Abstract
Background
Many genetic variants show highly robust associations with body mass index (BMI). However, the mechanisms through which genetic susceptibility to obesity operate are not well understood. Potentially modifiable mechanisms, including eating behaviors, are of particular interest to public health.
Objective
Here we explore whether eating behaviors mediate or modify genetic susceptibility to obesity.
Design
Genetic risk scores for BMI (BMI-GRS) were calculated for 3515 and 2154 adults in the Fenland and EDEN population-based cohort studies, respectively. The eating behaviors: emotional eating, uncontrolled eating and cognitive restraint, were measured using a validated questionnaire. The mediating effect of each eating behavior on the association between the BMI-GRS and measured BMI was assessed using the Sobel test. Additionally, we tested for interactions between each eating behavior and the BMI-GRS on BMI.
Results
The association between the BMI-GRS and BMI was mediated by both emotional eating (EDEN: P-Sobel=0.01; Fenland: P-Sobel=0.02) and uncontrolled eating (EDEN: P-Sobel=0.04; Fenland: P-Sobel=0.0006) in both sexes combined. Cognitive restraint did not mediate this association (P-Sobel>0.10), except amongst EDEN women (P-Sobel=0.0009). Cognitive restraint modified the relationship between the BMI-GRS and BMI amongst men (EDEN: P-interaction=0.0001; Fenland: P-interaction=0.04) and Fenland women (P-interaction=0.0004). By tertiles of cognitive restraint, the association between the BMI-GRS and BMI was strongest in the lowest tertile of cognitive restraint, and weakest in the highest tertile.
Conclusions
Genetic susceptibility to obesity was partially mediated by the ‘appetitive’ eating behavior traits (uncontrolled and emotional eating) and, in three of the four population groups studied, was modified by cognitive restraint. High levels of cognitive control over eating appear to attenuate the genetic susceptibility to obesity. Future research into interventions designed to support restraint may help to protect genetically susceptible individuals from weight gain.
Keywords: Eating behavior, Genetic risk score, Genetics, Obesity, BMI, Body mass index, Dieting
Introduction
There is a substantial genetic contribution to the determination of BMI. Heritability estimates from twin studies range from 47% to 90% (1) and the most recent GWAS meta-analysis identified 97 variants robustly associated with adult BMI (2). The possibility that genetic susceptibility to obesity is mediated or modified by eating behavior has not been fully explored.
Eating behaviors are partially heritable (3–5), emerge early in development and appear to be stable (6, 7). They are most often measured using validated questionnaires including the three factor eating questionnaire (TFEQ). The TFEQ assesses three subscales of eating behavior: cognitive restraint (CR), uncontrolled eating (UE) and emotional eating (EE). CR describes the intention to restrict food intake to influence shape or weight; UE describes a tendency to overeat accompanied by subjective loss of control; and EE indicates a tendency to overeat in response to dysphoric emotions. UE and EE are considered ‘appetitive traits’, whereas CR is thought to primarily represent an individual’s response to their weight (8).
The Behavioral Susceptibility Theory (BST) posits that genetic susceptibility to obesity is mediated by appetitive traits (9). Twin studies have demonstrated shared genetic influences on appetitive traits and weight in infancy (4) and many genes implicated by BMI-related variants show enriched expression in regions of the brain with an established role in the central regulation of eating (2). Moreover, MC4R genotype has been associated to UE (10) and EE (11); FTO to satiety responsiveness (12), food intake (13) and binge eating (14); NMB to disinhibited eating and hunger (15); and HTR2A to food reinforcement (16). However, inconsistencies found in some populations (17) mean further analyses are needed.
The possibility that eating behavior mediates genetic susceptibility to obesity has been examined in three previous studies: two in children (18, 19) and one in adults (20). One study reported mediation of a 28-locus BMI-GRS to BMI association by ‘satiety responsiveness’ amongst 2258 children (mean age: 9.9y) (18). The second study did not detect mediation of a 32-locus BMI-GRS to weight gain association by eating behavior amongst 652 children aged 6-8 years (19). The adult study tested for mediation of a 90-locus BMI-GRS to BMI association by UE and EE in two Finnish cohorts (20). Mediation by EE was detected in both cohorts and by UE in one cohort. A fourth study, in adults, reported associations between a 32-locus BMI-GRS with UE and EE, but did not explicitly test for mediation (21).
To our knowledge, no study to-date has examined mediation by CR or modification of the BMI-GRS to BMI association by any of the eating behaviors. Here we tested for both mediation and modification of genetic susceptibility to obesity by CR, UE and EE in two large, well-characterised population-based adult cohorts.
Subjects and Methods
Participants
The EDEN mother-child study is a prospective cohort that aims to assess pre- and postnatal determinants of childhood growth, development and health (22). Briefly, 2002 pregnant women were recruited in two French university hospitals before 24 weeks of amenorrhea. Exclusion criteria were: multiple pregnancies, known diabetes prior to pregnancy, illiteracy and planning to move outside the region in the next three years. At several occasions during their pregnancy and the child’s follow-up, mothers answered questionnaires about their own health and lifestyle. A clinical examination and a venous blood taking was organised at between 24 and 28 weeks of amenorrhea. Fathers were also invited to participate and to provide a venous blood sample at any time during the mother’s pregnancy. The study population for the present analysis comprised 2154 individuals (55.7% women) aged between 18 and 56 years with complete genotype and eating behavior data.
Ethics for EDEN
The study was approved by the Ethics Committee of the University Hospital of Kremlin-Bicêtre on December 12, 2002 and data files were declared to the National Committee for Processed Data and Freedom. Written consent was obtained from both parents.
