Abstract
Background:
There are sex differences in buprenorphine/naloxone clinical trials for opioid use. Whereas women have fewer opioid-positive urine samples, relative to men, a significant decrease in opioid-positive samples was found during treatment for men, but not women. In order to inform sex-based approaches to improve treatment outcomes, research is needed to determine if opioid use, and predictors of opioid use, differs between men and women during treatment.
Objectives:
To test for sex differences in opioid use during a buprenorphine/naloxone clinical trial and determine if sex differences exist in the associations between addiction-related problem areas and opioid use over the course of the trial.
Method:
This secondary data analysis of the National Drug Abuse Treatment Clinical Trials Network (CTN) 0003 examined sex differences (men=347, women=169) in opioid-positive samples in a randomized clinical trial comparing 7-day vs. 28-day buprenorphine/naloxone tapering strategies. Addiction-related problem areas were defined by Addiction Severity-Lite (ASI-L) domain composite scores.
Results:
Women were more likely than men to use opioids during the course of the buprenorphine/naloxone clinical trial (B=.33, p=.01) and medical issues were positively related to submitting an opioid-positive sample during treatment for women (B=1.67, p=.01). No ASI-L domain composite score was associated with opioid-positive samples during treatment for men.
Conclusion:
Women were more likely than men to use opioids during the course of the buprenorphine/naloxone clinical trial, and medical issues predicted opioid use during treatment for women but not men. Complementary treatment for medical problems during opioid replacement therapy may benefit women.
Keywords: Opioid dependence, Buprenorphine/naloxone, Addiction Severity Index, Sex differences
Introduction
In the US, over 2 million people are dependent on prescription opioids and approximately 591,000 are dependent on heroin (1). Although women regularly report lower rates of heroin use than men (1.6 per 1,000 vs. 3.6 per 1,000), women’s rate of heroin use doubled between 2002–2013 (2). While women endorse less lifetime (11.2% vs. 15.9%) and past-year (4.2% vs. 5.9%) use of prescription opioids compared to men, men and women do not differ in the percent (13.2%) of past-year use that meet the criteria for prescription opioid abuse or dependence (3). The gap is also narrowing for prescription opioid overdose deaths: between 1999–2010, women had a 400% increase in prescription opioid overdoses compared to a 265% increase for men (4). In 2015, over 11,000 women died as a result of an opioid overdose (5). As opioid use is rapidly escalating in women, it is critical to determine if opioid replacement therapy works equally well for women as it does for men.
Buprenorphine/naloxone is a medication used in the treatment of opioid dependence, with noted success across numerous clinical trials examining medication-assisted treatment and detoxification (6). Studies have found sex differences in buprenorphine/naloxone clinical trials for opioid use. Whereas women have had fewer opioid-positive urine samples, relative to men (2), other research has found a significant decrease in opioid-positive samples during treatment for men, but not women (7). When comparing buprenorphine/naloxone tapering strategies in this trial, no significant sex differences in opioid-positive samples were observed.
However, research on the effects of buprenorphine/naloxone has largely focused on end-point analyses (statistical analyses of groups at the end of treatment) and many opioid use disorder pharmacotherapy trials do not make use of the longitudinal data. Consequently, there is a lack of research examining sex differences in the trajectory of opioid use over the course of a clinical trial. Such examinations allow researchers to identify opioid use throughout the course of buprenorphine/naloxone clinical trials, determining if complementary treatments (e.g., psychosocial, behavioral, etc.) could be given to enhance overall treatment success. Recently, longitudinal assessments of opioid-positive samples found that women were significantly more likely than men to submit an opioid-positive sample over time in a randomized clinical trial that compared buprenorphine/naloxone tapering strategies using two different longitudinal modeling strategies (8, 9). While McPherson and colleagues (8, 9) found that sex as a covariate significantly predicted opioid-positive samples over time, this finding was never followed up on: parameters to determine if sex differences exist across treatment arms were not estimated, nor were predictors of opioid use tested. Thus, when examining longitudinal trajectories of opioid use, sex differences arise in opioid use during a buprenorphine/naloxone clinical trial; these differences are not seen when only end-point analyses are analyzed. Our first aim addresses this issue by testing sex differences in opioid use over the course of buprenorphine/naloxone treatment.
In addition to determining if opioid use differs between men and women during treatment, we must also test for variables that predict use during treatment. Defining sex differences in predictors of opioid use is critical to inform sex-based approaches to improve treatment outcomes. A global assessment of addiction-related problem areas in opioid-dependent patients may help highlight sex differences in opioid use in a clinical trial. The Addiction Severity Index-Lite (ASI-L) is often used to assess problems found in patients with alcohol and other issues (10, 11). Research with opioid-dependent individuals found that those with more medical, employment, and alcohol problems were more likely to submit opioid-positive samples over time (12), and women report more issues with drug, medical, psychological, family/social, and employment problems compared to men, whereas men have more problems with legal and alcohol-related issues (13). Similar results were found with methadone maintenance patients (14, 15). Therefore, attention to how sex differences in addiction-related problem areas may predict opioid use in clinical trials is crucial. A significant burden of issues related to substance use rests on women. If providers cannot meet the needs of women’s clinical characteristics, they will be less successful in treating opioid use disorders in women. We must determine the specific addiction-related problem areas that implicate the course of substance use treatment for women.
