Abstract
We report the case of a patient who had undergone injections of myoblasts in an infarct area 16 years before being referred for heart transplantation. The pathological examination of the explanted heart found persisting myotubes embedded in fibrosis. This finding supports the ability of myoblasts to survive in harsh environments, which can make them appealing candidates for transplantation in diseases requiring supply of new myogenic cells. Stem Cells Translational Medicine 2018;7:705–708
Keywords: Myoblasts, Heart failure, Clinical cell transplantation, Long‐term follow‐up
Significance Statement.
The finding of engrafted myotubes in a postinfarction scar in a patient who underwent cardiac transplantation 16 years after having received intramyocardial injections of autologous myoblasts supports the robustness of these cells, which could be relevant to their clinical use in muscular dystrophies or sphincter incontinence.
In June 2000, we initiated a phase I trial of autologous myoblast transplantation in patients with severe ischemic left ventricular dysfunction 1. The fourth patient of this series was a 39‐year‐old man who received 950 million myoblasts injected in 31 sites in a posterior postinfarction fibrous scar measured intraoperatively at 35 cm2, in combination with a double coronary artery bypass graft. He was then symptomatically improved over the next 9 years, with a left ventricular ejection fraction, which increased from 22% preoperatively to a peak value of 45% 1 year after the operation. From 2009 onward, his ejection fraction started to decline, and 1 year later, he was admitted in a local hospital for an acute coronary syndrome and underwent angioplasty of a new lesion of the left circumflex artery (not present in the preoperative coronary angiogram); the saphenous vein graft was found occluded while the internal mammary graft was fully patent. The patient then started to experience several episodes of heart failure and was only transiently improved by vagal stimulation. The worsening of this cardiac condition culminated in a cardiogenic shock in June 2016, which led to a pretransplantation check‐up. However, the repetition of episodes of heart failure precipitated the implantation of a left ventricular device as a bridge in December 2016, and 1 month later, he was finally transplanted. Although the operation was initially complicated with a primary graft dysfunction, the postoperative course gradually improved and the patient was finally discharged in a stable condition.
The explanted heart was sent to the pathology laboratory. Unexpectedly, several myotubes, embedded in fibrosis, were identified in 5 of 15 randomly sampled paraffin blocks of the postinfarction scarred myocardial tissue. They contained several nuclei (three to a dozen in a given section; Fig. 1), with occasional Z striations. Immunophenotyping of these myotubes was positive for the following markers: myosin heavy chain, the fast isoform (Fig. 1B, 1C) being largely predominant over the slow one (Fig. 1); the skeletal isoform of troponin T (Fig. 1); the myogenic differentiation factor myogenin (Fig. 1); and myoglobin. Some myotubes also expressed CD56 (N‐CAM, a marker of satellite cells, myoblasts, and myotubes; Fig. 1). Altogether, these features reflected the skeletal muscle origin of the cells that were identified in the scar. However, markers of quiescent myogenic cells (Pax7) or of early‐committed myoblasts (MyoD1) were not observed. The transplanted cells presented no replicating activity as they were negative for the expression of the proliferating cell nuclear antigen. The cardiac markers GATA4 and connexin 43 were not expressed either in the grafted area, although they were identified in the neighboring cardiac tissue. The intermediate filament desmin, which is normally present in both skeletal, smooth, and cardiac muscle cells, was accordingly expressed by single cells and myotubes in the injected area, vessel cells, and the neighboring cardiomyocytes. Nerve cells positive for PS100 and neurofilament antibodies were present in the injected area but did not project over the myogenic structures.
Figure 1.
