Abstract
Pancreatic cancer is an aggressive malignant disease and the efficacy of current treatments for unresectable diseases is quite limited despite recent advances. Gene therapy/virotherapy strategies may provide new options for treatment of various cancers including pancreatic cancer. Oncolytic adenovirus shows an antitumoral effect via its intratumoral amplification and strong cytocidal effect in a variety of cancers and it has been employed for the development of potent oncolytic virotherapy agents for pancreatic cancer. Our ultimate goal is to develop an oncolytic adenovirus enabling treatment of patients with advanced or spread diseases by systemic injection. Systemic application of oncolytic therapy mandates more efficient and selective gene delivery and needs to embody sufficient antitumor effect even with limited initial delivery to the tumor location. In this review, the current status of oncolytic adenoviruses from the viewpoints of vector design and potential strategies to overcome current obstacles for its clinical application will be described. We will also discuss the efforts to improve the antitumor activity of oncolytic adenovirus, in in vivo animal models, and the combination therapy of oncolytic adenovirus with radiation and chemotherapy.
Keywords: Oncolytic Adenovirus, Cox-2 promoter, Fiber-modified Ad, Pancreatic cancer, Adenovirus library screening
1. Why oncolytic adenoviruses for cancers?
New technology for the detection of cancer is advancing day by day, including anatomical/functional imaging and bio-markers[1]. Despite this progress, many cancer patients are found with advanced stage/spread disease, even at the time of initial diagnosis. At an advanced stage, the tumor can no longer be completely treated with a locoregional treatment such as surgery. The progression of cancer to metastatic stage thus imposes a major challenge to effective disease control. Specifically, pancreatic cancer represents a classic example of the difficulties faced upon treating this metastatic stage disease. Despite recent advances in cancer detection and treatment, neither early detection nor treatment of advanced lesions has led to the improvement of therapeutic outcomes in the patients. According to the American Cancer Society, for all stages of pancreatic cancer combined, the one-year relative survival rate is 29% and the five-year rate is 7%[2]. The highest cure rate occurs if the tumor is truly localized to the pancreas; however, this stage of disease accounts for less than 20% of cases.
The current major options for treatment of pancreatic cancer are a combination therapy of surgery and chemo/chemoradiation. Early stage pancreatic cancer can often be treated and even cured with surgery and chemo/radiation therapy. If surgery is not possible, the treatment option is chemo/chemoradiation therapy and palliative care. The most common chemotherapy agents used to treat late stage pancreatic cancer are FOLFIRINOX [FOL (folinic acid (Leucovorin)) + F (fluorouracil) + IRIN (irinotecan) + OX (oxaliplatin)] and nab-paclitaxel (Abraxane) + gemcitabine combination[3]. However, even with the strongest chemotherapy, remission of the metastatic or locally spread pancreatic cancer is rarely observed. In order to improve the clinical outcome of patients with advanced cancers, it is imperative to develop new approaches for treatment of these patients. Such demand is particularly high in the case of pancreatic cancer[4].
Gene therapy strategies provide new options for treatment of various cancers. Oncolytic virotherapy is a one of the most promising anti-cancer agents and, it has been employed for antitumoral potency via its intratumoral amplification and its strong oncolytic effect. Among them, herpes simplex virus (T-VEC, Talimogene laherparepvec, also known as OncoVEX GM-CSF), is showing positive outcomes in clinical trial and was recently approved by the US Food and Drug Administration (FDA) for use on unresectable melanoma[5,6]. Thus, oncolytic virotherapy is becoming increasingly popular for the treatment of many different forms of cancer.
Our group has been developing a series of oncolytic adenoviruses (OAds) in order to use for clinical application[7]. Many groups, including our own, have used adenoviruses (Ads) as a basis for the development oncolytic agents because of the many clinically beneficial attributes and the existing rich knowledge of the adenovirus vector system[7,8]. Adenovirus vectors are known for their high in vivo gene-delivery efficiency[9], a very desirable trait and a key requirement for anti-tumor effect. In contrast to enveloped viruses released from cells through budding, the lytic life cycle of Ad involves the infection, replication in, and eventual destruction of host cells[10]. This characteristic is directly exploitable for oncolysis. The Ad is genetically stable, and the virus genome does not integrate in to the target cell genome, meaning there is no genotoxicity[9]. Adenoviral infection is mediated by precise protein-protein interactions rather than lipid membrane fusion, which permit the configuration of stringent transductional targeting systems.
When we used the conventional Ad vector for cancer treatment, it raises some concerns, some of which are a relatively short duration of transgene expression and immunogenicity. Considering the required features for therapeutic application, these shortcomings become minor details compared to the significant benefits for its application in oncolytic virus development. The short duration of transgene expression is mitigated by the production of progeny virus in the tumor. Also, immunogenicity is not necessarily a problem considering the benefit of immunostimulation observed with T-VEC[5,6]. In this sense, the Ad is a highly suitable virus system for in vivo gene delivery applications and cancer therapy.
2. Replication-based control oncolytic adenoviruses
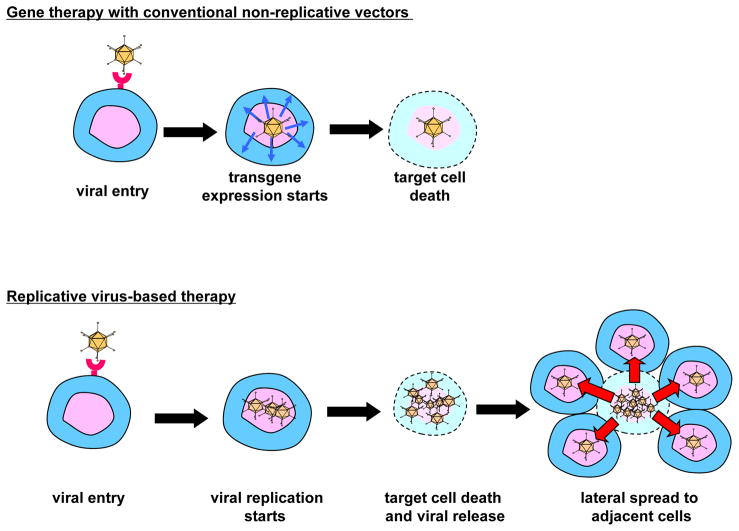
Conventionally, adenoviral gene therapy has been performed in a replication deficient system to avoid the possibility of toxicity resulting from adenoviral replication. To improve the antitumor efficacy without sacrificing specificity and safety, conditionally replicative adenoviruses (CRAds) have been developed. The basic concept of CRAds as oncolytic agents is that viruses replicate in tumor cells only and the subsequent lateral spread of progeny virus to surrounding tumor cells facilitates a dramatic amplification of the therapeutic effect leaving surrounding normal cells unharmed (Figure 1). To date, two types of CRAds have been designed to replicate selectively in tumor cells: mutation-based and cancer-specific promoter based.
