Abstract
Candida albicans (C. albicans) is the most prevalent opportunistic human pathogenic fungus and can cause mucosal membrane infections and invade the blood. In the oral cavity, it can ferment dietary sugars, produce organic acids and therefore has a role in caries development. In this study, we examined whether the polyphenol rich extractions Polyphenon from green tea (PPFGT) and Padma Hepaten (PH) can inhibit the caries-inducing properties of C. albicans. Biofilms of C. albicans were grown in the presence of PPFGT and PH. Formation of biofilms was tested spectrophotometrically after crystal violet staining. Exopolysaccharides (EPS) secretion was quantified using confocal scanning laser microscopy (CSLM). Treated C. albicans morphology was demonstrated using scanning electron microscopy (SEM). Expression of virulence-related genes was tested using qRT-PCR. Development of biofilm was also tested on an orthodontic surface (Essix) to assess biofilm inhibition ability on such appliances. Both PPFGT and PH dose-dependently inhibited biofilm formation, with no inhibition on planktonic growth. The strongest inhibition was obtained using the combination of the substances. Crystal violet staining showed a significant reduction of 45% in biofilm formation using a concentration of 2.5mg/ml PPFGT and 0.16mg/ml PH. A concentration of 1.25 mg/ml PPFGT and 0.16 mg/ml PH inhibited candidal growth by 88% and EPS secretion by 74% according to CSLM. A reduction in biofilm formation and in the transition from yeast to hyphal morphotype was observed using SEM. A strong reduction was found in the expression of hwp1, eap1, and als3 virulence associated genes. These results demonstrate the inhibitory effect of natural PPFGT polyphenolic extraction on C. albicans biofilm formation and EPS secretion, alone and together with PH. In an era of increased drug resistance, the use of phytomedicine to constrain biofilm development, without killing host cells, may pave the way to a novel therapeutic concept, especially in children as orthodontic patients.
1. Introduction
C. albicans is the most prevalent opportunistic human pathogenic fungus, which can cause infections of mucosal membranes (candidiasis) and invade the blood stream (candidemia) [1]. It is able to form biofilms on mucosal membranes as well as on implants [2]. Biofilm formation and virulence of C. albicans are related to its transition from the yeast to the hyphae morphotype, which represents a crucial step towards pathogenesis.
Hyphae provide structural integrity to biofilms [3]. C. albicans has been found in periodontal pockets in both the chronic and aggressive forms of periodontitis [4].
C. albicans is a common colonizer of carious lesions in children and in adolescents. It can ferment and/or assimilate some dietary sugars and produce organic acids in the dental plaque and therefore has a role in caries development [5]. An in vitro study revealed that the occurrence of caries in children was positively correlated with the frequency of oral candidal carriage [6].
C. albicans virulence is associated with the expression of extracellular polysaccharides (EPS), such as glucans and mannans. These EPS act as adhesins, which enable the adhesion of C. albicans to host epithelial cells. They are also required for host recognition [7].
Hyphal wall protein 1 (HWP1) is a well-characterized C. albicans cell-surface protein, required for hyphal development and yeast adhesion to epithelial cells. HWP1 is known to be required for C. albicans biofilm formation in vivo and thus may be an excellent therapeutic target [8].
EAP1 gene, which encodes a glycosylphosphatidylinositol-anchored glucan-cross-linked cell wall protein, and agglutinin-like sequence 3 (ALS3), which encodes a cell-surface glycoprotein, also have a role in adhesion and biofilm formation [9, 10].
Previous studies have shown that oral appliances increase the oral Candida carriage rate and cause significant population alterations [11, 12]. Moreover, it was found that the Candida counts increase during orthodontic treatment with fixed appliances [13–16].
C. albicans and additional catalase-rich microbiota may have the ability to scavenge oxidants and thus can protect catalase-negative anaerobes and facultative anaerobes cariogenic streptococci against peroxide and thus secure their survival in the oral cavity.
This oxidative scavenging ability is markedly potentiated by chlorhexidine [17].
Polyphenols are abundant micronutrients in our diet, which endow their beneficial effect as antioxidants mainly in the oral cavity, due to saliva which enhances their solubilization [18] and downstream also in the stomach [19].
