Abstract
Aims
To determine the risk of venous thromboembolism (VTE) defined as the combined endpoint of deep venous thrombosis (DVT) and pulmonary embolism (PE) among patients with psoriatic arthritis (PsA), psoriasis and rheumatoid arthritis (RA) compared with population controls.
Methods and results
A cohort study was conducted in a primary care medical record database in the UK with data from 1994–2014 among patients with PsA, RA, or psoriasis. Cox proportional hazards models were used to calculate the relative hazards for DVT, PE, and VTE. An interaction with disease modifying anti-rheumatic drugs (DMARD) was hypothesized a priori and was significant. Patients with PsA (n = 12 084), RA (n = 51 762), psoriasis (n = 194 288) and controls (n = 1 225 571) matched on general practice and start date were identified. Patients with RA (with and without a DMARD prescription) and patients with mild psoriasis had significantly elevated risks of VTE (HR 1.35, 1.29, and 1.07, respectively) after adjusting for traditional risk factors. Severe psoriasis and PsA prescribed a DMARD had an elevated but not statistically significant risk for VTE. Findings were similar for DVT. The age-and-sex-adjusted risk of PE was elevated in RA, severe psoriasis and PsA patients prescribed a DMARD.
Conclusion
While systemic inflammation is a risk factor for VTE, the risk of VTE compared with controls is different among patients with three different inflammatory disorders: RA, PsA, and psoriasis.
Keywords: Psoriatic arthritis , Psoriasis , Rheumatoid arthritis , Venous thromboembolism , Pulmonary embolus , Deep venous thrombosis
Introduction
Venous thromboembolism (VTE) is a relatively common and potentially fatal condition with an incidence of three cases per 1000 patient years.1–3 A number of risk factors for VTE, which includes both deep vein thrombosis (DVT) and pulmonary embolus (PE), have been identified. One such potential risk factor for VTE is systemic inflammation.4–6 This association is biologically plausible given the effects of systemic inflammation on platelet aggregation, coagulation cascade activation, stimulation of pro-coagulant activity of peripheral blood monocytes, endothelial damage, and potentially changes in blood flow.4,7 Previous studies have suggested an increased risk of VTE among patients with elevated serum c-reactive protein (CRP), interleukin (IL)-6, IL-8, monocyte chemotactic protein (MCP)-1 and tumor necrosis factor (TNF).4 However, it is unclear whether inflammation and the resulting cytokines are causal factors in the development of VTE.
Psoriasis, psoriatic arthritis (PsA) and rheumatoid arthritis (RA) are chronic inflammatory diseases. These disorders result in activation of both the Th1 and Th17 inflammatory pathways resulting in increased IL-6 and TNF and patients often have an elevated CRP. Chronic systemic inflammation in these disorders may result in accelerated atherosclerosis in patients with psoriasis, PsA and RA.8–10 However, less is known about whether these disorders are associated with VTE. A handful of studies have examined the risk of VTE in patients with RA and psoriasis.6,11–21 In a study by Kim et al in 2013, patients with RA had a 40% increase in VTE compared with patients without RA after adjustment for other known risk factors.13 Previous studies performed in psoriasis were highly heterogeneous and many did not optimally adjust for additional risk factors for VTE. To our knowledge, there are no studies to date examining the risk of VTE in PsA. If patients with these disorders have an increased risk of VTE, improved prevention and monitoring could improve morbidity and mortality from VTE in these patients.
We performed a population-based cohort study to examine whether psoriasis, PsA and RA are associated with an increased risk of VTE after adjustment for established risk factors for VTE. We hypothesized that all three inflammatory diseases are associated with increased risk of VTE (including PE and DVT) as compared with unexposed patients without these diseases.
Methods
Study Design: We performed a cohort study to examine the risk of incident VTE among patients with psoriasis, PsA, and RA.
Data Source: Data from The Health Improvement Network (THIN) in the United Kingdom (UK) between 1994 and January 2014 was used. THIN is representative of the UK population in terms of age, sex, geography, and medical conditions.22
Study Population: All patients with PsA, psoriasis or RA between the ages of 18 and 89 at the start date were included if they had observation time in THIN after Vision software implementation. Patients were excluded if they died or transferred out of the practice prior to the implementation of Vision software. Up to 5 unexposed controls were randomly selected for each patient with psoriasis, PsA and RA and were matched on practice and start date within the practice (defined as the latest of diagnosis date or 180 days after registration date). Matching is described in more detail in the Supplementary material online, Methods.
