluorescent protein; MSC, mesenchymal stem cell.
Enhanced Accumulation of Transplanted AT2R‐Overexpression MSCs in Injured Lung of ALI Mice
We have shown previously that Ang II are upregulated in the injured murine lung 9. To examine the in vivo effects of AT2R overexpression in MSCs, ALI was produced in mice by LPS instillation. Mice were randomly assigned to receive intravenous injection of MSCs or MSCs lentivirally transduced with GFP, AT2R‐GFP, or ShRNA‐AT2R‐GFP.
When ex vivo optical imaging was performed on the whole lung at organ level, a significant enhanced signal were detected in MSC‐AT2R group as compared to control MSC‐GFP group. In contrast, MSC‐ShAT2R group showed reduced signal compared with MSC‐Shcontrol group (Fig. 3A).
Figure 3.

MSCs migration toward injured lung after intravenous infusion at 24 and 72 hours. (A): ex vivo near infrared imaging of the lung. Near‐infrared color‐coded fluorescent images of organs of a mouse at 24 and 72 hours post‐MSC transplantation. (B): Fluorescence microscopy validation in recipient lungs. MSC with GFP (green) were observed in the lung tissue in both cases. Nuclei were stained with DAPI (blue). Scale bar = 20 μm. (C): Real‐time RT‐PCR assays for human‐specific sequences in the lung 24 and 72 hours after i.v. infusion of 1 × 105 MSCs (n = 3, * p < .05 vs. the MSC‐GFP group; # p < .05 vs. the MSC‐Shcontrol group). Abbreviations: AT2R, angiotensin II type 2 receptor; DAPI, 4′,6‐diamidino‐2‐phenylindole; GFP, green fluorescent protein; MSC, mesenchymal stem cell; PBS, phosphate buffered solution; RT‐PCR, reverse transcription‐polymerase chain reaction.
Fluorescence Microscopy was performed on the lung section at tissue level, a significant more GFP expression were observed in MSC‐AT2R group as compared to control MSC‐GFP group, suggesting that there are more MSC migration to the injured lung. However, the lung tissue of MSC‐ShAT2R group showed reduced GFP expression compared with MSC‐Shcontrol group, suggesting that AT2R downregulation reduce the MSC migration (Fig. 3B).
Real‐time RT‐PCR assays for human‐specific Alu sequences were performed to detect the human MSCs migration in lung of ALI at molecule level. A significant more Alu sequences expression were determined in MSC‐AT2R group as compared to control MSC‐GFP group. Whereas, MSC‐ShAT2R group showed reduced Alu sequences compared with MSC‐Shcontrol group (Fig. 3C).
These data indicate that AT2R overexpression can promote the migration of MSCs to injured lung of ALI mice.
Overexpression of AT2R‐Enhanced MSCs Inhibition of Lung Injury
Intra‐tracheal LPS resulted in inflammatory cell influx, alveolar wall thickness, pulmonary consolidation, and pulmonary hyaline membrane, suggesting a robust inflammatory response in the alveolus in mice. Simultaneous administration of MSC‐AT2R significantly attenuated the histology injury 24 and 72 hour after transfusion via tail vein (Fig. 4). Therapeutic effect on histology was considerably attenuated in MSC‐shAT2R group when compared to MSC‐Shcontrol group (Fig. 4).
Figure 4.

Histological evaluation of the therapeutic potential of AT2R genetically modified MSCs in ALI mice. (A, C): Hematoxylin and eosin staining images (×200) of lung sections in each group at 24 and 72 hours. (B, D): Quantitative analysis of the lung injury scores in each group at different time points (n = 5, * p < .05 vs. the NS + PBS group; # p < .05 vs. the LPS + PBS group; & p < .05 vs. the MSC‐GFP group; § p < .05 vs. the MSC‐Shcontrol group). Abbreviations: ALI, acute lung injury; AT2R, angiotensin II type 2 receptor; GFP, green fluorescent protein; LPS, lipopolysaccharide; MSC, mesenchymal stem cell; NS, normal saline; PBS, phosphate buffered solution.
