Abstract
Leaves from three varieties of Ficus deltoidea, colloquially termed small- (FDS), medium- (FDM), and big-type leaf (FDB), were subjected to water extraction. The crude extracts were fractionated using water (WF) and ethyl acetate (EAF). The phenolic and flavonoid content, antioxidant activity, and cytotoxicity of the fractions were investigated. The EAF had the highest phenolic and flavonoid content compared to the other FDS fractions. Conversely, the FDM crude extract had the highest phenolic and flavonoid content compared to the other FDM samples. Antioxidant activity was highest in the FDB crude extract. Ultra-high–performance liquid chromatography showed that two compounds, vitexin and coumaric acid, were present in the FDB crude extract. Additionally, the F. deltoidea leaves caused no signs of toxicity in a normal liver cell line. Our findings show that F. deltoidea varieties have excellent antioxidant activity with no cytotoxic effects on normal liver cells.
Keywords: Ficus deltoidea, Antioxidant, Flavonoids, Phenolic acid
Introduction
It has been widely reported that various plant phytochemicals have biological activity and have the potential to affect disease risk through complementary mechanisms. A large number of these phytochemicals, particularly polyphenols, have been identified and have a wide range of biological activities, including neuroprotective effects (Zhu et al., 2007); inflammatory and immune response regulation (Rotelli et al., 2003); and anti-fungal (Raj et al., 2001), anti-cancer, hypoglycemic (Sharma, Chandrajeet & Partha, 2008), anti-hyperlipidemic (Ghule et al., 2006), and anti-atherosclerotic effects (Kim et al., 2005a). Despite such phytochemical activity, one of the main research interests is investigating their antioxidant potential for preventing or delaying autoxidation by free radicals. Free radical autoxidation leads to macromolecule deterioration, especially of lipids, proteins, carbohydrates, and DNA (Carocho & Ferreira, 2013). The oxidation of these macromolecules, particularly in humans, leads to various illnesses, such as Alzheimer’s disease, cancer, and cardiovascular diseases.
Due to its wide medicinal uses, we selected Ficus deltoidea for the present study. Also known as mistletoe fig or Mas cotek, F. deltoidea is a shrub that is native to Southeast Asia. It is a well-known shrub, especially among the Malays, and is used to treat diabetes, headache, sore throat, and cold. Its leaves have been studied for their hypoglycemic, antinociceptive (Sulaiman et al., 2008), anti-inflammatory (Abdullah et al., 2009), and antioxidant (Hakiman & Maziah, 2009) activity. However, its antioxidant activity has not been fully elucidated due to the presence of various bioactive compounds that might contribute to different antioxidant capacities. These complex mixtures, especially in plant extracts, can interact synergistically, additively, or antagonistically in different assays (Wang et al., 2011; Colon & Nerin, 2016). According to Misbah, Abdul Aziz & Aminudin (2013), a combination of assays incorporating various mechanisms of action would be very helpful for providing complete information on the antioxidant capacity of a specific plant. Thus, the aim of the present study is to determine the antioxidant capacity of F. deltoidea leaves in different in vitro systems as well as to determine their cytotoxic effect on a normal liver cell line.
Methods
Sample preparation
The leaves of three varieties of F. deltoidea were obtained from a plantation in Rembau, Negeri Sembilan. The F. deltoidea varieties (small, FDS; medium, FDM; big, FDB) were deposited in the Herbarium, Rimba Ilmu, University of Malaya, Kuala Lumpur, and assigned individual voucher specimen numbers (KLU046467, KLU046469, KLU046471, respectively). The leaves were rinsed and air-dried at room temperature until they reached a constant weight and then ground into powder using a commercial blender. The powder was kept at −20 °C for further analysis.
Extraction and liquid–liquid fractionation
The dried leaf powder underwent extraction according to Misbah, Abdul Aziz & Aminudin (2013) to yield the crude extract. Then, the crude extracts were fractionated using partial liquid–liquid separation for finer separation of the plant constituents into fractions of different polarity. The process involved the use of two immiscible solvents of different polarities, i.e., water and ethyl acetate using the method established by Misbah, Abdul Aziz & Aminudin (2013) to yield the water and ethyl acetate fractions. Subsequent experiments were conducted using the FDS, FDM, and FDB crude extracts along with their respective water and ethyl acetate fractions.
Ultraviolet–visible (UV-Vis) spectroscopy
UV-Vis spectroscopy was used to distinguish the presence of phenolic components in the samples. The UV-Vis absorption pattern of phytoconstituents can be measured in very dilute solution against a solvent blank using a UV-Vis spectrophotometer. Sample solutions prepared in water were used for this analysis, and the spectra were recorded against a control (water). The wavelength maxima (λmax) of each samples was recorded.
Extract analysis assays
Folin-Ciocalteu assay
The total polyphenolic content (TPC) of the samples was determined using the Folin-Ciocalteu assay. Folin-Ciocalteu reagent (0.1 ml) was added to 1 µl sample and incubated for 5 min. Sodium carbonate (0.07 ml) was added to the mixture and left in the dark for 2 h. The absorbance of the mixture was measured at 765 nm using a microplate reader (BioTek, USA). Gallic acid (0–200 µg/ml) was used as the standard and was processed under similar conditions as above. The TPC in the samples was expressed as mg gallic acid equivalents (GAE)/g dry weight. All experiments were carried out in triplicate.
Aluminum chloride assay
Quercetin (0–100 µg/ml) was prepared to generate the standard curve. Sample (500 µl) or quercetin (1 mg/ml) was combined with 95% ethanol (1.5 ml), 10% aluminum chloride (0.1 ml), 1 M potassium acetate (0.1 ml), and distilled water (2.8 ml). The absorbance of the mixture was determined at 415 nm after 30-min incubation. The total flavonoid content (TFC) was expressed as mg quercetin equivalents (QE)/g dry weight. All analyses were performed in triplicate.
Cupric ion (Cu2+) reducing antioxidant capacity (CUPRAC) assay
The CUPRAC assay is the most commonly used assay for in vitro determination of the antioxidant activity of food elements, biological fluids, and also plant extracts. It uses copper (II)-neocuproine [Cu(II)-Nc] as the oxidizing agent to measure antioxidant activity close to physiological pH conditions. For this assay, 10 mM copper solution (1 ml) was mixed with 7.5 mM neocuproine (1 ml), 1 M ammonium acetate buffer (1 ml), and sample (1 ml), incubated for 30 min, and the absorbance of the mixture was determined at 450 nm. The samples and the positive control quercetin (0–1,000 µg/ml) were tested. All experiments were performed in triplicate.
2,2-Diphenyl-1-picryl-hydrazyl (DPPH) assay
We determined the radical scavenging activity of antioxidants in the samples and quercetin (positive control, 0–1,000 µg/ml) using DPPH. Samples (100 µl) were added to 600 µl DPPH reagent and mixed vigorously. The mixture was incubated in the dark for 30 min at room temperature, following which the decrease in absorbance was detected at 517 nm. The same procedure was repeated with the positive control. The absorbance of the radical without antioxidants was used as the negative control. The experiment was carried out in triplicate, and the percentage of inhibition (%) was calculated using the following formula: [absorbance(blank) –absorbance(sample)/absorbance(blank)] ×100.
