Abstract
Objective:
Quantification of vasculopathy severity and its impact on parenchymal hemodynamics is a necessary prerequisite for informing management decisions and evaluating intervention response in patients with moyamoya disease (MMD). The authors performed digital subtraction angiography and noninvasive structural and hemodynamic MRI, and they outline a new classification system for patients with moyamoya that they have named Prior Infarcts, Reactivity, and Angi- ography in Moyamoya Disease (PIRAMD).
Methods:
Healthy control volunteers (n = 11; age 46 +/− 12 years [mean +/− SD]) and patients (n = 25; 42 +/− 13.5 years) with angiographically confirmed moyamoya provided informed consent and underwent structural (T1-weighted, T2- weighted, FLAIR, MR angiography) and hemodynamic (T2*- and cerebral blood flow–weighted) 3-T MRI. Cerebrovascular reactivity (CVR) in the internal carotid artery territory was assessed using susceptibility-weighted MRI during a hy- percapnic stimulus. Only hemispheres without prior revascularization were assessed. Each hemisphere was considered symptomatic if localizing signs were present on neurological examination and/or there was a history of transient ischemic attack with symptoms referable to that hemisphere. The PIRAMD factor weighting versus symptomatology was optimized using binary logistic regression and receiver operating characteristic curve analysis with bootstrapping. The PIRAMD finding was scored from 0 to 10. For each hemisphere, 1 point was assigned for prior infarct, 3 points for reduced CVR, 3 points for a modified Suzuki Score ≥ Grade II, and 3 points for flow impairment in ≥ 2 of 7 predefined vascular territories. Hemispheres were divided into 3 severity grades based on total PIRAMD score, as follows: Grade 1, 0–5 points; Grade 2, 6–9 points; and Grade 3, 10 points.
Results:
In 28 of 46 (60.9%) hemispheres the findings met clinical symptomatic criteria. With decreased CVR, the odds ratio of having a symptomatic hemisphere was 13 (95% CI 1.1–22.6, p = 0.002). The area under the curve for individual PIRAMD factors was 0.67–0.72, and for the PIRAMD grade it was 0.845. There were 0/8 (0%), 10/18 (55.6%), and 18/20 (90%) symptomatic PIRAMD Grade 1, 2, and 3 hemispheres, respectively.
Conclusions:
A scoring system for total impairment is proposed that uses noninvasive MRI parameters. This scoring system correlates with symptomatology and may provide a measure of hemodynamic severity in moyamoya, which could be used for guiding management decisions and evaluating intervention response.
Keywords: moyamoya, cerebrovascular reactivity, magnetic resonance imaging, hemodynamic, vascular disorders
Introduction:
Moyamoya is a progressive steno-occlusive disease of the distal internal carotid arteries (ICA) and their proximal terminal branches. Patients most frequently present with ischemic stroke or recurrent transient ischemic attacks (TIAs). Quantification of vasculopathy severity and its impact on vascular morphology and parenchymal hemodynamics is a necessary prerequisite for informing management decisions and evaluating intervention response. Current management decisions use angiographic data combined with MRI evidence of infarct, along with perfusion studies based on PET or SPECT. Studies of hemodynamic reserve are likely a useful adjunct; however, current clinical methods require exogenous contrast administration and/or ionizing radiation. While such tools have improved our understanding of MMD, these methods are suboptimal for longitudinal monitoring of patients or assessing revascularization response owing to dose restrictions.
Cerebrovascular reactivity (CVR) is a well-documented and valuable surrogate marker of cerebrovascular reserve in patients with previously identified intravascular pathology1,9,10,12,13. In healthy parenchyma, CVR primarily derives from a large increase in cerebral blood flow (CBF) and volume (CBV) in response to a vasostimulatory agent such as carbon dioxide (i.e., hypercapnia). Significantly diminished or negative changes in CVR after during hypercapnia have previously been shown to correlate with regions affected by prior infarct and symptomatology1,26,30. However, owing to the relative novelty of hypercapnic CVR mapping using MRI compared to more established clinical measures such as acetazolamide SPECT, interpretation of hypercapnic CVR maps has not been standardized.
Here we outline an integrated neuroimaging-based severity classification system for moyamoya titled PIRAMD (Prior Infarcts, Reactivity, and Angiography in Moyamoya Disease). This scoring system accounts for functional measurements of hemodynamic impairment in moyamoya using BOLD MRI-weighted CVR, and therefore may be a comprehensive approach toward stratification of moyamoya severity.
Methods:
Patient selection
All procedures were followed in accordance with the ethical standards of the Vanderbilt University Institutional Review Board. Patients presenting with angiographically confirmed moyamoya between January 2011 and May 2015 underwent hemodynamic 3-T MRI (Philips) performed using body coil transmission and 12-channel sensitivity-encoding (SENSE) array neurovascular coil for reception. Patients were included in the study if cerebral digital subtraction angiography (DSA) was performed within 90 days of MRI, without any surgical intervention in the interval. Only hemispheres without a prior revascularization procedure were considered.