The Fenland cohort study is a population based cohort study of volunteers recruited from participating General Practices in Ely, Wisbech and the surrounding Cambridgeshire region in the UK between 2004 and 2015 (23). Eligible individuals were adults registered at a collaborating General Practice and residing in Cambridgeshire at the time of recruitment. Exclusion criteria were: clinically diagnosed diabetes mellitus, inability to walk unaided, terminal illness (life expectancy of ≤1 year at the time of recruitment), clinically diagnosed psychotic disorder, pregnancy or lactation. The study population of the present analysis comprised 3515 individuals (53.2% women; 98.5% self-reported white ethnicity) aged 35 to 64 years. All participants attended a visit to an MRC Epidemiology Unit testing centre where data was collected.
Ethics for Fenland
Written informed consent was attained from all participants and the study was approved by the Cambridge Local Research Ethics Committee.
Genotyping and BMI Genetic Risk Score (BMI-GRS) generation
In EDEN, DNA from the parents was genotyped for 27 BMI-related variants at the MRC Epidemiology Unit, Cambridge (iPLEX platform; Sequenom), as previously described (24). In Fenland, DNA was genotyped using the Affymetrix UK Biobank Axiom array. Missing genotypes and those not directly measured were imputed via IMPUTE version2 (25) based on the 1000 Genomes Project haplotype reference. All required BMI-related variants could be imputed with sufficient accuracy (imputation information value >0.4). Of the 97 BMI-related variants reported by Locke and colleagues in the most recent genome wide meta-analysis of BMI (2), 96 were appropriate for inclusion in the Fenland BMI-GRS. The excluded SNP, rs2033529 (nearest gene: TDRG1), was multi-allelic.
In both cohorts, a weighted genetic risk score for BMI (BMI-GRS) was generated for each participant. At each locus, the number of BMI increasing variants was multiplied by the effect estimate for the BMI-increasing variant from Locke et al (2) in Fenland and from Speliotes et al (26) in EDEN. The products across all 96 SNPs were then summed for each participant in Fenland and across 27 SNPs in EDEN.
The 27 loci that comprised the EDEN BMI-GRS are amongst the most strongly associated signals in the GWAS analysis used to weight the Fenland BMI-GRS (2). As such, they explain a larger proportion of the variance in BMI than the other 69 SNPs. The 96-locus BMI-GRS explains 4% of the variance in BMI in Fenland men and 1% in Fenland women. The 27-locus BMI-GRS explains 3% of the variance in BMI in EDEN men and 1% of the variance in EDEN women. The BMI-GRS in both cohorts was standardised (by z-score transformation) to produce comparable effect estimates in the two studies.
Eating behavior assessment
The most commonly used versions of the Three Factor Eating Questionnaire (TFEQ) are the 18-item (TFEQ-R18) (27) and 21-item (TFEQ-R21) versions. Both questionnaires assess eating behavior using 3 subscales: cognitive restraint (CR; 6 items), uncontrolled eating (UE; 9 items) and emotional eating (EE; 3 items in TFEQ-R18; 6 items in TFEQ-R21). The TFEQ-R21 was created by adding 3 items to the EE scale of the TFEQ-R18 in order to improve discrimination. The TFEQ-R18 was initially developed in an obese population (27) but has been validated for use in normal weight populations (28) and can accurately distinguish different patterns of eating behavior in the general population (29). The factor structure of the TFEQ-R21 has also been replicated in a population-based study of male Swedish twins (5).
In the EDEN cohort, parents completed the TFEQ-R21 at 2-y follow-up. In Fenland, the TFEQ-R18 was completed at baseline. In each study, subscale scores were generated and transformed to a 0 to 100 scale through the following equation: [((raw score – lowest possible raw score)/possible raw score range)*100] (29). The scores were then standardised to a mean of 0 and SD of 1. Cronbach’s alpha testing inter-correlations between individual questionnaire items within each eating behavior subscale were between 0.76 and 0.93 in EDEN, and between 0.75 and 0.87 in Fenland. These values suggest a high level of reliability of the questionnaire.
Body Mass Index assessment
In EDEN, maternal weight was measured to the nearest 0.1kg using electronic scales (Terraillon SL-351, Hanson Ltd, Hemel Hempstead, UK) at the 1-y and 3-y follow up study visits and paternal weight was measured at baseline. At 2-y follow up, mothers reported their current weight. Parental heights were, where possible (all women and the majority of men) measured to the nearest 0.2 cm using a wall-mounted stadiometer (Seca-206, Seca, Hamburg, Germany). Participants were barefoot and were asked to remove heavy items of clothing whilst measurements were taken. For mothers, as eating behavior was assessed at 2-y follow-up, we used, in order of priority: 2-y self-reported weight (55%), the mean of 1-y and 3-y measured weights (15%), or the 1-y measured weight (30%). Mother’s pre-pregnant weight was also self-reported by women at inclusion. For fathers, when anthropometric measurements during pregnancy were unavailable, we used self-reported height and weight at baseline (11%), or father’s height and weight reported by the mother at baseline (6%).
In Fenland, weight was measured at baseline to the nearest 0.1kg using electronic scales (TANITA model BC-418 MA; Tanita, Tokyo, Japan) and height was measured to the nearest 0.1cm using a wall-mounted stadiometer (SECA 240; Seca, Birmingham, UK). Participants were barefoot and were asked to remove heavy items of clothing whilst measurements were taken. In both cohorts, BMI was calculated as: [weight(kg)/height(m)2].
Statistical analyses
The association between the BMI-GRS and BMI was analysed in linear regression models with the BMI-GRS as the independent variable and BMI as the dependent variable. The models were adjusted for age, sex and, in the EDEN cohort, recruitment centre. This constituted the ‘base model’. If an eating behavior was associated with both the independent variable (BMI-GRS) and the dependent variable (BMI), we tested for the presence of mediation. Mediation is said to occur when a third variable lies on the causal pathway between an exposure (in this case, the BMI-GRS) and an outcome (in this case, BMI) (Supplemental Figure 1).
