Abstract
In this study, 50 tri‐substituted imidazoles (TIs), which are analogs of the small molecules TA‐01 and SB203580, were synthesized and screened for cardiomyogenic activities. Several TIs displayed cardiomyogenic activities when applied during the differentiation from days 3–5. The TIs did not affect the Wnt/β‐catenin pathway during cardiomyogenesis and the likely mechanism of action is through the inhibition of ALK5 of the TGFβ pathway. Interestingly, these TIs promoted the neural differentiation of human pluripotent stem cells (hPSCs) with a similar potency to that of the dual SMAD inhibitors SB431542/LDN‐193189 when dosed from days 1 to 9. The neural induction activities of the TIs correlated with their ALK5 inhibitory activities. This study reports the discovery of small molecule inhibitors of ALK5, which can promote the differentiation of hPSCs into cardiomyocytes or neural cells depending on the time of dosing, showing potential for the production of clinical‐grade cardiac/neural cells for regenerative therapy. Stem Cells Translational Medicine 2018;7:709–720
Keywords: Small molecule, Stem cells, Cardiac, Neural, Differentiation
Significance Statement.
The use of small molecules to induce the differentiation of stem cells has great potential. However, the mechanisms of differentiation are not well studied and the efficiency of differentiation is still not optimal. This article describes the development and use of 50 novel synthetic tri‐substituted imidazoles (TIs), to induce the cardiac differentiation of human pluripotent stem cells. For the first time, ALK5 of the TGFβ pathway was identified as the target for cardiomyogenesis as ALK5 inhibition correlated with the cardiomyogenic activities of the compounds. Interestingly, the same compounds were found to promote neural differentiation, also through ALK5 inhibition. These findings suggest a unique method for promoting cardiac and neural differentiation through a common mechanism, to provide clinical‐grade adult cells. This study will garner much interest from scientists who are interested in the use of small molecules for stem cells differentiation.
Introduction
One of the “holy grails” in regenerative medicine is the ability to create customized functionally active human cells to replace diseased or unhealthy cells in tissues and organs 1. As such, there has been much interest and progress in stem cell research, aimed at understanding the differentiation and expansion of various types of stem cells into cell‐specific lineages. Specifically, the use of small molecules that can interfere in signaling pathways that can control the differentiation of stem cells into various cell types is highly coveted due to the ease of application and quality control 2. The latter is due to the defined structure of small molecules that facilitates production and purification as compared with biologics, making the using of small molecules for large scale cultures of clinical‐grade differentiated cells for stem cell research or regenerative therapy attractive 3, 4. Indeed, a significant number of small molecule inducers have been reported for the differentiation of stem cells to different cell types, for example, cardiomyocytes, though it should be noted that the mechanisms of differentiation are still not well understood 5. In the context of cardiac differentiation, the accepted mechanisms involve the perturbation of Wnt/β‐catenin and TGFβ superfamily pathways 2, 6, 7, 8, 9, 10. The latter has also been implicated in the neural differentiation of human pluripotent stem cells (hPSCs). However, for neural differentiation, the inhibition of the TGFβ superfamily pathways should occur at early stages of differentiation to promote the formation of hPSC‐derived neuro‐ectoderm 11.
We recently reported the cardiomyogenic potential of substituted azoles that are analogs of the small molecule SB203580. The best inducer of cardiomyogenesis was TA‐01 (structure is shown in Supporting information Fig. S1) which showed a threefold increase in the percentage expression of NKX2‐5/GFP‐positive cells over DMSO when added from days 4 to 8 12. In this study, an expanded series of TA‐01 analogs with structural variations at the N1, C2, or N3 positions of imidazole (TI‐01 to TI‐50, structures are shown in Supporting information Table S1) were designed and synthesized. Their abilities in inducing the differentiation of hPSCs were evaluated in this study and the mechanism of action was also investigated.
Materials and Methods
Synthesis of Tri‐Substituted Imidazoles
Compound structures, details of synthesis and characterization data are described in the Supporting Information.
Kinase Assay to Determine the IC50 Values
The IC50 values against ALK5, TGFBR2, casein kinase 1 delta (CK1δ), and epsilon (CK1ε) of selected compounds were determined using LanthaScreen Eu Kinase binding assay (Life Technologies, Rockville, MD). The assays were performed following the manufacturers’ protocols in white 384‐well plates (Cat. No. 3572; Corning Life Sciences, Acton, MA). SB203580 and TA‐01 were tested as controls in all assays. The compounds were dissolved in DMSO (Hybri‐Max Cat. No. D2650; Sigma‐Aldrich, St. Louis, MO, 5 mM stocks) and diluted to a final concentration of 1% (vol/vol) DMSO for all assays. Each data point was carried out in triplicate. The assay was run according to the protocol developed by Invitrogen (Carlsbad, CA). Tecan Infinite M1000 Pro microplate reader was used for fluorescence measurements (LanthaScreen Eu kinase binding assay; excitation λ = 317 nm, emission λ = 665, 620 nm). Inhibition curves and IC50 values were generated by nonlinear regression analysis and data represent mean ± SEM. Results are included in Supporting information Table S1.
Kinase Assay to Determine the Percentage Inhibition
The percentage inhibition of ALK5, TGFBR2, CK1δ, and CK1ε at 1 μM of all compounds were determined using LanthaScreen Eu kinase binding assay (Life Technologies). All components of the assay were purchased from Invitrogen according to the recommendations listed in the respective protocols for ALK5, TGFBR2, CK1δ, and CK1ε. The assay was performed in white 384‐well plates (Cat. No. 3572; Corning). The compounds were dissolved in DMSO (Hybri‐Max Cat. No. D2650; Sigma‐Aldrich, 5 mM stocks) and diluted to a concentration of 3 μM in Kinase Buffer A [containing 3% (vol/vol) DMSO]. About 5 μl of this diluted solution was transferred to a well in the 384‐well plate. To the same well, 5 μl of kinase/antibody solution and 5 μL of tracer solution diluted was added according to the manufacturer's protocols. The assay was run according to the protocol developed by Invitrogen. Following incubation at room temperature for 1 hour, fluorescence measurements (excitation λ = 317 nm, emission λ = 665, 620 nm) were carried out using Tecan Infinite M1000 Pro microplate reader. Data analysis was carried out according to the manufacturer's protocols and each compound was carried out in triplicate. The percentage inhibition was calculated using the values obtained from the wells with no compound control (0% inhibition) and no kinase control (100% inhibition). Results are included in the Supporting information Table S1.
Culture of hPSCs for Cardiac and Neural Differentiation
HES‐3 NKX2‐5eGFP/w cells (this reporter cell line was kindly provided by Prof. David Elliot Monash University Australia) 13, IMR‐90, H1 cell lines and HES‐3 (46, XX, ES Cell International, Alameda, CA) were cultured feeder free with Essential 8 Media (Life Technologies) or mTeSR™1 (Stemcell Technologies, Vancouver, Canada). The reporter cell line was passaged mechanically (StemPro EZPassage tool, Invitrogen) as clumps for maintenance on tissue culture plates which was coated with Geltrex (Thermo Fisher Scientific, Waltham, MA). All media was refreshed daily and cultures were passaged every 3–4 days. Cultures were incubated at 37°C in a humidified atmosphere with 5% CO2.
