Summary
YAP and TAZ are highly related transcriptional regulators pervasively activated in human malignancies. Recent work indicates that, remarkably, YAP/TAZ are essential for cancer initiation or growth of most solid tumors. Their activation induces cancer stem cells attributes, proliferation, chemoresistance and metastasis. YAP/TAZ are sensors of the structural and mechanical features of the cell microenvironment. A number of cancer-associated extrinsic and intrinsic cues conspire to overrule the YAP-inhibiting microenvironment of normal tissues, including changes in mechanotransduction, inflammation, oncogenic signaling and inhibition of the Hippo pathway. Addiction to YAP/TAZ thus potentially represents a central cancer vulnerability that may be exploited therapeutically.
Introduction
Research on the biology and regulation of YAP/TAZ drew its initial inspiration from pioneering studies in Drosophila. YAP/TAZ are indeed orthologues of Drosophila Yorkie (Huang et al., 2005) and several of the biological traits now assigned to YAP/TAZ were in fact initially discovered in fly tissues in the context of some remarkable organ-overgrowth phenotypes initially observed in mutants of the Hippo cascade (see Box1).
The core Hippo kinase cassette.
Mammalian YAP and TAZ were discovered by M. Sudol and M. Yaffe (Kanai et al., 2000; Sudol, 1994), but their function started to be understood only after it became clear that they were homologue of Drosophila Yorkie, the nuclear mediator of the Drosophila Hippo cascade (Huang et al., 2005). In fact, Hippo pathway components were originally discovered in the fly before Yorkie, by means of genetic screens aimed at isolating genes regulating the growth of larval tissues (Harvey et al., 2003; Justice et al., 1995; Tapon et al., 2002; Wu et al., 2003; Xu et al., 1995; reviewed in Pan, 2010). These findings revealed that the Hippo pathway is a potent tumor-suppressor of fly tissues: mutations inactivating Hippo pathway components invariably cause overgrowth of larval tissues and the emergence of tumors. Yorkie was identified only in 2005 as Warts interacting protein (Huang et al., 2005).
The Hippo cascade is an evolutionary conserved module of two kinases, MST1/2 and LATS1/2 (corresponding to Drosophila’s Hippo and Warts, respectively). MST1/2, aided by its partner Sav1 (Salvador), stimulates LATS kinase activity by directly phosphorylating LATS1/2 and the LATS co-factor MOB1 (reviewed in Meng et al., 2016). Members of the MAP4K4 have been recently reported to substitute MST1/2 for LATS phosphorylation (Li et al., 2014; Meng et al., 2015). NF2 (or Merlin) is a potent upstream component of the Hippo cascade; in epithelial cells, this protein is located at cell-cell junctions where it strengthens adhesion and also serves as scaffold for the core Hippo kinases (Lallemand et al., 2003; Yin et al., 2013). Activated LATS1/2 directly phosphorylate YAP and TAZ, inhibiting them by causing their translocation in the cytoplasm and/or degradation (Meng et al., 2016). TAZ phosphorylation prominently impacts on TAZ stability, in part through formation of a LATS/CK1(ε/δ) phosphodegron leading to β-TrCP recognition, ubiquitination and proteosomal degradation (reviewed in Meng et al., 2016). YAP phosphorylation favors primarily its cytoplasmic localization through only partially understood mechanisms. LATS-mediated phosphorylation on YAPS127 (corresponding to mouse S112) generates a unique 14-3-3 binding site that has been long thought to mediate YAP cytoplasmic anchoring. This notion has been however challenged by recent genetic data with mouse knock-in strains, in which wild-type YAP has been substituted with a YAPS112A allele: this substitution is inconsequential for mammalian development and adult tissue homeostasis, ruling out YAP/14-3-3 association as main determinant of YAP regulation (Chen et al., 2015). It is possible that other YAP/TAZ cytoplasmic anchors may require LATS-phosphorylation. Moreover, YAP/TAZ integrate LATS-dependent and LATS-independent regulations making YAP/TAZ phosphorylation by LATS an important yet not an absolute determinant of their nuclear localization or stability (Aragona et al., 2013; Barry et al., 2013; Das et al., 2016; Dupont et al., 2011; Rashidian et al., 2015; Ren et al., 2010; Sorrentino et al., 2014; Wada et al., 2011; Wang et al., 2014).
Since these pioneering discoveries, the study of YAP/TAZ in mammalian tissues took off to become nowadays a burgeoning field. In several adult organs, YAP/TAZ appear ostensibly dispensable for normal homeostasis but critical to promote tissue repair upon injury (Azzolin et al., 2014; Bai et al., 2012; Cai et al., 2010; Chen et al., 2014; Lee et al., 2014; Taniguchi et al., 2015; Zanconato et al., 2015; Zhang et al., 2014b; Su et al., 2015). Moreover, YAP/TAZ activation is widespread in many human tumors, where YAP/TAZ have been shown to be essential for cancer initiation, progression or metastasis (reviewed in Part 2 of this review). The stark contrast between the inconsequentiality of YAP/TAZ inactivation for normal organ function and their absolute requirement for cancer development in the same organs is attractive, highlighting the possibility that targeting YAP/TAZ may display a large therapeutic window.
A theme that resonates in this review relates to one of most appealing aspects of YAP/TAZ biology, that is, their being transducers of the cell’s structural features, such as polarity, shape and cytoskeletal organization. In turn, these features are intimately connected to the cell’s location within the 3D architecture of tissues, including the attachment to other cells and to the surrounding extracellular matrix (ECM), and influenced by the chemical and physical features of cell’s microenvironement (Halder et al., 2012). Thus YAP/TAZ respond to cellular events reflecting changes that occur at the level of whole tissues, setting YAP/TAZ functions and regulations apart from classic growth-factor initiated signaling cascades.
This review is divided in three parts. We first outline how YAP/TAZ actually underlie many fundamental traits of cancer cells; in the second part, we review a large body of work on the specific roles of YAP/TAZ in different human tumors and corresponding mouse models. In the third part, we will detail the various mechanisms of YAP/TAZ regulation, including Hippo and non-Hippo dependent regulations. The reader should be aware of a potentially confounding semantic issue in this field: the phrase “Hippo pathway”, originally indicating a specific set of kinases such as Hippo/MST or LATS ultimately causing YAP/TAZ inhibitory phosphorylation, is in fact now often loosely used to indicate essentially any modality of YAP/TAZ control, and thus essentially as a proxy of “YAP/TAZ activity” itself. In contrast, here we remain adherent to the biochemical definition and thus our “Hippo signaling” invariably refers to the Hippo kinase module (Box 1). We finally close this paper with some future perspectives for YAP/TAZ in cancer biology.
Part 1. Overview of the Biological Properties of YAP/TAZ
YAP/TAZ are transcriptional coactivators that shuttle between the cytoplasm and the nucleus, where they recognize cognate cis-regulatory elements by interacting with other transcription factors, and in particularly TEA domain family members (TEAD) (reviewed in Piccolo et al., 2014). Association to TEAD is in fact essential for most, if not all, YAP/TAZ transcriptional effects (Chan et al., 2009; Zanconato et al., 2015; Zhao et al., 2008). YAP/TAZ/TEAD control the expression of their targets by binding to distant enhancers that contact their regulated promoters through DNA looping; from these enhancers, YAP/TAZ foster enhancer acetylation and recruitment of the transcriptional machinery (Galli et al., 2015; Stein et al., 2015; Zanconato et al., 2015).
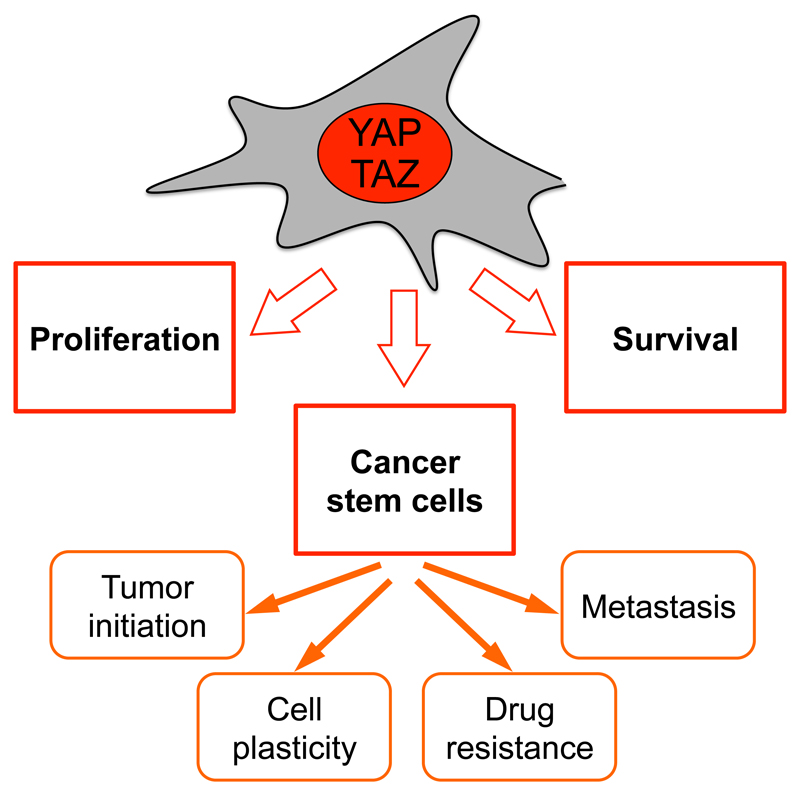
YAP/TAZ empower several of the key attributes of cancer cells (Figure 1) (Hanahan and Weinberg, 2011). To start, sustained activation of YAP/TAZ promotes aberrant cell proliferation (Camargo et al., 2007; Chan et al., 2008; Dong et al., 2007; Zhao et al., 2007). The mechanisms of this response have only recently been explored in cancer cells highlighting a broad transcriptional program connected to cell cycle progression downstream of YAP/TAZ (Kapoor et al., 2014; Zanconato et al., 2015). This program comprises activation or factors involved in replication licencing, DNA synthesis and repair, control of cyclins for S-phase entry and completion of mitosis. Notably, YAP/TAZ may indirectly reinforce their control over the cell cycle by inducing other proto-oncogenic transcription factors, such as c-Myc (Zanconato et al., 2015).
Figure 1. Schematic overview of YAP/TAZ functions in cancer cells.
See text for details.
Cancer cells typically display increased resistance to cell death. Increased YAP/TAZ levels can overcome anoikis induced when cells are detached from the substrate (Zhao et al., 2012). In line, YAP/TAZ transcriptionally upregulate Bcl2-family members (Rosenbluh et al., 2012), thus suppressing mitochondrial-induced apoptosis pathway, but can also overcome the alternative apoptosis cascade initiated by TNF-α and FAS ligands (Dong et al., 2007).
Tumors are made of distinct cell populations that are functionally and phenotypically heterogeneous. Intriguingly, YAP/TAZ are active in the tumor’s cancer-stem-cell (CSC) fraction, and functionally instrumental and required for CSC expansion (Bartucci et al., 2015; Basu-Roy et al., 2015; Cordenonsi et al., 2011; Hayashi et al., 2015; Song et al., 2014). YAP/TAZ actually reprogram non-stem tumor cells into cells with full-CSC attributes (Bartucci et al., 2015; Cordenonsi et al., 2011). This suggests that YAP/TAZ may molecularly define CSC populations so far defined only operationally, and inputs controlling YAP/TAZ may correspond to the so far poorly understood signals inducing CSCs in solid tumors. The connection with CSCs is consistent with additional cancer-associated traits regulated by YAP/TAZ (Figure 1). These include the ability to initiate tumor formation, to induce chemoresistance, metastasis and expand undifferentiated cell populations. As detailed below, attenuation of YAP or TAZ can restore sensitivity to chemotherapy and radiotherapy. Perhaps also connected to their role as CSC determinants, recent evidence also suggests that, in some contexts, YAP/TAZ constantly rejuvenate cancer cells by promoting basal levels of autophagy to avoid senescence (Garcia-Prat et al., 2016; Song et al., 2015a), while still opposing cell death caused by an excess of autophagy in the tumor bulk (Liang et al., 2014).
Tumors are increasingly perceived as complex tissues and tumor evolution can be understood only in light of the ever evolving heterotypic interactions between cancer cells and the genetically normal cells of the tumor stroma (Bissel and Hines, 2011). YAP/TAZ orchestrate this crosstalk from both compartments: in epithelial cells, YAP/TAZ favors the secretion of AREG (an EGF-like growth factor), of matricellular proteins such as Cyr61 and CTGF (also known to induce angiogenesis) (Piccolo et al., 2014), and of chemoattractants for T-cell suppressing myeloid cells favoring the installment of immune tolerance (Wang et al., 2016). In cancer-associated fibroblasts (CAFs), YAP/TAZ incite the production of inflammatory interleukins and deposition of a rigid extracellular matrix that, being a main upstream inducer of YAP/TAZ (Halder et al., 2012), in turn feedback on CAFs and epithelial cells alike to self-sustain YAP/TAZ activity (Calvo et al., 2013). Thus YAP/TAZ influence the chemical, physical and cellular composition of the tumor microenvironment.
