Abstract
Cancer is one of the leading causes of morbidity and mortality worldwide, with 1,688,780 new cancer cases and 600,920 cancer deaths projected to occur in 2017 in the U.S. alone. Conventional cancer treatments including surgical, chemo‐, and radiation therapies can be effective, but are often limited by tumor invasion, off‐target toxicities, and acquired resistance. To improve clinical outcomes and decrease toxic side effects, more targeted, tumor‐specific therapies are being developed. Delivering anticancer payloads using tumor‐tropic cells can greatly increase therapeutic distribution to tumor sites, while sparing non‐tumor tissues therefore minimizing toxic side effects. Neural stem cells (NSCs) are tumor‐tropic cells that can pass through normal organs quickly, localize to invasive and metastatic tumor foci throughout the body, and cross the blood‐brain barrier to reach tumors in the brain. This review focuses on the potential use of NSCs as vehicles to deliver various anticancer payloads selectively to tumor sites. The use of NSCs in cancer treatment has been studied most extensively in the brain, but the findings are applicable to other metastatic solid tumors, which will be described in this review. Strategies include NSC‐mediated enzyme/prodrug gene therapy, oncolytic virotherapy, and delivery of antibodies, nanoparticles, and extracellular vesicles containing oligonucleotides. Preclinical discovery and translational studies, as well as early clinical trials, will be discussed. Stem Cells Translational Medicine 2018;7:740–747
Keywords: Cellular therapy, Chemotaxis, Chemotherapy, Clinical translation, Gene delivery systems in vivo or in vitro, Glioma, Progenitor cells, Viral persistence
Significance Statement.
Cancer is a leading cause of death worldwide despite significant advances in biomedical research. Current cancer treatments are hindered by a lack of tumor specificity, which can lower their efficacy and cause undesirable side effects. Tumor‐specific penetration of freely delivered therapeutic agents is difficult to achieve, but stem cell‐mediated delivery can enable these agents to penetrate tumors, increasing exposure of the tumor while sparing non‐tumor tissues. In this review, we briefly describe the tumor tropism of neural stem cells and their use as therapeutic vehicles, providing representative examples of this state‐of‐the‐art approach in cancer therapy. Outlooks on novel therapeutic strategies and the probable direction of stem cell‐mediated cancer therapy in the near future are also discussed.
Introduction
There are more than 400,000 drugs that can eliminate tumor cells in vitro; however, cancer is still a leading cause of death (8.8 million deaths globally in 2015) 1, 2 in part due to our inability to selectively deliver adequate drug doses to residual tumor cells after surgery and radiation. Developing alternative strategies to selectively deliver sufficient amounts of anticancer agents to tumors is of utmost importance. One promising biological approach is to use live, migratory stem cells. Many types of stem cells exhibit inherent pathotropism toward tissue damage and inflammatory conditions, including tumors.
Although the precise molecular mechanisms underlying the tumor‐directed migration of stem cells are still being elucidated, tumors can be characterized as “wounds that never heal,” continuously releasing chemoattractants that recruit stem cells to the site of injury 3. These include cytokines (e.g., monocyte chemotactic protein‐1 4, stem cell factor 5, and hepatocyte growth factor 6), pro‐angiogenic growth factors (e.g., vascular endothelial growth factor [VEGF] 7), hypoxia‐inducible factors 8, extracellular matrix 9, and other inflammatory mediators 10. Stem cells express surface receptors responsive to these tumor‐derived factors (e.g., CCL2 4, c‐Kit 5, c‐Met 6, VEGF receptors 7, and hypoxia‐responsive receptors 8), which trigger their migration toward the tumor.
Tumor‐directed migration of various stem cell types was demonstrated initially in preclinical models of primary brain tumors 11, 12 and secondary brain metastases 13, 14, 15. An increasing number of preclinical studies have also demonstrated stem cell tropism to metastatic solid tumors outside the central nervous system (CNS), including ovarian 16, 17, pancreatic 18, lung 19, and breast cancers 19, as well as melanoma 20 and neuroblastoma 21. Routes of delivery have included intracerebral, intraventricular, intravenous, intranasal, and intraperitoneal 22, 23. Arming stem cells with antitumor agents ex vivo not only promises a higher degree of tumor specificity but also may increase the half‐lives of stem cell‐loaded therapeutics 24.
Stem cells at various stages of development have demonstrated tumor‐tropic properties, but most preclinical and clinical stem cell‐mediated approaches have used either transdifferentiated, immortalized, or freshly isolated multipotent progenitor cells (hematopoietic, mesenchymal, and neural stem cells [NSCs]) in an effort to avoid the potential tumorigenicity of pluripotent cell sources 25. Hematopoietic stem cells have been used to locally produce prodrug‐converting enzymes in breast, lung, and brain cancer settings 26, but have otherwise not been widely used for targeting of solid tumors due to their ability to promote tumor angiogenesis 27. Mesenchymal stem cells (MSCs) and NSCs have been more extensively investigated in the solid tumor setting. Efforts to use MSCs for tumor‐specific delivery of therapeutics are reviewed elsewhere 28, 29. In this concise review, we cover the preclinical and clinical use of NSCs as targeted delivery vehicles for anticancer agents. We also provide perspective on our translation of early NSC therapies to help guide future development efforts.