The current literature regarding the impact of sex on pharmacological treatments for opioid use disorders has not thoroughly tested sex differences in buprenorphine/naloxone clinical trials and has predominantly relied on end-of-treatment outcomes. Given the extent of the opioid crisis, we can no longer rely on what is known about other substances; we must conduct comprehensive tests of sex differences in use during treatment, as well as variables that predict use. The aims of this secondary data analysis of the National Drug Abuse Treatment Clinical Trials Network (CTN) 0003 study were to 1) test for sex differences in opioid use during a buprenorphine/naloxone clinical trial and 2) determine if sex differences exist in the associations between addiction-related problem areas (i.e., ASI-L domain composite scores) and opioid use over the course of the trial.
Methods
Participants and procedure
Participants were opioid-dependent men (n = 347) and women (n = 169) in the National Drug Abuse Treatment CTN 0003 study (16). Participants were randomized to a parallel-group, open-label study design for opioid-dependent individuals seeking treatment from 11 outpatient treatment facilities in 10 US cities, and 519 participants were stratified across maintenance dose and randomized to either the 7-day or 28-day tapering group (16). Data used in this study were from the end of the stabilization period (baseline n = 516) and the 4 subsequent weeks of treatment after randomization (week 1 n = 202, week 2 n = 431, week 3 n = 364, and week 4 n = 146). Please refer to the parent study (16) and screening study (13) for additional information on study protocol schema. The parent study was approved by each of the participating Institutional Review Boards and all participants gave written informed consent.
The mean age for the male sample was 36.40 (SD = 10.75) and 34.90 (SD = 9.76) for the female sample (nonsignificant difference, t(514) = 1.53, p = .13). Sixty-nine percent of the male sample was white and 12% was Black/African-American; 75.1% of the female sample was white and 8% was Black/African-American. Men (M =23.32, SD = 11.23) and women (M =23.01, SD = 12.33) did not differ on heroin use in the past 30 days (t(514) = 1.21, p = .23), but men had more lifetime years of use (M =7.22, SD = 8.74) compared to women (M =4.55, SD = 5.72), t(514) = 3.62, p < .001. Men and women did not differ in self-report or clinician-rated withdrawal scores (Ling et al., 2009). As reported in Ling et al. (2009), buprenorphine/naloxone tapering groups (7-day and 28-day tapering groups) did not differ in age, gender, heroin use (last 30 days or lifetime years of use), or self-report or clinician-rated withdrawal scores.
Measures
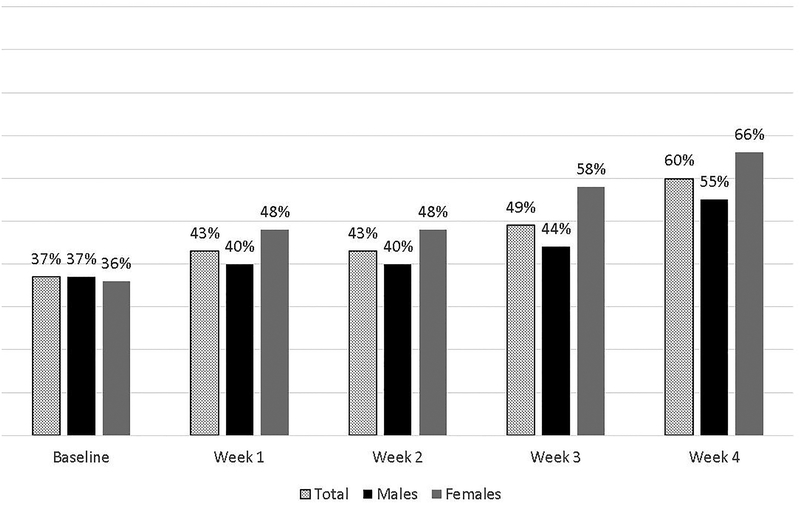
Buprenorphine/naloxone tapering groups were coded as −.5 = 7-day taper group (referent group), .5 = 28-day taper group, and sex was coded as −.5 = male (referent group), .5 = female. Age was recorded in years. Clinic visits for urine sample collection were scheduled once per week. Urine analysis results were coded as 0 = negative for opioid use and 1 = positive for opioid use. Figure 1 presents the percent of opioid-positive samples for the total sample and by sex over the course of the study.
Figure 1.
Percent of opioid-positive urine samples for the total sample and by sex.
