Abstract
Vascular endothelial cells (ECs) differentiated from pluripotent stem cells have enormous potential to be used in a variety of therapeutic areas such as tissue engineering of vascular grafts and re-vascularization of ischemic tissues. To date, various protocols have been developed to differentiate stem cells toward vascular ECs. However, current methods are still not sufficient to drive the distinct arterial venous differentiation. Therefore, developing refined method of arterial-venous differentiation is critically needed to address this gap. Here, we developed a biomaterial platform to mimic multivalent ephrin-B2/EphB4 signaling and investigated its role in the early arterial and venous specification of pluripotent stem cells. Our results show immobilized ephrinB2 or EphB4 on hydrogel substrates have a distinct effect on arterial venous differentiation by regulating several arterial venous markers. When in combination with Wnt pathway agonist or BMP4 signaling, the ephrin-B2/EphB4 biomaterial platform can create diverging EC progenitor populations, demonstrating differential gene expression pattern across a wide range of arterial and venous markers, as well as phenotypic markers such as anti-thrombotic, pro-atherogenic and osteogenic genes, that are consistent with the in vivo expression patterns of arterial and venous ECs. Importantly, this distinct EC progenitor population cannot be achieved by current methods of applying soluble factors or hemodynamic stimuli alone, illustrating that fine-tuning of developmental signals using the biomaterial platform offers a new approach to better control the arterial venous differentiation of stem cells.
Introduction
Vascular endothelial cells (ECs) differentiated from pluripotent stem cells have potential in a variety of therapeutic areas, however, there remain limitations with current methods in creating desired functional phenotypes, in particular arterial and venous ECs. Differentiation of phenotypic specific ECs can improve cellular functionality for specific applications including small diameter arterial vascular grafts and re-vascularization of ischemic tissues. Additionally, arteries and veins have varying levels of vulnerability to vascular diseases and different adaptation response to changes of hemodynamic conditions. Therefore, the ability to develop unique EC populations from pluripotent stem cells may also be utilized for in vitro disease modeling and drug screening for vascular-related medical problems.
Arterial and venous ECs have distinctive molecular and genetic profiles. Previously, it was believed that these variances were resulting from physiological biochemical and biomechanical environmental differences in the adult vascular system, such as flow rates and oxygen levels1. This was debunked when Wang et al. discovered molecular differences between arterial and venous ECs prior to the initiation of blood circulation2–5. Specifically, transmembrane protein EphB4 was observed solely expressed on venous ECs, conversely its ligand ephrinB2, is uniquely expressed on arterial ECs. Recent studies in vein graft adaptation suggested that adult venous ECs do not adapt to arterial ECs phenotypes after exposing to arterial hemodynamic conditions6, 7. We now believe that there is a genetic component to these phenotypes that can be detected within EC progenitor populations before physiological environmental differences are established.
To date, various protocols have been developed to differentiate stem cells toward vascular ECs8–13. Most in vitro approaches rely on soluble factors to guide differentiation. Common soluble factors include vascular endothelial growth factor (VEGF)10, 14–20, basic fibroblast growth factor 2 (bFGF2) 14, 16, 18, 21, 22, bone morphogenetic protein 4 (BMP4)14–16, 22–24 and small molecule Wnt agonists23, 25, 26. While there have been many successes in generic EC differentiation, there is limited work and modest outcomes for creating arterial venous EC populations. To promote arterial differentiation, hemodynamic flow condition and soluble factors (such as high concentrations of VEGF) were introduced to increase the expression of arterial markers15, 19, 27–32. Nevertheless, the improvement is moderate: there is up-regulation of only a few selected arterial markers without down-regulating venous markers, some even increase both the arterial and venous markers at the same time. These studies suggest that current methods of soluble factors and hemodynamic flows are insufficient to obtain distinct arterial venous differentiation. Furthermore, lacking of arterial markers are often taken as the default phenotypes for venous ECs. However, recent studies have shown that definitive venous EC phenotype is not the default pathway resulting from a lack of notch activation but in fact venous phenotype needs the active involvement of the COUP-TFII transcription factor33. Overall, arterial venous markers were never fully examined in stem cell derived ECs, raising the question on whether the divergent arterial venous phenotypes were actually achieved in previous studies. Therefore, developing refined method of arterial-venous differentiation is critically needed to address this gap.
