Abstract
Trichloroethylene (TCE) and tetrachloroethylene (PCE) are structurally similar chemicals that are metabolized through oxidation and glutathione conjugation pathways. Both chemicals have been shown to elicit liver and kidney toxicity in rodents and humans; however, TCE has been studied much more extensively in terms of both metabolism and toxicity. Despite their qualitative similarities, quantitative comparison of tissue- and strain-specific metabolism of TCE and PCE has not been performed. To fill this gap, we conducted a comparative toxicokinetic study where equimolar single oral doses of TCE (800 mg/kg) or PCE (1000 mg/kg) were administered to male mice of C57BL/6J, B6C3F1/J, and NZW/LacJ strains. Samples of liver, kidney, serum, brain, and lung were obtained for up to 36 hours after dosing. For each tissue, concentrations of parent compounds, as well as their oxidative and glutathione conjugation metabolites were measured and concentration-time profiles constructed. A multi-compartment toxicokinetic model was developed to quantitatively compare TCE and PCE metabolism. As expected, the flux through oxidation metabolism pathway predominated over that through conjugation across all mouse strains examined, it is 1,200–3,800 fold higher for TCE and 26–34 fold higher for PCE. However, the flux through glutathione conjugation, albeit a minor metabolic pathway, was 21-fold higher for PCE as compared to TCE. The degree of inter-strain variability was greatest for oxidative metabolites in TCE-treated and for glutathione conjugation metabolites in PCE-treated mice. This study provides critical data for quantitative comparisons of TCE and PCE metabolism, and may explain the differences in organ-specific toxicity between these structurally similar chemicals.
Keywords: trichloroethylene, tetrachloroethylene, toxicokinetics, glutathione conjugation, oxidation
1. Introduction
Trichloroethylene (TCE) and tetrachloroethylene (PCE) are structurally similar chlorinated olefins that are used in chemical manufacture, metal degreasing, and other industrial applications (U.S. EPA 2011a, b). TCE and PCE are high production volume chemicals and are ubiquitous in the environment (IARC 2014). Humans can be exposed to these chemicals via inhalation and ingestion (ATSDR 1997; Wu and Schaum 2000). In a National Health and Nutrition Examination Survey (2013–2014), the rate of detection for TCE and PCE in blood was 0.6% for TCE and 7.4% for PCE (CDC 2017). TCE and PCE are still prioritized for evaluation of the risks to human health and environment (U.S. EPA 2017).
There are differences in toxic effects of TCE and PCE in liver, kidney and other tissues (Cichocki et al. 2016). TCE is classified as “carcinogenic to humans” by US EPA (U.S. EPA 2011b) and IARC (Guha et al. 2012); while PCE is classified as “likely to be a human carcinogen” by US EPA (U.S. EPA 2011a) and as “probably carcinogenic to humans” by IARC (Guha et al. 2012). A comparative study of toxicodynamics of TCE and PCE in mice showed differences in the effects on liver and kidney, PCE perturbed more molecular pathways in mouse liver and kidney as compared to TCE (Zhou et al. 2017). However, there are no published reports of comparative analysis of toxicokinetics of TCE and PCE.
Upon absorption, TCE and PCE are metabolized through oxidative and glutathione conjugation pathways (Cichocki et al. 2016). Initial oxidation occurs on the double bond by cytochrome P450s (CYPs) to generate an epoxide, which can be further metabolized. Trichloroacetic acid (TCA) is a major oxidative metabolite of both TCE and PCE, and is a common urinary biomarker of exposure (Forkert et al. 2003; Volkel et al. 1998). The other oxidative metabolite, trichloroethanol (TCOH), is a TCE-specific metabolite that is formed through oxidation of TCE to chloral hydrate (CH), while PCE oxidation occurs through trichloroacetyl chloride (Chiu et al. 2007). Both TCE and PCE can enzymatically conjugate with glutathione to form dichloro- or trichloro-glutathione conjugates (DCVG or TCVG) (Lash et al. 2000). These can be further metabolized via hepatic or renal gamma-glutamyl transferase and di-peptidase to form corresponding cysteine conjugates, DCVC or TCVC, which are then n-acetylated via N-acetyltransferase to generate NAcDCVC or NAcTCVC, respectively. In addition, both NAcDCVC and NAcTCVC can be deacetylated via acylase to yield DCVC or TCVC, respectively. Apart from N-acetylation, DCVC and TCVC can be further bio-activated via cysteine conjugate β lyase to generate reactive thioketenes, or flavin-containing monooxygenase to form corresponding sulfoxides (Lash et al. 2014). These and other reactive species derived from glutathione conjugation are thought to be significant contributors to the nephrotoxicity of TCE and PCE (Lash et al. 2001a; Lash et al. 2003).
Quantitative estimation of inter-individual variability in metabolism is also a critical challenge in human health assessments of TCE and PCE (Cichocki et al. 2017b; Cichocki et al. 2016; Venkatratnam et al. 2017). The inter-strain variability in TCE metabolism has been characterized by using a multi-strain panel of inbred mice (Bradford et al. 2011) and the Collaborative Cross mouse population (Luo et al. 2018a; Venkatratnam et al. 2017; Venkatratnam et al. 2018). Mouse population-derived variability estimates for TCE metabolism closely matched population variability estimates previously derived from human toxicokinetic studies with TCE (Chiu et al. 2014). Likewise, the inter-individual variability in PCE metabolism has also been studied in the Collaborative Cross mouse population (Cichocki et al. 2017b), and a nonalcoholic steatohepatitis mouse model (Cichocki et al. 2017a). However, these studies of inter-strain variability in metabolism and toxicity were conducted separately for TCE or PCE and using doses that were not equivalent. Because of the close structural similarity of these chemicals and paucity of the available toxicokinetic data, a comparative study of equimolar doses was conducted concurrently using three inbred strains, selected based on variability observed across strains with respect to oxidative and glutathione conjugation metabolism for TCE (Bradford et al. 2011). The data from this study fill critical gaps in our understanding of the quantitative differences in TCE and PCE toxicokinetics.
2. Methods
2.1. Chemicals.
TCE (PN: 24254, ≥99%), PCE (PN: 270393, ≥99%), TCA (PN: T6399, ≥ 99%), TCOH (PN: T54801, ≥99%), 2-bromobutyric acid (PN: 147877, 97%), ethylbenzene (PN: E12508, 99%), methyl tert-butyl ether (PN: 443808, ≥99%), chloroform (PN: 650498, ≥99%), sulfuric acid (PN: 339741, ≥99%), sodium sulfate (PN: 239313, ≥ 99%), β-glucuronidase (PN: G0751, ≥300,000 units/ g solid), and sodium bicarbonate (PN: S6014, ≥99%) were obtained from Sigma Aldrich (St Louis, MO). Methanol (HPLC grade) was from Fischer Chemicals (Fair Lawn, NJ). S-(1,2-dichlorovinyl)-cysteine (DCVC, ≥98%), 2-(15N)amino-3-([1,2-dichloroethenyl]sulfanyl)(13C3)propanoic acid (DCVC*, purity ≥95%, isotopic purity ≥98.0%), S-(1,2-dichlorovinyl)-glutathione (DCVG, ≥99%), and 2-amino-5-[(2-([(13C)carboxy(13C)methyl] (15N)amino)-1-([1,2 dichloroethenyl]sulfanyl)-2-oxoethyl)amino]-5-oxopentanoic acid (DCVG*, purity ≥90%, isotopic purity ≥98%) were purchased from TLC Pharmaceutical Standards (Aurora, Canada). N-acetyl-S-(1,2-dichlorovinyl)-cysteine (NAcDCVC, 99%) and 3-([1,2-dichloroethenyl]sulfanyl)-2-[(1-13C, d3)ethanoyl amino]propanoic acid (NAcDCVC*, purity: 98%, isotopic purity: 99%), and NAcTCVC (purity: 99%) were obtained from Toronto Research Chemicals (Toronto, Canada). TCVG (purity: 99%), TCVC (purity: 98%), 2-Amino-5-[(1-{[(13C)carboxy(13C)methyl](15N)amino}−1-oxo-3-[(trichloroethenyl)sulfanyl]propan-2-yl)amino]-5-oxopentanoic acid (TCVG*, purity: 90%), 2-(15N)amino-3-[(trichloroethenyl)sulfanyl](13C3)propanoic acid (TCVC*, purity: 98%), and 2-[acetyl(15N)amino]-3-[(trichloroethenyl)sulfanyl] (13C3)propanoic acid (NAcTCVC*, purity: 99%) were synthesized by Dr. Avram Gold at the University of North Carolina at Chapel Hill. De-ionized water was generated via Arium®Pro Ultrapure Water System (Gottingen, Germany).