Single Embryoid Body‐Based Cardiac Differentiation Method
HES‐3 (NKX2–5eGFP/w reporter cell line) were dissociated with TrypLE (Invitrogen) and seeded at 1.5 × 104 cells/well in ultra‐low attachment 96‐well clear round bottom plates (Corning) in bSFS medium: DMEM (Life Technologies) supplemented with 2 mM l‐glutamine (Life Technologies), 0.182 mM sodium pyruvate (Life Technologies), 1% non‐essential amino acids (Life Technologies), 0.1 mM β‐mercaptoethanol, 5.6 mg/l transferrin (Life Technologies), 20 μg/l sodium selenite (Sigma‐Aldrich), 0.5% (w/vol) bovine serum albumin (Life Technologies), and 0.25% (w/vol) Hysoy (Sheffield Bioscience, Beloit, WI). Cells were forced to aggregate by spinning down at 800 rpm for 1 minute and incubated for 24 hours with 6 μM CHIR99021 (Selleck, Houston, TX), 7.5 μM Y27632 (Selleck) and 50 μg/ml Matrigel (BD, UK) at 37°C and 5% CO2 to allow EB formation. The medium was refreshed thereafter and the CHIR99021 concentration was lowered to 1.5 μM. 48 hours later the cells were supplied with fresh bSFS medium. On day 2 or day 3, EBs were stimulated with the respective inhibitor compounds (Supporting information Table S1) in DMSO (Sigma‐Aldrich) for 3 days. EBs were harvested for analysis on day 13.
Monolayer Cardiac Differentiation Method
HES‐3 (NKX2‐5eGFP/w reporter cell line), IMR‐90 and H1 cell lines were seeded in 6‐well flat bottom culture plates (Corning) and maintained for 5 days in Essential 8 Media until the cells were 70% confluent. On day 5, the cells are cultured in bSFS medium. Cells were treated with CHIR99021, Y27632, and Matrigel at the same concentration and time course as described in Single‐EB method. On day 3 cells were stimulated with the respective inhibitors (Supporting information Table S1) in DMSO (Sigma‐Aldrich) for 3 days. Cells were harvested on day 13 for analysis.
EB‐Based Image Analysis
On day 13, microscopic images of each EB were captured via a camera mounted on a Nikon Eclipse Ti microscope platform in phase contrast mode and with fluorescence imaging mode. Images were analyzed to objectively identify and quantify the green fluorescence protein (GFP) size of HES‐3 NKX2‐5eGFP/w, and the embryoid body (EB) size with Image J. GFP percentage was calculated following the equation: GFP percentage = (GFP area)/(EB area) The cultures on the platform were kept at 37°C with 5% CO2 via an on‐stage incubator.
Neural Induction of hPSCs (hESCs and hiPSCs)
hESC line HES‐3 (46, XX) or hiPSCs (IMR‐90) were harvested and dissociated by ACCUTASE (Millipore, Billerica, MA) and plated on geltrex‐coated plates (4 × 104 cells/well of 96‐well plate or 4 × 105 cells/well of 12‐well plate). hPSCs were fed with neural induction medium: 50% D‐MEM/F12 (Life Technologies, cat no. 10565–018), 50% Neurobasal Medium (life technologies, cat no. 21103–049), nonessential amino acids (NEAA; Thermo Scientific, 1:100), penicillin/streptomycin (P/S; Thermo Scientific, 1:100), 2 mM l‐Glutamine (Thermo Scientific, 1:100) N2 Supplement (Thermo Scientific, 100X), B27 Supplement Minus Vitamin A (Thermo Scientific, 50X). The test compounds were dissolved in neural induction medium and were added every second day. The dual SMAD inhibitors (LDN‐193189 0.2 μM and SB‐431542 10 μM) were used as positive control and DMSO as negative control. After 9 days of neural induction, induced neural progenitors were harvested for FACS analyses or transferred to geltrex‐coated plates for maintenance or further differentiation.
Maintenance of Neural Progenitor Cells
Cells were harvested and dissociated by ACCUTASE (Millipore) and plated on geltrex‐coated plates (4 x 10 5 cells/well of 6‐well plate). NPCs were fed with NPCs medium: 50% D‐MEM/F12 (Life Technologies), 50% Neurobasal Medium (Life Technologies), nonessential amino acids (NEAA), penicillin/streptomycin (P/S), 2 mM l‐Glutamine, N2 Supplement, B27 Supplement Minus Vitamin A, 20 ng/ml basic fibroblast growth factor (bFGF; Thermo scientific), and 20 ng/ml Epidermal growth factor (EGF; PeproTech GmbH, Rehovot, Israel) for maintenance.
Neuronal Differentiation
In total, 5.0–7.5 × 105 neural progenitor cells were firstly dissociated by ACCUTASE (Millipore) and seeded on geltrex‐coated six‐well plates. The minimal supportive medium used for the differentiating neural cells into neurons was neuronal differentiation medium consists of Neurobasal Medium (Life Technologies), nonessential amino acids (NEAA), penicillin/streptomycin (P/S), 2 mM l‐Glutamine, B27 Supplement Minus Vitamin A, containing 10 ng/ml of brain‐derived neurotrophic factor (BDNF; PeproTech) and 200 μM ascorbic acid (Sigma‐Aldrich). Differentiations were carried out for 4 weeks with media changes every 2–3 days. Differentiated cells were then replated on poly‐d‐lysine (PDL)/laminin‐coated coverslips for imaging.
Flow Cytometry
EBs or cells were harvested and dissociated into single cells using TrypLE Express (Life Technologies) or ACCUTASE (Millipore) for 7–10 minutes in a heating block (Thermomixer comfort, Eppendorf) at 37°C, 1200 rpm. Dissociated samples were pipetted through a 20 μM nylon mesh (Multi Screen, Millipore) and fixed in Fix and Perm solution medium A (Life Technologies) for 15 minutes at room temperature (RT). Cells were incubated with primary antibodies (Supporting information Table S2) in 1% bovine serum albumin (BSA) with 0.2 Triton X‐100 in PBS for 30 minutes. After that the cells were washed with blocking buffer 1% BSA in PBS, and incubated at 4°C for 20 minutes with diluted fluorescent secondary antibodies for conjugation of respective primary antibodies. Cells were washed with blocking buffer and analyzed on a flow cytometer (GUAVA easy Cyte 8HT, Millipore) using standard filter sets for the used secondary antibodies or green fluorescence protein of the HES‐3 NKX2‐5eGFP/w cell line.
Cell Count
Cell concentration was determined by the nuclei count method with DAPI and Acridine Orange using NucleoCounter NC‐3000 (Chemometec) according to the manufacturer's handbook. Expansion folds over DMSO of the growing were calculated by dividing cell number with the cell number from DMSO.
Immunofluorescence Staining of Cardiomyocytes
Differentiating cells were harvested and dissociated into single cells using TrypLE (Life Technologies) for 8 minutes on day 13. Single cells were cultured on Matrigel coated tissue culture plates for 4 days in bSFS medium. Cells were fixed with 4% paraformaldehyde and blocked for 1 hour in 3% BSA/PBS. Primary antibodies (Supporting information Table S2) were incubated overnight at 4°C in 0.2% Triton X‐100, 3% BSA/PBS. Thereafter, Alexa Fluor 594 and 647 conjugated goat anti‐mouse/rabbit secondary antibodies (Life Technologies) were incubated for 2 hours at 1:400 dilution in 3% BSA/PBS. Nuclear staining was counterstained by DAPI. Cell imaging was performed with Nikon Eclipse Ti microscope platform. Pseudo colors were added for a better visualization of the cyto‐antibody stains with Image J.
Immunofluorescence Staining of NPCs and Neurons
Differentiating cells were firstly dissociated using ACCUTASE (Millipore) into single cells for 8 minutes on day 9. The cells were replated and grown on PDL/laminin‐coated coverslips. At the time of assays, cells were rinsed with PBS once, and fixed by 4% PFA for 20 minutes at RT. The fixed cells were washed with PBS, and permeabilized by 0.5% Triton in PBS for 10 minutes. The cells were washed again and blocked in the blocking solution (5% BSA and 0.1% Triton in PBS) for 1 hour at room temperature (RT). Primary antibodies (Supporting information Table S2) were diluted in blocking solutions, and incubated with cells overnight at 4°C. After that, cells were washed three times with PBST (0.1% Tween‐20 in PBS), 10 minutes each. Diluted fluorescent secondary antibodies were applied for conjugation of respective primary antibodies and incubated for 1 hour in the dark. Then the coverslips were washed three times with PBST. Nuclear staining was counterstained by DAPI. The fluorescent images were acquired using Nikon Eclipse Ti microscope platform. Pseudo colors were added for a better visualization of the cyto‐antibody stains with Image J.