AT2R Overexpression Reduced the Permeability of Vessel in Injured Lung of ALI Mice
To explore the effect of MSC‐AT2R on the vessel permeability of ALI mouse, LWW/BW and Evans blue dye leakage in the lung were assessed. When compared with LPS group, MSC‐AT2R reduced LWW/BW and the Evans blue dye leakage 24 and 72 hours after transfusion. However, MSC‐ShAT2R increased LWW/BW and the Evans blue dye leakage 24 and 72 hours after transfusion (Fig. 5). These findings indicate that AT2R overexpression can effectively reduce the permeability of vessel in injured lung of ALI mice.
Figure 5.
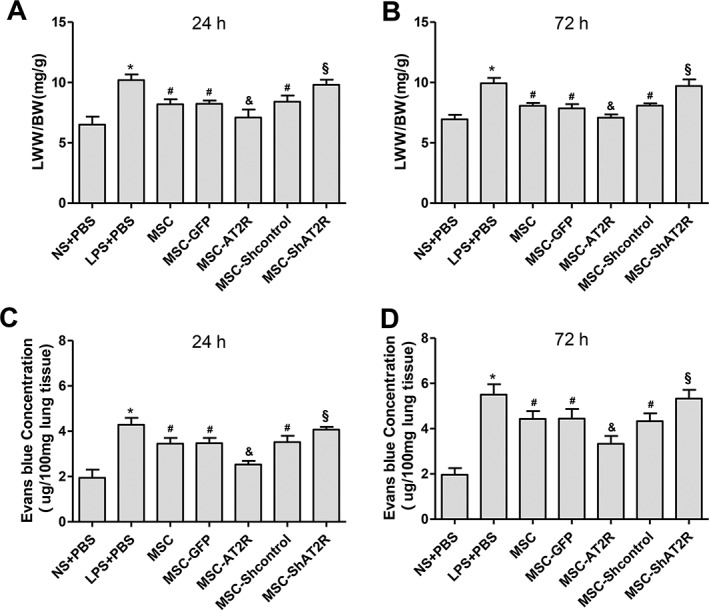
Effect of AT2R genetically modified MSCs on pulmonary capillary permeability. The pulmonary capillary permeability was assessed by LWW/BW (A, B) and Evan's blue dye leakage in lung (C, D) (n = 5, * p < .05 vs. the NS + PBS group; # p < .05 vs. the LPS + PBS group; & p < .05 vs. the MSC‐GFP group; § p < .05 vs. the MSC‐Shcontrol group). Abbreviations: AT2R, angiotensin II type 2 receptor; GFP, green fluorescent protein; LPS, lipopolysaccharide; MSC, mesenchymal stem cell; NS, normal saline; PBS, phosphate buffered solution.
Overexpression of AT2R Enhanced the Inhibition of Lung Tissue Inflammation in ALI
To study the role of MSC‐AT2R on the inflammatory response of ALI in mouse, MSC‐GFP and MSC‐AT2R were transplanted via tail vein 4 hours after LPS instillation. Lavage fluid from bronchial alveoli was collected 24 and 72 hours later. We measured the total cell counts, neutrophil counts, and the concentrations of proinflammatory cytokines IL‐1β and IL‐6 and anti‐inflammatory cytokine IL‐10 in BALF.
When compared with LPS group, there was a more significant reduction in the BALF concentrations of total cell counts and neutrophil counts in the MSC‐AT2R‐treated mice versus the MSC‐GFP‐treated mice (Fig. 6). However, MSC‐ShAT2R increased total cell counts and neutrophil counts compared with MSC‐Shcontrol‐treated mice (Fig. 6). Similarly, there was a more significant reduction in the BALF concentrations of IL‐1β (Fig. 7A, 7B) and IL‐6 (Fig. 7C, 7D) in the MSC‐AT2R‐treated mice versus the MSC‐GFP‐treated mice. However, the BALF concentration of IL‐10 (Fig. 7E, 7F) was significantly elevated in the MSC‐AT2R‐treated mice but not in the MSC‐GFP‐treated mice.