Preclinical Development of NSCs as Therapeutic Vehicles
Over the past 20 years, extensive research efforts have investigated the potential of NSC‐mediated therapeutic delivery. Tumor‐tropic NSCs are amenable to ex vivo manipulations that enable them to deliver a variety of anticancer agents selectively to tumor foci (Fig. 1). The advantages of NSC‐mediated delivery of these cancer therapies include more effective and selective delivery to and distribution throughout tumor foci, minimal immunogenicity, and limited off‐target effects, thereby resulting in lower toxicity to normal tissues. The efficacy of various NSC‐delivered anticancer agents has been demonstrated in preclinical tumor models, summarized in Table 1, showing much clinical promise 30. The different therapeutic cargo explored to date is discussed in the following section.
Figure 1.
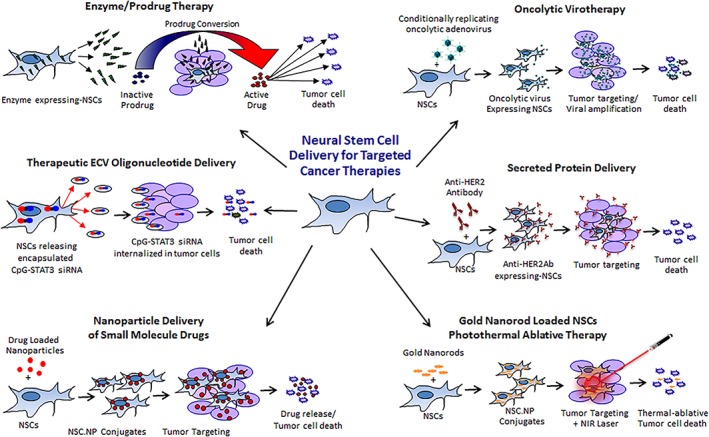
Engineering NSCs to deliver targeted anticancer payloads. Tumor‐tropic NSCs can be engineered to deliver anticancer agents selectively to tumor foci. Approaches explored include enzyme/prodrug gene therapy, oncolytic virotherapy, therapeutic protein delivery (antibody delivery shown as example), sustained‐release or stimuli‐responsive nanoparticle delivery, and extracellular vesicle oligonucleotide delivery. Abbreviation: NSC, neural stem cell.
Table 1.
NSC‐mediated cancer therapy: Preclinical in vivo studies
| Anticancer agent | Mechanism | Tumor model | NSC route of administration | Species: NSCs/tumor/host | Tumor location | Reference |
|---|---|---|---|---|---|---|
| HSV‐TK/GCV | GCV‐triphosphate cytotoxicity | Breast cancer to brain metastases | Intracerebral, intra‐arterial | Mouse/human/mouse | Metastatic | 31, 32 |
| 5‐FU | Inhibits TS | Glioma | Intratumoral | Human/human/mouse | Orthotopic brain | 11 |
| 5‐FU | Inhibits TS | Breast cancer | Intratumoral | Human/human/mouse | Orthotopic mammary fat pad | 33 |
| 5‐FU | Inhibits TS | Medulloblastoma | Intratumoral | Human/human/mouse | Orthotopic | 34 |
| SN‐38 | Inhibits topoisomerase | Medulloblastoma | Intratumoral | Human/human/mouse | Orthotopic cerebellar | 12 |
| SN‐38 | Inhibits topoisomerase | Neuroblastoma | Intravenous | Human/human/mouse | Metastatic | 21 |
| SN‐38 | Inhibits topoisomerase | Breast | Intravenous | Human/human/mouse | Orthotopic | 10 |
| SN‐38 | Inhibits topoisomerase | Pancreas | Intravenous | Human/human/mouse | Ectopic flank | 18 |
| SN‐38 | Inhibits topoisomerase | Lung | Intravenous | Human/human/mouse | Ectopic flank | 19 |
| CRAd‐S‐pk7 virus | Oncolysis | Glioma | Intratumoral | Human/human/mouse | Orthotopic brain | 35 |
| Trastuzumab | Inhibits HER2 | Breast to brain metastases | Intravenous | Human/human/mouse | Orthotopic brain | 36 |
| TRAIL | Induces apoptosis | Glioma | Intravenous | Human/human/mouse | Orthotopic brain | 37 |
| Osteoprogerin | Inhibits osteoclast‐mediated bone resorption | Neuroblastoma bone metastases | Intravenous | Human/human/mouse | Metastatic | 38 |
| Thrombospondin‐1 | Antiangiogenic | Glioma | Intracerebral | Human/human/mouse | Orthotopic brain | 39 |
| PEX | Blood vessel effector | Glioma | Intracranial | Human/human/mouse | Orthotopic brain | 40 |
| IL‐4 | Immune‐stimulatory | Glioma | Intracerebral | Mouse/mouse/mouse | Orthotopic brain | 37 |
| IL‐12 | Immune‐stimulatory | Glioma | Intracerebral | Mouse/mouse/mouse | Orthotopic brain | 24 |
| IL‐23 | Immune‐stimulatory | Glioma | Intracerebral | Mouse/mouse/mouse | Orthotopic brain | 41 |
| Iron/iron oxide magnetic nanoparticle + AMF | Physical disruption | Melanoma | Intravenous | Mouse/mouse/mouse | Orthotopic flank | 20 |
| Gold nanorods + NIR | Thermal ablation | Breast, bladder cancer | Intratumoral | Human/human/mouse | Ectopic flank | 42 |
| Docetaxel‐loaded NPs | Inhibits microtubules | Breast cancer | Intratumoral | Human/human/mouse | Orthotopic breast | 43 |
| Cisplatin‐loaded NPs | DNA damage | Ovarian cancer | Intraperitoneal | Human/human/mouse | Orthotopic ovarian | 16 |
| Doxorubicin‐loaded NPs | DNA intercalation | Glioma | Intracerebral | Human/human/mouse | Orthotopic brain | 44 |
Abbreviations: 5‐FU, 5‐fluorouracil; AMF, alternating magnetic field; GCV, ganciclovir; HER2, human epidermal growth factor receptor 2; HSV‐TK, herpes simplex virus thymidine kinase type 1; IL‐12, interleukin‐12; IL‐23, interleukin‐23; IL‐4, interleukin‐4; NIR, near infrared; NPs, nanoparticles; NSC, neural stem cells; PEX, hemopexin C domain autolytic fragment of matrix metalloproteinase‐2; SN‐38, irinotecan; TRAIL, tumor necrosis factor‐related apoptosis‐inducing ligand; TS, thymidylate synthase.