Cignal TGFβ Reporter (GFP) Assay
The Cignal TGFβ reporter (GFP) assay (Qiagen, CCS‐017G) was used to assess the effect of small molecules on the TGFβ pathway. This assay kit contains transfection‐ready SMAD reporter constructs as well as positive and negative controls. The SMAD reporter encodes the Monster GFP gene under the control of a minimal (m) CMV promoter and tandem repeats of the SMAD transcriptional response element (TRE). Cells (commercially available transfected fibroblast cells) were seeded into wells (5.0 × 104 cells/well) of a 96‐well plate (Corning) for introducing reporter constructs into MEF cells via reverse transfection according to the manufacturer's protocol (Cignal reporter assay handbook, section F. Transfection and treatment protocol for reporter assay + small molecules/organic compounds). For conditions with combinations of inhibitors and TGFβ3 activators, the cells were first exposed to the inhibitors for 30 minutes before TGFβ3 was added directly to the wells. Cells were incubated for 36 hours before the measurement of GFP expression. Nuclear staining was counterstained by DAPI. The fluorescent images were acquired using Nikon Eclipse Ti microscope platform. Pseudo colors were added for a better visualization of the stains with Image J.
Simple Western Analysis
Total protein was extracted with RIPA lysis and extraction buffer (Sigma Cat. No. R0278) with addition of Halt Protease and Phosphatase Single‐Use inhibitor cocktail (Thermo Scientific, 100X) according to the manufacturer's protocol. Protein quantification was done with Bio‐Rad DC Protein assay. Protein identification and quantification was carried out using a Simple Western system (Peggy Sue , Proteinsimpl, San Jose, CA), a fully automated western capillary based protein separation and detection system. Proteins were firstly separated through a gel matrix and immobilized to the capillary wall by photo‐activation and further identified by various primary antibodies (Supporting information Table S3) and subsequently detected by a horseradish peroxidase conjugated secondary antibody and a chemiluminescent substrate (ProteinSimple, San Jose, CA) 14. Protein expression were separated and analyzed by size following the manufacturer's protocol. The results were analyzed using Compass software (ProteinSimple, San Jose, CA).
Quantitative RT‐PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) from EBs following the supplier's protocol. Reverse transcription was carried out with 1 μg total RNA using SuperScript III (Invitrogen). Real‐time PCR was performed applying a standard two‐step amplification protocol on an ABI 7500 system (Applied Biosystem, Foster City, CA) to detect mRNA expression of Wnt3a (Forward: 5’tgttgggccacagtattcct3’, Reverse: 5’atgagcgtgtcactgcaaag3’, Sigma‐Aldrich). Relative expression values were obtained by normalizing Cτ values of the tested genes to the Cτ values of the house keeping gene GAPDH using the ΔΔCτ method 15.
Statistical Analysis
All experiments were performed at least three times. All data are expressed as the mean ± SEM using the statistical software GraphPad Prism, version 5 (GraphPad Software Inc., La Jolla, CA). Treatments were compared with their respective controls, and significant differences among the groups were determined using one way ANOVA followed by Tukey test. In addition, comparisons of two data sets were statistically analyzed with Student's t test. Statistical significance was indicated by *p < .05, **p < .001, and ***p < .0001, unless otherwise defined. A p value of >.05 is indicated as nonsignificant (N.S.). Bar graph represent mean± SEM, n = 3 (data obtained from three independent experiments).
Results
Evaluation of TIs for Their Abilities to Promote the Cardiomyogenesis of hPSCs
To assess the cardiomyogenic potential of the tri‐substituted imidazoles (TIs), a high‐efficiency method utilizing a single EB‐based cardiac differentiation was employed. In this method, CHIR99021 was added in the first 48 hours, followed by the addition of TIs from days 3 to 5 (Supporting Information Fig. S2A). On Day 13, the EBs were harvested and analyzed for NKX2‐5/GFP expression using image‐based microscopy (image examples are shown in Supporting Information Fig. S2B) 12, 16. From these studies, 11 compounds (TI‐14, TI‐15, TI‐16, TI‐20, TI‐21, TI‐24, TI‐25, TI‐26, TI‐27, TI‐33, and IWP‐2) were found to induce a higher GFP expression than the lead compound TA‐01 (Supporting Information Fig. S2C). Although this method of screening is relatively high‐throughput, there are potential limitations in quantifying the results as EB formation is strongly influenced by the permeability of the TIs and the permeability tests show that some TIs (e.g., TA‐01, TI‐15, and TI‐42) are less permeable as compared with IWR‐1 and CHIR99021 (Supporting Information Table S5). As such, a secondary assay based on a monolayer cardiac differentiation method was developed to evaluate the 19 compounds that were found to be cardiomyogenic based on the single EB screening studies. The workflow for the monolayer cardiac differentiation method is shown in Figure 1A. Similar to the protocol for the single EB‐based method, 6 μM of CHIR99021 was applied to the cells during the first 48 hours of differentiation, followed by the addition of TIs from days 3 to 5. On day 13, the cells were harvested and analyzed for the percentage of NKX2‐5/GFP positive cells using flow cytometric analysis. The effect of compounds on cell growth was also analyzed by counting the cell numbers on day 13. The results show that the compounds did not significantly affect cell growth over 13 days. In terms of cardiac differentiation, seven compounds (i.e., IWR‐1, TA‐01, TI‐15, TI‐21, TI‐24, TI‐29, and PF670642) were observed to have a positive effect on cardiomyogenesis as the percentage of induced NKX2‐5/GFP positive cells (~30%–40%) were significantly higher than the DMSO vehicle (at 14%). Four TIs, TI‐15 (at 38.5%), TI‐21 (at 31.5%), TI‐24 (at 32.5%), and TI‐29 (at 35.0%) showed comparable cardiomyogenic activities as compared with IWR‐1 (at 29.6%, Fig. 1B). Subsequently, the most potent compound TI‐15 was tested for its cardiomyogenic abilities at three different concentrations, 2.5, 5, and 10 μM. The results showed that TI‐15 exhibited the strongest cardiomyogenic activity at 5 μM concentration (Fig. 1C). Immunofluorescence staining of TI‐15 treated cells showed the expression of Troponin T (cTnT, Fig. 1D), myosin light chain 2a (MLC2a, Fig. 1E), and GFP/NKX2‐5 (Fig. 1F).
Figure 1.