Exposure Definitions: PsA, psoriasis, and RA were defined by the presence of at least one READ code consistent with these diseases.23 READ codes for psoriasis, PsA, and RA have been previously validated within the same or analogous large medical record databases.24–27 Some patients may have been diagnosed with two of the conditions (e.g. psoriasis and PsA). These patients were included in both cohorts (and had separate matched controls based on their start dates). In a sensitivity analysis, we examined alternative definitions of disease groups including restricting the group of patients with psoriasis to patients without a code for RA or PsA.
Outcome Definition: The outcomes of interest are VTE, PE, and DVT. The primary outcome was VTE, the combined endpoint of PE and DVT. The individual diagnoses (DVT and PE) were the secondary outcomes. Codes for VTE have been previously validated within the general practitioners research database (GPRD), a similar database to THIN.28
Covariates of Interest: All covariates of interest were measured prior to and including the index date. The potential confounders measured are listed in the see Supplementary material online, Methods. A priori we hypothesized a statistical interaction between disease status and disease modifying anti-rheumatic drugs (DMARD) use.
Person time calculation: The index date was the latest of diagnosis of psoriasis, PsA, or RA (diagnosis date for unexposed controls, as noted above, was the visit date within 6 months of the matched patient’s diagnosis date), start of therapy for those in the DMARD groups, 180 days after registration in the practice, and Vision software implementation date. Cohort time ended at earliest of development of the outcome of interest, transfer out of the practice, the date the practice stopped contributing to THIN, death, or the end of the study period.
Statistical Analysis: Statistical analyses were performed using STATA 13.0 (College Station, TX). Demographics and covariate distribution among the study groups were descriptively reported. The cumulative incidence of VTE was descriptively reported as the number of new events divided by the number of person years per group. Cox proportional hazards models were used to estimate the unadjusted and age-and-sex adjusted hazard ratios (HR) with 95% confidence intervals (CI). We next fit models that adjusted for additional covariates using a purposeful selection modelling approach.29 Covariates with biologically plausible relationships with the exposure and outcome or that had a P-value of <0.05 in the univariable model were added to create a full model. Covariates were then removed one at a time based on P-value and maintained in the model if P < 0.1 and removal resulted in a change in the point estimates of the main effects by >10–15%. The same modelling procedure was used for DVT and PE as secondary outcomes. Sensitivity analyses are described in the Supplementary material online, Methods.
Results
Patients with PsA (n = 12 084), RA (n = 51 762), and psoriasis (n = 194 288) were identified and matched to 1 225 571 controls. Among these groups, 53 and 61% of patients with PsA and RA respectively received a DMARD and 5.6% of patients with psoriasis received either phototherapy or a systemic therapy for psoriasis. Baseline characteristics are reported in Table 1. Rheumatoid arthritis patients were older and nearly 70% were female as opposed to the other three groups in which mean age was around 50 years and approximately half were female. Comorbidities (unadjusted for age and sex) were in general more common in RA patients although BMI was numerically greater in patients with PsA and severe psoriasis. Additional baseline demographics are provided in see Supplementary material online, Table S1.
Table 1.
Baseline demographics
| Control | PsA No DMARD | PsA DMARD | RA No DMARD | RA DMARD | Mild psoriasis | Severe psoriasis | ||
|---|---|---|---|---|---|---|---|---|
| n | 1 225 571 | 5688 | 6396 | 20 426 | 31 336 | 183 398 | 10 890 | |
| Age | Mean (SD) | 50.35 (17.84) | 50.65 (14.82) | 49.01 (13.60) | 62.54 (16.53) | 58.49 (14.58) | 46.57 (17.60) | 49.46 (15.44) |
| Female Sex | n (%) | 679 354 (55.43%) | 2800 (49.23%) | 3132 (48.97%) | 14 287 (69.95%) | 21 734 (69.36%) | 93 872 (51.18%) | 5 482 (50.34%) |