NSC‐Mediated Enzyme/Prodrug Therapy
Neural stem cells can deliver prodrug‐activating enzymes throughout tumor foci to convert inactive prodrugs into tumor‐toxic effector drugs. Various cells have been engineered to enhance the efficacy of five of the more than 50 different enzyme–prodrug combinations developed during the past two decades 28. Once generated, the effector drugs can initiate a “bystander effect,” affecting multiple surrounding tumor cells through diffusion, intercellular gap junctions, or endocytosis of apoptotic bodies released from dying cells. Genetically engineering cells to express prodrug‐converting enzymes also provides a critical safety switch that can eliminate the cells after their therapeutic effect has been actualized 45. Our laboratory has performed preclinical efficacy and safety/toxicity studies for two different modifications of a v‐myc immortalized clonal NSC line (HB1.F3.C1) 11, 21. In both cases, the NSCs were engineered to express prodrug‐converting enzymes for tumor‐localized chemotherapy production following intracerebral administration for recurrent high‐grade glioma patients. Preclinical efficacy and safety/toxicity studies enabled successful Investigational New Drug (IND) applications to the U.S. Food and Drug Administration (FDA). First, the NSCs were retrovirally transduced to stably express Escherichia coli cytosine deaminase (http://hb1.f3.cd21; CD‐NSCs), which converts the prodrug 5‐fluorocytosine (5‐FC) to the active chemotherapeutic 5‐fluorouracil (5‐FU) 11. These same NSCs were further modified to secrete a modified human carboxylesterase (hCE1m6; CE‐NSCs), which converts the prodrug irinotecan (IRN; CPT‐11) to its active metabolite SN‐38, a potent topoisomerase I inhibitor 46.
NSC‐Mediated Oncolytic Virotherapy
Oncolytic viruses can induce death of cancer cells regardless whether the cells are resistant to radio‐ or chemotherapy, and can stimulate immune system recognition of cancer cells as a result of exposure of tumor antigens after lysis. Although clinical trials to date have demonstrated the safety of oncolytic viruses, the efficacy of this approach has been limited by delivery hurdles such as rapid immune system inactivation of viruses, poor viral penetration of tumors, and the inability of the viruses to reach invasive foci that are separated from the main tumor mass by normal tissue 47, 48. In collaboration with Dr. Lesniak's group at the University of Chicago, we engineered our CD‐NSC line to deliver a conditionally replication‐competent adenovirus (CRAd‐Survivin‐pk7) that proliferates specifically in cells that overexpress survivin, a protein highly expressed in glioma cells (upregulated by radiation) but not in normal differentiated cells. Once the NSCs seed the virus into the invasive glioma sites, the virus continues to reproduce in tumor cells until normal tissue is reached and the effect ceases, resulting in reduced tumor burden and prolonged survival of mice bearing patient‐derived glioma xenografts 49, 50, 51. The minimal immunogenicity of the NSCs permits them to improve viral delivery and should enable repeat administrations.
NSC‐Mediated Therapeutic Protein Secretion
Neural stem cells can be transduced with integrating vectors so that they can stably release anticancer proteins, overcoming the short half‐lives of conventional delivery regimens. To date, several therapeutic proteins have been successfully engineered into NSCs, which have demonstrated anticancer effects when secreted in various preclinical carcinoma models.
Growth Factor‐Antagonists
We modified our CD‐NSC line to stably secrete a full‐length anti‐HER2 antibody (HER2Ab), which is functionally equivalent to trastuzumab 52. Preclinical in vivo experiments using HER2Ab‐overexpressing NSCs in a breast cancer brain metastasis mouse model demonstrated that intracerebral injection of HER2Ab‐NSCs significantly improved survival 36. The CD‐NSC line was also modified to stably secrete osteoprogerin, which can reduce osteolysis in bone tumors. Preclinical in vivo experiments in a neuroblastoma mouse model demonstrated a decrease in bone disease and slowed overall disease progression 38.
Tumor Necrosis Factor‐Related Apoptosis‐Inducing Ligand
Tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL) binds to death receptors preferentially overexpressed in cancer cells and induces apoptosis via activation of caspases. Shah et al. generated a secretable version of TRAIL that can be efficiently secreted from NSCs and used to induce apoptosis in glioma cell lines both in vivo and in vitro 53.
Interleukins
Interleukins exert antitumor effects via direct tumoricidal effects or positive modulation of the endogenous immune system. NSC‐mediated delivery of interleukins (IL‐4, IL‐12, and IL‐23) 24, 37, 41 has been used to improve antiglioma immune surveillance by activating cytotoxic lymphocytes and natural killer cells.