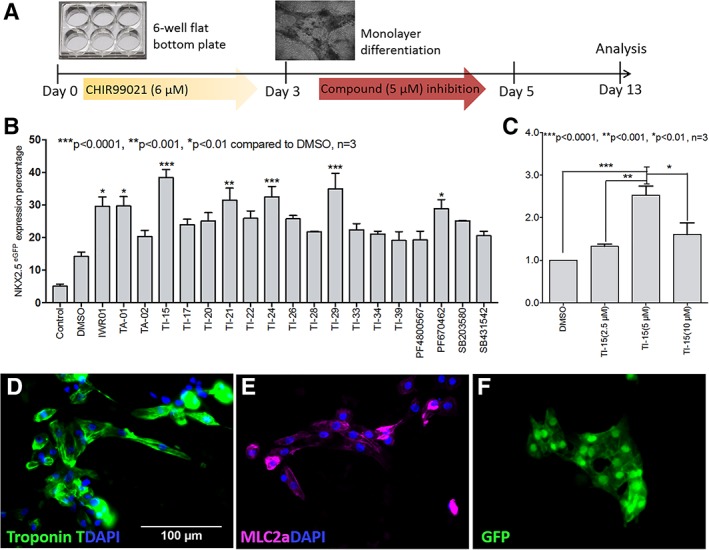
TIs promote the cardiac differentiation of hPSCs using a monolayer approach. (A): Workflow of the monolayer cardiac differentiation method. CHIR99021 (6 μM) was applied in the first 24 hours, and the concentration was reduced to 1.5 μM on day 2 and further reduced to 0 μM on day 3 (CHIR99021 was not added in the “control” condition). The test compounds were applied to the cells during days 3–5 at a 5‐μM concentration (IWR‐1: 2.5 μM). The measurements were obtained on day 13 using flow cytometry and cell counting. (B): The percentage of NKX2–5/GFP positive cells was obtained on day 13 using flow cytometric analysis. “Control” represents the condition with no CHIR99021 or test compound addition. (C) NKX2‐5/GFP positive cell expression was measured using cells treated with 2.5, 5, and 10 μM concentrations of TI‐15 and reported as a fold‐change over DMSO treatment. Statistical significance compared with the DMSO treatment in all figures is indicated as ***p < .0001, **p < .001, and *p < .01, n = 3. The data are presented as the mean ± SEM. Immunofluorescence staining images of cells treated with TI‐15 (5 μM, time course: days 3–5) captured on day 13 after staining for with cardiac markers: Troponin T is shown in green (D), myosin light chain 2a (MLC2a) is shown in pink (E), and NKX2–5/GFP is shown in green (F). The nuclei were counterstained using DAPI, shown in blue, in all three images. The bar scale applies to all three images (D–F).
TIs Do Not Inhibit the Wnt/β‐Catenin Pathway During Cardiomyogenesis
Simple Western analysis was subsequently carried out on the cells treated with TI‐15 (5 μM) using the monolayer cardiac differentiation method. Intriguingly as shown in Figure 2A, LRP5/6 and Dvl2 were not phosphorylated. This result was further supported by the low expression levels of cytosolic β‐catenin phosphorylation on Ser33/37/Thr41 and Ser45 which are due to the nonphosphorylation of LRP5/6. Moreover, the expression of β‐catenin phosphorylation on Ser552/675 and the downstream TCF‐1/LEF‐1 expression were observed but the expression levels did not change after the addition of TI‐15 (Fig. 2A). Thus, the evidence above strongly suggests that under these conditions, the Wnt/β‐catenin pathway is not affected by the TIs in the cardiomyogenesis process. The absence of the upstream activation of Wnt/β‐catenin pathway is postulated to be due to the absence of Wnt activators (e.g., Wnt3a). This in turn maybe an unintended consequence of the addition of CHIR99021 in the differentiation protocol. To investigate whether CHIR99021 suppresses the expression of Wnt3a, qPCR studies were carried out to study the effect of small molecules on Wnt3a expression over the first 5 days of the cardiac differentiation. From the results shown in Figure 2B, it was observed that on day 3 the expression of Wnt3a was suppressed after an initial 24 hours of CHIR99021 induction. This was not observed in cells not treated with CHIR99021 or with cells treated with imidazoles alone (SB203580 and TA‐01, Fig. 2B).
Figure 2.
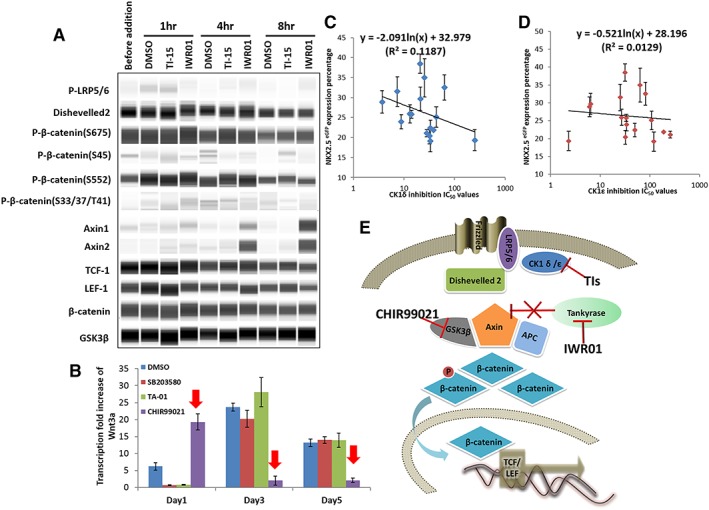
TIs did not act on the Wnt/β‐catenin pathway during cardiomyogenesis with CHIR99021 induction. (A): Simple Western analysis of cells treated with TI‐15 (5 μM), IWR‐1 (2.5 μM), and DMSO. Cells were treated with CHIR99021 in the first 48 hours. On day 3, the cells were harvested for analysis before the addition of the compounds and 1, 4, and 8 hours after treatment. Protein expression blots were cropped from digital images generated using Compass software (Proteinsimple, San Jose, CA). (B): Quantitative RT‐PCR analysis of Wnt3a expression in cells treated with DMSO, SB203580 (5 μM), TA‐01 (5 μM), and CHIR99021 (day 0: 6 μM, day 1: 1.5 μM, and day 3: 0 μM, red arrow). NKX2‐5/GFP‐positive cell percentage (shown in Fig. 1B) was plotted against IC50 values against CK1δ (C, R2 = 0.12) and CK1ε (D, R2 = 0.01), respectively. The data are presented as the mean ± SEM. (E): Proposed mechanism of how CHIR99021, IWR‐1, and TIs regulate the Wnt/β‐catenin signaling pathway during cardiomyogenesis.
These studies suggest that contrary to our previous assertion 12, the kinase target of TIs, CK1δ/ε, is unlikely to be critical for cardiac differentiation. When the cardiomyogenic activities of the compounds (shown in Fig. 1B) were plotted against the IC50 values of CK1δ/ε (shown in Supporting Information Table S1), no correlation was observed (Fig. 2C, D). In addition, IWR‐1 was used as a positive control and upregulation of Axin 1 and 2 were observed (Fig. 2A), suggesting the stabilization of the β‐catenin destructive protein complex 8. The proposed mechanisms underlying how CHIR99021, IWR‐1 and TIs regulate the Wnt/β‐catenin signaling pathway during cardiomyogenesis are depicted in Figure 2E.
TIs Induce the Cardiomyogenesis of hPSCs by Inhibiting ALK5 of the TGFβ Pathway
The studies above suggest that the TIs do not inhibit the Wnt/β‐catenin pathway and that the TIs may be important in the regulation of alternative cellular pathway(s) during cardiomyogenesis. When considering the other known effects of CHIR99021, apart from the suppression of Wnt3a expression and the activation of the Wnt/β‐catenin signaling, CHIR99021 is also known to activate the TGFβ superfamily pathways as is evidenced by the elevation of the expression of multiple SMAD proteins 17. Among the TGFβ superfamily pathways, the TGFβ signaling has been reported to play important roles in cardiomyogenesis, for example, in the specification of mesoderm toward cardiac mesoderm 2. Selected TGFβ‐selective inhibitors have been reported to promote the differentiation of uncommitted mesoderm to cardiomyocytes in ESCs when dosed during days 3–5 18. The information above coupled with the knowledge from kinase profiling studies that ALK5 of the TGFβ pathway is an off‐target of the lead compound TA‐01 suggest that ALK5 could potentially be involved in cardiomyogenesis 12. Totest this hypothesis, the IC50 values of the compounds against ALK5 were measured (results shown in Supporting Information Table S1) and plotted against their respective NKX2‐5/GFP percentage. A good correlation (n = 16, R 2 = 0.86, Fig. 3A) was observed, suggesting an association between ALK5 inhibition and cardiomyogenesis. To examine the function of the small molecules in the cardiac differentiation, the hESC‐3 cells were treated with selected compounds (IWR‐1, TA‐01, TI‐15, and DMSO as control) at two concentrations of TGFβ3 to activate the TGFβ pathway and counteract the function of the small molecules. The results (Supporting Information Fig. S3D) shows that with the addition of the TGFβ3, the efficiency of cardiac differentiation dropped, thus supporting the notion that the inhibition of TGFβ pathway is critical for cardiac differentiation.