| Body mass index | Mean (SD) | 26.32 (5.41) | 27.55 (5.58) | 28.45 (5.92) | 26.48 (5.51) | 26.66 (5.49) | 26.58 (5.54) | 27.97 (6.01) |
| Hospitalization | n (%) | 378 439 (30.88%) | 1648 (28.97%) | 2425 (37.91%) | 6114 (29.93%) | 11 190 (35.71%) | 49 046 (26.74%) | 4453 (40.89%) |
| Joint replacement | n (%) | 7108 (0.58%) | 36 (0.63%) | 58 (0.91%) | 578 (2.83%) | 1011 (3.23%) | 830 (0.45%) | 110 (1.01%) |
| Cancera | n (%) | 40 780 (3.33%) | 134 (2.36%) | 117 (1.83%) | 992 (4.86%) | 1202 (3.84%) | 4576 (2.50%) | 291 (2.67%) |
| Hypertension | n (%) | 246 995 (20.15%) | 1233 (21.68%) | 1418 (22.17%) | 6085 (29.79%) | 8731 (27.86%) | 30 438 (16.60%) | 2398 (22.02%) |
| Hyperlipidaemia | n (%) | 109 999 (8.98%) | 546 (9.60%) | 620 (9.69%) | 2110 (10.33%) | 3343 (10.67%) | 14 169 (7.73%) | 1176 (10.80%) |
| Diabetes | n (%) | 74 361 (6.07%) | 406 (7.14%) | 497 (7.77%) | 1782 (8.72%) | 2573 (8.21%) | 9789 (5.34%) | 940 (8.63%) |
| Peripheral vascular disease | n (%) | 17 599 (1.44%) | 81 (1.42%) | 62 (0.97%) | 573 (2.81%) | 621 (1.98%) | 2440 (1.33%) | 160 (1.47%) |
| Oral corticosteroid use | n (%) | 125 853 (10.27%) | 722 (12.69%) | 1444 (22.58%) | 4624 (22.64%) | 13 466 (42.97%) | 16 508 (9.00%) | 2565 (23.55%) |
| NSAID use | n (%) | 629 244 (51.34%) | 3983 (70.02%) | 5634 (88.09%) | 13 562 (66.40%) | 27 378 (87.37%) | 78 039 (42.55%) | 7435 (68.27%) |
| Hormone therapyb | n (%) | 364 202 (29.72%) | 1331 (23.40%) | 1764 (27.58%) | 4655 (22.79%) | 9578 (30.57%) | 46 846 (25.54%) | 3033 (27.85%) |
| Smoking | Never (n, %) | 588 707 (48.04%) | 2487 (43.72%) | 3001 (46.92%) | 9108 (44.59%) | 13 307 (42.47%) | 71 478 (38.97%) | 4090 (37.56%) |
| Past (n, %) | 261 781 (21.36%) | 1295 (22.77%) | 1204 (18.82%) | 4205 (20.59%) | 7017 (11.39%) | 52 702 (28.74%) | 2994 (27.49%) | |
| Current (n, %) | 246 383 (20.10%) | 1359 (23.89%) | 1774 (27.74%) | 4310 (21.10%) | 8517 (27.18%) | 39 759 (21.68%) | 3155 (28.97%) | |
| Missing (n, %) | 128 700 (10.50%) | 547 (9.62%) | 417 (6.52%) | 2803 (13.72%) | 2495 (7.96%) | 19 459 (10.61%) | 651 (5%) | |
| Cohort timec | Mean (SD) | 6.52 (4.79) | 6.16 (4.79) | 5.85 (4.45) | 5.77 (4.67) | 6.17 (4.50) | 6.24 (4.76) | 5.34 (4.14) |
Cancer includes haematologic and solid tumour malignancies.
Hormone therapy: oral contraceptives and hormone replacement therapy.
Time from index date to end date or the event of interest.
Approximately 2% of patients developed a DVT and 0.5% developed a PE during the mean follow up period of 6.3 years. The unadjusted incidence of VTE (combined), DVT, and PE (Table 2) were highest in patients with RA and similar in patients with severe psoriasis and PsA. Incidence declined over time among the unexposed (see Supplementary material online, Table S2). The unadjusted, age- and sex-adjusted and fully adjusted Cox proportional hazards models are presented in Table 3. The age- and sex-adjusted model for VTE suggested a significantly elevated risk for all disease groups. After adjusting for age, sex, and other known risk factors for VTE, patients with RA had the highest risk of VTE overall (No DMARD HR 1.29, 95%CI 1.18–1.39; DMARD HR 1.35, 95% CI 1.27–1.44). The HR for VTE for PsA and psoriasis were elevated but not statistically significant except for among patients with mild psoriasis (HR 1.07, 95%CI 1.03–1.12). The results for DVT were numerically similar to those for VTE overall. All groups had elevated HR but this was only statistically significant for RA and mild psoriasis (RA No DMARD HR 1.25, 95%CI 1.15–1.37; RA DMARD HR 1.29, 95%CI 1.20–1.38; mild psoriasis HR 1.09, 95%CI 1.05–1.14). All disease groups except mild psoriasis had a statistically significant increased risk for PE when accounting for age and sex. After adjusting for other risk factors, the HR remained elevated for the same groups but only RA and severe psoriasis were associated with a statistically significant increased risk for PE (RA No DMARD HR 1.45, 95%CI 1.23–1.72; RA DMARD HR 1.74, 95%CI 1.55–1.99; severe psoriasis HR 1.34, 95%CI 1.01–1.80). These results were robust to the sensitivity analyses (see Supplementary material online, Tables S3–S5). However, when age was used as a time scale, the HR for PsA increased to 1.30 (95%CI: 1.08–1.57) and 1.47 (95%CI: 1.23–1.75) for patients without and with a DMARD prescription respectively. Similarly, the HR for mild psoriasis increased to 1.18 (1.13–1.22).