Non-enzymatic lipid peroxidation (thiobarbituric acid–reactive substances, TBARS) assay
Fowl egg yolk homogenate was used as the lipid-rich medium and underwent non-enzymatic peroxidation when incubated with ferrous sulphate (FeSO4), which acts as a mediator for the initiation of lipid peroxidation. The yolk was separated from the albumin and the yolk membrane was removed. We used 0, 0.1, 0.5, 1, and 2 mg/ml sample and positive control were used. Samples (100 µl) were mixed with 500 µl buffered egg yolk (1%) and 100 µl FeSO4 (1 M) in a test tube. The mixture was incubated at 37 °C for 1 h, and then 250 µl trichloroacetic acid (TCA,15%) and 500 µl TBA (1%) were added to the mixture. Subsequently, the mixture was heated for 10 min at 100 °C and left to cool, centrifuged at 3,500 rpm for 10 min, and its absorbance was detected at 532 nm. Each experiment was carried out independently in triplicate. The percentage of inhibition (%) was calculated using the following formula: [absorbance(blank)—absorbance(sample)/absorbance(blank)] ×100.
Ferrous ion chelating (FIC, ferrozine) activity assay
The FIC activity assay was used to investigate the FIC capacity of the samples (Singh & Rajini, 2004). Briefly, 2 mM FeSO4 (0.005 ml) was mixed with 0–400 µg/ml sample (0.1 ml), followed by the addition of 5 mM ferrozine (0.02 ml). The absorbance of the reaction mixture was detected at 562 nm after 10-min incubation. A higher absorbance at 562 nm indicated the weaker FIC strength of the chelator. Ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) was used as the reference standard (Diaz et al., 2012). A blank, containing water, was also incorporated under the same conditions. The FIC capacity of the extracts (%) was estimated using the following equation: [(absorbance[blank] –absorbance[test])/absorbance(blank)] ×100, where absorbancetest is the absorbance of the reaction mixture containing the extract or ascorbic acid and absorbanceblank is the absorbance of the blank. All determinations were carried out in triplicate.
Ferricyanide (Prussian blue) assay
The reducing power of the crude extracts and fractions were measured using the method of Bursal & Koksal (2011) with a slight adjustment. The formation of the Prussian blue complex is due to the reduction of ferric ions (Fe3+) to ferrous ions (Fe2+), the absorbance of which can be detected at 700 nm. Sample (0, 0.1, 0.5, 1 mg/ml) was mixed with sodium phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (1%). The mixture was incubated at 50 °C for 20 min using a dry bath, followed by the addition of TCA (10%) and ferric trichloride (FeCl3, 0.1%) to the reaction mixture. Distilled water was used as the blank. The absorbance of the mixture was detected at 700 nm using a UV spectrophotometer.
Toxicity study
The inhibition of cell growth was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT is a tetrazolium salt that is cleaved into formazan crystals by succinate dehydrogenase and is only active in viable cells. A higher amount of formazan dye produced indicates a higher number of viable cells. WRL68 normal liver cells were seeded in 96-well culture plates (5 × 103 cells/well) and left to attach overnight at 37 °C in a 5% CO2 atmosphere. F. deltoidea leaf crude extracts and fractions were added to each well to yield final concentrations of 50, 100, 200, and 500 µg/ml. The cells were also treated with the same amount of vehicle (water) present in the plant extracts, and this was used as the negative control. Cytotoxicity was measured after 24-, 48-, and 72-h treatment by adding 10 µl MTT reagent (5 mg/ml) to the cells and incubating them for an additional 4 h. Finally, the medium and MTT reagent were discarded and replaced with 100 µl isopropanol. The absorbance was detected at 595 nm and the percentage of inhibition (%) was calculated as follows: [(total cells – viable cells)/total cells] ×100.
The median inhibitory concentrations (IC50) were determined. All experiments were carried out in three separate batches, each in triplicate.
Phytochemical analysis and identification
The sample with the most activity was subjected to ultra-high–performance liquid chromatography (UHPLC) identification. The sample was prepared using two hydrolysis methods: acidic and alkaline. The acidic hydrolysis was prepared by mixing 10 mg sample with 1.2 M hydrochloric acid (HCl) in 50% methanol, while alkaline hydrolysis was performed by mixing 10 mg sample with 0.5 M sodium hydroxide (NaOH) in 50% methanol. Both mixtures were heated for 2 h at 90 °C using a dry water bath (Labnet, USA), left to cool, and centrifuged at 5000 rpm for 20 min. The supernatant was filtered and stored at −20 °C until used.
The hydrolyzed samples were analyzed using a UHPLC system (Agilent, Santa Clara, CA, USA) comprising a dual wavelength absorbance detector, quaternary pumps, auto-injector with a 6-µl sample loop, and a column oven. Reverse-phase separations were carried out at 30 °C using a ZORBAX C18 column (Agilent, Santa Clara, CA, USA) (3. 9 × 50 mm). Trifluoroacetic acid (TFA) in water at pH 2.6 (solvent A) and acetonitrile (solvent B) were used as the mobile phase. The flow rate was maintained at 0.3 ml/min for a total run of 9 min, and the gradient program consisted of 5% to 15% solvent B for 3 min, 15% to 50% solvent B for 3 min, and 50% to 100% solvent B for 2 min, and it then was reduced to initial conditions for another 1 min. The eluted peaks were detected at 280 nm and 335 nm. The samples were diluted in solvent A to yield 5% methanol, and 3 µl sample was injected into the UHPLC system. All samples were prepared and analyzed in triplicate. The standards used in the UHPLC analysis were coumaric acid, catechin, gallic acid, and vitexin. Standard stock solutions of each compound were prepared in methanol at 1 mg/ml. A mixture of the standard solutions was prepared at various concentrations (100–500 ng, and 3 µl was injected into the UHPLC and run using the conditions described above. Peak identification was carried out by spiking the samples with the standards of possible compounds and by spectral analysis. The UV spectra of individual peaks were recorded in the 200–400-nm range. Data acquisition and processing were performed using a Lab Advisor chromatography manager (Agilent, Santa Clara, CA, USA).
Statistical analysis
The data were analyzed using the Excel statistical package for Windows software (Microsoft, USA); all analyses were done in triplicate. The results are expressed as the mean ± standard deviation (SD). The correlation coefficient was used to detect the relationship between the extracts’ phenolic content and the antioxidant activity. Statistical significance was calculated using Student’s t-test. Differences between means at the 95% confidence level (p < 0.05) were considered statistically significant as compared to the control.
Results
UV-Vis spectrophotometer analysis and polyphenol and flavonoid content in F. deltoidea leaf extracts and fractions
Table 1 shows the yield of extractible components and polyphenolic and flavonoid contents of the F. deltoidea leaf crude extracts and fractions. The yield of extractible components, expressed as g/100 g dried weight, ranged from 17 g/100 g dried weight (FDB crude extract) to 0.41 g/100 g dried weight (FDM ethyl acetate fraction). The TPC detected in the samples ranged 10–55 mg GAE/g dry weight. Among the crude extracts, the FDM crude extract had the highest phenolic content, followed by that of FDB and FDS. Fractionation of crude extracts causes changes in the phenolic content pattern. The FDS ethyl acetate fraction had the highest TPC (Table 1), followed by that of the FDS water fraction and FDS crude extract. FDM and FDB exhibited a different pattern whereby the crude extracts from both varieties had the highest TPC, followed by that of the water and ethyl acetate fractions. Contents in the water and ethyl acetate fractions, however, were not significantly different. The TFC was highest in the FDB ethyl acetate fraction compared to that of FDM and FDS. The findings show that, in all varieties tested, the ethyl acetate fraction had a higher concentration of flavonoids in comparison to the water fraction.
