Abstract
Objective
To evaluate the association between a clustering of cardio-metabolic risk factors in parents and the development of microalbuminuria (MA) in their offspring with childhood-onset type 1 diabetes (T1D).
Methods
The study population comprised 53 parents (mean age (±SD): 56.7±6.2 years) of 35 T1D young people with MA (MA+) and 86 parents (age: 56.1±6.3 years) of 50 matched offspring with normoalbuminuria (MA–), who underwent clinical, biochemical and cardiovascular imaging assessments. The primary study endpoint was the difference between parents from the MA+ and MA- groups in a cardio-metabolic risk score, calculated as the average value of the standardized measures (z-scores) for waist circumference, blood pressure, fasting glucose, insulin, HDL-cholesterol and triglycerides levels. Cardiovascular parameters, including carotid intima-media thickness (cIMT), flow-mediated dilatation (FMD) and pulse wave velocity (PWV), were also assessed. A DXA scan was performed to assess body composition.
Results
The cardio-metabolic risk score was significantly higher in parents of MA+ compared to parents of MA- offspring (mean [95% CI]: 1.066[0.076; 2.056] vs -0.268[-0.997; 0.460], p=0.03). Parents of MA+ offspring had slightly higher values of waist circumference, lipids, insulin and blood pressure, although only diastolic blood pressure was statistically different between the two groups (p=0.008). FMD, cIMT, PWV (all p>0.3) and DXA parameters (all p>0.2) were not significantly different between the two groups.
Conclusions
Parents of young offspring with childhood-onset T1D and MA showed an abnormal metabolic profile, reflected by a calculated risk score. The finding supports the role of a familial predisposition to risk of developing diabetic nephropathy.
Keywords: microalbuminuria, cardiometabolic, parents, type 1 diabetes
Introduction
Diabetic nephropathy (DN) and cardiovascular disease (CVD) are among the most common vascular complications of type 1 diabetes (T1D), which adversely influence the long-term prognosis of people with this disease [1–3]. Several studies have shown that premature atherosclerosis represents the main cause of morbidity and mortality in patients with T1D [1,4]. Although hard end-points, such as stroke and ischemic heart disease, are uncommon in children and adolescents with T1D, early subclinical structural and functional vascular abnormalities are often found in this age group [5,6]. Risk for CVD in patients with T1D is strongly associated with the presence of renal disease, as supported by the higher CVD morbidity and mortality in people with increased albumin excretion rates [7,8].
In addition, cardio-metabolic risk factors, such as increased blood pressure and lipid levels, visceral adiposity, insulin resistance, impaired glucose tolerance, and their combination, known as metabolic syndrome, are strong predictors for CVD risk in the general population and in people with T1D [9–11]. Based on these data, the investigation of specific cardio-metabolic risk factors and their clustering within families has become an appealing research area over the last years. A recent study on a large population of 12-year old children and their parents showed that a family history of CVD had a key impact on the cardio-metabolic profile of the offspring, thus highlighting the importance of identifying CVD risk factors within families for preventive strategies [9]. In adults with T1D a family history of hypertension or insulin resistance/type 2 diabetes has been associated with risk of DN in the offspring [12–15]. Some studies have also highlighted that a clustering of familial cardio-metabolic risk factors could have a stronger effect than individual factors on risk of DN [14].
Up to now, the association between familial cardio-metabolic risk factors and/or their clustering and risk of early stages of DN, such as microalbuminuria (MA), has not been fully assessed in families of young people with childhood-onset T1D. Previously, our group was able to replicate some of the findings reported in adults with T1D in young cohorts of children and adolescents with T1D, where parental blood pressure emerged to have an independent effect on MA risk in the offspring [16,17]. A similar association between parental history of hypertension and MA in the offspring was reported in other two previous studies [18,19]. In contrast, we were not able to show any effect of parental lipid levels on the risk of MA in the offspring [20].
The aim of this study was to investigate the association between an aggregation of cardio-metabolic risk factors in parents of young people with T1D and the occurrence of MA during adolescence in the offspring.
Methods
Study design
This was a nested case-control study within the Nephropathy Family Study (NFS) and Oxford Regional Prospective Study (ORPS) cohorts [21,22], where parents of probands with childhood-onset T1D were assessed. Cases were parents of T1D probands who had developed MA during childhood and adolescence, whereas controls were parents of probands who had not developed MA after a long follow-up. Once potential probands with MA were identified, two control probands without MA were selected within the same ORPS/NFS cohorts and were matched for age, sex and duration of diabetes with the probands with MA. Parents of both groups were approached by sending invitation letters together with information leaflets about the study.