Neurologic assessment
A neurologist (L.J.) retrospectively reviewed patient symptomatology derived from the electronic medical record. Symptomatic hemispheres were defined as those with either a history of recurrent localizable transient ischemic attacks or persistent neurological deficits (motor, sensory, and/or language) referable to the hemisphere. Psychological symptoms, deficits in concentration and memory, and/or headache were not included, given the potential ambiguity in localization
Imaging Protocol
BOLD imaging consisted of a T2*-weighted single-shot GRE Echo Planar Imaging (EPI) acquisition (slice thickness=5 mm, TR/TE=2000/35ms, FOV = 240×240 mm, spatial resolution=3×3×5 mm) across the entire brain. The experimental paradigm consisted of 5 total blocks each of 3 minutes duration, beginning and ending with the delivery of medical grade room air and interleaved with hypercapnic gas (5% CO2 / 95% O2) administration. We have recently quantified relationships between such hypercarbic hyperoxic and hypercarbic normoxic stimuli in healthy adults and patients with intracranial stenosis9 and have demonstrated abilities for this stimulus to be performed safely in a large volume of patients, provide contrast consistent with symptomatology and lateralizing disease, and correlate with perfusion reactivity upon appropriate post-processing. Gas delivery was performed using a custom non-rebreathing facemask, and core physiologic parameters including end tidal CO2 (EtCO2), heart rate, blood pressure, and arterial oxygen saturation were monitored throughout the experiment.
Radiologic Evaluation
Prior Infarct:
Each hemisphere under review was considered separately. Two board-certified neuroradiologists (M.K.S., T.L.D.) blinded to clinical status and hemodynamic findings reviewed FLAIR imaging acquired at the time of BOLD MRI to determine presence of infarct. T2-weighted imaging was also reviewed when available. For lacunar infarcts, a size criterion for T2 hyperintense lesions of greatest axial diameter ≥ 4 mm was used to separate prior infarcts from white matter changes17,23. The T1-weighted sequence was used to verify encephalomalacia when infarcts were suspected based on the FLAIR sequence.
Cerebrovascular Reactivity:
Healthy controls (n=11; 46±3.5yrs) and patients (n=23; age=41.0±3.0yrs) provided written informed consent and underwent structural (T1w, T2w, FLAIR, MRA) and hemodynamic (T2* and CBF-weighted) 3T MRI (Figure 1). The ICA territory was defined by a pre-determined mask (Figure 2) ICA-territory CVR was assessed using susceptibility-weighted MRI during a hypercapnic (ΔEtCO2 ~5mmHg; repeats=2) stimulus and normalized to cerebellar CVR. For each patient hemisphere (anterior circulation), the number of standard deviations by which CVR differed from the control mean CVR (Z-stastistics: mean=0.69; SD=0.19) was calculated.
Figure 1.
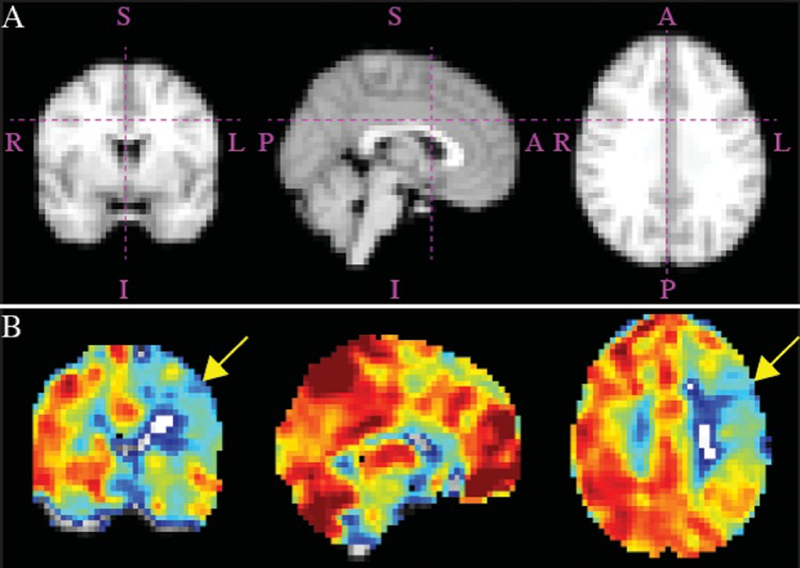
Admission MRI studies obtained in a patient with a symptomatic left hemisphere. Corresponding atlas maps for hemodynamic sections (a) and orthogonal representations of reactivity maps (b), demonstrating impairment in CVR in the left hemisphere (yellow arrows). Right hemisphere (asymptomatic) PIRAMD score: 0 (Grade 1); left hemisphere (symptomatic) PIRAMD score: 10 (Grade 3). A = anterior; I = inferior; L = left; P = posterior; R = right; S = superior. Figure is available in color online only.
Figure 2.