Part 2. YAP/TAZ in Human Tumors and Mouse Models
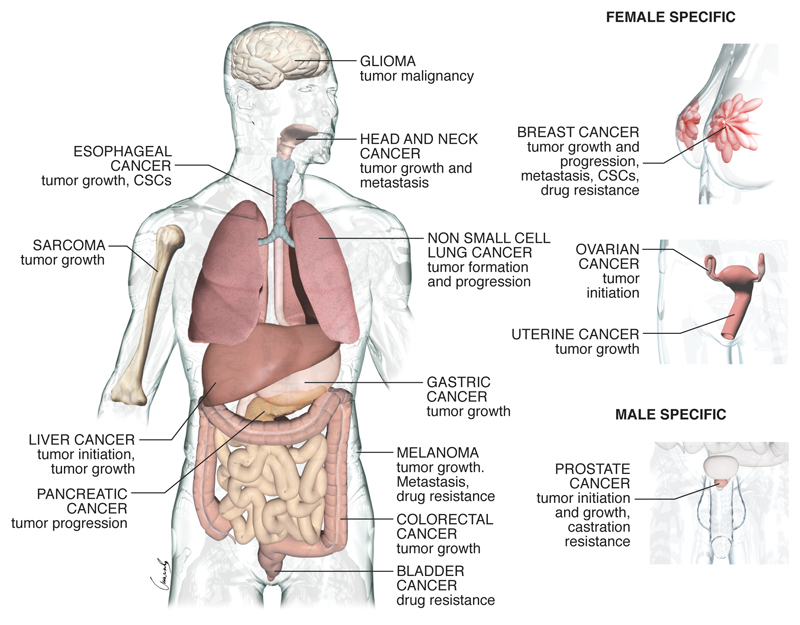
The above outline of the biological properties of YAP/TAZ necessarily summarizes general themes from the collective evidence obtained in distinct tumor types and experimental models. But what do we know about YAP and/or TAZ in the various types of human cancers, and what have we learned from their corresponding experimental models? (See Figure 2 and Table 1).
Figure 2. YAP/TAZ in human tumors.
Tumor types for which epidemiological data and functional evidence of YAP/TAZ activation have been reported.
Table 1. YAP/TAZ expression and functional relevance in human tumors and experimental models.
| Cancer Type | Genetic lesions affecting YAP/TAZ activity | Expression in human tumors | Expression in experimental systems | Loss-of-function phenotypes | Gain-of-function phenotypes |
|---|---|---|---|---|---|
| Prostate cancer (PCA) | None reported | IHC: YAP expression enriched in PCA with recurrence proclivity and castration resistance (Nguyen et al., 2015; Sheng et al., 2015; Zhang et al., 2015a) | IHC and gene expression: elevated nuclear YAP expression and YAP/TAZ target gene expression in PCA from conditional Pten -/-;Smad4-/- but not from conditional Pten-/- mouse models of PCA (Wang et al., 2016). | YAP knockdown impairs the growth of orthotopic xenotranspla nts of the human PCA cell line VCaP (Nguyen et al., 2015) | YAP overexpression in mouse prostate: development of age-related PCA (Nguyen et al., 2015) YAP overexpression in the human PCA cell line LNCaP: allows tumor formation in castrated mice, bypassing androgen addiction(Zhang et al., 2015a) |
| Cervical squamous cell carcinomas (CSCC) | Oncogenic strains of human papilloma-virus (HPV) (He et al., 2015) | IHC: high levels of YAP in CSCC compared to normal cervical tissue (He et al., 2015; Liu et al., 2013b; Xiao et al., 2014) | IHC: high levels of YAP in mouse cervical tumors induced by transgenic expression of HPV proteins E6/E7 (He et al., 2015) | YAP knockdown impairs the growth of subcutaneous xenografts of the CSCC cell line ME180 (He et al., 2015) | YAP overexpression promotes the growth of subcutaneous xenografts of the CSCC cell line ME180 (He et al., 2015) |
| Endometrial carcinoma (EMCA) | None reported | IHC: elevated YAP and TAZ nuclear staining in high grade EMCA; elevated YAP nuclear staining is prognostic of poor outcome (Romero-Perez et al., 2015; Tsujiura et al., 2014) | TAZ overexpression promotes the growth of subcutaneous xenografts of the EMCA cell line Ishikawa (Tsujiura et al., 2014) | ||
| Esophageal squamous cell carcinomas (ESCC), and esophageal adeno-carcinomas (EAC) | None reported | IHC: elevated YAP nuclear staining is a predictor of worse prognosis in ESCC and is associated with therapy resistance in EAC (Muramatsu et al., 2011; Song et al., 2015b; Yeo et al., 2012) | YAP overexpression confers CSC properties and resistance to chemotherapy to benign esophageal cells, and enhances subcutaneous tumor formation by the EAC cell line SKGT-4 (Song et al., 2014; Song et al., 2015b) | ||
| Urothelial carcinoma of the bladder (UCB) | None reported | IHC: elevated YAP expression correlates with high grade and lymph-node metastases, and is a predictor of worse prognosis and relapse. Cisplatin-resistant PDX samples derived from UCB display elevated YAP nuclear staining compared to cisplatin-sensitive PDX (Ciamporcero et al., 2016; Li et al., 2015b; Liu et al., 2013a) | Tumors arising from the UCB cell line T24 become cisplatin-sensitive after YAP-knockdown (Ciamporcero et al., 2016) | ||
| Medulloblastoma | IHC and gene expression: YAP expression is elevated in the SHH and WNT subtypes of medulloblastoma (Ellison et al., 2011; Fernandez et al., 2009) | YAP protein levels are upregulated in mouse models of medulloblastoma (Fernandez et al., 2009) | YAP overexpression in mouse cerebellar progenitor cells promotes their growth and confers resistance to IR, but is insufficient for medullo-blastoma formation (Fernandez et al., 2009) | ||
| Neuroblastoma | Elevated TAZ mRNA expression is prognostic of poor outcome (Wang et al., 2015a) | TAZ knockdown impairs the growth of subcutaneous xenografts of the neuro-blastoma cell line SK-N-AS (Wang et al., 2015a) | |||
| Meningioma | Associated with Neurofibrom atosis type 2 (germline NF2 mutants). Sporadic cases: NF2 mutations (60%) (Striedinger et al., 2008) | IHC: YAP is nuclear in 92% of NF2-mutant meningiomas (Baia et al., 2012; Striedinger et al., 2008; Tanahashi et al., 2015) | YAP overexpression confers the ability to form meningiomas to immortalized human arachnoid cells (Baia et al., 2012) | ||
| Schwannoma | Associated with Neurofibrom atosis type 2 (germline NF2 mutants). Sporadic cases: NF2 mutations (55%); LATS2 mutations (2%) (Oh et al., 2015) | IHC: YAP is nuclear in about 40% of schwannomas (Boin et al., 2014; Oh et al., 2015) | |||
| Melanoma | GNAQ and GNA11 mutations in uveal melanoma (UM) (about 67% of cases) (O'Hayre et al., 2013) | IHC: elevated YAP expression in a subset of cutaneous melanomas (CM) associated with poor prognosis; elevated YAP nuclear staining in UM samples (Feng et al., 2014; Menzel et al., 2014; Yu et al., 2014) | IHC: elevated YAP nuclear staining in melanoma induced by overexpression of oncogenic GNAQ (Feng et al., 2014; Yu et al., 2014) | YAP knockdown impairs the growth of subcutaneous xenografts of the UM cell line OMM1.3; YAP/TAZ knockdown impairs lung metastatic colonization of the CM cell line 1205Lu (Feng et al., 2014; Nallet-Staub et al., 2014) | |
| Squamous cell carcinoma of the skin (SCC) | None reported | IHC: YAP is nuclear in most SCC (Silvis et al., 2011) | IHC: YAP is nuclear in most SCC arising in mice after conditional deletion of α-E-catenin in the epidermis (Schlegelmilch et al., 2011) | YAP/TAZ are genetically required for formation of mouse skin tumors induced by chemical carcinogenesis (Zanconato et al., 2015) | |
| Head & neck squamous cell carcinoma (HNSCC) | None reported | IHC: elevated nuclear staining of YAP or TAZ correlates with tumor recurrence, resistance to radiotherapy and poor outcome (Li et al., 2015d) | YAP/TAZ knockdown impairs primary tumor growth and metastasis formation by the HNSCC cell line SCC2 after orthotopic transplantation (Hiemer et al., 2015) | ||
| Ovarian Cancer | None reported | IHC and gene expression: elevated nuclear YAP expression and YAP/TAZ target gene expression correlate with poor prognosis (Jeong et al., 2014; Xia et al., 2014; Zhang et al., 2011) | YAP overexpression confers the ability to form ovarian cancers to fallopian tube epithelial secretory cells (Hua et al., 2015) | ||
| Malignant Mesothelioma | Loss or mutation of NF2 (40%); loss or mutation of LATS1 (21%); loss or mutation of LATS2 (7%); loss or mutation of MST1 (16%); loss of MST2 (2%); loss of SAV1 (14%)((Bueno et al., 2016) | IHC: YAP levels are upregulated in about 70% of mesotheliomas (Fujii et al., 2012; Murakami et al., 2011) | YAP knockdown impairs the growth of mesothelioma cell lines | ||
| Osteosarcoma (OS) | None reported | IHC: YAP levels are upregulated in about 80% of OS compared to normal bone tissue(Chan et al., 2014; Zhang et al., 2013) | IHC: YAP levels are upregulated in mouse osteosarcoma induced by activation of the Shh pathway and loss of p53 in osteoblasts (Chan et al., 2014) | YAP knockdown impairs the growth of primary mouse OS cell lines and their in vivo tumorigenic abilities (Chan et al., 2014) | |
| Undifferentiated pleomorphic sarcomas (UPS) | Loss of NF2 (6%), SAV1 (8%) or LATS2 (16%) (Eisinger-Mathason et al., 2015) | IHC: elevated nuclear YAP staining in human UPS samples (Eisinger-Mathason et al., 2015) | IHC: elevated nuclear YAP staining in sarcomas arising by targeted activation of oncogenic KRas and loss of p53 in fibroblasts (Eisinger-Mathason et al., 2015) | YAP knockdown impairs the growth of subcutaneous xenografts of primary mouse UPS cell lines (Eisinger-Mathason et al., 2015) | |
| Kaposi’s sarcoma (KS) | vGPCR from Kaposi’s sarcoma-associated herpesvirus (KSHV) (Liu et al., 2015) | IHC: elevated nuclear YAP and TAZ staining in human KS samples (Liu et al., 2015) | |||
| Embryonic rhabdomyosarco ma (ERMS) | None reported | IHC: elevated nuclear YAP staining in human ERMS samples (Tremblay et al., 2014) | YAP knockdown reduces tumor growth after orthotopic transplantatio n of ERMS cells (Tremblay et al., 2014) | YAP overexpression in mouse satellite cells leads to ERMS formation (Tremblay et al., 2014) | |
| Epithelial hemangio-endothelioma (EHE) | Fusion of WWTR1 (encoding TAZ) with the CAMTA1 gene, encoding a constitutively nuclear and active version of TAZ; a minor subset of EHE carries a gene fusion between YAP1 and TFE3 (Antonescu et al., 2013; Errani et al., 2011; Tanas et al., 2015) | ||||
| Multiple Myeloma | Loss of YAP1 in 10% of tumors (Cottini et al., 2014) | low YAP1 expression is predictive of poor outcome (Cottini et al., 2014) | |||
| Acute myeloid leukemia (AML) | low YAP1 expression is predictive of poor outcome (Cottini et al., 2014) |
Lung cancer
Lung cancer includes several subtypes, including lung adenocarcinomas (LAC), lung squamous cell carcinomas (LSCC), large-cell lung carcinomas (LCLC), all generally defined as “non-small-cell lung cancer”(NSCLC). Immunohistochemistry (IHC) studies showed that elevated expression/nuclear localization of YAP or TAZ correlates with malignant features (e.g. high histological grade, late TNM stage, lymph-node metastasis) and poor patient outcome (Cheng et al., 2015; Noguchi et al., 2014; Su et al., 2012; Wang et al., 2010; Xie et al., 2012). In line, elevated expression of gene signatures for YAP/TAZ activity correlates with poor prognosis in datasets of NSCLC patients (Lau et al., 2014; Noguchi et al., 2014). In LAC mouse models, non-metastatic tumors induced by knock-in of oncogenic KrasG12D in alveolar cells display very weak YAP/TAZ staining, whereas high levels of nuclear YAP/TAZ can be found in metastatic LAC induced by combining oncogenic activation of KRas with biallelic loss of Tp53 or Lkb1 (Lau et al., 2014; Mohseni et al., 2014; Zhang et al., 2015b).