Figure 1. Non-replicative and replicative systems for cancer gene therapy.
Compared to conventional cancer gene therapy with replication deficient adenovirus vector system (upper panel), a replicative vector system (lower panel) permitting tumor-selective replication and exponential spread of the progeny vector laterally in the tumors facilitates a dramatic amplification of the therapeutic effect, while keeping non-target cell unaffected thanks to replication selectivity.
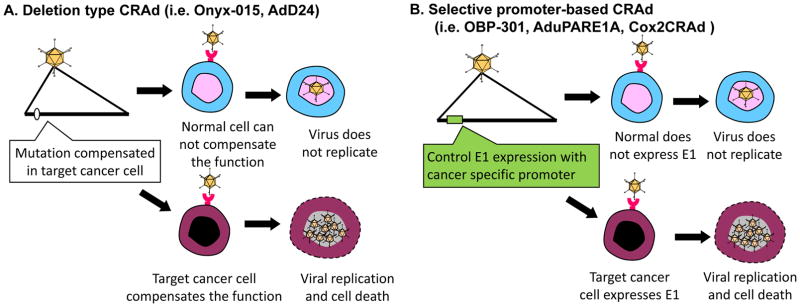
The first type of CRAds involved some mutations or deletion in the E1 region, which allowed replication in specific tumors only[11–13] (Figure 2A). The Ad mutant dl1520 (or ONYX-015) lacks the E1B region and was intended to achieve selective replication in cancer cells with mutated p53[11]. Also, AdΔ24 is another E1A-mutation type CRAd which theoretically restricts replication to cancer cells with mutated pRb[13].
Figure 2. Control mechanisms of oncolytic adenovirus.
(A). Deletion type CRAds: this type of CRAd has a mutation/deletion in a region crucial for viral replication. While cancer cells possess the cellular environment to compensate the missing function of the virus, normal cells do not have that capability. For example, ONYX-015 (dl1520) and AdΔ24 were designed to replicate only in p53 and pRb mutated cells, respectively. (B). Selective promoter-based CRAd: A tumor/tissue specific promoter controls the expression of viral genes crucial for replication. As a result, the virus can replicate only in cells in which the promoter is active. By using a promoter with a tumor-ON/normal cell-OFF profile, the replication can be restricted to cancer cells.
The second type of CRAds are driven by tumor-specific promoters (TSPs). This type of CRAds relies on cancer-specific, promoter-controlled transcription of the E1 region (Figure 2B). Since the E1A protein is necessary for Ad replication, promoter-controlled Ad can replicate only in cells where the controlling promoter is active. For example, OBP-301 was engineered to express E1A under the control of the human telomerase reverse transcriptase (hTERT) promoter, which is activated in various types of human cancer cells, including pancreatic cancer [14]. AduPARE1A virus drives the E1A gene under the control of the urokinase-type plasminogen activator receptor (uPAR) promoter and showed its selective replication and its strong antitumor activity in pancreatic cancer models [15,16]. Our group developed OAd controlled by cyclooxygenase-2 (Cox-2), Cox2-CRAd, for gastrointestinal cancers (e.g. pancreatic cancers[17], esophageal adenocarcinomas [18], and peritoneal dissemination of gastric cancer[19]). Also, we have recently generated a new CRAds that are targeted to Human Papilloma Virus (HPV)-positive head and neck squamous cell carcinomas (HNSCC). These CRAds included small deletions in the E1A region of the genome (Δ24 or CB016) intended to allow for selective replication in HPV-positive cells, and they demonstrated excellent in vitro and in vivo therapeutic effects[20].
As an emerging strategy for CRAd replication control, post-transcriptional control can also be used. Micro-RNAs (miRNA) are short RNAs expressed in the cells, which determine many aspects of the cell characteristics. By placing an miRNA binding sequence into the context of adenovirus E1A regions, replication can be restricted to the target cells (e.g. cancer cells)[21]. Also, the 5′ or 3′ UTR placed on E1A regions can enable cancer specificity[22,23]. The replication-selective Ad eliminates the cytocidal effect in non-cancer cells.
3. Adenoviral transductional targeting and its application to oncolytic viruses
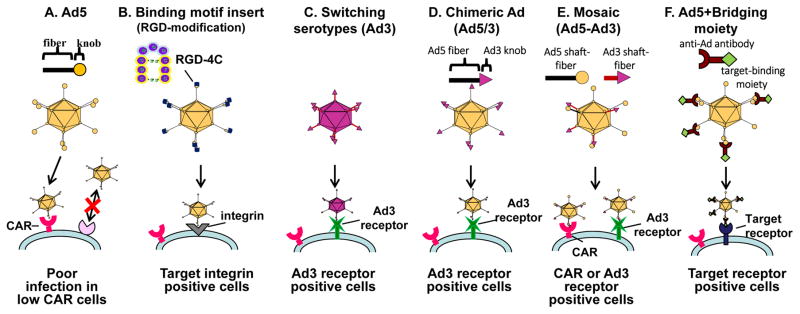
A lack of good strategy to control “binding and entry” of adenovirus vector (also known as transductional targeting) has been a major issue for the development of OAds. The OAd infectivity in many cancers (e.g. gastrointestinal cancers, pancreatic cancers, esophageal adenocarcinomas, ovarian cancer) is extremely low due to poor expression of the adenoviral primary receptor (Coxsackie adenovirus receptor, CAR), as reported by several groups, including our own[18,24]. Therefore, it is reasonable to develop a vector system that can transduce the target cells via another receptor. In order to solve this issue, our lab and several others have incorporated CAR-independent infection capabilities into OAd, as shown in Figure 3. Since the discovery that the “knob” domain within the Ad wild-type fiber region is responsible for CAR binding (Figure 3A) it has become a major target for infectivity enhancement. There are several ways to generate an infectivity-enhanced OAd: insertion of binding motif into Ad fiber (Figure 3B), switching subtype (Figure 3C), chimeric fiber (Figure 3D), mosaic fiber (Figure 3E), and bridging molecule (Figure 3F).
