Abstract
To maintain homeostasis, every cell must constantly monitor its energy level and appropriately adjust energy, in the form of ATP, production rates based on metabolic demand. Continuous fulfillment of this energy demand depends on the ability of cells to sense, metabolize, and convert nutrients into chemical energy. Mitochondria are the main energy conversion sites for many cell types. Cellular metabolic states dictate the mitochondrial size, shape, function and positioning. Mitochondrial shape varies from singular discrete organelles to interconnected reticular networks within cells. The morphological adaptations of mitochondria to metabolic cues are facilitated by the dynamic events categorized as transport, fusion, fission, and quality control. By changing their dynamics and strategic positioning within the cytoplasm, mitochondria carry out critical functions and also participate actively in inter-organelle cross-talk, assisting metabolite transfer, degradation, and biogenesis. Mitochondrial dynamics has become an active area of research because of its particular importance in cancer, metabolic diseases, and neurological disorders. In this review, we will highlight the molecular pathways involved in the regulation of mitochondrial dynamics and their roles in maintaining energy homeostasis.
Introduction
“The most successful creature on Earth is the mitochondria. A billion years ago, they swam into the first cells and they’ve been there ever since…Who’s to say our actions might not be motivated by trillions of mitochondria in our cells, for their own devious mitochondrial schemes? What we perceive as consciousness and free will, might merely be by-products of what our mitochondria are doing.”
(from Dance of the Ivory Madonna by Don Sakers)
Around one and a half billion years ago, mitochondria evolved from a free-living prokaryotic organism via symbiosis. Although this symbiotic merger occurred a long time ago, mitochondria remained as discrete organelles within the cytoplasm and played a critical role in eukaryotic cell evolution. Mitochondria, like bacteria, have a double membrane, contain ribosomes and DNA, proliferate by division, and constantly move and fuse to form a dynamic network. Almost all eukaryotic cells contain hundreds of mitochondria that work together to generate ATP, maintain calcium homeostasis, synthesize heme, phospholipids and extracellular signaling molecules including neurotransmitters, and also initiate the cell death process. Mitochondrial morphology and content vary highly across cells and tissues (Figure 1). The recent discovery that mitochondria interact with one another, combined with molecular insights into mitochondrial motility, have challenged the traditional view of mitochondria as singular organelles in the cytoplasm. The function of mitochondria is hard-wired to cellular metabolism, which is critical for energy homeostasis. However, our current understanding of the relationship between mitochondrial dynamics, positioning, function and metabolism is cursory at best.
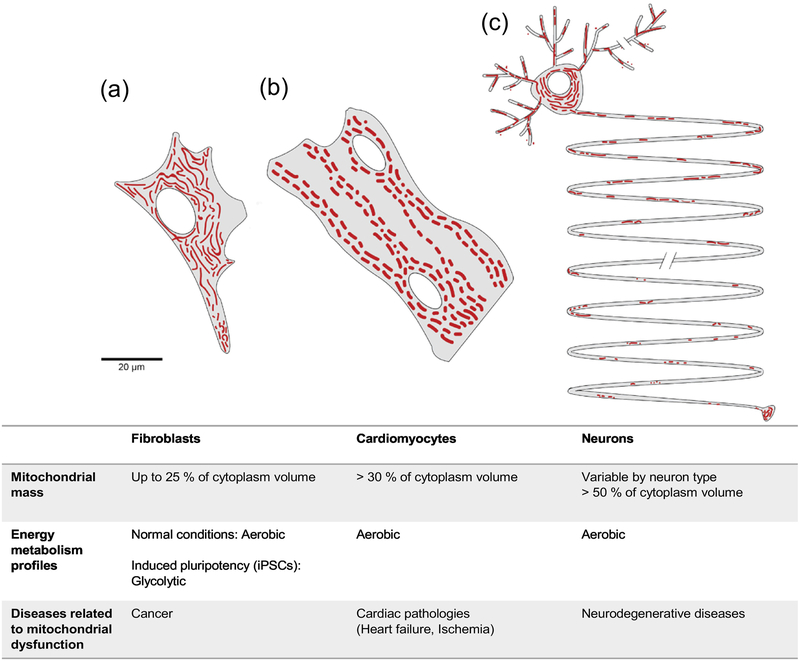
Figure 1.
Mitochondrial shape, number, function and distribution are tailored to match cell type-specific bioenergetic demands. Figures depicting the mitochondrial network (shown in red) in a (a) fibroblast, (b) cardiomyocyte, and (c) neuron. (a) In fibroblasts, mitochondria are evenly distributed throughout the cytoplasm, with a highly connected network around the nucleus and smaller sizes at the leading edges. In the healthy state, mitochondria are mostly elongated and fully branched. In normal fibroblasts, the bioenergetic profile is relatively aerobic, while pluripotent stem cells have a glycolytic profile [173]. (b) Cardiomyocytes have a dense and unusual distribution of mitochondria, aligned in parallel to the plasma membrane and nucleus. Mitochondria occupy at least 30% of the total cell volume, indicating the cellular energy dependency on OXPHOS [174]. (c) Neurons are highly polarized large cells with a vast cytoplasmic volume, highly branched dendrites and extraordinarily long axons. Mitochondria in the somatodendritic area are extensively connected and highly motile, while in axons most mitochondria are in a singular and stationary state (70%) [1]. The mitochondrial composition varies by the neurons subclasses, but occupancy of mitochondria is generally much higher than in other types of cells (~%50). Table summarizes the cell type-specific mitochondrial profiles.
The aim of this review is to provide an overview of the molecular pathways that give rise to mitochondrial shape and the reticular network in mammalian cells to maintain energy homeostasis. First, we will describe the key molecular machineries regulating mitochondrial dynamics, positioning, and quality. While there are many studies describing alterations of mitochondrial shape and dynamics in response to different stimuli or pathologies [1–5], our main focus will be on cellular metabolism-dependent regulations. Second, we will analyze mitochondrial dynamics within the context of a whole cell and interactions between mitochondria and other organelles. Lastly, we will discuss how dysregulation of mitochondrial dynamics contributes to different pathologies, including cancer, metabolic diseases and neurodegenerative disorders. Mitochondrial biology is still full of mysteries. However, given the central role of mitochondria in cellular metabolism, understanding how cellular cues tailor mitochondrial form, positioning and function in depth will reveal the molecular principles of energy homeostasis.
Regulation of Mitochondrial Dynamics
Mitochondria are governed by the molecular mechanisms regulating mitochondrial transport, fusion, fission and quality control. These mechanisms are highly conserved from yeast to mammalian cells. Here, we attempt to describe the key players that regulate mitochondrial dynamics (summarized in Figure 2) and the known upstream pathways coupling cellular metabolism to mitochondrial dynamics in cells.
Figure 2.
Summary of mitochondrial dynamics: (a) Mitochondrial transport on microtubules is mediated by the mitochondrial motor adaptor complex. The Mitochondrial protein, Miro, and the motor adaptor protein, Milton, links (+)- end directed motor protein, Kinesin-1 (KIF5), and dynein complex and facilitates the transport in anterograde and retrograde directions. Syntaphilin (SNPH) anchors mitochondria to microtubules and Myosin V to actin filaments (not shown in the figure). (b) Mitochondrial fusion is carried out by Mitofusin1, 2 (Mfn1, Mfn2), and MitoPLD, for the outer membrane, and long Opa1 (L-Opa1), for the inner membrane. The fusion events lead to elongated, or even extensively branched mitochondria, with a higher OXPHOS rate. (c) Drp1 gets recruited onto the mitochondrial outer membrane by Drp1 receptors, Mff and Fis1. Higherorder Drp1 assembly around the mitochondrion mediates the fission event. Excessive mitochondrial fission generates fragmented mitochondria, which is pivotal in mitochondrial inheritance, mitophagy and cell death. (d) Damaged or fragmented mitochondria are subjected to degradation by mitophagy. PTEN-induced putative kinase 1 (PINK1) accumulation on the damaged mitochondrial outer membrane, where it can bind to the E3 ligase Parkin, and induction of phosphoubiquitination, are the critical steps for the initiation of mitophagy. Autophagosomes can then selectively recognize damaged mitochondria to be degraded. NIX, the mammalian orthologue of Atg32, is another receptor on the mitochondrial outer membrane that introduces mitochondria to the autophagosome (not shown in figure). (e) Mitochondriaderived vesicles (MDVs) are vesicle cargoes that are transferred from mitochondria to other organelles. Cargoes containing oxidized mitochondrial proteins are usually shipped to lysosomes for degradation, whereas other cargoes that are targeted to the ER and peroxisomes mediate protein or lipid exchange for biogenesis purposes. Key molecular players and the functional significance of mitochondrial dynamics are summarized in the table.
Mitochondrial transport
In cells, mitochondria move, switch directions, temporarily pause, and remain stationary at designated areas. These regulated motions strategically position mitochondria in the cytoplasm to meet local energy and calcium buffering needs and regulate various signaling pathways. The requirement for mitochondrial transport in polarized large cells like neurons, which can be over a meter long in humans, and its impact on cellular functions are well-appreciated [1,6,7]. In contrast, the role of mitochondrial transport in typical cells ranging from 25–100 μm in length, has been presumed to be insignificant, although recent discoveries are beginning to change this view[8].
In all metazoan cells, long-distance mitochondrial transport is microtubule-based and mediated via motor/adaptor complex proteins (Figure 2a). The kinesin-1 family (also called KIF5 and kinesin heavy chain, or KHC) contains motor domains that drive mitochondrial movement towards the (+)-end of microtubules, whereas dynein/dynactin is responsible for (−)-end-directed movement. The mitochondrial adaptor proteins GTPase Miro (also called RhoT1/2) and Milton (also called TRAK1/2, OIP106/98) couple KHC or dynein/dynactin to mitochondria [9–12]. The mitochondrial motor/adaptor complex play a central role in regulating mitochondrial trafficking. While less well understood, other proteins like Kinesin 3 family members, Syntabulin, FEZ1 and Ran binding protein 2 can also affect mitochondrial transport [7]. Mitochondrial transport serves essential cellular functions, and therefore must be tightly regulated.
To achieve the requisite mitochondrial distribution, extracellular and intracellular signals regulate mitochondrial transport and docking. Although the cues may vary, including Ca2+, oxidative stress, damage and nutrients, they converge by targeting the mitochondrial motor/adaptor complex [13–16]. Miro participates in Ca2+-mediated mitochondrial arrest as it binds to cytosolic Ca2+ at EF-hand motifs, facilitating detachment of KHC from microtubules [13] and altering KHC-Miro-Milton interactions [16]. By sensing intracellular Ca2+ levels, Miro couples synaptic activity to mitochondrial transport and stops mitochondria at the regions with high Ca2+ in neurons [13,16]. Recently, Miro was also demonstrated to link mitochondria to the actin cytoskeleton through recruiting mitochondrial myosin Myo19, thereby mediating actin-dependent mitochondrial trafficking [17]. Syntaphilin (SNPH) protein is a neuron-specific docking protein, which anchors mitochondria to microtubules. According to the “Engine-Switch and Brake” model, elevation of local Ca2+ concentration causes KHC to bind SNPH instead of the Miro/Milton complex, leading to mitochondrial motility arrest on microtubules [18,19]. PTEN-induced putative kinase 1 (PINK1) is also known to interact with the Miro and Milton complex and phosphorylate Miro to assist E3 ligase Parkin-dependent degradation, which leads to mitochondrial motility arrest [20,21]. In addition, oxidative stress and cellular damage are other factors that control mitochondrial trafficking by affecting the Miro and Milton motor adaptor complex [22]. Intracellular Ca2+ and the JNK pathway play critical roles in this reactive oxygen species (ROS)-regulated mitochondrial transport.