Antiangiogenic Proteins
Neural stem cells are particularly attracted to angiogenic regions of tumors with high‐VEGF expression. NSCs engineered to provide sustained on‐site delivery of secretable antiangiogenic thrombospondin (TSP‐1) to tumor vasculature reduced tumor vessel density, inhibited tumor progression, and increased survival in glioma‐bearing mice 39. Consistent with these findings, NSCs engineered to secrete the hemopexin (PEX) fragment of matrix‐metalloprotease II caused a 44.8% decrease in tumor angiogenesis and a 90% reduction in tumor volume 40.
NSC‐Mediated Nanoparticle Delivery
NSCs are appealing for use as nanoparticle (NP) carriers to overcome suboptimal penetration, distribution, and retention of therapeutic NPs. NSCs maintain their tumor tropism when transporting either surface‐bound or internalized NPs. To date, two NSC‐NP treatment strategies for tumors have been explored, although efficacy convincing enough to pursue a clinical trial has not yet been achieved.
NSC‐NP Conjugates for Small‐Molecule Drug Delivery
Drug‐loaded NPs can be conjugated to the surface of NSCs or internalized before migrating to invasive tumor sites. The NSC‐NP platform offers potential for the selective release (triggered or sustained) of anticancer drugs at tumor sites. NSCs can distribute NPs to brain tumor foci in mice when injected adjacent to the tumor, into the contralateral hemisphere, or into the tail vein 44, 54. Furthermore, the NPs can be up to ∼fivefold larger than those typically used for cancer therapies, which translates into a >100‐fold increase in the loading potential of a typical small molecular weight chemotherapeutics. The utility of this approach has also been investigated in preclinical orthotopic breast 43 and ovarian cancer settings 16.
NSC‐NP Conjugates for Physical Tumor Destruction/Photothermal Ablative Therapy
Loading NSCs with inert, metallic, stimuli‐responsive NPs affords clinicians the ability to trigger the destruction of drug‐resistant tumors on demand. NSCs loaded with gold nanorods result in well‐distributed, tumor‐localized antennae that can convert bear infrared (near infrared [NIR]) light into heat capable of “burning” tumor tissue while avoiding collateral damage to surrounding healthy tissue 42. In cases where the tumor lies beyond the penetration limits of NIR light, NSCs can alternatively be loaded with iron oxide discs. When exposed to an alternating magnetic field, the discs induce physical disruption of the tumor 20.
NSC‐Secreted Exosomes as Carriers for Oligonucleotide Therapeutics
Oligonucleotide therapeutics (ONTs) can overcome the limitations of small‐molecule inhibitors of many molecular targets, such as oncogenic transcription factors. However, short circulatory half‐life and intratumoral penetration are still major hurdles in the clinical application of ONTs. NSCs secrete exosomes, which may enable the transfer of endogenous microRNAs, antigenic peptides, and perhaps even synthetic ONTs 55. Our preliminary studies suggest that NSCs can rapidly internalize exosome‐encapsulated ONTs and retain them for ≤3 days (unpublished data). We are still investigating whether NSC‐mediated ONT delivery improves access to glioma and tumor‐associated myeloid cells and thereby reduces tumor burden.
Summary of Preclinical Progress
At present, the most successful NSC‐mediated approaches that have advanced to the clinic involve cargo that exhibits a substantial bystander or amplification effect (i.e., prodrug‐converting enzymes and oncolytic viruses). NSCs can also potentially improve the efficacy of other therapeutic protein‐ or NP‐based cargo while minimizing off‐target toxicities. Further development will focus on optimizing the number and distribution of NSCs throughout loco‐regional and systemic solid tumor metastases. Furthermore, the majority of preclinical studies to date have involved immunodeficient xenograft models. Given the emerging importance of eliciting an antitumor immune response for effective treatments, studies in syngeneic and/or humanized mouse models will be important.
Clinical Development of NSCs as Therapeutic Vehicles
Despite these challenges, several academic institutions have begun the translational process for NSC‐mediated anticancer therapies. Table 2 lists both ongoing and complete clinical trials using cells to deliver therapeutic cargo to tumors. The lessons learned from these clinical trials are discussed in the following section, “Impact on Disease Treatment.” All human trials were performed with informed consent and were preceded by approval by both local institutional review board approval and the U.S. Department of Health and Human Services, U.S. Food and Drug Administration.
Table 2.
Cell‐mediated cancer therapy: Clinical trials
| Anticancer agent | Mechanism | Tumor targeted | Route of administration | Tumor‐tropic cell | http://clinicaltrials.gov ID | Reference |
|---|---|---|---|---|---|---|
| 5‐FU | Inhibits TS | Recurrent high‐grade glioma | Intracerebral | HB1.F3.CD21 | NCT01172964 | 56 |
| 5‐FU | Inhibits TS | Recurrent high‐grade glioma | Intracerebral | HB1.F3.CD21 | NCT02015819 | 56 |
| SN‐38 | Inhibits topoisomerase | Recurrent high‐grade glioma | Intracerebral | HB1.F3.CD21.hCE1m6 | NCT02192359 | 46 |
| HSV‐TK + GCV | GCV‐triphosphate cytotoxicity | Recurrent high‐grade glioma | Intracerebral | Nonmigratory fibroblasts | NCT00001328 | 57 |
| CRAd‐S‐pk7 virus | Oncolysis | Newly diagnosed glioma | Intracerebral | HB1.F3.CD21.CRAd‐S‐pk7 | NCT03072134 | 51 |
Abbreviations: 5‐FU, 5‐fluorouracil; GCV, ganciclovir; HSV‐TK, herpes simplex virus thymidine kinase type 1; SN‐38, irinotecan; TS, thymidylate synthase.