After the parents agreed to take part in the study and after they signed the informed consent they underwent a clinical, biochemical and cardiovascular imaging assessment, which were performed during two study visits. The study participants were admitted to the Wellcome Trust Clinical Research Facility (WTCRF) at Addenbrooke’s Hospital in Cambridge (UK) or at the Oxford Centre for Diabetes, Endocrinology and Metabolism (OCDEM) in Oxford (UK), for the first visit. The second visit, including vascular imaging assessments, was performed at the Vascular Physiology Unit, Great Ormond Street Hospital, in London (UK).
The study protocol was approved by the Ethics Committee in Cambridge, UK. The study was carried out in conformation with the spirit and the letter of the Declaration of Helsinki and in accordance with the guidance in Good Clinical Practice. Written signed informed consent was obtained from the study participants before the beginning of the study and any study assessment.
Study visits
Visit 1. The first outpatient visit included:
1. Collection of anthropometric parameters: Height was measured with a wall mounted electrical device; weight was taken with an electronic scale and BMI was calculated as weight/height squared (kg/m2). Waist circumference was measured midway between the rib cage and the superior border of the iliac crest while the subject was standing. Waist circumference was measured 3 times and the average of measures was calculated. Anthropometric assessments were performed by trained personnel, following the WHO recommendations.
2. Assessment of blood pressure: Blood pressure was taken in a supine position on both the right and left arms using an Omron M6 blood pressure machine (Omron healthcare, Netherlands) after ten minutes rest. Three readings were taken each 2 minutes apart and the mean value used in the analysis. Adult medium and large size cuffs were used depending on the size of the arm.
3. Collection of fasting blood samples for measuring glucose, insulin, lipids, HbA1c, CVD markers (high-sensitivity C-reactive protein (hs-CRP) and asymmetric dimethylarginine (ADMA)).
4. Collection of urine samples: All participants were asked to provide three consecutive early morning first-voided urine samples for the assessment of the albumin-creatinine ratio (ACR).
5. Questionnaires data: All parents were asked to fill in a questionnaire assessing history of smoking, high cholesterol and cholesterol lowering treatment, hypertension, antihypertensive treatment, cardiovascular events (coronary disease, myocardial infarction, and stroke), presence of diabetes.
6. Dual-energy x-ray absorptiometry (DXA): DXA scans were performed to assess body composition.
Visit 2:
The second study visit included cardiovascular imaging studies assessing carotid intima-media thickness (cIMT), endothelial function by flow-mediated dilatation (FMD) and arterial stiffness by pulse wave velocity (PWV).
All vascular scans were performed at the same vascular centre in London by the same sonographers, who underwent an accreditation and reproducibility training programme before scanning participants, and a single reader analyzed all the scans (HN).
cIMT: Ultrasound scanning for cIMT was performed with a Vivid 7 (GE Healthcare) using a L7, 5-10 MHz linear array broadband transducer probe. A standardized protocol was used to acquire longitudinal images of the common carotid artery on both the right and left sides. The neck was positioned at 45° to the horizontal and the probe held lateral to the neck. Loops of images were recorded in DICOM (Digital Imaging and Communications in Medicine) format for 10 heart cycles triggered on the R wave of the ECG. The bifurcation of the artery was positioned on the left side of the screen and a consistent zoom used so 2 cm of the vessel was viewed and recorded. Measurements were taken both at the right and left sides, and the mean between the two measurements was calculated and used for the analysis. All image analyses were carried out off-line using vascular research tools Carotid Analyser (Medical Imaging Applications, Iowa, USA). The cIMT value was measured 10mm posterior to the bifurcation in a 5mm section of the artery as a mean of 3 end diastolic cycles.
FMD: Prior to the FMD scan all participants were asked to avoid caffeine, vitamin C, strenuous exercise and tobacco for 6 hours; however, regular medications were continued. Measurements were taken in a quiet and temperature controlled room (20-24°C) and a skin temperature ≥30°C was required for the test to be carried out. The participants were rested for 10 minutes before examination. The right arm was placed in an arm rest fitted with a clamp to hold the ultrasound probe in position for the entire experiment. An automated blood pressure cuff was placed around the right arm, 1 cm below the antecubital fossa. B-mode ultrasound scans were obtained of the right brachial artery using an Aloka 5500 (Japan) equipped with a 7.5 MHz linear array transducer. The test was recorded on VHS and a PC using Imager programme in DICOM format. Ultrasound measurements were taken for 1 minute then the cuff was inflated to a pressure of 300mmHg for 5 minutes to restrict blood flow to the hand. After 5 minutes of blood flow ischemia the cuff was deflated and measurements taken for a further 5 minutes.
