Abstract
Background
Objective assessments of sedentary behavior and physical activity (PA) by using accelerometer-based wearable devices are ever expanding, given their importance in the global context of health maintenance. This study aimed to determine the reliability and validity of a new accelerometer-based analyzer (Fibion) for detecting different PAs and estimating energy expenditure (EE) during a simulated free-living day.
Methods
The study consisted of two parts: a reliability (n = 18) and a validity (n = 19) test. Reliability was assessed by a 45 min protocol of repeated sitting, standing, and walking (i.e., 3 × 15 min, repeated twice), using both Fibion and ActiGraph. Validity was assessed by a 12 h continuous sequence tasks of different types (sitting, standing, walking, and cycling) and intensities (light [LPA], moderate [MPA], and vigorous [VPA]) of PA. Two Fibion devices were worn on the thigh (FT) and in the pocket (FP), respectively and were compared with criteria measures, such as direct observation (criterion 1) and oxygen consumption by a portable gas analyzer, K4b2 (criterion 2).
Results
FT (intra-class correlation coefficients (ICCs): 0.687–0.806) provided similar reliability as the Actigraph (ICCs: 0.661–0.806) for EE estimation. However, the measurement error (ME) of FT compared to the actual time records indicated an underestimation of duration by 5.1 ± 1.2%, 3.8 ± 0.3% and 14.9 ± 2.6% during sitting, walking, and standing, respectively. During the validity test, FT but not FP showed a moderate agreement but lager variance with the criteria (1 and 2) in assessing duration of sitting, long sitting, LPA, MPA, and VPA (p > 0.05, ICCs: 0.071–0.537), as well as for EE estimation of standing, LPA, MPA, and VPA (p > 0.05, ICCs: 0.673–0.894).
Conclusions
FT provided similar reliability to that of the Actigraph. However, low correlations between subsequent measurements of both devices indicated large random MEs, which were somewhat diminished during the simulated 12 h real-life test. Furthermore, FT may accurately determine the types, intensities of PA and EE during prolonged periods with substantial changes in postures, indicating that the location of the accelerometer is essential. Further study with a large cohort is needed to confirm the usability of Fibion, especially for detecting the low-intensity PAs.
Keywords: Sedentary behavior, Motion, Posture allocation, Activity tracker
Introduction
Assessment of sedentary behavior and physical activity (PA) is ever expanding, given its importance in the global context of health maintenance (Ainsworth et al., 2011a; Hills, Mokhtar & Byrne, 2014). Especially, accelerometer-based wearable devices have become increasingly popular over the past decade. However, the different algorithms and output parameters make it difficult to determine their accuracy. Furthermore, the function and accuracy of the devices may significantly differ between the wearing locations (Loprinzi & Smith, 2017). For example, wrist-worn devices such as Fitbit and Jawbone have been shown to provide higher accuracy of steps but lower validity for EE, compared to indirect or direct calorimetry, accelerometry, and self-reported EE (Evenson, Goto & Furberg, 2015). In addition, waist- or thigh-based accelerometers, such as the ActivPAL monitor were designed to differentiate sitting/supine postures from standing, while it may not differentiate PA types (Steeves et al., 2015). Similarly, the IDEEA monitor can distinguish between 32 types of postures and gaits, and may also provide step counts and EE, but the complexity of the setup makes the device unfeasible for a wider use (Maffiuletti et al., 2008; Jiang & Larson, 2013).
Fibion (Fibion Inc, Jyväskylä, Finland) is a new three-axial lightweight (20 g, L × W × T = 30 × 32 × 10 mm) accelerometer-based device which was designed to follow orientation and movement of the thigh. Thus, it can be worn either on the thigh (FT) or Fibion worn in the Pocket of the trousers (FP). According to information provided by the manufacturer, it is able to detect no-wear time, to differentiate PA types (sitting, long sitting, standing, walking, and cycling) and intensities (light PA (LPA), moderate PA (MPA), and vigorous PA (VPA), as well as the associated EE. However, to the best of our knowledge, no objective data on its reliability and validity have been studied. Therefore, the aim of this study was to (1) assess the reliability of Fibion (FT) in a laboratory-based test-retest protocol, with comparison to ActiGraph (GT9X Link, Pensacola, FL, USA); (2) determine the validity of Fibion worn at two different locations (FT and FP) in differentiating PAs and estimating EE throughout a simulated 12 h free-living day.
Materials and Methods
Study design
The study included two protocols: investigating the reliability (n = 18) and validity (n = 19) of the Fibion accelerometer. All 37 participants were young and healthy volunteers, who had normal weight (i.e., BMI < 25 kg/m2) and were recreationally physically active. Exclusion criteria included acute and chronic diseases, which would prevent participants from prolonged sitting and/or standing or would interfere with the basic metabolic rate. All participants were informed of the study procedures and provided written informed consent prior to the testing. The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Shanghai Jiao Tong University (Approval number: ML16027).
Reliability protocol
A total of 18 young adults (eight females, 10 males; age 24.0 ± 2.8 years; BMI 22.8 ± 2.3 kg/m2) participated in the repeatability study. The protocol is a designed 45 min repeated test of walking, standing, and sitting, respectively (3 × 15 min, repeated twice, a total of 90 min). The participants were required to simultaneously wear Fibion and ActiGraph proximally on the left thigh, between the knee and the hip (Fig. S2A). However, the exact wearing location (i.e., medial or lateral) of each device was randomized but equally distributed among all participants. The ActiGraph was chosen because it is widely used and its validity and reliability have previously been studied (Welk, Schaben & Morrow, 2004; Esliger & Tremblay, 2006; Vanhelst et al., 2012; Lee, Kim & Welk, 2014; Aadland & Ylvisåker, 2015). Throughout the data recording, the participants were instructed to sit or stand still without shuffling their feet or repositioning their body, under observation by a research assistant. Walking was performed at a self-selected constant velocity. The procedure was strictly confined to the duration of 15 min, while any deviations from the protocol (i.e., uncommanded change of postures) were recorded.