First‐in‐Human Fibroblast‐Mediated Enzyme/Prodrug Therapy for Glioblastoma
This trial (1992–2010) was the first clinical attempt to treat malignant tumors in human beings by in vivo genetic manipulation of the tumor's genome. GBM tumors were directly injected with nonmigratory fibroblasts engineered to deliver a retrovirus that incorporated herpes‐thymidine kinase to tumor cells (NCT00001328). This trial underscored the need for migratory cells to distribute the therapeutic payload to tumor satellites.
First‐in‐Human NSC‐Mediated Enzyme/Prodrug Therapy for GBM
A first‐in‐human pilot safety/feasibility study was completed in 2013 to assess the safety of using genetically modified allogeneic NSCs for tumor‐selective enzyme/prodrug therapy (NCT01172964). Fifteen patients with recurrent GBMs received an intracerebral dose of CD‐NSCs at the time of resection or biopsy, followed by a 7‐day course of oral 5‐FC. Intracerebral microdialysis results demonstrated safety, non‐immunogenicity, and proof‐of‐concept for brain tumor‐localized conversion of 5‐FC to 5‐FU by CD‐NSCs 56. Brain autopsy data revealed that NSCs migrated to distant tumor sites and were non‐tumorigenic, demonstrating safety and proof‐of‐concept regarding the ability of NSCs to target tumor foci in the brain and locally produce chemotherapy. A phase I dose‐escalation, multiple‐treatment round study of CD‐NSCs in combination with 5‐FC and folinic acid (Leucovorin) to determine phase II recommended dose was completed in 2017 (NCT02015819).
Second‐Generation NSC‐Mediated Enzyme/Prodrug Therapy for GBM and Neuroblastoma
A phase I clinical trial for recurrent GBM patients is ongoing, similar to the previous protocol, using intracerebrally administered hCE1m6‐NSCs combined with intravenous IRN (NCT02192359). The primary objective of this dose‐escalation, multi‐treatment round study is to demonstrate safety and define the phase II NSC recommended doses. Secondary objectives include pharmacokinetics, immunogenicity, and cell‐fate correlative studies. Intravenous administration of hCE1m6‐NSCs in combination with IRN and temozolomide (TMZ) is planned for a phase I clinical trial for pediatric patients with metastatic neuroblastoma in 2018. This will be the first clinical trial in which therapeutic NSCs are administered intravenously for treatment of solid tumor metastases. The primary objectives are to demonstrate safety and define the phase II recommended dose.
First‐in‐Human NSC‐Mediated Oncoviral Therapy for GBM
A first‐in‐human trial of CRAd‐Survivn‐pk7‐loaded NSCs in combination with TMZ and radiation therapy is currently being conducted at Northwestern University and City of Hope in newly diagnosed GBM patients (NCT03072134). The primary objectives of this dose‐escalation trial are to establish safety and to determine the maximum tolerated CRAd‐Survivin‐pk7‐loaded NSC dose for phase II trials.
Summary of Clinical Progress
The results of glioma clinical trials to date with the allogeneic, clonal HB1.F3.CD21 NSC line have demonstrated safety, even when multiple‐treatment rounds are administered into the brain. The initial study, in which one round of treatment was given, demonstrated non‐immunogenicity and proof‐of‐principle for brain tumor‐localized NSC‐mediated prodrug conversion and long‐distance migration to tumor microsatellites. A first‐in‐human trial for metastatic neuroblastoma, in which NSCs will be administered intravenously, is pending. Once safety of intravenous NSC administration is established, the challenge will be to use therapeutic cargo that is potent and selective enough to more significantly reduce and eliminate tumor burden.
Impact on Disease Treatment
With sufficient development, NSC‐mediated therapies can revolutionize the way cancer patients are treated and significantly improve their quality of life during and after treatments. So far, the use of allogeneic, immortalized NSCs has provided a safe, economical, and reliable way to translate this platform technology into the clinic. Immortalized karyotypically normal, v‐myc immortalized NSCs have shown chromosomal and functional stability over multiple passages and good manufacturing practice scale‐up, and have demonstrated clinical safety and non‐tumorigenicity. Although allogeneic NSCs have no major histocompatibility complex (MHC) class 2 expression and low MHC class 1 expression, in early trials we are screening out patients with the same MHC class 1 expression as the allogeneic NSC line. Careful monitoring of potential immune responses after each dose in multiple treatment round studies is also important. In vitro allorecognition of NSCs by peripheral blood lymphocytes has been reported 58. In one study in which NSCs were injected into immunocompetent cotton rat and hamster models, the NSCs became vulnerable to immune‐mediated clearance within 3 days post‐transplantation 50. One possible alternative is the use of patient‐derived fibroblasts that have been transdifferentiated into tumor‐homing NSCs 59. This autologous approach could eliminate complications of immunosuppressive regimens and also prolong therapeutic NSC persistence to increase treatment durability 59. Much work is still needed to realize the full potential of NSC‐mediated cancer treatments.