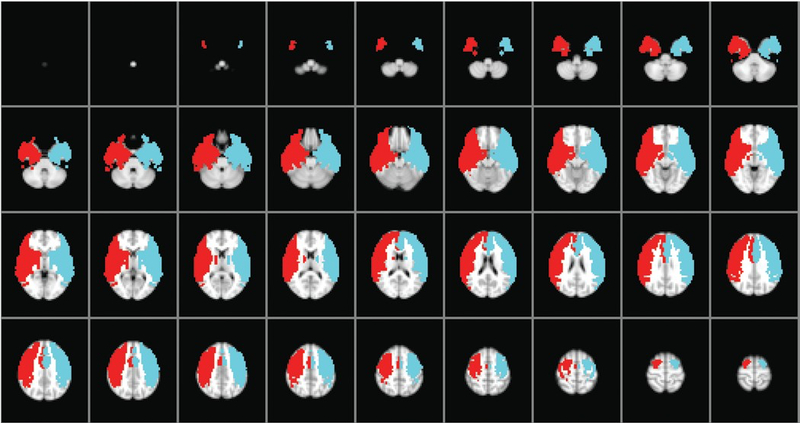
The ICA territory masks used to define right (red) and left (blue) regions of interest for assessment of CVR. Figure is avail- able in color online only.
Digital Subtraction Angiography:
Moyamoya changes on DSA were scored with a modified Suzuki Score (mSS), ranging from 0 to IV by two neuroradiologists (Table 1) with higher grades representing more severe disease.31 The mSS accounts for internal carotid, middle cerebral artery, and anterior cerebral artery disease, along with presence or absence of lenticulostriate collaterals. Regional collateralization on DSA was assessed via modification of a previously reported technique19,33 In brief, DSA was divided into 7 anatomic sites based on ASPECTS-defined regional vascular territories29,33: M1-M6 and basal ganglia (Figure 3). An interventional neurologist (M.F.) and a neuroradiologist (M.S.) working in tandem reviewed each territory on DSA for each patient and assessed whether regional blood flow was impaired or not for the ASPECTS-defined regions. A territory was considered impaired if there were no visible collaterals supplying the ischemic site or there were collaterals only to the periphery of the ischemic site. A territory was considered not impaired if collateral flow provided complete irrigation of the ischemic bed or if there was normal anterograde flow. Flow classification was made based on consensus. The total number of impaired territories (0–7) was counted for each hemisphere.
Table 1:
Modified Suzuki Score*
| Score | Description of classification |
|---|---|
| 0 | No evidence of disease |
| I | Mild-to-moderate stenosis around ICA bifurcation with absent or slightly developed ICA MMD |
| II | Severe stenosis around the ICA bifurcation or occlusion of either proximal anterior or MCA branches with well-developed ICA MMD |
| III | Occlusion of both anterior and MCA branches with well-developed ICA MMD (only a few of anterior or MCA branches or both are faintly opacified in antegrade fashion through meshwork of ICA MMD) |
| IV | Complete occlusion of both anterior and MCA branches with absent or small amount of ICA MMD (without opacification of either anterior or MCA branches in antegrade fashion) |
MMD = moyamoya disease.
Reprinted with permission from Strother MK, Anderson MD, Singer RJ, Du L, Moore RD, Shyr Y, et al: Cerebrovascular collaterals correlate with disease severity in adult North American patients with Moyamoya disease. AJNR Am J Neuroradiol 35:1318–1324, 2014.
Figure 3.
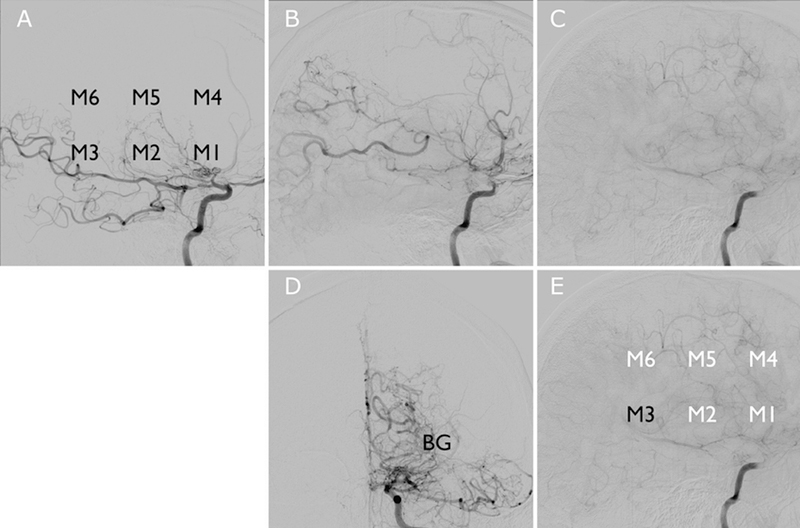
Lateral projections (a, early-; b, mid-; and c, delayed-phase sequences) from left ICA injection on DSA. The 7 DSA ter- ritories measured are labeled in the lower row from the same left ICA injection (D, AP projection; e, lateral projection); impaired regions with delayed perfusion from collaterals are labeled in white (E). BG = basal ganglia; M1–M6 = ASPECTS territories.