Mitochondrial positioning in cells can also be modulated based on the information received from the extracellular environment, such as nutrient and oxygen availability. Miro’s interaction with Hypoxia up-regulated mitochondrial movement regulator (HUMMR) protein regulates mitochondrial positioning based on oxygen levels [23]. When HUMMR function is compromised during hypoxia, mitochondrial transport rate is biased towards the soma in neurons, which reduces mitochondrial content in neuronal axons. Nutrient availability can affect mitochondrial transport rate and positioning via the cytoplasmic metabolic sensor enzyme O-GlcNAc Transferase (OGT). OGT catalyzes a reversible posttranslational modification of proteins by adding a GlcNAc sugar moiety to serine and threonine residues. The catalytic activity of OGT is regulated by intracellular UDP-GlcNAc concentrations, which fluctuate proportionally in response to nutrient flux through the hexosamine biosynthetic pathway [15,24]. OGT directly binds and O-GlcNAc-modifies Milton, which then results in mitochondrial motility arrest [25]. This mechanism allows mitochondria to be concentrated in glucose-enriched areas in neurons. So far, it is the only evidence of direct modulation of mitochondrial transport by nutrient availability through the motor-adaptor complex although, the exact molecular mechanisms of the arrest and its implications for cellular bioenergetics are yet to be elucidated.
Mitochondrial shape dynamics
Mitochondrial shape dynamics, facilitated by the coordinated actions of proteins and lipids, allows mitochondria to respond to external stimuli and contribute to cellular homeostasis for survival. In cells, mitochondrial shape ranges from discrete puncta to tubular networks. The balance of mitochondrial fusion and fission events determine the final spectrum of mitochondrial shapes. As mitochondria go through fusion and fission processes, mitochondrial size, function, distribution, quality, as well as their interaction with other subcellular organelles all change. Mitochondrial shape dynamics play critical roles in energy homeostasis as they can respond rapidly and directly to acute metabolic perturbations. For instance, upon nutrient starvation, mitochondria undergo a series of fusion events and their elongation significantly enhances bioenergetics efficiencies [26]. Also, changes in mitochondrial morphology, either elongation or fragmentation, are restored easily if the cells are rescued from metabolic insult [26–31].
Outer mitochondrial membrane (OMM) fusion and fission are regulated by several key evolutionary conserved proteins (Figure 2b and c). During mitochondrial outer membrane fusion, Mitofusin 1 and Mitofusin 2 (Mfn1 and Mfn2) are anchored to OMM at their C-termini, which then mediate the merging of separate OMMs by hydrolyzing GTP in homo- or heterotypic manners. Deletion of Mfn1 and Mfn2 in mice leads to developmental abnormalities and embryonic lethality due to mitochondrial fusion defects [32,33]. In addition to mitofusins, overexpression of MitoPLD promotes mitochondrial fusion whereas its ablation leads to mitochondrial fragmentation [34]. MitoPLD mediates the conversion of mitochondrial lipid cardiolipin (CL) to phosphatidic acid (PA), which will be discussed in detail in “Mitochondria and Endoplasmic Reticulum” section. The fission of mitochondrial OMM is regulated by the GTPase protein Dynamin related protein 1 (Drp1) and its receptor proteins Fis1, Mff, MiD49 and MiD51 [35]. Intracellular signaling pathways regulate the positioning of Drp1 on the OMM. Once recruited, Drp1 oligomerizes into a ring-like structure that wraps around the mitochondria, which is also marked by the presence of endoplasmic reticulum (ER) and actin cytoskeleton [36], constricts the mitochondrial membrane and triggers fission. Drp1 activity is modulated by post-translational modifications, such as phosphorylation, SUMOylation, and O-GlcNAcylation [37,38].
Dynamin-like GTPase Optic atrophy 1 (Opa1) plays a dual role in regulating inner mitochondrial membrane (IMM) fusion and fission [39,40]. Opa1 is encoded by a single gene and its proteolytic cleavage regulates the formation of long Opa1 (L-Opa1) and short Opa1 (SOpa1) isoforms. Cellular stress dependent Opa1 cleavage to S-Opa1 is catalyzed by OMA1 and YME1LA proteases [41]. While accumulation of S-Opa1 mediates mitochondrial fragmentation, L-Opa1 drives inner membrane fusion [40,41]. By responding to different cellular stimuli, Opa1 alters fusion-fission balance, mitochondrial cristae organization and the ATP synthesis rate [42].
In contrast to the mitofusin topology mentioned above, it has been recently revealed that the C-terminus of Mfn2 faces mitochondrial intermembrane spaces instead of the cytoplasm [43]. This paradigm-shifting topology study suggests that the redox-sensitive Cys residue of Mfn2 resides within intermembrane spaces, specifically responds to ROS signals and triggers Mfn2 dimerization. Future work is now required to understand how this structure and ROS-induced dimerization of Mfn2 will impact mitochondrial network regulation in cells to sustain energy homeostasis.
The metabolic state of a cell greatly impacts mitochondrial shape dynamics by coordinating transport, outer and inner mitochondrial fusion, and fission. Molecular mechanisms evolved for mitochondria to assess energy level and fine-tune ATP synthesis rates to resist nutrient stresses like fasting and a high fat diet. Starvation, or nutrient deprivation, induces transient elongation of mitochondria and tubular network formation to compensate for the shortage of nutrient availability by increasing ATP production efficiency [28,44]. To facilitate hyperfusion under stress, the mitochondrial fusion machinery protein Mfns are stabilized through deacetylation by sirtuin deacetylases SIRT1 and SIRT3 [26,45,46]. Moreover, the fission protein Drp1 is phosphorylated and inactivated by cAMP-protein kinase A (PKA) or AMP-activated protein kinase (AMPK) activity based on the AMP-to-ATP ratio [26,47,48]. Conversely, during nutrient oversupply, mitochondria are fragmented by the excess fission machinery activity, which also mediates the decline in bioenergetics [49]. AMPK also contributes to mitochondrial fission processes by activating Mff, the receptor of Drp1 on OMM, which is considered to be one of several stress-induced quality control mechanisms [31,49].
Because mitochondrial respiratory chain complexes are located in the inner mitochondrial membrane directly, cristae remodeling by Opa1 has a direct impact on oxidative phosphorylation (OXPHOS). Mice overexpressing Opa1 survive acute stress-induced ischemic muscle damage, due to Opa1 functioning as tightening rope for mitochondrial cristae structure, preventing the release of cytochrome C and apoptotic cell death [50]. While most studies evaluate Opa1 function within the context of apoptosis, Opa1 also plays a critical role in acute nutrient responses to sustain energy demand. The moderate overexpression of Opa1 ameliorates the phenotype of two mitochondrial disease (Complex I and IV deficiencies) mouse models [51]. When neonatal cardiac myocytes are exposed to high glucose, Opa1 gets OGlcNAcylated and inactivated, which leads to extensive mitochondrial elongation and dysfunction [52]. In addition, when mTORC1 activity is restricted in mouse liver cells, Opa1 is processed in a Mfn2-dependent manner, which mediates fragmentation of mitochondria, decreases cristae density, and most intriguingly, changes the structure of the mitochondria-ER contact site [53]. These results indicate that mitochondria adapt to postprandial transitions by orchestrating cristae remodeling together with mitochondria-ER assembly.
Mitochondrial quality control
Cells maintain their homeostasis by degrading detrimental or excessive cellular components that can be toxic to cellular health. The entire catabolic procedure is called autophagy, in which proteins or organelles are sequestered into the double-membrane and targeted to lysosomes for degradation upon membrane fusion [54,55]. The removal of damaged mitochondria by the autophagy process is referred as mitophagy (Figure 2d).
Mitochondrial quality control processes are essential for cellular health and are evolutionarily conserved from yeast to mammalian cells. In yeast, where the molecular mechanism of mitophagy was identified, binding of autophagy-related 32 (Atg32) to the OMM mediates recruitment of Atg8 which introduces mitochondria into autophagosomes [56,57]. In mammalian cells, it is well established that loss of the mitochondrial membrane potential triggers mitophagy as well as mitochondrial dysfunction. NIX and PINK1/Parkin pathways play critical role in targeted removal of damaged or dysfunctional mitochondria [54].
NIX is the mammalian orthologue of Atg32 in yeast, which works as a selective autophagy receptor. However, mitophagy can still happen even without NIX, implying that another pathway is responsible for selective degradation of mitochondria. It was found that the E3 ubiquitin ligase Parkin is translocated to dysfunctional mitochondria when phosphorylated by the mitochondrial kinase PINK1 [58–60]. Parkin accumulation on OMM mediates poly-ubiquitination-induced selective mitophagy. It is still a matter of debate whether NIX and Parkin orchestrate mitophagy in the same pathway. NIX was found to stimulate Parkin recruitment to depolarized mitochondria in MEF cells, indicating cooperative roles among mitophagy regulators, while basal mitophagy level was normal even in highly metabolic tissues in PINK1 knock-out mice [61,62]. Loss of Pink1 and Parkin in Drosophila minimally affected basal mitophagy across the organism [63]. The studies demonstrating the replaceable role of Pink1/Parkin in basal mitophagy indicates the existence of other molecular mechanisms by which damaged mitochondria can be selectively degraded. Further work is therefore needed to determine the conditions that trigger selective degradation of damaged mitochondria and physiological stimuli that activate Pink1/Parkin dependent mitophagy in vivo.
Synchronized regulation of mitochondrial transport, fission and mitophagy pathways ensures mitochondrial quality control. Mitochondrial damage activates PINK1/Parkin pathways which cause Miro-degradation-dependent mitochondrial motility arrest to quarantine damaged mitochondria [21]. The selective mitophagy of damaged mitochondria may occur locally in distal neuronal axons, primed by PINK1/Parkin pathways in cultured rat neurons [64]. While mitochondrial fragmentation itself is not sufficient to trigger mitophagy, it allows impaired mitochondria to be isolated from the network and promotes mitophagy. In pancreatic beta cells, a reduction in Opa1 activity ensures less fusion and the selective removal of damaged mitochondria with lower mitochondrial membrane potential [65]. The restriction in mitochondrial fusion prior to mitophagy, facilitated by Parkin-dependent ubiquitination and elimination of mitofusins, further supports the role of PINK1/Parkin in mitochondrial fission regulation [31,66].
Recent investigations into inner mitochondrial membrane protein Prohibitin 2 (PHB2) indicated that the protein works as a selective receptor for both Parkin-dependent and -independent mitophagy [67]. Due to its location on the IMM, PHB2 has only been considered a regulator of Opa-1-mediated cristae remodeling, but now it has been shown to carry out a plethora of roles as a mitochondrial functional regulator and mitophagy receptor simultaneously. In order for PHB2 to mediate mitophagy, proteasomal degradation of OMM is required to expose the LIR domain of PHB2, which then facilitates interaction between PHB2 and LC3, an autophagosomal cargo receptor protein. Also, deletion of phb-2 in male C. elegans leads to impaired sperm-derived mitochondrial degradation, resembling the phenotype observed in autophagy deficient embryos [68]. Paternal mitochondrial elimination was also affected by the disruption of mitochondrial dynamics in C. elegans embryos. Loss of the fzo-1 gene, which encodes Mfn2/Fzo1 in C. elegans and mediates mitochondrial fusion, results in fragmented and abnormal cristae formation in maternal mitochondria. This makes them indistinguishable to paternal mitochondria, causing a delay in selective removal of paternal mitochondria [69]. Furthermore, double-knockdown of fzo-1 and drp-1 genes greatly delays the paternal mitochondrial elimination by autophagosomes. These results highlight mechanisms of target selection in mitophagy.