Figure 3. Modification of adenovirus to achieve CAR-independent transduction.
To achieve CAR-independent transduction, several modification strategies have been employed in adenovirus. (A) Poor infectivity of CAR negative cells with conventional Ad system, (B) fiber modification, (C) switching serotypes, (D) chimeric, (E) mosaic, and (F) bridging molecule-based targeting.
One of the most successful extrinsic binding motifs for infectivity enhancements is the incorporation of the RGD-4C motif into the HI-loop of the fiber knob region[25,26] (Figure 3B). The RGD-4C motif is a partial peptide sequence of fibronectin identified by phage library screening[27]. When it was incorporated into the HI-loop of the fiber-knob region, the Ad vector showed CAR-independent infection of the target cells. Also, OAd with this motif showed an improved cytocidal effect in CAR negative cancer cell lines in vitro and in vivo[24,28].
Most Ad vectors to date are based on subtype 2 or 5. Both of them are using CAR for binding and run into the problem of poor transduction efficiency in cancer cells. Interestingly, there are other serotype Ad vectors that do not use CAR as their primary receptor. For example, Ad35 uses CD46[29], and Ad3 uses desmogrin-2 and CD46 as its receptor for initial binding[30]. Thus, the infection of these viruses is CAR-independent. There are several more approaches for changing tropism of adenoviral vectors. One approach is to make a vector fully based on alternate subtype vectors (Figure 3C), another is to design an Ad2/5 based vector with an alternate subtype’s binding domain incorporated (chimeric or mosaic) (Figure 3D and E), and the other approach is a bridging molecule-based method (Figure 3F). Switching subtype method has the advantage that all parts of the capsid consist of alternate subtype Ad proteins such as Ad3, resulting in distribution assumed to be identical to the parental virus. However, there is a risk of reduced virus replication and cytocidal effect in this approach because other subtype’s oncolysis is not necessarily as strong as that of Ad2/5. As for the bridging molecule-based method, it can achieve the precise selectivity embodied by employing a high affinity/specificity antibody (Ab), or by using a specific binding motif for the target moiety expressed on the cell surface [31–33]. While promising, it is impractical to incorporate the bridging molecule into an OAd system because effective incorporation of bridging molecules into progeny viruses is not easy [17]. In recognition of this fact, chimeric fiber approaches such as Ad5/3 (Ad5 vectors with the fiber knob domain of Ad3) are more frequently applied for OAds and chimeric OAds displays improved gene delivery and antitumor efficacy in many preclinical studies [18,28,34–36]. Additionally, ColoAd1 (also known as enadenotucirev, EnAd), a complex and highly potent chimeric Ad3/Ad11p virus, was generated by a novel “directed evolution” approach for its ability to kill colorectal cancer cells[37]. The viral Ad11p capsid is more resistant to elimination by human serum and blood cells than Ad5 [38] which may provide an advantage for systemic delivery. ColoAd1 virus is currently undergoing several early-phase clinical trials[39].
4. Making pancreatic cancer-targeted oncolytic adenovirus by the high-throughput screening of adenovirus library
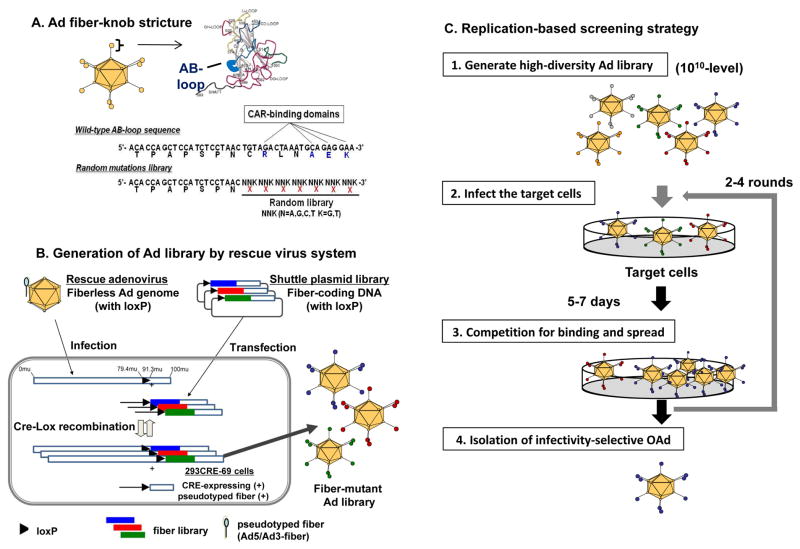
Although incorporation of several targeting motifs has been reported to increase the infectivity of replication deficient Ad in pancreatic cancer cells[40–42], the success rate of incorporating pre-identified targeting motifs into OAd has been low. To address this issue, we recently developed the high-throughput screening system using a high-diversity Ad library[43]. This system employs an Ad library with seven random amino acids instead of the CAR-binding domain in the adenoviral fiber knob region (Figure 4A) [43]. For constructing the high-diversity Ad library, we developed an Ad production system based on a newly designed rescue virus. This system enables high efficiency CRE-lox recombination between the shuttle plasmid coding Ad fiber library and the “genetically fiberless but pseudotyped” rescue virus (Figure 4B). This vector generation system accomplished a strikingly high library diversity (>1010 diversity), which permits the full coverage of seven random amino-acid library. Using this high-diversity library, we successfully isolated potent mesothelin-targeted OAd by replication based screening (Figure 4C) [43]. Mesothelin, also known as MSLN, is a cell surface glycoprotein that is highly expressed on pancreatic cancer, ovarian cancer, and mesothelioma[43,44]. The virus with the newly isolated MSLN-targeted OAd showed dramatic selectivity for MSLN-expressing pancreatic cancer cells in vitro and in vivo. The intravenously injected MSLN-targeted OAd showed an impressively strong antitumor effect, which was equivalent or stronger than that of intratumoral injection. These data indicate the possibility of systemic therapy with cancer-targeted OAd by selective infection mediated. In this sense, the library screening technology may have broad implications for the development of targeted gene delivery approaches.