Validity protocol
The validity protocol was performed by 19 young adults (9 females, 10 males; age 28.2 ± 3.8 years; BMI 21.0 ± 2.2 kg/m2), by using a 12 h guided sequence of tasks in the laboratory that simulated realistic daily activities. The participants were required to wear two Fibion devices (Fibion strapped to the front Thigh (FT) and worn in the pocket (FP)), as well as a portable gas analyzer (Cosmed K4b2; Cosmed, Rome, Italy) (Fig. S2B).
All participants were required to abstain from caffeine, alcohol intake, and unaccustomed exercise for 24 h prior to the measurements. The protocol commenced after resting metabolic rate was assessed in a fasting state following best practice guidelines (Compher et al., 2006). One supervisor and two assistants directly followed the participants in 4 h shifts. Specifically, the validity of the Fibion accelerometers was assessed using two criteria:
Criterion 1: 12 h guided sequence of tasks with direct observation
Direct observation of the designed 12 h guided sequence of tasks served as the criterion for detecting different types and intensities of PA (Table S1). The direct observation has been previously proven as a valid method, compared to indirect calorimetry (Lyden et al., 2014). In the present study, the 12 h guided sequence of tasks was designed to simulate an “ideal active working day,” according to current recommendations (Buckley et al., 2015). Thus, it included both computer-based office works (i.e., sitting, standing, walking, and cycling, etc.) and leisure activities in the afternoon (i.e., actually watching TV sitting on a sofa and exercising). In particular, walking was performed either within the laboratory or the hallway (short and long walks) or on the indoor track. Cycling was carried out on an ergometer at a self-paced intensity, which allowed participants to change the cadence as they do in the real-life conditions (i.e., commuting to/from work).
For the data collection, each of the tasks was coded as 0–12 based on the measurement logs of the direct observations, as was done previously (Lyden et al., 2017). The codes were subsequently separated into five different types (sitting, long sitting, standing, walking, and cycling) and three intensities (LPA, MPA, and VPA) of PA (Table S2). On the basis of the metabolic equivalent (MET; Ainsworth et al., 2011b), the PA intensities were defined as follows: long sitting was defined as uninterrupted sitting periods over 30 min. LPA was defined as any activities with an EE below three METs, excluding sitting. MPA was defined as activities with an EE between three and six METs. VPA was defined as activities over six METs. The entire 12 h measurement was recorded by video, to verify the correct timing of each criterion.
Criterion 2: indirect calorimetry
Indirect calorimetry served as the criterion for EE estimation. Pulmonary gas exchange of the participants was continuously measured throughout the 12 h guided sequence of tasks by a portable breath-by-breath gas analyzer (Cosmed K4b2, Rome, Italy). The K4b2 has previously been shown to be a valid and reliable device for estimating oxygen consumption (McLaughlin et al., 2001; Schrack, Simonsick & Ferrucci, 2010). Each participant was fitted with a rubber facemask (Hans-Rudolph, Kansas City, MO, USA), while the gas sensor was attached to a harvest and carried on the chest throughout the protocol (Fig. S2B). Prior to data recording, the device was calibrated according to the manufacturer’s guidelines (approximately 10 min before the start of the measurement). The facemask was removed from the face only during designated short breaks, that is, for food intake.
Data processing
Fibion data collected from the reliability and validity measurements were uploaded to the manufacturer’s web-browser-based online service (www.fibion.com/upload), in order to obtain detailed reports on PA types and intensities as well as the corresponding EE. The raw comma-separated values (CSV) files with minute-by-minute data from Fibion were subsequently exported for further analysis. Similarly, the obtained ActiGraph data were uploaded to the device-specific software (ActiLife 6) and subsequently exported to Microsoft Excel for further analyses. The ActiGraph data for each activity type (provided in seconds) were then synchronized with manual recordings. To obtain comparable data of EE, the breath-by-breath values collected with the K4b2 were averaged for the duration of each task and were expressed as average “kcals/min” of each PA type and a 12 h total EE (Tables S1 and S2). No data were removed from the reliability study. However, for the validity test, a total of 8.2 ± 2.3% of the data, both from Fibion and criteria, were excluded due to visible artifacts (i.e., originating from movement artifacts, errors in the assessment of EE by gas analysis, or meal breaks). Furthermore, validity data from two participants (ID 16, 17) of FT but not FP were removed entirely due to wrong positioning of the thigh strap, according to the retrospective check of the video recordings.
Statistical analyses
Reliability
The measurement precision of Fibion and ActiGraph was expressed as the coefficient of variation (“CV% = (RMS − SD)/mean × 100,” where the “RMS-SD” refers to the root mean square (RMS) of the standard deviation (SD) calculated from both measurements of each individual (i.e., for sitting, standing, and walking, respectively) and “mean” refers to the group mean (Brunzendorf & Behrens, 2007; Finni et al., 2007)). The CV% was classified as low (<10%), medium (10–20%), and high (20–30%) (Gomes, 2009). In addition, percent measurement error (ME) was calculated by the equation: “ME = (actual duration/estimated duration) × 100–100,” where the actual duration for each type was 15 min. Intra-class correlation coefficient (ICC) two-way ANOVA models were used to determine the random/individual errors between tests. ICC may be interpreted as low (<0.4), moderate (0.4–0.75), and high (>0.75), respectively (Cicchetti, 1994). The paired samples Student’s t-test was used to compare the difference between Fibion and ActiGraph. If data were not normally distributed even after log transformation, the Wilcoxon signed-rank test was used.