The causes of mitophagy may vary, such as mitochondrial damage, exercise, and starvation, but cellular stress triggered by nutrient imbalance mainly activates mitophagy in order to maintain energy homeostasis. The key cellular energy sensor AMPK is involved in autophagic procedures; as a substrate of AMPK, unc-51 like activating kinase 1 (ULK1) is directly phosphorylated to induce autophagy [70]. Loss of function studies in mammals further validated that AMPK and ULK1 are indispensable for functional mitophagy during starvation [71]. In contrast to pathways that trigger mitophagy, macroautophagy induced by starvation or mTOR silencing mediates cAMP accumulation which leads to PKA activation, phosphorylation of Drp1, and finally prevention of mitochondrial fission [72]. The elongated mitochondria spared from mitophagy show denser cristae and more effective ATP production rates, which indicates cellular fine-tuning of mitochondrial shape and network organization to maintain energy homeostasis.
Mitochondrial dynamics regulating organelle interactions
Cytoplasmic organelles display distinct morphologies and dynamics, which allow them to execute different biochemical tasks within the whole cell. Mitochondria are one of the largest organelles, occupy a significant portion of the cytoplasmic volume (Figure 1) and dynamically interact with other organelles including endoplasmic reticulum, lysosomes and peroxisomes (Figure 3). Here we summarize the rules governing these unique interactions.
Figure 3.
Scheme summarizing mitochondrial inter-organellar crosstalk. Mitochondria actively interact with other subcellular organelles. While communicating with other organelles, mitochondria continuously change their morphology, positioning and function based on the cellular metabolic need.
Mitochondria and Endoplasmic Reticulum
The endoplasmic reticulum (ER) forms a highly dynamic membranous network that is responsible for assorted cellular functions, such as lipid synthesis, protein folding, and Ca2+ storage and release. The dynamics between ER and mitochondria are tightly coupled as the two organelles share extensive contact sites and roles in cellular metabolism and Ca2+ homeostasis [73,74]. The contact sites for both organelles, called mitochondria-associated endoplasmic reticulum membranes (MAMs), not only mediate nutrient, ion, lipid and metabolite transport to mitochondria, but also participate in mitochondrial size control and downstream signals for cellular homeostasis.
Among mitochondrial dynamics proteins, Mfn2 is greatly involved in the formation of ER-mitochondria contact sites by directly tethering the two organelles. Indeed, silencing of Mfn2 leads to a reduction in Ca2+ uptake from ER as the distance between the organelles expands [75,76]. Controversially, acute down-regulation of Mfn2 increases ER-mitochondria contact and sensitizes cells to apoptotic stimuli by acting as a negative regulator of organelle apposition [77–79]. In addition, Mfn2 facilitates mitochondrial fusion to deliver metabolic cues to ER, functionally coupling the two dynamic organelles. Mitochondrial fragmentation by ablation of Mfn2 also plays a significant role in glucose homeostasis through pathways involving ER stress. Liver-specific deletion of Mfn2 in mice induces ER stress by generating ROS, leading to loss of insulin sensitivity and glucose intolerance [80]. The mitochondrial fission sites are marked by tubular ER and actin cytoskeleton, which play critical roles in self-assembly of the Drp1-Mff complex and membrane constriction. Mitochondrial fission organized at the MAM results in mitochondrial division, which is critical for mitochondrial inheritance and cellular adaptations to external stress [36]. ER-derived mitochondrial fission facilitates phospholipid transfer between the two organelles. ER provides phospholipids to mitochondria that are responsible for mitochondrial membrane structure, form and dynamics. As mentioned above, PA synthesized in the ER is transported to mitochondria at MAMs and converted to mitochondrial-specific phospholipid CL by multiple enzymatic reactions [81,82]. Initially CL is located in the IMM but it can be placed in the OMM and transformed back to PA by mitoPLD [34]. Interactions between dynamin-related GTPases like Drp1 and Opa1, and phospholipids imported from ER actively remodel mitochondrial membrane. ER and mitochondrial crosstalk is therefore necessary for both mitochondrial infrastructure and function.
As Ca2+ level affects numerous enzymatic activities within a cell, its regulation through ER and mitochondrial function contributes to metabolic homeostasis. For instance, Ca2+ concentration in mitochondria plays a critical role in promoting oxidative metabolism and activating ATPase, which leads to an overall increase in ATP synthesis rate by stimulating the TCA cycle [83,84]. To fine-tune cellular metabolism by preserving intracellular and mitochondrial Ca2+ levels, Ca2+ transfer between ER and mitochondria has to be tightly regulated. In addition to Drp1 recruitment at the site of ER-mediated mitochondrial fission as mentioned above, actin polymerization through ER-bound inverted formin 2 (INF2) mediates a rise in mitochondrial matrix Ca2+ level by increasing MAM surface area and mitochondrial Ca2+ uptake from the ER [85]. The mitochondrial Rho-GTPase Miro, separately from its commonly known role as part of the motor adaptor complex, is phosphorylated by Polo kinase and contacts the Ca2+ transporter at MAM, thus maintaining mitochondrial Ca2+ homeostasis [86]. The yeast homolog of Miro1, Gem1, facilitates mitochondria-ER connections and regulates ER-associated mitochondrial division [87]. Lastly, Drp1 stimulates mitochondrial fission by signaling from the anti-apoptotic protein Mcl-1 to protect mitochondria from hyperpolarization and Ca2+-mediated apoptosis [88,89]. These emerging data support the idea that mitochondrial dynamics at MAMs and Ca2+ signaling in both mitochondria and cytoplasm are key players in cellular metabolic homeostasis.
Mitochondrial-ER communication allows mitochondrial dynamics to adapt to nutrient availability and the metabolic state of a cell. Upon fasting and the postprandial glucose rise, MAM number is significantly reduced in mouse liver due to glucose-induced mitochondrial fission and the pentose phosphate-protein phosphatase 2A (PP-PP2A) pathway, which leads to mitochondrial respiration defects [90]. The insulin signaling pathway is also regulated by MAM integrity as well as signaling proteins present in MAMs; disrupting MAMs in hepatocytes impairs insulin signaling while Akt and mTORC2 share significant roles in retaining MAM and mitochondrial physiology [91,92]. Mitochondria-ER crosstalk ultimately works to restore balance after nutrient disruptions: during nutrient deprivation, elongated mitochondria prefer fatty acid-derived OXPHOS with more intimate crosstalk between ER and mitochondria followed by enhanced Ca2+ exchange, whereas high glucose supplies reduce MAM surfaces and fragmented mitochondria prefer to utilize glucose. In conclusion, MAMs facilitate ER-mitochondria Ca2+ exchange and provide a means to regulate signaling pathways based on nutrient availability, even though the underlying mechanisms of insulin sensing need to be elucidated.
Mitochondria and Lysosomes
Lysosomes have mainly been introduced as acidic organelles that degrade macromolecules from endocytotic and autophagic pathways. Recently however, studies indicate lysosomes as a metabolic hub facilitating metabolite storage and nutrient sensing, resembling mitochondrial functions [93]. Evidence of new forms of lysosome-mitochondrial crosstalk is also emerging as they share interconnected sites to shuttle amino acids, lipids, and Ca2+ in addition to communicating via mitophagy.
Lysosomes play a critical role in maintaining mitochondrial quality control by mitophagy. However, one interesting observation from many research groups is that malfunctions in mitochondria also cause lysosomal defects [94]. In mutant mice that lack essential mitochondrial proteins such as Opa1 and PINK1, large lysosomal vacuoles form, accumulating substrates inside the cells. In TFAM-deleted cells, biogenesis of lysosomes was increased, but newly synthesized lysosomes were less acidic and accumulated sphingomyelin and autophagy intermediates [95].These features - dysfunctional mitochondria and lysosomes with accumulated substrate and protein aggregates - are commonly observed in neurodegenerative diseases. Conversely, in a Gaucher-disease (GD) mice model, which recapitulates the well-known lysosomal storage disease, lysosomal defects impair autophagy and lead to the accumulation of dysfunctional and fragmented mitochondria, leading to Parkinson’s disease [96]. Recently, RAB7 GTPase, which was thought to specifically regulate lysosomal dynamics upon GTP hydrolysis, was found to mediate the interaction between mitochondria and lysosomes at the organelle contact sites [97]. Not only do the results suggest that these contact sites provide a means of binding the two organelles, but they also mark mitochondrial fission sites by recruiting RAB7 GTPase activating protein TBC1D15 via Fis1 on OMM, enabling bidirectional regulation of mitochondrial and lysosomal dynamics. Given the evidence, lysosomal and mitochondrial functions seem to be tightly associated for a multitude of cellular functions.
Active metabolite transfer takes place at lysosome-mitochondria contact sites, including lipids, amino acids, and ions [93,98]. Given that lysosomes function as amino acid-sensing organelles, these contact sites may also regulate mitochondrial metabolism, as amino acids are used as an indispensable carbon source for the TCA cycle. In fact, during amino acid starvation, lysosomal storage of essential amino acids is stimulated as well as lysosomal biogenesis by inactivated mTOR [99]. In addition, inactivated mTOR causes decreased translation of mitochondrial fission process protein 1 (MTFP1), which promotes mitochondrial fragmentation, resulting in elongated mitochondria [100]. More mitochondrial elongation and branching occurs with Drp1 inhibition by PKA in starved cells, as well as cristae reorganization with Opa1 [101]. All these mitochondrial dynamics help biogenesis of electron transport chain factors, such as ATP synthase, to promote cell survival [44,72]. This suggests a potential collaborative role of lysosomes and mitochondria on cell survival, which depends on mTOR signaling.
Mitochondrial-derived vesicles (MDVs) have recently been recognized as the product of localized mitochondrial dynamics that couple mitochondria quality control pathways to lysosomes (Figure 2e). McBride and colleagues elegantly demonstrated budding MDVs from mitochondria, destined to lysosomes for degradation purposes [102]. Unlike MDVs targeted to peroxisomes, MDVs headed to lysosomes contain oxidized mitochondrial proteins, including VDAC from OMM and the complex III core subunit from IMM. Instead of destruction of intact mitochondria by mitophagy, MDVs allow selective removal of old proteins to prevent further mitochondrial damage [103].
Mitochondria and Peroxisomes
Peroxisomes are now identified as indispensable organelles that harbor key metabolic pathways including β-oxidation of fatty acids, amino acid degradation, and ROS regulation. Both peroxisomes and mitochondria are dynamic organelles found ubiquitously in cells, where they collaborate to sustain metabolic homeostasis. Among the metabolic pathways, fatty acid β-oxidation is critically required for both mitochondria and peroxisome function. While mitochondria can degrade full-size fatty acids, peroxisomes are only capable of breaking down chain-shortened ones. Then, medium-sized fatty acid chains and acetyl-CoA from peroxisomes shuttle back to mitochondria through the contact sites. The molecular mechanisms regulating the physical interconnection of these two organelles still need to be elucidated [104,105].