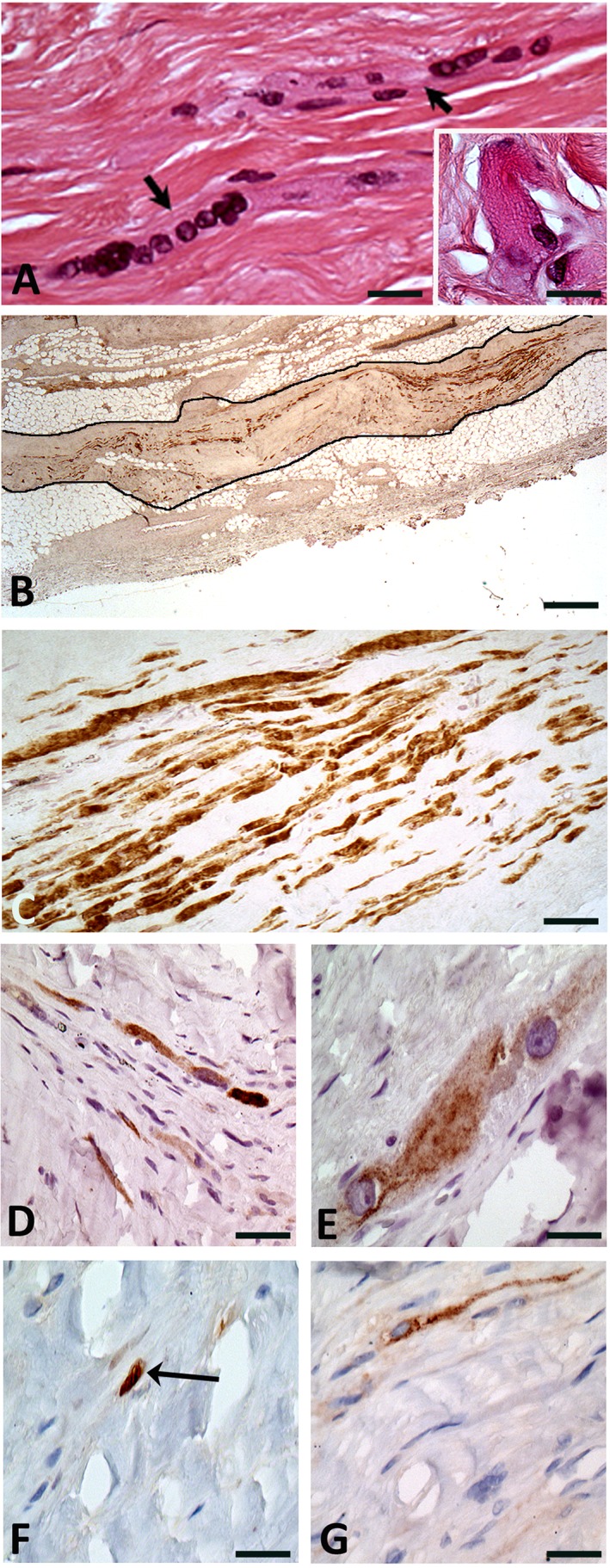
Histological and immunochemical findings in the native heart at the site of the cell‐transplanted myocardial infarction. (A): Multinucleated skeletal muscle cells/myotubes (arrows) embedded in fibrosis. Inset shows the Z‐bands in a skeletal muscle cell. Hematoxylin & Eosin staining. Original ×40 and ×100 for inset. (B): Large sub‐epicardial focus (black outline) of grafted skeletal muscle cells expressing the fast isoform of the skeletal muscle myosin heavy chain. Original ×2.5. (C): Strong expression of the fast isoform of the skeletal muscle myosin heavy chain (MYH1) by several cells. Although the density of skeletal muscle cells is high, they are separated by fibrosis. Original ×20. (D): Expression of the slow isoform of the skeletal muscle myosin heavy chain (MYH7) by a small proportion of the grafted cells. Original ×20. (E): Expression of the skeletal troponin T (TNNT3) fast isoform by the grafted skeletal muscle cells. Original ×40. (F): Expression of the myogenin transcription factor in nuclei of the grafted skeletal muscle cells (arrow). Original ×40. (G): Expression of CD56 by myotubes. Original ×40.
The identification of some cells engrafted 16 years earlier in a postinfarction scar is at variance with most of the published studies, which have reported that intramyocardially transplanted cells are usually short‐lived 2, 3. This exceptional longevity is likely due to the specific capacity of myoblasts to resist injurious stresses and was yet demonstrated by their presence in a skeletal muscle 18 months following transplantation in a patient with Duchenne muscular dystrophy 4. Likewise, in the heart, previous experimental data have shown persistence of intramyocardially delivered myoblasts at 3 months in rats 5 and until 1 year in a sheep model of myocardial infarction 6. Human data confirm these findings regardless of whether they were obtained from biopsy specimens taken either at the time of heart transplantation in patients previously implanted with an assist device combined with myoblast transplantation 7, 8 or during an autopsy 9. So far, however, the longest clinical follow‐up was limited to 17.5 months 9. The 16‐year follow‐up reported in the current observation is thus probably the longest one ever documented after any type of cell transplantation in the heart.