These IHC and gene-expression analyses were nicely complemented by several functional studies in NSCLC. YAP or TAZ knockdown in the human LAC cell line A549 impairs tumor formation after injection in the tail vein of nude mice (Lau et al., 2014; Noguchi et al., 2014). Genetic loss of Yap strongly reduces the number of tumors forming in the KrasG12D;Lkb1L/L mouse model; interestingly, most of the remaining lesions are benign and do not evolve in LAC or LSCC (Zhang et al., 2015b). YAP/TAZ activation might not be sufficient to promote NSCLC formation: expression of an active form of YAP in the mouse airway epithelium only causes hyperplasia of the tissue, but is per se insufficient to trigger tumor formation (Zhang et al., 2015b). However, constitutive expression of TAZ allows immortalized human bronchial cells to form tumors in immunocompromized mice (Noguchi et al., 2014; Zhou et al., 2011b) and ectopic YAP expression can promote progression of small adenomas to high grade LAC in the KrasG12D knockin mouse model (Lau et al., 2014; Zhang et al., 2015b).
Breast cancer
Breast cancer (BC) arises from the epithelial cells of the mammary gland and can be classified on the basis of their site of origin (ductal or lobular), their ability to grow in the lumen of the gland (carcinoma in situ), or in the stroma surrounding the gland (invasive carcinoma). Invasive ductal carcinomas (IDC), the most common forms of BC, can be further classified in hormone (estrogen receptor, ER, and/or progesterone receptor, PR) receptor positive BC (often referred as luminal BC), HER2-positive BC, and BC that are negative for all three receptors (triple-negative BC, TNBC). In datasets of BC patients, elevated expression of gene signatures for YAP/TAZ activity correlate with high histological grade, enrichment of stem cell signatures, metastasis proclivity and poor outcome (Cordenonsi et al., 2011; Di Agostino et al., 2015; Zanconato et al., 2015). In line, TAZ nuclear staining is highly enriched in high grade BC (Bartucci et al., 2015; Cordenonsi et al., 2011), and is prognostic of poor clinical outcome (Bartucci et al., 2015; Diaz-Martin et al., 2015). TAZ mRNA and protein expression are reported to be preferentially higher in TNBC than in the other BC subclasses (Diaz-Martin et al., 2015; Kim et al., 2015a; Li et al., 2015c; Skibinski et al., 2014). TAZ nuclear staining has been reported to be associated with low pathological complete response of a subset of HER2-positive BC patients treated with anti- HER2 (transtuzmab) neoadjuvant therapies (Vici et al., 2014).
Several studies reported no correlation between YAP IHC staining and clinical variables in BC cases (Kim et al., 2014; Sheen-Chen et al., 2012; Wang et al., 2012; Yuan et al., 2008), although the lack of resolution of these studies still allows for some BC subtypes to be stratified by YAP expression (Kim et al., 2015b). In the mouse mammary gland, nuclear YAP/TAZ are broadly induced in tumors caused by transgenic expression of HER2, Wnt1, or Polyoma-middleT (PyMT, molecularly resembling HER2 tumors)(Chen et al., 2014; Serrano et al., 2013).
Conditional knockout of YAP in the mammary gland greatly increases the latency and reduces the growth of mammary tumors arising in MMTV-PyMT mice (Chen et al., 2014). TAZ knockdown strongly reduces the number of tumor initiating cells of the malignant MCF10ACA1a human TNBC cell line and of primary human BC stem cells (Bartucci et al., 2015; Cordenonsi et al., 2011), also affecting the ability of primary BC cells to form distant metastasis (Bartucci et al., 2015). Of note, TAZ knockdown also inhibits tumor formation by the human luminal BC cell line MCF7 (Chan et al., 2008).
YAP overexpression in the mouse mammary gland causes defects in the terminal differentiation of secretory cells during lactation, but does not induce tumor formation (Chen et al., 2014). In contrast, YAP overexpression can confer lung metastatic abilities to otherwise benign mouse mammary tumor cell lines (Lamar et al., 2012). TAZ overexpression increases the content of tumor initiating cells in benign/low-grade human BC cell lines, and allows them to form high-grade cancers after injection in the mammary fat pads of immunocompromized mice (Cordenonsi et al., 2011). Consistently, TAZ overexpression confers metastatic abilities to low tumorigenic/non-stem primary human BC cells (Bartucci et al., 2015). Taken together, these studies indicate that TAZ, and possibly YAP, play an important role in breast cancer progression, favoring the acquisition of cancer stem cells features, thus promoting malignancy and metastatic relapse.
Colorectal cancer
Colorectal carcinoma (CRC) derives from benign tumors (polyps and adenomas) that arise from the mucosal epithelium of the lower tract of the digestive system (colon and rectum). Most CRC carry mutations leading to overactivation of the Wnt signaling pathway, among which the most frequent are mutations inactivating the APC tumor suppressor. High levels of expression of a gene signature for YAP activity has been found to be prognostic for bad outcome in four datasets of CRC patients, and correlated with cetuximab resistance (Lee et al., 2015; Yuen et al., 2013). This is in agreement with the reported correlation of high YAP and TAZ expression and nuclear localization with poor patients’ outcome (Kim et al., 2013a; Wang et al., 2013a; Wang et al., 2013b). YAP protein has also been found overexpressed and nuclearly localized in adenomas spontaneously developing in the small intestine of ApcMin/+ mice (Cai et al., 2015; Camargo et al., 2007; Gregorieff et al., 2015), and ectopically expressed in the hyperplastic Apc-/- villi (Azzolin et al., 2014).
Combined knockout of Yap and Taz suppresses the hyperplastic growth of the epithelium caused by loss of APC in mouse intestine (Azzolin et al., 2014). Consistently, conditional knockout of the sole Yap in the intestine of ApcMin/+ mice strongly reduces adenoma formation, significantly extending lifespans (Cai et al., 2015; Gregorieff et al., 2015). YAP and/or TAZ knockdown in human CRC cell lines suppresses their growth and ability to trigger tumor formation after injection in mice (Azzolin et al., 2012; Pan et al., 2012; Rosenbluh et al., 2012; Yuen et al., 2013). Thus, YAP/TAZ are required for formation and growth of intestinal tumors. However, forced YAP overexpression in the gut epithelium is not sufficient to induce tumor formation, and may in fact cause degeneration of the mucosa (Barry et al., 2013), possibly because an excess of cytoplasmic YAP may oppose the Wnt pathway (see below).
Liver cancer
Several subtypes of liver cancer exist: hepatocellular carcinoma (HCC), arising from hepatocytes; cholangiocarcinomas (CC) that derive from the bile ducts; and hepatoblastoma (HB), a pediatric tumor formed by immature liver cells. IHC studies on human HCC samples showed that elevated expression of YAP or TAZ correlates with poor tumor differentiation and is prognostic of bad outcome (Guo et al., 2015; Han et al., 2014; Kim et al., 2013b; Xiao et al., 2015; Xu et al., 2009). Moreover, high levels of YAP protein expression have also been detected in a majority of human CC and HB samples (Li et al., 2012; Tao et al., 2014). In a rat model of liver cancer, YAP protein levels are upregulated starting from precancerous lesions, but overt nuclear localization of YAP can be found only in fully developed HCC and CC (Perra et al., 2014).
Liver-specific YAP overexpression in transgenic mice leads to hepatomegaly and then to formation of liver tumors resembling human HCC (Dong et al., 2007). Similar results were obtained by delivering a phospho-mutant form of YAP in the mouse liver using transposon-mediated hydrodynamic transfection (Shen et al., 2015). Interestingly, hydrodynamic transfection of YAP in the mouse liver leads to development of HB when combined with an activated form of β-catenin (Tao et al., 2014). In line, YAP overexpression endows tumorigenic properties to the otherwise non-tumorigenic human hepatocyte cell line MHIA (Xu et al., 2011). YAP or TAZ knockdown strongly reduces subcutaneous tumor growth of human and mouse HCC cell lines (Xiao et al., 2015; Xiao et al., 2013; Zender et al., 2006). Genetic inactivation of Hippo pathway components also induces HCC and other types of liver cancer and YAP is genetically required for these phenotypes (Fitamant et al., 2015; Lee et al., 2010; Lu et al., 2010; Song et al., 2010; Zhang et al., 2010; Zhou et al., 2009).
Gastric cancer
The large majority of cases are gastric adenocarcinomas (GAC) that derive from the glandular epithelium of the stomach. YAP mRNA and protein levels are found upregulated in a relevant portion of GAC, and elevated YAP protein expression and nuclear localization correlate with bad patients’ outcome (Da et al., 2009; Hu et al., 2014; Kang et al., 2011; Song et al., 2012). Expression of YAP target genes, and of Yap mRNA itself, was found upregulated in a mouse model of GAC generated by infection with Helicobacter pylori, a common risk factor for GAC development in humans (Jiao et al., 2014). TAZ has been found highly expressed in human GAC of the singlet ring cell subtype (Yue et al., 2014). The requirement of YAP/TAZ for GAC growth has been revealed using a peptide (super-TDU) that inhibits YAP/TAZ interaction with TEADs; super-TDU inhibits subcutaneous growth of primary human GAC cell lines, and reduces GAC formation in mice after infection with H. pylori (Jiao et al., 2014). Conversely, reintroduction of YAP in the YAP-mutant GAC cells MKN45 enhances their growth as subcutaneous tumors (Kang et al., 2011).
Pancreatic cancer
The most common form of pancreatic cancer is pancreatic adenocarcinoma (PDAC), a very aggressive neoplasia that seems to arise from pancreatic exocrine cells through intermediate stages called pancreatic intraepithelial neoplasia (PanIN). By IHC on human pancreatic tissue samples, YAP and TAZ were found almost absent from normal acini, but moderately expressed and nuclearly localized in PanINs and in a subset of primary PDAC, whereas strong nuclear staining of YAP was found in metastases derived from PDACs (Diep et al., 2012; Morvaridi et al., 2015; Yang et al., 2015; Zhang et al., 2014b). Similarly, YAP/TAZ expression and nuclear localization can be detected in most PanINs and PDACs arising in mouse models of pancreatic cancer, and in tumors derived from human PDAC cell lines growing as xenografts (Diep et al., 2012; Morvaridi et al., 2015; Zhang et al., 2014b). Functionally, pancreas-specific Yap knockout abrogates tumor progression from early PanIN lesions to PDAC in a mouse model of pancreatic cancer (Zhang et al., 2014b).
Gliomas
Gliomas are the most common primary brain cancers and can be subdivided into several subtypes, most of which (astrocytomas, oligodendrogliomas, oligoastrocytomas) can be categorized as low-grade gliomas; high grade gliomas are very aggressive tumors that are classified as glioblastoma multiforme (GBM). By IHC, elevated TAZ levels are detected in the majority of GBM, and high TAZ mRNA levels are associated with reduced survival (Bhat et al., 2011; Tian et al., 2015). High levels of YAP expression are found in subsets of gliomas of all grades, and correlate with shorter survival of glioma patients (Orr et al., 2011). TAZ knockdown impairs tumor formation by primary GBM cell lines orthotopically injected in SCID mice (Bhat et al., 2011). Interestingly, TAZ overexpression in normal brain cells is per se insufficient to induce glioma formation, but can increase the malignancy of gliomas induced by a PDGF transgene (Bhat et al., 2011).
Available evidence concerning the role of YAP/TAZ in other tumors is outlined in Table 1.
Part 3. How are YAP/TAZ Induced in Cancer?
Hippo signaling in cancer: jack of all trades but master of none?
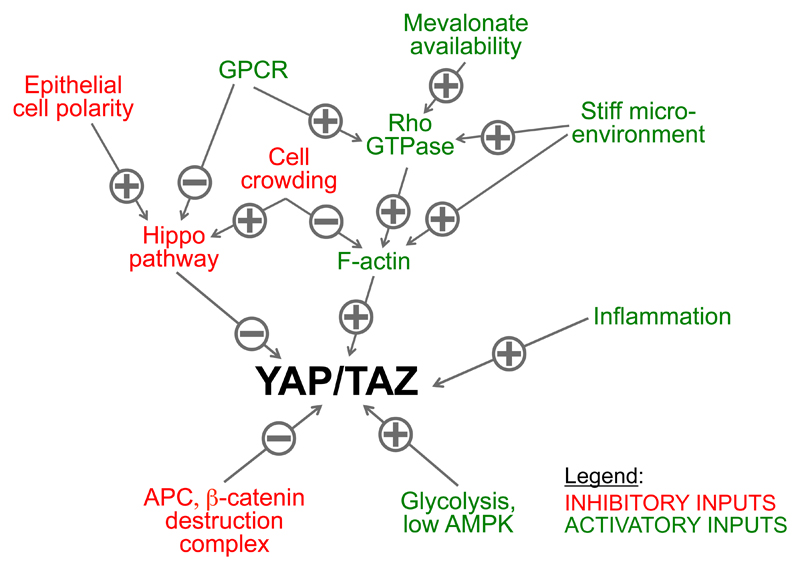
The Hippo pathway is the first discovered and thus best-understood modality to restrict YAP/TAZ activity (Box 1). For years, LATS-mediated phosphorylation has been the only known mechanisms to regulate YAP/TAZ. This has lent support to the notion that inactivation of Hippo pathway may be the culprit of YAP/TAZ stabilization and activation in cancer (Meng et al., 2016; Pan 2010). Yet, this assumption contrasts with the reality that Hippo pathway mutations are extremely rare in human tumors (Harvey et al., 2013). More puzzling is that, as detailed in Table 1 of this review, mutations in NF2, LATS1, or LATS2 can be selected in cancer but this occurs only in specific tumor histotypes, such as in mesothelioma, schwannomas and meningiomas. The fact that the same mutations in Hippo pathway components are not selected in other tumor types displaying high levels of YAP/TAZ activity appears, at face value, inconsistent with the idea that Hippo signaling is the only and dominant gatekeeper of YAP/TAZ activity in human tumors. Such idea would also require the Hippo pathway to be anti-correlated with the well-established activation/stabilization of YAP/TAZ detected in selected cell populations of normal tissues or within tumors; in contrast there is currently no evidence for such a patterned Hippo activity in normal or neoplastic adult tissues. Here we propose that the Hippo pathway is one of the tumor suppressor pathways keeping YAP/TAZ at bay in normal tissues, but neither the only nor invariably the most relevant one implicated in YAP/TAZ-driven tumorigenesis.