Validity
Paired difference tests (Student’s t-tests or Wilcoxon signed-rank test) and the ICC were used to determine the accuracy of Fibion compared to criterion 1 and 2. Bland–Altman plots were generated to examine the mean bias and limits of agreement (LOA, mean difference ±1.96 SD) of Fibion in comparison to the criteria for each PA type, intensity, and the 12 h total EE (Bland & Altman, 1986). All statistical analyses were performed with the R program (RStudio Team, 2018), and the level of significance was set at p < 0.05 (two-sided).
Results
Reliability
Table 1 presents the mean ± SD (ME, only for PA duration) of the repeated performances of Fibion (FT) and ActiGraph. The agreement of FT was moderate to good (CV%: 6.57–9.13; ICCs: 0.687–0.806) in all EE measures of sitting, standing, and walking, similar to the ActiGraph. FT accurately detected the duration of sitting (ME: 5.1 ± 1.2%), and walking (ME: 3.8 ± 0.3%), but not for standing (ME: 14.9 ± 2.6%). However, low correlations (ICCs: 0.189–0.459) were observed between subsequent measurements with both devices, especially in low-intensity PAs (sitting).
Table 1. Mean and standard deviation (%ME, only for PA duration) of repeatability measurements for Fibion worn on the thigh (FT) and ActiGraph (95% Confidence Interval).
| Sitting | Standing | Walking | |||||
|---|---|---|---|---|---|---|---|
| FT | ActiGraph | FT | ActiGraph | FT | ActiGraph | ||
| AT | 1st test ME | 14.40 ± 0.30 (4.22) | 14.09 ± 1.26 (7.40) | 13.36 ± 1.03 (13.00) | 13.83 ± 1.12 (9.20) | 14.45 ± 0.45 (4.00) | 14.86 ± 0.78 (3.8) |
| 2nd test ME | 14.18 ± 0.33 (5.85) | 13.98 ± 0.95 (8.20) | 12.98 ± 0.90 (16.07) | 13.89 ± 1.41 (11.00) | 14.48 ± 0.39 (3.60) | 14.60 ± 0.88 (5.20) | |
| CV% | 2.19 | 7.28 | 5.51 | 5.68 | 2.54 | 6.08 | |
| p | 0.031 | 0.587 | 0.112 | 0.486 | 0.760 | 0.390 | |
| ICC | 0.189 | 0.119 | 0.459 | 0.609 | 0.227 | −0.165 | |
| EE | 1st test | 1.28 ± 0.201 | 1.30 ± 0.211 | 1.73 ± 0.23 | 1.74 ± 0.25 | 3.40 ± 0.61 | 3.52 ± 0.54 |
| 2nd test | 1.33 ± 0.18 | 1.38 ± 0.21 | 1.70 ± 0.25 | 1.76 ± 0.24 | 3.44 ± 0.73 | 3.47 ± 0.63 | |
| CV% | 6.57 | 7.14 | 7.79 | 7.44 | 9.13 | 9.69 | |
| p | 0.081 | 0.014 | 0.058 | 0.523 | 0.551 | 0.766 | |
| ICC | 0.806 | 0.806 | 0.687 | 0.789 | 0.782 | 0.661 | |
Note:
AT, refers to the duration of activity types; EE, refers to the energy expenditure; CV%, coefficient of variation; ME, percent measurement error; ICC, intra-class correlation coefficient; p-values refer to the Student’s t-test or the Wilcoxon signed-rank test.
The bold p-values indicating significant differences between the compared two groups.
Validity
Table 2 presents the mean and SD of different PA durations (min) and EE (kcal/min) of Fibion compared with criterion 1 and 2.
Table 2. Mean and standard deviation of different PAs detection (time duration, min), specified-types of EE (kcal/min), and a 12 h total EE (kcals) measured with Fibion devices (FT and FP), compared to criterion 1 (direct observation) and criterion 2 (K4b2). (95% Confidence Interval).
| Criterion 1 n = 17 | FT n = 17 | ICC | p-Value | Criterion 1 n = 19 | FP n = 19 | ICC | p-Value | *p-Value n = 17 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PA types/intensities detection (min) | Sitting | 491.7 ± 10.2 | 487.5 ± 22.4 | 0.407 | 0.329 | 492.0 ± 9.7 | 411.4 ± 115.5 | −0.046 | 0.009 | 0.051 |
| LSit | 292.0 ± 20.1 | 283.0 ± 56.9 | 0.220 | 0.501 | 291.8 ± 19.0 | 199.6 ± 123.4 | 0.060 | 0.012 | 0.041 | |
| Standing | 80.1 ± 7.6 | 97.3 ± 17.6 | 0.204 | <0.000 | 80.3 ± 7.2 | 178.6 ± 121.9 | −0.036 | <0.000 | 0.040 | |
| Walking | 88.5 ± 10.7 | 71.2 ± 13.2 | 0.092 | <0.000 | 90.1 ± 11.2 | 74.7 ± 16.1 | 0.154 | <0.000 | 0.306 | |
| Cycling | 29.8 ± 0.5 | 23.0 ± 6.1 | 0.016 | <0.000 | 29.8 ± 0.5 | 17.5 ± 12.1 | −0.011 | <0.000 | 0.207 | |
| LPA | 158.0 ± 15.9 | 154.0 ± 13.8 | 0.537 | 0.240 | 160.3 ± 17.6 | 160.7 ± 16.4 | 0.650 | 0.900 | 0.009 | |
| MPA | 85.4 ± 28.7 | 76.8 ± 11.1 | 0.276 | 0.266 | 82.6 ± 30.0 | 59.1 ± 16.0 | 0.220 | 0.002 | <0.000 | |
| VPA | 18.9 ± 13.4 | 15.1 ± 7.6 | 0.071 | 0.320 | 18.5 ± 12.8 | 11.8 ± 6.4 | 0.376 | 0.014 | 0.159 | |
| TD | 700.0 ± 15.3 | 694.0 ± 20.7 | 0.638 | 0.121 | 702.1 ± 15.7 | 694.0 ± 18.6 | 0.534 | 0.055 | 0.854 |
Notes:
PA, physical activity; EE, energy expenditure; FT, Fibion worn on the thigh; FP, Fibion worn in the pocket; LSit, Long sitting (sitting periods over 30 min); LPA, light intensity PA (MET < 3, excluding sitting); MPA, moderate PA (3 < MET < 6); VPA, vigorous intensity PA (MET > 6); TD, total duration; 12 h EE, total EE during 12 h measurement protocol. ICC, Intra-class correlation coefficient; p-values refer to the Student’s t-test or the Wilcoxon signed-rank test.
p-Value = FT vs. FP.