PIRAMD Optimization and Analysis
Each component of PIRAMD was converted to a categorical variable and given a preliminary, simplified scoring system. The preliminary scoring system was optimized via simple logistic regression analysis and receiver operating characteristic (ROC) curve analysis using symptomatology as the dependent variable to determine a clinically valid and statistically significant stratification system. Acceptability criteria were p<0.05 for binary logistic regression analysis and area under the curve (AUC) >0.6 for ROC curve analysis. PIRAMD component grading is summarized in Table 2.
Table 2:
The PiramMD Scoring system
| Variable | Characteristics | Points |
|---|---|---|
| MRI | Prior infarct | 1.0 |
| CVR | Decreased | 3.0 |
| mSS | ≥ Grade II | 3.0 |
| Collaterals | ≥ 2 territories impaired | 3.0 |
PIRAMD Grading
The relative weight for each factor in the PIRAMD score was determined by using the factor’s odds ratio from simple logistic regression analysis. The lowest odds ratio was used as the baseline by which all other factors were scaled. The weighted scores for each component of PIRAMD were added together for a raw PIRAMD score. PIRAMD score was divided into three grades: PIRAMD 1, PIRAMD 2, and PIRAMD 3. Grade stratification was determined through optimization by ROC curve analysis and simple binary logistic regression analysis, considering PIRAMD score as a discrete independent variable for those purposes. ROC and binary logistic regression analyses were also repeated for PIRAMD grade. Acceptability criteria were p<0.01 for binary logistic regression analysis and area under the curve (AUC) >0.8 for ROC curve analysis.
PIRAMD Validation
Additional internal validation was conducted on the dataset via a bootstrap method. 95% confidence interval estimates for the binary logistic regression analyses were generated in SPSS using a bootstrap sample size of 1,000. Bootstrapped p values and confidence intervals were obtained and reported. ROC bootstrapping also was performed in SPSS using an adapted public domain macro (Available: http://gjyp.nl/marta/), with a bootstrap sample size of 1,000. Bootstrapped AUC and 95% confidence intervals were obtained and reported.
Results
Participants in Study
There were 25 moyamoya participants in the study, accounting for 46 hemispheres (Table 3). The mean age was 42 years, with a SD of 12 years. Most patients were female (20, 80%). There were 28 (60.9%) symptomatic hemispheres. The majority of patients had bilateral disease (22, 88%). Four of the 46 hemispheres analyzed (8.7%) had a prior contralateral revascularization surgery, but none had a prior ipsilateral revascularization surgery. The mean interval between MRI scan and diagnostic angiography was 30 days with a SD of 24.2 days.
Table 3:
Characteristics of patients and hemiespheres in study
| Characteristic | Value | ||
|---|---|---|---|
| By patient (n=25) | |||
| Demographic data | |||
| Age in yrs, mean ± SD | 42 ± 13.5 | ||
| Female sex | 20 (80%) | ||
| Race: | |||
| Asian/Pacific Islander | 3 (12%) | ||
| Black/African American | 8 (12%) | ||
| White/Caucasian, Hispanic/Latino | 1 (4%) | ||
| White/Caucasian, non-Hispanic/Latino | 13 (52%) | ||
| Clinical data | |||
| Bilateral moyamoya | 22 (88%) | ||
| Days between MRI and DSA, mean ± SD | 30 ± 24.2 | ||
| By hemisphere (n=46) | |||
| Clinical data | |||
| Symptomatic | 28 (60.9%) | ||
| Prior contralateral revascularization | 4 (8.7%) | ||
| Infarct: | |||
| No prior infarct | 16 (34.8%) | ||
| Prior infarct | 30 (65.2%) | ||
| CVR: | |||
| Normal/increased | 11 (23.9%) | ||
| Decreased | 35 (76.1%) | ||
| mSS: | |||
| 0 | 3 (6.5%) | ||
| I | 5 (10.9%) | ||
| II | 20 (43.5%) | ||
| III | 14 (30.4%) | ||
| IV | 4 (8.7%) | ||
| Number of collaterals impaired: | |||
| 0 | 10 (21.7%) | ||
| 1 | 1 (2.2%) | ||
| 2 | 6 (13.0%) | ||
| 3 | 10 (21.7%) | ||
| 4 | 10 (21.7%) | ||
| 5 | 3 (6.5%) | ||
| 6 | 3 (6.5%) | ||
| 7 | 3 (6.5%) | ||
| PIRAMD grade | |||
| 1 | 8 (17.4%) | ||
| 2 | 18 (39.1%) | ||
| 3 | 20 (43.5%) | ||
Structural MRI Data
Thirty (65.2%) hemispheres had a prior infarct on T2-weighted FLAIR imaging. With a prior infarct, the odds of having a symptomatic hemisphere were 4.6 times greater than without a prior infarct; however, the bootstrapped confidence interval estimates crossed unity and were not statistically significant (95% CI: 0.3–3.3, p=0.016, Table 4). AUC for prior infarct was 0.670 (95% CI: 0.539 – 0.802).