Green tea, extracted from Camellia sinensis, is a widely consumed beverage throughout the world, second only to water [20]. Green tea contains multiple polyphenolic catechin components, and epigallocatechin-3-gallate (EGCG) is the primary catechin accounting for 50–80% in a brewed cup [21]. Epicatechin-3-gallate (ECG) is the second most concentrated catechin component of green tea and is associated with its anti-inflammatory/antioxidant properties [22]. Other major catechins found in green tea include epicatechin (EC) and epigallocatechin (EGC).
Padma Hepaten is a polyphenolic formula derived from traditional Tibetan medicine, produced by Padma Inc. (Schwerzenbach, Switzerland). In Tibetan it is called “three fruits” and is composed of Chebulic myrobalan, amla fruit, and belleric myrobalan in the ratio 2:1:1 [23]. It was shown that Hepatic fibrosis is significantly ameliorated by PH administration [24]
Since polyphenols were previously shown to inhibit C. albicans biofilm formation [25, 26], the goal of this study was to examine whether PPFGT and PH have an inhibitory effect on C. albicans biofilm formation and can participate in the prevention of candidiasis, candidemia, and caries in the general population and in orthodontic patients.
2. Materials and Methods
2.1. Materials
Polyphenon 60 from green tea was purchased from Sigma- Aldrich (St. Louis, MO, USA) and Padma Hepaten was obtained from Padma Inc. (Schwerzenbach, Switzerland). Both powders were dissolved and diluted in brain-heart infusion (BHI) to different concentrations.
2.2. Biofilm Growth
C. albicans SC5314 cells, kept in a glycerol stock at −80°C, were thawed and incubated on BHI agar plates for 18 h at 37°C. The cells were then diluted in BHI broth to an optical density (OD) of 0.05 at 595 nm using the Tecan GENios machine (Tecan US, Durham, NC) in 96-well microtiter plates. 2% sucrose was added to each well. The growth medium was supplemented with different concentrations of the polyphenol rich PPFGT and PH. C. albicans without PPFGT and PH served as a control. The biofilms were allowed to develop for 48 h, at 5% CO2 at 37°C.
2.3. Optical Density of Grown Biofilms after Crystal Violet Staining
The biofilm was stained with crystal violet (CV) for 30 minutes, then the CV was washed and the biofilm was later washed three times with phosphate buffered saline (PBS). The remaining color was extracted using 33% acetic acid. Then, the OD was measured spectrophotometrically at 595 nm using the Tecan GENios machine (Tecan US, Durham, NC) [27].
2.4. Confocal Laser Scanning Microscopy (CLSM)
In order to quantify the biomass of C. albicans and its EPS, confocal laser scanning microscopy was used. The biofilm was prepared as described above but instead of wild type C. albicans SC5314, we used C. albicans SC5314 carrying the GFP reporter gene (C. albicans–GFP), kindly provided by J. Berman (Tel Aviv University, Israel).
Forty-eight-hour biofilms developed in the presence of PPFGT (1.25 mg/ml) and PH (0.16 mg/ml) at the minimal concentration, which inhibited the biofilm growth by 50% (MBIC50), were washed with PBS and incubated for 45 minutes with concanavalin A-Alexa Fluor 647 conjugate (Con A; 25 mg/ml) (Invitrogen, Carsbad, CA, USA). Con A (excitation wavelength 650 nm and emission at 668 nm) selectively binds to the glucose and mannose residues of fungal cell wall EPS [28]. Stained EPS and microorganisms were observed by a Zeiss LSM 510 CLS microscope (Carl Zeiss, Oberkochen, Germany). Three-dimensional images of the fungus and EPS distribution within the biofilm were constructed using Zen2009 software (Carl Zeiss, New York, USA). At least three random fields were observed and analyzed. The number of the cells, as well as individual EPS production by C. albicans, were calculated as a color-appropriated fluorescence intensity, using ImageJ v3.91 software (http://rsb.info.nih.gov/ij). The data is presented as the amount of cells as well as of the individual EPS production by C. albicans cells in each layer of the biofilm (10 μm). The percentage of total EPS production and total biomass in the biofilm formed in the presence of PH was calculated as the area under the curve (AUC) and compared to placebo control.
2.5. Morphology of the Biofilm
The assay was performed as described previously [29]. After washing, the biofilm was fixed in 4% formaldehyde for 1 hour at room temperature. The morphology of the cells in the biofilm formed in the presence of PPFGT was then visualized using an analytical Quanta 200 Environmental High-Resolution Scanning Electron Microscope (FEI, Eindhoven, Netherlands) at 200X–10,000X magnification. At least three random fields were observed and analyzed.