The bold p-values indicating significant differences between the compared two groups.
Criterion 1
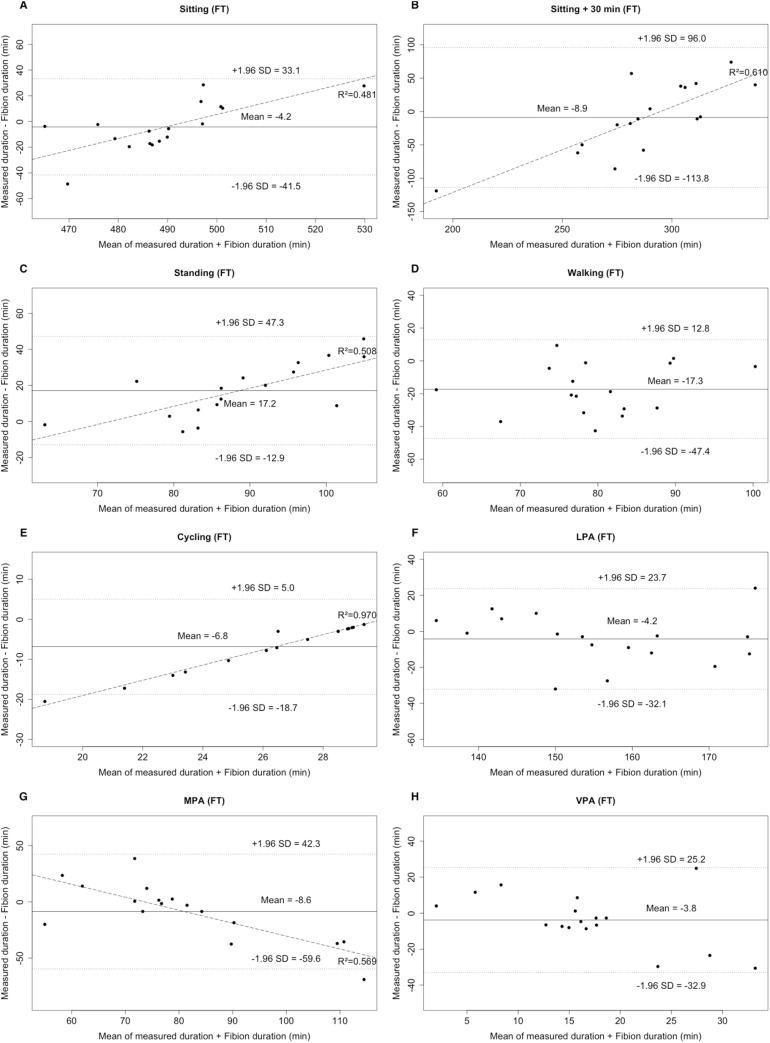
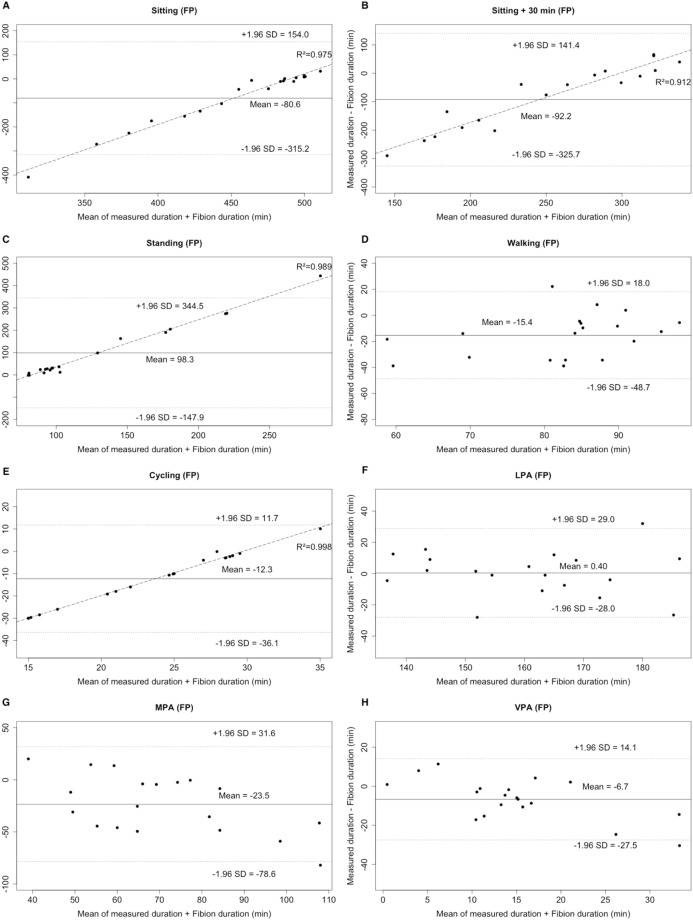
No significant differences were observed between FT and criterion 1 (i.e., PA duration) in sitting, long sitting, LPA, MPA, and VPA (all p > 0.05). By contrast, all types of FP (excepting LPA) significantly differed from criterion 1 (p < 0.05). When comparing FT and FP to the criterion 1, Bland–Altman plots revealed an individualized underestimation (positive mean bias) for standing and LPA, and an overestimation (negative mean bias) for all other PAs (Figs. 1 and 2). The LOA of PA durations ranged from −113.8 to 96.0 min for FT and from −325.7 to 344.5 min for FP. Furthermore, proportional errors were observed for FT (sitting, long sitting, standing, cycling, and MPA; (coefficient of determination) R2: 0.481–0.970) and FP (sitting, long sitting, and standing; R2: 0.912–0.989) and (Figs. 1 and 2). The low correlations (ICCs, FT: 0.016–0.638; FP: −0.046 to 0.650) also indicated that the MEs were random.