Changes in diameter in the brachial artery were measured off-line. Arterial diameter analysis was performed with an automated edge detection system, Brachial Tools (Iowa, USA). The software uses an automatic trace of the vessel lumen/wall interface in a 5 mm section of artery with clearly defined vessel walls throughout the examination. FMD was expressed as both the absolute difference between maximal and resting vessel diameters and as a percentage change of resting diameter. The velocity-time integral (VTI) of the pulse wave Doppler signal was used to calculate baseline blood flow and reactive hyperemia flow. FMD corrected for shear stress was obtained by dividing FMD by the hyperemic VTI [23].
PWV: Applanation tonometry SphygmoCor (AtCor Medical, Australia) was used to assess arterial stiffness. Participants were rested in a supine position for at least 15 minutes before PWV was measured. The tonometer was placed on the pulse points of left carotid and right femoral arteries in turn. The pulse pressure waveforms were recorded for 10 seconds and PWV was calculated in relation to ECG trace. Distances were measured between pulse points via the suprasternal notch to allow the accurate calculation of PWV by the SphygmoCor software.
Laboratory methods
Blood glucose was measured using an adaption of the hexokinase-glucose-6-phosphate dehydrogenase method on a Siemens Dimension analysers (RxL and EXL).
Plasma insulin concentrations were measured using a DAKO ELISA (DAKO Ltd., Denmark House, Angel Drove, Ely, Cambs, UK) according to the manufacturer’s instructions.
Lipids: measurements of total cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides were performed enzymatically on a Dimension RXL system (Dade Behring) using reagents and calibrants supplied by the manufacturer.
hsCRP: was measured in plasma samples by laser immunonephelometry (Dade Behring).
ADMA: was measured by stable isotope dilution electrospray tandem mass spectrometry, as previously described [24].
HbA1c: samples were centrally on a TOSOH G7 analyzer, using high-performance liquid chromatography and absorbance change detection and Diabetes Control and Complications Trial (DCCT) aligned methods.
Urinary albumin and creatinine: were measured centrally as previously described [25].
DXA
Data on body composition were gathered with a Lunar Prodigy machine using a constant pixel size of 1.2 x 1.2 cm and Lunar software programs (version 8.1, Lunar Corporation, Madison, WI). The effective radiation dose was 0.1 μSievert. The scan yielded measures of whole-body fat and fat-free mass (FFM) and therefore percent whole-body fat mass. All DXA scans were performed in compliance with local IR(ME)R Employer's Procedures.
Calculations
Cardio-metabolic risk score: A continuous clustered cardio-metabolic risk score (z-score) was computed. This score represents a summary variable for components of metabolic syndrome, which gives a standardized estimate and has been used in several studies [26–28]. The cardio-metabolic risk score was calculated based on previous studies and included the average value of age and sex-standardized measures (z-scores) for six outcomes: measures of central obesity (waist circumference), dyslipidaemia (triglycerides and HDL-cholesterol), hypertension (systolic and diastolic blood pressure) and glucose metabolism (fasting glucose and insulin). Each variable was standardized by calculating sex-specific z-scores (z-score= (individual value-population mean)/population SD). Subsequently, individual z scores were summed, after inverting HDL-cholesterol z scores and averaging systolic and diastolic blood pressure z scores. Adjustments were made to take into account presence of diabetes in the parents as well as active treatment for high blood pressure or dyslipidaemia, by adding a correcting factor, arbitrary chosen as 0.5 points when any of these factors was present.
Study endpoints
The primary study endpoint was defined as the difference in the continuous cardio-metabolic risk score between parents of MA positive and MA negative offspring.
The secondary endpoints were defined as differences between the two study groups in 1) the individual factors of the cardio-metabolic risk score: waist circumference, blood pressure, fasting lipids, fasting glucose, fasting insulin; 2) cIMT, FMD and PWV; 3) cardiovascular markers (hsCRP, ADMA, ACR); 4) body composition parameters obtained from the DXA scans (total lean and fat mass, regional fat mass distribution: gynoid, android, trunk and limbs fat mass).
Power calculation for the primary endpoint: Assuming a two sample t-test is performed at the 5% significance level (2-sided), 40 cases (parents of MA + offspring) and 80 controls (parents of MA- offspring) provides 80% power to detect a 0.55 unit difference in primary outcome metabolic z-score (assuming mean =0 and SD=1) between the groups.
Statistical methods
The cardio-metabolic risk score and the individual cardio-metabolic parameters and body composition measurements between parents of MA positive and MA negative offspring were compared using a two sample unpaired t-test (two tailed). In case of not normally distributed variables, a log base 10 transformation was applied before testing. Analysis of covariance was used when adjustments for potential confounding factors where required.
P values <0.05 were taken as statistically significant. Data are expressed as mean ± SD or median (interquartile range), unless otherwise stated. Statistical analysis was performed with SPSS version 23 software for Windows (SPSS, Chicago, IL).