Because molecular distinction of arterial-venous identity appears earlier than the onset of blood flow, in order to drive the proper arterial venous differentiation, it is critical to provide biomimetic signals of vascular development to guide the process. Importantly, most of these signals are not soluble factors, but presented as immobilized signals such as cell-cell and cell-matrix interactions. In this context, biomaterials are an excellent platform to explore immobilized biochemical signals and biophysical cues to help guide stem cell differentiation. Bioactive materials have been widely used in the fields of tissue engineering and regenerative medicine but far less applied towards guiding stem cell fate.
Here, we aim to develop a biomaterial platform to mimic these developmental signals to control stem cell differentiation. We first choose to mimic cell-cell ephrin-B2/EphB4 signaling through bioactive PEG hydrogels and investigate how it influences vascular differentiation. EphrinB2/EphB4 interactions play roles in a variety of biological systems including vascular development3, 34, brain/nervous system35–38 and bone remodeling39, 40. In vascular development, this signaling is important in angiogenesis and vascular patterning4, 34, 41–43. However, there has been no work to date investigating how these signals contribute to EC phenotype specification during stem cell differentiation. The ultimate goal of this study was to create diverging arterial venous EC progenitor populations. While neither ephrinB2/EphB4 immobilized cues nor soluble factors alone created diverging phenotypes, the combination of these two methods was successful in creating arterial enriched and venous enriched EC progenitor populations. We thoroughly explored their EC characteristics including tube formation assay, phenotypic related functional profiles and compared these selected stem cell derived ECs progenitors to in vivo arterial and venous ECs. Finally, further evaluation of ephrinB2-Fc hydrogel mechanism is explored to better understand how and when this signal influences vascular differentiation.
Materials and methods
ephrinB2-Fc/EphB4-Fc PEG-diacrylate hydrogel synthesis
EphrinB2-Fc (R&D Systems) and EphB4-Fc (R&D Systems) are dimers; each consists of two extracellular signaling mouse domain of the protein held together through the Fc domain of human IgG. To allow photocrosslinkable reaction of these bioactive signals, proteins were first reacted in phosphate buffer solution (PBS) at pH 8, for two hours in a 1:50 molar excess of acrylate-PEG-succinimidyl valerate (3400 Da, Laysan Bio) to obtain PEG-conjugated proteins: ephrinB2-Fc-PEGAc/EphB4-Fc-PEGAc. Successful conjugation was validated via SDS-PAGE. To create the hydrogel substrate for bioactive patterning, polyethylene glycol diacrylate (PEGDA, Laysan Bio, 3.4KDa) was combined with fibronectin (60 μg/mL) and photopolymerized within a 0.5mm thick mold using photoinitiator (300 mg/mL 2-dimethoxy-2-phenylacetophenone in n-vinyl pyrrolidone) and UV light (365nm, 3.5mW/cm2) for 120 seconds. Hydrogel samples were pressed out using a biopsies punch. Next, ephrinB2-Fc-PEGAc or EphB4-Fc-PEGAc solution (500 ng/mL) was pipetted onto the surface of hydrogels and conjugated with a second round of UV exposure for 60 seconds. Hydrogels were finally placed into well plates and unreacted species were washed away in PBS at 4°C overnight before stem cell seeding.
Vascular differentiation of mouse embryonic stem cells (mESCs)
For vascular differentiation, mESC were seeded at a seeding density of 2 × 105 cells/cm2 and cultured with differentiation media for seven days. Differentiation media was made up of α-MEM with L-glutamine (Life Technologies), 20% knockout serum replacement (Sigma), 100μg/mL penicillin/Streptomyocin (Sigma), 10mM non-essential amino acids (Life Technologies) and 50uM β-mercaptoethanol (Life Technologies). Growth factors were added to the basal media to induce a vascular differentiation using different combinations of VEGF (Peprotech, 30ng/mL), bFGF2 (Peprotech, 12.5ng/mL), GSK beta inhibitor chir99021 (Tocris Bioscience 4423, 3μM), or BMP4 (Peprotech, 12ng/mL). VEGF, bFGF2 and chir99021 was used as the standard media condition, unless otherwise stated. Medium was exchanged 50% every day over seven days.