To test for mediation, the ‘base model’ was adjusted for each eating behavior separately. The presence of mediation was established using the Sobel test (30, 31) and quantified by the mediation ratio ((β-β’)/ β), where β is the initial coefficient for BMI from the model BMI ~ BMI-GRS, age, (recruitment centre in EDEN) (Path c, Supplemental Figure 1) and β’ is the coefficient for BMI after the model is additionally adjusted for eating behavior (Path c’, Supplemental Figure 1).
Statistical modification is said to occur when the association between an exposure and an outcome differs depending on the level of a third variable (the modifier). To test whether the association between the BMI-GRS and BMI was modified by eating behavior, an interaction term (BMI-GRS*eating behavior score) was added to the base model for each eating behavior. Where modification was identified (P<0.05 for the interaction term), the BMI-GRS to BMI association was tested within tertiles of the eating behavior score. This step was performed in order to make clear any differential effects between groups detected by the interaction analysis.
Preliminary analyses showed that sex modified the relationship between the BMI-GRS and CR in Fenland (P-value for interaction term=0.02) but not UE (Fenland: P=0.34; EDEN: P=0.89) or EE (Fenland: P=0.26; EDEN: P=0.57). Therefore sex-stratified analyses were performed for CR in both cohorts. Additionally, CR showed evidence of a non-linear association with BMI (Supplemental Figure 2). Therefore, both CR and its quadratic term (CR*CR) were added to regression models when testing for mediation by CR.
Emotional eating and UE were highly correlated in both cohorts (Supplemental Table 1). To ascertain whether any mediating effects of the two eating behaviours were independent or occurred through a shared mechanism, analysis simultaneously accounting for both eating behaviours were conducted. First, the mediation effects were assessed in models where both eating behaviours were controlled for, representing the specific effects of one eating behaviour, whilst controlling for the other. Second, we modelled the mediation effects of the residuals from a model predicting EE from UE (and vice-versa). This was designed to reveal a mediation effect of the component of each eating behaviour that is independent of the other eating behaviour. Finally, in the EDEN study, sensitivity analyses were conducted using maternal pre-pregnant BMI to explore whether results in EDEN may be confounded by recent pregnancy.
Analyses were performed using SAS version 9.3 (SAS, Cary, NC) in EDEN, and Stata version 14 (StataCorp LCC, College Station, TX) in Fenland. Supplemental Figure 2 was produced using R Studio version 3.1.1 in EDEN and R version 3.3.1 in Fenland.
Results
The characteristics of the study populations are summarised in Table 1. The mean age of participants in Fenland (51 years in both sexes) was higher than in EDEN (men: 32 years; women: 30 years). The prevalence of obesity in Fenland (men: 24.1%; women: 21.6%) was approximately double that in EDEN (men: 9.0%; women: 11.4%). The Fenland participants also scored higher on all 3 eating behaviors than the EDEN participants. In both cohorts, women had higher scores for all eating behaviors than men. In both cohorts, EE and UE showed strong positive inter-correlations whilst their inter-correlations with CR were weaker (Supplemental Table 1).
Table 1. Characteristics of the EDEN and FENLAND cohorts.
| EDEN | FENLAND | |||
|---|---|---|---|---|
| Men (n=954) | Women (n=1200) | Men (n=1646) | Women (n=1869) | |
| Age (yrs) | 32.2 (5.6) | 29.9 (4.7) | 50.7 (7.3) | 50.9 (7.2) |
| BMI (kg/m2) | 25.2 (3.6) | 24.1 (4.8) | 27.6 (4.2) | 26.6 (5.3) |
| BMI status 1 | ||||
| Underweight | 0.7% (7) | 6.2% (74) | 0.4% (7) | 1.2% (22) |
| Normal weight | 51.6% (492) | 60.6% (727) | 27.2% (449) | 43.9% (821) |
| Overweight | 38.7% (369) | 21.8% (262) | 48.2% (793) | 33.3% (622) |
| Obese | 9% (86) | 11.4% (137) | 24.1% (397) | 21.6% (404) |
| Cognitive restraint (0-100 scale) | 20.7 (18.0) | 32.7 (21.2) | 35.5 (19.0) | 45.8 (19.1) |
| Emotional eating (0-100 scale) | 16.2 (21.1) | 34.5 (27.4) | 27.1 (24.8) | 42.2 (28.0) |
| Uncontrolled eating (0-100 scale) | 23.3 (18.5) | 23.4 (17.7) | 29.1 (17.5) | 31.0 (17.7) |
Values are mean (SD) or % (n)
WHO BMI categories: Underweight <18.5kg/m2; Normal weight 18.5-24.9kg/m2; Overweight ≥25kg/m2; Obese ≥30 kg/m2
Eating behavior and BMI
EE and UE showed positive linear associations with BMI in both cohorts (Table 2; Supplemental Figure 2). When both EE and UE were considered simultaneously, only EE remained associated with BMI in EDEN (EE: beta=1.08kg/m2 (95% CI: 0.85, -1.31); UE: beta=0.22kg/m2 (95% CI: -0.02, 0.46)). Although both eating behaviours maintained significant but reduced associations with BMI in Fenland (EE: beta=1.35kg/m2 (95% CI: 1.15, 1.55); UE: beta= 0.65kg/m2 (95% CI: 0.45, 0.85) (Supplemental Table 2).