Table 1. Yield of extractible components, polyphenolic and flavonoid contents of the extract and fractions of different varieties of Ficus deltoidea leaves.
| Sample | Extract/fraction | Yield of extractible components (g/100 g of dried weight) | Phenolic content* (mg GAE/g of dry weight) | Flavonoid content* (mg QE/g of dry weight) |
|---|---|---|---|---|
| FDS | Crude | 14.71 | 9.75 ± 0.70 | 0.65 ± 0.00 |
| Water fraction | 3.48 | 11.20 ± 1.74 | 5.05 ± 0.00 | |
| Ethyl acetate fraction | 0.59 | 54.46 ± 0.76 | 165.05 ± 0.01 | |
| FDM | Crude | 11.79 | 43.23 ± 0.45 | 163.47 ± 0.01 |
| Water fraction | 2.84 | 19.61 ± 0.87 | 61.37 ± 0.00 | |
| Ethyl acetate fraction | 0.41 | 16.42 ± 0.55 | 148.74 ± 0.00 | |
| FDB | Crude | 17 | 39.02 ± 1.95 | 101.37 ± 0.00 |
| Water fraction | 3.95 | 21.13 ± 0.43 | 59.79 ± 0.00 | |
| Ethyl acetate fraction | 0.68 | 18.09 ± 0.38 | 212.42 ± 0.00 |
Notes.
Results were expressed as means ± S.D. (n = 3).
- GAE
- gallic acid equivalent
- QE
- quercetin equivalent
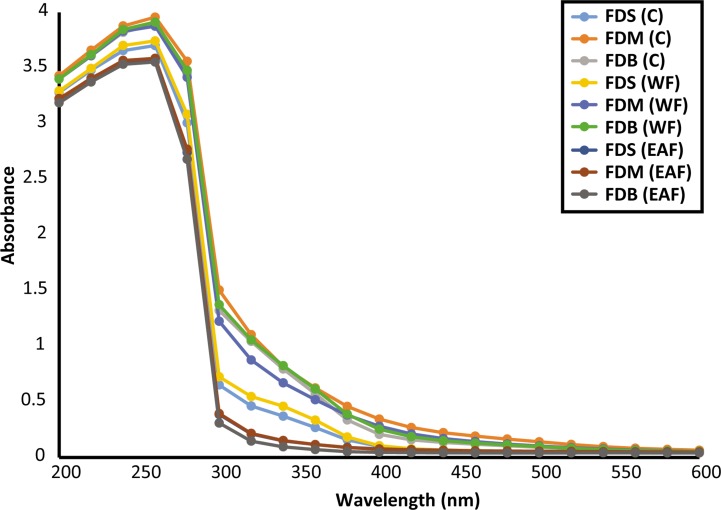
For further characterization of the phenolic compounds, the samples’ UV–Vis absorption spectra were assessed at 200–600 nm. Figure 1 shows that all samples had λmax of 250–300 nm, which may have been due to the presence of flavone/flavonol derivatives or anthocyanins, with absorbance values peaking at 4.
Figure 1. UV-vis spectra of crude and fractions of F. deltoidea leaves.
F. deltoidea leaf crude extract and fractions antioxidant activity
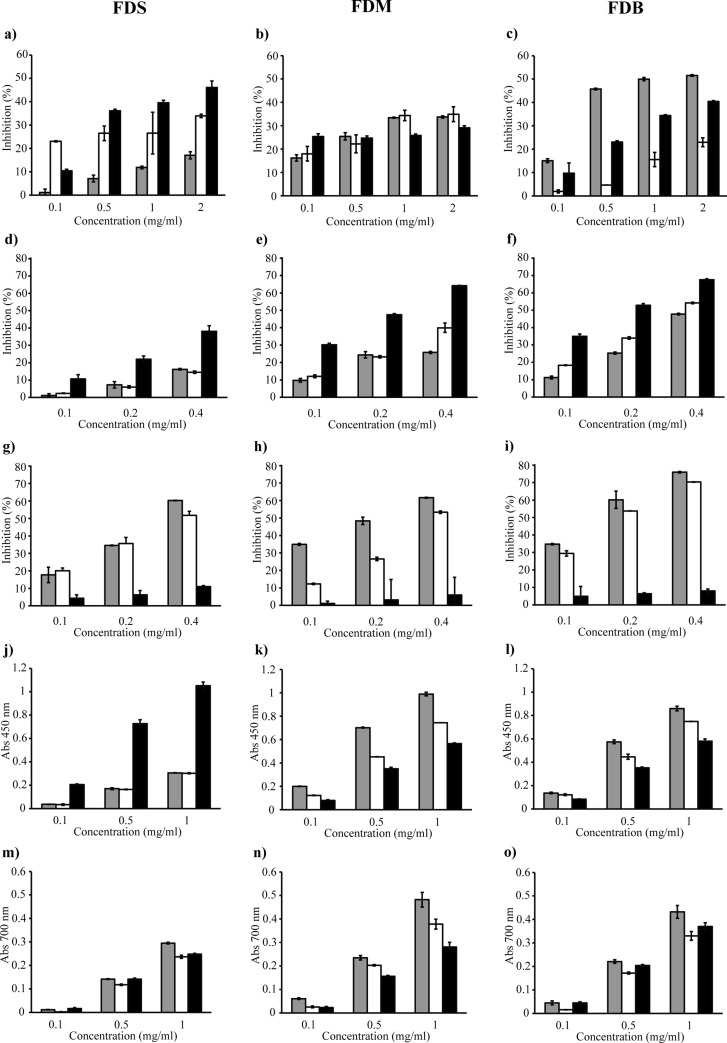
In this study, spectrophotometric TBA assay was used to evaluate the ability of the crude extracts and fractions to inhibit lipid peroxidation (Figs. 2A to 2C). Figure 2C demonstrates that, unlike the other samples, only the FDB crude extract caused at least 50% inhibition of lipid peroxidation.
Figure 2. FDS, FDM and FDB were tested for various antioxidant activities which are lipid peroxidation (A–C), DPPH radical scavenging (D–F), ferrozine (G–I), CUPRAC (J–L) and ferricyanide (M–O) assays.
Crude extract (grey bar) Water fraction (white bar) Ethyl acetate fraction (black bar).
In the DPPH assay, the discoloration of the reaction mixture reflects the potency of the antioxidant in the tested sample. Figures 2E and 2F shows that only FDB and FDM samples showed good inhibition of DPPH radicals, as both varieties inhibited at least 50% of the radicals. Fractionation of the crude extract helped improve the scavenging activity, as the FDB and FDM ethyl acetate fractions showed potent activity, with lower IC50 of 182 µg/ml and 223 µg/ml, respectively. This indicates that the compounds with strongest radical-scavenging ability in FDB and FDM are of medium polarity. However, both samples were not as effective as the positive control, quercetin. FDS crude extract and its fractions (Fig. 2D) had the weakest DPPH radical–scavenging activity, indicating the lack of hydrogen-donating ability. Overall, the samples’ DPPH radical–scavenging activity was in the order of FDB > FDM > FDS.