2.2. Animals and treatments.
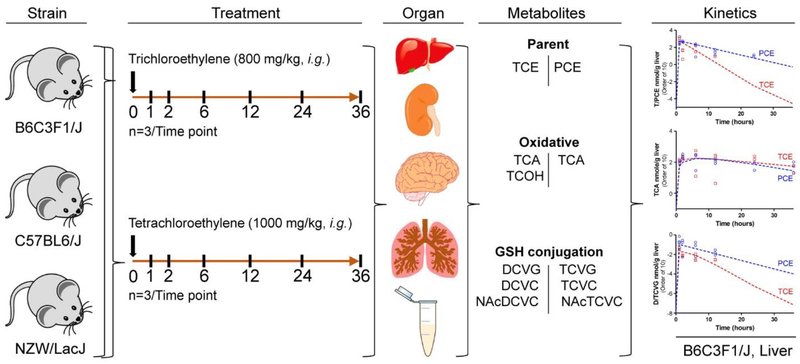
Adult male C57BL/6J, B6C3F1/J, and NZW/LacJ mice (6–7 weeks of age) were obtained from the Jackson laboratory (Bar Harbor, ME). Animals were housed in polycarbonate cages on Sani-Chips irradiated hardwood bedding (P.J. Murphy Forest Products, Montville, NJ), and fed with NTP-2000 (Zeigler Brothers, Gardners, PA) wafer diet and water ad libitum on a 12 h light-dark cycle. Animals were acclimated for one week, and then gavaged (Figure 1) with a molar-equivalent single dose (6 mmole/kg) of TCE or PCE in vehicle (5% Alkamuls El-620 in saline, 5 mL/kg). Mice were sacrificed with overdose of anesthesia (pentobarbital, 50 mg/kg i.p.) at 1, 2, 6, 12, 24, 36 h after dosing. The dose was selected based on other studies showing that it is well-tolerated in acute, sub-chronic, 90-day, and 2-year studies in mice (Buben and O’Flaherty 1985; Cichocki et al. 2017a; Cichocki et al. 2017b; National Toxicology Program 1977, 1990; Philip et al. 2007; Yoo et al. 2015c). Time points (1, 2, 6, 12, 24, 36 h) were selected to maximize the metabolic information from both oxidative and glutathione conjugation pathways (Kim et al. 2009). Organs (liver, kidney, brain, and lung) were rinsed in phosphate-buffered saline, blotted dry, weighed, and snap-frozen in liquid nitrogen. Serum was prepared using Z-gel tubes (Sarstedt, Nümbrecht, Germany) by centrifugation of blood collected from vena cava. All studies were conducted at and approved by the Institutional Animal Care & Use Committee at the University of North Carolina-Chapel Hill. Raw data for all phenotypes reported herein are available at the Mouse Phenome Database (https://phenome.jax.org/projects/Rusyn10 and https://phenome.jax.org/projects/Rusyn11).
Figure 1. Schematic representation of study design.
Male mice from one hybrid strain and two inbred strain (B6C3F1/J, C57BL/6J, and NZW/LacJ) were used in this study. Mice were intragastrically administered with a single dose of trichloroethylene or tetrachloroethylene (6 mmole/kg in 5% Alkamuls EL-620 (5 ml/kg)). Unchanged (TCE or PCE), oxidative (TCA and TCOH), and GSH conjugation metabolites (DCVG or TCVG, DCVC or TCVC, and NAcDCVC or NAcTCVC) were quantified in liver, kidney, brain, lung, and serum at various time points up to 36 hours (n=3 per time point/strain).
2.3. Quantification of TCE and PCE.
Tissue levels of TCE and PCE were quantified by a dynamic headspace gas chromatography-mass spectrometry as reported in (Cichocki et al. 2017b). Briefly, tissues (20 mg) were homogenized with 0.5 mL of ethylbenzene solution (0.5 μM in methanol). Homogenate was transferred to a 40 mL amber glass headspace vial containing 4 mL of deionized water, and analyzed via a dynamic headspace purge & trap GC-MS with single ion monitoring mode. The peak area ratio of TCE or PCE to ethylbenzene was used to construct an eight-point calibration curve (0, 0.015, 0.045, 0.137, 0.41, 1.23, 3.70, and 11.11 nmole spiked TCE or PCE) for quantitation of TCE or PCE.
2.4. Quantification of TCA.
Tissue levels of TCA were measured according to the US EPA method 815-B-03–002 (Domino et al. 2003) with slight modifications. Tissue samples (liver: 50 mg; kidney, brain, and lung: 30 mg; serum: 30 μL) were spiked with 11 nmole of 2-bromobutyric acid, homogenized in 1 mL of methanol:chloroform (1:1), and centrifuged at 14,000 g for 10 mins. The supernatant was mixed well with 1.5 mL of methanolic sulfuric acid (10%, v:v), and incubated in water batch at 55oC for 2 h to derive respective methyl esters. Thereafter, the derivative was mixed with 2 mL of methyl tert-butyl ether and 3 mL of sodium sulfate buffer (150 g/L). The collected upper layer was mixed well with 3 mL of saturated sodium bicarbonate. Again, the upper layer was collected, concentrated under nitrogen stream to a volume of ~20 μL, and analyzed via GC-MS. The peak area ratio of TCA to internal standards was used to construct an eight-point calibration curve (0, 4.1, 12.3, 37.0, 111.1, 333.3, 1000, and 3000 nmole spiked TCA) for quantitation of TCA.
2.5. Quantification of TCOH.
Tissue levels of total TCOH were determined as described elsewhere (Luo et al. 2018b). Briefly, liver (50 mg), kidney (30 mg), brain (30 mg), serum (30 μL), and lung (20 mg) tissues were homogenized with sodium acetate (0.1 M, pH 4.6), and mixed well with beta-glucuronidase (2000 units/ ml) in a thermomixer. After overnight incubation at 37oC, the homogenate was spiked with ethyl benzene (20 nmole), and then further incubated in 10% sulfuric acid in methanol (1.5 ml) at 50 °C for 1 h. Afterwards, the reaction mixture was evenly mixed with MTBE (2 mL) and sodium sulfate (3mL, 150 g/L), and then centrifuged at 2,500 g for 3 min. The extracted MTBE layer was neutralized by saturated sodium bicarbonate (3 mL), transferred to a new vial, and concentrated with nitrogen gas to ~20 μL for GC-MS analysis (Song and Ho 2003). Peak area ratio of TCOH to ethyl benzene was used to establish an eight-point calibration curve (0, 0.37, 1.11, 3.33, 10, 20, 30, and 60 nmole spiked TCOH) for quantification of total TCOH (free TCOH+TCOH-glucuronide (TCOG)).
2.6. Quantification of DCVG/TCVG, DCVC/TCVC, and NAcDCVC/NAcTCVC.
Tissue levels of DCVG/TCVG, DCVC/TCVC, and NAcDCVC/NAcTCVC were measured as detailed in (Luo et al. 2017; Luo et al. 2018a). In brief, tissue homogenates were subject to a liquid-liquid extraction with 400 μL of methanol:chloroform (1:1) and a solid-phase extraction (SPE) using a weak anion C-18 cartridge (Strata-X-AW, PN: 8E-S038-TGB, Phenomenex, Torrance, CA). Serum samples were mixed with methanol, centrifuged and the supernatant was collected. The supernatant was diluted with water and further processed by solid-phase extraction. The SPE eluent was dried under vacuum, and reconstituted with 50 μL of methanol: water (20:80) with 0.1 % acetic acid. Tissue levels of metabolites were determined by an eight-point calibration curve (0, 0.25, 0.5, 1.25, 2.5, 6.25, 18.75, and 31.25 pmole) using the peak area ratios of standards to isotopically-labeled internal standards via UPLC-MS/MS.