Table 2. Tests for mediation of the BMI-genetic risk score (BMI-GRS) to BMI association by Emotional eating and Uncontrolled eating.
| BMI-GRS to TFEQ | TFEQ to BMI | BMI-GRS to BMI | BMI-GRS to BMI (adj. for TFEQ) | Sobel test P-val. | Mediation ratio (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size (95%CI) | P-val. | Effect size (95%CI) | P-val. | Effect size (95%CI) | P-val. | Effect size (95%CI) | P-val. | |||
| Emotional eating | ||||||||||
| EDEN (n=2154) | 0.06 (0.01, 0.10) | 0.01 | 1.22 (1.04, 1.39) | <1×10-10 | 0.62 (0.44, 0.80) | <1×10-10 | 0.55 (0.38, 0.72) | 4×10-10 | 0.01 | 10.8% |
| Fenland (n=3515) | 0.04 (0.01, 0.07) | 0.02 | 1.78 (1.63, 1.94) | <1×10-10 | 0.70 (0.54, 0.85) | <1×10-10 | 0.63 (0.48, 0.78) | <1×10-10 | 0.02 | 10.0% |
| Uncontrolled eating | ||||||||||
| EDEN (n=2154) | 0.04 (0.00, 0.09) | 0.04 | 0.92 (0.74, 1.10) | <1×10-10 | 0.62 (0.44, 0.80) | <1×10-10 | 0.58 (0.40, 0.75) | 1×10-10 | 0.04 | 6.4% |
| Fenland (n=3515) | 0.06 (0.03, 0.09) | 5×10-4 | 1.50 (1.35, 1.65) | <1×10-10 | 0.70 (0.54, 0.85) | <1×10-10 | 0.61 (0.46, 0.76) | <1×10-10 | 6×10-4 | 12.0% |
Effect sizes and 95% confidence intervals are from least squares linear regression models. All models were adjusted for age, sex and (in EDEN) recruitment centre
The BMI-GRS and TFEQ scores were all standardised to z-scores (mean=0; SD=1)
Due to modification of the BMI-GRS to CR association by sex, CR was analysed in men and women separately. In both cohorts, in both men and women, there was a quadratic association between CR and BMI (Table 3, all P<1x10-3 for the quadratic term). At lower levels of CR, BMI appeared to be positively associated with CR, but at higher levels of CR, BMI values plateaued in EDEN and declined in Fenland (Supplemental Figure 2).
Table 3. Tests for mediation of the BMI-genetic risk score (BMI-GRS) to BMI association by Cognitive Restraint.
| BMI-GRS to TFEQ | TFEQ to BMI | BMI-GRS to BMI | BMI-GRS to BMI (adj. for TFEQ) | Sobel test P-val. | Mediation ratio (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size (95%CI) | P-val. | Effect size (95%CI) | P-val. | Effect size (95%CI) | P-val. | Effect size (95%CI) | P-val. | |||
| Cognitive restraint (linear term) | ||||||||||
| Men | ||||||||||
| EDEN (n=954) | 0.04 (-0.02, 0.10) | 0.21 | 0.98 (0.76, 1.20) | <1x10-10 | 0.37 (0.15, 0.60) | 1x10-3 | -- | -- | -- | -- |
| Fenland (n=1646) | 0.00 (-0.05, 0.05) | 0.94 | 0.14 (-0.06, 0.35) | 0.17 | 0.79 (0.60, 0.99) | <1x10-10 | -- | -- | -- | -- |
| Women | ||||||||||
| EDEN (n=1200) | 0.10 (0.04, 0.15) | 6x10-4 | 1.62 (1.36, 1.88) | <1x10-10 | 0.80 (0.53, 1.07) | 6x10-9 | 0.65 (0.39, 0.90) | <1x10-10 | 9x10-4 | 19.0% |
| Fenland (n=1869) | 0.07 (0.03, 0.12) | 9x10-4 | 0.10 (-0.15, 0.35) | 0.42 | 0.61 (0.38, 0.85) | 5x10-7 | -- | -- | -- | -- |
| Cognitive restraint (quadratic term) 1 | ||||||||||
| Men | ||||||||||
| EDEN (n=954) | -0.02 (-0.09, 0.04) | 0.51 | -0.34 (-0.55, -0.14) | 9x10-4 | 0.37 (0.15, 0.60) | 1x10-3 | -- | -- | -- | -- |
| Fenland (n=1646) | 0.00 (-0.01, 0.01) | 0.96 | -1.80 (-2.53, -1.07) | 2x10-6 | 0.79 (0.60, 0.99) | <1x10-10 | -- | -- | -- | -- |
| Women | ||||||||||
| EDEN (n=1200) | -0.01 (-0.08, 0.05) | 0.71 | -0.49 (-0.71, -0.27) | 1x10-5 | 0.80 (0.53, 1.07) | 6x10-9 | -- | -- | -- | -- |
| Fenland (n=1869) | -0.01 (-0.02, 0.01) | 0.31 | -2.16 (-3.02, -1.30) | 8x10-7 | 0.61 (0.38, 0.85) | 5x10-7 | -- | -- | -- | -- |
Effect sizes and 95% confidence intervals are from least squares linear regression models. All models were adjusted for age, sex and (in EDEN) recruitment centre
Effect estimates refer to the quadratic term. Models were additionally adjusted for the linear term
-- Not applicable. The eating behavior was not associated with both the BMI-GRS and BMI and, as such, could not be considered as a mediator of the association between the BMI-GRS and BMI
The BMI-GRS and TFEQ scores were all standardised to z-scores (mean=0; SD=1)
When stratified by BMI (BMI <25kg/m2 and BMI ≥25kg/m2), a positive linear association between CR and BMI was found amongst all groups with BMI<25kg/m2 (Fenland: men: beta=0.24kg/m2 (95% CI: 0.10, 0.37); P=7.0x10-4; women: beta=0.25kg/m2 (95% CI: 0.13, 0.36); P=2.0x10-5. EDEN: men: beta=0.57kg/m2 (95% CI: 0.43, 0.72); P<1.0x10-10; women: beta=0.69kg/m2 (95% CI: 0.55, 0.83); P<1.0x10-10). However, amongst overweight/obese participants (BMI ≥25kg/m2): an inverse association between CR and BMI was found in Fenland men (beta=-0.22kg/m2, 95% CI: -0.43, -0.002; P=0.05) and women (beta=-0.64kg/m2, 95% CI: -0.97, -0.33; P=8.3x10-5), and no association was found in EDEN men (beta=0.01kg/m2; 95% CI: -0.13, 0.16; P=0.85) or (women: beta=-0.08kg/m2, 95% CI: -0.28, 0.11; P=0.40).