Table 4:
Correlations with symptomatology
| Component | AUC | 95% CI | Subscore | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Prior Infarct | 0.670 | 0.539–0.802 | Present | 4.6 | 0.3–3.3 | 0.016 |
| CVR | 0.716 | 0.585–0.844 | Decreased | 13.0 | 1.1–22.6 | 0.002 |
| mSS | 0.678 | 0.560–0.793 | ≥ Grade II | 17.2 | 1.1–22.7 | 0.008 |
| Collaterals | 0.713 | 0.593–0.836 | ≥ 2 Territories | 13.0 | 1.1–22.7 | 0.006 |
| PIRAMD Grade | 0.845 | 0.735–0.956 | 2 3 |
NA NA |
20.4–22.5 22.3–42.4 |
0.004 0.004 |
Hemodynamic MRI Data
After normalizing ICA territory CVR by total cerebellar CVR, 35 (76.1%) patients had reduced normalized CVR relative to the control cohort. With CVR lower than control CVR, the odds of having a symptomatic hemisphere were 13 times greater than when normal or increased CVR was present (95% CI: 1.1–22.6, p=0.002). AUC for CVR was 0.716 (95% CI: 0.585 – 0.844).
Angiographic Data
Thirty-eight (82.6%) hemispheres were mSS of Grade II or greater. With a mSS in this range, the odds of having a symptomatic hemisphere were 17.2 times greater than Grade 0–1 mSS (95% CI: 1.1–22.7, p=0.008). AUC for mSS was 0.678 (95% CI: 0.560 – 0.793).
With regard to collateral flow impairment, 35 (76.1%) hemispheres had 2–7 territories impaired; With 2–7 impaired territories, the odds of having a symptomatic hemisphere were 13 times greater than 0–1 territories impaired (95% CI: 1.1–22.7, p=0.006). AUC for mSS was 0.713 (95% CI: 0.593 – 0.836).
Development of PIRAMD Score
Following the simple analysis of the individual PIRAMD factors, the relative weighting for each factor was determined by scaling the odds ratio for prior infarct (4.6), as this had the lowest odds ratio. The PIRAMD scoring system is summarized in Table 2. Scores were added to achieve the hemisphere’s PIRAMD score, and in this way, PIRAMD was scored from 0 to 10, with increasing score representing increasing impairment (Figure 4).
Figure 4.
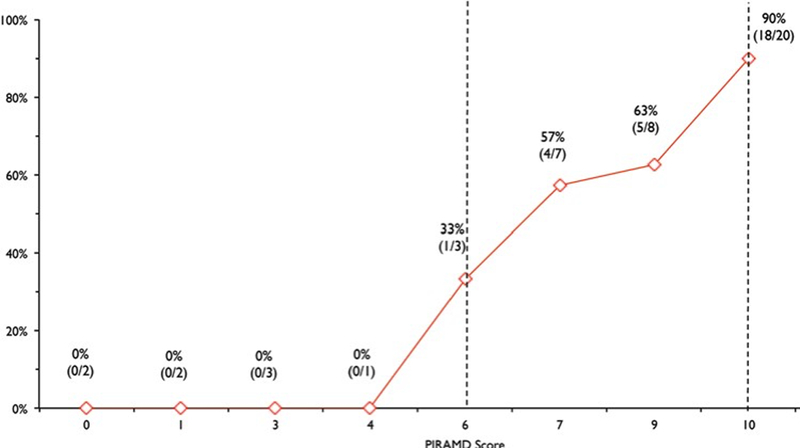
Graph showing the PIRAMD grade versus proportion of patients who were symptomatic. Vertical dashed lines representPIRAMD Grade 2 (≥ 6) and Grade 3 (10) demarcations, respectively. Figure is available in color online only.
PIRAMD Grade
Hemispheres were divided into three severity grades based on total PIRAMD score: Grade 1: 1–5 points; Grade 2: 6–9 points; and Grade 3: 10 points. There were 0/8 (0%), 8/18 (55.6%), and 18/20 (90%) symptomatic PIRAMD 1, 2, and 3 hemispheres, respectively (Figure 5). AUC for PIRAMD grade (1–3) was 0.845 (95% CI: 0.735 – 0.956). AUC for PIRAMD score was 0.860 (95% CI: 0.746–0.974).
Figure 5.
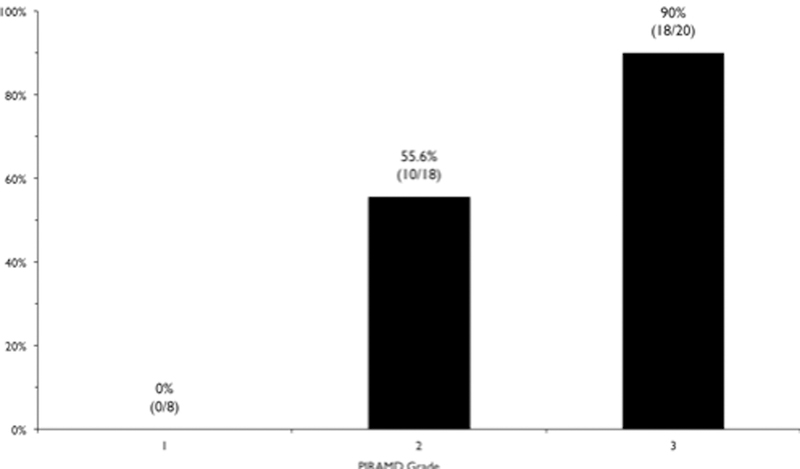
Bar graph showing the PIRAMD grade versus symptomatology. There were 0/8 (0%), 10/18 (55.6%), and 18/20 (90%) symptomatic PIRAMD Grade 1, 2, and 3 hemispheres, respectively.