The metal chelating ability of the samples were tested using the ferrozine assay, where lower absorbance indicates stronger FIC strength of the tested sample. Figures 2G to 2I shows that all crude extracts exhibited good FIC activity. In fact, the FDB crude extract (Fig. 2I) exhibited the highest FIC activity by far, with a chelating IC50 of 150 µg/ml. Generally, the Fe2+ chelating activity of the crude extracts was in the order of FDB > FDM > FDS. All water fractions also showed FIC activity; however, the IC50 values were rather high compared to their respective crude extracts. In addition, all ethyl acetate fractions exhibited very low FIC activity, with no IC50. Even though the extracts did not chelate Fe2+ as strongly as EDTA, they demonstrated noteworthy chelating properties.
In the CUPRAC assay, a higher absorbance indicates a higher Cu2+-reducing power. Figures 2J to 2L shows that all samples reacted with the reagent. The FDS ethyl acetate fraction exhibited the most Cu2+-reducing activity, while the FDS crude extract and water fraction had the lowest activity (Fig. 2J). A similar pattern of activity was observed for FDM and FDB (Figs. 2K and 2L) in the order of crude extract > water fraction > ethyl acetate fraction. The reducing activity of both the FDM and FDB crude extracts might have been due to the presence of various phytochemicals that interacted synergistically. On the other hand, fractionation causes the loss of reducing activity, which could be observed in both the FDM and FDB fractions.
The ferricyanide assay is a reducing power assay based on the ability of test samples to reduce yellow Fe3+ to blue Fe2+. The resulting blue color is considered linearly connected to the total reducing capacity of electron-donating antioxidants. Figures 2M to 2O shows that the reducing activity could be divided into three types: high, moderate, and low. The extracts that exhibited the highest reducing activity were the FDM crude extract (0.482), followed by the FDB crude extract (0.432). The samples that showed moderate activity were the FDM water fraction (0.378), FDB ethyl acetate fraction (0.370), and FDB water fraction (0.330). The samples that exhibited low reducing abilities were in the order of FDS crude extract > FDM ethyl acetate fraction > FDS ethyl acetate fraction > FDS water fraction.
Correlation coefficient between antioxidant assays
Table 2 shows the relationship between the TPC and TFC and the antioxidant activities of each sample. Strong interaction was observed between the TPC and Cu2+-reducing activity (R2 = 0.9161, p < 0.01) and between the TFC and DPPH radical–scavenging activity (R2 = 0.7765, p < 0.05). There were moderate correlations between the TFC and Cu2+-reducing activity (R2 = 0.5867, p < 0.01) and between the TFC and lipid peroxidation activity (R2 = 0.5225, p < 0.05). In contrast, the TPC was poorly correlated with DPPH radical–scavenging and metal-chelating activity (R2 = 0.1697, p < 0.05; R2 = − 0.0213).
Table 2. Correlation analyses between phenolic (PC) and flavonoid content (FC) and antioxidant activities of the crude extracts and fractions of F deltoidea leaves.
| DPPH assay | Cuprac assay | Ferrozine assay | Ferricyanide assay | Lipid peroxidation assay | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | |
| PC | 0.1697* | 0.9161** | −0.0213 | 0.3255** | 0.6658 |
| FC | 0.7765* | 0.5867** | −0.6321* | 0.3358** | 0.5225* |
Notes.
Data with p value < 0.05 were considered significant.
Data with p value < 0.01 were considered significant.
Effects of F. deltoidea leaf extracts on WRL68 cell growth
Normal liver cell, WRL68 was used for toxicity evaluation. Table 3 summarizes the results of the cytotoxic activity of the F. deltoidea leaf extracts. The data are expressed as the IC50 for all incubation times. No IC50 was detected for FDS and FDM even up to 72-h incubation. On the other hand, the FDB crude extract inhibited 50% of the cells after 72-h incubation, with an IC50 of 340 µg/ml. The WRL68 cells were also more sensitive to the FDB water fraction, which had IC50 of 375 µg/ml, 300 µg/ml, and 227 µg/ml after 24-, 48-, and 72-h incubation, respectively.
Table 3. Cytotoxicity of the extracts of F. deltoidea leaves against WRL68 cells.
| Sample | Extract/fraction | Incubation | ||
|---|---|---|---|---|
| 24 hr (IC50 - µg/ml) | 48 hr (IC50 - µg/ml) | 72 hr (IC50 - µg/ml) | ||
| FDS | Crude | N/D | N/D | N/D |
| Water fraction | N/D | N/D | N/D | |
| Ethyl acetate fraction | N/D | N/D | N/D | |
| FDM | Crude | N/D | N/D | N/D |
| Water fraction | N/D | N/D | N/D | |
| Ethyl acetate fraction | N/D | N/D | N/D | |
| FDB | Crude | N/D | N/D | 347.67 ± 2.52 |
| Water fraction | 378.3 ± 5.51 | 306.7 ± 7.37 | 224 ± 21.17 | |
| Ethyl acetate fraction | N/D | N/D | N/D | |
Notes.
The experiment was conducted in a 96-well plate, each in triplicate. Cells were allowed to attach for 24 h after seeding. WRL68 cells were treated with various concentrations of the extracts of F.deltoidea crude extracts and fractions for 24, 48 and 72 h.
Results were expressed as means ± S.D. (n = 3).
IC50 = concentration of plant extracts (μ g/ml) that inhibited 50% of the cells.
N/D, no inhibition detected
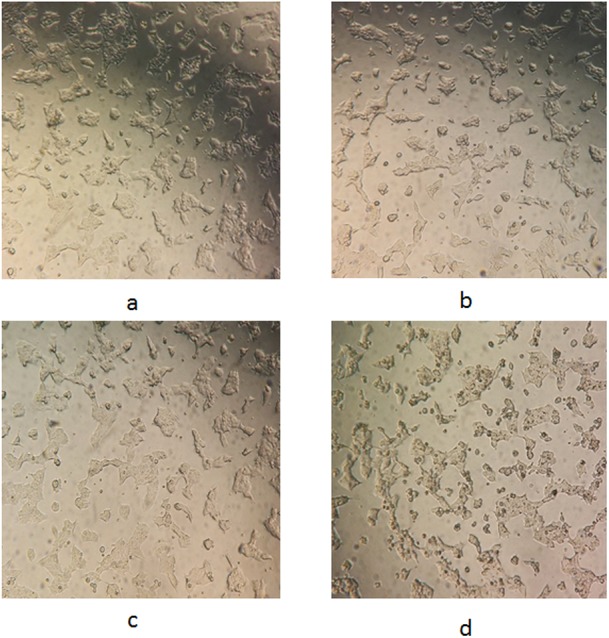
The morphological changes of the WRL68 cells following treatment with the F. deltoidea leaf extracts were observed using a phase contrast microscope after 72-h incubation. Figure 3 shows that there was an obvious difference between the untreated (Fig. 3A) and treated (Figs. 3B–3D) cells. The distinct changes observed in the cells treated with the FDB crude extract (Fig. 3D) included shrinkage, rounding, and detachment from the surface of the wells. These alterations became increasingly noticeable as the dose increased, but were not observed in the control cells. In contrast, the FDS and FDM extracts (Figs. 3B and 3C) showed no indications of cytotoxicity, as no morphological changes were observed.