The ASI-L baseline data was used in this study. The ASI-L is a standardized semi-structured clinical interview that offers clinical information for treatment planning and assesses severity profiles in the following domains: medical, employment, alcohol, drug, psychological, legal, and family/social (11). ASI-L domain composite scores range from .00 (no problem) to 1.00 (most severe). The ASI-L has been shown to have adequate to good internal consistency (depending on the domain), good test-retest reliability, independence across the domain composite scores, and agreement with the longer version of the ASI (11). The ASI-L was administered in CTN 0003 by trained, bachelor’s level research staff members. ASI-L composite scores were grand-mean centered in the statistical analyses.
Statistical analyses
Latent growth modeling (LGM) using the logit link function was used to examine the trajectory of opioid-positive samples (binary outcome, using CATEGORICAL ARE syntax) at baseline and the following 4 weeks of treatment (5 time points) using full information robust maximum likelihood estimation (FIMLR). LGM allows for the assessment of individual opioid use over time within the structural equation modeling framework (19). The growth factors, or random effects, include the intercept and slope (trajectory or growth) latent factors. Therefore, we modeled repeated measures of opioid-positive samples from baseline to week 4 of treatment.
In examining the percent of crude and estimated opioid-positive samples over time (Figure 1), trajectories appeared linear, and this was verified with larger Bayesian Information Criteria (BIC) values for quadratic latent growth models (total sample BIC = 1899.31, male sample BIC = 1287.78, female sample BIC = 642.42) compared to the linear models presented in the Results section. While the primary study used positive urine analysis imputation for missing data (16), this secondary data analysis utilized FIMLR to estimate parameters using all available data and assuming data was missing at random (44.6% missing data across opioid urinalysis assessments). We have shown that positive urine analysis imputation can result in an inflated treatment effect with this clinical trial (17), and our previous methodological work has demonstrated that FIMLR is an effective missing data strategy with similar results when compared to missing-not-at-random approaches, specifically for treatment and sex differences in this clinical trial (CTN-0003) (9). In all models, the intercept (baseline opioid-positive samples) predicted the slope (opioid-positive samples over time). To estimate a LGM, intercepts are constrained to 1 and the linear slope is indicated by slope parameter constraints 0, 1, 2, 3, and 4.
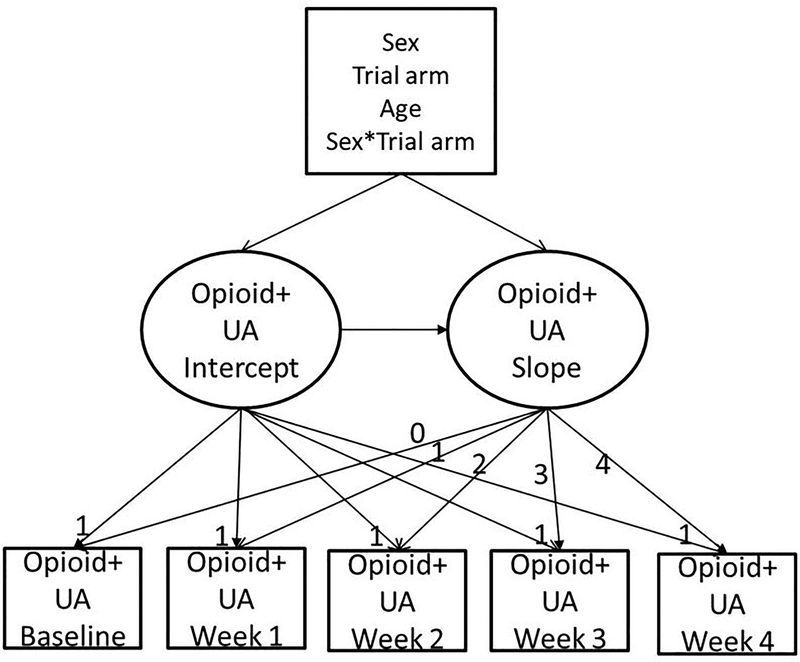
To test Aim 1, we modeled opioid-positive samples over the course of the trial, regressing the growth factors on age, sex, taper trial arm, and a sex*taper trial arm interaction term (model 1). A schematic presentation of this model is presented in Figure 2. To test Aim 2, we added in the model parameters to assess sex differences in ASI-L domain composite scores and opioid use over the course of the trial, regressing the growth factors on the ASI-L domain composite scores and sex*ASI-L domain composite score interaction terms (medical*sex, employment*sex, alcohol*sex, drug*sex, legal*sex, psychological*, and family*sex interaction terms; model 2). Post hoc analyses following interaction follow-up procedures (20) were planned for statistically significant interaction terms to assess the magnitude of the associations by sex, whereby we regressed the growth factors on the ASI-L domain composite scores in sex specific opioid-positive sample LGMs (model 3 for women, model 4 for men).
Figure 2.
Schematic representation of the linear latent growth model of opioid-positive urinalysis (opioid+UA) over time in a randomized clinical trial that compared buprenorphine/naloxone tapering strategies (7-day and 28-day). Intercept and slope parameters are constrained to indicate the linear model.