Isolation of ECs from mESC
After the differentiation period, cells were harvested for endothelial cell sorting. In short, media was removed from wells and cells were incubated with Accutase (Stem Cell Technologies) for several minutes until detached. Cells were removed from each well and centrifuged at 300 RPM for three minutes. Cells were re-suspended in minimal volume of blocking buffer (PBS with 5% FBS) for ten minutes and then incubated with primary rat anti-mouse Flk1 antibody (1μg/million cells, BD Bioscience) for 20–30 minutes. Next, samples were washed in 10 mL of blocking buffer and centrifuged at 300 RPM for ten minutes. Anti-rat magnetic beads (Miltenyi Biotec) were incubated with single cell suspension for 15 minutes, following Miltenyi Biotec manufacturer’s protocol. Again, cells were washed with 10 mL of PBS and centrifuged at 300 RPM for ten minutes. Using magnetic column and holder, single cell suspension was added over the top of the equilibrated column, allowing for positively selected cells to bind to the magnetic resin. After removal of unlabeled cells, the column was removed from the magnetic field, allowing for positively selected cells to elute from the column. Cells were plated on fibronectin-coated well plates or flow chambers in post sorting medium of EGM-2 (Lonza) with additional 7.5% FBS.
Tube forming assay
Matrigel (Corning) tube forming assay was performed following manufacture protocol. In short, Flk1+ sorted cells were seeded with 5 × 104 cells in matrigel coated 48 well plates. EGM-2 media containing no growth factors was added into each well at low volume. Tube formation was observed 12–16 hours after seeding. Live staining (calcein AM, Thermo Fisher Scientific) was used for fluorescent imaging.
Low density lipoprotein (LDL) uptake analysis
LDL uptake was performed via manufacturer protocol. Briefly, Alexa Flour 594 acetylated-LDL (Molecular Probes) was added into media at a concentration of 10 μg/mL and incubated for four hours at 37°C. Wells were washed twice to remove soluble acetylated-LDL and fluorescent images were taken to observe uptake of fluorescently labeled acetylated-LDL.
Alizarin red and alkaline phosphatase analysis
To evaluate osteogenic potential of arterial and venous Flk1 populations, cells were cultured under osteogenic differentiation media (Lonza) for seven days. Calcium deposition and alkaline phosphatase staining were performed. For calcium deposition, cells were washed twice in PBS without magnesium and calcium and fixed in 4% paraformaldehyde for 10 minutes. After two washes of PBS, Alizarin Red staining was performed based on manufacturer’s instructions (American MasterTech Sci.). For alkaline phosphatase staining, cells were washed twice in PBS and fixed for one minute with 4% paraformaldehyde. Wells were washed in PBS and stained with SigmaFast BCIP/NBT following Sigma manufacturer protocol.
Flow set-up for dynamic culture
Flk1 sorted cells were seeded into fibronectin coated flow chamber micro-slide VI0.4 (ibidi) following the manufacturer protocol. Static conditions required media change every 12 hours. Post sort media was used for both flow and static conditions, EGM-2 with additional 7.5% FBS. For dynamic culture, a continuous flow circuit was created with media reservoirs, pulsatile pump, cylindrical bubble trap and cell-seeded flow chambers. Each flow chamber was run in parallel with individual media reservoirs. Flow rate was ramped up to 5 dynes/cm2 over the first 12 hours and then held constant for 24 hours. Finally, cells were lysed for RT-PCR analysis.
Statistical analysis
Experiments were performed in biological triplicates, unless otherwise stated. Data reported as biological replicated mean with error bars representing standard error. Statistical significance was determined by one-way ANOVA for multiple treatment experiments. Tukey’s HSD post hoc test was used determine which samples differed from each other only if ANOVA rejected the null hypothesis and returned p value < 0.05. Experiments with only two treatment groups were evaluated with an unpaired t-test to determine with significance considered at p < 0.05.