2.7. Toxicokinetic model.
A multi-compartment model to describe the toxicokinetics of TCE and PCE was developed based on the model reported in Kim et al. (2009). A compendium of differential equations with first-order kinetics was used to describe the metabolism, and excretion of TCE or PCE and their metabolites from oxidative and glutathione conjugation pathways (Figure 2). The model also includes formation of reactive species from cysteine conjugates. Additionally, we assumed that the ingested dose of TCE or PCE was rapidly absorbed into the blood and distributed to other tissues. Unlike physiologically-based pharmacokinetic (PBPK) models that use the partition coefficient to describe the ratio of concentration in blood and tissues, this model utilized a “lumped” compartment to represent the total quantity of each compound in the body. To describe the quantity of TCE or PCE and its metabolites in tissues, we created fraction parameters that individually distribute the cumulative dose in the lumped compartment, which can be represented as, QT = (fSrm + fLiv + fKid + fBrn + fLng + fOth), where QT is the quantity of parent compounds (or its metabolites). The fraction parameters include serum (fSrm), liver (fLiv), kidney (fKid), brain (fKid), lung (fLng), and all other tissues (fOth), respectively. The quantity of a chemical can be calculated by multiplying the total quantity and distributed fraction such as, QLiv = QT ∙ fLiv. Then, we can estimate the concentration by using tissue weights measured in this study and the reference blood volume. To investigate the disposition of TCE and PCE metabolites, we estimated the final cumulative dose in the compartments of all elimination (TCE/PCE, TCA, TCOH, and GSH conjugation) and bio-activation routes (the dotted boxes shown in Figure 2). The estimations followed the mass-balance rule, where the final cumulative dose from elimination and bio-activation routes was equal to the initial oral dose of TCE/PCE. The percentage of excreted TCE/PCE, TCA, TCOH, and GSH conjugation was obtained by dividing the excreted amount of individual compound with the given oral dose. We estimated metabolism, excretion, and fraction parameters using a Bayesian approach, assuming non-informative priors for each parameter, and estimating posterior distributions using Markov chain Monte Carlo simulations.
Figure 2. Toxicokinetics analyses of TCE and PCE metabolites.
The model is composed of one parent compartment (TCE or PCE), up to two oxidative compartments (TCA and TCOH), three GSH conjugation compartments (DCVG or TCVG, DCVC or TCVC, and NAcDCVC or NAcTCVC), and one bio-activation compartment (“Unidentified reactive species”). The first order kinetics of formation of TCA, TCOH, D/TCVG, D/TCVC, NAcD/TCVC, and reactive species were described by rate constants KTCA, KTCOH, KGSH, KCys, KNAc, and Kbio accordingly. The first order elimination kinetics of TCE or PCE, TCA, TCOH, and NAcDCVC or NAcTCVC was described by KeT/PCE, KeTCA, KeTCOH, and KeNAc. Median estimates of selected rate constants are shown. All rate constants are listed in Table 1.
2.8. Statistical analysis.
The fitness of toxicokinetic models developed in this study was examined by using Spearman (ρ) and Pearson (r) correlation. The correlation was deemed to be statistically significant if p<0.05. The area under curve within the study period (AUC0–36) was calculated based on a trapezoidal rule by using the R software (v 3.3.3).
3. Results
Levels of parent compounds (TCE and PCE), as well as their oxidative (TCA and TCOH) and glutathione conjugation metabolites (DCVG, DCVC, NAcDCVC, TCVG, TCVC, and NAcTCVC) [see comparative schematics of metabolism for TCE and PCE in (Cichocki et al. 2016; Luo et al. 2018b)] were quantified in liver, kidney, brain, lung and serum of male mice of three strains across a range of time points. These data were used to develop toxicokinetic models for TCE and PCE metabolism.
3.1. Model evaluation
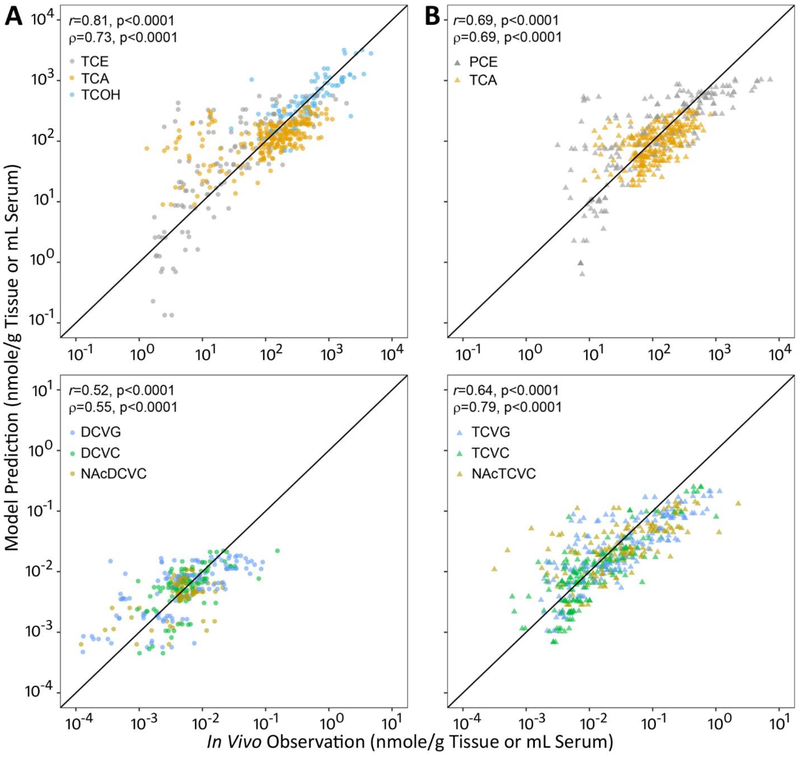
The model (Figure 2) fits well to oxidative (r=0.69–0.81; ρ=0.69–0.73) and glutathione conjugation (r=0.52–0.64; ρ =0.55–0.79) metabolites of TCE or PCE in multiple tissues of male C57BL/6J, B6C3F1/J, and NZW/LacJ mice (Figure 3). With respect to each parent chemical and their oxidative metabolites, the model demonstrated higher performance in TCE-treated mice (r= 0.81; ρ =0.73) as compared to PCE-treated mice (r=0.69; ρ =0.69). For glutathione conjugation metabolites, the model fits better in PCE-treated mice (r=0.64; ρ =0.79) than in TCE-treated mice (r=0.52; ρ =0.55).
Figure 3. Global evaluation of toxicokinetic model fit for TCE (A), PCE (B), and their respective metabolites.
Pearson (r) and spearman (ρ) correlation were used to evaluate the model fit. The correlation was considered statistically significant if p−value was lower than 0.05.