Several examples of physical interactions between mitochondria and peroxisomes are emerging, although the existing studies are confined to few cell types. When extracted from rat liver cells, mitochondria and peroxisomes are co-purified [106]. In yeast, peroxisomes localize within mitochondria-ER junctions alongside Pex11, a peroxisome biogenesis factor participating in peroxisomal division and proliferation [107]. Interestingly, mitochondrial fission factor Drp1 is associated with both organelles as Drp1 is found juxtaposed in elongated peroxisomes [108]. Fis1, Mff, and Pex11 are the receptors that bind Drp1 to the mitochondrial OMM and peroxisomal membranes respectively, leading to the organellar fission [107,109,110]. In addition, the PINK1/Parkin pathway takes part in this process by recruiting Drp1 machinery to the organelles in Drosophila, thereby changing their morphologies [31].
In addition to physical contact sites, the two organelles are also thought to be functionally connected. Since both organelles are sensitive to cellular redox homeostasis, they communicate redox stress signal to each other. For instance, disruption of peroxisomal lipid and ROS metabolism leads to mitochondrial redox stress by disturbing the redox balance. In Zellweger syndrome, which is characterized by reduced functional peroxisomes, loss of peroxisomal biogenesis and function mediates mitochondrial dysfunction. The direct impact of peroxisomal dysfunction was studied in mouse Pex5 −/− hepatocytes, resulting in impaired respiration, depleted mtDNA, and upregulation of mitochondrial biogenesis by PGC-1α [111].
Moreover, MDVs, which have recently been fully investigated as another contributor to mitochondrial dynamics, are another way of bonding mitochondrial and peroxisomal functions. Among various types of cargos, some of the MDVs are targeted to peroxisomes [112]. The generation of MDVs is independent of Drp1, and a small fraction of MDVs specifically targeted to peroxisomes contain mitochondria-anchored protein ligase (MAPL). This shows that the specificity of MDVs participating in interorganellar crosstalk as components of MDVs targeted to ER and lysosome are totally different [113]. Furthermore, recent studies indicate that some MDVs bud off as pre-peroxisomal structures with essential peroxisomal membrane proteins like Pex3 and Pex14, then mature as they fuse to the ER and complete peroxisome biogenesis [114].
Abnormalities of Mitochondrial Dynamics in Diseases
Defects in mitochondrial functions can have deleterious effects on cellular health and survival. Most mitochondrial proteins are encoded by the nuclear genome, including those that facilitate mitochondrial dynamics. Mutations in these nuclear and mitochondrial encoded genes cause monogenic disorders with distinct mitochondrial pathologies. Mutations in some of the mitochondrial dynamics-associated genes have been described for monogenic disorders such as Charcot-Marie-Tooth Disease (Mfn2 mutations), Dominant Optic Atrophy (Opa1 mutations) and Parkinson’s disease (Pink1 and PARKIN mutations). However, there is growing evidence that abnormal mitochondrial dynamics contribute to other diseases that are not classically considered as either mitochondrial or monogenic, such as cancer, metabolic and neurodegenerative disorders.
Cancer
According to observations of Otto Warburg from almost a century ago, cancer cells primarily utilize glucose fermentation as a main energy source, even in the presence of oxygen, which is now known as aerobic glycolysis [115]. In his seminal work, he showed that mitochondria are dysfunctional in cancer cells and postulated that mitochondrial defects are the leading cause of tumorigenesis. In fact, however, mitochondria are fully functional in parallel to aerobic glycolysis, and the abnormal metabolic character of tumors is now collectively referred as the “Warburg effect” [116,117]. There is growing evidence indicating the mitochondrial role in cancer cell formation and tumorigenesis [118,119].
Not only are intact mitochondria indispensable for tumorigenesis, but also ATP produced by regional mitochondria at the leading edge is necessary for cancer cells to sustain their high energy demand for invasion and metastasis. Recently, Caino et al. showed that an alternatively spliced version of SNPH is localized to cancer cell mitochondria and gradually downregulated as tumorigenesis progresses, since its loss promotes repositioning of active mitochondria to the cell periphery required for cellular motility [120]. The role of mitochondrial dynamics in adjusting the balance between cell proliferation and migration in cancer cells has been established by many studies, including disruption in Opa1, Drp1, and Miro 1; all of which resulted in reduction of the migration abilities of cancer cells [121,122]. Specifically, in breast cancer cells, mitochondria were found to be more fragmented due to an upregulation of Drp1 and downregulation of Mfn1 expression. The role of Drp1 in tumor growth was also highlighted in brain tumor initiating cells, with its activity stimulated by CDK5 phosphorylation and further regulating downstream AMPK pathway [123]. Mitochondria are also involved in preventing cancer development by inducing cellular apoptosis. In addition to the regulation of mitochondrial bioenergetics by a non-canonical Wnt5a pathway, a canonical Wnt/β-catenin pathway controls melanoma oncogenesis by changing cellular metabolism and remodeling mitochondrial shape to promote apoptosis [124]. These findings reveal that mitochondrial shape actively responds to alterations in cellular metabolism and contribute to tumorigenesis. Therefore, the proteins regulating mitochondrial dynamics could be potential targets for anti-cancer therapies.
Metabolic disorders
Metabolic diseases are caused by alterations in normal metabolic processes, leading to inability to adapt to a new energy status, simply stated as “metabolic inflexibility” [125]. Tissues from individuals with metabolic diseases, like obesity and type 2 diabetes mellitus (T2DM), have defects in tailoring cellular bioenergetics to changes in nutrient availability; including impaired fatty acid oxidation patterns during starvation and following exercise, as well as an inability to switch from lipid oxidation to glucose oxidation after feeding. Insulin resistance hampers the substrate shift in metabolism, and prevent tissues, including skeletal muscle, liver, and adipose, from storing energy efficiently [126]. There are many molecular mechanisms implicated in insulin resistance and fuel selections, but most of them indicate inflexibility in mitochondria to efficiently utilize nutrient sources as the primary reason [127]. Considering the role of mitochondria as a metabolic hub, disruptions in mitochondrial function and dynamics may directly contribute to metabolic diseases.
Mitochondrial dysfunction is a common feature of skeletal muscle tissue in obesity and diabetes. Reduced expression of genes encoding mitochondrial proteins, due to altered activity of the mitochondrial transcription factors PGC1α and PGC1β, is suggested as the molecular mechanism underlying this pathology [128,129]. Mfn2-deleted animal models further support the role of mitochondrial dynamics in metabolic homeostasis in vivo. Liver-specific ablation of Mfn2 causes mitochondrial dysfunction, ER stress, and enhanced generation of reactive oxygen species, which then leads to impaired insulin signaling and glucose intolerance [80]. Also, pancreatic beta cell-specific Opa1 deficiency induces abnormal insulin secretion in response to glucose and leads to subsequent hyperglycemia in mice [130]. Both in animal disease models and in humans, insulin resistance coincides with mitochondrial shape anomalies. However, this phenotype does not necessarily mean that impaired mitochondrial dynamics are the only factor that induces insulin resistance; specifically, mitochondrial defects in lipid metabolism and oxidative stress response directly mediate insulin resistance as observed in beta cell hyperplasia and obesity.
Chronic high blood glucose levels, or hyperglycemia, is a distinctive feature of diabetes. Chronically high glucose levels often cause increased activity of the major metabolic sensor enzyme OGT. Elevated O-GlcNAcylation of mitochondrial proteins is now emerging as a main contributor to mitochondrial dysfunction in diabetic tissues [38,118,131,132]. Using T2DM mouse cardiomyocytes as a model, Gawlowski et al. identified that Drp1 is O-GlcNAcylated and that its function is modulated by the change in phosphorylation state. This then leads to an increase in the active form of Drp1 as well as fragmented mitochondria with decreased membrane potential. Opa1 also gets deactivated by O-GlcNAcylation [38]. Interestingly, the Hart group recently described diabetes-associated dysregulation of mitochondrial O-GlcNAcylation cycling, OGT mislocalization to the mitochondrial matrix, and mitochondrial dysfunction in diabetic rat cardiomyocytes [133]. Taken together, O-GlcNAcylation and other nutrientdependent regulatory pathways control overall mitochondrial shape, function, and positioning, and their disruption is associated with metabolic disorders.
Development
Cellular adaptations to ever changing environment also occurs as cells progress through different developmental stages in tissue. Cells undergo massive transformation in their structures and functions during development, including mitochondrial dynamics, biogenesis and mitophagy [134–136]. During cell division, mitochondria disassociate from microtubules by “shedding” kinesin and dynein motor proteins, and this process is regulated by the phosphorylation of the motor adaptor protein Milton. Mitochondrial enrichment at the cell periphery facilitates accurate chromosome segregation during mitosis [137]. Selective removal of mitochondria from developing erythrocytes and destruction of paternal mtDNA utilize pathways required for mitochondrial quality control (Figure 1d) [138].
Somatic cells in early developmental stages, or even progenitor and stem cells, are highly engaged in glycolytic energy metabolism. In fact, pluripotent stem cells, like iPSCs or embryonic stem cells, are known to favor hypoxic conditions, restricting oxygen consumption and ROS production by subduing mitochondrial OXPHOS [139,140]. The glycolytic nature of stem cells makes them highly sensitive to glucose level. Therefore they express more glucose transporters than older cell types to sustain excessive glucose demand and compensate for reduced mitochondrial function [139]. Once differentiation begins, however, the self-renewing properties are lost due to drastic changes in ROS-mediated processes and the cellular metabolism shifts towards mitochondrial OXPHOS. Recent findings highlight that mitochondrial dynamics mediate this transition during neural stem cell fate (NSC) decision [141]. Deletion of Opa1 or Mfn1,2 disrupts the self-renewal capacity of the stem cells followed by a limitation in adult neurogenesis and cognitive defects. In addition to mitochondrial structure, mitochondrial function is also involved in stem cell lineage regulation, as well as in inhibiting adult neurogenesis deficits [136]. Interestingly, the Ca2+-dependent GTPase Miro was also identified as a regulator of NSC lineage development and a critical player in Ca2+ homeostasis maintenance [86]. The molecular pathways directly regulating mitochondrial dynamics are now accepted as the key players in mitochondrial inheritance during cell division, metabolic transitions from glycolytic to oxidative, and the removal of unwanted mitochondria during differentiation. The distribution of newly generated mitochondria upon mitochondrial biogenesis also requires fusion, fission, and transport.
Aging
Mitochondrial dynamics are essential in maintaining a healthy pool of mitochondria in cells, particularly in highly polarized cells like neurons. With aging, both mitochondrial dynamics and cellular metabolism decline [142]. The mitochondrial dysfunction in aging is mainly caused by the accumulation of mtDNA mutations, a decrease in mitochondrial enzyme activities and respiratory capacity, restricted dynamics, an increase in ROS, and a reduction in mitochondrial biogenesis [143–147]. Age-dependent decline in mitochondrial functions may also serve as an adaptive response to prevent accelerated tissue decay. Aging-associated changes in mitochondrial dynamics perturb energy homeostasis. Mice with a triple knockout of the mitochondrial dynamic proteins Mfn1, Mfn2, and Drp1 in cardiomyocytes develop aging symptoms including heart failure, mitochondrial senescence, and impaired mitophagy faster than other single knockout mouse models [145]. Conversely, in the case of healthy aging, AMPK and dietary restriction help to maintain metabolic homeostasis by mitochondrial fusion with coordination of peroxisomes, which lead to a longer life span in C. elegans [146]. Overall, further studies are needed to distinguish mitochondria’s emerging role in aging and aging-related pathologies, either as a cause or a consequence.