Results
In total 139 parents of 85 T1D offspring (35 offspring with MA (MA positive) and 50 T1D offspring without MA (MA negative)) agreed to take part in the study and attended the two study visits. The 139 parents (63 fathers and 76 mothers) included 53 parents of MA positive and 86 parents of MA negative offspring. For 54 offspring both parents agreed to take part in the study, whereas for the remaining 31 only one parent agreed to take part in the study (22 mothers and 9 fathers) (Table 1). The general characteristics of the offspring are reported in Table 2.
Table 1. Numbers and characteristics of recruited parents within the ORPS/NFS cohorts.
| Total | MA+ group | MA- group | |
|---|---|---|---|
| Probands | 85 | 35 | 50 |
| Total parents | 139 | 53 | 86 |
| Fathers | 63 | 21 | 42 |
| Mothers | 76 | 32 | 44 |
| Both parents recruited | 54 | 18 | 36 |
| Only mothers | 22 | 14 | 8 |
| Only fathers | 9 | 3 | 6 |
Table 2. Characteristics of offspring with microalbuminuria (MA+ group) and normoalbuminuria (MA- group).
| MA+ group | MA- group | P value | |
|---|---|---|---|
| N | 35 | 50 | |
| Sex (Males) | 8 (23%) | 16 (32%) | 0.5 |
| Age at T1D diagnosis (years) | 9.3 ± 3.2 | 8.8 ± 3.3 | 0.5 |
| Age at MA onset (years) | 17.3 ± 4.5 | ||
| T1D duration at MA onset (years) | 8.1 ± 4.6 | ||
| Age at last visit (years) | 19.1 ± 3.0 | 20.1 ± 3.5 | 0.2 |
| Mean HbA1c (%) | 9.9 ± 1.6 | 9.0 ± 1.2 | 0.006 |
| BMI (Kg/m2) | 23.5 ± 3.8 | 22.4 ± 3.2 | 0.19 |
| Systolic blood pressure (mmHg) | 117.3 ± 9.7 | 113.2 ±10.7 | 0.08 |
| Diastolic blood pressure (mmHg) | 72.8 ± 6.2 | 66.8 ± 7.2 | <0.001 |
| logACR | 0.495 ± 0.298 | -0.735 ± 0.157 | <0.001 |
Data are mean ± SD. ACR: albumin:creatinine ratio; MA: microalbuminuria; T1D: type 1 diabetes BMI, Blood pressure and logACR are mean values during the whole follow up period
Cardio-metabolic risk score in the parents: MA positive vs MA negative group
Parents of MA positive offspring were similar in age, height, and BMI compared with parents of MA negative offspring (Table 3).
Table 3. Clinical and biochemical characteristics of the parents of MA positive vs MA negative offspring.
| MA+group | MA-group | P value | |
|---|---|---|---|
| N | 53 | 86 | |
| Sex (fathers) | 21 (39.6%) | 42 (48.8%) | 0.3 |
| Age (years) | 56.7 ± 6.2 | 56.1 ± 6.3 | 0.5 |
| Height (cm) | 167.8 ± 8.7 | 170.7 ± 9.0 | 0.06 |
| Weight (Kg) | 79.8 ± 16.5 | 80.3 ± 17.4 | 0.9 |
| BMI (Kg/m2) | 28.3 ± 5.3 | 27.4 ± 4.7 | 0.3 |
| Waist circumference (cm) | 95.0 ± 14.0 | 93.0 ± 14.1 | 0.08 |
| Systolic blood pressure (mmHg) | 126.2 ± 13.1 | 124.3 ± 13.2 | 0.3 |
| Diastolic blood pressure(mmHg) | 76.5 ± 8.2 | 72.8 ± 7.5 | 0.005 |
| Glucose (mmol/l) | 5.2 ± 1.7 | 5.1 ± 0.9 | 0.7 |
| Insulin (pmol/l) | 37.0 [20.0-57.7] | 34.0 [22.0-51.0] | 0.6 |
| HDL cholesterol (mmol/l) | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.2 |