3.2. Toxicokinetics of TCE and PCE metabolites in multiple mouse tissues
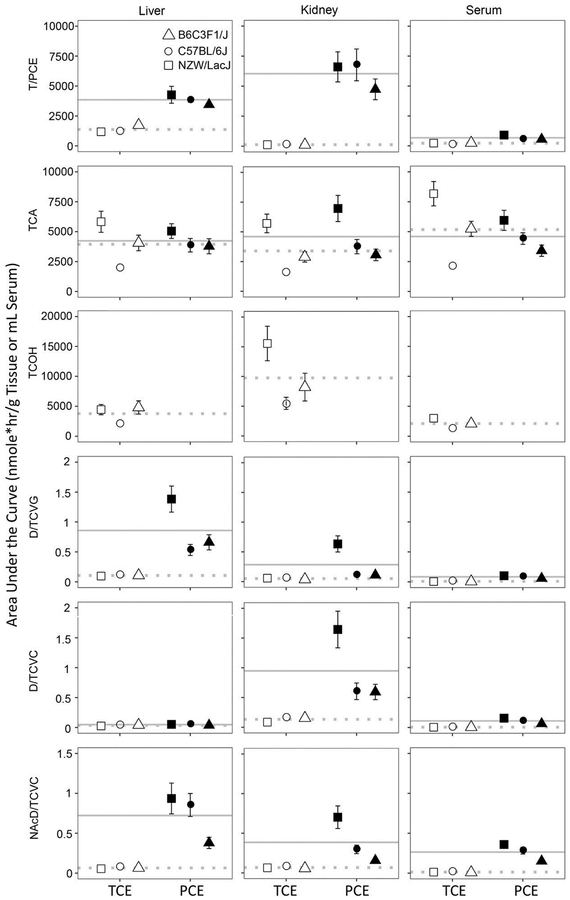
Based on the model outputs, we compared the toxicokinetics of TCE and PCE metabolites in multiple tissues of male B6C3F1/J, C57BL/6J, and NZW/LacJ mice. Figure 4 and Supplemental Table 1 provide data on AUCs (in nmole*hr/g of tissue or mL of serum) for each compound, tissue and strain. We found that tissue levels of TCE were lower than those of PCE; this was most pronounced in kidney. Specifically, the AUC0–36 of TCE was 39.8% for serum, 43.9% for liver, 29.0% for brain, 36.2% for lung, and 2.0% for kidney, as compared to the AUC0–36 values for PCE in the same tissues of B6C3F1/J mice. TCE elimination rate constant was 2.8-fold higher than that for PCE (Table 1, Supplemental Figure 1). These data are concordant with the results from previous studies (Cichocki et al. 2017a; Yoo et al. 2015c) and consistent with the fact that TCE is metabolized and eliminated more rapidly than PCE. In addition, saturation of the metabolism of PCE at dose of 1000 mg/kg (Buben and O’Flaherty 1985) may also contribute to the higher AUC0–36 of PCE and lower Ke, pce compared to those of TCE.
Figure 4. The model-predicted AUCs (0–36hr) of parent compound (TCE or PCE), oxidative metabolites (TCA and TCOH), and GSH conjugation metabolites (D/TCVG, D/TCVC, and NAcD/TCVC) in liver, kidney, and serum of male B6C3F1/J (Δ), C57BL/6J (○), and NZW/LacJ (□) mice.
Data points are shown as mean ± standard deviation. Open symbols represent TCE, and closed symbols PCE. The grey lines represent the population means of TCE-treated (dashed line) and PCE-treated (solid line) groups.
Table 1.
Toxicokinetic parameter estimates for TCE and PCE metabolism.
| TCE | PCE | ||
|---|---|---|---|
| Oxidation | T/PCE→TCA | 0.034±0.010 | 0.014±0.003 |
| T/PCE→TCOH | 0.13±0.04 | — | |
| GSH conjugation | T/PCE→D/TCVG | (3.5±1.1)*10−6 | (460±230)*10−6 |
| D/TCVG→D/TCVC | 0.30±0.07 | 31.9±20.6 | |
| D/TCVC→NAcD/TCVC | 73.4±17.4 | 43.2±28.7 | |
| D/TCVC→Reactive species | 1.69±0.45 | 37.4±19.2 | |
| Elimination | T/PCE→ | 0.49±0.12 | 0.17±0.04 |
| TCA→ | 0.05±0.02 | 0.08±0.02 | |
| TCOH→ | 0.49±0.13 | — | |
| NAcT/DCVC→ | 23.8±10.5 | 16.4±10.1 |
First order rate constants are expressed as mean ± SD, with units of h−1. Data are average of all strains used in this study. Strain-specific rate constants are provided in Supplemental Table 3.
Upon absorption, TCE and PCE are metabolized primarily via oxidation to TCA and/or TCOH (TCOH is a TCE-specific metabolite). Tissue levels of TCA were largely comparable between TCE- and PCE-treated mice. Except for C57BL/6J mice, the AUC0–36 of TCA in TCE-treated mice was 1.2–1.7 fold higher in serum, liver, brain, and lung, but similar in kidney as compared to PCE-treated mice. The formation rate constant of TCA (KTCA) in TCE-treated mice was 2.4 fold higher than that in PCE-treated mice. The elimination rate constant (Ke) of TCA in TCE-treated mice was 62.5% of that in PCE- treated mice (Table 1). The more rapid formation of TCA concurs with the faster elimination of TCE, as compared to PCE-treated mice. With regard to the TCE-specific metabolite TCOH, it was generally most abundant in kidney. Interestingly, the AUC0–36 of TCOH was 35.7–60% for serum, 22.7–53.1% for brain, 49.8–68.7% for lung, but 266–322% for kidney compared to those of TCA. These findings showed that generation of TCOH and TCOG is more efficient in kidney, which is concordant with the higher expression of UDP-glucuronosyltransferases and alcohol dehydrogenases in mouse kidney (Yue et al. 2014).
The yield through glutathione conjugation pathway was approximately 0.033%−3.3% of that through oxidative pathway for TCE and PCE, where the flux through glutathione conjugation pathway was even smaller in TCE-treated mice compared to PCE-treated mice. In liver, the AUC0–36 values for glutathione conjugates in TCE-treated mice were 7.8–20.4% of those in PCE-treated mice, with a net difference of 0.43–1.12 nmole*hr/g liver. In kidney, the AUC0–36 values for cysteine conjugates in TCE-treated mice were 7.5%–41.7% of those in PCE treated mice, with a net difference of 0.33–1.47 nmole*hr/g kidney. The formation rate constant in TCE-treated mice was 0.76% for glutathione conjugates, 0.94% for cysteine conjugates, but 170% for n-acetylcysteine conjugates as compared to PCE-treated mice. At the same time, the elimination constant was 1.5-fold higher for n-acetylcysteine conjugates in TCE-treated mice as compared to PCE-treated mice. These results (Table 1) suggest that the detoxification of cysteine conjugates (e.g., metabolism to n-acetylcysteine conjugates) is more efficient than the toxification of cysteine conjugates (e.g., metabolism to reactive species) for TCE compared to PCE, where a higher percentage of cysteine conjugates would be converted to reactive species in PCE-treated mice. Tissue-specific variation of these glutathione conjugation metabolites was similar between TCE-treated and PCE-treated mice. The highest level of the glutathione conjugates was found in liver and the highest level of the cysteine conjugates was found in kidney, in accord with recent studies (Luo et al. 2017; Luo et al. 2018a; Yoo et al. 2015a; Yoo et al. 2015b).
We also examined the inter-strain variability in formation of TCE and PCE metabolites based on the model-derived AUCs (Supplemental Table 2) and toxicokinetic model-derived rate constants (Supplemental Table 3). The AUC values across strains were similar for parent compounds (geometric standard deviation, GSD=1.10−1.46 for TCE, and =1.02−1.30 for PCE). For oxidative metabolites (TCA and TCOH), the inter-strain variability was larger in TCE treated mice (GSD=1.24−2.04) as compared to PCE-treated mice (GSD=1.06−1.52). However, for glutathione conjugation metabolites, the inter-strain variability was more prominent in PCE treated mice (GSD=1.12−2.77) as compared to TCE treated mice (GSD=1.04−1.92).
3.3. Disposition of TCE and PCE in male B6C3F1/J, C57BL/6J, and NZW/Lac mice
The fractions of administered TCE or PCE that were metabolized to oxidative or glutathione conjugation metabolites in each strain at steady state were also estimated (Figure 5). Most of each parent compound (TCE and PCE) remained unmetabolized until excretion, the fraction of the total administered dose ranged from 61.7% to 84.3% for TCE, and from 90.3% to 93.5% for PCE. The unmetabolized fractions of TCE and PCE are likely cleared via exhalation, as shown in mice orally dosed with 500 mg/kg 14C-labelled PCE where exhalation fraction of PCE was 82.6% (Schumann et al. 1980). Excreted TCA accounted for 3.5%−7.1% of the total dose for TCE, and 6.4−9.4% for PCE. With respect to the TCE-specific oxidative metabolite TCOH, it accounted for 12.2−31.2% of the total dose for TCE.
Figure 5. Predicted disposition of TCE (A), PCE (B), and their respective metabolites in male B6C3F1/J, C57BL/6J, and NZW/LacJ mice.