Figure 3.
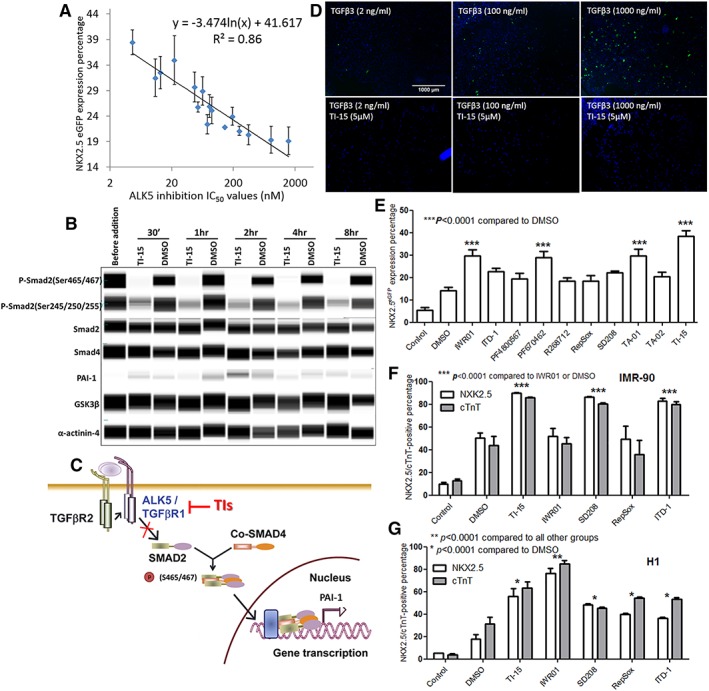
Cardiomyogenesis was induced by inhibiting ALK5 of the TGFβ pathway. (A): The NKX2‐5/GFP‐positive cell percentage (shown in Fig. 1B) was plotted against the ALK5 IC50 values (Supporting Information Table S1), n = 16, R 2 = 0.86. The data are presented as the mean ± SEM. (B): Simple Western analysis of the TGFβ signaling pathway. Cells were treated with TI‐15 (5 μM) and DMSO and subsequently harvested for analysis before compound addition on day 3 and at 30 minutes and 1, 2, 4, and 8 hours after addition. α‐Actinin‐4 and GSK3β were used as loading controls. Protein expression blots were cropped from digital images generated using Compass software (Proteinsimple, San Jose, CA). (C): The proposed mechanism of TIs inhibiting the ALK5 of the TGFβ pathway. (D): Immunofluorescence images of SMAD eGFP/w reporter cell line treated with TI‐15 (5 μM), followed by the addition of TGFβ3 at three different concentrations (2, 100, and 1000 ng/ml, bottom images) against cells treated with the same concentrations of TGFβ3 only (top images). GFP is shown in green. The nuclei were counterstained using DAPI, shown in blue. Bar scale applies to all images. (E): The percentage of NKX2‐5/GFP‐positive cells (HES‐3 cell line) induced by commercially available inhibitors of the TGFβ and Wnt/β‐catenin signaling pathways (time course: days 3–5, concentration: 5 μM for test compounds and 2.5 μM for IWR‐1) measured on day 13 using flow cytometry. IMR‐90 cells (F) and H1 cells (G) were treated with IWR‐1, TI‐15, and TGFβ inhibitors (time course: days 3–5, concentration: 5 μM for test compounds, and 2.5 μM for IWR‐1) and measured on day 13 for NKX2‐5/GFP and cTnT expression. “Control” represents the condition with no CHIR99021 or test compounds addition. The data is presented as the mean ± SEM, n = 3.
Simple Western analysis was performed to examine the effect of the TIs on the TGFβ pathway. From the phosphoprotein analysis, the phosphorylation of SMAD2 protein at Ser 465/467 was found to be significantly reduced in the presence of TI‐15 (Fig. 3B), thus causing the accumulation of SMAD2–SMAD4 complexes in the nucleus to be inhibited as depicted in Figure 3C. However, the downstream PAI‐1 expression in the nucleus was weakly detected (Fig. 3B).
To verify that the TIs induced the inhibition of nuclear TGFβ, the TGFβ signaling output was measured using a nuclear SMAD/GFP reporter cell line. The immunofluorescence images showed that in the presence of TI‐15, the numbers of GFP‐positive cell were reduced as compared with DMSO‐treated controls (Fig. 3D). High magnification cell images are shown in the Supporting Information Figure S3B,C.
We also examined the effects of other chemically distinct inhibitors of the TGFβ pathway on cardiomyogenesis by treating hPSCs with the following commercially available TGFβ inhibitors: ITD‐1, R268712, RepSox, and SD208. The cardiac differentiation abilities of these compounds were compared with that obtained from treatment with Wnt/β‐catenin inhibitors (IWR‐1, PF4800567, and PF670642) and 2,4,5‐tri‐substituted compounds (TA‐01, TA‐02, and TI‐15). From our studies, ITD‐1, a selective inhibitor of the TGFβ signaling pathway 18, was observed to induce 25% of GFP/NKX2‐5‐positive cells in HES‐3 cell lines (Fig. 3E), ~80% NKX2‐5/cTnT‐positive cells in IMR‐90 cells (Fig. 3F) and ~40% in H1 cells (Fig. 3G). Selective ALK5 inhibitors, R268712 19, RepSox 20, and SD208 21 failed to induce cardiomyogenesis in HES‐3 cells (Fig. 3E) but promoted cardiac differentiation in IMR‐90 and H1 cells (Fig. 3F,G). In addition, IWR‐1 did not increase the GFP/NKX2‐5‐positive cell percentage or the cTnT‐positive cell percentage in IMR‐90 cells as compared with H1 and HES‐3 (Fig. 3F,G). These data indicate that, although the effects are cell line dependent, the TGFβ inhibitors can promote the cardiac differentiation of hPSCs.
TIs Promote the Neural Differentiation of hPSCs Through the Inhibition of ALK5
The TGFβ superfamily pathways have been reported to be important at various stages of neural differentiation 11, 22, 23, 24. In view of this, we examined the ability of the TIs to affect the neural differentiation of stem cells. Fifteen imidazoles (TAs and TIs) and four commercially available TGFβ inhibitors were selected based on their ALK5 inhibitory activities and applied to hPSCs from days 1 to 9 at concentrations of 5 and 10 μM. The neural differentiation abilities of these molecules were compared with those of dual SMADs inhibitors containing LDN‐193189 and SB431542 25, 26, 27. On day 9 of differentiation, the population of Nestin‐positive cells was measured.