Figure 4. Novel system for adenovirus vector production.
(A) Structure of Ad library with random 7 amino acid in the AB-loop of fiber-knob region. (B) High- diversity Ab library was produced with the newly developed rescue virus system by CRE-lox recombination in CRE-expressing, fiber-transcomplementing cell line (293CRE-69cell). The shuttle plasmid coding fiber library has a single loxP site in the upstream of the fiber gene. The produced library viruses were harvested 48 hours after shuttle plasmid transfection and rescue virus infection. (C)the strategy of replication-based Ad library screening [43].
5. Possibility of systemic administration
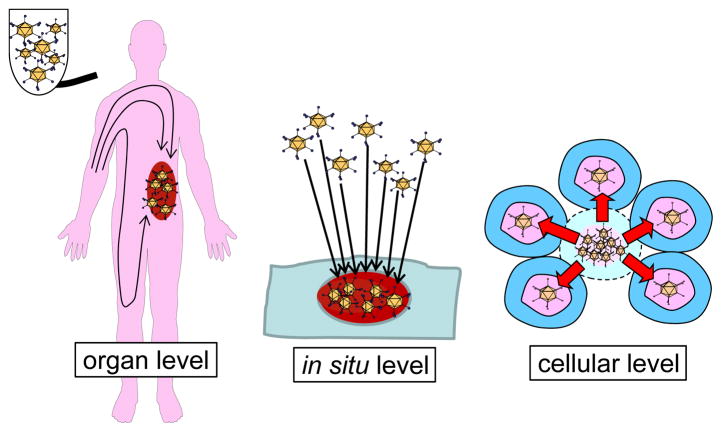
The ultimate goal of cancer genetherapy/virotherapy development is to develop a device enabling treatment of patients with advanced or spread diseases by systemic injection. In systemic application, the potency and selectivity of the oncolytic viruses can be defined at three levels. The first is organ level targeting which allows the administered virus to be delivered selectively to the target tumor region. The second is in situ level targeting enabling selective infection of the cancer cells in the tumor site. The third is replication level control to provide cancer cell selective viral replication (Figure 5). The vast majority of oncolytic viruses historically have had only replication-level targeting for cancer selectivity[7,8] because achieving transductional targeting embodying organ-level and in situ-level targeting in OAd has not been easy.
Figure 5. Oncolytic virus targeting at three levels.
The oncolytic viruses can be targeted at 3 levels: the first is organ level targeting which allows the administered virus delivered selectively to the target tumor region, the second is in situ level targeting enabling selective infection of the cancer cells, and the third is replication level control to provide cancer cell selective viral replication.
Development of transductional targeting of OAd (targeting by selective infection) is most challenging aspect, but it has many benefits. Transductional targeting of OAd should potentiate antitumor effect by improving the initial tumor site localization after systemic administration. One such example is the behavior of fiber-modified OAd in human pancreatic cancer xenografts after systemic administration. As we described above, the mesothelin (MSLN)-targeted OAd was identified by Ad library screening and this virus exhibited a strong therapeutic effect in a pancreatic cancer subcutaneous xenograft model by systemic injection[43]. In addition, the liver sequestration of the transductionally-targeted OAd with the re-designed AB-loop was more than one order of magnitude lower than that with wild type Ad after intravenous injection. This indicates that targeting at the level of transduction helps reduce various adverse effects by decreasing distribution to the normal organs (e.g. innate immune response and hepatotoxicity). Intravenously administered Ad localizes principally to the liver, which limits delivery of Ad to tumor targets and also elicits hepatotoxicity. At this point, our library system is capable of identifying very promising OAds for the systemic therapy of pancreatic cancer via transductional tumor targeting by increasing cancer cell selective transduction and reducing sequestration by non-target cells.
Moreover, there are other studies of systemic treatment of chimeric Ad5/3 OAd in an orthotopic pancreatic cancer model. Here, the Ad5/3 modified vector showed significantly higher tumor transduction following systemic delivery[28]. In addition, the systemic administration of COX-2 controlled OAd with Ad5/3 modification had an anti-tumor effect as strong as that of the positive control wild-type virus that exhibits maximal (but non-selective) replication[28]. Considering Ads with the Ad5/3 fiber infect human cells very efficiently[18] but does not infect host (mouse) cells[45], these data in this artificial model of suitable transductional targeting indicate that appropriate vector targeting based on fiber-knob modification realizes tumor transduction and potent therapeutic effect in more challenging systemic therapy.
6. Improvement of the in vivo model
In order to advance vector development, valid in vivo experimental systems are critical for further understanding OAd functionality. In particular, a convenient in vivo experimental system for the analyses of OAd replication/toxicity and virus-host interaction is urgently needed. To date, most in vivo experiments of OAd have been performed with human cancer cell xenografts in immuno-deficient mice. However, the stringent species selectivity of adenoviridae replication does not permit human Ad to replicate in conventional rodent cells. This biology greatly limits the ability to conduct virus replication-related studies in syngeneic models in the leading experimental animals (i.e. mice). Cotton rat, syrian hamster, and pig permit productive human Ad replication[46–48] and the fact that these are the only small to medium size animal model systems permissive for human Ad replication emphasizes the importance, especially in the context of toxicological studies[49], but it is not completely clear how closely viral replication in this system resembles that in humans.