Pie charts are used to provide a relative comparison among various metabolites as predicted by the model in each strain. Parent compounds (black) or their oxidative metabolites (TCOH, dark gray; TCE, light gray) are shown on the left with the GSH onjugation fraction magnified to the right of the main pie chart. For GSH conjugate pie charts, the fraction of reactive species formed is represented by a yellow slice.
With respect to the glutathione conjugation metabolism, the overall flux to conjugation was less than 0.3% of the administered dose for both chemicals. However, it was 20-fold higher in PCE-treated mice (0.19%–0.30%) as compared to TCE-treated mice (0.010%–0.013%). Importantly, the estimated fraction of the reactive species formation from cysteine conjugates ranged from 2.1% to 2.4% for TCE, but from 46.7% to 52.6% for PCE.
4. Discussion
The most notable finding of this comparative study of toxicokinetics of TCE and PCE in mice is the observation of considerable differences in metabolism between these structurally-similar compounds. A larger portion of the parent compound undergoes metabolism in TCE-treated mice (15.7%−38.3%) as compared to PCE-treated mice (6.6%–9.7%), a finding that provides empirical data in strong support of the estimates from PBPK models (Chiu et al. 2014; Chiu and Ginsberg 2011). The more efficient oxidative metabolism of TCE as compared to PCE is also concordant with previous animal studies (Cichocki et al. 2017a; Green and Prout 1985; Larson and Bull 1992) and PBPK modeling estimates (Chiu et al. 2014; Chiu and Ginsberg 2011; Chiu et al. 2009).
Among the most notable findings of this study is the observation of high levels of conjugates of metabolites of PCE in the kidney. Previous PBPK models were developed using only limited data for glutathione conjugation pathway and suggested that the overall flux through the glutathione conjugation pathway was comparable between TCE and PCE (Tables 2 and 3). To the contrary, the model estimates for activation of cysteine conjugates to reactive metabolites presented herein showed that this process is more efficient in PCE-treated mice as compared to TCE-treated mice. In this study, we show that even though the glutathione conjugation pathway only accounts for less than 0.3% of the total dose of TCE and PCE, the metabolic flux through glutathione conjugation is approximately 21-fold higher for PCE compared to TCE. Higher levels of glutathione conjugation metabolites were previously reported for PCE as compared to TCE in studies in mice (Cichocki et al. 2017a; Luo et al. 2017; Luo et al. 2018a); however, the dissimilarities in dose and times of metabolite detection made direct comparisons difficult. Therefore, our findings are significant with respect to providing direct evidence for considerable differences in toxicokinetics between TCE and PCE and are consistent with the observations that PCE is more extensively metabolized via glutathione conjugation that TCE (U.S. EPA 2011a).
Table 2.
Comparative analysis of toxicokinetics of TCE metabolites in mice, rats, and humans.
| Study | Dose† | Route | Species | Strains | Sex | TCA(%)‡ | TCOH (%) | GSH conjugation (%) | Oxidation to GSH | |
|---|---|---|---|---|---|---|---|---|---|---|
| B6C3F1/J | 5.8 | 21 | 0.0131 | 2045.8 | ||||||
| This study | 800 | oral | mice | C57BL/6J | M | 3.5 | 12 | 0.0129 | 1201.6 | |
| NZW/LacJ | 7.1 | 31 | 0.0104 | 3663.5 | ||||||
| Chiu et al 2014‖ | 2100 | oral | mice | B6C3F1/J | M | 4.4 | — | 0.065 | 290 | |
| Chiu et al 2009‖ | 1000 | oral | mice | — | — | 90 | 0.2 | 450 | ||
| Larson and Bull 1992 | 600 | oral | mice | B6C3F1/J | M | 3.9 | 31 | — | — | |
| Fisher et al. 1991‖ | 368 | inhalation | mice | B6C3F1/J | M | 7 | — | — | — | |
| Green and Prout 1985 | 500 | oral | mice | B6C3F1/J | M | 7.2* | 91.1* | — | — | |
| Dekant et al. 1984 | 200 | stomach tube | mice | NMRI | F | 0.1* | 94.3* | — | — | |
| Chiu et al 2009‖ | 100 | inhalation | rat | — | — | 40 | 0.06 | 666.7 | ||
| Bernauer et al 1996 | 160 | inhalation | rat | Wistar | M&F | — | — | — | 2562¶ | |
| Larson and Bull 1992 | 600 | oral | rat | Sprague-Dawley | M | 4.1 | 23.2 | — | — | |
| Fisher et al. 1991‖ | 505 | inhalation | rat | F344 | M | 6 | — | — | — | |
| Commandeur 1990 | 400 | i.p. | rat | Wistar | M | — | — | 0.0026¶ | — | |
| Green and Prout 1985 | 500 | oral | rat | Osborne-Mendal | M | 6.3* | 90.1* | — | — | |
| Dekant et al. 1984 | 200 | stomach tube | rat | Wistar | F | 15.3* | 73.6* | — | — | |
| Chiu et al 2009‖ | 1 | inhalation | human | — | M | 38 | 5 | 7.6 | ||
|
Chiu et al 2006 |
1 | inhalation | human | — | M | 1–2 | 16–28 | — | — | |
| Bernauer et al 1996 | 160 | inhalation | human | — | M | — | — | — | 7163¶ | |
|
Monster 1979 |
70 | inhalation | human | — | M | 21 | 43 | — | — | |
| Monster 1976 | 70 | inhalation | human | — | M | 18 | 39 | — | — | |
Dose units: ppm for inhalation route; mg/kg for other routes.
Percentage of administered TCE excreted in urine (% were estimated for this study).
Estimates were derived from a PBPK model.
Percentage of total urinary metabolite calculated based on radioactivity.
Used excreted n-acetyl cysteine conjugates to represent GSH conjugation of TCE.
Table 3.
Comparative analysis of toxicokinetics of PCE metabolites in mice, rats, and humans.
| Study | Dose† | Route | Species | Strains | Sex | TCA (%)‡ | GSH conjugation (%) | Oxidation to GSH |
|---|---|---|---|---|---|---|---|---|
| B6C3F1/J | M | 6.4 | 0.187 | 34.2 | ||||
| This study | 800 | oral | mice | C57BL/6J | M | 6.6 | 0.253 | 26.1 |
| NZW/LacJ | M | 9.4 | 0.298 | 31.5 | ||||
| Cichocki et al 2017 | 300 | oral | mice | C57BL/6J | M | 5.9 | 0.79 | 7.5 |
| 10 | inhalation | mice | — | — | 12 | 0.02 | 600 | |
| 100 | oral | mice | — | — | 35 | 0.07 | 500 | |
| Chiu et al 2011‖ | 10 | inhalation | rat | — | — | 3.9 | 0.2 | 19.5 |
| 100 | oral | rat | — | — | 8.9 | 0.6 | 14.8 | |
| 40 | — | — | 316.4 | |||||
| Volkel et al 1998 | 20 | inhalation | rat | Wistar | M&F | — | — | 540.6 |
| 10 | — | — | 623.6 | |||||
| Chiu et al 2011‖ | 10 | inhalation | human | — | — | 0.98 | 9.4 | 0.1 |
| 100 | oral | human | — | — | 1.8 | 18 | 0.1 | |
| Chiu et al 2006 | 1 | inhalation | human | — | M | 0.2–0.8 | — | — |
| 40 | — | — | — | 96.3 | ||||
| Volkel et al 1998 | 20 | inhalation | human | — | M&F | — | — | 89.1 |
| 10 | — | — | — | 107.7 | ||||
| Monster 1979 | 72 | inhalation | human | — | M | 2 | — | — |
Dose units: ppm for inhalation route; mg/kg for other routes.
Percentage of administered TCE excreted in urine (% were estimated for this study).
Estimates were derived from a PBPK model.