Two lines of data support the idea that Hippo and LATS kinases as key suppressors of YAP/TAZ oncogenic properties in mammalian tissues. The first is that, an increase in YAP phosphorylation levels (e.g. at S127 and other residues) occurs in cultured cells in association to YAP/TAZ inhibition by several upstream signals, and these biochemical findings, including some from our own group (Cordenonsi et al., 2011), have been interpreted as Hippo/LATS serving as unique bottleneck/integrator for YAP/TAZ regulation (Meng et al., 2016).
Here we would like to suggest a more cautionary interpretation of these data, as LATS kinase activity, by itself, should not be automatically considered as surrogate of LATS functional relevance. Experimental inactivation of LATS1/2 is in fact inconsequential, or only partially relevant, for key modalities of YAP/TAZ regulation (Aragona et al., 2013; Azzolin et al., 2012; Das et al., 2016; Dupont et al., 2011; Enzo et al., 2015; Feng et al., 2014; Gailite et al., 2015; Li et al., 2016; Mo et al., 2015; Schlegelmilch et al., 2011; Silvis et al., 2011; Sorrentino et al., 2014; Taniguchi et al., 2015). These include cell mechanotransduction, Rho-GTPase signaling, inflammation, metabolism and, at least in part, contact inhibition and GPCR signaling (detailed below, see also Figure 3). For example, LATS-mediated YAP/TAZ phosphorylation levels do raise in cells seeded in soft ECM, although this YAP/TAZ inhibition is LATS1/2 independent (see below, next chapter). The fact that these same signals do also entail changes in YAP phosphorylation is consistent with a role of LATS1/2 as reinforcing mechanism to ensure complete YAP/TAZ turn-off irrespectively of the primary cause of YAP/TAZ inactivation.
Figure 3. Inputs regulating YAP/TAZ activity in cancer cells.
See text for details.
A second argument consistent with the view that the Hippo pathway is central for YAP/TAZ regulation in cancer is that, in mouse models, inactivation of Hippo pathway components causes some dramatic overgrowth phenotypes ultimately leading, at least in liver and intestine, to tumor development (Chen et al., 2015; Heallen et al., 2011; Lee et al., 2010; Lu et al., 2010; Song et al., 2010; Zhang et al., 2010; Zhou et al., 2009; Zhou et al., 2011a). However, liver tumors emerge with a longer latency in Hippo knockout mice than in mice expressing an inducible form of YAP, in line with the notion that Hippo signaling needs to cooperate with other signals even in these paradigm tissues. Tissue damage may be one of these essential inputs: in the Sav1 mutant intestine, YAP/TAZ-driven tumorigenesis is greatly increased by intestinal damage (Cai et al., 2010) and Medzhitov and colleagues insightfully noticed that latent inflammation is always present and actually required for YAP/TAZ activity in the liver of mice bearing Hippo pathway mutations, likely as a consequence of globally disturbed organ differentiation, microbial infiltration and systemic metabolic changes (Su et al., 2015). Moreover, MST-inactivation in isolated single liver cells, as opposed to the whole tissue, is insufficient to trigger cell transformation (Su et al., 2015).
The skin is a third paradigm tissue in which YAP overexpression in adult basal keratinocytes causes overgrowth and tumorigenesis. Of note, the effect of YAP gain-of-function has been demonstrated by transplanting a patch of YAP-expressing skin into immunocompromised mice, that is, also in the context of a wounded tissue (Schlegelmilch et al., 2011). Notably, YAP/TAZ activation is essential for skin tumor formation after application of the skin carcinogenesis protocol (entailing mutagenesis and inflammation) (Zanconato et al., 2015). However, and in stark contrast to the liver scenario, inactivation of the Hippo pathway in the adult skin is inconsequential, causing neither overgrowth nor tumorigenesis (Schlegelmilch et al., 2011); this indicates that Hippo/LATS-independent mechanisms are at play to regulate YAP/TAZ pro-growth and tumorigenic effects in the skin. It is worth noting that genetic inactivation of Hippo pathway components in knockout mice is insufficient to trigger tumor formation in multiple tissues, such as mammary gland, lung, heart, kidney, pancreas and nervous system (Chen et al., 2014; George et al., 2012; Heallen et al., 2011; Lavado et al., 2013; Lin et al., 2015a; Reginensi et al., 2013; Schlegelmilch et al., 2011).
In light of the available data, we favor the view that activation of YAP/TAZ protumorigenic effects requires the concurrence of distinct events, such as a YAP/TAZ proficient cellular environment conveyed through Hippo independent mechanisms as well as relieve of YAP/TAZ repression from LATS phosphorylation (Figure 3). The relative importance of these steps (and their potential crosstalk) may vary in different contexts.
Additionally, tumor-associated pathways not directly involved in YAP/TAZ regulation may be required for activation of the malignant properties of YAP/TAZ, at least in some contexts. For example, it has been recently shown that YAP/TAZ operate by binding to complex enhancer elements where they require transcriptional cooperation with other transcription factors (Galli et al., 2015; Stein et al., 2015; Zanconato et al., 2015; Nguyen et al., 2015), and these may in turn be targeted by cancer-relevant pathways distinct from those directly activating YAP/TAZ. For example, AP-1 factors - known to be activated by multiple oncogenes - are a pervasive presence in a large fraction of the YAP/TAZ-bound enhancers (Liu et al., 2016; Stein et al., 2015; Verfaillie et al., 2015; Zanconato et al., 2015), and required for YAP/TAZ-driven oncogenic growth in mammary tumor cells (Zanconato et al., 2015).
YAP/TAZ and mechanotransduction
Living cells are sophisticated mechanical entities, in which cell shape is deeply interwoven to cell function. Cell structure ultimately relies on an equilibrium of forces acting on the F-actin cytoskeleton: cell respond to external forces, such as pulling forces from neighboring cells or from the surrounding ECM by changing the tension and organization of the F-actin cytoskeleton (Halder et al., 2012). In this way, cells are informed of their location within tissue niches, perceive their own shape and the 3D architecture of the whole tissue, and are able to stay tuned with changes in rigidity, geometry and composition of the ECM, all with nanometer accuracy. These structural, mechanical features inform cells on proliferation vs. differentiation, or on the need to preserve stem cell attributes depending on tissue needs. In normal epithelial organs, tissue architecture has been suggested as an overarching tumor suppressor (Bissell and Hines, 2011), strictly confining mechanical strains only to specific tissue niches. However, mechanical signals become progressively aberrant in cancer because of progressive deviations from normal tissue organization, inflammation, increased compression forces, changes in ECM composition and overall increase in ECM rigidity (Butcher et al., 2009). These changes initiate early, even before frank neoplasia could be detected, and can epigenetically switch cell behavior from normal to pre-neoplastic (reviewed in Halder et al., 2012).
Mechanical inputs represent a central pillar in the control of YAP/TAZ activity (Aragona et al., 2013; Dupont et al., 2011). YAP and TAZ are directly regulated by ECM stiffness, cell shape and cytoskeletal tension (Figure 3) (reviewed in Gaspar and Tapon, 2014; Low et al., 2014)). For example, when cells are cultured on stiff ECM, YAP and TAZ become nuclear and transcriptionally active, whereas they are inhibited and relocalized in the cytoplasm in cells that are forced to remain attached to a small adhesive area, or cultured on a soft ECM (Dupont et al., 2011). This regulation depends on the Rho family of small GTPase, ROCK and integrity of the actomyosin cytoskeleton. Interestingly, mechanotransduction controls YAP/TAZ in a manner largely independent from LATS (Aragona et al., 2013; Dupont et al., 2011; Sorrentino et al., 2014). For example, loss of LATS1/2 is unable to revive YAP/TAZ activity in cells that are mechanically inhibited (Aragona et al., 2013; Dupont et al., 2011), a finding we have recently confirmed genetically by using Lats1 and Lats2 double knockout mouse embryonic fibroblasts (MEFs): in these cells, YAP/TAZ remains fully sensitive to changes in cell shape induced by cell density, ECM compliance and F-actin integrity (Chang et al, in preparation). Clearly, there is potential for crosstalk between LATS- and cytoskeletal-mediated control of YAP/TAZ. For example, in Drosophila, the LATS1/2 homologue Warts has been shown to regulate F-actin dynamics by phosphorylating ENA (a controller of F-actin capping) (Lucas et al., 2013) and, in mammalian cells, LATS1/2 phosphorylate AMOT (another F-actin regulator) (Mana-Capelli et al., 2014), collectively raising the prospect that LATS may feedback on the YAP/TAZ cytoskeletal/mechanotransduction cascade. In turn, cytoskeletal inhibition is also reinforced by LATS-mediated YAP phosphorylation (Aragona et al., 2013; Zhao et al., 2012). That said, the mechanistic details by which cell mechanics control YAP/TAZ remains an open quest for the field.
Mechanotransduction is intimately interwoven to cytoskeletal dynamics and thus not surprisingly F-actin regulators, such as F-actin capping and severing proteins, have been recently causally linked to YAP/TAZ control (Aragona et al., 2013; Fernandez et al., 2011; Sansores-Garcia et al., 2011). Intriguingly, conditional inactivation of the two main actin-severing proteins, ADF and cofillin, has been shown to induce increased cellular tension and dramatic organ overgrowths occurring in just days (Kanellos et al., 2015). The functional involvement of YAP in these phenotypes remains however to be determined.
Mechanotransduction is integral to life of all cells, including cells of the neoplastic stroma. These include cancer-associated fibroblasts (CAFs), responsible for synthesis of collagen and other ECM proteins and of the enzymes essential for their remodeling (see also Figure 4). As such, CAFs are involved in the generation of a mechanically aberrant tumor microenvironment. In addition, CAFs secrete interleukins and other chemokines, in turn constantly modifying the chemical and physical tumor niches and their cellular composition. It has been recently shown that YAP is a mechanically activated inducer of CAF function, and a marker for CAFs from early tumor stages (Calvo et al., 2013). In CAFs, ECM stiffening and contractility activate YAP through Src (Calvo et al., 2013), as Src may promote YAP nuclear functions through direct YAP phosphorylation, fostering TEAD association (Rosenbluh et al., 2012). In turn, raising YAP function in CAFs would turn on contractility and restructuring of the ECM, locking fibroblast in a mechanically self-sustaining YAP/TAZ feed-forward loop. The identification of YAP/TAZ as downstream effectors of cellular mechanotransduction has thus potentially relevant implications in cancer, linking the disturbed tissue integrity by which pathologists microscopically define tumors to the molecular mechanisms of malignant transformation.
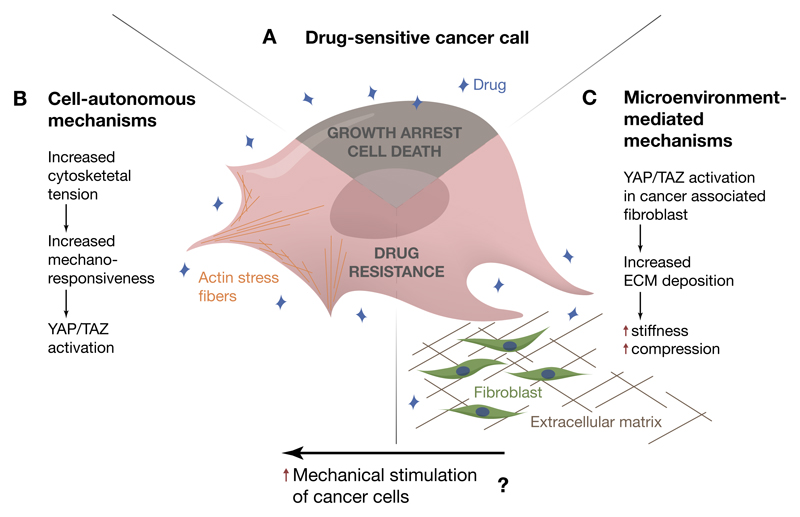
Figure 4. The onset of drug resistance is accompanied by YAP/TAZ activation.