Neurodegenerative disease
Dysfunctional mitochondria have been observed as early features of several neurodegenerative diseases. Clinical studies on neuropathologies provide further evidence that defects in mitochondrial quality control and shape regulation directly lead to common neurodegenerative diseases. Mfn2 was observed as the primary gene mutated in Charcot-Marie-Tooth (CMT) type 2A patients with visual impairment. Similarly, Opa1 mutations are commonly detected in Dominant optic atrophy type 1 [148,149]. These mutations disrupt the mitochondrial network and mtDNA maintenance [150,151]. Mutations in Drp1 induce impaired mitochondrial and peroxisomal fission, leading to abnormal brain development [152]. The motor adaptor protein Milton mutations are also associated with neurodevelopmental defects in humans [153].
In addition to patient mutations, studies utilizing the neurodegenerative disease models also suggest that defects in synaptic mitochondria function, caused by accumulation of damaged mitochondria and dysregulation of mitochondrial dynamics, are the underlying cause of neurodegeneration (also recently reviewed by Misgeld and Schwarz) [1,6,151]. Mitochondrial dynamics are essential for synapse formation, synaptic activity regulation and overall neuronal survival. Enhancing mitochondrial fission by Drp1 overexpression promotes synaptogenesis in embryonic hippocampal neurons [154]. The role of Drp1 in synaptic transmission is further supported by studies in Drosophila neuromuscular junctions showing that mutants with impaired Drp1 have less mitochondria present at synapses as well as defects in neurotransmitter release in response to intense stimuli [155]. Neuron-specific deletion of mitochondrial motor adaptor complex proteins Miro and Milton in mice leads to neuronal dysfunction and cell death, which demonstrate that defects in mitochondrial transport are sufficient to trigger neurodegeneration [153,156].
Proteins involved in mitochondrial quality control, Pink1 and Parkin, are also mutated in familial forms of Parkinson’s Diseases (PD). The reduction of mitophagy efficiency by Parkin mutations causes an accumulation of dysfunctional mitochondrial and mitochondrial network fragmentation, which is also observed in vulnerable dopamine neurons in PD [157]. These all may lead to reduced energy supply at the distant axonal terminals due to mitochondrial deficiency. As important as it is to distribute mitochondria down the axons, retrograde movement of stressed mitochondria back to the somatodendritic region is also a mechanism that neurons use to cope with neurodegenerative stress. Damaged neuronal mitochondria can be removed by local degradation or shipping of intact or small vesicles containing mitochondrial proteins retrogradely to somatodendritic regions for destruction [64,158,159]. While dividing cells have a chance to eliminate older malfunctioning mitochondria entirely, terminally differentiated cells, like neurons, have to sustain the mitochondria that they have for their lifetime. Thus, pathways regulating mitochondrial dynamics (Figure 2) are a key protection mechanism.
Studies with Alzheimer’s disease (AD) mouse models, in both drosophila and mice, note abnormal elongation of mitochondria due to excess stabilization of F-actin, preventing Drp1 localization on OMM [160,161]. Amyloid-beta overproduction, another representative feature of AD, also perturbs expression of mitochondrial dynamics-related proteins, including Drp1, Fis1, and Opa1 [162]. The molecular mechanisms enhancing the mitochondrial transport rate may also facilitate neuronal regeneration [163–166]. While the exact molecular mechanisms of age-dependent neurodegenerative diseases are mostly unknown, abnormal distribution, function and morphology of mitochondria may exaggerate neuronal degeneration, leading to earlier onset of the diseases.
Concluding Remarks
Trillions of mitochondria throughout the human body work together to sustain life. Since the discovery of mitochondria in the 19th century, the field of mitochondrial biology has evolved drastically. Application of live-cell imaging techniques together with mitochondria-tagged fluorescent proteins allows us to observe mitochondria as a complex and highly dynamic reticular network instead of simply a kidney shaped singular unit. While their complex coordinated dynamics as a network fascinates us, as they move and go through fusion and fission cycles, we are just beginning to understand the complex molecular pathways regulating these behaviors. Although much remains unknown about how cells customize mitochondrial network properties, with respect to other organelles, and based on their unique needs, the centrality of mitochondrial dynamics to metabolic homeostasis is well-defined. Regarding the meaning of mitochondrial dynamics for a cell, we still have much to learn, particularly for the following topics:
Regulation of the mitochondrial life cycle:
Mitochondrial content and morphology are tightly regulated in cells to adapt the ATP production capacity depending on the incoming nutrient sources and energy demand. Mitochondrial shape and form changes are important regulators of the mitochondrial life cycle [2]. Like everything, mitochondrial proteins have a finite life-span. To maintain a healthy mitochondrial network, damaged mitochondria and proteins have to be removed and replaced with new ones. While segregation of damaged mitochondria requires fission, recruitment of newly generated proteins or mitochondria utilizes fusion machineries. For the number of mitochondria to remain constant, their turnover rate has to be coupled with mitochondrial biogenesis. Despite ongoing progress regarding this topic, many open questions remain: How are mitochondrial dynamics orchestrated throughout the whole cell? How do cells keep track of how many mitochondria to generate? How do nuclei identify mitochondrial content, especially in highly polarized cells such as neurons? What are the molecular players that regulate cell type-specific mitochondrial shape and content?
Plotting intracellular energy landscape:
Cell polarity is a fundamental feature of many cell types with respect to both tissues and organs. Regulation of mitochondrial dynamics allows coupling of mitochondrial bioenergetics to cellular metabolism. However, not only the amount of ATP produced, but the positioning of mitochondria in polarized cells also define intracellular energy landscapes. It has long been accepted that in a typical cell (ranging from 25–75 μm) the intracellular ATP level is homogenous. However, it has recently been shown that the positioning of mitochondria defines local energy gradients even in small cells such as fibroblasts [8]. Local energy levels are critical for a plethora of cellular functions such as neuronal activity, cell migration, tumor cell invasion, wound healing, and immunity [121,167–169]. Intracellular transport and the positioning of mitochondria shape spatiotemporal heterogeneities in ATP distribution, and understanding how mitochondria in cells know where to go, where to stop and which function to carry out based on the metabolic state of the cell to sustain energy homeostasis will be the next challenge.
Facilitating intercellular communications:
Intriguingly, transcellular transport of mitochondria has recently been reported, indicating that mitochondria can be relocated to facilitate intercellular communication. In cardiac muscle, mitochondria are transported between cardiomyocytes and myofibroblasts to support myocyte survival [170]. Similarly, in the central nervous system, extracellular mitochondrial transport is prevalent between neurons and glia [171,172]. Conversely, neurons can release damaged axonal mitochondria to be taken up and degraded by nearby astrocytes [171]. These findings demonstrate a collaboration between neighboring cells to preserve a healthy mitochondrial pool at the tissue level. Therefore, mitochondria dynamics are not only restricted to single cell activity but have the potential to play a more expansive role in governing intracellular networks, tissues, organs, and even wholebody metabolism.
New discoveries about the interactions between mitochondria and other organelles, between different cell types, and their shape and function under different metabolic states leaves us with even more questions on how cells tailor mitochondrial dynamics to sustain energy homeostasis. Considering the essential role of mitochondrial dynamics in health and disease, a full understanding of the underlying molecular pathways may ultimately inspire novel metabolic therapies.
Research Highlights.
-
-
Mitochondrial functions are hard-wired to cellular metabolism, which is critical for energy homeostasis.
-
-
The morphological adaptations of mitochondria to cellular metabolism are facilitated by dynamic events categorized as transport, fusion, fission and quality control.
-
-
Mitochondrial dynamics support energy homeostasis by regulating strategic positioning of mitochondria within the cytoplasm and inter-organelle cross-talk, metabolite transfer, mitochondrial degradation and biogenesis.
-
-
Dysregulation of mitochondrial dynamics leads to diseases including cancer, metabolic and neurological disorders.
Acknowledgements
We thank Pekkurnaz Lab members, Meghan J. Rossi and Dr. Matthew Banghart for their insightful comments. Research in the laboratory of G.P. is supported by the University of California San Diego institutional funds, Parkinson’s foundation (PF-JFA-1888) and NIH (R35GM128823). S.B.Y. is funded by the NIH Training Grant T32GM007240.