Results
Validation of ephrinB2-Fc and EphB4-Fc immobilized PEG hydrogels
To mimic ephrinB2 and EphB4 signaling, PEG hydrogels were synthesized and surface modified with either ephrinB2-Fc or EphB4-Fc. Proteins were reacted with heterobifunctional PEG acrylate linkers (PEGAc) via NHS ester chemistry (Fig. 1a). An increase in protein molecular weight by SDS-PAGE validates the addition of PEGAc (Fig. 1b). To investigate whether these modifications affected protein bioactivity, a ligand-receptor ephrinB2-EphB4 binding assay was preformed, comparing unmodified and PEGAc modified proteins (Fig. 1c). Both PEGAc modified proteins measure no significant change in binding efficiency compared to the positive control of unmodified ephrinB2-Fc/EphB4-Fc, but overall are still able to engage the affinity binding of ephrin-B2/EphB4 pairs. Negative control demonstrates limited non-specific protein adsorption to BSA blocked surface. EphrinB2-Fc-PEGAc and EphB4-Fc-PEGAc successfully conjugated to PEG diacrylate (PEGDA) hydrogel surface, illustrated using a photo patterning technique (Fig. 1d). To validate the stability of these bioactive hydrogels over time we monitored the presence of fluorescently tagged ephrinB2-Fc and EphB4-Fc after surface immobilization, and observed no loss of protein over two weeks (Fig. 1e). For simplicity, further studies refer to PEGDA hydrogels surface immobilized with ephrinB2-Fc-PEGAc or EphB4-Fc-PEGAc as ephrinB2-Fc gel or EphB4-Fc gel, respectively.
Figure 1. Development of bioactive ephrinB2-Fc/EphB4-Fc hydrogels for vascular differentiation.
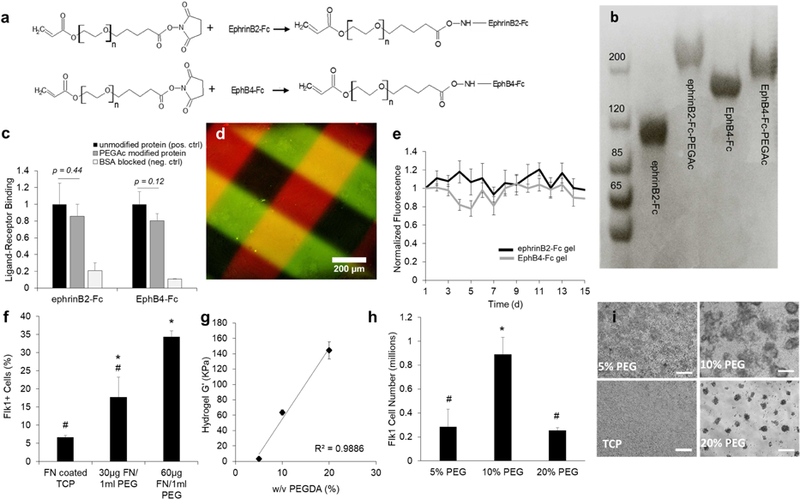
(a) Chemical reaction for modified recombinant ephrinB2-Fc/EphB4-Fc with PEG-acrylate linkers (PEGAc) via lysine targeted NHS chemistry. (b) Modified PEGAc proteins yield increased molecular weight compared to unmodified protein, illustrated with SDS PAGE. (c) EphrinB2-EphB4 ligand-receptor binding assay for unmodified (positive control) and PEGAc modified ephrinB2-Fc and EphB4-Fc (n=3) (unpaired t-test): EphrinB2-Fc protein samples measured binding efficiency to EphB4-Fc substrate; EphB4-Fc protein samples measured binding efficiency to ephrinB2-Fc substrate. Negative control used to measure non-specific protein absorption to BSA blocked surface (d) EphrinB2-Fc-PEGAc (red) and EphB4-Fc-PEGAc (green) capable of photo patterning on PEGDA hydrogels, scale bar 200µm. (e) Surface immobilized ephrinB2-Fc-PEGAc and EphB4-Fc-PEGAc remain intact on hydrogels surface over two weeks. Values are mean ± standard deviation of three individual hydrogels. (f) Higher fibronectin concentrations within the hydrogel lead to increased percentage of Flk1+ cells after seven-day differentiation with VEGF, bFGF2 and chir99021 media. Values are mean ± standard error of three biological replicates from flow cytometry analysis. *, # p<0.05 (ANOVA). (g) Hydrogel stiffness can be controlled by changing the PEGDA monomer concentration within the precursor gel solution. Values report G’ mean ± standard deviation from three individual hydrogel samples. (h) Number of sorted Flk1+ cells after day seven of differentiation on differing hydrogel stiffness with 60 μg/mL of fibronectin and in media containing VEGF, bFGF2 and chir99021. Values report mean ± standard error of three biological replicates from. *, # p<0.05 (ANOVA). (i) Phase contrast images represent mESC differentiation morphology on different hydrogel stiffness, scale bar 200 μm.