Table 2.
Incidence of venous thromboembolism per 10 000 person years
| Control | PsA No DMARD | PsA DMARD | RA No DMARD | RA DMARD | Mild psoriasis | Severe psoriasis | ||
|---|---|---|---|---|---|---|---|---|
| VTE | Events n(%) | 30 356 (2.5%) | 159 (2.8%) | 158 (2.5%) | 851 (4.2%) | 1479 (4.7%) | 179 317 (2.2%) | 10 634 (2.4%) |
| Person Years | 7 850 777 | 34 358 | 36 726 | 114 193 | 187 008 | 1 125 926 | 57 139 | |
| Incidencea | 38.67 | 46.28 | 43.02 | 74.52 | 79.08 | 36.25 | 44.80 | |
| DVT | Events n(%) | 25 490 (2.1%) | 130 (2.3%) | 123 (1.9%) | 702 (3.4%) | 1162 (3.7%) | 3474 (1.9%) | 205 (1.9%) |
| Person Years | 7 866 680 | 34 431 | 36 843 | 114 716 | 188 064 | 1 127 771 | 57 303 | |
| Incidencea | 32.40 | 37.76 | 33.39 | 61.19 | 61.79 | 30.80 | 35.77 | |
| PE | Events n(%) | 6066 (0.5%) | 39 (0.7%) | 44 (0.7%) | 186 (0.9%) | 393 (1.3%) | 757 (0.4%) | 65 (0.6%) |
| Person Years | 7 972 070 | 34 957 | 37 242 | 117 283 | 192 129 | 1 142 243 | 57 928 | |
| Incidencea | 7.60 | 11.16 | 11.81 | 15.86 | 20.45 | 6.63 | 11.22 |
Incidence is per 10 000 person years. The previously reported rate of VTE in the UK population was 30/10 000 person years.
VTE, venous thromboembolism; DVT, deep venous thrombosis; PE, pulmonary embolism.
Table 3.
Hazard ratios for incident venous thromboembolism
| Unadjusted |
Age/Sex adjusted |
Fully adjusted |
||||
|---|---|---|---|---|---|---|
| HR | CI | HR | CI | HR | CI | |
| Venous thromboembolism | ||||||
| PsA—No DMARD | 1.20 | 1.03–1.40 | 1.23 | 1.05–1.44 | 1.07 | 0.88–1.29 |
| PsA—DMARD | 1.12 | 0.96–1.31 | 1.27 | 1.09–1.49 | 1.10 | 0.92–1.31 |
| RA—No DMARD | 1.93 | 1.81–2.07 | 1.30 | 1.21–1.39 | 1.29 | 1.18–1.39 |
| RA—DMARD | 2.06 | 1.95–2.17 | 1.63 | 1.55–1.72 | 1.35 | 1.27–1.44 |
| Mild Psoriasis | 0.94 | 0.91–0.97 | 1.05 | 1.02–1.08 | 1.07 | 1.03–1.12 |
| Severe Psoriasis | 1.17 | 1.04–1.33 | 1.28 | 1.13–1.45 | 1.13 | 0.99–1.30 |
| Deep venous thrombosis | ||||||
| PsA—No DMARD | 1.17 | 0.98–1.39 | 1.20 | 1.01–1.43 | 1.07 | 0.87–1.31 |
| PsA—DMARD | 1.04 | 0.87–1.24 | 1.17 | 0.98–1.40 | 1.02 | 0.83–1.24 |
| RA—No DMARD | 1.90 | 0.76–2.04 | 1.28 | 1.19–1.38 | 1.25 | 1.15–1.37 |
| RA—DMARD | 1.91 | 1.81–2.03 | 1.52 | 1.43–1.61 | 1.29 | 1.20–1.38 |
| Mild Psoriasis | 0.95 | 0.92–0.99 | 1.06 | 1.03–1.10 | 1.09 | 1.05–1.14 |
| Severe Psoriasis | 1.11 | 0.97–1.28 | 1.21 | 1.06–1.39 | 1.09 | 0.93–1.27 |
| Pulmonary embolism | ||||||
| PsA—No DMARD | 1.47 | 1.07–2.02 | 1.49 | 1.08–2.03 | 1.24 | 0.85–1.84 |
| PsA—DMARD | 1.58 | 1.18–2.13 | 1.84 | 1.36–2.47 | 1.41 | 1.00–2.03 |
| RA—No DMARD | 2.11 | 1.82–2.44 | 1.39 | 1.20–1.61 | 1.45 | 1.23–1.72 |
| RA—DMARD | 2.73 | 2.46–3.02 | 2.22 | 2.01–2.46 | 1.74 | 1.55–1.99 |
| Mild psoriasis | 0.87 | 0.81–0.94 | 0.98 | 0.90–1.05 | 0.97 | 0.89–1.06 |
| Severe psoriasis | 1.53 | 1.20–1.95 | 1.69 | 1.32–2.16 | 1.34 | 1.01–1.80 |
The fully adjusted models include age, sex, history of cancer, hypertension, hospitalization in the baseline period, joint replacement in the baseline period, liver disease, oral glucocorticoids, NSAIDs, COPD, drinking and smoking in the baseline period.