From the results shown in Figure 4A, it was observed that the percentage of Nestin positive cells for DMSO vehicle was approximately 25%. The LDN‐193189/SB431542 positive controls gave rise to approximately 60% Nestin positive cells, which was 15% higher than when SB431542 (10 μM) and LDN‐193189 (0.2 μM) were tested separately. These results showed that the combined use of SB431542 and LDN‐193189 induced more NPCs than when they were used individually, suggesting a synergistic inhibitory action of both the BMP and TGFβ signaling pathways. Among the TIs tested, TI‐21, 24, and 29 (56–68% of Nestin‐positive cells, Fig. 4A), which were previously identified to be the most potent TIs in inducing cardiomyogenesis, were also found to be the most potent compounds in inducing neural differentiation, with comparable activities as the LDN‐193189/SB431542 positive controls (Fig. 4A). Although these three TIs are potent ALK5 inhibitors (TI‐21 IC50 = 10.9 nM, TI‐24 IC50 = 13.1 nM, TI‐29 IC50 = 22.1 nM, Supporting Information Table S1), their neural differentiation abilities may not be solely due to the ALK5 inhibition but may also be associated with their inhibitory activities against the BMP pathway. From the kinase profiling of TA‐01 12, strong binding affinity against BMPR1B kinase (POC at 10 μM = 6.2) was reported 12 and this kinase is involved in the BMP pathway 28. Thus, it was postulated that the mechanism for the induction of neural differentiation by the TIs is through the inhibition of both the TGFβ and BMP pathways. However, it should be noted that the two commercially available ALK5 selective inhibitors, R268712 and SD208 also displayed similar activities (~60% Nestin‐positive cells, Fig. 4A) as the positive controls although these compounds do not have inhibitory activities against the BMP pathway.
Figure 4.
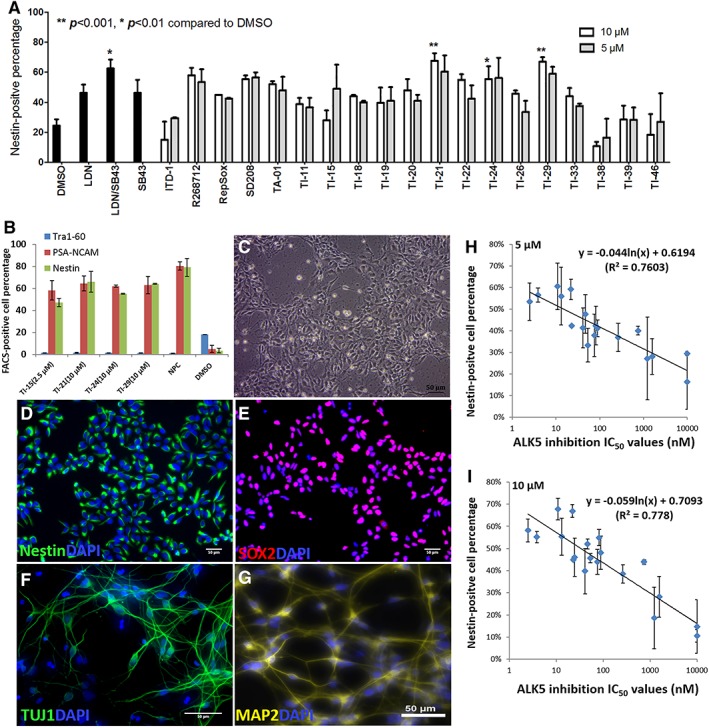
TIs induce the neural differentiation of hPSCs through the inhibition of ALK5. (A): Screening of 19 compounds at 5 and 10 μM concentrations using a 9‐day monolayer neural differentiation method. Percentage of Nestin‐positive cells was measured on day 9 using flow cytometric analysis. A combination of SB341542 (10 μM)/LDN‐193189 (0.2 μM) was used as a positive control. DMSO vehicle was used as a negative control. The data is presented as the mean ± SEM, n = 3. Statistical significance compared with the DMSO treatment is indicated as **p < .001 and *p < .01. (B): The expression of Tra1–60, PSA‐NCAM, and Nestin were measured on day 9 by flow cytometry on cells treated with TI‐15 (2.5 μM), T‐21 (10 μM), TI‐24 (10 μM), and TI‐29 (10 μM). The expression of these three markers on NPCs and DMSO‐treated cells was also measured as controls. The data is presented as the mean ± SEM, n = 3. (C): Microscopic image of cells treated with TI‐21 (5 μM) captured on day 9. Day 9 images of immunofluorescence cell staining against the following stem cell markers: Nestin (image D) and SOX2 (image E). Immunofluorescence cell staining (further differentiated for 4 weeks after induction with TI‐21 from days 1 to 9) against the following neuronal markers: β‐III tubulin (TUJ1, F) and MAP2 (G). The nuclei were counterstained using DAPI, shown in blue (D–E). The Nestin‐positive cell percentage among cells treated with TIs at 5 μM (H, n = 18, R 2 = 0.76) and 10 μM (I, n = 19, R 2 = 0.78) concentrations were plotted against the ALK5 IC50 values (Supporting information Table S1). The data is presented as the mean ± SEM.
In terms of cell growth, from the results shown in Supporting Information Figure S4A, three compounds were identified to inhibit cell proliferation, that is, ITD‐1, TI‐15, and TI‐38. Among these compounds, TI‐15 was the most potent compound identified previously in inducing cardiomyogenesis. Its ability to induce neural differentiation may be affected by the low cell proliferation after treatment with this compound. Optimization of conditions show that TI‐15 inhibited cell growth at both 5 and 10 μM concentrations (Supporting Information Fig. S4A) but not at the concentration of 2.5 μM (Supporting Information Fig. S4B).
The four most potent TIs for the induction of neural differentiation, TI‐15, TI‐21, TI‐24, and TI‐29, were assessed for their neural differentiation abilities again using multiple markers, that is, Nestin, PSA‐NCAM, and Tra1–60. Apart from the two markers for NPCs, Tra1–60 is a human stem cell pluripotency marker which indicates the pluripotency of the cells 29. In this test, NPCs were used as positive control and DMSO was used as negative control. From the results shown in Figure 4B, results observed from the PSA‐NCAM and the Nestin markers are similar, supporting the reliability of Nestin as a NPC marker. Moreover, the percentage of Tra1–60 positive cells was observed to be low in cells treated with TIs, similar to the NPC control, but much lower as compared with the DMSO treatment, indicating that the cells treated with TIs have differentiated and lost their pluripotency.
To test the hypothesis that the neural differentiation induced by the TIs was also through ALK5 inhibition, the percentage of Nestin positive cells (shown in Fig. 4A) was plotted against the IC50 values against ALK5. From the results shown in Fig. 4H, J, correlations were observed when the compounds were dosed at both 5 μM (n = 18, R 2 = 0.76) and 10 μM (n = 19, including SB431542, R 2 = 0.78) concentrations, suggesting that the mechanism for the induction of neural differentiation by the TIs is through the inhibition of ALK5. TI‐15 was not included in the plots as cell proliferation is inhibited by this compound at 5 and 10 μM.
To characterize the NPCs that were derived from hESCs following the induction of TIs, immunofluorescence staining was carried out on the cells treated with TI‐21, TI‐25, and TI‐29 at 5 μM as well as TI‐15 (2.5 μM) over 9 days. The NPC and stem cell markers, Nestin, and SOX2 were used to characterize the structure of the NPCs generated 30. The representative microscopic image and immunofluorescence staining images of cells induced by TI‐21 over 9 days are shown in Figure 4. From the microscopic image (Fig. 4C), the cell population was homogeneous and the morphology of NPCs was observed. Nestin expression was observed in the cytosolic fraction of cells as expected in NPCs (green in Fig. 4D). The expression of SOX2 was found in the nucleus of cells (red in Fig. 4E). These results confirmed that the cells induced are NPCs. Furthermore, cells treated with TI‐21 were further differentiated in neuronal differentiation medium. After 4 weeks, differentiated neurons were observed based on the expression of the neuron‐specific markers β‐III tubulin (TUJ1, Fig. 4F) and MAP2 (Fig. 4G). Similar effects were observed when TIs were applied to induce the neural differentiation of IMR‐90 cells (Supporting Information Fig. S4C–E).