(A-C) A) Drug sensitive cancer cells. The mechanism of YAP/TAZ activation in response to molecularly targeted therapies involves cell-autonomous events (B), such as cytoskeletal rearrangements leading to increased mechanosensitivity, as well as cell extrinsic events (C), such as remodeling of the extracellular microenvironment. Possibly, these two mechanisms cooperate to induce YAP/TAZ nuclear accumulation in cancer cells, leading to uncontrolled cell survival and proliferation in spite of drug administration.
YAP/TAZ mechanotransduction and drug resistance
Tumor cells with activated YAP/TAZ display resistance to chemotherapeutic drugs. This trait is part of a repertoire of CSC characteristics endowed to cancer cells by YAP/TAZ. Indeed, TAZ sustains survival of breast cancer stem cells treated with conventional chemotherapeutics, such as paclitaxel and doxorubicin, both in vitro and in experimental models (Bartucci et al., 2015; Cordenonsi et al., 2011). As discussed above, YAP/TAZ activation further confers protection against DNA-damaging agents - including cisplatin, UV, radiation - in a broad number of tumor types (Baia et al., 2012; Cheng et al., 2015; Ciamporcero et al., 2016; Fernandez et al., 2012; Hall et al., 2010; Mao et al., 2014).
YAP/TAZ also promote resistance to molecularly targeted therapies (Figure 4). Lin and colleagues reported that YAP promotes resistance to RAF and MEK inhibitors in several tumor cell lines harboring BRAF, KRAS or NRAS activating mutations (Lin et al., 2015c). IHC analysis in human melanoma specimens with BRAF mutation confirmed that high YAP expression is anti-correlated to responsiveness to RAF/MEK inhibitors. Thus, even though YAP/TAZ themselves are not primary oncogenic lesions, they act as survival inputs for tumor cells, dampening the efficacy of oncogene-targeting drugs.
Understanding the mechanisms leading to YAP activation during the acquisition of drug resistance is thus essential to develop more effective synthetic lethal strategies, or re-awaken drug sensitivity. Very recent studies have highlighted YAP/TAZ-regulation by mechanotransduction as central for empowering chemoresistance (Figure 4). Kim and colleagues showed that two events take place in BRAF-mutant melanoma cells as they acquire resistance to the BRAF-inhibitor vemurafenib: increase of the polymerization of actin stress fibers and YAP/TAZ accumulate in the nucleus of resistant cells (Kim et al., 2016). Thus, YAP activation seems to be the consequence of increased mechano-sensitivity. Consistently, the responsiveness of HER2-positive breast cancer cells to lapatinib is higher on soft ECM, whereas a stiff ECM would limit the anti-proliferative effect of lapatinib in a YAP/TAZ-dependent manner (Lin et al., 2015b). Of note, in vivo studies have demonstrated that BRAF inhibitors do not only act in melanoma cells but also in the surrounding tumor fibroblasts, activating them to produce a stiff, collagen-rich extracellular matrix (ECM) (Hirata et al., 2015). An intriguing hypothesis is that these two events - the cell-autonomous increase in responsiveness to ECM rigidity and the stiffening of the microenvironment - might occur simultaneously and concur to the activation of YAP in therapy-resistant cancer cells (Zanconato and Piccolo, 2015).
The recent progress on immune checkpoint therapies for treatment of melanoma and other cancer types raises questions as to how immune tolerance is installed in the tumor infiltrate. One mechanism is recruitment of a population of myeloid cells that are potent suppressors of T-cells by chemoattractive cytokines, such as CXCL5. Remarkably, CXCL5 expression is promoted by YAP in cancer cells (Wang et al., 2016). It is thus tempting to speculate that attenuation of YAP/TAZ mechanotransduction may blunt, at once, a triple threat of CSCs: tumor regeneration, chemoresistance and escape from immune surveillance.
Regulation by contact inhibition
A classic “social-like” behavior displayed by epithelial cells cultured in 2D or 3D is contact-inhibition of proliferation (CIP). It is thought that this represents a proxy of the growth-suppressive tissue microenvironment, and a form of “crowd” control that is disrupted during tumorigenesis. CIP occurs gradually, slowing down proliferation when cells initiate cell-cell contact, but causing complete growth arrest only when cells reach a stationary, postconfuent cell density as it occurs when spatial/physical constrains impede any further spreading or enlargement of the monolayer. Guan and colleagues first discovered that inhibition of YAP/TAZ is the key underlying mechanism of contact-inhibition (Zhao et al., 2007). But by what mechanism? The Hippo kinases are activated during the initial steps of contact inhibition, as revealed by an increased phospho-YAPS127 read-out in cells experiencing an “all around” cell-cell contact (Aragona et al., 2013; Zhao et al., 2007); in these conditions, LATS1/2 inactivation increases nuclear YAP/TAZ levels, and LATS1/2 knockdown rescues cadherin-induced proliferative decline (Kim et al., 2011). That said, reaching confluency typically leaves cells with sufficient YAP/TAZ activity to sustain proliferation. Growth-arrest in postconfluent cells in fact requires biomechanical inhibition of YAP/TAZ, as crowding imposes cells to be attached to a progressively smaller adhesive ECM area until a critical threshold is reached, below which YAP/TAZ are completely inactivated (Aragona et al., 2013). This step is independent from LATS1/2, but can be rescued by raising mechanotransduction, either by inducing distortions of the cell monolayer or by inhibiting the remodeling of the F-actin cytoskeleton from a “strained” state to one associated to “softer” cellular conditions (Aragona et al., 2013; Benham-Pyle et al., 2015). Consistently, raising cellular mechanotransduction by ECM stiffening disables CIP, allowing contact-independent uniform proliferation (Kim and Asthagiri, 2011). Moreover, YAP/TAZ mechanotrasduction is also activated when E-cadherin cell-cell junctions are mechanically stretched: application of strain to quiescent, contact inhibited, dense cell monolayers induces rapid YAP nuclear accumulation and YAP-driven cell cycle reentry (Benham-Pyle et al., 2015).
α-catenin is a component of adherens junctions and a relevant suppressor of YAP/TAZ activity in several tissues (Herr et al., 2014; Li et al., 2015a; Schlegelmilch et al., 2011; Silvis et al., 2011). Mechanistically, α-catenin operates independently of the Hippo/LATS kinases (Li et al., 2016; Schlegelmilch et al., 2011; Silvis et al., 2011). Interestingly, it has been recently proposed that loss-of-α-catenin imposes a remodeling of the cellular adhesion complexes in favor of integrin/Src signaling leading to YAP activation by phosphorylation in tyrosine residues (Li et al., 2016).
Control by cell polarity and EMT
Loss of cell polarity is a hallmark of cancer and an initial step in Epithelial-to-Mesenchymal transition (EMT), a transdifferentiation program whereby epithelial cell acquire a more spread, migratory and fibroblast-like shape. Intriguing, this morphological transition also empowers cancer stem cell properties in tumor cells (Hanahan and Weinberg, 2011). Remarkably, EMT induces and requires YAP/TAZ for triggering cancer stemness and metastasis (Cordenonsi et al., 2011). This has been connected to the role of Scribble (Scrib), a protein that organizes basolateral cell polarity along with other two tumor suppressors, DLG and LGL (that is, the Scribble complex). For YAP/TAZ regulation, Scribble operates as membrane-localized adaptor for LATS and MST, leading to LATS activation and YAP/TAZ phosphorylation (Cordenonsi et al., 2011). That said, it remains to be formally demonstrated that SCRIB entirely operates through LATS1/2 or also other pathways.
The role of Scribble as regulatory node of YAP/TAZ goes beyond EMT. Scribble mediates the tumor suppressing effects of the tumor suppressor LKB1 that stabilizes the Scribble/Hippo kinase complex by phosphorylating Par1 (Mohseni et al., 2014). Moreover, inducing membrane localization of Scribble by LIFR signaling is sufficient to blunt YAP/TAZ and suppress metastasis in breast cancer (Chen et al., 2012).
The Scribble complex plays a prominent role in human malignancies: it is inactivated or delocalized away from cell membranes in a broad variety of tumors (including colon, breast, cervical, prostate and lung) and Scrib+/- mice are tumor prone (Martin-Belmonte and Perez-Moreno, 2011; Pearson et al., 2011; Zhan et al., 2008). In addition to LKB1 defects or classic EMT-inducing signals such as TGF-β, a number of distinct cues may induce Scribble inactivation. Importantly, YAP/TAZ are not only downstream of EMT but also active inducers of EMT (Shao et al., 2014; Zhao et al., 2007), opening the possibility of sophisticated self-sustaining loops between cell polarity and other YAP/TAZ regulations.
“Wounds that never heals….”: regulation by tissue damage and inflammation
YAP/TAZ are fundamental for tumorigenesis but remarkably dispensable for the homeostasis of most adult tissues, which makes them appealing targets of cancer therapy. The role of YAP/TAZ in cancer is, however, not a cancer-specific event, as it recapitulates an equally essential function of YAP/TAZ for physiological tissue repair. In the intestine, for example, YAP/TAZ are dispensable in the unperturbed adult intestine but critical for intestinal wound healing (Azzolin et al., 2014; Cai et al., 2010; Taniguchi et al., 2015).
After injury of an epithelial organ, restoration of epithelial integrity occurs rapidly and includes loss of contact inhibition, spreading over the ECM and loss of epithelial polarity at the wound margins. As per the above discussion, all these inputs are well known potential inducers of YAP/TAZ activity. In addition, tissue repair is intimately associated to inflammation, with recruitment of myeloid cells that secrete proinflammatory cytokines, such as IL6, in turn able to induce robust proliferation and migration of epithelial cells. Recent work in transgenic mice showed that overexpression of the general IL-co-receptor gp130 in the intestinal epithelium induces hyperproliferative intestinal crypts displaying aberrant differentiation but resistant to mucosal damage (Taniguchi et al., 2015). These inflammatory responses are entirely YAP-dependent and associated to YAP stabilization and nuclear translocation. Thus, YAP activation serves as link between inflammation and epithelial repair. Importantly, injury/inflammation-driven YAP activation may represent a general requirement for YAP-induced proliferation in both tissue repair and cancer.
YAP activation at inflammation/injury sites occurs largely through Hippo/LATS-independent mechanisms, through activation of Src kinases (Taniguchi et al., 2015). Elevation of YAP/TAZ levels in Hippo mutant mice is unable, by itself, to trigger early onset intestinal tumorigenesis, but this occurs only after intestinal damage (Cai et al., 2010). A similar scenario presents itself in the liver, where upregulation of YAP in individual cells of the liver is inconsequential in absence of a permissive signal given by inflammatory cytokines (Su et al., 2015). YAP activation may thus explain why chronic inflammatory diseases in humans can increase risks of tumorigenesis. In the liver, for example, an excess of bile accumulation in cholestatic diseases triggers YAP-driven proliferation and HCC development (Anakk et al., 2013; Bai et al., 2012; Su et al., 2015).
Inflammation has been described as an “enabling” factor for many capabilities of cancer cells in virtually all neoplastic lesions. The duality of inflammation as promoter of tumorigenesis and tissue repair - and the recognition of the similarities between physiological and neoplastic inflammation - evokes the original recognition by R. Virchow that tumors are “wound that never heal” (Virchow, 1858). It is remarkable that YAP/TAZ activation is emerging as a common element of these classic connections and invariable traits of neoplastic lesions.
Regulation by Oncogenic lesions
APC mutations and Wnt signaling
Constitutive activation of the Wnt pathway is a main oncogenic stimuli for distinct tissues (Clevers, 2006). Wnts are secreted growth factors that interact with membrane receptors, Frizzled and LRP5/6, leading to the inactivation of a cytoplasmic protein complex, named the β-catenin destruction complex. Within this complex, two central scaffold proteins, Axin1/2 and adenomatous polyposis coli (APC), recruit the kinase glycogen synthase kinase-3 (GSK3) to phosphorylate β-catenin promoting its degradation (Clevers, 2006).
Recent studies found that, in the absence of Wnt stimulation, cytoplasmic YAP/TAZ contribute to the function of the destruction complex (Azzolin et al., 2014) (Figure 3). YAP/TAZ bind directly Axin, APC and β-catenin (Azzolin et al., 2014; Cai et al., 2015; Imajo et al., 2012), causing their sequestration in the complex, and also causing TAZ to interact with β-TrCP, leading to its degradation (Azzolin et al., 2014; Azzolin et al., 2012). Therefore, Wnt stimulation, depletion of Axin1/2 or of APC release YAP/TAZ from such cytoplasmic sites and promote YAP/TAZ nuclear accumulation and target genes transcription (Azzolin et al., 2014; Azzolin et al., 2012; Byun et al., 2014)(Figure 3); as expected, Wnt activation of YAP/TAZ can be blocked by the Tankyrase inhibitor XAV939, a compound that promotes Axin1/2 stabilization (Azzolin et al., 2012). In line, YAP/TAZ are detected in the nucleus of most cells composing Wnt1-induced mammary tumors (Park et al., 2015; Serrano et al., 2013), and are largely upregulated and nuclear in colon adenomas of ApcMin/+ mice, and in the hyperplastic epithelium forming after conditional knockout of Apc in the intestine (Azzolin et al., 2014; Cai et al., 2015; Camargo et al., 2007; Gregorieff et al., 2015). Functionally, as detailed above for CRC, YAP/TAZ are required downstream to APC mutations for uncontrolled proliferation of the intestinal epithelium and colon tumorigenesis (Azzolin et al., 2014; Cai et al., 2015; Gregorieff et al., 2015)(Figure 3). In CRC cells bearing inactivating APC mutations, β-catenin and TAZ cooperate to promote cell growth, although TAZ may specifically endow CRC cells with clonogenic properties (Azzolin et al., 2012). Moreover, in CRC cells with hyper-active β-catenin, YAP is required to foster β-catenin dependent cancer cell survival (Rosenbluh et al., 2012). Consistently, concomitant activation of YAP/TAZ and β-catenin in the mouse intestine leads to formation of adenomas, reminiscent of those induced by Apc mutations (Cai et al., 2015).