In this experiment the biofilm was grown on polyvinyl chloride (PVC) orthodontic surfaces (Essix) to check whether biofilm inhibition can also occur on orthodontic surfaces. Prior to biofilm formation, the PVC was disinfected by immersion in ethanol 70% overnight.
2.6. Quantitative Real-Time RT-PCR Analysis of C. albicans Specific Genes in the Biofilm
The assay was performed similarly to that described previously [29]. The biofilm was grown in the absence and presence of 1.25 mg/ml PPFGT and 0.16 mg/ml PH in 6-well plates under the conditions described above. After washing with PBS, biofilm cells were removed from the bottom of the plates with a sterile scraper and disrupted in a FastPrep Cell Disrupter (Bio101, Savant Instruments, Inc., NY, USA). Total RNA was extracted from the biofilm using Tri-Reagent (Sigma-Aldrich). RNA concentration was determined spectrophotometrically using a Nanodrop ND-1000 Instrument (Wilmington, DE, USA). Two micrograms of template was reverse-transcribed with Super Script First Strand (Invitrogen, Life Technologies, Carlsbad, CA, USA). The integrity and purity of the RNA were assessed using an Agilent 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA, USA). Expression of C. albicans biofilm formation and stress associated genes (hwp1, eap1, and als3) was analyzed. The relative expression levels of the target genes were analyzed using an ABI-Prism 7300 Instrument (Applied Biosystems, Foster City, CA, USA). Platinum SYBR Green PCR Master Mix (Invitrogen) was used to monitor the amplified product in real time, following the manufacturer's protocol. Primers for the tested genes are listed in Supplementary Table S1. Three independent experiments were performed. To normalize the results, we used the expression of 18s rRNA.
2.7. Statistical Analysis
The statistical analysis was performed using Student's one tailed t-test (equal variances assumed) with a significance level of P < 0.05.
3. Results
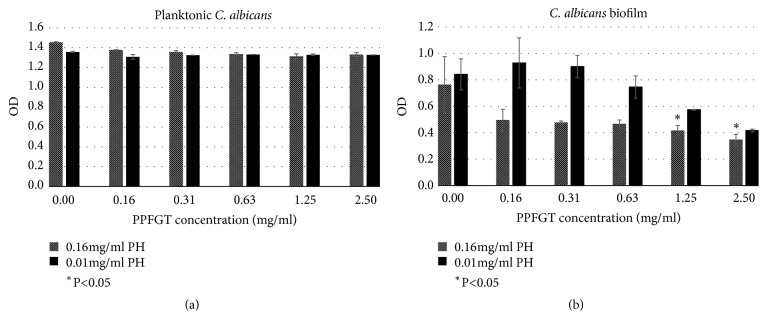
PPFGT inhibited C. albicans biofilm growth (Figure 1). While there was a dose-dependent inhibition on biofilm cells, there was no such an effect on planktonic cells. Concentrations of 1.25 and 2.5 mg/ml PPFGT significantly inhibited biofilm formation. Those same concentrations did not inhibit planktonic growth. The addition of higher concentration of PH (0.16 mg/ml instead of 0.01 mg/ml) resulted in a stronger inhibition on the biofilm formation, suggesting a combined effect between the two substances.
Figure 1.
Total biomass of planktonic and biofilm cells of C. albicans stained with crystal violet. (a) OD of planktonic C. Albicans with increasing concentrations of PPFGT. (b) OD of biofilm of C. albicans with increasing concentrations of PPFGT. Error bars indicate the standard deviation (n=96).