| Triglycerides (mmol/l) | 0.8 [0.8-1.6] | 0.9 [0.7-1.4] | 0.2 |
| ADMA (nmol/L) | 465.5 ± 65.1 | 456.2 ± 58.2 | 0.4 |
| hsCRP (mg/L) | 0.96 [0.41-1.73] | 1.01 [0.52-1.70] | 0.9 |
| ACR (mg/mol) | 0.56 [0.44-0.75] | 0.58 [0.45-0.88] | 0.9 |
| Hypertension treatment, n (%) | 12 (22.6) | 9 (10.5) | 0.08 |
| Dyslipidemia treatment, n (%) | 10 (18.9) | 10 (11.6) | 0.3 |
| Diabetes, n (%) | 2 (3.8) | 7 (8.1) | 0.5 |
Data are mean±SD for normal distributed variable or median [Interquartile range] for non-normal distributed variables. P values are adjusted for parental age and sex. ACR: albumin creatinine ratio, ADMA: asymmetric dimethylarginine, hsCRP: high sensitivity C-reactive protein, MA: microalbuminuria
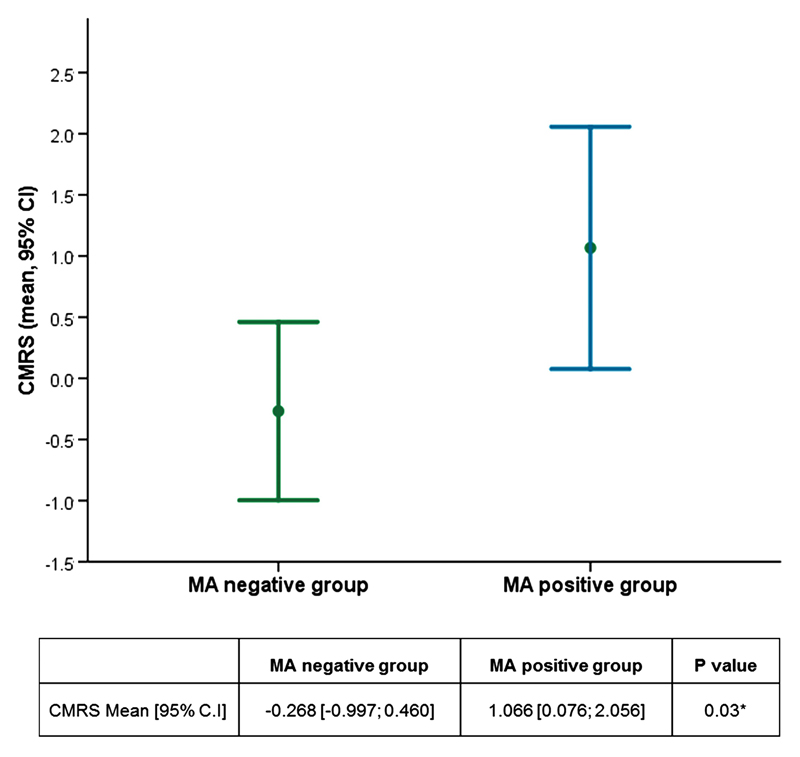
A significant difference was found in the cardio-metabolic risk score between parents of MA positive offspring and parents of MA negative offspring, with higher values in the MA positive group: mean [95% CI]: 1.066 [0.076; 2.056] vs -0.268 [-0.997; 0.460], p= 0.03 (Figure 1).
Figure 1. Cardio-metabolic risk score (CMRS) in parents of MA positive and MA negative offspring.
Parents of MA positive offspring tended to have higher values of the individual components of the cardio-metabolic score, such as waist circumference, blood pressure and insulin, although only the difference in diastolic blood pressure reached statistical significance (Table 3).
Cardiovascular imaging in parents of MA positive vs MA negative offspring
There were no statistically significant differences in the circulating cardiovascular markers ADMA and hsCRP between parents of MA positive and MA negative offspring. Similarly, urinary ACR was similar between the two groups (Table 3).
No significant differences were found in the direct cardiovascular measurements including cIMT, FMD and PWV, before and after adjusting for potential confounders such as age and sex (Table 4).
Table 4. Cardiovascular assessments and body composition (DXA) in parents of microalbuminuria (MA) positive vs MA negative offspring.
| MA+ Group (n 53) | MA- Group (n 86) | P value | |
|---|---|---|---|
| Cardiovascular measures | |||
| cIMT (mm) | 0.66 ± 0.14 | 0.64 ± 0.11 | 0.3 |
| Absolute FMD (mm) | 0.22 ± 0.12 | 0.24 ±0.11 | 0.5 |
| FMD (%) | 6.11 ± 3.33 | 6.77 ± 3.56 | 0.3 |
| FMD/VTI | 0.24 ± 0.52 | 0.22 ±0.24 | 0.7 |
| PWV (m/sec)* | 7.95 ± 1.86 | 7.49 ± 1.60 | 0.7 |
| DXA measures | |||
| Total Lean mass, kg | 46.2 ± 12.2 | 49.0 ± 11.4 | 0.2 |
| Total Fat mass, Kg | 28.3 ± 10.3 | 27.4 ± 10.2 | 0.6 |
| Fat mass android, Kg | 3.0 ± 1.3 | 4.8 ± 1.7 | 0.2 |
| Fat mass gynoid, Kg | 5.1 ± 1.6 | 4.9 ± 1.7 | 0.4 |
| Fat mass trunk, Kg | 16.3 ± 6.2 | 15.4 ± 6.4 | 0.4 |
| Fat mass limbs, Kg | 11.5 ± 3.9 | 11.2 ± 4.4 | 0.6 |
P values are adjusted for parental age and sex.