Nuclear localization of YAP/TAZ is by itself an event that depletes the destruction complex favoring β-catenin nuclear localization; for example nuclear translocation of YAP/TAZ induced by strong mechanical stimuli - such as those produced by inflammation, fibrosis, or stretching epithelial monolayers - has been recently shown to be sufficient to be followed by β-catenin activation even without overt presence of Wnt ligands (Benham-Pyle et al., 2015; Nowell et al., 2015). Thus, the mechanical control of YAP/TAZ can feedback into the same pathways that control tissue patterning, providing a mean for the cell's structural features to attain a high-order control over growth factor signaling.
Finally, YAP/TAZ could be also activated by non-canonical Wnt signaling, involving Frizzled/ROR/Gα12-13 signaling rather than LRP5/6 (Park et al., 2015). The involvement of this Wnt signaling branch for YAP/TAZ activation in cancer remains unclear.
Lkb1 signaling
Lkb1 is a master tumor suppressor that is frequently mutated in NSCLC and cervical carcinoma. In normal cells, Lkb1 activates two independent pathways: a) in combination with starvation, Lkb1 activates AMPK and related kinases that, in turn, inactivate the mTOR complex; b) Lkb1 activates MARK proteins in the basal compartment of epithelial cells, facilitating YAP inhibition through the Scribble regulatory module (Mohseni et al., 2014). YAP/TAZ are activated by loss-of-Lkb1 in mouse models of NSCLC, and are required to sustain tumor progression (Mohseni et al., 2014; Zhang et al., 2015b).
G-protein coupled receptors (GPCR)
The discovery of RhoGTPases as global regulators of YAP/TAZ activity prompted the intriguing discovery that soluble ligands signaling through GPCR and Rho can promote YAP/TAZ nuclear localization and transcriptional activities (Yu et al., 2012) (Figure 3). This pathway also involves the cytoskeleton, LATS-mediated phosphorylation and distinct G-protein α-subunits. The latters are found mutated in various types of cancers, suggesting they can work as oncogenes (O'Hayre et al., 2013). A strong evidence supporting this notion is the fact that the genes coding for Gαq and Gα11 are frequently mutated in uveal melanoma (about 2/3 of cases). Indeed, overexpression of a mutant form of Gαq in melanocytes promotes melanoma formation in transgenic mice, and Gαq knockdown in a human UM cell line impairs their ability to form subcutaneous tumors in nude mice (Feng et al., 2014; Yu et al., 2014). Two recent studies showed that overexpression of oncogenic Gαq promotes nuclear localization of YAP, both in cell cultures and in vivo. How oncogenic Gαq activates YAP is unclear, as one study claimed that Gαq ultimately impact on LATS-mediated phosphorylation of YAP (Yu et al., 2014), while the other suggested that Gαq promotes changes in the actin cytoskeleton involving AMOT, but functionally independent on LATS (Feng et al., 2014).
Regulation by viral proteins
Human Papilloma Virus (HPV)
Infection by oncogenic strains of HPV is the leading cause of cervical carcinomas. This is mainly caused by the expression of two viral proteins, E6 and E7, which produce multiple effects in the infected cells. Among these, E6 proteins from oncogenic HPVs promote stabilization of YAP protein (He et al., 2015). This could explain the upregulation of YAP protein detected in mouse cervical tumors induced by transgenic expression of oncogenic E6/E7.
Kaposi sarcoma-associated herpesvirus (KSHV)
Kaposi’s sarcoma is a specific form of soft-tissue sarcoma that is induced by KSHV infection; as such it typically occurs in immunodeficient patients. The genome of KSHV contains a viral GPCR (vGPCR). KSHV infection or overexpression vGPCR can trigger YAP/TAZ activation in human cells through a pathway that involves activation of Gαq/11 and Gα12/13 (Liu et al., 2015). In line, YAP/TAZ knockdown strongly inhibits vGPCR-induced cell growth, and tumor growth of Kaposi’s sarcoma cells injected subcutaneously in nude mice.
Regulation by cancer metabolism
Cancer cells modify their metabolic profile to adjust energy production to their increased need of biosynthetic building blocks, such as lipids, amino acids and nucleosides. In turn, cell metabolism must feedback on the signals and transcription factors inducing cell division, to ensure that this process initiate only when the cell’s metabolic conditions can sustain cell proliferation. New evidence indicates that YAP/TAZ participate in these feedbacks (Figure 3). The first demonstration related to mevalonate pathway, synthesizing cholesterol and other metabolites. Inhibiting this pathway blunts YAP/TAZ function in cultured cells (Sorrentino et al., 2014; Wang et al., 2014). The discovery of Rho-GTPases as key determinants of YAP/TAZ function indicated a mechanism: Rho, just like other small-GTPases, is activated by prenylation, a post-translational modification attaching a lipid chain for membrane anchoring. These lipids are generated by the mevalonate pathway, and reducing their bioavailability may represent a strategy to curb YAP/TAZ activity in vivo. A number of very safe FDA-approved drugs already exists to limit this pathway for the prevention of cardiovascular diseases or other diseases, such as Statins and Bisphosphonates, and it is encouraging that in large epidemiological studies patients treated with Statins are less prone to develop tumors and that mevalonate inhibitors can oppose the growth of HNSCC cells in mice (Li et al., 2015d; Sorrentino et al., 2014; Wang et al., 2014).
Glycolysis is typically boosted in cancer cell as part of the “Warburg effect” and this regulates YAP/TAZ activity. One mechanism entails phospho-fructo-kinase (PFK1), an enzyme that controls the first rate-limiting step of glycolysis and whose expression is induced in malignancies. PFK1 has been shown to associate to TEAD in the nucleus, facilitating YAP/TEAD association (Enzo et al., 2015). The AMPK kinase provides another mechanism by which glycolysis, and more generally energy metabolism, may feedback on YAP/TAZ: in this model, AMPK is induced by low energy conditions and attenuates YAP/TAZ function (DeRan et al., 2014; Mo et al., 2015; Wang et al., 2015b). Intriguingly, chemical AMPK inducers, such as the anti-diabetic drugs Metformin and AICAR, have been shown to partially attenuate expression of YAP/TAZ-dependent target genes. Although AMPK knockouts do not cause tissue/organ overgrowth in mammals, such connection exists in Drosophila (Gailite et al., 2015). Mechanistically, AMPK may act on YAP directly, causing its phosphorylation and inhibition of TEAD association or indirectly, through LASTs. Yet, YAP inhibition by AMPK occurs unabated in both LATS-mutant fly embryos and in LATS1/2 knockout MEFs, indicating that AMPK works, at least in part, independently of LATS1/2 (Gailite et al., 2015; Mo et al., 2015).
YAP/TAZ cooperation with AP1 and other nuclear factors
Transcription factors do not work in isolation, as control of gene expression is achieved through combinatorial interactions between different transcription factors bound at neighboring cognate sites on cis-regulatory DNA elements. YAP/TAZ conform to this general rule and integrate with other nuclear factors to unfold their gene-expression programs. By ChIP-Seq analyses in a number of different tumor types, YAP/TAZ cooperate with AP-1 factors (Activator Protein 1), dimers of the prototypic oncogenes Jun and Fos (Stein et al., 2015; Zanconato et al., 2015). YAP/TAZ/TEAD and AP-1 are co-recruited on the majority of YAP/TAZ-bound enhancers (Liu et al., 2016; Stein et al., 2015; Verfaillie et al., 2015; Zanconato et al., 2015), and synergistically activate target genes to promote cell proliferation and transformation (Zanconato et al., 2015; Liu et al., 2016). In prostate cancer, ERG, a transcription factor of the ETS family, is frequently activated in prostate cancer due to chromosomal rearrangements. Nguyen et al. found that ERG and YAP/TEAD share a wide set of target genes (Nguyen et al., 2015).
YAP/TAZ are generally considered as transcriptional co-activators and TEAD factors have been shown to be required for YAP/TAZ transcriptional responses in most cellular contexts (Bhat et al., 2011; Chan et al., 2009; Galli et al., 2015; Kapoor et al., 2014; Stein et al., 2015; Xia et al., 2014; Zanconato et al., 2015; Zhao et al., 2008), to the extent that inhibiting YAP/TAZ-TEAD interaction represents one of the most promising avenues for anti-YAP/TAZ therapeutic intervention (Chen et al., 2010; Liu-Chittenden et al., 2012; Pobbati et al., 2015; Tian et al., 2010).
TEAD factors have been shown to bind proteins other than YAP/TAZ. Vgl-like-4 (VGLL4) is an interesting point in case: it competes with YAP for binding to TEAD and, by overexpression, it can oppose YAP-induced tumor growth (Jiao et al., 2014; Koontz et al., 2013; Zhang et al., 2014a). Low VGLL4 levels may thus sensitize cellular responsiveness to YAP/TAZ inducing signals, as it has been reported in gastric and lung cancer (Jiao et al., 2014; Zhang et al., 2014a). Moreover, a synthetic peptide mimicking the TEAD binding domain in VGLL4 showed some therapeutic activity in gastric cancer (Jiao et al., 2014).
Perspectives and Open Issues
The above presentation indicates that many genetic or epigenetic inputs actually converge on sustaining nuclear YAP/TAZ activities in cancer. That said, as exemplified by the role of YAP/TAZ in chemoresistance, even the most aggressive cancer cells cannot fully autonomously sustain YAP/TAZ, as they still rely on physical and structural inputs generated by a proficient microenvironment. YAP/TAZ are normally inactive in normal tissues, but in early neoplastic lesions, YAP/TAZ can be re-activated by cell shape distortions that accompany tissue disorganization or attachment to an increasingly abnormal and rigid ECM. In addition, cancer cells may display an intrinsically enhanced mechanoresponsiveness, enabling sustainment of YAP/TAZ through mechanical inputs that would be below threshold for normal cells. As such, intrinsic (oncogenic lesions, disturbed Hippo signaling etc.) and biomechanical cues must conspire to wreak havoc spatial control of YAP/TAZ. Why tumors need to exploit different cues for activating the same transcription factor is unclear, but this may be linked to the need of bypassing molecularly distinct gatekeepers, for example one ensuring lower level of total YAP/TAZ, and one restricting their nuclear/transcriptional functions. Thus, cancer cells may use different combinations of such inputs, in a manner that may change from tumor to tumor, and even between different areas of the same lesion.
A better understanding of these events may suggest innovative strategies to treat cancer, for example inducing “normalization” of tumor cells by reverting them from a more malignant to a benign, differentiated phenotype. This is not so far fetched: after all, inactivation of Hippo pathway components causes organ to grow well beyond their normal size up to a ostensible preneoplastic state; and yet this is a reversible condition, as when the YAP/TAZ inducing stimuli is interrupted, the big organ shrinks back to its correct size, losing the excess of cells by apoptosis (Pan, 2010). How the organ normalizes itself after overgrowth is mysterious; a likely possibility is that while the organ recoils to its correct size, local mechanical cues may instruct death or survival of each individual cell with great specificity.
There is also correlative evidence that cells that retain elevated YAP levels, but are no longer in a YAP-proficient environment (e.g., after resolution of inflammation/tissue repair, or transition from a rigid to a more pliant ECM), may take care of themselves: under these conditions, YAP may turn from a tumor inducer to a tumor suppressor, causing elimination of that same cell (Su et al., 2015). Clearly, being able to engineer the cell microenvironment and flip the power of YAP/TAZ against the tumor would represent an elegant strategy for tumor eradication.
YAP/TAZ are required in cancer and wound healing, but not for normal homeostasis of adult tissues. Understanding the nature of this puzzling difference is another very important issue for the field. Although YAP/TAZ have been discussed as potentially associated to normal somatic stem cells, genetic evidence do not support this conclusion, at least in vivo. In contrast, YAP/TAZ might turn out essential for a different event: de-novo generation of new stem cell-like cells starting from more differentiated cells. It is known that this conversion is exploited by tissues when they are in immediate need of new stem cells to counteract tissue damage, for example after exhaustion of “professional” somatic SCs (Blanpain and Fuchs, 2014). Cancer is also a disease of disturbed cell differentiation as much as it is of aberrant cell proliferation. Data from different types of human cancer and mouse models at least suggest that acquisition of an immature, stem-like state is a prerequisite for tumor progression (Blanpain and Fuchs, 2014). Expression of YAP/TAZ reprograms non-stem tumor cells into cells with cancer stem cell characteristics (Cordenonsi et al., 2011). Such ability of YAP/TAZ to induce transdifferentiation prior to tumorigenesis has been also noticed in YAP-expressing transgenic livers, turning hepatocytes into biliary progenitors (Yimlamai et al., 2014). The ability of YAP/TAZ to induce cell fate plasticity intriguingly resonates with the ability of common oncogenes to trigger lineage-infidelity (Van Keymeulen et al., 2015). Clearly more work is required to validate these scenarios.