For better understanding of the biology of replicative Ad in the matched host settings, syngeneic models have been proposed. One current model is based on the hamster cancer cell line syngeneic graft in Syrian hamsters[46,50]. Another approach is to employ conditionally replicative canine Ads to treat spontaneous dog osteosarcoma[51,52]. These unique models would provide valuable information about an oncolytic agent in its natural host, and such data would be uniquely translatable to human context. Thus, experiments with non-human, non-mouse models have critical relevance to the analyses of host specific phenomena, such as immunity.
7. Further improvement for clinical feasibility
A reasonable approach to strengthen the anti-tumor effect of the OAd is expressing a transgene with an anti-tumor effect from the oncolytic virus. This approach has been taken in a wide variety of oncolytic viruses including Ad and vaccinia[53,54]. One interesting example with Ad is interferon (IFN)-α. It has been known that IFN-α has a strong anti-tumor effect and has the ability to sensitize the tumoricidal effects of chemotherapeutic agents (e.g. 5-FU) and radiotherapy[55–58]. Particularly, in the field of pancreatic cancer, the adjuvant chemotherapy, immunotherapy (IFN), and external-beam radiation for resected pancreatic ductal adenocarcinoma (PDAC)[59–62] and a multicenter phase II trial (5-FU, cisplatin, and IFN-α in conjunction with radiation therapy) confirmed the efficacy of IFN-based chemoradiation for PDAC[55]. However, despite encouraging survival results and immunological data, clinical trials have defined several problems impairing the clinical utility of IFN-α for pancreatic cancer patients: i) Systemic toxicity of IFN-α, and ii) Insufficient delivery and unsustainable levels of IFN-α in the tumor site due to rapid degradation of the cytokine in blood circulation and low vascularity [63,64].
In the context of IFN expression from Ad, different from many other viruses whose replication is remarkably suppressed by IFNs, intrinsic class I IFN expression from the infected cancer cells did not hamper Ad replication in the tumor. As a result, IFN expressing Ad vectors have been made at high titer[65], and OAd with IFN-α showed efficient replication in pancreatic cancer cells[53,66]. In this way, OAd with IFN-α has a unique benefit for its application to pancreatic cancers. Also, many OAds with various anti-cancer and immunomodulatory molecules, such as GM-CSF and interleukin-12 (IL-12), have shown promising results[67,68]. In particular, the phase III trial of T-VEC (genetically modified herpes simplex virus expressing GM-CSF) demonstrated improvements in durable response rate and a trend toward improved overall survival compared to GM-CSF alone, which led to the approval by the FDA of its use in advanced melanoma patients[5]. T-VEC has also been tested in phase I, II, and III clinical trials for the treatment of pancreatic cancer, soft-tissue sarcomas, and head and neck cancer.
8. Combination therapy with oncolytic viruses
Combination therapies involving multiple chemotherapies and radiation have been performed in many cancers[69–71]. Likewise, combination therapy is possible and promising with OAds[72]. Particularly, the combination of OAds with radiation is not only clinically, but also biologically interesting. Clinically, an early version OAd (ONYX-015) showed very nice clinical response in head and neck tumors when treated with combination therapy with external radiation and 5-FU[69]. In basic biology aspects, Ad E4 region has the ability to suppress double strand break repair in order to avoid concatemerization of linear double strand DNA[73–75]. Since double strand break repair is a major process for the recovery from radiation damage[76], its suppression directly links to radio-sensitization of the infected cells. Conditionally replicating OAd as well as those armed with therapeutic gene can also be combined with cisplatin to enhance the overall therapeutic effects in hepatocellular carcinoma[77], head and neck cancer[78,79], and cervical cancer[80]. Several studies have reported the combination therapy of gemcitabine and OAd. For example, oncolytic mutants lacking the anti-apoptotic E1B19K gene showed increased pancreatic cancer cell killing in combination with gemcitabine by enhancing drug-induced apoptosis[81]. Ad5/3-Δ24 was used in combination with gemcitabine in ovarian cancer cells, synergistic interactions were observed that resulted in enhanced cell killing[82]. More recently, the combination therapy of oncolytic viruses and immune-checkpoint inhibitor such as anti- CTLA-4 antibody and anti-PD-1 antibody has demonstrated promising results. For example, Ad5/3-Δ24-based OAd coding for anti-CTLA4 antibody has been tested in several cancer cell lines, and a direct anti-CTLA-4-mediated pro-apoptotic effect was observed in vitro and in vivo[83]. The combination therapy with reovirus and anti-PD-1 antibody that showed prolonged survival in in vivo melanoma model[84]. Therefore, the combination of oncolytic virus and immune-checkpoint inhibitor will be an appealing strategy. While it is promising, combinatory approach can sometimes be a double-edged sword because proper evaluation of the combination effect is not that simple: it is crucial to determine appropriate timing, dosing and sequence schedules of each agent. However, once it is established, it may make a big impact for clinical efficacy in various cancers.
9. Summary
By overcoming poor infectivity and enforcing selectivity, a series of OAds showing significant therapeutic effect have been developed. We are currently pursuing clinical translation of one of these vectors in pancreatic cancer. Therapeutic transgene configuration and combination with chemoradiation are the directions being developed as promising logical extensions of previous efforts. These combinations have started to generate outcomes which are possibly translatable into clinical usage. Treatment of non-locoregional diseases by systemic administration of the oncolytic virus has been a dream of many gene-/viro- therapy researchers. Such a strategy is being developed by actualization of transductional targeting of OAds. Many impactful works in recent days, including ours, has put OAd much closer to clinical realization of impactful therapeutic modality in pancreatic cancer patients.
Acknowledgments
The scientific works in this review article by our lab was partly supported by NIH/NCI R01CA196215 and CA168448.