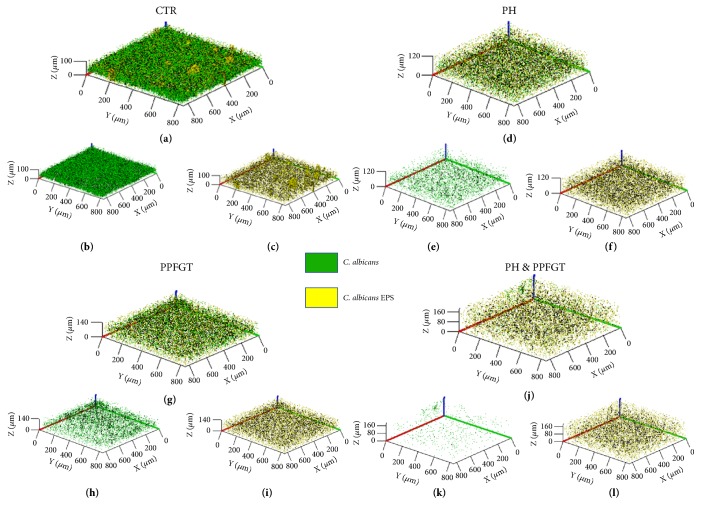
The 3D image of the reconstructed biofilm layers (Figures 2(a)-2(l)) visualizes the strong inhibition that PPFGT, PH, and their combination have on biofilm formation and EPS secretion by C. albicans. The treated biofilm consisted of less candida cells and less EPS and that the use of the two polyphenols together yielded the strongest inhibition.
Figure 2.
Biofilm 3D reconstruction of confocal laser scanning microscopy. Computerized 3D reconstruction (X, Y, and Z axis) of the biofilm layers (in µm), recorded using CLSM and generated by the zen2009 software. (a) Control candida cells and EPS. (b) Candida cells only. (c) EPS only. (d) PH treated biofilm, both Candida cells and EPS. (e) Candida cells only. (f) EPS only. (g) PPFGT treated biofilm, both Candida cells and EPS. (h) Candida cells only. (i) EPS only. (j) PH and PPFGT treated biofilm, both Candida cells and EPS. (k) Candida cells only. (l) EPS only.
Figures 3(a)-3(c) provide a numerical analysis of these results. The bell-shaped charts characterize the Candida and EPS counts in the different layers of the biofilm. The highest counts are in the middle layers of the biofilm. The number of Candida cells and the EPS was reduced dramatically by the treatment with PPFGT and PH, and most significantly when they were combined. The cells growth was inhibited by 88% and the EPS production by 74% when the polyphenol mix was used at concentrations of 0.16 mg/ml PH and 1.25 mg/ml PPFGT.
Figure 3.
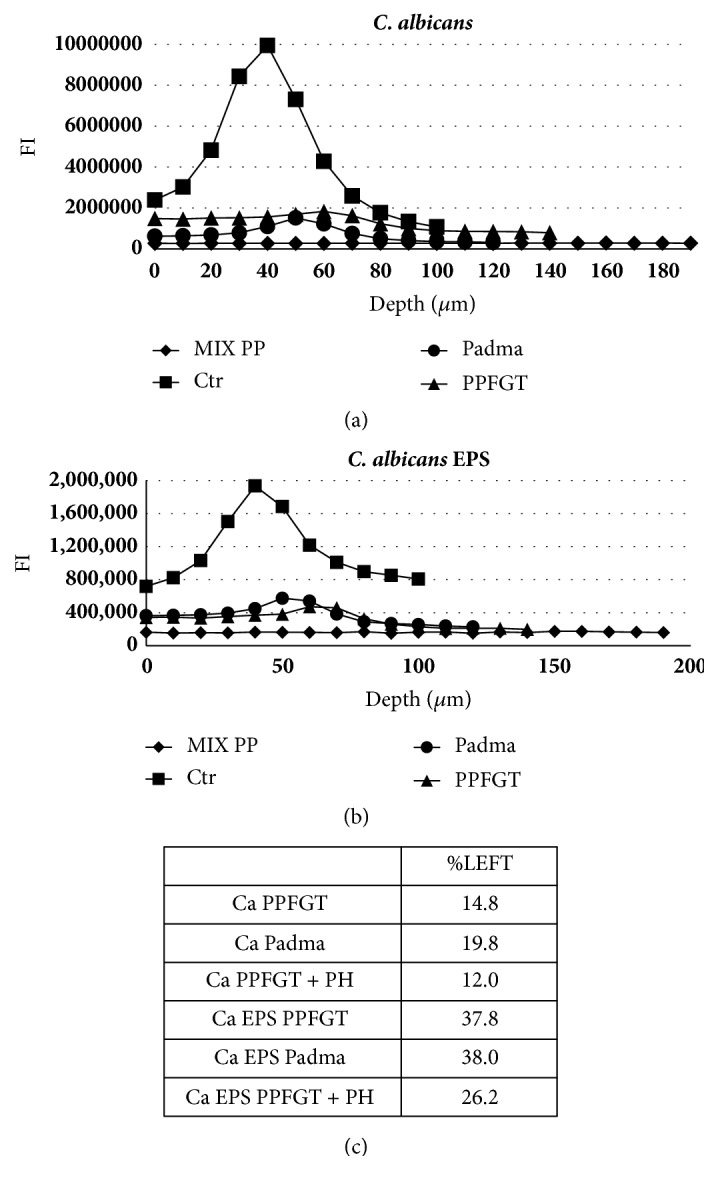
Quantification of the confocal laser scanning microscopy results. Charts (a) + (b) show the fluorescence intensity (FI) in each layer of the biofilm depth. (a) Cells quantification of the control and the treated groups. (b) EPS quantification of the control and the treated groups. (c) A table summing up the percentage of cells and EPS left after the various treatments relative to the control.