A third aspect that should deserve attention is the mechanisms by which YAP/TAZ select their downstream transcriptional programs and transcriptional partners, as this may reveal new players and mechanisms by which YAP/TAZ orchestrate cancer cell behavior and suggest new targets for therapy.
Acknowledgments
We thank all members of SP lab for discussion. FZ is supported by a fellowship from Italian Association for Cancer Research (AIRC). This work is supported by AIRC Special Program Molecular Clinical Oncology “5 per mille” and an AIRC PI-Grant to S.P and by Epigenetics Flagship project CNR-Miur grants to S.P. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 670126-DENOVOSTEM).
Footnotes
Author Contributions
All authors collected and discussed the material, FZ prepared figures and MC the table. All authors contributed to write the MS.
References
- Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. Bile acids activate YAP to promote liver carcinogenesis. Cell reports. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, Chen HW, Pathan N, Krausz T, Dickson BC, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes, chromosomes & cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baia GS, Caballero OL, Orr BA, Lal A, Ho JS, Cowdrey C, Tihan T, Mawrin C, Riggins GJ. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Molecular cancer research : MCR. 2012;10:904–913. doi: 10.1158/1541-7786.MCR-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, Basilico C. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nature communications. 2015;6 doi: 10.1038/ncomms7411. 6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes & development. 2011;25:2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344 doi: 10.1126/science.1242281. 1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boin A, Couvelard A, Couderc C, Brito I, Filipescu D, Kalamarides M, Bedossa P, De Koning L, Danelsky C, Dubois T, et al. Proteomic screening identifies a YAP-driven signaling network linked to tumor cell proliferation in human schwannomas. Neuro-oncology. 2014;16:1196–1209. doi: 10.1093/neuonc/nou020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS, Chirieac LR, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nature Genetics. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB, Hong JH. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell death and differentiation. 2014;21:854–863. doi: 10.1038/cdd.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes & development. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes & development. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857–4866. doi: 10.1038/onc.2013.433. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284:14347–14358. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC, Ma L. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chan SW, Zhang X, Walsh M, Lim CJ, Hong W, Song H. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes & development. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Xie R, Wang W, Cai J, Choi KS, David KK, Huang B, Yabuta N, Nojima H, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes & development. 2015;29:1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Zhang Z, Rodriguez-Barrueco R, Borczuk A, Liu H, Yu J, Silva JM, Cheng SK, Perez-Soler R, Halmos B. Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget. 2015 doi: 10.18632/oncotarget.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciamporcero E, Shen H, Ramakrishnan S, Yu Ku S, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti S, et al. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene. 2016;35:1541–1553. doi: 10.1038/onc.2015.219. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri A, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da CL, Xin Y, Zhao J, Luo XD. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World J Gastroenterol. 2009;15:4055–4061. doi: 10.3748/wjg.15.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Fischer RS, Pan D, Waterman CM. YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, myosin II- and phospho-YAP independent pathway during ECM mechanosensing. J Biol Chem. 2016 doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell reports. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Agostino S, Sorrentino G, Ingallina E, Valenti F, Ferraiuolo M, Bicciato S, Piazza S, Strano S, Del Sal G, Blandino G. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO reports. 2015;17(2):188–201. doi: 10.15252/embr.201540488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Martin J, Lopez-Garcia MA, Romero-Perez L, Atienza-Amores MR, Pecero ML, Castilla MA, Biscuola M, Santon A, Palacios J. Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer. Endocrine-related cancer. 2015;22:443–454. doi: 10.1530/ERC-14-0456. [DOI] [PubMed] [Google Scholar]
- Diep CH, Zucker KM, Hostetter G, Watanabe A, Hu C, Munoz RM, Von Hoff DD, Han H. Down-regulation of Yes Associated Protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One. 2012;7:e32783. doi: 10.1371/journal.pone.0032783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Eisinger-Mathason TS, Mucaj V, Biju KM, Nakazawa MS, Gohil M, Cash TP, Yoon SS, Skuli N, Park KM, Gerecht S, Simon MC. Deregulation of the Hippo pathway in soft-tissue sarcoma promotes FOXM1 expression and tumorigenesis. Proc Natl Acad Sci U S A. 2015;112:E3402–3411. doi: 10.1073/pnas.1420005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. The EMBO journal. 2015;34:1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes, chromosomes & cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehogassociated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes & development. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LA, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, Nahle Z, Kenney AM. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitamant J, Kottakis F, Benhamouche S, Tian HS, Chuvin N, Parachoniak CA, Nagle JM, Perera RM, Lapouge M, Deshpande V, et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell reports. 2015 doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Toyoda T, Nakanishi H, Yatabe Y, Sato A, Matsudaira Y, Ito H, Murakami H, Kondo Y, Kondo E, et al. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209:479–494. doi: 10.1084/jem.20111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailite I, Aerne BL, Tapon N. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc Natl Acad Sci U S A. 2015;112:E5169–5178. doi: 10.1073/pnas.1505512112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, Gurung B, Pepe-Mooney B, Zhang T, Geeven G, Gray NS, de Laat W, et al. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Mol Cell. 2015;60:328–337. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Current opinion in cell biology. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- George NM, Day CE, Boerner BP, Johnson RL, Sarvetnick NE. Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol. 2012;32:5116–5128. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yapdependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Guo Y, Pan Q, Zhang J, Xu X, Liu X, Wang Q, Yi R, Xie X, Yao L, Liu W, Shen L. Functional and clinical evidence that TAZ is a candidate oncogene in hepatocellular carcinoma. Journal of cellular biochemistry. 2015;116:2465–2475. doi: 10.1002/jcb.25117. [DOI] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, Hilsenbeck SG, Orsulic S, Goode S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang LJ, Li M, Ying X, Zhu Q. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 2014;2014 doi: 10.1155/2014/261365. 261365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, Fukushima Y, Ishimoto T, Kuroki H, Nitta H, Hashimoto D, et al. An Imbalance in TAZ and YAP Expression in Hepatocellular Carcinoma Confers Cancer Stem Cell-like Behaviors Contributing to Disease Progression. Cancer Res. 2015;75:4985–4997. doi: 10.1158/0008-5472.CAN-15-0291. [DOI] [PubMed] [Google Scholar]
- He C, Mao D, Hua G, Lv X, Chen X, Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7:1426–1449. doi: 10.15252/emmm.201404976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr KJ, Tsang YH, Ong JW, Li Q, Yap LL, Yu W, Yin H, Bogorad RL, ahlman JE, Chan YG, et al. Loss of alpha-catenin elicits a cholestatic response and impairs liver regeneration. Scientific reports. 2014;4 doi: 10.1038/srep06835. 6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemer SE, Zhang L, Kartha VK, Packer TS, Almershed M, Noonan V, Kukuruzinska M, Bais MV, Monti S, Varelas X. A YAP/TAZRegulated Molecular Signature Is Associated with Oral Squamous Cell Carcinoma. Molecular cancer research : MCR. 2015;13:957–968. doi: 10.1158/1541-7786.MCR-14-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xin Y, Xiao Y, Zhao J. Overexpression of YAP1 is correlated with progression, metastasis and poor prognosis in patients with gastric carcinoma. Pathol Oncol Res. 2014;20:805–811. doi: 10.1007/s12253-014-9757-y. [DOI] [PubMed] [Google Scholar]
- Hua G, Lv X, He C, Remmenga SW, Rodabough KJ, Dong J, Yang L, Lele SM, Yang P, Zhou J, et al. YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells. Oncogene. 2015 doi: 10.1038/onc.2015.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. The EMBO journal. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Kim SB, Sohn BH, Park YY, Park ES, Kim SC, Kim SS, Johnson RL, Birrer M, Bowtell DS, et al. Activation of YAP1 is associated with poor prognosis and response to taxanes in ovarian cancer. Anticancer Res. 2014;34:811–817. [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. The EMBO journal. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellos G, Zhou J, Patel H, Ridgway RA, Huels D, Gurniak CB, Sandilands E, Carragher NO, Sansom OJ, Witke W, et al. ADF and Cofilin1 Control Actin Stress Fibers, Nuclear Integrity, and Cell Survival. Cell reports. 2015;13:1949–1964. doi: 10.1016/j.celrep.2015.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2130–2139. doi: 10.1158/1078-0432.CCR-10-2467. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim SH, Lee OJ, Huang SM, Kwon JL, Kim JM, Kim JY, Seong IO, Song KS, Kim KH. Differential expression of Yes-associated protein and phosphorylated Yes-associated protein is correlated with expression of Ki-67 and phospho-ERK in colorectal adenocarcinoma. Histol Histopathol. 2013a;28:1483–1490. doi: 10.14670/HH-28.1483. [DOI] [PubMed] [Google Scholar]
- Kim GJ, Kim H, Park YN. Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS One. 2013b;8:e75449. doi: 10.1371/journal.pone.0075449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Jung WH, Koo JS. Expression of Yes-associated protein (YAP) in metastatic breast cancer. Int J Clin Exp Pathol. 2015a;8:11248–11257. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. Journal of cell science. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Kim J, Hong H, Lee SH, Lee JK, Jung E, Kim J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. The EMBO journal. 2016;35:462–78. doi: 10.15252/embj.201592081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Jung WH, Koo JS. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol. 2014;7:3224–3234. [PMC free article] [PubMed] [Google Scholar]
- Kim T, Yang SJ, Hwang D, Song J, Kim M, Kyum Kim S, Kang K, Ahn J, Lee D, Kim MY, et al. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nature communications. 2015b;6 doi: 10.1038/ncomms10186. 10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes & development. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, Beede AM, Montoro DT, Sinkevicius KW, Walton ZE, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. The EMBO journal. 2014;33:468–481. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, He Y, Pare J, Neale G, Olson EN, Giovannini M, Cao X. Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators. Development. 2013;140:3323–3334. doi: 10.1242/dev.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, Lim DS. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Lee SS, Kim SB, Sohn BH, Lee HS, Jang HJ, Park YY, Kopetz S, Kim SS, Oh SC, Lee JS. Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:357–364. doi: 10.1158/1078-0432.CCR-14-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ Regulate Skin Wound Healing. The Journal of investigative dermatology. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- Li H, Wolfe A, Septer S, Edwards G, Zhong X, Abdulkarim AB, Ranganathan S, Apte U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, van Roy F, Radice GL. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res. 2015a;116:70–79. doi: 10.1161/CIRCRESAHA.116.304472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. alphaE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes & development. 2016;30:798–811. doi: 10.1101/gad.274951.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yu Z, Chen SS, Li F, Lei CY, Chen XX, Bao JM, Luo Y, Lin GZ, Pang SY, Tan WL. The YAP1 oncogene contributes to bladder cancer cell proliferation and migration by regulating the H19 long noncoding RNA. Urol Oncol. 2015b;33:427 e421–410. doi: 10.1016/j.urolonc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Li YW, Shen H, Frangou C, Yang N, Guo J, Xu B, Bshara W, Shepherd L, Zhu Q, Wang J, et al. Characterization of TAZ domains important for the induction of breast cancer stem cell properties and tumorigenesis. Cell Cycle. 2015c;14:146–156. doi: 10.4161/15384101.2014.967106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang W, Qi B, Qiu J, Song X, Ye J, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol. 2015d;9:1091–1105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yao E, Chuang PT. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev Biol. 2015a;403:101–113. doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, LaBarge MA. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Molecular biology of the cell. 2015b;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015c;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yu FX, Kim YC, Meng Z, Naipauer J, Looney DJ, Liu X, Gutkind JS, Mesri EA, Guan KL. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2015;34:3536–3546. doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ, Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013a;13:349. doi: 10.1186/1471-2407-13-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu Y, Gao H, Meng F, Yang S, Lou G. Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int J Gynecol Cancer. 2013b;23:735–742. doi: 10.1097/IGC.0b013e31828c8619. [DOI] [PubMed] [Google Scholar]
- Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, et al. Tead and AP1 Coordinate Transcription and Motility. Cell reports. 2016;14:1169–1180. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & development. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588(16):2663–70. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EP, Khanal I, Gaspar P, Fletcher GC, Polesello C, Tapon N, Thompson BJ. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. The Journal of cell biology. 2013;201:875–885. doi: 10.1083/jcb.201210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mana-Capelli S, Paramasivam M, Dutta S, McCollum D. Angiomotins link F-actin architecture to Hippo pathway signaling. Molecular biology of the cell. 2014;25:1676–1685. doi: 10.1091/mbc.E13-11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F, Meng S, Wang Y, Yuan Z, Bi W. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468–1474. doi: 10.1038/onc.2013.88. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2011;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes & development. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nature communications. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel M, Meckbach D, Weide B, Toussaint NC, Schilbach K, Noor S, Eigentler T, Ikenberg K, Busch C, Quintanilla-Martinez L, et al. In melanoma, Hippo signaling is affected by copy number alterations and YAP1 overexpression impairs patient survival. Pigment Cell Melanoma Res. 2014;27:671–673. doi: 10.1111/pcmr.12249. [DOI] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nature cell biology. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nature cell biology. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvaridi S, Dhall D, Greene MI, Pandol SJ, Wang Q. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Scientific reports. 2015;5:16759. doi: 10.1038/srep16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T, Akatsuka S, Horio Y, Hida T, Kondo Y, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T, Inazawa J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389–398. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- Nallet-Staub F, Marsaud V, Li L, Gilbert C, Dodier S, Bataille V, Sudol M, Herlyn M, Mauviel A. Pro-Invasive Activity of the Hippo Pathway Effectors YAP and TAZ in Cutaneous Melanoma. The Journal of investigative dermatology. 2014;134:123–132. doi: 10.1038/jid.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, Bolouri H, Kutyavin VI, Morrissey C, True LD, et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Saito A, Horie M, Mikami Y, Suzuki HI, Morishita Y, Ohshima M, Abiko Y, Mattsson JS, Konig H, et al. An integrative analysis of the tumorigenic role of TAZ in human non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:4660–4672. doi: 10.1158/1078-0432.CCR-13-3328. [DOI] [PubMed] [Google Scholar]
- Nowell CS, Odermatt PD, Azzolin L, Hohnel S, Wagner EF, Fantner GE, Lutolf MP, Barrandon Y, Piccolo S, Radtke F. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nature cell biology. 2015;18:168–80. doi: 10.1038/ncb3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JE, Ohta T, Satomi K, Foll M, Durand G, McKay J, Le Calvez-Kelm F, Mittelbronn M, Brokinkel B, Paulus W, Ohgaki H. Alterations in the NF2/LATS1/LATS2/YAP Pathway in Schwannomas. J Neuropathol Exp Neurol. 2015;74:952–959. doi: 10.1097/NEN.0000000000000238. [DOI] [PubMed] [Google Scholar]
- Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Developmental Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Li S, Chi P, Xu Z, Lu X, Huang Y. Lentivirus-mediated RNA interference targeting WWTR1 in human colorectal cancer cells inhibits cell proliferation in vitro and tumor growth in vivo. Oncology reports. 2012;28:179–185. doi: 10.3892/or.2012.1751. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HB, Perez-Mancera PA, Dow LE, Ryan A, Tennstedt P, Bogani D, Elsum I, Greenfield A, Tuveson DA, Simon R, Humbert PO. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J Clin Invest. 2011;121:4257–4267. doi: 10.1172/JCI58509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra A, Kowalik MA, Ghiso E, Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore E, Roncalli M, Giordano S, Columbano A. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61:1088–1096. doi: 10.1016/j.jhep.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiological reviews. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- Pobbati AV, Han X, Hung AW, Weiguang S, Huda N, Chen GY, Kang C, Chia CS, Luo X, Hong W, Poulsen A. Targeting the Central Pocket in Human Transcription Factor TEAD as a Potential Cancer Therapeutic Strategy. Structure. 2015;23:2076–2086. doi: 10.1016/j.str.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian J, Le Scolan E, Ji X, Zhu Q, Mulvihill MM, Nomura D, Luo K. Ski regulates Hippo and TAZ signaling to suppress breast cancer progression. Science signaling. 2015;8:ra14. doi: 10.1126/scisignal.2005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Zhang L, Jiang J. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol. 2010;337:303–312. doi: 10.1016/j.ydbio.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Perez L, Garcia-Sanz P, Mota A, Leskela S, Hergueta-Redondo M, Diaz-Martin J, Lopez-Garcia MA, Castilla MA, Martinez-Ramirez A, Soslow RA, et al. A role for the transducer of the Hippo pathway, TAZ, in the development of aggressive types of endometrial cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28:1492–1503. doi: 10.1038/modpathol.2015.102. [DOI] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. The EMBO journal. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nature communications. 2013;4 doi: 10.1038/ncomms3976. 2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen-Chen SM, Huang CY, Tsai CH, Liu YW, Wu SC, Huang CC, Eng HL, Chan YC, Ko SF, Tang RP. Yes-associated protein is not an independent prognostic marker in breast cancer. Anticancer Res. 2012;32:3321–3325. [PubMed] [Google Scholar]
- Shen S, Guo X, Yan H, Lu Y, Ji X, Li L, Liang T, Zhou D, Feng XH, Zhao JC, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25:997–1012. doi: 10.1038/cr.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X, Li WB, Wang DL, Chen KH, Cao JJ, Luo Z, He J, Li MC, Liu WJ, Yu C. YAP is closely correlated with castration-resistant prostate cancer, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol Med Rep. 2015;12:4867–4876. doi: 10.3892/mmr.2015.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Science signaling. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski A, Breindel JL, Prat A, Galvan P, Smith E, Rolfs A, Gupta PB, Labaer J, Kuperwasser C. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell reports. 2014;6:1059–1072. doi: 10.1016/j.celrep.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Cheong JH, Kim H, Noh SH, Kim H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827–3834. [PubMed] [Google Scholar]
- Song Q, Mao B, Cheng J, Gao Y, Jiang K, Chen J, Yuan Z, Meng S. YAP enhances autophagic flux to promote breast cancer cell survival in response to nutrient deprivation. PLoS One. 2015a;10:e0120790. doi: 10.1371/journal.pone.0120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, Heallen TR, Xiao L, Hofstetter WL, Weston B, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74:4170–4182. doi: 10.1158/0008-5472.CAN-13-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015b;21:2580–2590. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nature cell biology. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, Agarinis C, Schmelzle T, Bouwmeester T, Schubeler D, Bauer A. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015;11:e1005465. doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, VandenBerg SR, Baia GS, McDermott MW, Gutmann DH, Lal A. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–1212. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LL, Ma WX, Yuan JF, Shao Y, Xiao W, Jiang SJ. Expression of Yes-associated protein in non-small cell lung cancer and its relationship with clinical pathological factors. Chin Med J (Engl) 2012;125:4003–4008. [PubMed] [Google Scholar]
- Su T, Bondar T, Zhou X, Zhang C, He H, Medzhitov R. Two-signal requirement for growth-promoting function of Yap in hepatocytes. Elife. 2015;4 doi: 10.7554/eLife.02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152. [PubMed] [Google Scholar]
- Tanahashi K, Natsume A, Ohka F, Motomura K, Alim A, Tanaka I, Senga T, Harada I, Fukuyama R, Sumiyoshi N, et al. Activation of Yes-Associated Protein in Low-Grade Meningiomas Is Regulated by Merlin, Cell Density, and Extracellular Matrix Stiffness. J Neuropathol Exp Neurol. 2015;74:704–709. doi: 10.1097/NEN.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Tanas MR, Ma S, Jadaan FO, Ng CK, Weigelt B, Reis-Filho JS, Rubin BP. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene. 2015 doi: 10.1038/onc.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger S, et al. Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Tian T, Li A, Lu H, Luo R, Zhang M, Li Z. TAZ promotes temozolomide resistance by upregulating MCL-1 in human glioma cells. Biochemical and biophysical research communications. 2015;463:638–643. doi: 10.1016/j.bbrc.2015.05.115. [DOI] [PubMed] [Google Scholar]
- Tian W, Yu J, Tomchick DR, Pan D, Luo X. Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc Natl Acad Sci U S A. 2010;107:7293–7298. doi: 10.1073/pnas.1000293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M, Judson RN, Thway K, Nadal G, Selfe JL, et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. 2014;26:273–287. doi: 10.1016/j.ccr.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Tsujiura M, Mazack V, Sudol M, Kaspar HG, Nash J, Carey DJ, Gogoi R. Yes-associated protein (YAP) modulates oncogenic features and radiation sensitivity in endometrial cancer. PLoS One. 2014;9:e100974. doi: 10.1371/journal.pone.0100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Lee MY, Ousset M, Brohee S, Rorive S, Giraddi RR, Wuidart A, Bouvencourt G, Dubois C, Salmon I, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015;525:119–23. doi: 10.1038/nature14665. [DOI] [PubMed] [Google Scholar]
- Verfaillie A, Imrichova H, Atak ZK, Dewaele M, Rambow F, Hulselmans G, Christiaens V, Svetlichnyy D, Luciani F, Van den Mooter L, et al. Decoding the regulatory landscape of melanoma reveals TEADS as regulators of the invasive cell state. Nature communications. 2015;6 doi: 10.1038/ncomms7683. 6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici P, Mottolese M, Pizzuti L, Barba M, Sperati F, Terrenato I, Di Benedetto A, Natoli C, Gamucci T, Angelucci D, et al. The Hippo transducer TAZ as a biomarker of pathological complete response in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Oncotarget. 2014;5:9619–9625. doi: 10.18632/oncotarget.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. Die cellularpathologie in ihrer begründung auf physiologische und pathologische gewebelehre. Berlin: A. Hirschwald; 1858. [PubMed] [Google Scholar]
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, Wen W, Zhu Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013a;8:e65539. doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang Q, Xu Z, An Q, Jiang D, Wang L, Liang B, Li Z. TAZ promotes epithelial to mesenchymal transition via the upregulation of connective tissue growth factor expression in neuroblastoma cells. Mol Med Rep. 2015a;11:982–988. doi: 10.3892/mmr.2014.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nature cell biology. 2015b;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer. 2012;48:1227–1234. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie C, Li Q, Xu K, Wang E. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013b;34:2169–2174. doi: 10.1007/s13277-013-0751-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A. 2014;111:E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M, Fan HY. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9:e91770. doi: 10.1371/journal.pone.0091770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Jiang N, Zhou B, Liu Q, Du C. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015;106:151–159. doi: 10.1111/cas.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Wu L, Zheng H, Li N, Wan H, Liang G, Zhao Y, Liang J. Expression of Yes-associated protein in cervical squamous epithelium lesions. Int J Gynecol Cancer. 2014;24:1575–1582. doi: 10.1097/IGC.0000000000000259. [DOI] [PubMed] [Google Scholar]
- Xiao W, Wang J, Ou C, Zhang Y, Ma L, Weng W, Pan Q, Sun F. Mutual interaction between YAP and c-Myc is critical for carcinogenesis in liver cancer. Biochemical and biophysical research communications. 2013;439:167–172. doi: 10.1016/j.bbrc.2013.08.071. [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu ZH, Xu F, Zhao HY. Prognostic significance of TAZ expression in resected non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:799–807. doi: 10.1097/JTO.0b013e318248240b. [DOI] [PubMed] [Google Scholar]
- Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, Poon RT, Zender L, Lowe SW, Hong W, Luk JM. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F, Fu K, Chen Y, Solheim J, Singh PK, et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6:36019–36031. doi: 10.18632/oncotarget.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo MK, Kim SH, Kim JM, Huang SM, Kim MR, Song KS, Kim KH. Correlation of expression of phosphorylated and non-phosphorylated Yesassociated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a Korean population. Anticancer Res. 2012;32:3835–3840. [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell death and differentiation. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]
- Yue G, Sun X, Gimenez-Capitan A, Shen J, Yu L, Teixido C, Guan W, Rosell R, Liu B, Wei J. TAZ is highly expressed in gastric signet ring cell carcinoma. Biomed Res Int. 2014;2014 doi: 10.1155/2014/393064. 393064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen HF, McCrudden CM, Huang YH, Tham JM, Zhang X, Zeng Q, Zhang SD, Hong W. TAZ expression as a prognostic indicator in colorectal cancer. PLoS One. 2013;8:e54211. doi: 10.1371/journal.pone.0054211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nature cell biology. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Piccolo S. Eradicating tumor drug resistance at its YAP-biomechanical roots. The EMBO journal. 2015 doi: 10.15252/embj.201593584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM, Fu K, Datta K, Palermo N, Chen Y, Dong J. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015a;35:1350–1362. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gao Y, Li F, Tong X, Ren Y, Han X, Yao S, Long F, Yang Z, Fan H, et al. YAP Promotes Malignant Progression of Lkb1-Deficient Lung Adenocarcinoma through Downstream Regulation of Survivin. Cancer Res. 2015b;75:4450–4457. doi: 10.1158/0008-5472.CAN-14-3396. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014a;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Science signaling. 2014b;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB, group AS. Bowtell DD, Harvey KF. The Hippo pathway transcriptional coactivator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Li B, Shen L, Shen Y, Chen XD. The role and clinical significance of YES-associated protein 1 in human osteosarcoma. Int J Immunopathol Pharmacol. 2013;26:157–167. doi: 10.1177/039463201302600115. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & development. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011a;108:E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS, Yang X. TAZ is a novel oncogene in non-small cell lung cancer. Oncogene. 2011b;30:2181–2186. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]