PWV was also adjusted for blood pressure.
Data are mean±SD. cIMT: carotid intima-media thickness, FMD: flow mediated dilatation, PWV: pulse wave velocity; VTI: velocity time integral
Body composition in parents of MA positive vs MA negative offspring
No significant difference in total fat and lean mass, gynoid, android, limbs and trunk fat mass were found between parents of Ma positive and MA negative children (Table 4).
Discussion
In the present nested case-control study within the ORPS/NFS cohorts, a clustering of parental cardio-metabolic risk factors, expressed as a cardio-metabolic risk score, was assessed in relation to the development of MA in the offspring. The main study finding was that the cardio-metabolic risk score was higher in parents of offspring who developed MA during adolescence compared to parents of those who did not develop this complication.
Although glycemic control is an important risk factor for renal and other vascular complications of T1D, it does not entirely explain risk, particularly during puberty, when hormonal and metabolic changes, together with lifestyle, environmental exposures and genetic/familial factors may also significantly contribute to the overall complication risk [5,6]. Previously, our group and others have shown that during adolescence, in addition to poor glycemic control, factors associated with increased albumin excretion and presence of MA are high blood pressure, dyslipidemia, inflammatory markers, hyperfiltration [5,6]. Inherited cardio-metabolic risk factors, such as lipid, blood pressure, insulin resistance, obesity, have also been suggested as potential contributing factors to the overall risk of developing MA and DN [12–16]. One way for exploring the role of inherited factors is assessing them within families. Previous family studies in adults with T1D have reported an association between parental CVD risk factors, such as hypertension, insulin resistance and type 2 diabetes, as well as their clustering, and presence of nephropathy in the offspring [12–15]. Limited data are available for young people with childhood-onset T1D [16–20]. In two previous studies, we showed an independent effect of parental blood pressure, assessed by 24-hour ambulatory blood pressure monitoring, on urinary albumin excretion in young offspring with T1D [16,17]. In contrast, parental lipid levels, although being an independent predictor of the same trait in the offspring, did not emerge to be a contributing factor for variations in albumin excretion in offspring from the same cohorts [17,20].
In the present study we aimed to extend these findings and assess the effect of a clustering of cardio-metabolic risk factors on MA risk. Interestingly, the standardized cardio-metabolic risk score we used in the study emerged to be higher in parents of offspring with MA. This association could be explained by a similar genetic background influencing cardio-metabolic profiles in the parents and risk of MA in the offspring. An alternative explanation might be the effect of sharing lifestyle behaviors between parents and offspring, which might be a key target for preventive strategies, or the effect of shared sociodemographic influences.
Over the last years the use of a continuous cardio-metabolic risk score has been suggested and proved to be a valid tool for epidemiological and clinical studies [26–29]. In particular, a continuous cardio-metabolic risk score can offer some advantages compared to the use of dichotomous variables, such as presence or absence of metabolic syndrome. One important advantage is a higher statistical power. In addition, a continuous score has been shown to better reflect the fact that cardiovascular risk is a progressive function of several risk factors, thus removing problems related to the application of arbitrary cutoffs [29]. In our study the use of the cardio-metabolic score also allowed us to make adjustments for specific treatments, such as for hypertension/dyslipidemia or for presence of diabetes, which can be common in adult populations, and which can influence comparisons of individual data on lipids, blood pressure, glucose/insulin levels.
Most of the individual components of the cardio-metabolic risk score taken individually tended to be higher in parents of offspring with MA than in parents of offspring without this complication, but only the difference in diastolic blood pressure reached statistical significance. The latter finding confirms previous observations in young people with T1D, where parental blood pressure or a history of hypertension was one of the main familial risk factors associated risk of MA in the offspring [12,16–19].
In the present study, no significant differences were found in circulating markers of CVD such as hsCRP, ADMA or ACR. Similarly, no differences were found in direct measures of vascular structure and function. This could be due to lack of sufficient power to detect changes in these parameters, for which differences could be subtler than those seen in the cardio-metabolic risk score, and therefore might emerge only with larger sample sizes. Another potential explanation for the lack of differences in these specific CVD measures could be that they might emerge later at an older age in the parents. Indeed, compared to previous studies assessing families of patients with T1D, parents in the present study were younger, with a mean age of around 56 years.
A major strength of the present study was that parents underwent direct assessments during the study visits, including collection of anthropometric measurements, blood samples for measuring circulating markers of CVD, direct imaging measures of vascular structure and function. In contrast, previous studies have been mainly based on collections of family history of cardiovascular events through questionnaires or direct interviews [13,30].