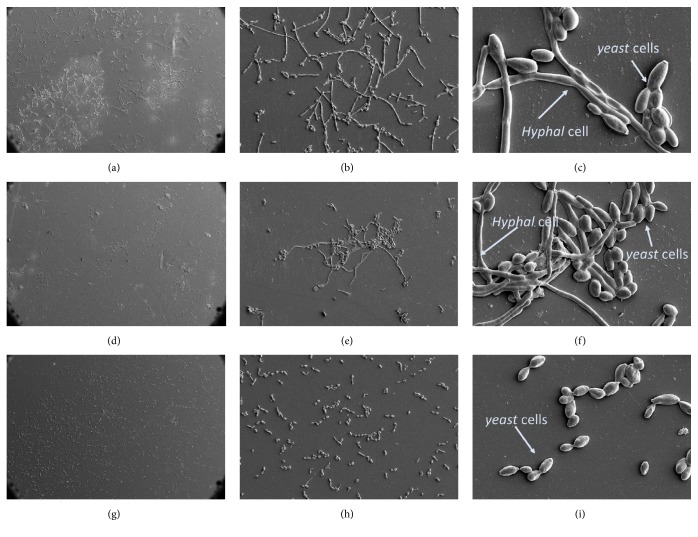
The SEM results (Figure 4) indicated a reduction in cell number in the PPFGT group (Figures 4(d)-4(f)) in comparison to the control group (Figures 4(a)-4(c)). Figures 4(g)-4(i) show the morphology of C. albicans treated with both polyphenols. The treated group contains more yeast shaped cells and less hyphal cells, suggesting that PPFGT with PH may affect C. albicans morphology switching ability, which is associated with its virulence.
Figure 4.
Biofilm morphology on orthodontic PVC using SEM. Morphology of the biofilm using SEM. (a) Control group at X200 magnification, (b) at X1,000, and (c) at X5,000. (d) PPFGT treated C. albicans at X200 magnification, (e) at X1,000, and (f) at X5,000. (g) PPFGT + PH treated at X200 magnification, (h) at X1,000, and (i) at X5,000.
The gene expression results (Figure 5) indicate that PPFGT, PH, and their combination inhibited the als3 gene; the most significant reduction was obtained using PH. PH and the PH-PPFGT combination also inhibited eap1. All of the treatments inhibited hwp1 expression, PPFGT most significantly. Surprisingly, the combination of the two polyphenols did not yield the strongest reduction in the genes expression.
Figure 5.
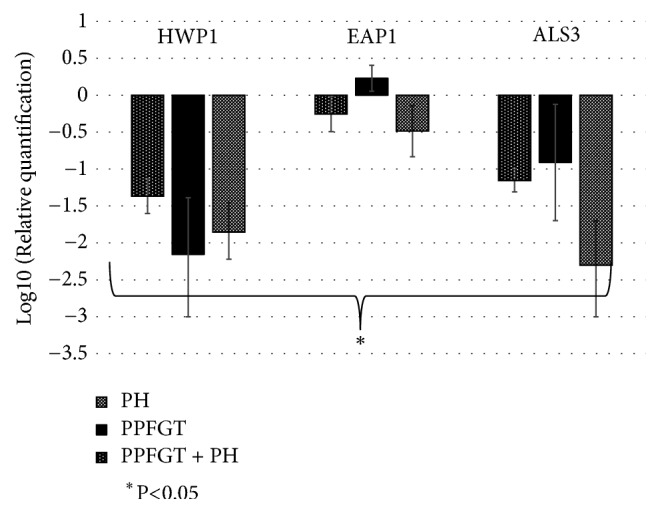
Relative expression of selected genes. Relative expression of the virulence associated genes (logarithmic scale), based on three independent experiments; error bars indicate standard deviation.