Abbreviations
- KHC
kinesin heavy chain
- SNPH
Syntaphilin
- PINK1
PTEN-induced putative kinase 1
- ROS
reactive oxygen species
- HUMMR
hypoxia up-regulated mitochondrial movement regulator
- OGT
OGlcNAc transferase
- OMM
outer mitochondrial membrane
- Mfn
mitofusin
- CL
cardiolipin
- PA
phosphatidic acid
- Drp1
Dynamin related protein 1
- ER
endoplasmic reticulum
- Opa1
Optic atrophy 1
- IMM
inner mitochondrial membrane
- PKA
cAMP-protein kinase A
- AMPK
AMP-activated protein kinase
- OXPHOS
oxidative phosphorylation
- Atg
autophagyrelated
- PHB2
prohibin 2
- ULK1
unc-51 like activating kinase
- MAM
mitochondria-associated endoplasmic reticulum membrane
- INF2
inverted formin
- PP-PP2A
pentose phosphate-protein phosphatase 2A
- GD
Gaucher disease
- MTFP1
mitochondrial fission process protein
- MDV
mitochondrial derived vesicle
- mtDNA
mitochondrial DNA
- PGC-1
Peroxisome proliferatoractivated receptor gamma coactivator 1
- MAPL
mitochondria-anchored protein ligase
- T2DM
type 2 diabetes mellitus
- iPSC
induced pluripotent stem cell
- CMT2A
Charcot-Marie-Tooth (CMT) type 2A
- PD
Parkinson diseases
- AD
Alzheimer’s disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- [1].Misgeld T, Schwarz TL, Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture, Neuron. 96 (2017) 651–666. doi: 10.1016/j.neuron.2017.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liesa M, Shirihai OS, Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure, Cell Metab. 17 (2013) 491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sebastián D, Palacín M, Zorzano A, Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging, Trends Mol. Med 23 (2017) 201–215. doi: 10.1016/j.molmed.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [4].Mishra P, Chan DC, Metabolic regulation of mitochondrial dynamics, J. Cell Biol 212 (2016) 379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rambold AS, Pearce EL, Mitochondrial Dynamics at the Interface of Immune Cell Metabolism and Function, Trends Immunol. 39 (2018) 6–18. doi: 10.1016/j.it.2017.08.006. [DOI] [PubMed] [Google Scholar]
- [6].Sheng Z-H, Cai Q, Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration, Nat. Rev. Neurosci 13 (2012). doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwarz TL, Mitochondrial trafficking in neurons, Cold Spring Harb. Perspect. Med 3 (2013) 1–16. doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schuler M-H, Lewandowska A, Di Caprio G, Skillern W, Upadhyayula S, Kirchhausen T, Shaw JM, Cunniff B, Miro1-mediated mitochondrial positioning shapes intracellular energy gradients required for cell migration, Mol. Biol. Cell 28 (2017) 2159–2169. doi: 10.1091/mbc.E16-10-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL, Axonal transport of mitochondria to synapses depends on Milton, a novel Drosophila protein, Neuron. 36 (2002) 1063–1077. doi: 10.1016/S0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- [10].Fransson Å, Ruusala A, Aspenström P, The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking, Biochem. Biophys. Res. Commun 344 (2006) 500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- [11].Brickley K, Smith MJ, Beck M, Stephenson FA, GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: Association in vivo and in vitro with kinesin, J. Biol. Chem 280 (2005) 14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- [12].van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, Hoogenraad CC, TRAK/Milton Motor-Adaptor Proteins Steer Mitochondrial Trafficking to Axons and Dendrites, Neuron. 77 (2013) 485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- [13].Wang X, Schwarz TL, The Mechanism of Ca2+-Dependent Regulation of Kinesin-Mediated Mitochondrial Motility, Cell. 136 (2009) 163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Debattisti V, Gerencser AA, Saotome M, Das S, Hajnóczky G, ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak, Cell Rep. 21 (2017) 1667–1680. doi: 10.1016/j.celrep.2017.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL, Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase, Cell. 158 (2014) 54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].MacAskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT, Miro1 Is a Calcium Sensor for Glutamate Receptor-Dependent Localization of Mitochondria at Synapses, Neuron. 61 (2009) 541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].López‐Doménech G, Covill‐Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT, Miro proteins coordinate microtubule‐ and actin‐dependent mitochondrial transport and distribution, EMBO J. 37 (2018) e96380. doi: 10.15252/embj.201696380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH, Docking of Axonal Mitochondria by Syntaphilin Controls Their Mobility and Affects Short-Term Facilitation, Cell. 132 (2008) 137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y, Sheng ZH, Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport, J. Cell Biol 202 (2013) 351–364. doi: 10.1083/jcb.201302040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ, Pink1 forms a multiprotein complex with miro and milton, linking Pink1 function to mitochondrial trafficking, Biochemistry. 48 (2009) 2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, Lavoie MJ, Schwarz TL, PINK1 and Parkin target miro for phosphorylation and degradation to arrest mitochondrial motility, Cell. 147 (2011) 893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liao PC, Tandarich LC, Hollenbeck PJ, ROS regulation of axonal mitochondrial transport is mediated by Ca2+and JNK in Drosophila, PLoS One. 12 (2017) 1–21. doi: 10.1371/journal.pone.0178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Y, Lim S, Hoffman D, Aspenstrom P, Federoff HJ, Rempe DA, HUMMR, a hypoxia- and HIF-1α-inducible protein, alters mitochondrial distribution and transport, J. Cell Biol. 185 (2009) 1065–1081. doi: 10.1083/jcb.200811033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O, Cross Talk Between O-GlcNAcylation and Phosphorylation:Roles in Signaling, Transcription, and Chronic Disease, (2012) 825–858. doi: 10.1146/annurev-biochem-060608-102511.Cross. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iyer SPN, Hart GW, Roles of the Tetratricopeptide Repeat Domain in O-GlcNAc Transferase Targeting and Protein Substrate Specificity, J. Biol. Chem 278 (2003) 24608–24616. doi: 10.1074/jbc.M300036200. [DOI] [PubMed] [Google Scholar]
- [26].Lee J-Y, Kapur M, Li M, Choi M-C, Choi S, Kim H-J, Kim I, Lee E, Taylor JP, Yao T-P, MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria, J. Cell Sci 127 (2014) 4954–4963. doi: 10.1242/jcs.157321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dietrich MO, Liu ZW, Horvath TL, XMitochondrial dynamics controlled by mitofusins regulate agrp neuronal activity and diet-induced obesity, Cell. 155 (2013) 188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC, SlP-2 is required for stress-induced mitochondrial hyperfusion, EMBO J. 28 (2009) 1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ramírez S, Gómez-Valadés AG, Schneeberger M, Varela L, Haddad-Tóvolli R, Altirriba J, Noguera E, Drougard A, Flores-Martínez Á, Imbernón M, Chivite I, Pozo M, Vidal-Itriago A, Garcia A, Cervantes S, Gasa R, Nogueiras R, Gama-Pérez P, Garcia-Roves PM, Cano DA, Knauf C, Servitja JM, Horvath TL, Gomis R, Zorzano A, Claret M, Mitochondrial Dynamics Mediated by Mitofusin 1 Is Required for POMC Neuron Glucose-Sensing and Insulin Release Control, Cell Metab. 25 (2017) 1390–1399.e6. doi: 10.1016/j.cmet.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [30].Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodríguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M, Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance, Cell. 155 (2013) 172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ, The PINK1/Parkin pathway regulates mitochondrial morphology., Proc. Natl. Acad. Sci. U. S. A 105 (2008) 1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC, Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development, J. Cell Biol 160 (2003) 189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen H, McCaffery JM, Chan DC, Mitochondrial Fusion Protects against Neurodegeneration in the Cerebellum, Cell. 130 (2007) 548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- [34].Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA, A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis, Nat. Cell Biol 8 (2006) 1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- [35].Loson OC, Song Z, Chen H, Chan DC, Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission, Mol. Biol. Cell 24 (2013) 659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK, ER tubules mark sites of mitochondrial division, Science (80-. ) 334 (2011) 358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chang C-R, Blackstone C, Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1, Ann. N. Y. Acad. Sci 1201 (2010) 34–39. doi:doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR, Hoshijima M, Dillmann W, Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes, J. Biol. Chem 287 (2012) 30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J, Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion, J. Cell Biol 186 (2009) 793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ban T, Ishihara T, Kohno H, Saita S, Ichimura A, Maenaka K, Oka T, Mihara K, Ishihara N, Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin, Nat. Cell Biol 19 (2017) 856–863. doi: 10.1038/ncb3560. [DOI] [PubMed] [Google Scholar]
- [41].Ishihara N, Fujita Y, Oka T, Mihara K, Regulation of mitochondrial morphology through proteolytic cleavage of OPA1, EMBO J. 25 (2006) 2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Norton M, Ng ACH, Baird S, Dumoulin A, Shutt T, Mah N, Andrade-Navarro MA, McBride HM, Screaton RA, ROMO1 is an essential redox-dependent regulator of mitochondrial dynamics, Sci. Signal 7 (2014) 1–10. doi: 10.1126/scisignal.2004374. [DOI] [PubMed] [Google Scholar]
- [43].Mattie S, Riemer J, Wideman JG, McBride HM, A new mitofusin topology places the redox-regulated C terminus in the mitochondrial intermembrane space, J. Cell Biol (2017) jcb.201611194. doi: 10.1083/jcb.201611194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J, Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation, Proc. Natl. Acad. Sci 108 (2011) 10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ahn B-H, Kim H-S, Song S, Lee IH, Liu J, Vassilopoulos A, Deng C-X, Finkel T, A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis, Proc. Natl. Acad. Sci 105 (2008) 14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Oanh NTK, Park YY, Cho H, Mitochondria elongation is mediated through SIRT1-mediated MFN1 stabilization, Cell. Signal 38 (2017) 67–75. doi: 10.1016/j.cellsig.2017.06.019. [DOI] [PubMed] [Google Scholar]
- [47].Li J, Wang Y, Wang Y, Wen X, Ma XN, Chen W, Huang F, Kou J, Qi LW, Liu B, Liu K, Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction, J. Mol. Cell. Cardiol 86 (2015) 62–74. doi: 10.1016/j.yjmcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- [48].Kang SWS, Haydar G, Taniane C, Farrell G, Arias IM, Lippincott-Schwartz J, Fu D, AMPK Activation prevents and reverses drug-induced mitochondrial and hepatocyte injury by promoting mitochondrial fusion and function, PLoS One. 11 (2016) 1–24. doi: 10.1371/journal.pone.0165638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Toyama EQ, Herzig S, Courchet J, Lewis TL Jr., Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ, AMP-activated protein kinase mediates mitochondrial fission in response to energy stress, 351 (2016) 275–282. doi: 10.1007/978-3-319-43589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L, The Opa1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage, Cell Metab. 21 (2015) 834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M, Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models, Cell Metab. 21 (2015) 845–854. doi: 10.1016/j.cmet.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, Dillmann WH, Makino A, Suarez J, Gawlowski T, Han W, Wang H, Bt S, Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes, (2011) 1296–1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sood A, Jeyaraju DV, Prudent J, Caron A, Lemieux P, McBride HM, Laplante M, Tóth K, Pellegrini L, A Mitofusin-2–dependent inactivating cleavage of Opa1 links changes in mitochondria cristae and ER contacts in the postprandial liver, Proc. Natl. Acad. Sci 111 (2014) 16017–16022. doi: 10.1073/pnas.1408061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Youle RJ, Narendra DP, Mechanisms of mitophagy, Nat. Rev. Mol. Cell Biol 12 (2011) 9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gomes LC, Scorrano L, Mitochondrial morphology in mitophagy and macroautophagy, Biochim. Biophys. Acta - Mol. Cell Res 1833 (2013) 205–212. doi: 10.1016/j.bbamcr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- [56].Okamoto K, Kondo-Okamoto N, Ohsumi Y, Mitochondria-Anchored Receptor Atg32 Mediates Degradation of Mitochondria via Selective Autophagy, Dev. Cell 17 (2009) 87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- [57].Kraft C, Peter M, Hofmann K, Selective autophagy: Ubiquitin-mediated recognition and beyond, Nat. Cell Biol 12 (2010) 836–841. doi: 10.1038/ncb0910-836. [DOI] [PubMed] [Google Scholar]