All primary statistical analyses were conducted in Mplus, Version 7.11 (20) using FIMLR, and BIC is reported for the models (note that only BIC is reported because the longitudinal manifest variables in the LGM are dichotomous and FIMLR was used in order to obtain model convergence). Unstandardized regression coefficients are reported because the outcome (opioid-positive samples) is binary.
Results
Sex differences across opioid-positive samples
Results of the LGM on the total sample (BIC = 1872.03) found that men were more likely than women to submit opioid-positive samples at baseline (B = −1.18, p = .02), whereas women were more likely than men to submit opioid-positive samples over the course of the trial (unstandardized regression coefficient B = .33, p = .01). Those in the 7-day taper trial arm were more likely than the 28-day taper trial arm to submit opioid-positive samples over the course of the trial (B = −.23, p < .001), and those with lower baseline use were more likely to submit opioid-positive samples over the course of the trial (B = −.12, p = .001). The sex*taper trial arm interaction term was significant at baseline (B = .70, p = .01), with women in the 28-day taper more likely to submit opioid-positive samples at baseline. However, the sex*taper trial arm interaction term was statistically nonsignificant over the course of trial (B = −.11, p = .12), indicating no sex difference in opioid use across tapering strategies. Please refer to Table 1 (Model 1) for parameter estimates for the total sample.
Table 1.
Parameter estimates for linear latent growth models for opioid-positive samples at baseline and 4 weeks of treatment
| Independent variable | Dependent variable | Unstandardized estimate (B) | 95% CI | P-value | |
|---|---|---|---|---|---|
| Model 1: Total sample |
|||||
| --- | UA intercept | .53 | −1.26, 2.32 | .56 | |
| --- | UA slope | .77 | .19, 1.25 | .01 | |
| Sex | UA intercept | −1.18 | −1.22, −.21 | .02 | |
| Trial arm | UA intercept | .35 | −.14, .85 | .16 | |
| Age (years) | UA intercept | −.04 | −.08, .002 | .06 | |
| Trial arm*sex | UA intercept | .70 | .17, 1.23 | 01 | |
| Sex | UA slope | .33 | .09, .58 | .01 | |
| Trial arm | UA slope | −.23 | −.35, −.10 | <.001 | |
| Age (years) | UA slope | −.003 | −.01, .01 | .64 | |
| Trial arm*sex | UA slope | −.11 | −.25, .02 | .12 | |
| UA intercept | UA slope | −.12 | −.19, −.05 | .001 |
Buprenorphine/naloxone tapering groups were coded as −.5 = 7-day taper group (referent group), .5 = 28-day taper group, and sex was coded as −.5 = male (referent group), .5 = female; ASI-L composite scores were grand-mean centered.
ASI-L as predictors of opioid-positive samples
To test sex differences in the relationship between ASI-L domain composite scores and opioid use trajectories, ASI-L*sex interaction terms were added to the model as predictors of opioid-positive samples. Results indicated significant sex by ASI-L composite score interaction terms for 1) ASI-L medical composite score*sex on the intercept (B = −2.04, p = .03) and slope (B = 0.63, p < .01) and 2) ASI-L family composite score*sex on the intercept (B = −2.75, p = .05; model 2). These results indicate that men with higher ASI-L medical and family composite scores were more likely to submit opioid-positive samples at the start of the trial, while women with higher ASI-L medical composite scores were more likely to submit opioid-positive samples at over the course of the trial (i.e., during treatment).
In order to determine sex differences in the magnitude of the relationship between ASI-L medical and family composite scores with opioid use during the trial, post hoc analyses were performed, and the sample was split by sex for the remaining analyses.
ASI-L as predictors of opioid-positive samples in the female sample
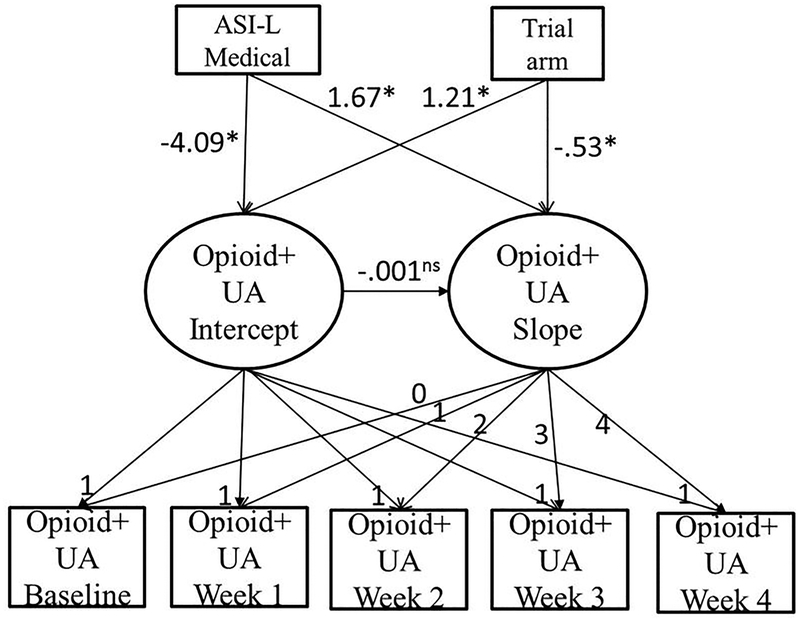
For women, taper trial arm results remained the same as previously reported, where those in the 28-day taper trial arm were significantly more likely to submit opioid-positive samples at baseline (B = 1.21, p = .003) and those in the 7-day taper trial arm were significantly more likely to submit opioid-positive samples over the course of the trial (B = −.53, p = .001, BIC = 638.53). Those with higher ASI-L medical composite scores were less likely to submit opioid-positive samples at baseline (B = −4.09, p < .01) but were more likely to submit opioid-positive samples over the course of the trial (B = 1.67, p = .001). No other ASI-L domain composite scores were associated with baseline opioid-positive samples or submissions of opioid-positive samples over time. Please refer to Table 2 (Model 3) for parameter estimates for the female sample. Figure 3 shows the primary results of this model for the female sample.