Discussion
Venous thromboembolism is a relatively common condition that may result in significant morbidity and mortality. In this population-based cohort study, patients with PsA, RA, and psoriasis had an elevated incidence of VTE compared with patients without these common inflammatory disorders after adjusting for age and sex. The age- and sex-adjusted risk for PE was significantly elevated compared with controls except for patients with mild psoriasis. Patients with RA prescribed a DMARD and severe psoriasis had higher point estimates for the outcomes of interest than those without a DMARD prescription suggesting level of systemic inflammation may play a role. Furthermore, our data suggest that RA and mild psoriasis are independent risk factors for VTE after adjusting for other known risk factors for VTE. Comparison among these diseases is important for understanding how these diseases are similar and different, particularly in terms of burden and consequences of systemic inflammation.30
To our knowledge this is the first study to evaluate the risk of VTE in PsA. The age- and sex-adjusted HR for PE among patients with PsA were significantly increased but this was not statistically significant after adjusting for other known risk factors. This was possibly related to the small number of events and insufficient power to detect a difference between patients with and without PsA. (When all patients with PsA were pooled, the fully adjusted HR for PE was 1.32, 1.02–1.72). Psoriatic arthritis is a highly heterogeneous disease: some patients may have a single joint involved and others have severe polyarticular disease. It may be that only those with the most active or debilitating disease have an elevated risk leading to a wide confidence interval.
Few studies have examined the risk of VTE among patients with psoriasis but all report an increased risk.14 Ramagopalan et al. examined VTE hospitalizations among patients with a variety of chronic immune-mediated conditions compared with patients with ‘minor medical or surgical’ reasons for hospitalization.11 They found a relative risk (RR) of VTE of 1.65 (1.23–2.17) and 1.57 (1.37–1.79) in patients with psoriasis and RA respectively after indirect standardization for age category, sex, region of residence, deprivation score, and calendar year. In a nationwide cohort study in Denmark, Ahlehoff et al. analysed data from approximately 35 000 patients with incident psoriasis.31 The RR of VTE was elevated in both mild psoriasis patients and severe psoriasis with RR 1.35 (95%CI 1.21–1.49) and RR 2.06 (1.63–2.61), respectively. This article did not adjust for VTE risk factors including BMI, smoking and alcohol. Lutsey et al. examined the risk of VTE among 859 women with psoriasis in the Women’s Health Initiative and found an increased risk for VTE (RR 1.39, 1.00–1.93) after adjusting for education, smoking, BMI, DM and HRT use.32 Thus, our study is the first study of psoriasis patients, both men and women, to examine the risk for VTE compared with internal controls after accounting for established risk factors for VTE.
Previous studies have found an increased risk of VTE in RA, including one from a similar population in THIN.15,16 Choi et al. examined the risk of VTE among patients with RA in THIN using an incident disease cohort of patients with RA prescribed a DMARD (similar to our RA-DMARD group). In addition to the use of an incidence cohort, other differences include defining VTE as a medical code for VTE plus a prescription for an anticoagulant and dates of inclusion were slightly different (1986–2010 compared with 1994–2014 in our study). The HR for both PE (1.74 vs. 2.16) and VTE were attenuated in our study (1.35 compared to 2.14). In Choi et al., the risk was highest in the one year after RA diagnosis and then steadily declined. We used a prevalence cohort in our study. While we didn’t find significantly different results when we examined the incident cohort by the same definition as Choi et al., the examination of all time (rather than just the first year) may have decreased the HR overall. On the other hand, our results were strikingly similar to results published by Kim et al. who examined the risk or VTE among patients with RA in an administrative database (HR 1.4; 95% CI 1.1–1.7).13 In this sense, the inclusion of patients with RA served as an internal positive control and provides face validity for the remainder of the results. Additionally, Kim et al. examined the effect of prescriptions for DMARDs on the risk for VTE and found that patients initiating a TNF inhibitor had a higher risk for VTE compared with those prescribed a non-biologic DMARD (e.g. methotrexate) but this was only significant in the first time window after prescribing. Similar to Choi et al., this analysis likely reflects the high systemic inflammation in incident disease. As inflammation decreases, so too does the risk for VTE.33 We were unable to repeat similar analyses as biologic DMARDs are prescribed by consultants in the UK and are thus not uniformly available in THIN.