- [58].Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ, PINK1 is selectively stabilized on impaired mitochondria to activate Parkin, PLoS Biol 8 (2010). doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J, Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin, Nature. 441 (2006) 1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- [60].Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N, Ubiquitin is phosphorylated by PINK1 to activate parkin, Nature. 510 (2014) 162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- [61].Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, Yin XM, Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkinubiquitin-p62-mediated mitochondrial priming, J. Biol. Chem 285 (2010) 27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].McWilliams TG, Prescott AR, Montava-Garriga L, Ball G, Singh F, Barini E, Muqit MMK, Brooks SP, Ganley IG, Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand, Cell Metab. (2018) 439–449. doi: 10.1016/j.cmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee JJ, Sanchez-Martinez A, Zarate AM, Benincá C, Mayor U, Clague MJ, Whitworth AJ, Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin, J. Cell Biol 217 (2018) 1613–1622. doi: 10.1083/jcb.201801044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL, Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin, J. Cell Biol 206 (2014) 655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS, Fission and selective fusion govern mitochondrial segregation and elimination by autophagy, EMBO J 27 (2008) 433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AHV, Taanman JW, Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy, Hum. Mol. Genet 19 (2010) 4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wei Y, Chiang WC, Sumpter R, Mishra P, Levine B, Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor, Cell. 168 (2017) 224–238. e10. doi: 10.1016/j.cell.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sato M, Sato K, Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos, Science. 334 (2011) 1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- [69].Wang Y, Zhang Y, Chen L, Liang Q, Yin XM, Miao L, Kang BH, Di. Xue, Kinetics and specificity of paternal mitochondrial elimination in Caenorhabditis elegans, Nat. Commun 7 (2016) 1–15. doi: 10.1038/ncomms12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kim J, Kundu M, Viollet B, Guan KL, AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1, Nat. Cell Biol 13 (2011) 132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Egan DF, Shackelford DB, Mihaylova MM, Gelino S, a Kohnz R, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ, Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy, Science (80-. ) 331 (2011) 456–461. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gomes LC, Di Benedetto G, Scorrano L, During autophagy mitochondria elongate, are spared from degradation and sustain cell viability, Nat. Cell Biol 13 (2011) 589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Phillips MJ, Voeltz GK, Structure and function of ER membrane contact sites with other organelles, Nat. Rev. Mol. Cell Biol 17 (2016) 69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kameoka S, Adachi Y, Okamoto K, Iijima M, Sesaki H, Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics, Trends Cell Biol. 28 (2018) 67–76. doi: 10.1016/j.tcb.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].De Brito OM, Scorrano L, Mitofusin 2 tethers endoplasmic reticulum to mitochondria, Nature. 456 (2008) 605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- [76].Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, Serafini A, Semenzato M, Herkenne S, Hernández-Alvarez MI, Zorzano A, De Stefani D, Dorn GW, Scorrano L, Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum–mitochondria tether, Proc. Natl. Acad. Sci 113 (2016) 11249–11254. doi: 10.1073/pnas.1606786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang PTC, Garcin PO, Fu M, Masoudi M, St-Pierre P, Pante N, Nabi IR, Distinct mechanisms controlling rough and smooth endoplasmic reticulum contacts with mitochondria, J. Cell Sci 128 (2015) 2759–2765. doi: 10.1242/jcs.171132. [DOI] [PubMed] [Google Scholar]
- [78].Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P, Mitofusin 2 ablation increases endoplasmic reticulum–mitochondria coupling, Proc. Natl. Acad. Sci 112 (2015) E2174–E2181. doi: 10.1073/pnas.1504880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P, On the role of Mitofusin 2 in endoplasmic reticulum–mitochondria tethering, Proc. Natl. Acad. Sci 114 (2017) E2266–E2267. doi: 10.1073/pnas.1616040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Oresic M, Pich S, Burcelin R, Palacin M, Zorzano A, Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis, Proc. Natl. Acad. Sci 109 (2012) 5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P, An ERMitochondria Tethering Complex Revealed by a Synthetic Biology Screen, Science (80-. ) 325 (2009) 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang M, Mileykovskaya E, Dowhan W, Gluing the respiratory chain together: Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane, J. Biol. Chem 277 (2002) 43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- [83].Jouaville LS, Pinton P, Bastianutto C, a Rutter G, Rizzuto R, Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming., Proc. Natl. Acad. Sci. U. S. A 96 (1999) 13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].De Marchi U, Thevenet J, Hermant A, Dioum E, Wiederkehr A, Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells, J. Biol. Chem 289 (2014) 9182–9194. doi: 10.1074/jbc.M113.513184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chakrabarti R, Ji WK, Stan RV, de Sanz JJ , Ryan TA, Higgs HN, INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division, J. Cell Biol 217 (2018) 251–268. doi: 10.1083/jcb.201709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee S, Lee KS, Huh S, Liu S, Lee DY, Hong SH, Yu K, Lu B, Polo Kinase Phosphorylates Miro to Control ER-Mitochondria Contact Sites and Mitochondrial Ca2+Homeostasis in Neural Stem Cell Development, Dev. Cell 37 (2016) 174–189. doi: 10.1016/j.devcel.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, Nunnari J, ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast, Elife. 2013 (2013) 1–16. doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH, The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals, J. Biol. Chem 280 (2005) 33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- [89].Morciano G, Giorgi C, Balestra D, Marchi S, Perrone D, Pinotti M, Pinton P, Mcl-1 involvement in mitochondrial dynamics is associated with apoptotic cell death, Mol. Biol. Cell 27 (2016) 20–34. doi: 10.1091/mbc.E15-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Theurey P, Tubbs E, Vial G, Jacquemetton J, Bendridi N, Chauvin MA, Alam MR, Le Romancer M, Vidal H, Rieusset J, Mitochondria-associated endoplasmic reticulum membranes allow adaptation of mitochondrial metabolism to glucose availability in the liver, J. Mol. Cell Biol 8 (2016) 129–143. doi: 10.1093/jmcb/mjw004. [DOI] [PubMed] [Google Scholar]
- [91].Ozcan L, Wong CCL, Li G, Xu T, Pajvani U, Park SKR, Wronska A, Chen BX, Marks AR, Fukamizu A, Backs J, Singer HA, Yates JR, Accili D, Tabas I, Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity, Cell Metab. 15 (2012) 739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Betz C, Stracka D, Prescianotto-baschong C, Frieden M, Demaurex N, mTOR complex 2-Akt signaling at mitochondria associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 12526. doi: 10.1073/pnas.1302455110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ballabio A, The awesome lysosome, EMBO Mol. Med 8 (2016) 73–76. doi: 10.15252/emmm.201505966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Demers-Lamarche J, Guillebaud G, Tlili M, Todkar K, Bélanger N, Grondin M, P’Nguyen A, Michel J, Germain M, Loss of mitochondrial function impairs Lysosomes∗, J. Biol. Chem 291 (2016) 10263–10276. doi: 10.1074/jbc.M115.695825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Baixauli F, Acín-Pérez R, Villarroya-Beltrí C, Mazzeo C, Nuñez-Andrade N, Gabandé-Rodriguez E, Ledesma MD, Blázquez A, Martin MA, Falcón-Pérez JM, Redondo JM, Enríquez JA, Mittelbrunn M, Mitochondrial respiration controls lysosomal function during inflammatory t cell responses, Cell Metab. 22 (2015) 485–498. doi: 10.1016/j.cmet.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, V Schapira AH, Duchen MR, Mitochondria and quality control defects in a mouse model of gaucher disease - Links to parkinson’s disease, Cell Metab. 17 (2013) 941–953. doi: 10.1016/j.cmet.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wong YC, Ysselstein D, Krainc D, Mitochondria–lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis, Nat. 2018 554 (2018) 382–386. doi: 10.1038/nature25486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hönscher C, Mari M, Auffarth K, Bohnert M, Griffith J, Geerts W, van der Laan M, Cabrera M, Reggiori F, Ungermann C, Cellular metabolism regulates contact sites between vacuoles and mitochondria, Dev. Cell 30 (2014) 86–94. doi: 10.1016/j.devcel.2014.06.006. [DOI] [PubMed] [Google Scholar]
- [99].Abu-Remaileh M, Wyant GA, Kim C, Laqtom NN, Abbasi M, Chan SH, Freinkman E, Sabatini DM, Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes, Science (80-. ) 358 (2017) 807–813. doi: 10.1126/science.aan6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, Pearl D, Siddiqui N, Strack S, McGuirk S, St-Pierre J, Larsson O, Topisirovic I, Vali H, McBride HM, Bergeron JJ, Sonenberg N, mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1, Mol. Cell 67 (2017) 922–935. e5. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- [101].Ouellet M, Guillebaud G, Gervais V, Lupien St-Pierre D, Germain M, A novel algorithm identifies stress-induced alterations in mitochondrial connectivity and inner membrane structure from confocal images, PLoS Comput. Biol 13 (2017) 1–23. doi: 10.1371/journal.pcbi.1005612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM, A vesicular transport pathway shuttles cargo from mitochondria to lysosomes, Curr. Biol 22 (2012) 135–141. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- [103].Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM, Reconstitution of Mitochondria Derived Vesicle Formation Demonstrates Selective Enrichment of Oxidized Cargo, PLoS One. 7 (2012). doi: 10.1371/journal.pone.0052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cohen Y, Klug YA, Dimitrov L, Erez Z, Chuartzman SG, Elinger D, Yofe I, Soliman K, Gärtner J, Thoms S, Schekman R, Elbaz-Alon Y, Zalckvar E, Schuldiner M, Peroxisomes are juxtaposed to strategic sites on mitochondria, Mol. BioSyst 10 (2014) 1742–1748. doi: 10.1039/C4MB00001C. [DOI] [PubMed] [Google Scholar]
- [105].Wanders RJA, Waterham HR, Ferdinandusse S, Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum, Front. Cell Dev. Biol 3 (2016) 1–15. doi: 10.3389/fcell.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Islinger M, Lüers GH, Zischka H, Ueffing M, Völkl A, Insights into the membrane proteome of rat liver peroxisomes: Microsomal glutathione-S-transferase is shared by both subcellular compartments, Proteomics. 6 (2006) 804–816. doi: 10.1002/pmic.200401347. [DOI] [PubMed] [Google Scholar]
- [107].Mattiazzi Ušaj M, Brložnik M, Kaferle P, Žitnik M, Wolinski H, Leitner F, Kohlwein SD, Zupan B, Petrovič U, Genome-wide localization study of yeast pex11 identifies peroxisomemitochondria interactions through the ERMES complex, J. Mol. Biol 427 (2015) 2072–2087. doi: 10.1016/j.jmb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Koch A, Thiemann M, Grabenbauer M, Yoon Y, McNiven MA, Schrader M, Dynamin-like protein 1 is involved in peroxisomal fission, J. Biol. Chem 278 (2003) 8597–8605. doi: 10.1074/jbc.M211761200. [DOI] [PubMed] [Google Scholar]
- [109].Yoon Y, Krueger EW, Oswald BJ, Mcniven A, a Mcniven M, The Mitochondrial Protein hFis1 Regulates Mitochondrial Fission in Mammalian Cells through an Interaction with the Dynamin-Like Protein DLP1 The Mitochondrial Protein hFis1 Regulates Mitochondrial Fission in Mammalian Cells through an Interaction with, Mol. Cell. Biol 23 (2003) 5409–5420. doi: 10.1128/MCB.23.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Koch A, Yoon Y, Bonekamp NA, McNiven MA, Schrader M, A Role for Fis1 in Both Mitochondrial and Peroxisomal Fission in Mammalian Cells, Mol. Biol. Cell 16 (2005) 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Peeters A, Shinde AB, Dirkx R, Smet J, De Bock K, Espeel M, Vanhorebeek I, Vanlander A, Van Coster R, Carmeliet P, Fransen M, Van Veldhoven PP, Baes M, Mitochondria in peroxisome-deficient hepatocytes exhibit impaired respiration, depleted DNA, and PGC-1α independent proliferation, Biochim. Biophys. Acta - Mol. Cell Res 1853 (2015) 285–298. doi: 10.1016/j.bbamcr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- [112].Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM, Cargo-Selected Transport from the Mitochondria to Peroxisomes Is Mediated by Vesicular Carriers, Curr. Biol 18 (2008) 102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- [113].Sugiura A, McLelland G-L, Fon EA, McBride HM, A new pathway for mitochondrial quality control: mitochondrial-derived vesicles, EMBO J. 33 (2014) 2142–2156. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sugiura A, Mattie S, Prudent J, Mcbride HM, Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes, Nature. 542 (2017) 251–254. doi: 10.1038/nature21375. [DOI] [PubMed] [Google Scholar]