Optimizing hydrogel composition for vascular differentiation
To establish a PEGDA hydrogel composition that can support stem cell culture for vascular differentiation, different stiffness and fibronectin concentrations were investigated. PEGDA hydrogels were synthesized with different concentrations of fibronectin. Tissue culture plastic (TCP) coated with fibronectin was used as a control substrate. After seven days under differentiation media conditions, the percent of Flk1 positive cells was evaluated by flow cytometry. There was a significant increase in Flk1 population with hydrogels containing 60 µg/mL fibronectin concentration compared to 30 μg/mL and TCP control (Fig. 1f). Next, we investigating how altering the stiffness of the gel effected vascular differentiation. PEG hydrogels were synthesized at 5%, 10% and 20% (w/v) which resulted in stiffness moduli (G’) of 3.4 kPa, 64 kPa and 144 kPa, respectively (Fig. 1g). The highest number of Flk1+ cells was captured at 10% w/v (Fig. 1h). Phase contrast images illustrate representative cell growth on different hydrogel stiffness on day three of differentiation (Fig. 1i). In particular, the softest hydrogel, 5% w/v, showed strong cell attachment and a growth pattern similar to 2D surface. On the other hand, in the stiffest hydrogel tested, 20% w/v, mESCs did not spread on the substrate but formed 3D cell aggregates over time. Finally, the 10% w/v hydrogel substrate provided strong cell attachment and also promoted both 2D and 3D growth which led to the highest amount of Flk1+ cells. For remaining experiments, PEG hydrogels were synthesized at 10% w/v with 60 μg/mL fibronectin in order to support mESC culture and vascular differentiation.
Investigation of media compositions for arterial venous differentiation
Different growth factor combinations have been proven successful for endothelial differentiation of embryonic stem cells. In order to investigate which media composition allows for high vascular efficiency, we cultured mESCs on PEG hydrogel substrate (10% w/v with 60 μg/mL fibronectin) under different media compositions: 1) VEGF, bFGF2 and chir99021; 2) VEGF, bFGF2 and BMP4; or 3) VEGF, bFGF2, BMP4 and chir99021. After seven days of differentiation, cells were harvested, stained for Flk1 and analyzed by flow cytometry. All conditions were positive for vascular differentiation ranging from 30–50% efficiency (Supplemental Fig. S1). To evaluate if these soluble factors play a role in arterial venous specification, Flk1+ cells were sorted on seven day of differentiation and immediately lysed for gene expression analysis. Results illustrate a few arterial venous markers were affected by the media composition (Fig. 2a,b). Specifically, media containing VEGF, bFGF2 and chir99021 had higher expression of arterial marker Dll4, Notch4 (Fig. 2a), and venous marker COUP-TFII (Fig. 2b). VEGF, bFGF2 and BMP4 media had higher expression of arterial marker Hey1 and ephrinB2 (Fig. 2a). Although the addition of soluble BMP4 and/or chir99021 did influence arterial venous markers, overall these conditions were unable to create distinctly diverging arterial venous EC populations. Interestingly, mESC differentiation with media containing all soluble factors (VEGF, bFGF2, BMP4 and chir99021), did not upregulate any of the arterial venous markers that are influenced by BMP4 or chir99021 alone, suggesting the complex interaction of WNT and BMP pathway. The method of simply adding or subtracting soluble factors are unlikely to drive the distinct arterial venous differentiation.
Figure 2. Media composition and bioactive hydrogel regulate arterial venous differentiation.