One potential limitation of the study was the sample size which was not particularly high, although a power calculation was performed and we were able to recruit a sufficient number of subjects to detect the expected differences in the primary outcome. As stated above, it could be that the power was not enough to detect subtler changes in direct measures of vascular structure and function. Another potential study limitation is related to the fact that we were not able to recruit complete mother-father pairs for all offspring. This would have allowed a more detailed analysis of potential differences between the effects of maternal vs paternal cardio-metabolic risk factors on MA risk in the offspring. The lack of an assessment of the offspring in their early adulthood, at the time when the parents were assessed, also needs to be acknowledged. However, the main study aim was to assess whether parental cardio-metabolic risk factors were more common among young people with T1D who developed MA during pubertal years.
Conclusions
This study showed that MA in young offspring with childhood-onset T1D was associated with an abnormal cardio-metabolic profile in the parents, mainly driven by abnormal blood pressure, thus highlighting that the familial influence on risk of developing renal complications previously described in adult patients with diabetes, exists also in young people with an early onset of T1D. These findings underline the importance of considering familial cardio-metabolic risk factors when one is estimating the renal risk in young offspring with diabetes. In particular, the characterization of familial risk factors predisposing to the development of MA could help in identifying patients at a higher risk of developing diabetic complications, who might require more intensive preventive and treatment strategies to prevent their development and/or slow their progression.
Future studies are required to explore whether the reported association is related to a common genetic background or might be related to shared lifestyle/sociodemographic factors, which could be targeted to reduce DN in offspring with T1D.
Acknowledgments
The study was supported by a grant from Diabetes UK (09/0003859).
PHT was financially supported by Academy of Finland (decision 130171); The Diabetes Research Foundation, Finland; Foundation for Pediatric Research, Finland; The Alma and K. A. Snellman Foundation, Oulu, Finland and The Finnish Medical Foundation.
We are extremely grateful to the study participants and the staff at the Wellcome Trust Clinical Research facility, Addenbrooke’s Hospital, Cambridge and at the Oxford Centre for Diabetes, Endocrinology and Metabolism (OCDEM) in Oxford, in particular S. Rous and N. McRobert. We also acknowledge the National Institute for Health Research Cambridge
Biomedical Research Centre, the Core Biochemical Assay Laboratory (CBAL), MRC Metabolic Diseases Unit [MRc_MC_UU_12012/5] for analysis of the samples and the the laboratory assistance of A. Watts and K. Whitehead.
We are extremely grateful for the support of L. Watson and P. R. Murgatroyd in body composition assessments in Cambridge and S. Betty and F. Karpe for the DXA assessments in Oxford.
We would like also to acknowledge the statistical support of R. Parker, Centre for Applied Medical Statistics, Institute of Public Health, University of Cambridge, UK.
Footnotes
Conflict of interest: none
References
- 1.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan E, Cardwell CR, Black CJ, McCance DR, Patterson CC. Excess mortality in Type 1 diabetes diagnosed in childhood and adolescence: a systematic review of population-based cohorts. Acta Diabetol. 2015;52:801–7. doi: 10.1007/s00592-014-0702-z. [DOI] [PubMed] [Google Scholar]
- 3.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59:3216–22. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130:1110–30. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 5.Marcovecchio ML, Tossavainen PH, Dunger DB. Status and rationale of renoprotection studies in adolescents with type 1 diabetes. Pediatr Diabetes. 2009;10:347–55. doi: 10.1111/j.1399-5448.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 6.Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes. 2014;15:18–26. doi: 10.1111/pedi.12112. [DOI] [PubMed] [Google Scholar]
- 7.Groop P-H, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen V-P, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–8. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–25. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berentzen NE, Wijga AH, van Rossem L, Koppelman GH, van Nieuwenhuizen B, Gehring U, et al. Family history of myocardial infarction, stroke and diabetes and cardiometabolic markers in children. Diabetologia. 2016;59:1666–74. doi: 10.1007/s00125-016-3988-2. [DOI] [PubMed] [Google Scholar]