Ongoing development work for the glioma trials is focused on improving NSC administration to optimize tumor coverage. We estimate that each intratumoral administration results in up to 25%–50% tumor coverage 30, suggesting a need to develop repeat administration strategies, alternative administration routes, or an self‐amplifying therapeutic payload. Ongoing investigations are assessing the relative effectiveness of interventricular 60 and intranasal 22 administration. Alternatively, we are considering the use of a hydrogel delivery matrix placed in the resection cavity to protect and increase viability of NSCs implanted, and maximize the NSC/tumor interface 53. Until NSC administration is optimized, the best clinical efficacy will likely be observed using cargo with the largest bystander effect, or self‐amplifying replication‐competent oncolytic viruses. While initial trials have focused on local and loco‐regional disease settings, they will soon expand into the peripheral setting with the upcoming neuroblastoma trial. Pulmonary bypass may limit the type of therapeutic cargo that can effectively be delivered intravenously to gene‐based therapies, which hold little risk of off‐target deposition given that administered NSCs only remain viable if they have reached the tumor site.
While the intended role of tumor‐tropic NSCs is to deliver and/or produce a therapeutic within the tumor, NSCs are complex biological machines that may have additional unintended effects on tumor progression (positive or negative) if they persist or replicate within the tumor environment for an extended period of time. The duration of NSC persistence within the tumor depends on several factors, including post‐transplantation survival efficiency, immune recognition, and tumorigenicity of the NSCs. Like most other cell therapies, post‐transplantation survival efficiency of NSCs is currently low (<10%), so new efforts are underway to equip them with viability‐promoting antioxidants 61, antiapoptotic transgenes 62, or support matrices 53.
As the duration of therapeutic NSC persistence increases, it will be important to gain more insight into how NSCs impact the immune microenvironment within the tumor. The immunosuppressive properties of NSCs have been reported in animal models of experimental autoimmune encephalomyelitis, in which NSCs secrete molecules that inhibit the proliferation and activation of T‐cells, antigen‐presenting cells, and microglia, and can even induce apoptosis in blood‐borne, CNS‐infiltrating, pro‐inflammatory T helper type 1 cells 63. However, the net impact that NSCs have on the tumor immune microenvironment remains to be elucidated. Early clinical studies using tumor‐tropic NSCs within tumor settings were performed without collecting complete immune profiles, and the majority of preclinical therapeutic efficacy studies have been evaluated in immunodeficient xenograft models. It has been reported that NSCs delivering oncolytic viral payloads can release pro‐inflammatory immunomodulators, IL‐6, and tumor necrosis factor α, which are associated with triggering an innate immune response, but NSCs also release the immunosuppressive cytokine IL‐10 49. NSCs can also decrease oncolytic virus‐mediated astroglial activation within nude mouse brains, but no measurable effects with regard to macrophage infiltration have been observed 49. And no effects of the NSCs on adaptive immune cells within the context of a tumor model have yet been reported. If NSCs are determined to have an overall immunosuppressive effect within the tumor, it may be necessary to engineer them with immune‐stimulating cytokines (e.g., IL‐4, IL‐12, and TRAIL) or secretable nucleotide sequences.
Tumorigenicity of tumor‐tropic NSCs has not yet been a problem, despite it being a substantial regulatory concern when the first‐in‐human allogeneic NSC‐mediated CD/5‐FC trial for recurrent glioma (NCT01172964) was initiated in 2010. Concerns arose after donor‐derived tumors developed in settings in which NSCs were intended to engraft and provide sustained therapeutic benefit (i.e., a preclinical peripheral nerve injury model 64 and a clinical case of ataxia telangiectasia in which the NSC source was not adequately defined 65). Through these studies, it became apparent that each NSC source (clone or pool) must be characterized for genetic and functional stability over time and passage, as well as tumortropism. Recent clinical results using the allogeneic clonal HB1.F3.CD NSC line, which is extensively characterized to be chromosomally stable, have demonstrated non‐tumorigenicity 11, 56.
Future work will focus on developing more effective methodologies to image NSC biodistribution within patients. In addition, NSC‐mediated cancer therapy is capable of synchronizing the delivery of multiple anticancer agents, which will likely afford more robust therapeutic efficacy against dynamic and heterogeneous tumors that acquire resistance to single‐agent treatment approaches. Improved efficacy of combinatorial MSC‐mediated therapies has been confirmed 26, but this approach has yet to be explored fully using NSC delivery vehicles.
Author Contributions
R.M.: collection, assembly, and interpretation of references; manuscript organization and writing. M.H.: collection of references, manuscript organization. J.B.‐C.: collection of references, manuscript editing. A.A.M.: figure preparation. K.S.A.: collection, assembly, and interpretation of references; manuscript organization and writing.
Disclosure of Potential Conflicts of Interest
R.M. discloses patent holder and research funding. K.A. discloses employment/Advisory role/stock ownership with CSO, TheraBiologics, Inc.; intellectual property with City of Hope and Harvard Patents. All other authors disclosure no potential conflict of interest.
Acknowledgements
Financial support was provided by the Ben & Catherine Ivy Foundation, the Rosalinde and Arthur Gilbert Foundation, the Alvarez Charitable Foundation, the Anthony F. and Susan M. Markel Foundation, STOP Cancer, City of Hope, H.N. & Frances Berger Foundation, Norman & Melinda Payson Fellowship, and NIH‐NCI grants R01 CA198076, R01 FD004816, R43 CA86768, R44 CA8678, and P30 CA03357 NIH‐NINDS U01NS082328. K.S.A. is a director and officer of TheraBiologics Inc.