Figure 1. Bland–Altman plots for the duration of different PA types (A–E: sitting, long sitting, standing, walking, cycling) and intensities (F–H: LPA, MPA, VPA) for Fibion worn on the thigh (FT).
The middle line shows the mean difference between FT and criterion 1 and the dashed lines indicate the limits of agreement (±1.96 × SD of the different scores). Linear regression lines were fitted for proportional error.
Figure 2. Bland–Altman plots for the duration of different PA types (A–E: sitting, long sitting, standing, walking, cycling) and intensities (F–H: LPA, MPA, VPA) for Fibion worn in the pocket (FP).
The middle line shows the mean difference between FP and criterion 1 and the dashed lines indicate the limits of agreement (±1.96 × SD of the different scores). Linear regression lines were fitted for proportional error.
Criterion 2
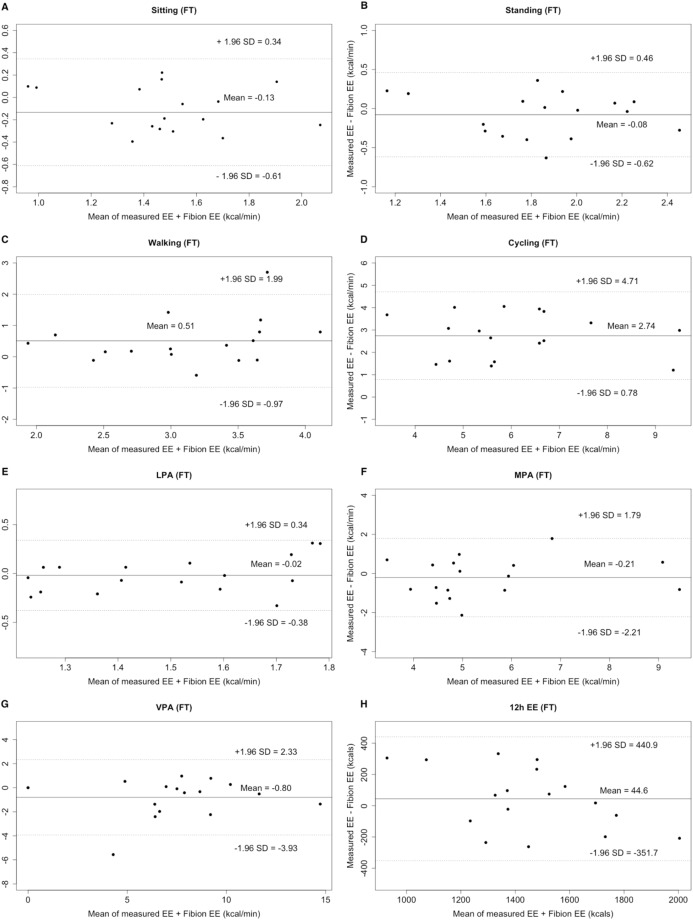
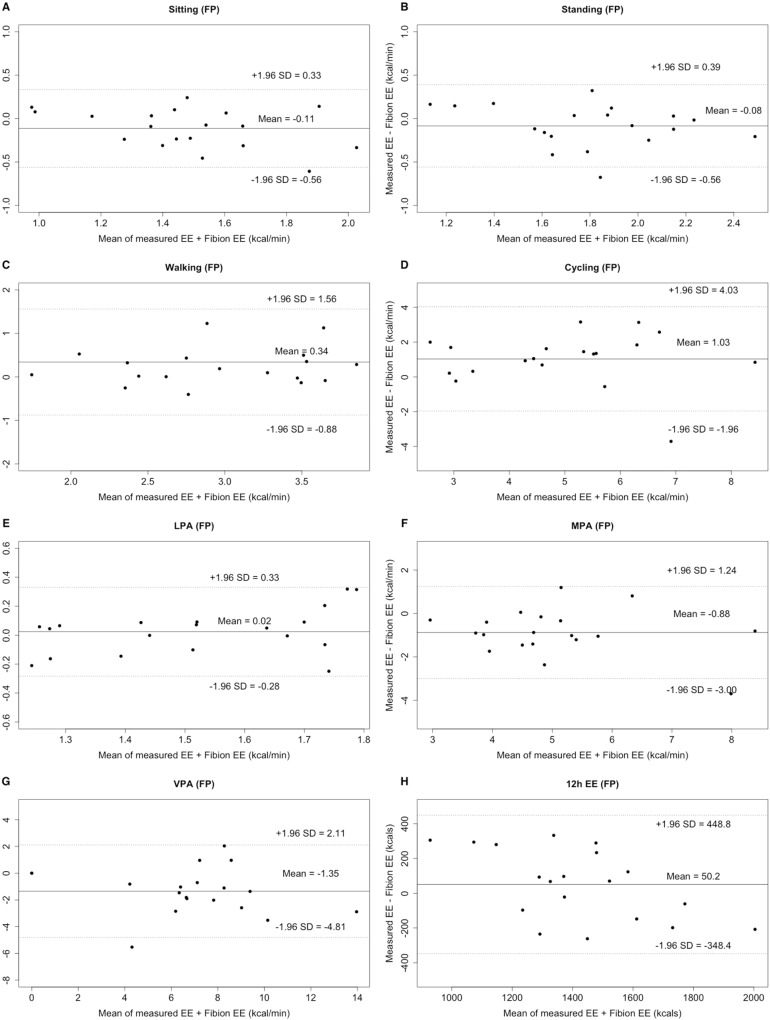
The EE of standing, LPA, MPA, VPA, as well as the overall 12 h EE did not differ between FT and criterion 2 (all p > 0.05; ICCs: 0.363–0.894). However, all other activities significantly differed between FP and criterion 2, except for sitting, standing, LPA, and 12 h EE (Table 2). Bland–Altman plots revealed an individualized underestimation for walking, cycling, and the 12 h EE, as well as an overestimation for sitting, standing, LPA, MPA, and VPA, when comparing FT to the criterion 2 (LOA: −3.93 to 4.71 kcal/min, Fig. 3). Moreover, an individualized underestimation for walking, cycling, LPA, and 12 h EE and an overestimation for sitting, standing, MPA, and VPA were observed, when comparing FP to the criterion 2 (LOA: −4.81 to 4.03 kcal/min, Fig. 4).