Figure 6.

Effect of AT2R genetically modified MSCs on inflammatory cell influx into the LPS‐injured alveolus. Total cell counts (A, B) and neutrophils counts (C, D) in BALF at 24 and 72 hours was assessed after engineered MSCs transfusion after LPS‐induced acute lung injury (n = 5, * p < .05 vs. the NS + PBS group; # p < .05 vs. the LPS + PBS group; & p < .05 vs. the MSC‐GFP group; § p < .05 vs. the MSC‐Shcontrol group). Abbreviations: AT2R, angiotensin II type 2 receptor; BALF, Bronchoalveolar lavage fluid; GFP, green fluorescent protein; LPS, lipopolysaccharide; MSC, mesenchymal stem cell; NS, normal saline; PBS, phosphate buffered solution.
Figure 7.
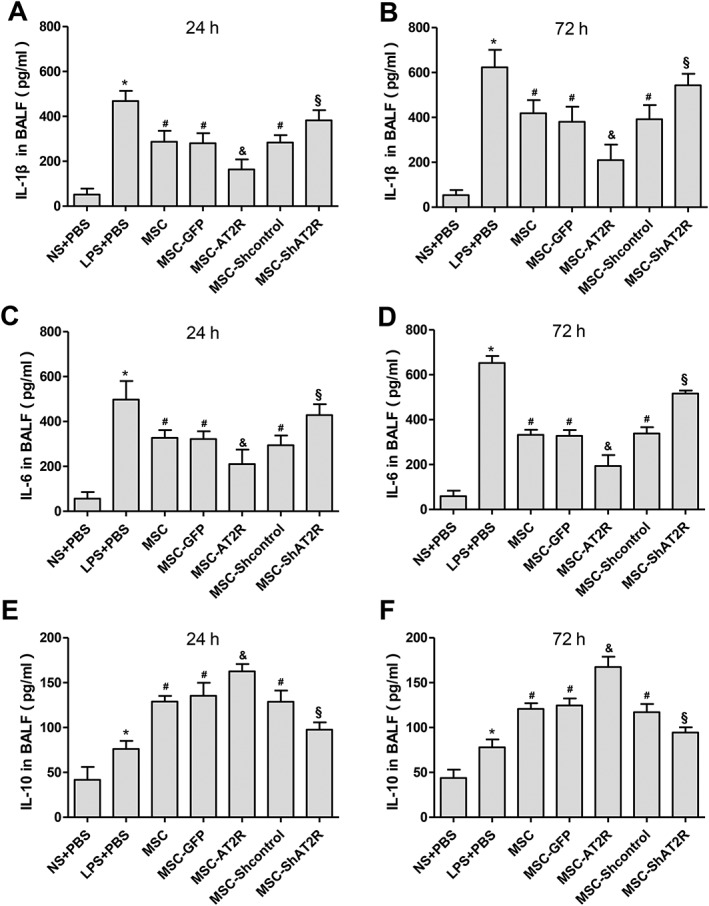
Effect of AT2R genetically modified MSCs on BAL cytokine. C57BL/6 mice were injected with engineered MSCs and control MSCs 4 hours post LPS‐injection, and lavage fluid from bronchial alveoli was removed 24 and 72 hours later. (A, C, E): levels of IL‐1β, IL‐6, and IL‐10 in BAL fluid at 24 hour were assayed by ELISA. (B, D, F): levels of IL‐1β, IL‐6, and IL‐10 in BAL fluid at 72 hours were assayed by ELISA (n = 5, * p < .05 vs. the NS + PBS group; # p < .05 vs. the LPS + PBS group; & p < .05 vs. the MSC‐GFP group; § p < .05 vs. the MSC‐Shcontrol group). Abbreviations: AT2R, angiotensin II type 2 receptor; BAL, Bronchoalveolar lavage; ELISA, enzyme‐linked immunosorbent assay; GFP, green fluorescent protein; LPS, lipopolysaccharide; MSC, mesenchymal stem cell; NS, normal saline; PBS, phosphate buffered solution.