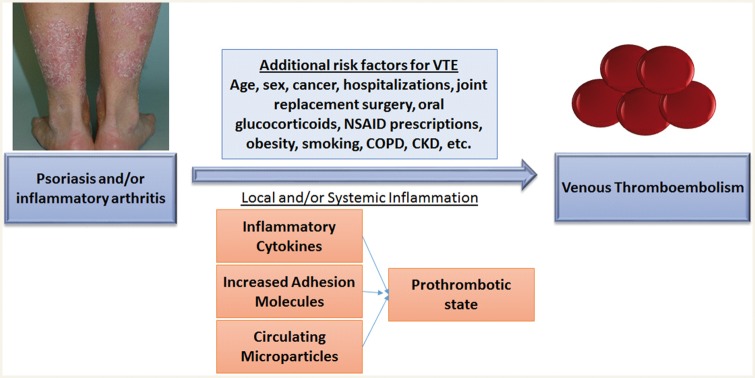
Virchow used the triad of vascular injury, hypercoagulation, and venous stasis to describe the basic mechanisms leading to thrombosis. Systemic inflammation may affect at least two of three of these components. Upregulation of endothelial adhesion molecules and infiltration of activated inflammatory monocytes has been observed in the arterial walls of patients with systemic lupus erythematosus suggesting early vascular damage as a result of systemic inflammation.7 Inflammation also has a direct impact on the coagulation cascade; thrombogenic factors upregulated by inflammation lead to hypercoagulation34 and systemic inflammation, such as the inflammation seen in PsA, RA, and psoriasis, may lead to platelet aggregation and clot formation (see conceptual model in Figure 1).35 Previous studies have also found increased circulating microparticles from endothelial cells and platelets in patients with psoriasis36 which may contribute to increased cardiovascular risk and potentially VTE as well. Overall, in the present study, the HR for VTE were lower in magnitude than the risk for major cardiovascular adverse cardiovascular events found in a previous study within the same cohort.8 Additionally, this risk may be less than the risk conferred by Factor V Leiden, a prevalent thrombophilic condition present in 3–5% of the population, although this has not been examined.
Figure 1.
Conceptual model. We hypothesized that psoriasis, psoriatic arthritis (PsA) and rheumatoid arthritis (RA) are associated with venous thromboembolism (VTE) as a result of local and/or systemic inflammation which then triggers prothrombotic pathways. Additional risk factors for VTE are also shown and were adjusted for in our analyses; they may be directly related to the risk for VTE or may act as confounders. In this model, we hypothesize local and/or systemic inflammation is a mediator or on the causal pathway between psoriasis or inflammatory arthritis and VTE. This hypothesis was not tested in this study. NSAID, non-steroidal anti-inflammatory drugs; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
Beyond inflammation, other potential risk factors for VTE include obesity, non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroid use.37–40 Patients with psoriasis and PsA have been found to be more obese than the general population and patients with RA and PsA more frequently use NSAIDs and corticosteroids. While each was associated with VTE in the multivariable models, there was not a statistically significant interaction with any one of these risk factors in our study (data not shown). However, in the multivariable models including NSAIDs as a covariate, NSAIDs were independently associated with VTE (HR 1.33, data not shown). This has previously been described but warrants future study as the majority of patients in this population use these medications.37
Strengths of this study include the use of a large population-based medical record database, broadly representative of the UK general population, the relatively large number of patients with PsA, psoriasis and RA, validation of the codes for the exposures and outcomes, and an unexposed cohort sampled from the general population. Additionally, the incidence rate for VTE found in our control population is nearly identical to reports from previous studies as noted in Table 2 (30/10 000 PYs in the UK, 38/10 000 PYs in our unexposed control population).1 Limitations of the study include the risk of exposure misclassification (e.g. patients diagnosed with PsA may in reality have psoriasis and osteoarthritis). However, previous validation studies for PsA, RA, and psoriasis found high positive predictive values for the codes (85–95%) using GP confirmation.24–27 Lack of direct measures of disease activity limited the assessment of active inflammation and the relationship with VTE. Similarly, not all laboratory results are available and only approximately 20% of our cohort had CRP and/or sedimentation rate results. Over the counter medications are not available in THIN. Biologic medications are not often recorded by general practitioners as they are prescribed by specialists or consultants. This limits our ability to examine the effect of these medications on the risk of VTE. Finally, there may be other unmeasured confounders, such as physical activity level and/or level of disability, that are not measured in a medical record database.