- [115].Warburg O, The Metabolism of Carcinoma Cells, J. Cancer Res 9 (1925) 148–163. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- [116].Shim H, Chun YS, Lewis BC, V Dang C, A unique glucose-dependent apoptotic pathway induced by c-Myc, Proc. Natl. Acad. Sci 95 (1998) 1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Crabtree HG, Observations on the carbohydrate metabolism of tumours., Biochem. J 23 (1929) 536–45. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, Pesdar EA, Sobol M, Filimonenko A, Stuart S, Vondrusova M, Kluckova K, Sachaphibulkij K, Rohlena J, Hozak P, Truksa J, Eccles D, Haupt LM, Griffiths LR, Neuzil J, Berridge MV, Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA, Cell Metab. 21 (2015) 81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- [119].Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GRS, Chandel NS, Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity, Proc. Natl. Acad. Sci 107 (2010) 8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Caino MC, Seo JH, Aguinaldo A, Wait E, Bryant KG, Kossenkov AV, Hayden JE, Vaira V, Morotti A, Ferrero S, Bosari S, Gabrilovich DI, Languino LR, Cohen AR, Altieri DC, A neuronal network of mitochondrial dynamics regulates metastasis, Nat. Commun 7 (2016) 1–11. doi: 10.1038/ncomms13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y, Mitochondrial dynamics regulates migration and invasion of breast cancer cells, Oncogene. 32 (2013) 4814–4824. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Desai SP, Bhatia SN, Toner M, Irimia D, Mitochondrial localization and the persistent migration of epithelial cancer cells, Biophys. J 104 (2013) 2077–2088. doi: 10.1016/j.bpj.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z, Fang X, Shi Y, Ferguson AN, Kashatus DF, Bao S, Rich JN, Mitochondrial control by DRP1 in brain tumor initiating cells, Nat. Neurosci 18 (2015) 501–510. doi: 10.1038/nn.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Brown K, Yang P, Salvador D, Kulikauskas R, Ruohola-Baker H, Robitaille AM, Chien AJ, Moon RT, Sherwood V, WNT/β-catenin signaling regulates mitochondrial activity to alter the oncogenic potential of melanoma in a PTEN-dependent manner, Oncogene. 36 (2017) 3119–3136. doi: 10.1038/onc.2016.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Theurey P, Rieusset J, Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases, Trends Endocrinol. Metab 28 (2017) 32–45. doi: 10.1016/j.tem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- [126].Goodpaster BH, Sparks LM, Metabolic Flexibility in Health and Disease, Cell Metab. 25 (2017) 1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Muoio DM, Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock, Cell. 159 (2014) 1253–1262. doi: 10.1016/j.cell.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Mootha VK, Lindgren CM, Eriksson K, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC, PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes, Nat. Genet 34 (2003) 267–273. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- [129].Liu R, Jin P, Yu L, Wang Y, Han L, Shi T, Li X, Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle, PLoS One. 9 (2014) 1–8. doi: 10.1371/journal.pone.0092810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song W-J, Sereda S, Clerc P, Polster BM, Aja SM, Pletnikov MV, Kensler TW, Shirihai OS, Iijima M, Hussain MA, Sesaki H, The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells, Mol. Biol. Cell 22 (2011) 2235–2245. doi: 10.1091/mbc.E10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhao L, Feng Z, Yang X, Liu J, The regulatory roles of O-GlcNAcylation in mitochondrial homeostasis and metabolic syndrome, Free Radic. Res 50 (2016) 1080–1088. doi: 10.1080/10715762.2016.1239017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Tan EP, Villar MT, Lezi E, Lu J, Selfridge JE, Artigues A, Swerdlow RH, Slawson C, Altering O-linked β -N-acetylglucosamine cycling disrupts mitochondrial function, J. Biol. Chem 289 (2014) 14719–14730. doi: 10.1074/jbc.M113.525790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Banerjee PS, Ma J, Hart GW, Diabetes-associated dysregulation of O -GlcNAcylation in rat cardiac mitochondria, Proc. Natl. Acad. Sci 112 (2015) 6050–6055. doi: 10.1073/pnas.1424017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Agostini M, Romeo F, Inoue S, V Niklison-Chirou M, Elia AJ, Dinsdale D, Morone N, Knight RA, Mak TW, Melino G, Metabolic reprogramming during neuronal differentiation, Cell Death Differ. 23 (2016) 1502–1514. doi: 10.1038/cdd.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jin G, Xu C, Zhang X, Long J, Rezaeian AH, Liu C, Furth ME, Kridel S, Pasche B, Bian XW, Lin HK, Atad3a suppresses Pink1-dependent mitophagy to maintain homeostasis of hematopoietic progenitor cells article, Nat. Immunol 19 (2018) 29–40. doi: 10.1038/s41590-017-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Beckervordersandforth R, Ebert B, Schäffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Hölter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, li Ming G, Toni N, Jessberger S, Song H, Lie DC, Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis, Neuron. 93 (2017) 560–573. e6. doi: 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chung JYM, Steen JA, Schwarz TL, Phosphorylation-Induced Motor Shedding Is Required at Mitosis for Proper Distribution and Passive Inheritance of Mitochondria, Cell Rep. 16 (2016) 2142–2155. doi: 10.1016/j.celrep.2016.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ashrafi G, Schwarz TL, The pathways of mitophagy for quality control and clearance of mitochondria, Cell Death Differ. 20 (2013) 31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhang C, Skamagki M, Liu Z, Ananthanarayanan A, Zhao R, Li H, Kim K, Biological Significance of the Suppression of Oxidative Phosphorylation in Induced Pluripotent Stem Cells, Cell Rep. 21 (2017) 2058–2065. doi: 10.1016/j.celrep.2017.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Folmes CDL, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A, Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming, Cell Metab. 14 (2011) 264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper ME, Park DS, Slack RS, Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program, Cell Stem Cell. 19 (2016) 232–247. doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- [142].Sun N, Youle RJ, Finkel T, The Mitochondrial Basis of Aging, Mol. Cell 61 (2016) 654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wei Y, Lee H, Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging, Exp. Biol. Med 227 (2008) 671–682. [DOI] [PubMed] [Google Scholar]
- [144].Chan DC, Mitochondria: Dynamic Organelles in Disease, Aging, and Development, Cell. 125 [DOI] [PubMed] [Google Scholar]; (2006) 1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [Google Scholar]
- [145].Song M, Franco A, Fleischer JA, Zhang L, Dorn GW, Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence, Cell Metab. 26 (2017) 872–883. e5. doi: 10.1016/j.cmet.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, Laboy R, Hirschey MD, Mair WB, Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling, Cell Metab. 26 (2017) 884–896. e5. doi: 10.1016/j.cmet.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI, Aging-Associated Reductions in AMP-Activated Protein Kinase Activity and Mitochondrial Biogenesis, Cell Metab. 5 (2007) 151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, De Jonghe P, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battologlu E, Polyakov AV, Timmerman V, Schröder JM, Vance JM, Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A, Nat. Genet 36 (2004) 449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- [149].Züchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, Hamilton SR, Van Stavern G, Krajewski KM, Stajich J, Tournev I, Verhoeven K, Langerhorst CT, De Visser M, Baas F, Bird T, Timmerman V, Shy M, Vance JM, Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2, Ann. Neurol 59 (2006) 276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- [150].Hudson G, Amati-Bonneau P, Blakely EL, Stewart JD, He L, Schaefer AM, Griffiths PG, Ahlqvist K, Suomalainen A, Reynier P, McFarland R, Turnbull DM, Chinnery PF, Taylor RW, Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: A novel disorder of mtDNA maintenance, Brain. 131 (2008) 329–337. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- [151].Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP, Nuclear gene OPA1, encoding a mitochondrial dynaminrelated protein, is mutated in dominant optic atrophy, Nat. Genet 26 (2000) 207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- [152].Waterham HR, Koster J, van Roermund CWT, Mooyer PAW, Wanders RJA, Leonard JV, A Lethal Defect of Mitochondrial and Peroxisomal Fission, N. Engl. J. Med 356 (2007) 1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- [153].Barel O, Christine V Malicdan M, Ben-Zeev B, Kandel J, Pri-Chen H, Stephen J, Castro IG, Metz J, Atawa O, Moshkovitz S, Ganelin E, Barshack I, Polak-Charcon S, Nass D, Marek-Yagel D, Amariglio N, Shalva N, Vilboux T, Ferreira C, Pode-Shakked B, Heimer G, Hoffmann C, Yardeni T, Nissenkorn A, Avivi C, Eyal E, Kol N, Glick Saar E, Wallace DC, Gahl WA, Rechavi G, Schrader M, Eckmann DM, Anikster Y, Deleterious variants in TRAK1 disrupt mitochondrial movement and cause fatal encephalopathy, Brain. 140 (2017) 568–581. doi: 10.1093/brain/awx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Li Z, Okamoto KI, Hayashi Y, Sheng M, The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses, Cell. 119 (2004) 873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [155].Verstreken P, Ly CV, Venken KJT, Koh TW, Zhou Y, Bellen HJ, Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions, Neuron. 47 (2005) 365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- [156].Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler M-H, Smith NK, Macfarlane J, Saunders G, Palmer CA, Debattisti V, Koshiba T, Pulst S, Feldman EL, Hajnoczky G, Shaw JM, Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease, Proc. Natl. Acad. Sci 111 (2014) E3631–E3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sterky FH, Lee S, Wibom R, Olson L, Larsson N-G, Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo, Proc. Natl. Acad. Sci 108 (2011) 12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lin MY, Cheng XT, Tammineni P, Xie Y, Zhou B, Cai Q, Sheng ZH, Releasing Syntaphilin Removes Stressed Mitochondria from Axons Independent of Mitophagy under Pathophysiological Conditions, Neuron. 94 (2017) 595–610. e6. doi: 10.1016/j.neuron.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Maday S, Wallace KE, Holzbaur ELF, Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons, J. Cell Biol 196 (2012) 407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].DuBoff B, Götz J, Feany MB, Tau Promotes Neurodegeneration via DRP1 Mislocalization In Vivo, Neuron. 75 (2012) 618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP, Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission, Curr. Biol 15 (2005) 678–683. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- [162].Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X, Amyloidoverproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins, Proc. Natl. Acad. Sci 105 (2008) 19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Cartoni R, Norsworthy MW, Bei F, Wang C, Li S, Zhang Y, Gabel CV, Schwarz TL, He Z, Erratum: The Mammalian-Specific Protein Armcx1 Regulates Mitochondrial Transport during Axon Regeneration (Neuron (2016) 92(6) (1294–1307) (S0896627316308340) (10.1016/j.neuron.2016.10.060)), Neuron. 94 (2017) 689. doi: 10.1016/j.neuron.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Cartoni R, Pekkurnaz G, Wang C, Schwarz TL, He Z, A high mitochondrial transport rate characterizes CNS neurons with high axonal regeneration capacity, PLoS One. 12 (2017) 1–12. doi: 10.1371/journal.pone.0184672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Han SM, Baig HS, Hammarlund M, Mitochondria Localize to Injured Axons to Support Regeneration, Neuron. 92 (2016) 1308–1323. doi: 10.1016/j.neuron.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH, Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits, J. Cell Biol 214 (2016) 103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Campello S, Lacalle RA, Bettella M, Mañes S, Scorrano L, Viola A, Orchestration of lymphocyte chemotaxis by mitochondrial dynamics, J. Exp. Med 203 (2006) 2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa-Bellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, Sanchez-Madrid F, Miro-1 Links Mitochondria and Microtubule Dynein Motors To Control Lymphocyte Migration and Polarity, Mol. Cell. Biol 34 (2014) 1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mills KM, Brocardo MG, Henderson BR, APC binds the Miro/Milton motor complex to stimulate transport of mitochondria to the plasma membrane, Mol. Biol. Cell 27 (2016) 466–482. doi: 10.1091/mbc.E15-09-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Shen J, Zhang J-H, Xiao H, Wu J-M, He K-M, Lv Z-Z, Li Z-J, Xu M, Zhang Y-Y, Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis, Cell Death Dis 9 (2018). doi: 10.1038/s41419-017-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Davis CO, Kim K-Y, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N, Transcellular degradation of axonal mitochondria, Proc. Natl. Acad. Sci 111 (2014) 9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH, Transfer of mitochondria from astrocytes to neurons after stroke, Nature. 535 (2016) 551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhang J, Nuebel E, Wisidagama DRR, Setoguchi K, Hong JS, Van Horn CM, Imam SS, Vergnes L, Malone CS, Koehler CM, Teitell MA, Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells, Nat. Protoc 7 (2012) 1068–1085. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Piquereau J, Caffin F, Novotova M, Lemaire C, Veksler V, Garnier A, Ventura-Clapier R, Joubert F, Mitochondrial dynamics in the adult cardiomyocytes: Which roles for a highly specialized cell?, Front. Physiol 4 May (2013) 1–12. doi: 10.3389/fphys.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]