These findings indicate that AT2R overexpression can effectively downregulate the inflammatory response of ALI.
Discussion
In the present study, our data show that AT2R‐overexpression MSCs exhibit higher chemotactic activity. The enhanced migration ability of MSC‐AT2R observed in vitro was also present on injection of these cells into the murine lung after ALI. A greater number of AT2R‐MSCs accumulated within lung tissue, and those cells showed increased migratory capacity toward the area of injury compared to the control MSCs group. More importantly the injury level of the AT2R‐MSCs‐injected group was significantly reduced compared to the control group. On the contrary, AT2R downregulated MSCs significantly decreased the migration ability of MSCs in vitro and migration toward the lung in vivo and diminished the protective effects of MSCs on the injured lung. Our results demonstrate that overexpression of AT2R enhance the MSCs migration to lung in ALI mice and may provide a new therapeutic strategy for the injured lung.
Mounting preclinical evidence indicates that MSCs are effective in ARDS model 22 and the mechanisms underlying the therapeutic benefit include MSCs release of paracrine factors, elaboration of exosomes and microvesicles, and transfer of mitochondria 23. However, most paracrine factors have a relatively short half‐life, and their beneficial effects are confined to the tissue within which they are released 19. Moreover, both microvesicles and exosomes interact with their target cells via either ligand‐receptor signaling pathways or internalization by phagocytosis, endocytosis, and direct membrane fusion, leading to the delivery of their molecular cargo to the recipient cells 24, 25. Many studies also demonstrated that mitochondria transfer required direct cell contact through formation of gap junctions 26 or tunneling nanotubes 27, 28. More importantly, there are also reports showing that the MSC secretome was not effective 15 or was not effective as whole cell therapy 29, suggesting that some effects of MSCs appear to require the cells to be present. Several studies have shown that modifying MSCs before their systemic infusion improved the homing of MSCs to injured tissues and led to better functional outcomes 30, 31. Therefore, facilitating MSCs migration to injured lung tissue might improve the efficacy of MSCs in ARDS treatment.
Our approach to lung repair is enhanced by the ability to genetically engineer stem cells addressing, in this case, the problem of cell migration. The stem cell‐based strategies engineering stem/progenitor cells with higher amount of chemokine receptors to enhance the migration and homing ability of MSCs include preconditioning with chemical compounds, cytokines and growth factors or hypoxia, genetic modifications 32. Because the way of genetic modifications could achieve stable and long‐term expression of targeted factors 33 and as shown in our previous study, Ang II enhance the migration of MSCs in an AT2R dependent way. Thus, we overexpressed AT2R in MSCs, using lentiviral vector, results in increased MSCs homing to injured lung of ARDS mice, which is consistent with existing literature using same genetic modifications strategy 19, 34, 35. As a result, lung retention of MSCs genetically enhanced to overexpress AT2R are superior to that of control MSCs expressing reporter genes alone.
Our study demonstrated a significant beneficial effect from MSCs transplantation in LPS induced ALI. We found that MSC‐AT2R reduced lung inflammation and protein permeability, which prevented the formation of pulmonary edema as measured by the LWW/BW and EVLW. Moreover, MSC‐AT2R had a significantly greater effect on reducing the influx of inflammatory cells, both total cells and neutrophils, and inflammatory cytokines IL‐1β and IL‐6 and elevating an anti‐inflammatory cytokine IL‐10 in BALF of mice injured with LPS than MSC‐GFP. These results indicated that high AT2R expression promoted immunomodulatory functions of MSCs in vivo. Take together, pathological damage was less severe when MSC‐AT2R rather MSC‐GFP were transplanted, indicating protective effects for the injured lung. In contrast, MSC‐ShAT2R increased lung inflammation and protein permeability, the influx of inflammatory cells and cytokines, and reduced anti‐inflammatory cytokine thereby resulting more severe pathological damage than MSC‐Shcontrol. However, whether the effect on lung repair is due to cell transdifferentiation of MSCs from cell transplantation or to paracrine mediators/microvesicles and exosomes released by MSCs requires further investigation.