Histologically, the engrafted cells displayed typical patterns of myotubes and expressed the fast (glycolytic) isoform of myosin heavy chain even though they may have incurred milieu‐induced phenotypic changes accounting for some expression of the slow‐type myosin heavy chain isoform, characterized by an oxidative metabolism. This finding suggests that some cells could rely on both glycolytic and oxidative metabolic pathways, which might have endowed them with a survival advantage in this environment. Mechanistically, the persisting engraftment of myoblasts in a cardiac milieu intrinsically different from their specific one suggests that transplanted myoblasts and graft‐derived myotubes may survive for years without the support of a classically organized myogenic niche, although the negativity of Pax‐7 staining suggests that the grafted myoblasts did not regenerate quiescent satellite cells, possibly because of the lack of supportive basal laminae under which they normally reside. The engrafted myotubes were found insulated from the neighboring cardiomyocytes and did not express connexin 43, which confirms the lack of an electrical integration at the graft‐host interface 10. This pattern has been held responsible for the arrhythmias reported after myoblast transplantation in the heart. Indeed, our patient presented an early postoperative arrhythmic storm, which led to the implantation of an internal cardioverter‐defibrillator. No further noticeable arrhythmic episodes were observed during the follow‐up despite the persisting engraftment of some of the cells. It is possible that aside from the cellular phenotype, the mode of cell delivery, which was based on multiple intramyocardial injections, has played a role in the genesis of these early postprocedural arrhythmias. as a recent clinical study reported the absence of electrical instability when myoblasts were delivered under the form of epicardial sheets 11. Mechanistically, the finding that myofiber bundles were embedded in scar tissue makes it unlikely that they contributed to improve heart contractility. However, if one assumes that, in combination with the coronary artery bypass, myoblast grafting may have helped in stabilizing the patient's cardiac condition for 9 years, it is conceivable that the underlying mechanism of action was paracrine, because myoblasts have been shown to release factors involved in several pathways including preservation of matrix architecture 12, decrease in fibrosis 13 and apoptosis 14, stimulation of angiogenesis 14, and mitigation of oxidative stress 15. Of note, in the last computed tomography scan, which was performed 6 months before heart transplantation, the seven inferior wall segments corresponding in part to the infarcted myoblast‐transplanted area were assessed as hypokinetic (i.e., not completely akinetic, in contrast to other areas of the heart). Although these data must be interpreted very cautiously, they may suggest that the paracrine effects of the transplanted cells possibly contributed to slightly improve the contractility of the target area, more likely by acting on the still viable peri‐infarct tissue rather than by actually reducing the size of the core infarct. We acknowledge, however, that in the absence of serial measurements of infarct size throughout the 16‐year follow‐up, no definite conclusions can be drawn regarding the precise mechanism of action of the transplanted cells.
Although this observation is unlikely to rekindle interest in grafting myoblasts in patients with severe heart failure, it supports the robustness of these cells, reflected by their survival in a hostile, poorly vascularized environment and in the absence of any observed innervation. As such, the current findings could be relevant to current or future applications of skeletal muscle cell‐derived therapy in muscular dystrophies 16, 17, 18 or urinary 19, 20 or anal sphincter incontinence 21.