Figure 3. Bland–Altman plots of specified-types of EE (A–G: sitting, standing, walking, cycling, LPA, MPA, and VPA, kcal/min) and 12 h total EE (H, kcal) for Fibion worn on the thigh (FT).
The middle line shows the mean difference between FT and criterion 2 and the dashed lines indicate the limits of agreement (±1.96 × SD of the different scores).
Figure 4. Bland–Altman plots of specified-types of EE (A–G: sitting, standing, walking, cycling, LPA, MPA, and VPA, kcal/min) and 12 h total EE (H, kcal) for Fibion worn in the pocket (FP).
The middle line shows the mean difference between FP and criterion 2 and the dashed lines indicate the limits of agreement (±1.96 × SD of the different scores).
Discussion
The current study consisted of two parts: a reliability and a validity protocol. We primarily found that Fibion wore on the thigh (FT) showed similar reliability to the Actigraph. However, low correlations between subsequent measurements with both devices indicated large random MEs, especially in low-intensity PAs (sitting). Furthermore, FT but not FP accurately detected different types (sitting and long sitting) and intensities (LPA, MPA, and VPA) of PA and EE (standing, LPA, MPA, VPA, and 12 h total EE), compared to both of the direct observation of the 12 h task guided sequence of tasks (criterion 1) and indirect calorimetry (criterion 2). But as such, FP differed significantly for all PA types and intensities (excepting LPA) when compared to both of the criteria, indicating that the location of the accelerometer is an essential factor.
The validity and reliability of ActiGraph have previously been well documented (Welk, Schaben & Morrow, 2004; Esliger & Tremblay, 2006; Vanhelst et al., 2012; Lee, Kim & Welk, 2014; Aadland & Ylvisåker, 2015). In line with the present results of the ActiGraph, FT provided relatively small marginal error by a low CV% (all <10%). However, the larger variance of ICCs (−0.165 to 0.609) indicated larger individual errors of the measurements for both FT and ActiGraph. The larger individual errors are likely due to the relatively shorter (i.e., 3 × 15 min) duration of our measurements compared to previous studies, which typically utilized durations of multiple hours up to several weeks (Aadland & Ylvisåker, 2015; Loprinzi & Smith, 2017; Hibbing et al., 2018; Arguello et al., 2018). Consequently, reliability design with longer duration may be further needed for Fibion.
Apart from the reliability test, the second aim of this study was to test the validity of the Fibion worn at two different positions (FT and FP), to determine different types, intensities of PA and EE throughout a simulated 12 h working day. Previous studies generally tended to use short protocols or isolated tasks for the validation of accelerometer-based gadgets (Duffield et al., 2004; Sun, Schmidt & Teo-Koh, 2008; Powell et al., 2016). However, shorter protocols are inevitability unable to capture the infinite number of activities in realistic free-living environments. Thus, our protocol provided a more advanced design to simulate extended periods of realistic activities of daily living with self-selected speeds and cadences, to reflect the current PA recommendations and to avoid excessive sitting (Buckley et al., 2015). Throughout this protocol, FT was found to provide a moderate differentiation for the duration of sitting, long sitting, LPA, MPA, and VPA. However, the LOA provided by the Bland–Altman plots were rather large, especially for FP. Moreover, the MEs appeared to be random, with low correlations being observed in sitting and standing tasks. As the magnitude of the ME seemed to be independent of the PA types, they were likely to be caused by the algorithm of the device rather than by movement artifacts, and this was also reported by other studies (Lee, Kim & Welk, 2014; Kooiman et al., 2015).
A unique feature of the present study was to compare the EE assessed by Fibion with that assessed by a portable gas analyzer during the prolonged simulated real-life protocol. Since the indirect calorimetry was used as a gold standard, we were able to define LPA, MPA, and VPA in an accurate manner, whereas, other studies used constant values for individual PA intensities (Adam Noah et al., 2013; Sasaki et al., 2015; Price et al., 2016; Lyden et al., 2017; Montoye et al., 2017). Interestingly, we did not find differences in standing, LPA, MPA, VPA, and 12 h EE between FT and oxygen consumption. This implied that the data of Fibion seemed to be valid, at least when compared to other methods of EE estimation. However, large differences were observed in the agreement between FT and PT, indicating poor agreements for the detection of PA types and intensities when Fibion was worn in the pocket (Fig. S1). It is possible that the Fibion devices did not consistently remain in the recommended location of the frontal part of the thigh, or participants wore rather loose shorts that allowed the device to shift inside the pocket. This indicated that the proper location of the accelerometer is essential for accurately assessing PA and EE for daily use.
When interpreting the findings of this study, one should bear in mind that the participants were relatively young and were selected as a convenience sample from a university and the nearby communities, which limited the generalizability of the results. Furthermore, walking and cycling were performed indoors in this study, and they might differ from the outdoor environments. Last, the laboratory-based setting of the validity study was also a limitation, given that the execution of tasks may differ between laboratory-based and free-living conditions, even though all efforts were made to simulate natural living conditions.