The main limitation of this study is its focus on the short‐term (24 and 72 hours) therapeutic effects of AT2R‐MSCs treatment because the proportion of accumulation in lung decreased from about 35% early after transplantation to 2% or less by day 10 36. Data on the long‐term effects (e.g., survival rate) are lacking and will require further study. This study is focused on migration of AT2R overexpression MSCs towards the injured lung as a novel target to enhance MSC therapeutic effect in vivo. We do not report here the effect of AT2R gene modification on MSCs differentiation or paracrine. Moreover, experimental ARDS was induced by endotoxin, and our results cannot be extrapolated to other models or to human ARDS. In addition, the distribution of MSCs in other tissues is not detected. Therefore, we cannot determine the effect of MSCs overexpressing AT2R on other organs or tissues. Further work is still needed.
Conclusion
In summary, our efforts to genetically manipulate MSCs ex vivo by enhancing the expression of AT2R show that overexpression of AT2R enhances MSC migration to injured lung. Meanwhile, the presence of more MSC at the site of injury reduce the permeability of pulmonary endothelial, downregulate the inflammation reaction in ALI, and promote the restoration of injured lung. This approach has advanced our understanding of Ang II‐AT2R affecting stem cell migration and may offer a new strategy for improved stem cell therapy for ARDS patients in future.
Author Contributions
X.P.X.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing. L.L.H., S.L.H., and J.B.H.: collection and assembly of data, data analysis and interpretation. H.L.H., J.Y.X., J.F.X., and A.R.L.: Data analysis and interpretation, manuscript writing. S.Q.L., L.L., Y.Z.H., F.M.G., and Y.Y.: conception and design, data analysis and interpretation. H.B.Q.: conception and design, data analysis and interpretation, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supplementary Table 1 Designed sequences target to different sites in human AT2R mRNA
Supplementary Table 2 Designed sense and antisense of shRNAs based on three target sequences in human AT2R
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81372093, 81471843, 81501705, and 81571874), Jiangsu Provincial Key Medical Discipline (Laboratory) (ZDXKA2016025), Jiangsu Provincial Medical Talent (ZDRCA2016082), and the Fundamental Research Funds for the Central Universities (2242018K40167).
References
- 1. Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell‐based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med 2017;196:266‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson CL, Soeder Y, Dahlke MH. Concise review: Mesenchymal stromal cell‐based approaches for the treatment of acute respiratory distress and sepsis syndromes. Stem Cells Translational Medicine 2017;6:1141‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson JG, Liu KD, Zhuo H et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir Med 2015;3:24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rustad KC, Gurtner GC. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care 2012;1:147‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nitzsche F, Muller C, Lukomska B et al. Concise review: MSC adhesion cascade‐insights into homing and transendothelial migration. Stem Cells 2017;35:1446‐1460. [DOI] [PubMed] [Google Scholar]
- 6. Lee RH, Pulin AA, Seo MJ et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti‐inflammatory protein TSG‐6. Cell Stem Cell 2009;5:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zambelli V., Grassi A., Bellani G. Role of the Renin‐Angiotensin System in ARDS In: Vincent JL, eds. Annual Update in Intensive Care and Emergency Medicine. Berlin, Heidelberg: Springer, 2012:171‐181. [Google Scholar]
- 8. Imai Y, Kuba K, Rao S et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He H, Liu L, Chen Q et al. Mesenchymal stem cells overexpressing angiotensin‐converting enzyme 2 rescue lipopolysaccharide‐induced lung injury. Cell Transplant 2015;24:1699‐1715. [DOI] [PubMed] [Google Scholar]
- 10. Xu XP, He HL, Hu SL et al. Ang II‐AT2R increases mesenchymal stem cell migration by signaling through the FAK and RhoA/Cdc42 pathways in vitro. Stem Cell Res Ther 2017;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Honczarenko M, Le Y, Swierkowski M et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006;24:1030‐1041. [DOI] [PubMed] [Google Scholar]