Author Contributions
M.C.: provision of study material, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; M.‐C.B.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; J.‐T.V.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.‐P.M., M.D., and J.L.: provision of study material or patient data, data analysis and interpretation, manuscript writing, final approval of manuscript; G.S.: collection and/or assembly of data, data analysis and interpretation (imaging); P.B.: provision of study material collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.A.H. and P.M.: provision of study material and management of the patient, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1. Menasché P, Hagège AA, Scorsin M et al. Myoblast transplantation for heart failure. Lancet 2001;357:279–280. [DOI] [PubMed] [Google Scholar]
- 2. Seeger FH, Zeiher AM, Dimmeler S. Cell‐enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med 2007;4(suppl 1):S110–S113. [DOI] [PubMed] [Google Scholar]
- 3. Abdelwahid E, Kalvelyte A, Stulpinas A et al. Stem cell death and survival in heart regeneration and repair. Apoptosis Int J Program Cell Death 2016;21:252–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skuk D, Goulet M, Roy B et al. First test of a “high‐density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: Eighteen months follow‐up. Neuromuscul Disord 2007;17:38–46. [DOI] [PubMed] [Google Scholar]
- 5. Reinecke H, MacDonald GH, Hauschka SD et al. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol 2000;149:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghostine S, Carrion C, Souza LC et al. Long‐term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation 2002;106:I131–I136. [PubMed] [Google Scholar]
- 7. Pagani FD, DerSimonian H, Zawadzka A et al. Autologous skeletal myoblasts transplanted to ischemia‐damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol 2003;41:879–888. [DOI] [PubMed] [Google Scholar]
- 8. Fujita T, Sakaguchi T, Miyagawa S et al. Clinical impact of combined transplantation of autologous skeletal myoblasts and bone marrow mononuclear cells in patients with severely deteriorated ischemic cardiomyopathy. Surg Today 2011;41:1029‐1036. [DOI] [PubMed] [Google Scholar]
- 9. Hagège AA, Carrion C, Menasché P et al. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet 2003;361:491–492. [DOI] [PubMed] [Google Scholar]
- 10. Leobon B, Garcin I, Menasché P et al. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A 2003;100:7808–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyagawa S, Domae K, Yoshikawa Y et al. Phase I clinical trial of autologous stem cell–sheet transplantation therapy for treating cardiomyopathy. J Am Heart Assoc 2017;6:e003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farahmand P, Lai TYY, Weisel RD et al. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation 2008;118:S130–S137. [DOI] [PubMed] [Google Scholar]
- 13. Shintani Y, Fukushima S, Varela‐Carver A et al. Donor cell‐type specific paracrine effects of cell transplantation for post‐infarction heart failure. J Mol Cell Cardiol 2009;47:288–295. [DOI] [PubMed] [Google Scholar]
- 14. Perez‐Ilzarbe M, Agbulut O, Pelacho B et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail 2008;10:1065–1072. [DOI] [PubMed] [Google Scholar]
- 15. Siltanen A, Nuutila K, Imanishi Y et al. The paracrine effect of skeletal myoblasts is cardioprotective against oxidative stress and involves EGFR‐ErbB4 signaling, cystathionase, and the unfolded protein response. Cell Transplant 2016;25:55–69. [DOI] [PubMed] [Google Scholar]
- 16. Périé S, Trollet C, Mouly V et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: A phase I/IIa clinical study. Mol Ther J Am Soc Gene Ther 2014;22:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skuk D, Tremblay JP. Intramuscular cell transplantation as a potential treatment of myopathies: Clinical and preclinical relevant data. Expert Opin Biol Ther 2011;11:359–374. [DOI] [PubMed] [Google Scholar]
- 18. Skuk D, Tremblay JP. Cell therapy in muscular dystrophies: Many promises in mice and dogs, few facts in patients. Expert Opin Biol Ther 2015;15:1307–1319. [DOI] [PubMed] [Google Scholar]
- 19. Blaganje M, Lukanović A. Ultrasound‐guided autologous myoblast injections into the extrinsic urethral sphincter: Tissue engineering for the treatment of stress urinary incontinence. Int Urogynecology J 2013;24:533‐535. [DOI] [PubMed] [Google Scholar]
- 20. Peters KM, Dmochowski RR, Carr LK et al. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J Urol 2014;192:469–476. [DOI] [PubMed] [Google Scholar]
- 21. Frudinger A, Pfeifer J, Paede J et al. Autologous skeletal‐muscle‐derived cell injection for anal incontinence due to obstetric trauma: A 5‐year follow‐up of an initial study of 10 patients. Colorectal Dis 2015;17:794‐801. [DOI] [PubMed] [Google Scholar]