Conclusions
Fibion located on the thigh (FT) provided similar reliability to that of the Actigraph. However, low correlations between subsequent measurements of both devices indicated large random MEs, which were somewhat diminished during the simulated 12 h real-life test. Furthermore, FT may accurately determine the types, intensities of PA and EE during prolonged periods with substantial changes in postures, indicating that the location of the accelerometer is essential. Further study with a large cohort is needed to confirm the usability of Fibion, especially for detecting the low-intensity PAs.
Supplemental Information
PA = physical activity.
The “Activity” was defined as all activity types except sitting.
(Photo by Tao Zhang).
Acknowledgments
The authors would like to thank Dr. Olli Tikkanen and Dr. Noora Ronkainen for their help to the study design and measurement instructions. Also, the authors would like to thank Fang Hui Cai and Tao Zhang for the measurement and data collection process.
Funding Statement
This study was supported by a grant from the Chinese Postdoctoral Science Foundation (2016M590352/OT) and the Shanghai Jiao Tong University Zhiyuan Foundation (Grant CP2014013). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Dr. Olli Tikkanen is co-founder in Fibion Inc. The authors declare that they have no competing interests.
Author Contributions
Yanxiang Yang performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Moritz Schumann conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Shenglong Le performed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Shulin Cheng conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Shanghai Jiao Tong University (Approval number: ML16027).
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Aadland & Ylvisåker (2015).Aadland E, Ylvisåker E. Reliability of the Actigraph GT3X+ accelerometer in adults under free-living conditions. PLOS ONE. 2015;10(8):e0134606. doi: 10.1371/journal.pone.0134606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam Noah et al. (2013).Adam Noah J, Spierer DK, Gu J, Bronner S. Comparison of steps and energy expenditure assessment in adults of Fitbit Tracker and Ultra to the Actical and indirect calorimetry. Journal of Medical Engineering & Technology. 2013;37(7):456–462. doi: 10.3109/03091902.2013.831135. [DOI] [PubMed] [Google Scholar]
- Ainsworth et al. (2011a).Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 compendium of physical activities. Medicine & Science in Sports & Exercise. 2011a;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Ainsworth et al. (2011b).Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 compendium of physical activities. Medicine & Science in Sports & Exercise. 2011b;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Arguello et al. (2018).Arguello D, Andersen K, Morton A, Freedson PS, Intille SS, John D. Validity of proximity sensor-based wear-time detection using the ActiGraph GT9X. Journal of Sports Sciences. 2018;36(13):1502–1507. doi: 10.1080/02640414.2017.1398891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland & Altman (1986).Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;327(8476):307–310. doi: 10.1016/s0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- Brunzendorf & Behrens (2007).Brunzendorf J, Behrens R. How to type test the coefficient of variation of an indication. Radiation Protection Dosimetry. 2007;123(1):21–31. doi: 10.1093/rpd/ncl078. [DOI] [PubMed] [Google Scholar]
- Buckley et al. (2015).Buckley JP, Hedge A, YATES T, Copeland RJ, Loosemore M, Hamer M, Bradley G, Dunstan DW. The sedentary office: an expert statement on the growing case for change towards better health and productivity. British Journal of Sports Medicine. 2015;49(21):1357–1362. doi: 10.1136/bjsports-2015-094618. [DOI] [PubMed] [Google Scholar]
- Cicchetti (1994).Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6(4):284–290. doi: 10.1037//1040-3590.6.4.284. [DOI] [Google Scholar]
- Compher et al. (2006).Compher C, Frankenfield D, Keim N, Roth-Yousey L, Evidence Analysis Working Group Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. Journal of the American Dietetic Association. 2006;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Duffield et al. (2004).Duffield R, Dawson B, Pinnington HC, Wong P. Accuracy and reliability of a Cosmed K4b2 portable gas analysis system. Journal of Science and Medicine in Sport. 2004;7(1):11–22. doi: 10.1016/S1440-2440(04)80039-2. [DOI] [PubMed] [Google Scholar]
- Esliger & Tremblay (2006).Esliger DW, Tremblay MS. Technical reliability assessment of three accelerometer models in a mechanical setup. Medicine & Science in Sports & Exercise. 2006;38(12):2173–2181. doi: 10.1249/01.mss.0000239394.55461.08. [DOI] [PubMed] [Google Scholar]
- Evenson, Goto & Furberg (2015).Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. International Journal of Behavioral Nutrition and Physical Activity. 2015;12(1):159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finni et al. (2007).Finni T, Hu M, Kettunen P, Vilavuo T, Cheng S. Measurement of EMG activity with textile electrodes embedded into clothing. Physiological Measurement. 2007;28(11):1405–1419. doi: 10.1088/0967-3334/28/11/007. [DOI] [PubMed] [Google Scholar]
- Gomes (2009).Gomes FP. Curso De estatística Experimental. Fifteenth Edition. Vol. 15. Piracicaba: SP; 2009. [Google Scholar]
- Hibbing et al. (2018).Hibbing PR, Lamunion SR, Kaplan AS, Crouter SE. Estimating energy expenditure with ActiGraph GT9X inertial measurement unit. Medicine & Science in Sports & Exercise. 2018;50(5):1093–1102. doi: 10.1249/MSS.0000000000001532. [DOI] [PubMed] [Google Scholar]