Discussion
PIRAMD is a simple scoring system for total impairment in MMD, which utilizes non-invasive functional MRI parameters in addition to angiography. PIRAMD was found to correlate well with symptomatology (AUC=0.860). There were 0/8 (0%), 8/18 (55.6%), and 18/20 (90%) symptomatic PIRAMD 1, 2, and 3 hemispheres, respectively.
Patients with MMD are heterogeneous with regard to their clinical presentations and outcomes; however angiographic studies alone may not be sufficient to understand patient pathophysiology. Clinical severity does not follow a perfect correlation with angiography, as proximal occlusions may be completely compensated by robust pial and lenticulostriate auto-collateralization. In contrast, uncompensated mild stenosis may portend a severe course21,31. In some cases, angiography may not even correlate with hemodynamic impairment4.
As surgical candidacy is weighed heavily by imaging appearance and symptomatology, efforts to stratify patients for intervention are critical. A contemporary issue in the management of patients with MMD is the selection of impaired individuals who are likely to benefit from surgical revascularization. CVR may be predictive of outcome, and may be useful in noninvasive monitoring of such patients15,20,24,28 Han et al. have shown that post-revascularization CVR correlated with graft patency and clinical outcomes in MMD15. In intracranial stenosis in general, Mandell showed that patients with impaired CVR were more likely to have hemodynamic normalization post-revascularization25.
There has been a robust effort in the field to quantify and understand hemodynamic impairment in MMD. CVF and CBV increase in the early stages of impairment, and oxygen extraction fraction (OEF) increases when CBF cannot increase sufficiently to meet oxygen demands22. The current methods available for assessing these impairments include PET, SPECT, Xenon-CT, dynamic perfusion CT, DSC-MRI, ASL-MRI, and Doppler ultrasound (see Table 1 in Lee et al.22). However, many of these methods (e.g. PET, SPECT, Xenon-CT, and dynamic perfusion CT) require ionizing radiation exposure and/or administration of exogenous contrast (e.g. gadolinium, which causes renal failure in up to 2% of cases3 ). Diagnostic angiography carries risk, with a complication rate as high as 1.2% in the Asymptomatic Carotid Atherosclerosis Study11, in addition to potential dose-dependent radiation-induced skin injuries32.
MRI uses blood oxygenation level as an endogenous contrast agent, and therefore does not require exogenous contrast or ionizing radiation. It can be acquired serially during routine structural MRI for patients, and therefore holds promise as a noninvasive, readily available, and valid adjunct to routine imaging of MMD patients. Additionally, MR offers improved spatial (3–5 mm isotropic) and temporal (2–3s) resolution and may be more clinically available compared to PET and SPECT, especially in non-specialized hospitals.
In patients with intracranial disease, secondary reductions in cerebral perfusion pressure may initially be compensated for via an increase in cerebral blood volume (CBV) and CBF2,7.To assess this autoregulatory capacity, a vasostimulus such as carbogen can be administered. Carbogen serves to increase the arterial partial pressure of O2 and CO2, CBF, and CBV, and in turn increases blood oxygenation. The resulting increase in the ratio of oxyhemoglobin compared to deoxyhemoglobin will lead to an increase in T2*-weighted MRI signal. The magnitude of this change in the BOLD signal, or CVR, reflects the ability of vessels to regulate CBF and CBV, indicating how close parenchyma is to failing to meet the hemodynamic demand. This permits an endogenous signal to be measured rather than having to rely upon exogenous contrasts or acetazolamide. Carbogen is a safe substance for CVR measurement; in our experience with 92 consecutive patients, carbogen elicited no short-term neurological events, and longer term (2-year) events fell within the expected range for patients with intracranial stenosis9.
Although the role of CVR in predicting long-term stroke risk is not yet completely known, numerous studies have established a strong correlation between CVR and intracranial vascular disease9,16. In patients with moyamoya, strong inverse relationships between mean CVR and both Suzuki score and the presence of collateral vessels have been identified,4,18. Moyamoya patients refractory to medical management undergoing surgical revascularization have been shown to demonstrate post-surgical revascularization improvements in CVR in regions which were previously compromised 15,25,27.
Using Xenon-CT with acetazolamide challenge in 40 patients (80 hemispheres), Czabanka et al. created a similar scoring mechanism for MMD severity6. Compromised cerebrovascular reserve capacity was defined with Xenon-CT as CBF decreased by greater than 5% after acetazolamide challenge. Although for methodological reasons we were unable to compare PIRAMD to the Czabanka system directly, the AUC was similar between populations (0.80 vs. 0.86). While that study has laid the foundation for using CVR in moyamoya severity stratification, the technical innovations that have occurred since then have lead us to conclude that PIRAMD might be a more favorable scoring system for patients, particularly in the United States, where there is not widespread xenon use due to concerns in the literature and from the FDA related to reports of respiratory side effects with xenon.5 Symptomatic classification was more conservative in our study. Whereas Czabanka et al. considered psychological or headache symptoms as bilaterally symptomatic, these were not counted in our study for difficulty in localization. This may account for the apparent difference in prevalence of symptomatic hemispheres between cohorts (61% in our study, 68% in the study by Czabanka et al.).