Table 2.
Parameter estimates for linear latent growth models for opioid-positive samples at baseline and 4 weeks of treatment, with Addiction Severity-Lite domain composite scores as predictors.
| Independent variable | Dependent variable | Unstandardized estimate (B) | 95% CI | P-value | |
|---|---|---|---|---|---|
| Model 2: Total sample |
|||||
| --- | UA intercept | .77 | −1.22, 2.75 | .45 | |
| --- | UA slope | .87 | .22, 1.52 | .01 | |
| Sex | UA intercept | −.09 | −.64, .45 | .74 | |
| Trial arm | UA intercept | .54 | .03, 1.05 | .04 | |
| Age (years) | UA intercept | −.04 | −.09, .01 | .12 | |
| Medical*sex | UA intercept | −2.04 | −3.95, −.20 | .03 | |
| Employment*sex | UA intercept | .02 | −1.60, 1.64 | .98 | |
| Alcohol*sex | UA intercept | 1.48 | −4.81, 7.62 | .66 | |
| Drug*sex | UA intercept | 5.93 | −2.94, 14.79 | .19 | |
| Legal*sex | UA intercept | −.98 | −4.93, 2.97 | .63 | |
| Psychological*sex | UA intercept | 1.06 | −1.67, 3.79 | .45 | |
| Family/social*sex | UA intercept | −2.75 | −5.48, −.03 | .05 | |
| Sex | UA slope | .13 | −.01, .27 | .07 | |
| Trial arm | UA slope | −.23 | −.36, −.10 | <.001 | |
| Age (years) | UA slope | −.01 | −.02, .01 | .33 | |
| Medical*sex | UA slope | .63 | .17, 1.10 | .01 | |
| Employment*sex | UA slope | .31 | −.08, .70 | .12 | |
| Alcohol*sex | UA slope | −1.13 | −3.22, .97 | .29 | |
| Drug*sex | UA slope | −1.38 | −3.44, .67 | .19 | |
| Legal*sex | UA slope | −.64 | −1.67, .40 | .23 | |
| Psychological*sex | UA slope | −.65 | −1.35, .05 | .07 | |
| Family/social*sex | UA slope | .31 | −.27, .88 | .30 | |
| UA intercept | UA slope | −.12 | −.20, −.05 | .001 |
ASI-L composite scores were included in the model to define the interaction terms. Interaction terms are denoted with an * between variables names. Buprenorphine/naloxone tapering groups were coded as −.5 = 7-day taper group (referent group), .5 = 28-day taper group, and sex was coded as −.5 = male (referent group), .5 = female; ASI-L composite scores were grand-mean centered.
Figure 3.
Schematic representation or primary results of the linear latent growth model of opioid-positive urinalysis (opioid+UA) over time in a randomized clinical trial that compared buprenorphine/naloxone tapering strategies (7-day and 28-day) in the female sample. Unstandardized regression coefficients are reported. ASI-L = Addiction Severity Index-Lite; *p<.05; ns statistically non-significant.