Figure 3. Representative images for morphological changes of WRL68 cells after 72 h of incubation without (A) and with treatment of FDS (B), FDM (C) and FDB (D) at the highest concentration (original magnification:10×).
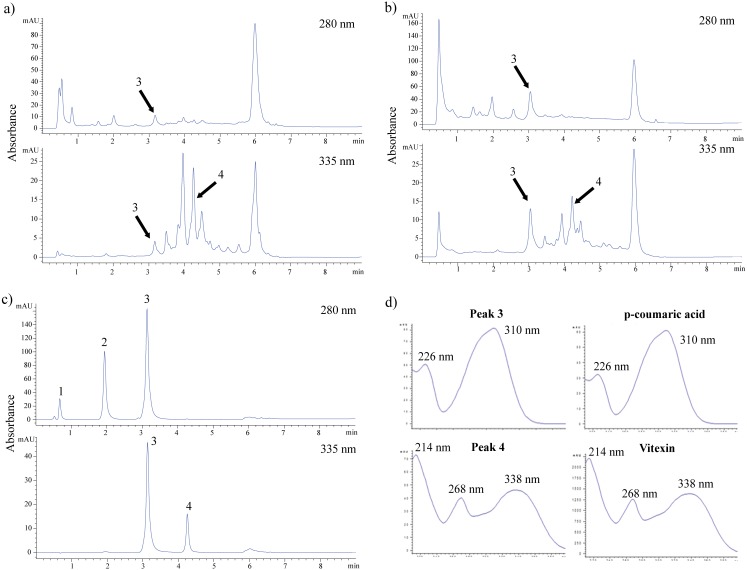
UHPLC phytochemical analysis of F. deltoidea leaves
The sample with good activity in most antioxidant assays was subsequently subjected to UHPLC for phytochemical identification. Figure 4C depicts the separation of a standard mixture of four flavonoids and phenolic acids. Good dissolution was obtained in a short separation time of 9 min. Gallic acid, catechin, and p-coumaric acid were detected at 280 nm. The absorbance at 335 nm was used to detect the presence of coumaric acid and vitexin.
Figure 4. HPLC chromatograms of crude water extract of FDB in acid (A), alkaline (B) hydrolysis and standard solution mixture (C). (D) UV-spectra of peak 3 and 4 corresponding to p-coumaric acid and vitexin.
These compounds were detected at a total run of 9 min by using two different wavelength at 280 and 335 nm. Standard solution mixture consists of gallic acid (1), catechin (2), p-coumaric acid (3) and vitexin (4).
Figure 4A and 4B show the chromatogram of the FDB crude extract under acidic and alkaline hydrolysis conditions, respectively. Two peaks were positively identified based on their retention time, UV spectra, and commercial standards spiking test. Coumaric acid was present in a higher concentration under alkaline conditions as compared to acidic conditions. In contrast, there was a higher amount of vitexin following acidic hydrolysis rather than alkaline hydrolysis. Figure 4D shows that the UV spectrum of p-coumaric acid was characterized by the presence of two maxima at 226 nm and 310 nm, while the vitexin spectrum consisted of a prominent band at 214 nm and 268 nm, with a shoulder at the 338 nm region.
Discussion
In the present study, water was used as a medium for extracting the hydrophilic antioxidants present in F. deltoidea leaves. This is of interest, as typically in the preparation of food and nutraceuticals, aqueous plant extracts are nutritionally more useful and have apparent benefit in relation to safety. On top of that, this is also a similar method as how the plant extract was prepared and consumed traditionally. Many researchers have found that the physiological functions of natural foods can be associated with the presence of phenolic components. Furthermore, flavonoids have various biological properties, such as anti-bacterial, anti-inflammatory, anti-viral, and anti-thrombotic effects. Therefore, it is reasonable to determine the TPC and TFC contents of the F. deltoidea leaf extracts. Three most commonly found varieties of F. deltoidea were evaluated in this study and the results show that some of our findings differ from that of others. Pushpanathan & Nithyanandam (2015) reported that the F. deltoidea leaf TPC was 96.225 mg GAE/100 g dried weight (water extract), 225.917 mg GAE/100 g dried weight (80% methanol), and 264.765 mg/100 g dried weight (80% ethanol), which was lower than that detected in our sample. By contrast, Mun et al. (2017) detected higher F. deltoidea leaf TPC compared that in the present study, i.e., 368.42 ± 6.37 mg GAE/g (aqueous extract), 295.03 ± 16.65 mg GAE/g (methanol extract), and 263.45 ± 5.28 mg/g (ethanol extract). By contrast, we detected higher TFC as compared to others (Hakiman & Maziah, 2009; Dzolin et al., 2010; Soib et al., 2015). Thus, it is difficult to compare the results obtained from different investigations, as differences may arise for various reasons. Differing extraction methods are a factor in the varied TPC and TFC determinations between studies. Various methods can be used to extract plant compounds. Parameters such as the nature and volume of the solvent, temperature, and time can affect compound extraction (Soong & Barlow, 2004; Maisuthisakul & Pongsawatmanit, 2004). Moreover, the presence of interfering substances in plant extracts, such as lipophilic compounds, sugar, ascorbic acid, and aromatic amines may contribute to the variations in TPC estimation between studies (Ikram et al., 2009; Khan, Bakht & Shafi, 2016). It is also important to note that the TPC and TFC evaluation methods are based on the general structure of phenolics and flavonoids; and hence, complexity of the compounds and structural modifications that could have occurred during growth process may lead to variations. The choice of using different varieties also will results in differences in term of compound extracted, their types and quantity. Each of this Ficus variety differs in term of morphology and growth behavior and requirements, all of which that may contribute to compound variations. Eight varieties of F. deltoidea can be found in Malaysia; thus, genetic and geographical origins may also affect the chemical composition between plants (Zimisuhara et al., 2015; Chen, Wang & Chen, 2014).
Many methods can be used to demonstrate the antioxidant activity of plant extracts. However, no single assay can establish the complete antioxidant potential of such compounds, as multiple reactions and mechanisms are involved in the antioxidative processes (González-Centeno et al., 2013). Hence, we tested the antioxidant activity of F. deltoidea leaves using several assays involving different mechanisms. Fractionation of the crude extracts led to the loss of antioxidant activity, especially for FDM and FDB. The finding suggests that the bioactive components in the crude extracts may act synergistically to produce the antioxidant effects, and fractionation might have eliminated some of the compounds (Zahin, Aqil & Ahmad, 2010). In fact, the crude extracts’ TPC and TFC (Table 1) correlated well with the antioxidant activity (Fig. 2), but not that of the fractions. Furthermore, the potential antioxidants in both FDM and FDB were mainly high-polarity compounds. Antioxidant activity was also observed for the FDS ethyl acetate fraction, which was also due to the presence of high TPC and TFC. This suggests that medium-polarity compounds contribute more to that particular activity. Based on these observations, we could see differences in antioxidants capability showed by the varieties evaluated; FDB and FDM demonstrated a more similar pattern contrasting with FDS. This may also suggests that antioxidant effects of F.deltoidea could be contributed by variety of compounds other than the commonly known phenolics. Nevertheless, Table 2 shows the lack of correlation between the TPC and TFC and the antioxidant activity. Thus, highlighting that the phenolic and flavonoid compounds were not major contributors to the antioxidant activity of F. deltoidea leaves. Our findings are in agreement with the studies of Norra (2011) and Mun et al. (2017). The differing antioxidant activity of the samples was most probably due to the differing phenolic content and phenolic composition. In some instances, samples with lower phenolic and flavonoid content had higher antioxidant activity. In plant extracts, the types and amounts of phenolics and flavonoids present does not necessarily affect the antioxidant activity; in fact, it also depends on the degree of polymerization, concentration, and the synergistic interaction between the diverse chemical structures of the antioxidants and the antioxidant assays (Stratil, Klejdus & Kubaan, 2006; Sulaiman et al., 2011). Furthermore, antioxidants can exert their protective effects at different stages of oxidation and through different mechanisms (Parejo et al., 2002; Wang et al., 2011; Chanudom et al., 2014).