We find it interesting that a milder correlation with symptomatology in our study was found with prior infarct. This is not to say that prior infarct is not important, as it indicates that significant hemodynamic impairment has already occurred. In fact, we found post-hoc that 22/28 (78.6%) of symptomatic hemispheres had an infarct. There was, however, a high rate of clinically silent infarcts (8/30 infarct, 26.7%), particularly in the watershed distribution. There was considerable collinearity with other PIRAMD components; if a prior infarct was present, in all but two cases (93.3%), impairment in another PIRAMD component was also present. Unsurprisingly, with prior infarct the mean PIRAMD score was 8.5 versus 6.2 without (p=0.013). However, for the hemispheres without infarct (especially the 21% of symptomatic hemispheres without infarct), the PIRAMD score adds more clarity to hemodynamic impairment than just infarct assessment alone. Patients without infarct may have other impairments that increase the PIRAMD score, and this may weigh more heavily on the decision to revascularize.
Therefore, it is useful to complement this information with functional measures of impairment. The angiographic assessment of collateral perfusion is a strong contributor to PIRAMD score, as it allows a functional, dynamic assessment of territory perfusion. However, it is invasive and requires radiation, which may limit its use in serial monitoring. Thus, we offer a functional complement of parenchymal impairment via BOLD MRI8. The measurement of CVR using MRI provides a noninvasive method for assessing hemodynamic instability. We found that reduced CVR carried a 13-fold increase in odds of being symptomatic. When synthesizing angiographic, structural, and hemodynamic MRI evidence of impairment via the PIRAMD score, the probability of being symptomatic can be calculated. We found that hemispheres were asymptomatic until PIRAMD score reached 6, at which a precipitous proportional rise in symptomatology occurred (Figure 4).
The PIRAMD severity grades may be useful in counseling patients for further management. In particular, PIRAMD 3 patients, if not already symptomatic, might have a high risk of becoming symptomatic, and surgery should be more strongly considered. In contrast, PIRAMD 1 patients might not become symptomatic, and conservative management with serial monitoring may be recommended. Of course, while reduced CVR may be associated with greater stroke risk14, the long-term risks of PIRAMD grades have not yet been evaluated, and would need to be assessed in larger prospective studies. Nonetheless, the strong correlation between PIRAMD score and symptomatology suggests it may still have a role in patient stratification.
Limitations
This study is limited by its retrospective nature and small sample size, necessitating univariate analysis. While we included patients if DSA was performed within 90 days, it would have been more ideal to have these studies obtained concurrently; the mean interval between angiography and MRI was 30 days. Though we performed a limited internal validation via bootstrapping, external validation with a larger number of patients is needed. This study addresses historic symptom risk, and does not predict future symptoms or postoperative response, and therefore we are continuing to evaluate this prospectively.
Conclusion
A scoring system for total impairment in moyamoya is proposed which utilizes non-invasive MRI parameters along with conventional angiography. This scoring system was found to correlate with symptomatology and may provide an objective measure of hemodynamic severity in moyamoya, which could be used for guiding management decisions and evaluating intervention response.
Acknowledgments:
None
Disclosure:
This work was supported by the National Institutes of Health (5R01NS078828–02). T.R.L. received funding via the Medical Student Summer Research Fellowship from the American Association of Neurological Surgeons. A portion of the findings were presented as an oral abstract at the 2015 AANS/CNS Joint Cerebrovascular Section Annual Meeting on February 9th, 2015 in Nashville, TN.
Prior presentation: A portion of the findings herein were presented as an oral abstract at the 2015 AANS/CNS Joint Cerebrovascular Section Annual Meeting on February 9th, 2015 in Nashville, TN.