The toxicokinetic profiles for TCE and PCE metabolites are modified by dose, species, and administration routes (Tables 2 and 3) (Bernauer et al. 1996; Chiu et al. 2014; Chiu and Ginsberg 2011; Chiu et al. 2007; Chiu et al. 2009; Cichocki et al. 2017a; Commandeur and Vermeulen 1990; Dekant et al. 1984; Fisher et al. 1991; Green and Prout 1985; Larson and Bull 1992; Monster et al. 1976; Monster and Houtkooper 1979; Volkel et al. 1998). Inter-species differences in toxicokinetics of TCE and PCE metabolites have also been reported. In general, mice are thought to be more efficient in oxidative metabolism, but less efficient in glutathione conjugation as compared to rats and humans, whether in TCE-treated or PCE-treated mice (Chiu and Ginsberg 2011; Chiu et al. 2009). However, our results show that the quantitative differences in metabolism between rats and mice may not be as large as the previously modeled estimates. The ratio of oxidative metabolites to glutathione conjugation metabolites reported in this study is closer to that predicted in rats (Chiu and Ginsberg 2011). The measurement of glutathione conjugates in rats and humans would be of great interests to address the inter-species differences in glutathione conjugation of TCE and PCE.
It has been postulated that the reactive species generated from cysteine conjugates of chlorinated solvents, are the critical nephrotoxic metabolites in animals and humans (Lash and Parker 2001; Lash et al. 2007; Lash et al. 2001b; Lash et al. 2002). Thus, the difference in flux through glutathione metabolites of TCE and PCE reported herein needs to be reconciled with the evidence for kidney toxicity and carcinogenicity of TCE and PCE (Cichocki et al. 2016). For the parent compounds, the strength of evidence for human kidney cancer is greater for TCE as compared to PCE (IARC 2014). Similarly, non-cancer effects were found at lower doses of TCE as compared to PCE (U.S. EPA 2011a, b). Studies that used more sensitive markers of kidney toxicity, such as Kim-1 (Luo et al. 2018b; Yoo et al. 2015b) and gene expression (Zhou et al. 2017), also confirmed that TCE is more toxic to the kidney as compared to PCE when dose is taken into account.
A different picture emerges from studies in rats and mice that examined kidney toxicity of directly administered cysteine conjugates of TCE and PCE in a variety of acute, sub-acute and sub-chronic exposure scenarios (Birner et al. 1997; Shirai et al. 2012; Vaidya et al. 2003). Toxicity of DCVC and TCVC to the kidney is firmly established. Most studies reported effects at doses above 10 mg/kg, with some adverse effects observable as doses as low as 1 mg/kg (Shirai et al. 2012). It should be noted, however, that all of these studies did not use sensitive measures of kidney injury and relied on relatively insensitive histopathology and serum markers. Furthermore, not many studies conducted a comparative analysis of the effects of DCVC and TCVC, one study showed that TCVC is more nephrotoxic than DCVC when equimolar doses were compared (Birner et al. 1997). In our study, the overall flux of glutathione conjugation after equimolar treatment of TCE or PCE corresponds to 0.13–0.17 mg/kg and 2.9–4.5 mg/kg, respectively. Comparisons of these fluxes to other studies of oral administration of DCVC and TCVC entail a number of uncertainties related to oral absorption, first pass through the liver, and distribution to the kidney. Nonetheless, the fact that we observed nephrotoxicity at glutathione conjugation fluxes below doses where it was observed after direct administration raises the possibility that alternative mechanisms of nephrotoxicity may also contribute to those observed with TCE and PCE treatment.
One such additional mechanism, albeit highly controversial (Caldwell and Keshava 2006), is through TCOH, a metabolite that is specific to TCE (Chiu et al. 2007). We found that upon administration of TCE, TCOH levels were the highest in kidney among the tissues examined, suggesting that this can be another mechanism for kidney toxicity of TCE. The enzymes associated with generation of TCOH (i.e., ADHs) and its conversion to TCOG (i.e., UGTs) have high activity in kidney, which may account for the high total TCOH level observed in kidney. To date, there is no consensus on TCOH-induced kidney toxicity, but it may be resulting through perturbation of formate metabolism. The oxidative metabolites of TCE, TCA and TCOH, are known to mediate formic aciduria in rats (Yaqoob et al. 2013) and mice (Lock et al. 2017). It was also reported that TCE, but not TCOH, can induce elevated renal injury markers in rats after 12 weeks of exposure (Yaqoob et al. 2014), even though both chemicals can cause formic aciduria in rats and mice. However, with chronic exposure up to 52 weeks, TCOH has been associated with higher formic acid excretion, cell replication, protein accumulation, and increased incidence of basophilic tubules in the cortex of rat kidney (Green et al. 2003). Although TCOH alone does not sufficiently explain the full range of renal effects after TCE exposure (U.S. EPA 2011b), the high level of TCOH found in the kidney is consistent with the hypothesis that TCE-induced formic aciduria may contribute to chronic renal injury observed in rats and mice, a mechanism that is absent in PCE-treated mice.
The inter-strain variability in metabolism of TCE (Bradford et al. 2011; Luo et al. 2018a; Venkatratnam et al. 2017; Yoo et al. 2015a; Yoo et al. 2015b) and PCE (Cichocki et al. 2017a; Cichocki et al. 2017b) has been demonstrated in mice. We selected three strains for this study, B6C3F1/J, C57BL/6J, and NZW/LacJ, based on their known differences in serum levels of TCA and glutathione conjugates of TCE (Bradford et al. 2011). We posited that these strains would be informative of the range of inter-strain variability in metabolism of TCE and PCE. Indeed, the inter-strain variability was observed; it was larger for TCA, but smaller for glutathione conjugates in TCE-treated mice as compared to PCE-treated mice. The inter-strain variability of TCA levels in liver was recently examined by using the Collaborative Cross mouse population with a single oral dose of TCE or PCE, where the GSD was 2.24 for TCE-treated mice (Venkatratnam et al. 2017) and 1.64 for PCE-treated mice (Cichocki et al. 2017b). Our study confirms these differences.
We note that this study is not without limitations. First, the doses used in this study (6 mmoles/kg) are orders of magnitude higher than those expected in the general human population; still, the doses used herein are in the range of doses used in other animal studies, including sub-chronic and chronic animal bioassays with both TCE and PCE (National Toxicology Program 1990). Second, the oxidative metabolism of PCE is likely saturated at these high oral doses (Buben and O’Flaherty 1985). Saturation of oxidation leads to greater relative formation of glutathione conjugates, which may have greater human relevance given evidence that at low doses glutathione conjugation metabolites of TCE in rodents was approximately 1% of that in humans (Chiu et al. 2009). Still, some caution is warranted in interpreting our comparative analysis due to non-linearities at high doses, so understanding of toxicokinetics at lower doses is an area of future research focus that would benefit from more sensitive methods for measuring oxidative and glutathione conjugation metabolites, as well as use of PBPK modeling to address non-linearities due to metabolic saturation. Our recent analytical methods (Luo et al. 2017; Luo et al. 2018a) can be applied in the dose range of 10–100 mg/kg, but additional improvements to the sensitivity of these methods are needed. Third, the use of one hybrid and two inbred mouse strains may not be representative for the inter-strain variability of T/PCE metabolites. The selection of these three strains was based on the crude estimate of the inter-strain variability (Bradford et al. 2011). A population-based mouse model, such as the Collaborative Cross mouse population, would advance our knowledge of inter-strain variability in metabolism and toxicity of T/PCE. Finally, the presumed bio-activation of cysteine conjugates necessitates an additional compartment in the current model. However, further experimental data (e.g., measurement of dichloroacetic acid [DCA] or unidentified reactive metabolites) would be useful to confirm our predictions. DCA in particular is of interest because it is a multispecies hepatocarcinogen and can be formed via multiple pathways from TCE and PCE, but there have been long-standing concerns about its measurement due to the potential for ex vivo formation (Chiu et al. 2006). Still, this study is the first to systematically compare oxidative and GSH conjugative metabolism between TCE and PCE. Our next step will be updating the PBPK models for TCE and PCE, which is of importance for the health risk assessments of TCE and PCE.
5. Conclusions
In summary, this study provides a comparative analysis for the metabolisms between TCE and PCE. We show that one atom replacement of chlorine can substantially affect the metabolism via both oxidative and glutathione conjugation pathways. The qualitative and quantitative differences between TCE and PCE metabolites, as well as the tissue-specific distribution of metabolites, can shed light on the differences between TCE- and PCE-induced toxicities.