The BMI-GRS and eating behavior
In both cohorts, in both sexes combined, the BMI-GRS was positively associated with EE (Fenland: P=0.02; EDEN: P=0.01) and UE (Fenland: P=5x10-4; EDEN: P=0.04). In men, the BMI-GRS was not related to CR (linear and quadratic CR terms: P>0.20). In women in both cohorts, the BMI-GRS was positively associated with the linear CR term (P<1x10-3) but not to the quadratic CR term (P>0.30).
Individual SNP to eating behaviour associations are generally underpowered. However, in Fenland, nine SNPs showed nominally significant (P<0.05) associations with EE (six positive), eight with UE (five positive) and five with CR (one positive) (Supplemental Table 5). In EDEN, four SNPs showed nominally significant associations with EE (three positive), three with UE (one positive) and two with CR (two positive) (Supplemental Table 6).
Mediation by eating behaviors
In both cohorts, UE and EE partially mediated the relationship between the BMI-GRS and BMI (Table 2). For EE, the mediation ratio (Sobel test P-value) was 11% (P=0.01) in EDEN and 10% (P=0.02) in Fenland. For UE, the corresponding values were 6% (P=0.04) in EDEN and 12% (P=<0.0006) in Fenland. Controlling for UE, EE did not independently mediate the BMI-GRS to BMI association in either cohort (Supplemental Table 4). Controlling for EE, UE did not mediate the association in EDEN, but was a mediator in Fenland (Supplemental Tables 2 and 3).
The quadratic CR term did not meet the pre-defined conditions to be analysed as a mediator (see Methods) as it was not associated with the BMI-GRS and BMI. Despite non-linearity of the association between CR and BMI, amongst EDEN women only, the linear CR term was associated with both the BMI-GRS and BMI. In this group only, the linear CR term appeared to mediate the association between the BMI-GRS and BMI (mediation ratio=19%; Sobel test P-value=0.0009). However, we do not consider CR to be a true mediator between the BMI-GRS and BMI (see discussion). Analysis of pre-pregnancy BMI in EDEN women, instead of 2-y post-partum BMI, did not substantially alter the mediation analysis results (Supplemental Table 4).
Modification by eating behaviors
We found a significant interaction between CR and the BMI-GRS on BMI amongst men in both cohorts (EDEN: P-interaction=0.0001; Fenland: P-interaction=0.04) and amongst women in Fenland (P=0.0004) but not amongst women in EDEN (P-interaction=0.14). Neither UE nor EE showed evidence of interaction with the BMI-GRS.
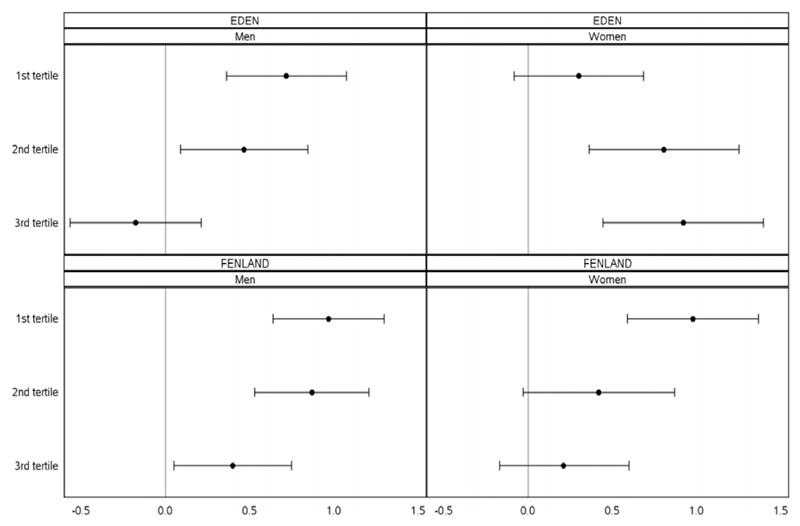
For all groups showing evidence of interaction, when participants were grouped into tertiles by CR score, the association between the BMI-GRS and BMI was strongest in the lowest tertile of CR and weakest in the highest tertile (Figure 1).
Figure 1.
Effect modification of the BMI-GRS to BMI association by cognitive restraint. The first tertile refers to the lowest tertile of cognitive restraint and the third tertile refers to the highest.
Discussion
In two large population-based cohort studies, we show that the association between a polygenic genetic risk score for BMI (BMI-GRS) and BMI was both mediated and modified by eating behavior. Specifically, the appetitive traits (UE and EE) partially mediated genetic susceptibility obesity and, with the exception of EDEN women, CR modified this genetic predisposition. Our findings are based on 3515 predominantly British adults aged 35-64 years in the Fenland study and 2154 French adults aged 18-56 years in the EDEN study.
In both our cohorts, UE and EE demonstrated positive linear associations with BMI. Whilst both analyses were cross-sectional, these findings are consistent with non-genetic reports that EE (32, 33) and UE-related behaviors, including disinhibited eating (34), are positively associated with prospective changes in BMI. Our mediation results for UE and EE corroborate a growing body of evidence in support of the Behavioral Susceptibility Theory of obesity (BST) (9). Previous evidence in support of BST includes the finding that obesity-related genetic variants are expressed in areas of the brain involved in the regulation of food intake (2) and reported relationships between single BMI-related variants and appetitive aspects eating behavior (11, 12, 15, 16). Explicit testing for mediation by appetitive traits has only previously been reported in one study in adults and two studies in children. Our analyses suggest the mediating effects of UE and EE act through a common mechanism, likely related to food responsiveness.