Despite the good antioxidant activity in different assay systems, determining the extracts’ toxicity, especially in a normal cell line, was essential. Here, we tested the cytotoxicity of the F. deltoidea leaf crude extracts and fractions on the WRL68 normal liver cell line. The liver is the main site of various metabolism activities in the human body, thus it is of interest to test the extracts’ cytotoxicity using this cell line. To the best of our knowledge, ours is the first study to analyze the cytotoxicity of F. deltoidea leaves in a normal liver cell line. As both FDM and FDS showed no signs of cytotoxicity (Table 3), this could be interpreted as a positive sign in that they are relatively non-toxic, rendering them safe for consumption as an alternative medicine and health supplement. However, the US National Cancer Institute (NCI) states that a plant extract is considered toxic if it causes cytotoxicity with IC50 < 20 µg/ml (Shafaei et al., 2014). Thus, FDB also can be considered non-toxic to WRL68 cells. The samples were also found to be non-toxic on MRC5 (human lung fibroblast) and Chang (liver) cell lines (Data S2). Another study also reported that F. deltoidea leaves are not toxic to normal cell lines, particularly human umbilical vein endothelial cells (HUVEC) and neuroblastoma cells (SH-SY5Y) (Dzolin et al., 2010; Shafaei et al., 2014). These data are also concurrent with Ilyanie, Wong & Choo (2011), who reported that F. deltoidea leaf extract does not induce liver and kidney toxicity in streptozotocin (STZ)-induced diabetic rats. However, F. deltoidea leaves are toxic to other cell lines, especially human cancer cell lines, such as HL-60 (leukemia), DU145 (prostate cancer), HCT116 (colorectal carcinoma), and MDA-MB-231 (hormone-resistant breast cancer) (Soib et al., 2015; Shafaei et al., 2014; Norrizah et al., 2012). In contrast, Lee et al. (2011) found that F. deltoidea leaf did not cause toxicity in MCF-7 breast cancer cells. These reports show that F. deltoidea leaves have different toxicity effects on different cell types.
Based on the consistent antioxidant activity shown in each antioxidant assay and because it was non-toxic to normal cells, the FDB crude extract was subjected to UHPLC identification. Acid- and alkaline-catalyzed hydrolysis were used to release flavonoids and phenolic acids from their bound forms for easier identification. Coumaric acid and vitexin were detected using a method developed in our laboratory. The presence of vitexin in the F. deltoidea leaves was in accordance with that reported previously (Abdullah et al., 2009; Omar, Mullen & Crozier, 2011; Choo et al., 2012). In the present study, vitexin (peak 4) was easily detected in acidic medium at 335 nm (Fig. 4A). Moreover, several peaks could not be identified at retention times of 3.43 min, 3.92 min, and 4.43 min. These peaks showed UV maxima at 335 nm and 270 nm, suggesting that these compounds are flavonoids with apigenin derivatives. On the other hand, vitexin and other peaks showed sign of degradation in alkaline medium (Fig. 4B), as lower amounts of vitexin were detected. Figure 4B shows that alkaline hydrolysis was a good medium for liberating p-coumaric acid (peak 3), as p-coumaric acid is degraded in acidic conditions (Verma, Hucl & Chibbar, 2009; Waszkowiak & Gliszczyńska-Świgło, 2016). This may explain the lower amount of p-coumaric acid we detected following acid hydrolysis. Previous work on the phytochemical composition of F. deltoidea leaves has also reported the presence of other compounds such as ursolic acid, epicatechin, naringenin, catechin, epigallocatechin, luteolin, and coumaroylquinic acid (Shafaei et al., 2014; Omar, Mullen & Crozier, 2011). Several researchers have also found vitexin in other plant species such as Acer palmatum, Vitex agnus castus, Trigonella foenum-graecum L., Arum dioscoridis, and Codiaeum variegatum. This flavone is a compound that contributes to the antioxidant activity of these plants, particularly for the DPPH radical scavenging, lipid peroxidation, and ferric reducing assays (Kim et al., 2005b; Gokbulut et al., 2010; Uguzlar, Maltas & Yildiz, 2012; Khole et al., 2014; Hassan et al., 2014). p-Coumaric acid is a phenolic acid that prevents lipid peroxidation and scavenges DPPH radicals (Kiliç & Yeşilouğlou, 2013; Shairibha & Rajadurai, 2014; Widowati et al., 2016). Thus, it is suggested that the FDB crude extract activity could have been due to the presence of vitexin and p-coumaric acid.
There have been very few studies on F. deltoidea compounds and their effects on biological activity. Lupeol in F. deltoidea leaves exhibited antibacterial activity when tested on three bacteria: Escherichia coli, Bacillus subtilis, and Staphylococcus aureus (Suryati et al., 2011). Vitexin and isovitexin have also been detected in F. deltoidea leaves and have antidiabetic properties by inhibiting α-glucosidase activity in STZ-induced diabetic rats (Choo et al., 2012). Hanafi et al. (2017) found that a mixture of compounds comprising oleanolic acid, botulin, and lupeol in the active fraction of F. deltoidea showed better anti-proliferative activity compared to individual compounds. The active fraction exerted its anti-proliferative properties by increasing the expression of Bax and Smac/DIABLO (diablo IAP-binding mitochondrial protein) and downregulating the expression of Bcl-2 in PC3 prostate cancer cells, which leads to apoptosis.
Conclusion
Through in vitro assays involving different mechanisms, such as radical scavenging, metal chelation, reduction, and suppression of the initiation of radical formation, the present study findings show that F. deltoidea leaf varieties demonstrate potential as a good source of antioxidants. This study also showed that FDB is having a better potential to be further developed and used as nutraceutical agent comparative to other F. deltoidea varieties (FDM and FDS). The presence of coumaric acid and vitexin in the extracts may contribute to the antioxidative action of the plant, suggesting that the phenolic and flavonoid compounds present in the extracts could be responsible for its beneficial effects. Furthermore, the extracts are safe for consumption because they did not cause toxicity in the WRL68 normal liver cell line.
Supplemental Information
Acknowledgments
The authors would like to acknowledge Dr. Shatrah Othman and Dr. Nurshamimi Nor Rashid for the cell culture facilities in their laboratory, and the staff of the High Impact Research (HIR) Center for the UHPLC facility.