- 10.Thorn LM, Forsblom C, Wadén J, Saraheimo M, Tolonen N, Hietala K, et al. Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2009;32:950–2. doi: 10.2337/dc08-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han TS, Hart CL, Haig C, Logue J, Upton MN, Watt GCM, et al. BMJ Open. Vol. 5. British Medical Journal Publishing Group; 2015. Contributions of maternal and paternal adiposity and smoking to adult offspring adiposity and cardiovascular risk: the Midspan Family Study; p. e007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagerudd JA, Tarnow L, Jacobsen P, Stenman S, Nielsen FS, Pettersson-Fernholm KJ, et al. Predisposition to essential hypertension and development of diabetic nephropathy in IDDM patients. Diabetes. 1998;47:439–44. doi: 10.2337/diabetes.47.3.439. [DOI] [PubMed] [Google Scholar]
- 13.Thorn LM, Forsblom C, Wadén J, Söderlund J, Rosengård-Bärlund M, Saraheimo M, et al. Effect of parental type 2 diabetes on offspring with type 1 diabetes. Diabetes Care. 2009;32:63–8. doi: 10.2337/dc08-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorn LM, Forsblom C, Fagerudd J, Pettersson-Fernholm K, Kilpikari R, Groop P-H, et al. Clustering of risk factors in parents of patients with type 1 diabetes and nephropathy. Diabetes Care. 2007;30:1162–7. doi: 10.2337/dc06-2033. [DOI] [PubMed] [Google Scholar]
- 15.Hadjadj S, Péan F, Gallois Y, Passa P, Aubert R, Weekers L, et al. Different patterns of insulin resistance in relatives of type 1 diabetic patients with retinopathy or nephropathy: the Genesis France-Belgium Study. Diabetes Care. 2004;27:2661–8. doi: 10.2337/diacare.27.11.2661. [DOI] [PubMed] [Google Scholar]
- 16.Marcovecchio ML, Tossavainen PH, Acerini CL, Barrett TG, Edge J, Neil A, et al. Maternal but not paternal association of ambulatory blood pressure with albumin excretion in young offspring with type 1 diabetes. Diabetes Care. 2010;33:366–71. doi: 10.2337/dc09-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz CJ, Dalton RN, Selwood M, Dunger DB, Neil HAW, Oxford Regional Prospective Study Group Paternal phenotype is associated with microalbuminuria in young adults with Type 1 diabetes mellitus of short duration. Diabet Med. 2004;21:246–51. doi: 10.1111/j.1464-5491.2004.01123.x. [DOI] [PubMed] [Google Scholar]
- 18.Lévy-Marchal C, Sahler C, Cahané M, Czernichow P, GECER Study Group Risk factors for microalbuminuria in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2000;13:613–20. doi: 10.1515/jpem.2000.13.6.613. [DOI] [PubMed] [Google Scholar]
- 19.Rudberg S, Stattin EL, Dahlquist G. Familial and perinatal risk factors for micro- and macroalbuminuria in young IDDM patients. Diabetes. 1998;47:1121–6. doi: 10.2337/diabetes.47.7.1121. [DOI] [PubMed] [Google Scholar]
- 20.Marcovecchio ML, Tossavainen PH, Heywood JJ, Dalton RN, Dunger DB. An independent effect of parental lipids on the offspring lipid levels in a cohort of adolescents with type 1 diabetes. Pediatr Diabetes. 2012;13:463–9. doi: 10.1111/j.1399-5448.2012.00860.x. [DOI] [PubMed] [Google Scholar]
- 21.Amin R, Widmer B, Prevost aT, Schwarze P, Cooper J, Edge J, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. 2008;336:697–701. doi: 10.1136/bmj.39478.378241.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcovecchio MLL, Dalton RNN, Schwarze CPP, Prevost ATT, Neil HAW a W, Acerini CLL, et al. Ambulatory blood pressure measurements are related to albumin excretion and are predictive for risk of microalbuminuria in young people with type 1 diabetes. Diabetologia. 2009;52:1173–81. doi: 10.1007/s00125-009-1327-6. [DOI] [PubMed] [Google Scholar]
- 23.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–9. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 24.Marcovecchio ML, Widmer B, Turner C, Dunger DB, Dalton RN. Asymmetric dimethylarginine in young people with Type1 diabetes: A paradoxical association with HbA1c. Diabet Med. 2011;28:685–91. doi: 10.1111/j.1464-5491.2011.03252.x. [DOI] [PubMed] [Google Scholar]
- 25.Marcovecchio ML, Dalton RN, Prevost AT, Acerini CL, Barrett TG, Cooper JD, et al. Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care. 2009;32:658–63. doi: 10.2337/dc08-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 27.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care. 2005;28:1195–200. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 28.Franks PW, Ekelund U, Brage S, Wong M-Y, Wareham NJ. Does the association of habitual physical activity with the metabolic syndrome differ by level of cardiorespiratory fitness? Diabetes Care. 2004;27:1187–93. doi: 10.2337/diacare.27.5.1187. [DOI] [PubMed] [Google Scholar]
- 29.Wijndaele K, Beunen G, Duvigneaud N, Matton L, Duquet W, Thomis M, et al. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care. 2006;29:2329. doi: 10.2337/dc06-1341. [DOI] [PubMed] [Google Scholar]
- 30.Harjutsalo V, Katoh S, Sarti C, Tajima N, Tuomilehto J. Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes. 2004;53:2449–54. doi: 10.2337/diabetes.53.9.2449. [DOI] [PubMed] [Google Scholar]