References
- 1.Hall NC, Zhang J, Povoski SP, Martin EW, Knopp MV. New developments in imaging and functional biomarker technology for the assessment and management of cancer patients. Expert Rev Med Devices. 2009;6:347–351. doi: 10.1586/erd.09.21. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. [Accessed Oct 20, 2016];Cancer Facts & Figures. 2016 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- 3.Gall TMH, Tsakok M, Wasan H, Jiao LR. Pancreatic cancer: current management and treatment strategies. Postgrad Med J. 2015;91:601–607. doi: 10.1136/postgradmedj-2014-133222. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M. Conditionally replicative adenovirus for gastrointestinal cancers. Expert Opin Biol Ther. 2004;4(8):1241–1250. doi: 10.1517/14712598.4.8.1241. [DOI] [PubMed] [Google Scholar]
- 5.Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 6.Killock D. Skin cancer: T-VEC oncolytic viral therapy shows promise in melanoma. Nat Rev Clin Oncol. 2015;12:438. doi: 10.1038/nrclinonc.2015.106. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemany R, Balagué C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 9.Hallenbeck PL, Chang YN, Hay C, Golightly D, Stewart D, Lin J, Phipps S, Chiang YL. A novel tumor-specific replication-restricted adenoviral vecor for gene theraoy for hepatocellular carcinoma. Hum Gene Ther. 1999;10(10):1721–1733. doi: 10.1089/10430349950017725. [DOI] [PubMed] [Google Scholar]
- 10.Shenk T. Adenoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. Vol. 2. Lippincott-Raven; Philadelphia, PA: 1996. pp. 2111–2148. [Google Scholar]
- 11.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 12.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawakins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 13.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 14.Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N, Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 15.Huch M, Gros A, José A, González JR, Alemany R, Fillat C. Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models. Neoplasia. 2009;11:518–528. doi: 10.1593/neo.81674. 4 p following 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobrevals L, Mato-Berciano A, Urtasun N, Mazo A, Fillat C. uPAR-controlled oncolytic adenoviruses eliminate cancer stem cells in human pancreatic tumors. Stem Cell Res. 2014;12:1–10. doi: 10.1016/j.scr.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, Curiel DT. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 18.Davydova J, Le LP, Gavrikova T, Wang M, Krasnykh V, Yamamoto M. Infectivity-enhanced cyclooxygenase-2-based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res. 2004;64:4319–4327. doi: 10.1158/0008-5472.CAN-04-0064. [DOI] [PubMed] [Google Scholar]
- 19.Kosaka T, Davydova J, Ono HA, Akiyama H, Hirai SI, Ohno S, Takeshita F, Aoki K, Ochiya T, Yamamoto M, Kunisaki C, Endo I. Imaging and antitumoral effect of a cyclo-oxygenase 2-specific replicative adenovirus for small metastatic gastric cancer lesions. Anticancer Res. 2015;35:5201–5210. [PubMed] [Google Scholar]
- 20.LaRocca CJ, Han J, Salzwedel AO, Davydova J, Herzberg MC, Gopalakrishnan R, Yamanoto M. Oncolytic adenoviruses targeted to human papilloma virus-positive head and neck squamous cell carcinomas. Oral Oncol. 2016;56:25–31. doi: 10.1016/j.oraloncology.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ylösmäki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol. 2008;82:11009–11015. doi: 10.1128/JVI.01608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoff-Khalili M, Rivera A, Nedeljkovic-Kurepa A, DeBenedetti A, Li XL, Odaka Y, Podduturi J, Sibley DA, Siegal GP, Stoff A, Young S, Zhu ZB, Curiel DT, Mathis JM. Cancer-specific targeting of a conditionally replicative adenovirus using mRNA translational control. Breast Cancer Res Treat. 2008;108:43–55. doi: 10.1007/s10549-007-9587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed A, Thompson J, Emiliusen L, Murphy S, Beauchamp RD, Suzuki K, Alemany R, Harrington K, Vile RG. A conditionally replicating adenovirus targeted to tumor cells through activated RAS/P-MAPK-selective mRNA stabilization. Nat Biotechnol. 2003;21:771–777. doi: 10.1038/nbt835. [DOI] [PubMed] [Google Scholar]
- 24.Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva E, Blackwell JL, Reynolds PN, Li H, Parameshwar M, Vickers SM, Jaffee EM, Huibregtse K, Curiel DT, Dmitriev I. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 25.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao VJ, Ozawa MG, Varner AS, Kasman IM, Chanthery YH, Pasqualini R, Arap W, McDonald DM. Antiangiogenic therapy decreases integrin expression in normalized tumor blood vessels. Cancer Res. 2006;66(5):2639–2249. doi: 10.1158/0008-5472.CAN-05-1824. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez PJ, Vickers SM, Ono HA, Davydova J, Takayama K, Thompson TC, Curiel DT, Bland KI, Yamamoto M. Optimization of conditionally replicative adenovirus for pancreatic cancer and its evaluation in an orthotopic murine xenograft model. Am J Surg. 2008;195:481–490. doi: 10.1016/j.amjsurg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Gaggar A, Shayakhmetov DM, Liszewski MK, Atkinson JP, Lieber A. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirena D, Lilienfeld B, Eisenhut M, Kälin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann N Y Acad Sci. 1999;886:158–171. doi: 10.1111/j.1749-6632.1999.tb09409.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Huang J, Hirai S, Kuroki M, Kuroki M, Watanabe N, Tomihara K, Kato K, Hamada H. Carcinoembryonic antigen-targeted selective gene therapy for gastric cancer through FZ33 fiber-modified adenovirus vectors. Clin Cancer Res. 2006;12:3803–3813. doi: 10.1158/1078-0432.CCR-06-0024. [DOI] [PubMed] [Google Scholar]