Funding Statement
This work was supported from the funding of a Postgraduate Research Grant (PG046/2014B) granted by the University of Malaya and the University of Malaya High Impact Research (HIR) (UM.0000105/HIR.C3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Noor Nazirahanie Abrahim performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Puteri Shafinaz Abdul-Rahman and Norhaniza Aminudin conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Abdullah et al. (2009).Abdullah Z, Hussain K, Ismail Z, Mat Ali R. Anti-inflammatory activity of standardised extracts of leaves of three varieties of Ficus deltoidea. International Journal of Pharmaceutical and Clinical Research. 2009;1(3):100–105. [Google Scholar]
- Bursal & Koksal (2011).Bursal E, Koksal E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.) Food Research International. 2011;44:2217–2221. doi: 10.1016/j.foodres.2010.11.001. [DOI] [Google Scholar]
- Carocho & Ferreira (2013).Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food and Chemical Toxicology. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Chanudom et al. (2014).Chanudom L, Bhoopong P, Khwanchuea R, Tangpong J. Antioxidant and antimicrobial activities of aqueous and ethanol crude extracts of 13 Thai traditional plants. International Journal of Current Microbiology and Applied Sciences. 2014;3(1):549–558. [Google Scholar]
- Chen, Wang & Chen (2014).Chen W, Wang X, Chen F. Characterization of nine traditional Chinese plant extracts with specific acid dissociation constants by UV-Vis spectrophotometry. Analytical Methods. 2014;6:581–588. doi: 10.1039/C3AY40946E. [DOI] [Google Scholar]
- Choo et al. (2012).Choo CY, Sulong NY, Man F, Wong TW. Vitexin and isovitexin from the leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. Journal of Ethnopharmacology. 2012;142:776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- Colon & Nerin (2016).Colon M, Nerin C. Synergistic, antagonistic and additive interactions of green tea polyphenols. European Food Research and Technology. 2016;242:211–220. doi: 10.1007/s00217-015-2532-9. [DOI] [Google Scholar]
- Diaz et al. (2012).Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. BMC Chinese Medicine. 2012;7:26. doi: 10.1186/1749-8546-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzolin et al. (2010).Dzolin S, Syed Aris SR, Ahmad R, Mat Zain M. Radical scavenging and neurotoxicity of four varieties of Ficus deltoidea. 2010 International conference on science and social research (CSSR 2010); 2010. pp. 11–15. [Google Scholar]
- Ghule et al. (2006).Ghule BV, Ghante MH, Saoji AN, Yeole PG. Hypolipidemic and antihyperlidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Indian Journal of Experimental Biology. 2006;44:905–909. [PubMed] [Google Scholar]
- Gokbulut et al. (2010).Gokbulut A, Ozhan O, Karacaoglu M, Sarer E. Radical scavenging activity and vitexin content of Vitex agnus-castus leaves and fruits. FABAD Journal of Pharmaceutical Sciences. 2010;35:85–91. [Google Scholar]
- González-Centeno et al. (2013).González-Centeno MR, Jourdes M, Femenia A, Simal S, Rosselló C, Teissedre PL. Characterization of polyphenols and antioxidant potential of white grape pomace byproducts (Vitis vinifera L.) Journal of Agricultural and Food Chemistry. 2013;61:11579–11587. doi: 10.1021/jf403168k. [DOI] [PubMed] [Google Scholar]
- Hakiman & Maziah (2009).Hakiman M, Maziah M. Non enzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus deltoidea accessions. Journal of Medicinal Plants Research. 2009;3(3):120–131. [Google Scholar]
- Hanafi et al. (2017).Hanafi MMM, Afzan A, Yaakob H, Aziz R, Sarmidi MR, Wolfender JL, Prieto JM. In vitro pro-apoptotic and anti-migratory effects of Ficus deltoidea L. Plant extracts on the human prostate cancer cell lines PC3. Frontiers in Pharmacology. 2017;8:895. doi: 10.3389/fphar.2017.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan et al. (2014).Hassan EM, Hassan RA, El-Toumy SA, Mohamed SM, Omer EA. Phenolic metabolites and antioxidant activity of Codiaeum variegatum CV. spirale. Journal of Pharmacy Research. 2014;88(5):619–623. doi: 10.13140/2.1.3450.1761. [DOI] [Google Scholar]
- Ikram et al. (2009).Ikram EHK, Khoo HE, Mhd Jalil AM, Ismail A, Idris S, Azlan A, Mohd Nazri HS, Mat Diton NA, Mohd Mokhtar RA. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. Journal of Food Composition and Analysis. 2009;22:388–393. doi: 10.1016/j.jfca.2009.04.001. [DOI] [Google Scholar]
- Ilyanie, Wong & Choo (2011).Ilyanie Y, Wong TW, Choo CY. Evaluation of hypoglycemic activity and toxicity profiles of the leaves of Ficus deltoidea in rodents. Journal of Complementary and Integrative Medicine. 2011 doi: 10.2202/1553-3840.1469. Epub ahead of print Feb 10 2011. [DOI] [PubMed] [Google Scholar]
- Khan, Bakht & Shafi (2016).Khan W, Bakht J, Shafi M. Evaluation of polyphenol content in different parts of Physalis ixocarpa. Pakistan Journal of Botany. 2016;48(3):1145–1151. [Google Scholar]
- Khole et al. (2014).Khole S, Chatterjee S, Variyar P, Sharma A, Devasagayam TPA, Ghaskadbi S. Bioactive constituents of germinated fenugreek with strong antioxidant potential. Journal of Functional Foods. 2014;6:270–279. doi: 10.1016/j.jff.2013.10.016. [DOI] [Google Scholar]
- Kiliç & Yeşilouğlou (2013).Kiliç I, Yeşilouğlou Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochima Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;115:719–724. doi: 10.1016/j.saa.2013.06.110. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2005b).Kim JH, Lee BC, Kim JH, Sim GS, Lee DH, Lee KE, Yun YP, Pyo HB. The isolation and antioxidative effects of vitexin from Acer palmatum. Archives of Pharmacal Research. 2005b;28(2):195–202. doi: 10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2005a).Kim DH, Sung JJ, Chung IS, Lee YH, Kim DK, Kim SH, Kwon BM, Jeong TS, Park MH, Seoung NS, Baek NI. Ergosterol peroxide from flowers of Erigeron annuus L. as an anti-atherosclerosis agent. Archives of Pharmacal Research. 2005a;28(5):541–545. doi: 10.1007/BF02977755. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2011).Lee SW, Wee W, Yong JFS, Syamsumir DF. Characterization of antioxidant, antimicrobial, anticancer property and chemical composition of Ficus deltoidea Jack. leaf extract. Journal of Biologically Active Products from Nature. 2011;1(1):1–6. doi: 10.1080/22311866.2011.10719067. [DOI] [Google Scholar]
- Maisuthisakul & Pongsawatmanit (2004).Maisuthisakul P, Pongsawatmanit R. Effect of sample preparation methods and extraction time on yield and antioxidant activity from kradonbok (Careya sphaerica Roxb.) leaves. Kasetsart Journal (Natural Science) 2004;38:8–14. [Google Scholar]