- Hills, Mokhtar & Byrne (2014).Hills AP, Mokhtar N, Byrne NM. Assessment of physical activity and energy expenditure: an overview of objective measures. Frontiers in Nutrition. 2014;1:5. doi: 10.3389/fnut.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang & Larson (2013).Jiang Y, Larson JL. IDEEA activity monitor: validity of activity recognition for lying, reclining, sitting and standing. Frontiers of Medicine. 2013;7(1):126–131. doi: 10.1007/s11684-012-0236-0. [DOI] [PubMed] [Google Scholar]
- Kooiman et al. (2015).Kooiman TJM, Dontje ML, Sprenger SR, Krijnen WP, Van Der Schans CP, De Groot M. Reliability and validity of ten consumer activity trackers. BMC Sports Science, Medicine and Rehabilitation. 2015;7(1):24. doi: 10.1186/s13102-015-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Kim & Welk (2014).Lee J-M, Kim Y, Welk GJ. Validity of consumer-based physical activity monitors. Medicine & Science in Sports & Exercise. 2014;46(9):1840–1848. doi: 10.1249/MSS.0000000000000287. [DOI] [PubMed] [Google Scholar]
- Loprinzi & Smith (2017).Loprinzi PD, Smith B. Comparison between wrist-worn and waist-worn accelerometry. Journal of Physical Activity and Health. 2017;14(7):539–545. doi: 10.1123/jpah.2016-0211. [DOI] [PubMed] [Google Scholar]
- Lyden et al. (2017).Lyden K, Keadle SK, Staudenmayer J, Freedson PS. The activPALTM accurately classifies activity intensity categories in healthy adults. Medicine & Science in Sports & Exercise. 2017;49(5):1022–1028. doi: 10.1249/MSS.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden et al. (2014).Lyden K, Petruski N, Mix S, Staudenmayer J, Freedson P. Direct observation is a valid criterion for estimating physical activity and sedentary behavior. Journal of Physical Activity and Health. 2014;11(4):860–863. doi: 10.1123/jpah.2012-0290. [DOI] [PubMed] [Google Scholar]
- Maffiuletti et al. (2008).Maffiuletti NA, Gorelick M, Kramers-De Quervain I, Bizzini M, Munzinger JP, Tomasetti S, Stacoff A. Concurrent validity and intrasession reliability of the IDEEA accelerometry system for the quantification of spatiotemporal gait parameters. Gait & Posture. 2008;27(1):160–163. doi: 10.1016/j.gaitpost.2007.01.003. [DOI] [PubMed] [Google Scholar]
- McLaughlin et al. (2001).McLaughlin JE, King GA, Howley ET, Bassett DR, Jr, Ainsworth BE. Validation of the COSMED K4 b2 portable metabolic system. International Journal of Sports Medicine. 2001;22(4):280–284. doi: 10.1055/s-2001-13816. [DOI] [PubMed] [Google Scholar]
- Montoye et al. (2017).Montoye AHK, Pivarnik JM, Mudd LM, Biswas S, Pfeiffer KA. Evaluation of the activPAL accelerometer for physical activity and energy expenditure estimation in a semi-structured setting. Journal of Science and Medicine in Sport. 2017;20(11):1003–1007. doi: 10.1016/j.jsams.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Powell et al. (2016).Powell C, Carson BP, Dowd KP, Donnelly AE. The accuracy of the SenseWear Pro3 and the activPAL3 Micro devices for measurement of energy expenditure. Physiological Measurement. 2016;37(10):1715–1727. doi: 10.1088/0967-3334/37/10/1715. [DOI] [PubMed] [Google Scholar]
- Price et al. (2016).Price K, Bird SR, Lythgo N, Raj IS, Wong JYL, Lynch C. Validation of the Fitbit one, Garmin Vivofit and Jawbone UP activity tracker in estimation of energy expenditure during treadmill walking and running. Journal of Medical Engineering & Technology. 2016;41(3):208–215. doi: 10.1080/03091902.2016.1253795. [DOI] [PubMed] [Google Scholar]
- RStudio Team (2018).RStudio Team . RStudio: Integrated Development for R. Boston: RStudio, Inc; 2018. [Google Scholar]
- Sasaki et al. (2015).Sasaki JE, Hickey A, Mavilia M, Tedesco J, John D, Keadle SK, Freedson PS. Validation of the Fitbit wireless activity tracker for prediction of energy expenditure. Journal of Physical Activity and Health. 2015;12(2):149–154. doi: 10.1123/jpah.2012-0495. [DOI] [PubMed] [Google Scholar]
- Schrack, Simonsick & Ferrucci (2010).Schrack JA, Simonsick EM, Ferrucci L. Comparison of the Cosmed K4b2 portable metabolic system in measuring steady-state walking energy expenditure. PLOS ONE. 2010;5(2):e9292. doi: 10.1371/journal.pone.0009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves et al. (2015).Steeves JA, Bowles HR, McClain JJ, Dodd KW, Brychta RJ, Wang J, Chen KY. Ability of Thigh-Worn ActiGraph and activPAL monitors to classify posture and motion. Medicine & Science in Sports & Exercise. 2015;47(5):952–959. doi: 10.1249/MSS.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Schmidt & Teo-Koh (2008).Sun DX, Schmidt G, Teo-Koh SM. Validation of the RT3 accelerometer for measuring physical activity of children in simulated free-living conditions. Pediatric Exercise Science. 2008;20(2):181–197. doi: 10.1123/pes.20.2.181. [DOI] [PubMed] [Google Scholar]
- Vanhelst et al. (2012).Vanhelst J, Baquet G, Gottrand F, Béghin L. Comparative interinstrument reliability of uniaxial and triaxial accelerometers in free-living conditions. Perceptual and Motor Skills. 2012;114(2):584–594. doi: 10.2466/03.26.PMS.114.2.584-594. [DOI] [PubMed] [Google Scholar]
- Welk, Schaben & Morrow (2004).Welk GJ, Schaben JA, Morrow JR., Jr Reliability of accelerometry-based activity monitors: a generalizability study. Medicine and Science in Sports and Exercise. 2004;36(9):1637–1645. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PA = physical activity.
The “Activity” was defined as all activity types except sitting.
(Photo by Tao Zhang).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.