Konttinen et al tested for mediation of the association between a 90-locus BMI-GRS and BMI by UE and EE in two Finnish cohorts: adults aged 25-74 years in the DILGOM study (19.6% of men and 22.9% of women were obese) and twins aged 21-26 years in the FinnTwin12 study (5.7% of men and 4.7% of women were obese) (20). In both cohorts they measured eating behavior using the TFEQ-R18. They reported partial mediation by EE in both cohorts and by UE in DILGOM, but did not test for mediation by CR. Corroborating these findings, we report mediation by EE and UE in both our cohorts. The lack of mediation by UE in FinnTwin12 may be the result of low levels of obesity or the young age of participants relative to the 3 other cohorts. Consistent with our results, Konttinen et al also reported sex differences in UE and EE, with women scoring more highly, but no interaction between the BMI-GRS and sex on BMI (20).
Eating behavior traits in children are not directly comparable to those in adults. However, 2 studies directly testing mediation by appetitive traits in children have been conducted. Mediation of a 28-locus BMI-GRS to BMI association by satiety responsiveness (SR) was demonstrated amongst 2258 children aged 8 to 11 years in one study (18). Only the Adult Eating Behavior Questionnaire (developed in 2016) measures SR in adults (35). As such, this finding is not directly comparable to the results of our study. However, SR measures feelings of hunger and fullness and may be considered broadly consistent with items on the UE scale of the TFEQ. A second study in 662 children aged 6-8y did not detect mediation of a 32-locus BMI-GRS to weight gain association by eating behavior, measured by the Children’s Eating Behavior Questionnaire (19, 36). It is possible either that these relationships are not evident in this age group or that the sample size was not sufficient to detect an effect.
Our results suggest that the relationships between CR, the BMI-GRS and BMI are distinct from those of UE and EE. The association between CR and BMI appeared to be quadratic in all groups. Amongst underweight or normal weight participants (<25kg/m2), there was a positive linear association, whilst amongst overweight and obese adults (BMI ≥25kg/m2), the association was negative in Fenland women and non-significant in all other groups.
We do not conclude that CR is a positive mediator of the genetic susceptibility to higher BMI, as indicated by EDEN women. Indeed, longitudinal evidence suggests that CR is more likely to be a consequence than a cause of elevated BMI (39, 40). We speculate that CR might represent a response to increasing BMI amongst normal weight individuals. This is supported by evidence from other studies suggesting that CR is often motivated by a desire to prevent weight gain, rather than to instigate weight loss (37). Conversely, the abandonment of restraint might contribute to overweight and obese, explaining the quadratic association in our study. Longitudinal data with repeated assessments of eating behavior and BMI are needed to better understand this relationship.
In support of a limiting effect of CR on BMI, we show for the first time that CR modifies the association between the BMI-GRS and BMI. In all groups showing an interaction between the BMI-GRS and CR on BMI (men in both cohorts and women in Fenland), the effect of the BMI-GRS on BMI was strongest amongst those with the lowest levels of CR and was weakest amongst those with the highest levels of CR. This novel finding suggests that CR could help to protect genetically susceptible individuals from excessive weight gain. In a previous study, higher scores for restraint over eating were found in non-obese adults who reported a history of obesity during childhood or adolescence compared to non-obese adults who did not report a history of obesity (41). This speculatively suggests that CR might be effectively controlling the weight of these individuals in adulthood, despite a propensity to weight gain and that dietary restraint might be beneficial to weight control under specific circumstances.
The main strengths of the present study include the corroboration of findings across two large, well phenotyped, population-based cohorts each containing validated measures of eating behavior. The main limitation of our study is the cross-sectional design of the analyses. Further research is needed to confirm the directions of the relationships between eating behavior traits and BMI, especially for CR. Further, whilst the results in the two cohorts are broadly consistent, no modifying effect of CR was identified amongst women in the EDEN cohort. Eating behaviors and BMI were assessed in EDEN women at 2 years post-partum, a time when cognitive restraint and weight are plausibly still altered by pregnancy. The results were similar when using self-reported pre-pregnant BMI and it remains uncertain why CR might have a different relationship with BMI in this group.
In summary, our findings suggest that genetic susceptibility to obesity is partially mediated by appetitive traits and is modified by CR. Intervention studies that aim to modify eating behavior should test whether increasing CR might help to mitigate genetic susceptibility to obesity.
Supplementary Material
Acknowledgements
The EDEN study is supported by: Fondation pour la Recherche Médicale (FRM), French Ministry of Research: Federative Research Institutes and Cohort Program, INSERM Human Nutrition National Research Program, and Diabetes National Research Program (through a collaboration with the French Association of Diabetic Patients (AFD)), French Ministry of Health, French Agency for Environment Security (AFSSET), French National Institute for Population Health Surveillance (InVS), Paris–Sud University, French National Institute for Health Education (INPES), Nestlé, Mutuelle Générale de l’Education Nationale (MGEN), French speaking association for the study of diabetes and metabolism (ALFEDIAM), National Agency for Research (ANR non thematic program), National Institute for Research in Public health (IRESP: TGIR cohorte santé 2008 program).
The Fenland Study is supported by the Medical Research Council (MC_U106179471). This work was supported by the Medical Research Council [Unit Programme numbers MC_UU_12015/2 and MC_UU_12015/1]. Genotyping was supported by the Medical Research Council (MC_PC_13046). We are grateful to all the volunteers for their time and help and to the General Practitioners and practice staff for assistance with recruitment. We thank the Fenland Study Investigators, Fenland Study Co-ordination team and the Epidemiology Field, Anthropometry, Data and Laboratory teams. Biochemical assays were performed by the National Institute for Health Research, Cambridge Biomedical Research Centre, Core Biochemistry Assay Laboratory, and the Cambridge University Hospitals NHS Foundation Trust, Department of Clinical Biochemistry.