Supplementary Material
6. Acknowledgements
The authors wish to show gratitude to Drs. Anthony H. Knap, Terry Wade, Stephen Sweet, and Thomas J. McDonald at Texas A&M University for providing access to the analytical instrumentation. The authors also thank Dr. Chimeddulam Dalaijamts for fruitful discussions. This work was supported, in part, by grants from the U.S. EPA (STAR RD83561202) and National Institutes of Health (F32 ES026005), and institutional support from Texas A&M University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR. (1997) Toxicological profile for trichloroethylene (TCE), Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services, Atlanta, GA. [PubMed] [Google Scholar]
- Bernauer U, Birner G, Dekant W and Henschler D (1996) Biotransformation of trichloroethene: dose-dependent excretion of 2,2,2-trichloro-metabolites and mercapturic acids in rats and humans after inhalation. Arch Toxicol 70, 338–346. [DOI] [PubMed] [Google Scholar]
- Birner G, Bernauer U, Werner M and Dekant W (1997) Biotransformation, excretion and nephrotoxicity of haloalkene-derived cysteine S-conjugates. Arch Toxicol 72, 1–8. [DOI] [PubMed] [Google Scholar]
- Bradford BU, Lock EF, Kosyk O, Kim S, Uehara T, Harbourt D, DeSimone M, Threadgill DW, Tryndyak V, Pogribny IP, Bleyle L, Koop DR and Rusyn I (2011) Interstrain differences in the liver effects of trichloroethylene in a multistrain panel of inbred mice. Toxicol Sci 120, 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buben JA and O’Flaherty EJ (1985) Delineation of the role of metabolism in the hepatotoxicity of trichloroethylene and perchloroethylene: a dose-effect study. Toxicol Appl Pharmacol 78, 105–122. [DOI] [PubMed] [Google Scholar]
- Caldwell JC and Keshava N (2006) Key issues in the modes of action and effects of trichloroethylene metabolites for liver and kidney tumorigenesis. Environ Health Perspect 114, 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2017) National Health and Nutrition Examination Survey 2013–2014. CDC. [Google Scholar]
- Chiu WA, Campbell JL, Clewell HJ, Zhou YH, Wright FA, Guyton KZ and Rusyn I(2014) Physiologically-Based Pharmacokinetic (PBPK) Modeling of Inter-strain Variability in Trichloroethylene Metabolism in the Mouse. Environ Health Perspect 122, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WA and Ginsberg GL (2011) Development and evaluation of a harmonized physiologically based pharmacokinetic (PBPK) model for perchloroethylene toxicokinetics in mice, rats, and humans. Toxicol Appl Pharmacol 253, 203–234. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Micallef S, Monster AC and Bois FY (2007) Toxicokinetics of inhaled trichloroethylene and tetrachloroethylene in humans at 1 ppm: empirical results and comparisons with previous studies. Toxicol Sci 95, 23–36. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Okino MS and Evans MV (2009) Characterizing uncertainty and population variability in the toxicokinetics of trichloroethylene and metabolites in mice, rats, and humans using an updated database, physiologically based pharmacokinetic (PBPK) model, and Bayesian approach. Toxicol Appl Pharmacol 241, 36–60. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Okino MS, Lipscomb JC and Evans MV (2006) Issues in the pharmacokinetics of trichloroethylene and its metabolites. Environ Health Perspect. 114, 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki JA, Furuya S, Konganti K, Luo YS, McDonald TJ, Iwata Y, Chiu WA, Threadgill DW, Pogribny IP and Rusyn I (2017a) Impact of Nonalcoholic Fatty Liver Disease on Toxicokinetics of Tetrachloroethylene in Mice. J Pharmacol Exp Ther 361, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki JA, Furuya S, Venkatratnam A, McDonald TJ, Knap AH, Wade T, Sweet S, Chiu WA, Threadgill DW and Rusyn I (2017b) Characterization of Variability in Toxicokinetics and Toxicodynamics of Tetrachloroethylene Using the Collaborative Cross Mouse Population. Environ Health Perspect 125, 057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichocki JA, Guyton KZ, Guha N, Chiu WA, Rusyn I and Lash LH (2016) Target Organ Metabolism, Toxicity, and Mechanisms of Trichloroethylene and Perchloroethylene: Key Similarities, Differences, and Data Gaps. J Pharmacol Exp Ther 359, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commandeur JN and Vermeulen NP (1990) Identification of N-acetyl(2,2-dichlorovinyl)- and N-acetyl(1,2-dichlorovinyl)-L-cysteine as two regioisomeric mercapturic acids of trichloroethylene in the rat. Chem Res Toxicol 3, 212–218. [DOI] [PubMed] [Google Scholar]
- Dekant W, Metzler M and Henschler D (1984) Novel metabolites of trichloroethylene through dechlorination reactions in rats, mice and humans. Biochem Pharmacol 33, 2021–2027. [DOI] [PubMed] [Google Scholar]
- Domino MM, Pepich BV, Munch DJ, Fair PS and Xie Y (2003) Method 552.3: Determination of haloacetic acids and dalapon in drinking water by liquid-liquid microextraction, derivatization, and gas chromatography with electron capture detection Office of Ground Water and Drinking Water, Cincinnati, OH. [Google Scholar]
- Fisher JW, Gargas ML, Allen BC and Andersen ME (1991) Physiologically based pharmacokinetic modeling with trichloroethylene and its metabolite, trichloroacetic acid, in the rat and mouse. Toxicol Appl Pharmacol 109, 183–195. [DOI] [PubMed] [Google Scholar]
- Forkert PG, Lash L, Tardif R, Tanphaichitr N, Vandevoort C and Moussa M (2003) Identification of trichloroethylene and its metabolites in human seminal fluid of workers exposed to trichloroethylene. Drug Metab Dispos 31, 306–311. [DOI] [PubMed] [Google Scholar]
- Green T, Dow J and Foster J (2003) Increased formic acid excretion and the development of kidney toxicity in rats following chronic dosing with trichloroethanol, a major metabolite of trichloroethylene. Toxicology 191, 109–119. [DOI] [PubMed] [Google Scholar]
- Green T and Prout MS (1985) Species differences in response to trichloroethylene. II. Biotransformation in rats and mice. Toxicol Appl Pharmacol 79, 401–411. [DOI] [PubMed] [Google Scholar]
- Guha N, Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K and International Agency for Research on Cancer Monograph Working Group. (2012) Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol 13, 1192–1193. [DOI] [PubMed] [Google Scholar]
- IARC. (2014) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (Vol. 106): Trichloroethylene, Tetrachloroethylene and Some Other Chlorinated Agents. 106.