- 33.Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wang H, Yumul R, Gao W, Gambotto A, Morita T, Baker A, Shayakhmetov D, Lieber A. Transduction of liver metastases after intravenous injection of Ad5/35 or Ad35 vectors with and without factor X-binding protein pretreatment. Hum Gene Ther. 2009;20:621–629. doi: 10.1089/hum.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liikanen I, Monsurrò V, Ahtiainen L, Raki M, Hakkarainen T, Diaconu I, Escutenaire S, Hemminki O, Dias JD, Cerullo V, Kanerva A, Pesonen S, Marzioni D, Colombatti M, Hemminki A. Induction of interferon pathways mediates in vivo resistance to oncolytic adenovirus. Mol Ther. 2011;19:1858–1866. doi: 10.1038/mt.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K, Ranki T, Oksanen M, Holm SL, Haavisto E, Karioja-Kallio A, Laasonen L, Partanen K, Ugolini M, Helminen A, Karli E, Hannuksela P, Pesonen S, Joensuu T, Kanerva A, Hemminki A. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–84. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, Thorne S, Reid T, Ni S, Lieber A, Fisher K, Seymour L, Rubanyi GM, Harkins RN, Hermiston TW. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Y, Seymour L, Fisher K. Activity of a group B oncolytic adenovirus (ColoAd1) in whole human blood. Gene Ther. 2014;21:440–443. doi: 10.1038/gt.2014.2. [DOI] [PubMed] [Google Scholar]
- 39.Boni V, De La Portilla F, Cubillo A, Gil-Martin M, Calvo E, Salazar R, Santos C, Sanchez-Gastaldo A, Prados S, Sanjuan X, Bozada JM, Duran H, Jurado M, Ellis C, Alvis S, Beadle J, Fisher K, Blanc C, Garcia-Carbonero R. A phase 1 mechanism of action study of intra-tumoural (IT) or intravenous (IV) administration of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus in colon cancer patients undergoing resection of primary tumour. Ann Oncol. 2014;25(suppl 4):iv368. [Google Scholar]
- 40.Joung I, Harber G, Gerecke KM, Carroll SL, Collawn JF, Engler JA. Improved gene delivery into neuroglial cells using a fiber-modified adenovirus vector. Biochem Biophys Res Commun. 2005;328:1182–1187. doi: 10.1016/j.bbrc.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 41.Nicklin SA, Von Seggern DJ, Work LM, Pek DC, Dominiczak AF, Nemerow GR, Baker AH. Ablating adenovirus type 5 fiber–CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol Ther. 2001;4:534–542. doi: 10.1006/mthe.2001.0489. [DOI] [PubMed] [Google Scholar]
- 42.Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat Med. 2002:751–755. doi: 10.1038/nm720. [DOI] [PubMed] [Google Scholar]
- 43.Miura Y, Yamasaki S, Davydova J, Brown E, Aoki K, Vickers S, Yamamoto M. Infectivity-selective oncolytic adenovirus developed by high-throughput screening of adenovirus-formatted library. Mol Ther. 2013;21:139–148. doi: 10.1038/mt.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frierson HF, Moskaluk CA, Powell SM, Zhang H, Cerilli LA, Stoler MH, Cathro H, Hampton GM. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 2003;34:605–609. doi: 10.1016/s0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 45.Davydova J, Brown EJ, Selwyn M, Vickers MY. New model for testing safety of oncolytic fiber-modified conditionally repricative adenovirus designed for pancriatic cancer. Gastroenterology. 2008:A453. [Google Scholar]
- 46.Clyde WAJ. Experimental models for study of common respiratory viruses. Environ Health Perspect. 1980;35:107–112. doi: 10.1289/ehp.8035107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jogler C, Hoffmann D, Theegarten D, Grunwald T, Überla K, Wildner O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol. 2006;80:3549–3558. doi: 10.1128/JVI.80.7.3549-3558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wold WS, Toth K. Chapter three--Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv Cancer Res. 2012;115:69–92. doi: 10.1016/B978-0-12-398342-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 49.Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer JM, Shashkova EV, Kuppuswamy M, Dhar D, Thomas MA, Tollefson AE, Zumstein LA, Wold WS, Toth K. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 2009;16:644–654. doi: 10.1038/cgt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, Wold WS. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 51.Hay J. “Man’s best friend”: a new model system for cancer therapeutics? Mol Ther. 2003;7:144–145. doi: 10.1016/s1525-0016(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 52.Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, Wang M, Desmond RA, Keriel A, Barnett B, Baker HJ, Siegal GP, Curiel DT. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 53.Shashkova EV, Kuppuswamy MN, Wold WSM, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, Roh MS, Je JE, Yoon JH, Thorne SH, Kirn D, Hwang TH. Systemic armed oncolytic and immunologic therapy for cancer with JX-594 a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt J, Patrut EM, Ma J, Jäger D, Knaebel HP, Büchler MW, Märten A. Immunomodulatory impact of interferon-alpha in combination with chemoradiation of pancreatic adenocarcinoma (CapRI) Cancer Immunol Immunother. 2006;55:1396–1405. doi: 10.1007/s00262-006-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talpaz M, Chernajovsky Y, Troutman-Worden K, Wetzler M, Kantarjian H, Gutterman JU, Kurzrock R. Interferon-stimulated genes in interferon-sensitive and -resistant chronic myelogenous leukemia patients. Cancer Res. 1992;52:1087–1090. [PubMed] [Google Scholar]
- 58.Holsti LR, Mattson K, Niiranen A, Standertskiöld-Nordenstam CG, Stenman S, Sovijärvi A, Cantell K. Enhancement of radiation effects by alpha interferon in the treatment of small cell carcinoma of the lung. Int J Radiat Oncol. 1987;13:1161–1166. doi: 10.1016/0360-3016(87)90189-1. [DOI] [PubMed] [Google Scholar]
- 59.Picozzi VJ, Abrams R, Decker P, Traverso W, O’Reilly EM, Greeno E, Martin RC, Wilfong LS, Rothenberg ML, Posner MC, Pisters PW American College of Surgeons Oncology Group. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2011;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 61.Picozzi VJ, Traverso LW. The Virginia Mason approach to localized pancreatic cancer. Surg Oncol Clin N Am. 2004;13:663–674. doi: 10.1016/j.soc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Picozzi VJ, Pisters PWT, Vickers SM, Strasberg SM. Strength of the evidence: Adjuvant therapy for resected pancreatic cancer. J Gastrointest Surg. 2008;12:657–661. doi: 10.1007/s11605-007-0446-y. [DOI] [PubMed] [Google Scholar]