BLG and EADC contributed equally to this work. BH and KOO equally supervised this work. BLG, MAC, BH and KOO designed the research. EADC, BLG, BH and KOO wrote the manuscript. BLG, EADC, YAK and FRD analysed the data. SB, NGF, SJG, NJW oversaw the Fenland study. BH and MAC oversaw the EDEN study. SB, NGF, SJG, NJW were responsible for data collection in Fenland. BH, KC, YP and MAC were responsible for data collection in EDEN. All authors reviewed drafts, provided critical feedback, approved the final manuscript and were responsible for the final content of the paper.
Sources of support: The Fenland Study is supported by the Medical Research Council (MC_U106179471) (grant holder: NJW). This work was supported by the Medical Research Council [Unit Programme numbers: MC_UU_12015/2 (grant holder: NJW) MC_UU_12015/1 (grant holder KOO)]. Genotyping was supported by the Medical Research Council (MC_PC_13046). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The EDEN study authors received no specific funding for this work
Abbreviations
- BST
Behavioral susceptibility theory
- BMI
Body mass index
- BMI-GRS
Genetic risk score for body mass index
- CR
Cognitive restraint
- EE
Emotional eating
- GRS
Genetic risk score
- UE
Uncontrolled eating
Footnotes
The authors report no conflict of interest
Contributor Information
Blandine de Lauzon-Guillain, INSERM, UMR1153 Epidemiology and Biostatistics Sorbonne Paris Cité Center (CRESS), ORCHAD Team, Paris, F-75014 France; Paris Descartes University France.
Karine Clément, Institute of Cardiometabolism and Nutrition, ICAN, F-75013, Paris, France; INSERM, UMRS 1166, Nutriomic team 6, Paris, F-75013 France; Sorbonne Universités, UPMC Université Paris 06, UMRS1166.
Simon J Griffin, MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Institute of Metabolic Science, Cambridge, UK; Department of Public Health and Primary Care, Institute of Public Health, University of Cambridge, United Kingdom.
Yves Akoli Koudou, INSERM, UMR1153 Epidemiology and Biostatistics Sorbonne Paris Cité Center (CRESS), ORCHAD Team, Paris, F-75014 France.
Véronique Pelloux, Institute of Cardiometabolism and Nutrition, ICAN, F-75013, Paris, France; INSERM, UMRS 1166, Nutriomic team 6, Paris, F-75013 France; Sorbonne Universités, UPMC Université Paris 06, UMRS1166.
Nicholas J Wareham, MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Institute of Metabolic Science, Cambridge, UK.
Ken K Ong, MRC Epidemiology Unit, University of Cambridge School of Clinical Medicine, Institute of Metabolic Science, Cambridge, UK.
References
- 1.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne) 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Kaprio J, Silventoinen K, Perola M. The Three-Factor Eating Questionnaire, body mass index, and responses to sweet and salty fatty foods: a twin study of genetic and environmental associations. Am J Clin Nutr. 2008;88:263–71. doi: 10.1093/ajcn/88.2.263. [DOI] [PubMed] [Google Scholar]
- 4.Llewellyn CH, van Jaarsveld CH, Plomin R, Fisher A, Wardle J. Inherited behavioral susceptibility to adiposity in infancy: a multivariate genetic analysis of appetite and weight in the Gemini birth cohort. Am J Clin Nutr. 2012;95:633–9. doi: 10.3945/ajcn.111.023671. [DOI] [PubMed] [Google Scholar]
- 5.Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. Am J Clin Nutr. 2005;81:564–9. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH, Wardle J. Continuity and stability of eating behaviour traits in children. Eur J Clin Nutr. 2008;62:985–90. doi: 10.1038/sj.ejcn.1602855. [DOI] [PubMed] [Google Scholar]
- 7.Craigie AM, Lake AA, Kelly SA, Adamson AJ, Mathers JC. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas. 2011;70:266–84. doi: 10.1016/j.maturitas.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Carnell S, Benson L, Wardle J. Eating behaviours in obesity. In: Akabas S, Lederman SA, Moore BJ, editors. Textbook of obesity: Biological, psychological and cultural influences. Oxford: John Wiley & Sons Ltd; 2012. [Google Scholar]
- 9.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88:22–9. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 10.Vega JA, Salazar G, Hodgson MI, Cataldo LR, Valladares M, Obregon AM, Santos JL. Melanocortin-4 Receptor Gene Variation Is Associated with Eating Behavior in Chilean Adults. Ann Nutr Metab. 2016;68:35–41. doi: 10.1159/000439092. [DOI] [PubMed] [Google Scholar]
- 11.Horstmann A, Kovacs P, Kabisch S, Boettcher Y, Schloegl H, Tonjes A, Stumvoll M, Pleger B, Villringer A. Common genetic variation near MC4R has a sex-specific impact on human brain structure and eating behavior. PLoS One. 2013;8:e74362. doi: 10.1371/journal.pone.0074362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 13.Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Bruning JC, Nolan PM, Ashcroft FM, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–92. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micali N, Field AE, Treasure JL, Evans DM. Are obesity risk genes associated with binge eating in adolescence? Obesity (Silver Spring) 2015;23:1729–36. doi: 10.1002/oby.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouchard L, Drapeau V, Provencher V, Lemieux S, Chagnon Y, Rice T, Rao DC, Vohl MC, Tremblay A, Bouchard C, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. 2004;80:1478–86. doi: 10.1093/ajcn/80.6.1478. [DOI] [PubMed] [Google Scholar]