In summary, we found an increased age and sex adjusted risk for VTE in patients with RA, psoriasis and PsA and this risk remained significantly elevated after adjustment for established VTE risk factors in RA and mild psoriasis. Our article has advantages over the previous analyses including comparison among three inflammatory diseases, identification and analysis of PsA as separate from severe psoriasis, and a more generalizable approach relevant for all patients with psoriasis, PsA, or RA. Thus, patients with one of these diseases and another risk factor for VTE such as general surgery, major trauma, immobility or oral contraceptives may warrant additional monitoring, and clinicians may counsel such patients on the use of compression stockings or consider VTE prophylaxis. Finally, clinicians should be aware of the increased risk of VTE and consider the diagnosis of PE or DVT in patients presenting with acute shortness of breath or acute lower extremity swelling.
Supplementary Material
Acknowledgements
We thank Yihui Jiang for administrative support.
Funding
Pfizer to the trustees of the University of Pennsylvania (J.M.G.). National Institutes of Health (K23 AR063764 to A.O.). National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR068433 to J.T.). National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (K24 AR064310 to J.M.G.). National Psoriasis Foundation to Z.C. Medical dermatology fellowship from the National Psoriasis Foundation and NIH Training (T32-GM075766 to M.N.). Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation or approval of the manuscript; or decision to submit the manuscript for publication. Pfizer participated in reviewing the manuscript only.
Conflict of interest: A.O. has received has consulted for Novartis and Pfizer. J.T. has an investigator-initiated research grant from Pfizer and has received payment for continuing medical education work related to psoriasis. J.M.G. served as a consultant for Abbvie, Astrazeneca, Celgene Corp, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis Corp, Endo, Valeant, and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Amgen, Eli Lilly, Janssen, Novartis Corp, Regeneron, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis. ZC serves as the site-PI for a study from Regeneron.
Footnotes
See page 3615 for the editorial comment on this article (doi: 10.1093/eurheartj/ehx251)
References
- 1. Walker AJ, Card TR, West J, Crooks C, Grainge MJ.. Incidence of venous thromboembolism in patients with cancer—a cohort study using linked United Kingdom databases. Eur J Cancer 2013;49:1404–1413. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol 2015;12:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldhaber SZ, Bounameaux H.. Pulmonary embolism and deep vein thrombosis. Lancet 2012;379:1835–1846. [DOI] [PubMed] [Google Scholar]
- 4. Fox EA, Kahn SR.. The relationship between inflammation and venous thrombosis: a systematic review of clinical studies. Thromb Haemost 2005;94:362–365. [DOI] [PubMed] [Google Scholar]
- 5. van den Oever IA, Sattar N, Nurmohamed MT.. Thromboembolic and cardiovascular risk in rheumatoid arthritis: role of the haemostatic system. Ann Rheum Dis 2014;73:954–957. [DOI] [PubMed] [Google Scholar]
- 6. Matta F, Singala R, Yaekoub AY, Najjar R, Stein PD.. Risk of venous thromboembolism with rheumatoid arthritis. Thromb Haemost 2009;101:134–138. [PubMed] [Google Scholar]
- 7. Silvestri E, Scalera A, Emmi G, Squatrito D, Ciucciarelli L, Cenci C, Tamburini C, Emmi L, Di Minno G, Prisco D.. Thrombosis in autoimmune diseases: a role for immunosuppressive treatments? Semin Thromb Hemost 2016; 42:650–661. [DOI] [PubMed] [Google Scholar]
- 8. Ogdie A, Yu Y, Haynes K, Love T, Maliha S, Jiang Y, Troxel AB, Hennessy S, Kimmel SE, Margolis DJ, Choi HK, Mehta NN, Gelfand JM.. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: apopulation-based cohort study. Ann Rheum Dis 2015;74:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelfand J, Neimann A, Shin D, Wang X, Margolis D, Troxel A.. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735–1742. [DOI] [PubMed] [Google Scholar]
- 10. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano A.L., Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren VM. Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ.. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Medical 2011;9. DOI: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holmqvist ME, Neovius M, Eriksson J, Mantel Ä, Wållberg-Jonsson S, Jacobsson LT, Askling J.. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA 2012;308:1350–1356. [DOI] [PubMed] [Google Scholar]
- 13. Kim SC, Schneeweiss S, Liu J, Solomon DH.. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P.. Psoriasis and risk of venous thromboembolism: a systematic review and meta-analysis. QJM 2014;107:793–797. [DOI] [PubMed] [Google Scholar]
- 15. Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL.. Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol 2014;33:297–304. [DOI] [PubMed] [Google Scholar]
- 16. Choi HK, Rho YH, Zhu Y, Cea-Soriano L, Avina-Zubieta JA, Zhang Y.. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis 2013;72:1182–1187. [DOI] [PubMed] [Google Scholar]
- 17. Liang KP, Liang KV, Matteson EL, McClelland RL, Christianson TJ, Turesson C.. Incidence of noncardiac vascular disease in rheumatoid arthritis and the relationship to extraarticular disease manifestations. Arthritis Rheum 2006;54:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL.. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum 2012;64:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zöller B, Li X, Sundquist J, Sundquist K.. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow up study from Sweden. Lancet 2012;379:244–249. [DOI] [PubMed] [Google Scholar]
- 20. Chung WS, Peng CL, Lin CL, Chang YJ, Chen YF, Chiang JY, Sung FC, Kao CH.. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis 2014;73:1774–1780. [DOI] [PubMed] [Google Scholar]
- 21. Lee JJ, Pope JE.. A meta-analysis of the risk of venous thromboembolism in inflammatory rheumatic disease. Arthritis Res Ther 2014;16:435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cegedim Strategic Data. The Health Improvement Network: our data. http://csdmruk.cegedim.com/our-data/our-data.shtml (2 October 2013).