- 12. Chen QH, Liu AR, Qiu HB et al. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res Ther 2015;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JW, Krasnodembskaya A, McKenna DH et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013;187:751‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu S, Li J, Xu X et al. The hepatocyte growth factor‐expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther 2016;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devaney J, Horie S, Masterson C et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015;70:625‐635. [DOI] [PubMed] [Google Scholar]
- 16. Wang XY, Ju S, Li C et al. Non‐invasive imaging of endothelial progenitor cells in tumor neovascularization using a novel dual‐modality paramagnetic/near‐infrared fluorescence probe. PloS One 2012;7:e50575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu L, He H, Liu A et al. Therapeutic effects of bone marrow‐derived mesenchymal stem cells in models of pulmonary and extrapulmonary acute lung injury. Cell Transplant 2015;24:2629‐2642. [DOI] [PubMed] [Google Scholar]
- 18. McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real‐time PCR. Cytotherapy 2003;5:7‐18. [DOI] [PubMed] [Google Scholar]
- 19. Han J, Lu X, Zou L et al. E‐prostanoid 2 receptor overexpression promotes mesenchymal stem cell attenuated lung injury. Hum Gene Ther 2016;27:621‐630. [DOI] [PubMed] [Google Scholar]
- 20. Cai SX, Liu AR, Chen S et al. Activation of Wnt/beta‐catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther 2015;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Hafko R, Villapol S, Nostramo R et al. Commercially available angiotensin II At(2) receptor antibodies are nonspecific. PloS One 2013;8:e69234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monsel A, Zhu YG, Gennai S et al. Cell‐based therapy for acute organ injury: Preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014;121:1099‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matthay MA, Pati S, Lee JW. Concise review: Mesenchymal stem (stromal) cells: Biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells 2017;35:316‐324. [DOI] [PubMed] [Google Scholar]
- 24. Collino F, Deregibus MC, Bruno S et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PloS One 2010;5:e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan A, Farber EL, Rapoport AL et al. Transfer of microRNAs by embryonic stem cell microvesicles. PloS One 2009;4:e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Islam MN, Das SR, Emin MT et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18:759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson MV, Morrison TJ, Doherty DF et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 2016;34:2210‐2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmad T, Mukherjee S, Pattnaik B et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 2014;33:994‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayes M, Curley GF, Masterson C et al. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator‐induced lung injury. Intensive Care Med Exp 2015;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang K, Zhao X, Kuang C et al. Overexpression of SDF‐1alpha enhanced migration and engraftment of cardiac stem cells and reduced infarcted size via CXCR4/PI3K pathway. PloS One 2012;7:e43922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu N, Patzak A, Zhang J. CXCR4‐overexpressing bone marrow‐derived mesenchymal stem cells improve repair of acute kidney injury. Am J Physiol Renal Physiol 2013;305:F1064‐F1073. [DOI] [PubMed] [Google Scholar]
- 32. Naderi‐Meshkin H, Bahrami AR, Bidkhori HR et al. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol Int 2015;39:23‐34. [DOI] [PubMed] [Google Scholar]
- 33. Mangi AA, Noiseux N, Kong D et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195‐1201. [DOI] [PubMed] [Google Scholar]
- 34. Yang JX, Zhang N, Wang HW et al. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem 2015;290:1994‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nowakowski A, Walczak P, Lukomska B et al. Genetic engineering of mesenchymal stem cells to induce their migration and survival. Stem Cells Int 2016;2016:4956063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gholamrezanezhad A, Mirpour S, Bagheri M et al. In vivo tracking of 111In‐oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol 2011;38:961‐967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Designed sequences target to different sites in human AT2R mRNA
Supplementary Table 2 Designed sense and antisense of shRNAs based on three target sequences in human AT2R