4. Discussion
C. albicans biofilm infections are an escalating clinical problem. Two consequences of biofilm growth with profound clinical implications are the markedly enhanced resistance to antimicrobial agents and the protection from host defenses [30].
From a dental point of view, C. albicans not only produces acids which causes caries, but also has a symbiotic relationship with caries-associated bacteria such as S. mutans. This symbiotic relationship allows the microorganisms to create an enhanced biofilm both in vitro and in vivo. Coinfected animals displayed higher levels of infection and microbial carriage within plaque biofilms compared to animals infected with either species alone. Furthermore, coinfection synergistically enhanced biofilm virulence, leading to aggressive onset of the disease with formation of rampant carious lesions [31].
In the present study, PH and PPFGT treatment affected C. albicans in three important manners; it reduced the number of the cells and the quantity of the EPS and inhibited the morphologic transition associated with the fungus' pathogenic capacity.
Another important finding is the specific inhibition of the polyphenols on biofilm sessile cells but not on planktonic cells. The killing of planktonic cells by medications such as azoles may result in a selection and enrichment of drug-resistant strains. Another consideration which favors biofilm inhibition is that drugs penetrate poorly into biofilms and without treatment directed at the biofilm the response is poor and temporary [32].
Shown in Figure 1 PPFGT dose-dependently inhibited the biofilm formation of C. albicans. This reduction was significant using concentrations of 1.25 mg/ml and higher. The addition of 0.16 mg/ml PH rather than 0.01 mg/ml resulted in a stronger reduction in biofilm generation. Therefore, we concluded that the two polyphenols have an additive effect on the inhibition of C. albicans biofilm formation.
This additive effect is shown also in Figures 2 and 3, describing the CLSM results.
The use of the two polyphenols together yielded the strongest inhibition on biofilm formation and EPS secretion by 88% and 74%, respectively.
Our SEM results in Figure 4 demonstrate that the morphogenic transition from yeast cells to hyphal cells may be impaired by the use of the PH+PPFGT combination. In other studies, Farnesoic acid was found to be an autoregulator of the morphogenic transition from yeast to hyphal form, produced and secreted by C. albicans. This autoregulator induces the transition from yeast to hypha in low concentrations but inhibits it in higher concentrations [33]. Resveratrol, a polyphenol found in grapes, and honey flavonoids also impaired this morphological alteration [34, 35].
In Figure 5, we have shown a significant downregulation in the virulence associated genes- als3, eap1, and hwp1. In similar works, curcumin, a polyphenol from the turmeric spice, showed a significant downregulation on the als3 and hwp1 genes [26]. In a different study, curcumin caused a downregulation of als3 and an upregulation of the eap1 gene [36]. An administration of 16 μg/ml Thiazolidinedione-8 also resulted in an inhibition of the als3 and hwp1 genes [29]. In comparison, our research shows a reduced expression of all these three genes using PH and the PH + PPFGT combination. The use of PPFGT alone resulted in an upregulation of the eap1 gene and downregulation of als3 and hwp1.
Our SEM results (Figure 4) also demonstrated the capacity of the polyphenols to inhibit biofilm growth on an orthodontic PVC surface. This finding, we believe, is critical since oral appliances increase the oral candida carriage rate and cause a significant population alterations [12]. To our knowledge, this is the first study showing a possible mechanism for C. albicans inhibition on orthodontic appliances using natural agents.
PH and PPFGT showed a combined mechanism of action for the inhibition of C. albicans biofilm. We believe that this research may pave the way to reveal more herbal combinations for increased efficacy for C. albicans biofilm inhibition. In addition, PH and PPFGT might have an inhibitory effect also on biofilms formed by other microorganisms; therefore, future research is required.
5. Conclusion
PH and PPFGT administration dose-dependently and synergistically inhibits C. albicans biofilm growth in vitro, its ability to secrete EPS, and the yeast to hypha morphogenic change, which is crucial for the fungus virulence. It influences the total biomass of the biofilm, the morphology of the biofilm, and caries-associated properties of the biofilm, as well as the expression of the hwp1, eap1, and als3 virulence associated genes. Therefore, PPFGT and PH may take part in the fight against C. albicans infections and ever-growing drug resistance and in caries prevention in the general population, as well as in orthodontic patients.