- 23. Chishom J. The Read clinical classification. BMJ 1990;300:1092.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson DJ, Rhodes T, Bing C, Guess HA.. Lower risk of thromboembolic cardiovascular events with Naproxen among patients with rheumatoid arthritis. Arch Intern Med 2002;162:1105–1110. [DOI] [PubMed] [Google Scholar]
- 25. Garcia Rodriguez LA, Tolosa LB, Ruigomez A, Johansson S, Wallander MA.. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol 2009;38:173–177. [DOI] [PubMed] [Google Scholar]
- 26. Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, Troxel AB, Gelfand JM.. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011;164:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogdie A, Alehashemi S, Love T, Jiang Y, Haynes K, Hennessy S, Choi HK, Gelfand JM.. Validity of psoriatic arthritis and capture of disease modifying antirheumatic drugs in The Health Improvement Network. Pharmacoepidemiol Drug Saf 2014;23:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawrenson R, Todd J, Leydon G, Williams T, Farmer R.. Validation of the diagnosis of venous thromboembolism in general practice database studies. Pharmacoepidemiol Drug Saf 2000;49:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bursac Z, Gauss CH, Williams DK, Hosmer DW.. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3. DOI: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coates LC, FitzGerald O, Helliwell PS, Paul C.. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: Is all inflammation the same? Semin Arthritis Rheum 2016;46:291–304. [DOI] [PubMed] [Google Scholar]
- 31. Ahlehoff O, Gislason GH, Lindhardsen J, Charlot MG, Jørgensen CH, Olesen JB, Bretler DM, Skov L, Torp-Pedersen C, Hansen PR.. Psoriasis carries an increased risk of venous thromboembolism: a Danish nationwide cohort study. PLoS One 2011;6:e18125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lutsey OL, Prizment AE, Folsom AR.. Psoriasis is associated with a greater risk of incident venous thromboembolism: the Iowa Women’s Health Study. J Thromb Haemost 2012;10:708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim SC, Solomon DH, Liu J, Franklin JM, Glynn RJ, Schneeweiss S.. Risk of venous thromboembolism in patients with rheumatoid arthritis: initiating disease-modifying antirheumatic drugs. Am J Med 2015;128:539.e7–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zezos P, Kouklakis G, Saibil F.. Inflammatory bowel disease and thromboembolism. World J Gastroenterol 2014;20:13863–13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Douglas KM, Kitas GD, Kitas GD.. Platelet function in rheumatoid arthritis: arthritic and cardiovascular implications. Rheumatol Int 2011;31:153–164. [DOI] [PubMed] [Google Scholar]
- 36. Takeshita JlM ER, Krishnamoorthy P, Moore J, Rogers WT, Zhang L, Gelfand JM, Mehta NN.. Endothelial cell-, platelet-, and monocyte/macrophage-derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J Am Heart Assoc 2014;3:e000507.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ungprasert P, Srivail N, Wijarnpreecha K, Charoenpong P, Knight EL.. Non-steroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford) 2015;54:736–742. [DOI] [PubMed] [Google Scholar]
- 38. J Johannesdottir SA, Horvath-Puho E, Dekkers OM, Cannegieter SC, Jorgensen JO, Ehrenstein V, Vandenbroucke JP, Pedersen L, Sorensen HT.. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–753. [DOI] [PubMed] [Google Scholar]
- 39. Stuijver DJ, Majoor CJ, van `Zaane B, Souverein PC, de Boer A, Dekkers OM, Buller HR, Gerdes VE.. Use of oral glucocorticoids and the risk of pulmonary embolus: a population-based case-control study. Chest 2013;143:1337–1342. [DOI] [PubMed] [Google Scholar]
- 40. Streiff MB. Predicting the risk of recurrent venous thromboembolism. J Thromb Thrombolysis 2015;39:353–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.