- 16.Carr KA, Lin H, Fletcher KD, Sucheston L, Singh PK, Salis RJ, Erbe RW, Faith MS, Allison DB, Stice E, et al. Two functional serotonin polymorphisms moderate the effect of food reinforcement on BMI. Behav Neurosci. 2013;127:387–99. doi: 10.1037/a0032026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valette M, Bellisle F, Carette C, Poitou C, Dubern B, Paradis G, Hercberg S, Muzard L, Clement K, Czernichow S. Eating behaviour in obese patients with melanocortin-4 receptor mutations: a literature review. Int J Obes (Lond) 2013;37:1027–35. doi: 10.1038/ijo.2012.169. [DOI] [PubMed] [Google Scholar]
- 18.Llewellyn CH, Trzaskowski M, van Jaarsveld CH, Plomin R, Wardle J. Satiety mechanisms in genetic risk of obesity. JAMA Pediatr. 2014;168:338–44. doi: 10.1001/jamapediatrics.2013.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinsbekk S, Belsky D, Guzey IC, Wardle J, Wichstrom L. Polygenic Risk, Appetite Traits, and Weight Gain in Middle Childhood: A Longitudinal Study. JAMA Pediatr. 2016;170:e154472. doi: 10.1001/jamapediatrics.2015.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konttinen H, Llewellyn C, Wardle J, Silventoinen K, Joensuu A, Mannisto S, Salomaa V, Jousilahti P, Kaprio J, Perola M, et al. Appetitive traits as behavioural pathways in genetic susceptibility to obesity: a population-based cross-sectional study. Sci Rep. 2015;5 doi: 10.1038/srep14726. 14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornelis MC, Rimm EB, Curhan GC, Kraft P, Hunter DJ, Hu FB, van Dam RM. Obesity susceptibility loci and uncontrolled eating, emotional eating and cognitive restraint behaviors in men and women. Obesity (Silver Spring) 2014;22:E135–41. doi: 10.1002/oby.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heude B, Forhan A, Slama R, Douhaud L, Bedel S, Saurel-Cubizolles MJ, Hankard R, Thiebaugeorges O, De Agostini M, Annesi-Maesano I, et al. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol. 2016;45:353–63. doi: 10.1093/ije/dyv151. [DOI] [PubMed] [Google Scholar]
- 23.de Lucia Rolfe E, Loos RJ, Druet C, Stolk RP, Ekelund U, Griffin SJ, Forouhi NG, Wareham NJ, Ong KK. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–52. doi: 10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Zhao JH, Luan J, Luben RN, Rodwell SA, Khaw KT, Ong KK, Wareham NJ, Loos RJ. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. Am J Clin Nutr. 2010;91:184–90. doi: 10.3945/ajcn.2009.28403. [DOI] [PubMed] [Google Scholar]
- 25.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Magi R, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlsson J, Persson LO, Sjostrom L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 2000;24:1715–25. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 28.Anglé S, Engblom J, Eriksson T, Kautiainen S, Saha MT, Lindfors P, Lehtinen M, Rimpela A. Three factor eating questionnaire-R18 as a measure of cognitive restraint, uncontrolled eating and emotional eating in a sample of young Finnish females. Int J Behav Nutr Phys Act. 2009;6:41. doi: 10.1186/1479-5868-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys J-M, Karlsson J, Ducimetière P, Charles MA. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. The Journal of nutrition. 2004;134:2372–2380. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon DP, Warsi G, Dwyer JH. A Simulation Study of Mediated Effect Measures. Multivariate Behav Res. 1995;30:41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobel ME. Asymptotic intervals for indirect effect in structural equations models. In: Leinhart S, editor. Sociological methodology. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- 32.Dohle S, Hartmann C, Keller C. Physical activity as a moderator of the association between emotional eating and BMI: evidence from the Swiss Food Panel. Psychol Health. 2014;29:1062–80. doi: 10.1080/08870446.2014.909042. [DOI] [PubMed] [Google Scholar]
- 33.Koenders PG, van Strien T. Emotional eating, rather than lifestyle behavior, drives weight gain in a prospective study in 1562 employees. J Occup Environ Med. 2011;53:1287–93. doi: 10.1097/JOM.0b013e31823078a2. [DOI] [PubMed] [Google Scholar]
- 34.Chaput JP, Leblanc C, Perusse L, Despres JP, Bouchard C, Tremblay A. Risk factors for adult overweight and obesity in the Quebec Family Study: have we been barking up the wrong tree? Obesity (Silver Spring) 2009;17:1964–70. doi: 10.1038/oby.2009.116. [DOI] [PubMed] [Google Scholar]
- 35.Hunot C, Fildes A, Croker H, Llewellyn CH, Wardle J, Beeken RJ. Appetitive traits and relationships with BMI in adults: Development of the Adult Eating Behaviour Questionnaire. Appetite. 2016;105:356–63. doi: 10.1016/j.appet.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–70. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 37.Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res. 2005;13:797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- 38.Lowe MR. Dieting: proxy or cause of future weight gain? Obes Rev. 2015;16(Suppl 1):19–24. doi: 10.1111/obr.12252. [DOI] [PubMed] [Google Scholar]
- 39.Chavance M, Escolano S, Romon M, Basdevant A, de Lauzon-Guillain B, Charles MA. Latent variables and structural equation models for longitudinal relationships: an illustration in nutritional epidemiology. BMC Med Res Methodol. 2010;10:37. doi: 10.1186/1471-2288-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snoek HM, van Strien T, Janssens JM, Engels RC. Restrained eating and BMI: a longitudinal study among adolescents. Health Psychol. 2008;27:753–9. doi: 10.1037/0278-6133.27.6.753. [DOI] [PubMed] [Google Scholar]
- 41.Bellisle F, Clement K, Le Barzic M, Le Gall A, Guy-Grand B, Basdevant A. The Eating Inventory and body adiposity from leanness to massive obesity: a study of 2509 adults. Obes Res. 2004;12:2023–30. doi: 10.1038/oby.2004.253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.