Acknowledgments
The authors would like to thank Dr. Mark Tarshish for his excellent work with the CLSM.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Professor Steinberg holds the Chair of H. Leslie Levine in Oral Pathology and Dental Medicine.
Conflicts of Interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Supplementary Materials
A list of primers sequences used in this study.
References
- 1.Kim J., Sudbery P. Candida albicans, a major human fungal pathogen. Journal of Microbiology. 2011;49(2):p. 171. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 2.Cuéllar-Cruz M., López-Romero E., Villagómez-Castro J. C., Ruiz-Baca E. Candida species: New insights into biofilm formation. Future Microbiology. 2012;7(6):755–771. doi: 10.2217/fmb.12.48. [DOI] [PubMed] [Google Scholar]
- 3.Finkel J. S., Mitchell A. P. Genetic control of Candida albicans biofilm development. Nature Reviews Microbiology. 2011;9(2):109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urzua B., Hermosilla G., Gamonal J., et al. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Medical Mycology. 2008;46(8):783–793. doi: 10.1080/13693780802060899. [DOI] [PubMed] [Google Scholar]
- 5.Klinke T., Kneist S., de Soet J. J., et al. Acid production by oral strains of Candida albicans and lactobacilli. Caries Research. 2009;43(2):83–91. doi: 10.1159/000204911. [DOI] [PubMed] [Google Scholar]
- 6.Raja M., Hannan A., Ali K. Association of oral candidal carriage with dental caries in children. Caries Research. 2010;44(3):272–276. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 7.Calderone R. A., Fonzi W. A. Virulence factors of Candida albicans. Trends in Microbiology. 2001;9(7):327–335. doi: 10.1016/S0966-842X(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 8.Nobile C. J., Nett J. E., Andes D. R., Mitchell A. P. Function of Candida albicans adhesin hwp1 in biofilm formation. Eukaryotic Cell. 2006;5(10):1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F., Svarovsky M. J., Karlsson A. J., et al. Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukaryotic Cell. 2007;6(6):931–939. doi: 10.1128/EC.00049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X., Oh S.-H., Cheng G., et al. Als3 and als8 represent a single locus that encodes a candida albicans adhesin; functional comparisons between als3p and als1p. Microbiology. 2004;150(7):2415–2428. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- 11.Hägg U., Kaveewatcharanont P., Samaranayake Y. H., Samaranayake L. P. The effect of fixed orthodontic appliances on the oral carriage of Candida species and Enterobacteriaceae. European Journal of Orthodontics. 2004;26(6):623–629. doi: 10.1093/ejo/26.6.623. [DOI] [PubMed] [Google Scholar]
- 12.Arendorf T., Addy M. Candidal carriage and plaque distribution before, during and after removable orthodontic appliance therapy. Journal of Clinical Periodontology. 1985;12(5):360–368. doi: 10.1111/j.1600-051X.1985.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 13.Gündüz Arslan S., Akpolat N., Kama J. D., Özer T., Hamamci O. One-year follow-up of the effect of fixed orthodontic treatment on colonization by oral candida. Journal of Oral Pathology & Medicine. 2008;37(1):26–29. doi: 10.1111/j.1600-0714.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 14.Addy M., Shaw W. C., Hansford P., Hopkins M. The effect of orthodontic appliances on the distribution of Candida and plaque in adolescents. British Journal of Orthodontics. 1982;9(3):158–163. doi: 10.1179/bjo.9.3.158. [DOI] [PubMed] [Google Scholar]
- 15.Brusca M. I., Chara O., Sterin-Borda L., Rosa A. C. Influence of different orthodontic brackets on adherence of microorganisms in vitro. The Angle Orthodontist. 2007;77(2):331–336. doi: 10.2319/0003-3219(2007)077[0331:IODOBO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Saloom H. F., Mohammed-Salih H. S., Rasheed S. F. The influence of different types of fixed orthodontic appliance on the growth and adherence of microorganisms (in vitro study) Journal of Clinical and Experimental Dentistry. 2013;5(1):e36–e41. doi: 10.4317/jced.50988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsburg I., Koren E., Feuerstein O., Zogakis I. P., Shalish M., Gorelik S. Chlorhexidine markedly potentiates the oxidants scavenging abilities of Candida albicans. Inflammopharmacology. 2015;23(5):271–281. doi: 10.1007/s10787-015-0245-0. [DOI] [PubMed] [Google Scholar]