ASI-L as predictors of opioid-positive samples in the male sample
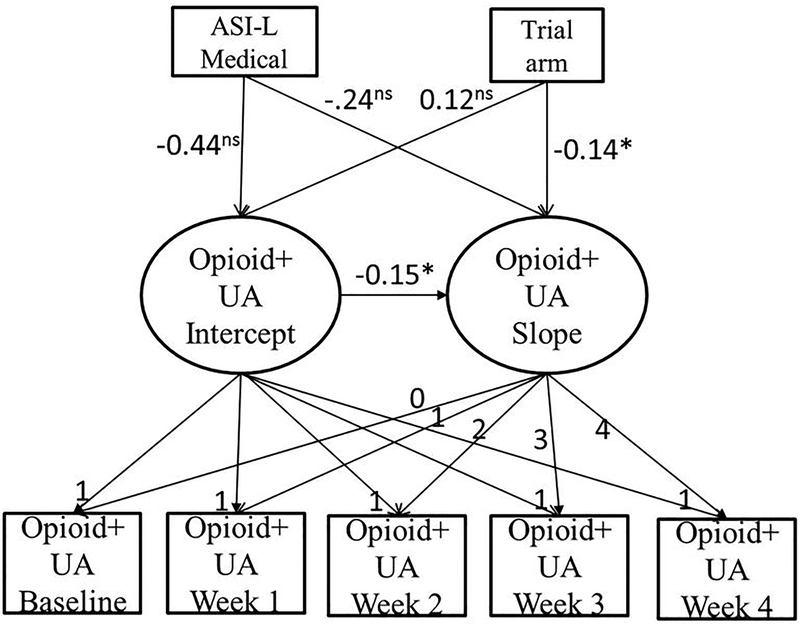
In the male sample, taper trial arm results remained the same as previously reported, where those in the 7-day taper trial arm were significantly more likely to submit opioid-positive samples over the course of the trial (B = −.14, p < .05), and men with lower baseline use were more likely to submit opioid-positive samples over time (B = −.15, p < .001) (BIC = 1267.74). No ASI-L domain composite scores were associated with baseline opioid-positive samples or submission of opioid-positive samples over the course of the trial for men. Please refer to Table 2 (Model 4) for parameter estimates for the male sample. Figure 4 shows the primary results of this model for the male sample.
Figure 4.
Schematic representation or primary results of the linear latent growth model of opioid-positive urinalysis (opioid+UA) over time in a randomized clinical trial that compared buprenorphine/naloxone tapering strategies (7-day and 28-day) in the male sample. Unstandardized regression coefficients are reported. ASI-L = Addiction Severity Index-Lite; *p<.05; ns statistically non-significant.
Discussion
This secondary data analysis of the National Drug Abuse Treatment CTN 0003 study examined sex differences in buprenorphine/naloxone taper trial arms (7-day and 28-day tapering groups) and examined ASI-L domain composite scores across sex and their associations with opioid use trajectories over the course of the clinical trial. We found that women were more likely than men to use opioids during the course of the buprenorphine/naloxone clinical trial, and medical issues are positively related to submitting an opioid positive urinalysis during treatment for women but not men. Therefore, sex differences do indeed exist in this opioid use treatment trial, and in what predicts use during treatment, demonstrating an additional burden and unmet needs in women. Women are quicker to enter treatment and therefore have fewer years of substance use yet are underrepresented in drug treatment (21). In addition, women are more likely to have other health problems, to have sought previous drug treatment, to have suffered sexual abuse or other physical abuse, and to have attempted suicide (22). Women also have poorer treatment participation and retention than men in mixed-gender treatment settings (23). A consensus exists that women have different needs and deficient gender-specific services lead to barriers to care (24). Treatment that focuses on problems more common to substance-abusing women is effective (25). As our findings demonstrate a significant increase in opioid use in relation to medical problems for women, complementary treatment for medical problems at the start of the opioid replacement therapy may help increase overall treatment success. Our results have the potential to significantly impact treatment and advance our goal of improving outcomes for men and women. This study is both significant and timely as the President’s Commission on Combating Drug Addiction and the Opioid Crisis continues to seek information on opioid use specific to women. Additional research is needed to determine if medical problems in women are consistently associated with opioid use across additional clinical trials, and how this finding can be used to develop gender-specific therapies. Time to initial relapse across sex (via time-to-event analyses) is also crucial to determine the exact timing of first use of opioids while in treatment to assist with the timing of complementary treatment.
We also found that both women and men in the 7-day taper trial arm were significantly more likely than those in the 28-day taper trial arm to submit an opioid-positive sample over the course of the trial. This is consistent with previous longitudinal analyses of opioid-positive samples over the course of the trial (8, 9), and in contrast to end-point analyses (end-of-taper) of opioid-positive samples (16, 17). This may be due to the fact that participants in the 28-day trial arm were still on medication over the course of the repeated measures tested, while those in the 7-day taper trial arm were in non-medication weeks (i.e., observation only). Therefore, we may be seeing relapse in the 7-day taper trial arm, and this is supported by a figure previously published on this data that showed a greater number of opioid-positive samples in the 7-day taper group compared to the 28-day taper group towards the end of the trial (8). While our longitudinal findings differ from end-point analyses, both approaches are complementary in that the end-point analyses answer the primary research question of CTN 0003 (what is the effect of a 7-day vs. 28-day taper schedule on opioid-free urine samples at the end of each taper), while this study further examines what is occurring over time in the trial in regards to opioid-positive samples, when one taper has ended and another is still slowly tapering down. An additional difference to note is that the primary paper imputed a positive urine analysis for missing data (16) while we used FIMLR estimation to estimate parameters using all available data, a missing data technique that has been advocated over single imputation techniques (9).