- 63.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodallec M, Vilgrain V, Couvelard A, Rufat P, O’Toole D, Barrau V, Sauvanet A, Ruszniewski P, Menu Y. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006;6:77–85. doi: 10.1159/000090026. [DOI] [PubMed] [Google Scholar]
- 65.Ohashi M, Yoshida K, Kushida M, Miura Y, Ohnami S, Ikarashi Y, Kitade Y, Yoshida T, Aoki K. Adenovirus-mediated interferon alpha gene transfer induces regional direct cytotoxicity and possible systemic immunity against pancreatic cancer. Br J Cancer. 2005;93:441–449. doi: 10.1038/sj.bjc.6602713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaRocca CJ, Han J, Gavrikova T, Armstrong L, Oliveira AR, Shanley R, Vickers SM, Yamamoto M, Davydova J. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery. 2015;157:888–898. doi: 10.1016/j.surg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, Nokisalmi P, Raki M, Laasonen L, Särkioja M, Rajecki M, Kangasniemi L, Guse K, Helminen A, Ahtiainen L, Ristimäki A, Räisänen-Sokolowski A, Haavisto E, Oksanen M, Karli E, Karioja-Kallio A, Holm SL, Kouri M, Joensuu T, Kanerva A, Hemminki A. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 68.Poutou J, Bunuales M, Gonzalez-Aparicio M, Garcia-Aragoncillo E, Quetglas JI, Casado R, Bravo-Perez C, Alzuguren P, Hernandez-Alcoceba R. Safety and antitumor effect of oncolytic and helper-dependent adenoviruses expressing interleukin-12 variants in a hamster pancreatic cancer model. Gene Ther. 2015;22:696–706. doi: 10.1038/gt.2015.45. [DOI] [PubMed] [Google Scholar]
- 69.Reboul F, Serin D, Martin D, Plat F. Combination radiotherapy and chemotherapy in cancer of the pancreas. Review of the literature and prospects. Bull Cancer. 1990;77:275–281. [PubMed] [Google Scholar]
- 70.Noordhuis MG, Eijsink JJH, Roossink F, de Graeff P, Pras E, Schuuring E, Wisman GB, de Bock GH, van der Zee AG. Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo)radiation: a systematic review. Int J Radiat Oncol. 2011;79:325–334. doi: 10.1016/j.ijrobp.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 71.Whistance RN, Blazeby JM. Systematic review: quality of life after treatment for upper gastrointestinal cancer. Curr Opin Support Palliat Care. 2011;5:37–46. doi: 10.1097/SPC.0b013e3283436ecb. [DOI] [PubMed] [Google Scholar]
- 72.Nelson AR, Davydova J, Curiel DT, Yamamoto M. Combination of conditionally replicative adenovirus and standard chemotherapies shows synergistic antitumor effect in pancreatic cancer. Cancer Sci. 2009;100:2181–2187. doi: 10.1111/j.1349-7006.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carson CT, Orazio NI, Lee DV, Suh J, Bekker-Jensen S, Araujo FD, Lakdawala SS, Lilley CE, Bartek J, Lukas J, Weitzman MD. Mislocalization of the MRN complex prevents ATR signaling during adenovirus infection. EMBO J. 2009;28:652–662. doi: 10.1038/emboj.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lakdawala SS, Schwartz R, Ferenchak K, Carson CT, McSharry BP, Wilkinson GW, Weitzman MD. Differential requirements of the C terminus of Nbs1 in suppressing adenovirus DNA replication and promoting concatemer formation. J Virol. 2008;82:8362–8372. doi: 10.1128/JVI.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carson CT, Schwartz R, Stracker TH, Lilley CE, Lee DV, Weitzman MD. The Mre11 complex is required for ATM activation and the G 2/M checkpoint. EMBO J. 2003;22:6610–6620. doi: 10.1093/emboj/cdg630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsieh JL, Lee CH, Teo ML, Lin YJ, Huang YS, Wu CL, Shiau AL. Transthyretin-driven oncolytic adenovirus suppresses tumor growth in orthotopic and ascites models of hepatocellular carcinoma. Cancer Sci. 2009;100:537–545. doi: 10.1111/j.1349-7006.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ganesh S, Gonzalez-Edick M, Gibbons D, Ge Y, VanRoey M, Robinson M, Jooss K. Combination therapy with radiation or cisplatin enhances the potency of Ad5/35 chimeric oncolytic adenovirus in a preclinical model of head and neck cancer. Cancer Gene Ther. 2009;16:383–392. doi: 10.1038/cgt.2008.90. [DOI] [PubMed] [Google Scholar]
- 79.McNally LR, Rosenthal EL, Zhang W, Buchsbaum DJ. Therapy of head and neck squamous cell carcinoma with replicative adenovirus expressing tissue inhibitor of metalloproteinase-2 and chemoradiation. Cancer Gene Ther. 2009;16:246–255. doi: 10.1038/cgt.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsu KF, Wu CL, Huang SC, Hsieh JL, Huang YS, Chen YF, Shen MR, Chung WJ, Chou CY, Shiau AL. Conditionally replicating E1B-deleted adenovirus driven by the squamous cell carcinoma antigen 2 promoter for uterine cervical cancer therapy. Cancer Gene Ther. 2008;15:526–534. doi: 10.1038/cgt.2008.37. [DOI] [PubMed] [Google Scholar]
- 81.Leitner S, Sweeney K, Oberg D, Davies D, Miranda E, Lemoine NR, Halldén G. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin Cancer Res. 2009;15:1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raki M, Kanerva A, Ristimaki A, Desmond RA, Chen DT, Ranki T, Sarkioja M, Kangasniemi L, Hemminki A. Combination of gemcitabine and Ad5/3-Delta24, a tropism modified conditionally replicating adenovirus, for the treatment of ovarian cancer. Gene Ther. 2005;12:1198–1205. doi: 10.1038/sj.gt.3302517. [DOI] [PubMed] [Google Scholar]
- 83.Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, Escutenaire S, Kanerva A, Pesonen S, Löskog A, Cerullo V, Hemminki A. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19:988–998. doi: 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- 84.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, Shim KG, Diaz RM, Pandha H, Harrington K, Coffey M, Melcher A, Vile R. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther. 2016;24:166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]