Bibliography
- 1.Arteaga DF, Strother MK, Faraco CC, Jordan LC, Ladner TR, Dethrage LM, et al. : The vascular steal phenomenon is an incomplete contributor to negative cerebrovascular reactivity in patients with symptomatic intracranial stenosis. J Cereb Blood Flow Metab:2014 [DOI] [PMC free article] [PubMed]
- 2.Blicher JU, Stagg CJ, O’Shea J, Ostergaard L, MacIntosh BJ, Johansen-Berg H, et al. : Visualization of altered neurovascular coupling in chronic stroke patients using multimodal functional MRI. J Cereb Blood Flow Metab 32:2044–2054, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briguori C, Colombo A, Airoldi F, Melzi G, Michev I, Carlino M, et al. : Gadolinium-based contrast agents and nephrotoxicity in patients undergoing coronary artery procedures. Cathet Cardiovasc Intervent 67:175–180, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Calamante F, Ganesan V, Kirkham FJ, Jan W, Chong WK, Gadian DG, et al. : MR perfusion imaging in Moyamoya Syndrome: potential implications for clinical evaluation of occlusive cerebrovascular disease. Stroke 32:2810–2816, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Carlson AP, Brown AM, Zager E, Uchino K, Marks MP, Robertson C, et al. : Xenon-enhanced cerebral blood flow at 28% xenon provides uniquely safe access to quantitative, clinically useful cerebral blood flow information: a multicenter study. AJNR Am J Neuroradiol 32:1315–1320, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czabanka M, Peña-Tapia P, Schubert GA, Heppner FL, Martus P, Horn P, et al. : Proposal for a new grading of Moyamoya disease in adult patients. Cerebrovasc Dis 32:41–50, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. : Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 125:595–607, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Donahue MJ, Ayad M, Moore R, van Osch M, Singer R, Clemmons P, et al. : Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J Magn Reson Imaging:2013 [DOI] [PMC free article] [PubMed]
- 9.Donahue MJ, Dethrage LM, Faraco CC, Jordan LC, Clemmons P, Singer R, et al. : Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 45:2335–2341, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donahue MJ, Strother MK, Hendrikse J: Novel MRI approaches for assessing cerebral hemodynamics in ischemic cerebrovascular disease. Stroke 43:903–915, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study:JAMA 273:1421–1428, 1995 [PubMed] [Google Scholar]
- 12.Faraco CC, Strother MK, Dethrage LM, Jordan L, Singer R, Clemmons PF, et al. : Dual echo vessel-encoded ASL for simultaneous BOLD and CBF reactivity assessment in patients with ischemic cerebrovascular disease. Magn Reson Med:2014 [DOI] [PMC free article] [PubMed]
- 13.Gao Y-Z, Zhang J-J, Liu H, Wu G-Y, Xiong L, Shu M: Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer’s disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Curr Neurovasc Res 10:49–53, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, et al. : Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43:2884–2891, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han JS, Abou-Hamden A, Mandell DM, Poublanc J, Crawley AP, Fisher JA, et al. : Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke 42:3047–3054, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Han JS, Mikulis DJ, Mardimae A, Kassner A, Poublanc J, Crawley AP, et al. : Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke 42:1261–1269, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, et al. : Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 126:424–432, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Heyn C, Poublanc J, Crawley A, Mandell D, Han JS, Tymianski M, et al. : Quantification of Cerebrovascular Reactivity by Blood Oxygen Level–Dependent MR Imaging and Correlation with Conventional Angiography in Patients with Moyamoya Disease. AJNR Am J Neuroradiol 31:862–867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP: Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 35:1340–1344, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kohno K, Oka Y, Kohno S, Ohta S, Kumon Y, Sakaki S: Cerebral blood flow measurement as an indicator for an indirect revascularization procedure for adult patients with moyamoya disease. Neurosurgery 42:752–757; discussion 757–758, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y: Radiological Findings, Clinical Course, and Outcome in Asymptomatic Moyamoya Disease Results of Multicenter Survey in Japan. Stroke 38:1430–1435, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Zaharchuk G, Guzman R, Achrol A, Bell-Stephens T, Steinberg GK: Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg Focus 26:E5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longstreth WT, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR: Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 55:1217–1225, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, et al. : Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 39:2021–2028, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Mandell DM, Han JS, Poublanc J, Crawley AP, Fierstra J, Tymianski M, et al. : Quantitative measurement of cerebrovascular reactivity by blood oxygen level-dependent MR imaging in patients with intracranial stenosis: preoperative cerebrovascular reactivity predicts the effect of extracranial-intracranial bypass surgery. AJNR Am J Neuroradiol 32:721–727, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markus H, Cullinane M: Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124:457–467, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Li M, Jiao LQ, Zhang HQ, Ling F: Contralateral cerebral hemodynamic changes after unilateral direct revascularization in patients with moyamoya disease. Neurosurg Rev 34:347–353; discussion 353–354, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Mikulis DJ, Krolczyk G, Desal H, Logan W, Deveber G, Dirks P, et al. : Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J Neurosurg 103:347–355, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Pexman JHW, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. : Use of the Alberta Stroke Program Early CT Score (ASPECTS) for Assessing CT Scans in Patients with Acute Stroke. AJNR Am J Neuroradiol 22:1534–1542, 2001 [PMC free article] [PubMed] [Google Scholar]
- 30.Siero JCW, Hartkamp NS, Donahue MJ, Harteveld AA, Compter A, Petersen ET, et al. : Neuronal activation induced BOLD and CBF responses upon acetazolamide administration in patients with steno-occlusive artery disease. Neuroimage 105:276–285, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strother MK, Anderson MD, Singer RJ, Du L, Moore RD, Shyr Y, et al. : Cerebrovascular Collaterals Correlate with Disease Severity in Adult North American Patients with Moyamoya Disease. AJNR Am J Neuroradiol:2014 [DOI] [PMC free article] [PubMed]
- 32.Vano E, Fernandez JM, Sanchez RM, Martinez D, Ibor LL, Gil A, et al. : Patient radiation dose management in the follow-up of potential skin injuries in neuroradiology. AJNR Am J Neuroradiol 34:277–282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK: Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 42:2485–2491, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