References
- 1. Jemal A, Siegel R, Xu J et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kendall SE, Najbauer J, Johnston HF et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3‐kinase signaling. Stem Cells 2008;26:1575–1586. [DOI] [PubMed] [Google Scholar]
- 4. Magge SN, Malik SZ, Royo NC et al. Role of monocyte chemoattractant protein‐1 (MCP‐1/CCL2) in migration of neural progenitor cells toward glial tumors. J Neurosci Res 2009;87:1547–1555. [DOI] [PubMed] [Google Scholar]
- 5. Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest 2004;113:1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heese O, Disko A, Zirkel D et al. Neural stem cell migration toward gliomas in vitro. Neuro Oncol 2005;7:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt NO, Przylecki W, Yang W et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia 2005;7:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao D, Najbauer J, Garcia E et al. Neural stem cell tropism to glioma: Critical role of tumor hypoxia. Mol Cancer Res 2008;6:1819‐1829. [DOI] [PubMed] [Google Scholar]
- 9. Ziu M, Schmidt NO, Cargioli TG et al. Glioma‐produced extracellular matrix influences brain tumor tropism of human neural stem cells. J Neurooncol 2006;79:125–133. [DOI] [PubMed] [Google Scholar]
- 10. Zhao D, Najbauer J, Annala AJ et al. Human neural stem cell tropism to metastatic breast cancer. Stem Cells 2012;30:314–325. [DOI] [PubMed] [Google Scholar]
- 11. Aboody KS, Najbauer J, Metz MZ et al. Neural stem cell‐mediated enzyme/prodrug therapy for glioma: Preclinical studies. Sci Transl Med 2013;5:184ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutova M, Shackleford GM, Khankaldyyan V et al. Neural stem cell‐mediated CE/CPT‐11 enzyme/prodrug therapy in transgenic mouse model of intracerebellar medulloblastoma. Gene Ther 2013;20:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aboody KS, Najbauer J, Schmidt NO et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol 2006;8:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seol HJ, Jin J, Seong DH et al. Genetically engineered human neural stem cells with rabbit carboxyl esterase can target brain metastasis from breast cancer. Cancer Lett 2011;311:152–159. [DOI] [PubMed] [Google Scholar]
- 15. Hong SH, Lee HJ, An J et al. Human neural stem cells expressing carboxyl esterase target and inhibit tumor growth of lung cancer brain metastases. Cancer Gene Ther 2013;20:678–682. [DOI] [PubMed] [Google Scholar]
- 16. Cao P, Mooney R, Tirughana R et al. Intraperitoneal administration of neural stem cell‐nanoparticle conjugates targets chemotherapy to ovarian tumors. Bioconjug Chem 2017;28:1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuroki LM, Jin X, Dmitriev IP et al. Adenovirus platform enhances transduction efficiency of human mesenchymal stem cells: An opportunity for cellular carriers of targeted TRAIL‐based TR3 biologics in ovarian cancer. PLoS One 2017;12:e0190125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi SS, Yoon K, Choi SA et al. Tumor‐specific gene therapy for pancreatic cancer using human neural stem cells encoding carboxylesterase. Oncotarget 2016;7:75319‐75327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yi BR, Kim SU, Choi KC. Co‐treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT‐11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget 2014;5:12835–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rachakatla RS, Balivada S, Seo GM et al. Attenuation of mouse melanoma by A/C magnetic field after delivery of bi‐magnetic nanoparticles by neural progenitor cells. ACS Nano 2010;4:7093–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutova M, Goldstein L, Metz M et al. Optimization of a neural stem‐cell‐mediated carboxylesterase/irinotecan gene therapy for metastatic neuroblastoma. Mol Ther Oncolytics 2017;4:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balyasnikova IV, Prasol MS, Ferguson SD et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol Ther 2014;22:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mader EK, Maeyama Y, Lin Y et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res 2009;15:7246–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehtesham M, Kabos P, Kabosova A et al. The use of interleukin 12‐secreting neural stem cells for the treatment of intracranial glioma. Cancer Res 2002;62:5657–5663. [PubMed] [Google Scholar]
- 25. Lee AS, Tang C, Rao MS et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 2013;19:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei J, Blum S, Unger M et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell 2004;5:477–488. [DOI] [PubMed] [Google Scholar]
- 27. Corsten MF, Shah K. Therapeutic stem‐cells for cancer treatment: Hopes and hurdles in tactical warfare. Lancet Oncol 2008;9:376–384. [DOI] [PubMed] [Google Scholar]
- 28. Mooney R, Abdul Majid A, Batalla J et al. Cell‐mediated enzyme prodrug cancer therapies. Adv Drug Deliv Rev 2017;118:35–51. [DOI] [PubMed] [Google Scholar]
- 29. Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev 2012;64:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barish ME, Herrmann K, Tang Y et al. Human neural stem cell biodistribution and predicted tumor coverage by a diffusible therapeutic in a mouse glioma model. Stem Cells Translational Medicine 2017;6:1522–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang J, Lam DH, Goh SS et al. Tumor tropism of intravenously injected human‐induced pluripotent stem cell‐derived neural stem cells and their gene therapy application in a metastatic breast cancer model. Stem Cells 2012;30:1021–1029. [DOI] [PubMed] [Google Scholar]
- 32. Bagci‐Onder T, Du W, Figueiredo JL et al. Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain 2015;138:1710‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joo KM, Park IH, Shin JY et al. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol Ther 2009;17:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SK, Kim SU, Park IH et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res 2006;12:5550–5556. [DOI] [PubMed] [Google Scholar]