- 18.Ginsburg I., Koren E., Shalish M., Kanner J., Kohen R. Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity. Archives of Oral Biolog. 2012;57(10):1327–1334. doi: 10.1016/j.archoralbio.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Gorelik S., Ligumsky M., Kohen R., Kanner J. A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. The FASEB Journal. 2008;22(1):41–46. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- 20.Dreosti I. E. Bioactive ingredients: antioxidants and polyphenols in tea. Nutrition Reviews. 1996;54(11):S51–S58. doi: 10.1111/j.1753-4887.1996.tb03819.x. [DOI] [PubMed] [Google Scholar]
- 21.Khan N., Afaq F., Saleem M., Ahmad N., Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Research. 2006;66(5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 22.Rice-Evans C. A., Miller N. J., Bolwell P. G., Bramley P. M., Pridham J. B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Research. 1995;22(4):375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 23.Choedon T., Kumar V. Medicinal plants used in the practice of tibetan medicine. Recent Progress in Medicinal Plants. 2012;34:385–402. doi: 10.1007/s00281-012-0312-1. [DOI] [Google Scholar]
- 24.Ginsburg I., Koren E., Horani A., et al. Amelioration of hepatic fibrosis via padma hepaten is associated with altered natural killer t lymphocytes. Clinical & Experimental Immunology. 2009;157(1):155–164. doi: 10.1111/j.1365-2249.2009.03936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Bagieh N. H., Al Bageah H. N. Effect of tannic acid on growth and acid production of candida albicans. Pakistan Oral & Dental Journal. 2014;34(3) doi: 10.1007/s11596-014-1284-2. [DOI] [Google Scholar]
- 26.Shahzad M., Sherry L., Rajendran R., Edwards C. A., Combet E., Ramage G. Utilising polyphenols for the clinical management of Candida albicans biofilms. International Journal of Antimicrobial Agents. 2014;44(3):269–273. doi: 10.1016/j.ijantimicag.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Christensen G. D., Baldassarri L., Simpson W. A. Methods in Enzymology. Elsevier; 1995. [38] methods for studying microbial colonization of plastics; pp. 477–500. [DOI] [PubMed] [Google Scholar]
- 28.Chandra J., Kuhn D. M., Mukherjee P. K., Hoyer L. L., McCormick T., Ghannoum M. A. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. Journal of Bacteriology. 2001;183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman M., Ginsburg I., Al-Quntar A., Steinberg D. Thiazolidinedione-8 alters symbiotic relationship in c. Albicans-s. Mutans dual species biofilm. Frontiers in Microbiology. 2016;7(140) doi: 10.3389/fmicb.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mah T. F., O'Toole G. A. Mechanisms of biofilm resistance to antimicrobial agents. Trends in Microbiology. 2001;9(1):34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 31.Falsetta M. L., Klein M. I., Colonne P. M., et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infection and Immunity. 2014;82(5):1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rautemaa R., Ramage G. Oral candidosis–clinical challenges of a biofilm disease. Critical Reviews in Microbiology. 2011;37(4):328–336. doi: 10.3109/1040841x.2011.585606. [DOI] [PubMed] [Google Scholar]
- 33.Oh K.-B., Miyazawa H., Naito T., Matsuoka H. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in candida albicans. Proceedings of the National Acadamy of Sciences. 2001;98(8):4664–4668. doi: 10.1073/pnas.071404698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canonico B., Candiracci M., Citterio B., et al. Honey flavonoids inhibit candida albicans morphogenesis by affecting DNA behavior and mitochondrial function. Future Microbiology. 2014;9(4):445–456. doi: 10.2217/fmb.14.17. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto-Shibayama K., Sato Y., Azuma T. Resveratrol impaired the morphological transition of candida albicans under various hyphae-inducing conditions. Journal of Microbiology and Biotechnology. 2010;20(5):942–945. doi: 10.4014/jmb.0911.11014. [DOI] [PubMed] [Google Scholar]
- 36.Alalwan H., Rajendran R., Lappin D. F., et al. The anti-adhesive effect of curcumin on Candida albicans biofilms on denture materials. Frontiers in Microbiology. 2017;8:p. 659. doi: 10.3389/fmicb.2017.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A list of primers sequences used in this study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.