Limitations
There are several limitations to this study. Our results are not generalizable to other clinical trials; our goal was to highlight potential sex differences in this buprenorphine/naloxone clinical trial to raise awareness to such differences when opioid maintenance therapy is considered. Future research may explore any potential artifacts of this data as there is a possibility that sex differences in the slope may be an artifact of sex differences at baseline in the 7-day taper group. Differential retention across tapering groups in this clinical trial is another limitation, which is to be expected as the 7-day taper group was in a non-medication phase, as is differing results based on how missing data was handled (positive urine analysis imputation for missing data (16) vs. maximum likelihood estimation used in this study and in other published work (9). While urine screening results are a valid indicator of use and standard operating procedures in clinical and research settings, future research should also utilize self-report data. In addition, the drug, legal, and employment ASI-L domains had adequate internal consistency values which may have reduced the strengths of the relationships with opioid-positive samples. Lastly, as this was secondary data analysis, this clinical trial was not designed to answer our specific research questions on opioid use over the course of the clinical trial; however our results present important associations demonstrating sex differences in opioid use and predictors of opioid use during treatment.
Conclusions
In testing for sex differences in opioid use in a buprenorphine/naloxone clinical trial, women were more likely than men to use opioids during the course of the trial, and medical issues predicted opioid use during treatment for women but not men. Our findings suggest that additional tests of sex differences in buprenorphine/naloxone clinical trials is warranted to further verify that women have increased opioid use during treatment. In addition, sex-specific risk factors such as medical problems may assist in developing more precise treatment strategies to couple with buprenorphine/naloxone therapy. Our findings demonstrate an increase in opioid use during treatment in relation to medical problems for women, suggesting that complementary treatment for medical problems during opioid replacement therapy would benefit women. Failure to address these needs may result in less successful opioid treatment for women.
Table 3.
Post hoc parameter estimates for linear latent growth models for opioid-positive samples at baseline and 4 weeks of treatment by sex, with Addiction Severity-Lite domain composite scores as predictors.
| Independent variable | Dependent variable | Unstandardized estimate (B) | 95% CI | P-value | |
|---|---|---|---|---|---|
| Model 3: Female sample |
|||||
| --- | UA intercept | 2.53 | −.54, 5.61 | .11 | |
| --- | UA slope | 2.17 | 1.10, 3.25 | <.001 | |
| Trial arm | UA intercept | 1.21 | .42, 2.00 | .003 | |
| Age (years) | UA intercept | −.02 | −.10, .06 | .62 | |
| Medical | UA intercept | −4.09 | −6.85, −1.33 | .004 | |
| Employment | UA intercept | 1.39 | −.85, 1.33 | .22 | |
| Alcohol | UA intercept | −2.38 | −11.59, 6.84 | .61 | |
| Drug | UA intercept | 5.17 | −7.59, 17.94 | .43 | |
| Legal | UA intercept | −1.45 | −7.25, 4.35 | .62 | |
| Psychological | UA intercept | −.26 | −4.00, 3.47 | .89 | |
| Family/social | UA intercept | −2.54 | −5.91, 1.08 | .18 | |
| Trial arm | UA slope | −.53 | −.96 −.21 | .001 | |
| Age (years) | UA slope | −.02 | −.05, .01 | .15 | |
| Medical | UA slope | 1.67 | .48, 2.87 | .01 | |
| Employment | UA slope | .55 | −.26, 1.36 | .18 | |
| Alcohol | UA slope | −2.14 | −6.68, 2.40 | .36 | |
| Drug | UA slope | −.30 | −4.67, 4.07 | .89 | |
| Legal | UA slope | −1.32 | −3.65, 1.01 | .27 | |
| Psychological | UA slope | −.84 | −2.27, .61 | .26 | |
| Family/social | UA slope | .10 | −.97, 1.18 | .85 | |
| UA intercept | UA slope | .03 | −.17, .22 | .80 | |
| Model 4: Male sample |
|||||
| --- | UA intercept | −.28 | −2.69, 2.14 | .82 | |
| --- | UA slope | .39 | −.41, 1.12 | .36 | |
| Trial arm | UA intercept | .12 | −.52, .74 | .77 | |
| Age (years) | UA intercept | −.05 | −.12, .01 | .09 | |
| Medical | UA intercept | −.44 | −2.94, 2.06 | .73 | |
| Employment | UA intercept | 1.68 | −.54, 3.90 | .14 | |
| Alcohol | UA intercept | −5.52 | −12.15, 1.12 | .10 | |
| Drug | UA intercept | −4.52 | −14.48, 5.44 | .37 | |
| Legal | UA intercept | .61 | −3.70, 4.91 | .78 | |
| Psychological | UA intercept | −2.46 | −6.05, 1.13 | .18 | |
| Family/social | UA intercept | 2.48 | −1.22, 6.18 | .19 | |
| Trial arm | UA slope | −.14 | −.28, −.03 | .04 | |
| Age (years) | UA slope | −.001 | −.02, .01 | .83 | |
| Medical | UA slope | −.24 | −.74, .26 | .35 | |
| Employment | UA slope | −.10 | −.55, .35 | .67 | |
| Alcohol | UA slope | −.20 | −1.56, 1.16 | .77 | |
| Drug | UA slope | 2.05 | −.07, 4.17 | .06 | |
| Legal | UA slope | −0.05 | −89, .79 | .91 | |
| Psychological | UA slope | .46 | −.30, 1.22 | .24 | |
| Family/social | UA slope | −.48 | −1.20, 1.12 | .19 | |
| UA intercept | UA slope | −.15 | −.23, −.08 | <.001 |
Buprenorphine/naloxone tapering groups were coded as −.5 = 7-day taper group (referent group), .5 = 28-day taper group, and sex was coded as −.5 = male (referent group), .5 = female; ASI-L composite scores were grand-mean centered.