- 35. Kim JW, Robert Kane J, Young JS et al. Neural stem cell‐mediated delivery of oncolytic adenovirus. Curr Protoc Hum Genet 2015;85:13.11.1–13.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanojia D, Balyasnikova IV, Morshed RA et al. Neural stem cells secreting anti‐HER2 antibody improve survival in a preclinical model of HER2 overexpressing breast cancer brain metastases. Stem Cells 2015;33:2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benedetti S, Pirola B, Pollo B et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 2000;6:447–450. [DOI] [PubMed] [Google Scholar]
- 38. Sims TL Jr, Hamner JB, Bush RA et al. Neural progenitor cell‐mediated delivery of osteoprotegerin limits disease progression in a preclinical model of neuroblastoma bone metastasis. J Pediatr Surg 2009;44:204–210. discussion 210‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Eekelen M, Sasportas LS, Kasmieh R et al. Human stem cells expressing novel TSP‐1 variant have anti‐angiogenic effect on brain tumors. Oncogene 2010;29:3185–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SK, Cargioli TG, Machluf M et al. PEX‐producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res 2005;11:5965–5970. [DOI] [PubMed] [Google Scholar]
- 41. Yuan X, Hu J, Belladonna ML et al. Interleukin‐23‐expressing bone marrow‐derived neural stem‐like cells exhibit antitumor activity against intracranial glioma. Cancer Res 2006;66:2630–2638. [DOI] [PubMed] [Google Scholar]
- 42. Mooney R, Roma L, Zhao D et al. Neural stem cell‐mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS Nano 2014;8:12450–12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mooney R, Weng Y, Garcia E et al. Conjugation of pH‐responsive nanoparticles to neural stem cells improves intratumoral therapy. J Control Release 2014;191:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng Y, Morshed R, Cheng SH et al. Nanoparticle‐programmed self‐destructive neural stem cells for glioblastoma targeting and therapy. Small 2013;9:4123–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li W, Xiang AP. Safeguarding clinical translation of pluripotent stem cells with suicide genes. Organogenesis 2013;9:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Metz MZ, Gutova M, Lacey SF et al. Neural stem cell‐mediated delivery of irinotecan‐activating carboxylesterases to glioma: Implications for clinical use. Stem Cells Translational Medicine 2013;2:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cattaneo R, Miest T, Shashkova EV et al. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat Rev Microbiol 2008;6:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hartkopf AD, Fehm T, Wallwiener M et al. Oncolytic viruses to treat ovarian cancer patients—A review of results from clinical trials. Geburtshilfe Frauenheilkd 2012;72:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmed AU, Thaci B, Alexiades NG et al. Neural stem cell‐based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther 2011;19:1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thaci B, Ahmed AU, Ulasov IV et al. Pharmacokinetic study of neural stem cell‐based cell carrier for oncolytic virotherapy: Targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther 2012;19:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tobias AL, Thaci B, Auffinger B et al. The timing of neural stem cell‐based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Translational Medicine 2013;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frank RT, Edmiston M, Kendall SE et al. Neural stem cells as a novel platform for tumor‐specific delivery of therapeutic antibodies. PLoS One 2009;4:e8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kauer TM, Figueiredo JL, Hingtgen S et al. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat Neurosci 2011;15:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mooney R, Weng Y, Tirughana‐Sambandan R et al. Neural stem cells improve intracranial nanoparticle retention and tumor‐selective distribution. Future Oncol 2014;10:401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han C, Sun X, Liu L et al. Exosomes and their therapeutic potentials of Stem Cells. Stem Cells Int 2016;2016:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Portnow J, Synold TW, Badie B et al. Neural stem cell‐based anticancer gene therapy: A first‐in‐human study in recurrent high‐grade glioma patients. Clin Cancer Res 2017;23:2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 2000;11:2389–2401. [DOI] [PubMed] [Google Scholar]
- 58. Ubiali F, Nava S, Nessi V et al. Allorecognition of human neural stem cells by peripheral blood lymphocytes despite low expression of MHC molecules: Role of TGF‐beta in modulating proliferation. Int Immunol 2007;19:1063‐1074. [DOI] [PubMed] [Google Scholar]
- 59. Bagó JR, Okolie O, Dumitru R et al. Tumor‐homing cytotoxic human induced neural stem cells for cancer therapy. Sci Transl Med 2017;9(375). Article number: eaah6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown CE, Alizadeh D, Starr R et al. Regression of glioblastoma after chimeric antigen receptor T‐cell therapy. N Engl J Med 2016;375:2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim CK, Ahmed AU, Auffinger B et al. N‐acetylcysteine amide augments the therapeutic effect of neural stem cell‐based antiglioma oncolytic virotherapy. Mol Ther 2013;21:2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mooney R, Majid A, Mota D, Aramburo S et al. Aboody, Bcl‐2 overexpression improves survival and efficacy of neural stem cellmediated enzyme prodrug therapy. Stem Cells Int 2018; Article ID 7047496, 13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pluchino S, Zanotti L, Rossi B et al. Neurosphere‐derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005;436:266–271. [DOI] [PubMed] [Google Scholar]
- 64. Radtke C, Redeker J, Jokuszies A et al. In vivo transformation of neural stem cells following transplantation in the injured nervous system. J Reconstr Microsurg 2010;26:211–212. [DOI] [PubMed] [Google Scholar]
- 65. Amariglio N, Hirshberg A, Scheithauer BW et al. Donor‐derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLOS Med 2009;6:e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]