Discussion
In this study, an expanded series of imidazoles (TIs) were designed and synthesized based on the structures of the lead compounds TA‐01 and SB203580, both of which were reported to exert cardiomyogenic activities 12, 31. Our previous studies have shown that the cardiomyogenic activities of tri‐substituted azoles were not due to p38α MAPK inhibition but was likely to be due to the inhibition of the Wnt/β‐catenin pathway via the interaction with the CK1δ/ε during the post mesodermal stage (days 4–8) 12. Based on the biphasic and time dependent role of Wnt/β‐catenin signaling in cardiomyogenesis 1, a high‐efficiency single EB‐based cardiac differentiation method with the addition of CHIR99021 was established and employed in the present study for the preliminary screening of TIs. This was then followed by a secondary screen using a monolayer cardiac differentiation assay.
A previous study suggested that SB203580 and TA‐01 inhibited CK1δ/ε of the Wnt/β‐catenin pathway leading to the dephosphorylation of Dishevelled2. The latter was initially phosphorylated as a result of LRP5/6 activation in the presence of Wnt activators (e.g., Wnt3a) 12. Consequently, the β‐catenin destructive protein complex, comprising APC, Axin, and GSK3β, was stabilized, thus preventing the translocation of β‐catenin into the nucleus 12. However, in this study, the Simple Western analysis showed only low levels of cytosolic β‐catenin phosphorylation at Ser33/37/Thr41 and Ser 45, suggesting that the initial addition of CHIR99021 did not activate the upstream Wnt/β‐catenin pathway at the LRP5/6 level. This is contradictory to our earlier report, and suggests that the TIs did not inhibit the Wnt/β‐catenin pathway during cardiomyogenesis. Hence, the cardiomyogenic abilities of these molecules must be via the regulation of alternative cellular pathway(s). Potential kinase targets were narrowed down to ALK5 and TGFBR2 for two reasons: first, these kinases are involved in the TGFβ pathway, which also plays a biphasic role in cardiomyogenesis in a similar manner to the Wnt/β‐catenin pathway 18. Second, CHIR99021 upregulates the TGFβ superfamily pathway by upregulating the phosphorylation of multiple SMAD proteins 17. In addition, Willems et al. demonstrated that the small molecule cardiomyogenesis inducer ITD‐1 inhibited the TGFβ pathway by promoting TGFβ type II receptor (TGFBR2) degradation 18. Although TA‐01 also exhibited strong binding affinity against TGFBR2 (percentage of control at 10 μM TA‐01: 1.6) 12, the percentage inhibition against TGFBR2 at 1‐μM concentrations for all the compounds (Supporting Information Table S1) did not correlate with the cardiomyogenic activities of the compounds (e.g., TI‐15: 80% < TI‐34: 81% < TA‐01: 91%). Thus, the possible target for the induction of cardiomyogenesis by TIs is through the inhibition of ALK5.
Although the overall levels of cardiomyogenesis induction were below 40% in HES‐3 cells, the results of this study showed that selected compounds can induce high cardiomyogenic conversion in other human pluripotent cell lines: TI‐15, SD208, and ITD‐1 induced approximately 90% of NKX2‐5 and cTnT expression in the IMR‐90 cell line (Fig. 3F), and IWR‐1 induced approximately 80% in H1 cell lines (Fig. 3G). Further studies are needed to explore the cardiomyogenic potential of TIs on other human pluripotent cell lines.
In neural differentiation, the SMAD signaling inhibitors Noggin and SB431542 act synergistically to induce the highly efficient neural conversion of hPSCs 24. SB431542 is a selective TGFβ signaling inhibitor with no effect on BMP signaling 27, whereas Noggin inhibits BMP signaling. A later study replaced Noggin with the small molecule BMP inhibitor, LDN‐193189 25. In this study, the application of SB431542/LDN‐193189 induced approximately 70% of Nestin‐positive cells, which was approximately 20% higher than that observed by induction with either SB431542 or LDN‐193189 alone, suggesting that SB431542 and LDN‐193189 compensated for each other in the inhibition of both BMP and TGFβ pathways, resulting in high neural induction activities. As BMPR1B is an off‐target of TA‐01 12, it is highly likely that TIs can inhibit both TGFβ and BMP pathways during neural differentiation. Moreover, the two selective ALK5 inhibitors R268712 and SD208 exhibited neural induction activities similar to TI‐21, 24, 29, and the SB431542/LDN‐193189 combination. In addition, the neural induction activities of TIs were not reinforced by the addition of LDN‐193189 (Supporting Information Fig. S4F). Therefore, we suggest that ALK5/TGFβ inhibition may work upstream of both TGFβ and BMP inhibition during neural differentiation.
TI‐15 was the most potent compound in inducing cardiomyogenesis and the most potent ALK5 inhibitor among the TIs. The induction of TI‐15 during days 3–5 of cardiac differentiation does not inhibit cell growth, whereas earlier induction during days 2–5 showed inhibition of cell proliferation (data not shown). The inhibition of cell growth due to this compound was also observed during neural differentiation when TI‐15 was dosed from days 1 to 9. We concluded that TI‐15 inhibits the growth of hPSCs when added during the first 2 days of differentiation, which is the time window for the gastrulation of hPSCs. This effect was not observed with the other TIs.
The compounds synthesized in our study allowed for a systematic study of the mechanism of action. From the structure activity relationship (SAR) analysis, TI‐15 is approximately 10‐times more potent that TA‐01 in the inhibition of ALK5. This could be due to the different sizes of chlorine and fluorine. Specifically, the chlorine atom is approximately two times the size of fluorine atom (E s value of Cl is −0.97 while E s value of F is −0.46) 32. Interestingly, substituting the fluoro substituents for methoxy substituents at the 2‐ and 6‐positions (TI‐18) show a large drop in ALK5 inhibitory activity (IC50: 747 nM, Supporting Information Table S1). The steric parameter for a methoxy substituent is close in value to that of a fluoro group but has different electronic properties as the methoxy group is an electron donating group as compared with the electron withdrawing fluoro substituent (the OMe group has E s value of −0.55; σp is −0.28 vs. 0.15 for F) 32, 33. We also observed that TIs with electron withdrawing groups at the 2‐ and 6‐positions of the C2 phenyl ring generally are good inhibitors of ALK5 (e.g., TI‐19, TI‐21, TI‐24, and TI‐29, Supporting Information Table S1), and are also the most potent compounds in inducing cardiac and neural differentiation. Modifying other substituents of TA‐01 (e.g., TI‐01 to TI‐13, TI‐36 to TI‐47, Supporting Information Table S1) led to a loss in potency. From the above SAR analysis, we posit that the potency of a compound as an ALK‐5 inhibitor can be improved if the substituents at the C2 phenyl ring are both sterically bulky and are electron withdrawing in nature.
In the 1,4,5‐trisubstituted series of imidazoles, all of these imidazoles lost their potency in the inhibition of ALK5 or in their ability to induce the differentiation. As the 1,4,5‐trisubstituted imidazoles are structurally different to the 2,4,5‐trisubstituted imidazoles (e.g., the absence of an N‐H in the former structures), these observations suggest the sensitivity of the ALK5 binding pocket to structural modifications.
Four TIs (TI‐15, TI‐21, 24, and 29) were identified as the most potent compounds in inducing both the cardiac and neural differentiation of hPSCs. We propose that the induction of both differentiation pathways occurs through a common mechanism, that is, the inhibition of ALK5 in the TGFβ pathway. However, the fate of the hPSCs is strongly dependent on the time window in which the TIs are added. Specifically, the induction of cardiomyogenesis initially requires the formation of the mesoderm which is facilitated by the application of CHIR99021. This is followed by the subsequent inhibition of ALK5 by TIs during days 3–5 which promotes the differentiation of cardiac progenitors from the mesoderm. In neural differentiation, TIs inhibit ALK5 since day 1, thus promoting the formation of neuroectoderm (Fig. 5).
Figure 5.