Acknowledgments
Funding
This project was supported by a grant to the Clinical Trials Network Pacific Northwest Node (award number 5 U10 DA013714-10).
References
- 1.Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16–4984, NSDUH Series H-51). 2016.
- 2.Jones HE, Fitzgerald H, Johnson RE. Males and females differ in response to opioid agonist medications. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2005;14(3):223–33. Epub 2005/07/16. [DOI] [PubMed] [Google Scholar]
- 3.Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addictive behaviors. 2010;35(11):1001–7. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. Vital Signs: Prescription Painkiller Overdoses. A Growing Epidemic, Especially Among Women. July 2013; Available from: http://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html.
- 5.Kaiser Family Foundation. Opioid Overdose Deaths by Gender. 2017; Available from: http://www.kff.org/other/state-indicator/opioid-overdose-deaths-by-gender/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D.
- 6.Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2004;14(3):209–16. Epub 2004/04/02. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug and alcohol dependence. 1995;40(1):17–25. Epub 1995/11/01. [DOI] [PubMed] [Google Scholar]
- 8.McPherson S, Barbosa-Leiker C, McDonell M, Howell D, Roll J. Longitudinal missing data strategies for substance use clinical trials using generalized estimating equations: an example with a buprenorphine trial. Human psychopharmacology. 2013;28(5):506–15. Epub 2013/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPherson S, Barbosa-Leiker C, Mamey MR, McDonell M, Enders CK, Roll J. A ‘missing not at random’ (MNAR) and ‘missing at random’ (MAR) growth model comparison with a buprenorphine/naloxone clinical trial. Addiction. 2015;110(1):51–8. Epub 2014/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. The Journal of nervous and mental disease. 1985;173(7):412–23. Epub 1985/07/01. [DOI] [PubMed] [Google Scholar]
- 11.Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug and alcohol dependence. 2007;87(2–3):297–302. Epub 2006/10/19. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SM, Dweik D, McPherson S, Roll JM. Association between hepatitis C virus and opioid use while in buprenorphine treatment: preliminary findings. The American journal of drug and alcohol abuse. 2015;41(1):88–92. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, et al. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. The American journal of drug and alcohol abuse. 2011;37(5):313–23. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown LS Jr., Alterman AI, Rutherford MJ, Cacciola JS, Zaballero AR. Addiction Severity Index scores of four racial/ethnic and gender groups of methadone maintenance patients. Journal of substance abuse. 1993;5(3):269–79. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 15.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. Journal of substance abuse treatment. 1992;9(3):199–213. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 16.Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104(2):256–65. Epub 2009/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherson S, Barbosa-Leiker C, Burns GL, Howell D, Roll J. Missing Data in Substance Abuse Treatment Research: Current Methods and Modern Approaches. Experimental and clinical psychopharmacology. 2012;20(3):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbosa-Leiker C, McPherson S, Mamey MR, Burns GL, Roll J. Psychometric Properties of the Adjective Rating Scale for Withdrawal across treatment groups, gender, and over time. Journal of substance abuse treatment. 2014;46(2):251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith W, Tisak J. Latent Curve Analysis. Psychometrika. 1990;55(1):107–22. [Google Scholar]
- 20.Muthén LK, Muthén BO. Mplus User’s Guide. Sixth Edition ed. Los Angeles: Muthén & Muthén; 1998-2015. [Google Scholar]
- 21.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and alcohol dependence. 2004;74(3):265–72. Epub 2004/06/15. [DOI] [PubMed] [Google Scholar]
- 22.NIDA. NIDA Notes: Gender Differences in Drug Abuse Risks and Treatment. September, 2000; Available from: http://archives.drugabuse.gov/NIDA_Notes/NNVol15N4/Tearoff.html.
- 23.McCaul ME, Svikis DS, Moore RD. Predictors of outpatient treatment retention: patient versus substance use characteristics. Drug and alcohol dependence. 2001;62(1):9–17. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 24.Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. Journal of addictive diseases. 2010;29(2):200–16. Epub 2010/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug and alcohol dependence. 2007;86(1):1–21. Epub 2006/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]