- Misbah, Abdul Aziz & Aminudin (2013).Misbah H, Abdul Aziz A, Aminudin N. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complementary and Alternative Medicine. 2013;13:118. doi: 10.1186/1472-6882-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun et al. (2017).Mun HS, Mamat AS, Aslam MS, Ahmad MS. Total phenolic content and anti-oxidant potential of Ficus deltoidea using green and non-green solvents. Journal of Pharmaceutical Negative Results. 2017;8:15–19. doi: 10.4103/0976-9234.204913. [DOI] [Google Scholar]
- Norra (2011).Norra I. Free radical scavenging activity and phenolic content of Ficus deltoidea accessions MFD4 and MFD6 leaves. Journal of Tropical Agriculture and Food Science. 2011;39(1):85–92. [Google Scholar]
- Norrizah et al. (2012).Norrizah JS, Norizan A, Sharipah Ruzaina SA, Dzulsuhaimi D, Nurul Hidayah MS. Cytotoxicity activity and reproductive profiles of male rats treated with methanolic extracts of Ficus deltoidea. Research Journal of Medicinal Plant. 2012;6(2):197–202. doi: 10.3923/rjmp.2012.197.202. [DOI] [Google Scholar]
- Omar, Mullen & Crozier (2011).Omar MH, Mullen W, Crozier A. Identification of proanthocyanidin dimers and trimers, flavone c-glycosides, and antioxidants in Ficus deltoidea, a Malaysian herbal tea. Journal of Agricultural and Food Chemistry. 2011;59(4):1363–1369. doi: 10.1021/jf1032729. [DOI] [PubMed] [Google Scholar]
- Parejo et al. (2002).Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. Journal of Agricultural and Food Chemistry. 2002;50:6882–6890. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- Pushpanathan & Nithyanandam (2015).Pushpanathan K, Nithyanandam R. Antioxidant potential of Malaysian medicinal plant. Journal of Engineering Science and Technology. 2015;Special Issue:138–150. [Google Scholar]
- Raj et al. (2001).Raj NK, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian Journal of Pharmacology. 2001;33:2–16. [Google Scholar]
- Rotelli et al. (2003).Rotelli AE, Guardia T, Juárez AO, De la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacological Research. 2003;48:601–606. doi: 10.1016/S1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]
- Shafaei et al. (2014).Shafaei A, Muslim NS, Nassar ZD, Aisha AFA, Abdul Majid AMS, Ismail Z. Antiangiogenic effect of Ficus deltoidea Jack standardised leaf extracts. Tropical Journal of Pharmaceutical Research. 2014;13(5):761–768. doi: 10.4314/tjpr.v13i5.16. [DOI] [Google Scholar]
- Shairibha & Rajadurai (2014).Shairibha SMR, Rajadurai M. Anti-diabetic effect of p-coumaric acid on lipid peroxidation, antioxidant status and histopathological examinations in streptozotocin-induced diabetic rats. International Journal of Integrative Sciences, Innovationa and Technology. 2014;3(5):1–11. [Google Scholar]
- Sharma, Chandrajeet & Partha (2008).Sharma B, Chandrajeet B, Partha R. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food and Chemical Toxicology. 2008;46:2376–2383. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Singh & Rajini (2004).Singh N, Rajini PS. Free radical scavenging activity of an aqueous extract of potato peel. Food Chemistry. 2004;85(4):611–616. doi: 10.1016/j.foodchem.2003.07.003. [DOI] [Google Scholar]
- Soib et al. (2015).Soib HH, Ware I, Yaakob H, Mukrish H, Sarmidi MR. Antioxidant and anti-cancer actvity of standardized extracts of three varieties of Ficus deltoidea’s leaves. Jurnal Teknologi (Science & Engineering) 2015;77(3):19–25. [Google Scholar]
- Soong & Barlow (2004).Soong YY, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry. 2004;88:411–417. doi: 10.1016/j.foodchem.2004.02.003. [DOI] [Google Scholar]
- Stratil, Klejdus & Kubaan (2006).Stratil P, Klejdus B, Kubaan V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. Journal of Agricultural and Food Chemistry. 2006;54:607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- Sulaiman et al. (2008).Sulaiman MR, Hussain MK, Zakaria ZA, Somchit MN, Moin S, Mohamad AS, Israf DA. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79:557–561. doi: 10.1016/j.fitote.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Sulaiman et al. (2011).Sulaiman SF, Sajak AAA, Ooi KL, Supriatno, Seow EM. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. Journal of Food Composition and Analysis. 2011;24:506–515. doi: 10.1016/j.jfca.2011.01.020. [DOI] [Google Scholar]
- Suryati et al. (2011).Suryati, Nurdin H, Dachriyanus HJ, Lajis MN. Structure elucidation of antibacterial compound from Ficus deltoidea Jack leaves. Indonesian Journal of Chemistry. 2011;11(1):67–70. doi: 10.22146/ijc.626. [DOI] [Google Scholar]
- Uguzlar, Maltas & Yildiz (2012).Uguzlar H, Maltas E, Yildiz S. Screening of phytochemicals and antioxidant activities of Arum dioscoridis seeds. Journal of Food Biochemistry. 2012;36(3):285–291. doi: 10.1111/j.1745-4514.2010.00537.x. [DOI] [Google Scholar]
- Verma, Hucl & Chibbar (2009).Verma B, Hucl P, Chibbar RN. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chemistry. 2009;116:947–954. doi: 10.1016/j.foodchem.2009.03.060. [DOI] [Google Scholar]
- Wang et al. (2011).Wang S, Meckling KA, Marcone MF, Kakuda Y, Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. Journal of Agricultural and Food Chemistry. 2011;59:960–968. doi: 10.1021/jf1040977. [DOI] [PubMed] [Google Scholar]
- Waszkowiak & Gliszczyńska-Świgło(2016).Waszkowiak K, Gliszczyńska-Świgło A. Binary ethanol-water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. European Food Research and Technology. 2016;242:777–786. doi: 10.1007/s00217-015-2585-9. [DOI] [Google Scholar]
- Widowati et al. (2016).Widowati W, Fauziah N, Herdiman H, Afni M, Afifah E, Kusuma HSW, Nufus H, Arumwardana S, Rihibiha DD. Antioxidant and anti aging assyas of Oryza sativa extracts, vanillin and coumaric acid. Journal of Natural Remedies. 2016;16(3):88–99. doi: 10.18311/jnr/2016/7220. [DOI] [Google Scholar]
- Zahin, Aqil & Ahmad (2010).Zahin M, Aqil F, Ahmad I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010;703:99–107. doi: 10.1016/j.mrgentox.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2007).Zhu JTT, Choi RCY, Chu GKY, Cheung AWH, Gao QT, Jun LI, Jiang ZY, Dong TTX, Tsim KWK. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing β-Amyloid-induced cell death. Journal of Agricultural and Food Chemistry. 2007;55(6):2438–2445. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]
- Zimisuhara et al. (2015).Zimisuhara B, Valdiani A, Shaharuddin NA, Qamaruzzaman F, Mahmood M. Structure and principal componenet analyses reveal an intervarietal fusion in Malaysian mistletoe fig (Ficus deltoidea Jack) populations. International Journal of Molecular Science. 2015;16:14369–14394. doi: 10.3390/ijms160714369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.