- Kim S, Kim D, Pollack GM, Collins LB and Rusyn I (2009) Pharmacokinetic analysis of trichloroethylene metabolism in male B6C3F1 mice: Formation and disposition of trichloroacetic acid, dichloroacetic acid, S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine. Toxicol Appl Pharmacol 238, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JL and Bull RJ (1992) Species differences in the metabolism of trichloroethylene to the carcinogenic metabolites trichloroacetate and dichloroacetate. Toxicol Appl Pharmacol 115, 278–285. [DOI] [PubMed] [Google Scholar]
- Lash LH, Chiu WA, Guyton KZ and Rusyn I (2014) Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity. Mutat Res Rev Mutat Res 762, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Fisher JW, Lipscomb JC and Parker JC (2000) Metabolism of trichloroethylene. Environ Health Perspect 108 Suppl 2, 177–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Hueni SE and Putt DA (2001a) Apoptosis, necrosis, and cell proliferation induced by S-(1,2-dichlorovinyl)-L-cysteine in primary cultures of human proximal tubular cells. Toxicol Appl Pharmacol 177, 1–16. [DOI] [PubMed] [Google Scholar]
- Lash LH and Parker JC (2001) Hepatic and renal toxicities associated with perchloroethylene. Pharmacol Rev 53, 177–208. [PubMed] [Google Scholar]
- Lash LH, Putt DA, Huang P, Hueni SE and Parker JC (2007) Modulation of hepatic and renal metabolism and toxicity of trichloroethylene and perchloroethylene by alterations in status of cytochrome P450 and glutathione. Toxicology 235, 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH, Putt DA, Hueni SE, Krause RJ and Elfarra AA (2003) Roles of necrosis, Apoptosis, and mitochondrial dysfunction in S-(1,2-dichlorovinyl)-L-cysteine sulfoxide-induced cytotoxicity in primary cultures of human renal proximal tubular cells. J Pharmacol Exp Ther 305, 1163–1172. [DOI] [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Krause RJ and Parker JC (2001b) Renal and hepatic toxicity of trichloroethylene and its glutathione-derived metabolites in rats and mice: sex-, species-, and tissue-dependent differences. J Pharmacol Exp Ther 297, 155–164. [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Sicuri AR and Parker JC (2002) Renal toxicity of perchloroethylene and S-(1,2,2-trichlorovinyl)glutathione in rats and mice: sex- and species-dependent differences. Toxicol Appl Pharmacol 179, 163–171. [DOI] [PubMed] [Google Scholar]
- Lock EA, Keane P, Rowe PH, Foster JR, Antoine D and Morris CM (2017) Trichloroethylene-induced formic aciduria in the male C57 Bl/6 mouse. Toxicology 378, 76–85. [DOI] [PubMed] [Google Scholar]
- Luo YS, Cichocki JA, McDonald TJ and Rusyn I (2017) Simultaneous detection of the tetrachloroethylene metabolites S-(1,2,2-trichlorovinyl) glutathione, S-(1,2,2-trichlorovinyl)-L-cysteine, and N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine in multiple mouse tissues via ultra-high performance liquid chromatography electrospray ionization tandem mass spectrometry. J Toxicol Environ Health A 80, 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YS, Furuya S, Chiu W and Rusyn I (2018a) Characterization of inter-tissue and inter-strain variability of TCE glutathione conjugation metabolites DCVG, DCVC, and NAcDCVC in the mouse. J Toxicol Environ Health A 81, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YS, Furuya S, Soldatow VY, Kosyk O, Yoo HS, Iwata Y and Rusyn I (2018b) Metabolism and toxicity of trichloroethylene and tetrachloroethylene in cytochrome P450 2E1 knockout and humanized transgenic mice. Toxicol Sci in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AC, Boersma G and Duba WC (1976) Pharmacokinetics of trichloroethylene in volunteers, influence of workload and exposure concentration. Int Arch Occup Environ Health 38, 87–102. [DOI] [PubMed] [Google Scholar]
- Monster AC and Houtkooper JM (1979) Estimation of individual uptake of trichloroethylene, 1,1,1-trichloroethane and tetrachloroethylene from biological parameters. Int Arch Occup Environ Health 42, 319–323. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. (1977) Bioassay of tetrachloroethylene for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser 13, 1–83. [PubMed] [Google Scholar]
- National Toxicology Program. (1990) Carcinogenesis Studies of Trichloroethylene (Without Epichlorohydrin) (CAS No. 79-01-6) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser 243, 1–174. [PubMed] [Google Scholar]
- Philip BK, Mumtaz MM, Latendresse JR and Mehendale HM (2007) Impact of repeated exposure on toxicity of perchloroethylene in Swiss Webster mice. Toxicology 232, 1–14. [DOI] [PubMed] [Google Scholar]
- Schumann AM, Quast JF and Watanabe PG (1980) The pharmacokinetics and macromolecular interactions of perchloroethylene in mice and rats as related to oncogenicity. Toxicol Appl Pharmacol 55, 207–219. [DOI] [PubMed] [Google Scholar]
- Shirai N, Ohtsuji M, Hagiwara K, Tomisawa H, Ohtsuji N, Hirose S and Hagiwara H (2012) Nephrotoxic effect of subchronic exposure to S-(1,2-dichlorovinyl)-L-cysteine in mice. J Toxicol Sci 37, 871–878. [DOI] [PubMed] [Google Scholar]
- Song JZ and Ho JW (2003) Simultaneous detection of trichloroethylene alcohol and acetate in rat urine by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 789, 303–309. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. (2011a) Toxicological Review of Tetrachloroethylene (CAS No. 127-18-4): In Support of Summary Information on the Integrated Risk Information System (IRIS). U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. (2011b) Toxicological Review of Trichloroethylene (CAS No. 79-01-6): In Support of Summary Information on the Integrated Risk Information System (IRIS).
- U.S. EPA. (2017) EPA Names First Chemicals for Review Under New TSCA Legislation.
- Vaidya VS, Shankar K, Lock EA, Bucci TJ and Mehendale HM (2003) Renal injury and repair following S-1, 2 dichlorovinyl-L-cysteine administration to mice. Toxicol Appl Pharmacol 188, 110–121. [DOI] [PubMed] [Google Scholar]
- Venkatratnam A, Furuya S, Kosyk O, Gold A, Bodnar W, Konganti K, Threadgill DW, Gillespie KM, Aylor DL, Wright FA, Chiu WA and Rusyn I (2017) Collaborative Cross Mouse Population Enables Refinements to Characterization of the Variability in Toxicokinetics of Trichloroethylene and Provides Genetic Evidence for the Role of PPAR Pathway in Its Oxidative Metabolism. Toxicol Sci 158, 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatratnam A, House JS, Konganti K, McKenney C, Threadgill DW, Chiu WA, Aylor DL, Wright FA and Rusyn I (2018) Population-based dose-response analysis of liver transcriptional response to trichloroethylene in mouse. Mamm Genome 29, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkel W, Friedewald M, Lederer E, Pahler A, Parker J and Dekant W (1998) Biotransformation of perchloroethene: dose-dependent excretion of trichloroacetic acid, dichloroacetic acid, and N-acetyl-S-(trichlorovinyl)-L-cysteine in rats and humans after inhalation. Toxicol Appl Pharmacol 153, 20–27. [DOI] [PubMed] [Google Scholar]
- Wu C and Schaum J (2000) Exposure assessment of trichloroethylene. Environ Health Perspect. 108 Suppl 2, 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob N, Evans A, Foster JR and Lock EA (2014) Trichloroethylene and trichloroethanol-induced formic aciduria and renal injury in male F-344 rats following 12 weeks exposure. Toxicology 323, 70–77. [DOI] [PubMed] [Google Scholar]
- Yaqoob N, Evans AR and Lock EA (2013) Trichloroethylene-induced formic aciduria: effect of dose, sex and strain of rat. Toxicology 304, 49–56. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Bradford BU, Kosyk O, Shymonyak S, Uehara T, Collins LB, Bodnar WM, Ball LM, Gold A and Rusyn I (2015a) Comparative analysis of the relationship between trichloroethylene metabolism and tissue-specific toxicity among inbred mouse strains: liver effects. J Toxicol Environ Health A 78, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HS, Bradford BU, Kosyk O, Uehara T, Shymonyak S, Collins LB, Bodnar WM, Ball LM, Gold A and Rusyn I (2015b) Comparative analysis of the relationship between trichloroethylene metabolism and tissue-specific toxicity among inbred mouse strains: kidney effects. J Toxicol Environ Health A 78, 32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HS, Cichocki JA, Kim S, Venkatratnam A, Iwata Y, Kosyk O, Bodnar W, Sweet S, Knap A, Wade T, Campbell J, Clewell HJ, Melnyk SB, Chiu WA and Rusyn I (2015c) The Contribution of Peroxisome Proliferator-Activated Receptor Alpha to the Relationship Between Toxicokinetics and Toxicodynamics of Trichloroethylene. Toxicol Sci 147, 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, Lacerda de Sousa B, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Ramalho Santos M, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B and Mouse EC (2014) A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YH, Cichocki JA, Soldatow VY, Scholl EH, Gallins PJ, Jima D, Yoo HS, Chiu WA, Wright FA and Rusyn I (2017) Comparative Dose-Response Analysis of Liver and Kidney Transcriptomic Effects of Trichloroethylene and Tetrachloroethylene in B6C3F1 Mouse. Toxicol Sci 160, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.