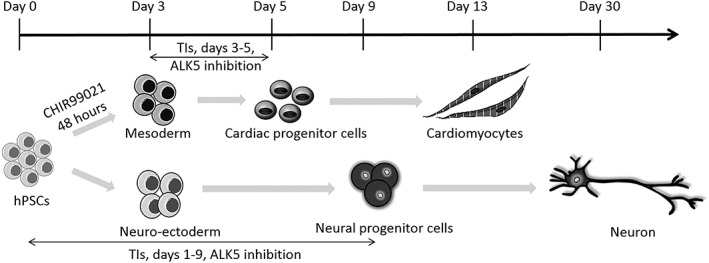
The mechanisms by which TIs induce both the cardiac and neural differentiation of hPSCs.
Conclusion
In conclusion, TIs were discovered to promote the cardiac and neural differentiation of hPSCs. The proposed mechanism of TIs inhibiting ALK5 in the TGFβ pathway is not only an alternative to current theories for stem cell differentiation but also unifies the mechanisms of small molecule inducers of cardiac and neural differentiation. Critically our studies provide a single method to control the fate of stem cell differentiation that will be useful to provide clinical‐grade adult cells for future cardiomyocyte and neural cell therapies, drug testing, and cell tissue models.
Author Contributions
Q.Z.: Conception and design, collection and assembly of data, data analysis, interpretation and make figures, manuscript writing; F.L.: Conception and design, data analysis and interpretation, provision of study materials; M.L.: Conception and design, collection and assembly of data; T.L.W.: Collection and assembly of data; S.K.W.O.: Conception and design, administrative support, final approval of manuscript; C.L.L.C.: Conception and design, administrative support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
Q.Z, F.L., S.K.W.O, and C.L.L.C. disclosed as a patent holder. The other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Acknowledgments
This work was financially supported through a grant from the Singapore Ministry of Education (MOE; grant R148‐000‐146‐133). We thank Ms Alice X. T. Xu from the Department of Pharmacology and Toxicology, University of Toronto, for assistance with the immunofluorescence assay. We thank the Drug Development Unit, National University of Singapore, for performing the parallel artificial membrane permeability assay. We also thank Dr Gloryn Chia from Bioprocessing Technology Institute, A*STAR for revising the manuscript.
Contributor Information
Steve K.W. Oh, Email: steve_oh@bti.a-star.edu.sg
Christina L.L. Chai, Email: phacllc@nus.edu.sg.
References
- 1. Parikh A, Wu J, Blanton RM et al. Signaling pathways and gene regulatory networks in cardiomyocyte differentiation. Tissue Eng Part B Rev 2015;21:377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schade D, Plowright AT. Medicinal chemistry approaches to heart regeneration. J Med Chem 2015;58:9451–9479. [DOI] [PubMed] [Google Scholar]
- 3. Kochegarov A. Small molecules for stem cells. Expert Opin Ther Pat 2009;19:275–281. [DOI] [PubMed] [Google Scholar]
- 4. Minami I, Yamada K, Otsuji TG et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine‐ and xeno‐free conditions. Cell Rep 2012;2:1448–1460. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Li W, Laurent T et al. Small molecules, big roles: The chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci 2012;125:5609–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davidson KC, Adams AM, Goodson JM et al. Wnt/beta‐catenin signaling promotes differentiation, not self‐renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA 2012;109:4485–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu J, Ma Z, Hsieh J‐C et al. Structure–activity relationship studies of small‐molecule inhibitors of Wnt response. Bioorg Med Chem Lett 2009;19:3825–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen B, Dodge ME, Tang W et al. Small molecule‐mediated disruption of Wnt‐dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009;5:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hao J, Daleo MA, Murphy CK et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One 2008;3:e2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kattman SJ, Witty AD, Gagliardi M et al. Stage‐specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011;8:228–240. [DOI] [PubMed] [Google Scholar]
- 11. Smith JR, Vallier L, Lupo G et al. Inhibition of activin/nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol 2008;313:107–117. [DOI] [PubMed] [Google Scholar]
- 12. Laco F, Low JL, Seow J et al. Cardiomyocyte differentiation of pluripotent stem cells with SB203580 analogues correlates with Wnt pathway CK1 inhibition independent of p38 MAPK signaling. J Mol Cell Cardiol 2015;80:56–70. [DOI] [PubMed] [Google Scholar]
- 13. Elliott DA, Braam SR, Koutsis K et al. NKX2‐5eGFP/w hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods 2011;8:1037–1040. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen U, Squaglia N, Boge A et al. The Simple Western: a gel‐free, blot‐free, hands‐free Western blotting reinvention. Nat Methods 2011;8:v–vi. [Google Scholar]
- 15. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative CT method. Nat Protoc 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 16. Elliott DA, Braam SR, Koutsis K et al. NKX2‐5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods 2011;8:1037–1040. [DOI] [PubMed] [Google Scholar]
- 17. Lian X, Hsiao C, Wilson G et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 2012;109:E1848–E1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willems E, Cabral‐Teixeira J, Schade D et al. Small molecule‐mediated TGF‐beta type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell 2012;11:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terashima H, Kato M, Ebisawa M et al. R‐268712, an orally active transforming growth factor‐beta type I receptor inhibitor, prevents glomerular sclerosis in a Thy1 nephritis model. Eur J Pharmacol 2014;734:60–66. [DOI] [PubMed] [Google Scholar]
- 20. Gellibert F, Woolven J, Fouchet MH et al. Identification of 1,5‐naphthyridine derivatives as a novel series of potent and selective TGF‐beta type I receptor inhibitors. J Med Chem 2004;47:4494–4506. [DOI] [PubMed] [Google Scholar]
- 21. Kapoun AM, Gaspar NJ, Wang Y et al. Transforming growth factor‐beta receptor type 1 (TGFbetaRI) kinase activity but not p38 activation is required for TGFbetaRI‐induced myofibroblast differentiation and profibrotic gene expression. Mol Pharmacol 2006;70:518–531. [DOI] [PubMed] [Google Scholar]
- 22. Zhou J, Su P, Li D et al. High‐efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor beta superfamily receptors. Stem Cells 2010;28:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Y, Zhang H, Yung A et al. ALK5‐dependent TGF‐β signaling is a major determinant of late‐stage adult neurogenesis. Nat Neurosci 2014;17:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chambers SM, Fasano CA, Papapetrou EP et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009;27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kriks S, Shim J‐W, Piao J et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson/'s disease. Nature 2011;480:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanvitale CE, Kerr G, Chaikuad A et al. A new class of small molecule inhibitor of BMP signaling. PLoS One 2013;8:e62721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inman GJ, Nicolas FJ, Callahan JF et al. SB‐431542 is a potent and specific inhibitor of transforming growth factor‐beta superfamily type I activin receptor‐like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 2002;62:65–74. [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol 2005;284:1–11. [DOI] [PubMed] [Google Scholar]
- 29. Schopperle WM, DeWolf WC. The TRA‐1‐60 and TRA‐1‐81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells 2007;25:723–730. [DOI] [PubMed] [Google Scholar]
- 30. Liao MC, Diaconu M, Monecke S et al. Embryonic stem cell‐derived neural progenitors as non‐tumorigenic source for dopaminergic neurons. World J Stem Cells. 2014;6:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaur M, Ritner C, Sievers R et al. Timed inhibition of p38MAPK directs accelerated differentiation of human embryonic stem cells into cardiomyocytes. Cytotherapy 2010;12:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charton M. Steric effects. 7. Additional υ constants. J Org Chem 1976;41:2217–2220. [Google Scholar]
- 33. Bromilow J, Brownlee RTC, Lopez VO et al. Para‐substituent C‐13 chemical shifts in substituted benzenes. 1. Updating the .sigma.R0 scale and analysis of aprotic solvent effects. J Org Chem 1979;44:4766–4770. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