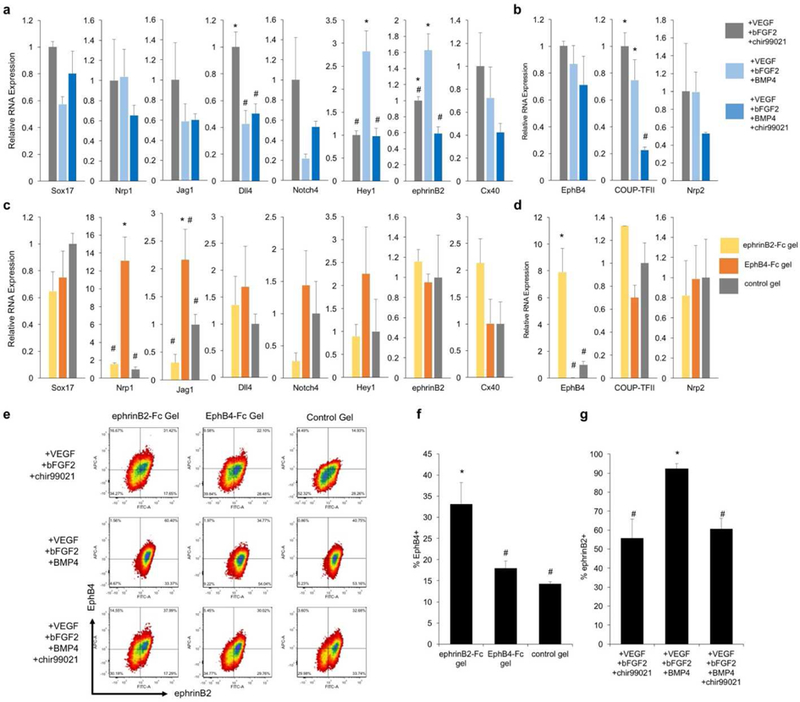
(a,b) Relative gene expression of Flk1+ sorted cell population differentiated on control gel in three media conditions: VEGF, bFGF2 and chir99021 media (grey), VEGF, bFGF2 and BMP4 media (light blue) or VEGF, bFGF2, BMP4 and chir99021 media (dark blue) for (a) arterial and (b) venous marker expression. RNA expression is relative to VEGF, bFGF2 and chir99021 media condition. Values are mean ± standard error of three biological replicates. *, # p <0.05 (ANOVA). (c,d) Relative gene expression of Flk1+ sorted cell population derived from ephrinB2-Fc gel (yellow), EphB4-Fc gel (orange) or control gel (grey) combined with VEGF, bFGF2 and chir99021 media for (c) arterial and (d) venous markers. RNA expression values are relative to control gel. Values are mean ± standard error of three biological replicates. *, # p<0.05 (ANOVA). (e) Representative FACS plots of Flk1+ sorted populations stained for ephrinB2 (x-axis) and EphB4 (y-axis) illustrating the combinations of three hydrogels and three media compositions. (f) Percent of EphB4+ cells within Flk1+ population derived from ephrinB2-Fc, EphB4-Fc or control gel with VEGF, bFGF2, chir99021 media. Values are mean ± standard error of three biological replicates obtained from FACs analysis. *, # p<0.05 (ANOVA). (g) Percent of ephrinB2+ cells within Flk1+ sorted derived from VEGF, bFGF2, chir99021 media, VEGF, bFGF2, BMP4 media, or VEGF, bFGF2, BMP4, chir99021 media on control gels. Values are mean ± standard error of three biological replicates collected from FACs analysis. *, # p<0.05 (ANOVA).
EphrinB2-Fc and EphB4-Fc hydrogels affect vascular and phenotypic differentiation
Next, we wanted to evaluate if ephrinB2/EphB4 signaling played a role in arterial venous EC phenotypes. First, for comparison, we cultured HUVECs on ephrinB2-Fc or EphB4-Fc gels, and found they have little effect to modulate arterial venous marker expression (Supplemental Fig. S3), suggesting that mature ECs do not respond to these signals to change their arterial venous specification. We then cultured mESCs on ephrinB2-Fc gel, EphB4-Fc gel or control gel. Flk1+ cells were sorted on day seven of differentiation and analyzed by qPCR. Noticeably, several markers are differentially regulated under ephrinB2-Fc gel or EphB4-Fc gel. Specifically, EphB4-Fc gel upregulated arterial markers Nrp1, Jag1 (Fig. 2c). Conversely, ephrinB2-Fc gel resulted in higher expression of venous marker EphB4 (Fig. 2d). Importantly, these alterations of gene expression were not observed when soluble ephrinB2-Fc/EphB4-Fc was added directly to the media (Supplemental Fig. S4), suggesting immobilization of ephrinB2/EphB4 signals is critical for its activity. To further examine the effect of ephrinB2/EphB4 gel on arterial venous specification, sorted Flk1+ populations were evaluated for cell membrane ephrinB2/EphB4 protein expression by flow cytometry. It is clear that ephrinB2-Fc gel resulted in an upward shift of the Flk1+ cells towards more EphB4+. This upregulation is evident under all three media compositions (Fig. 2e,f). On the other hand, EphB4 gel has no such effect. From the ephrinB2/EphB4 plots, we also found that media compositions influence ephrinB2 protein expression, in agreement with gene expression measurements (Fig. 2a). In particular, media containing VEGF, bFGF2 and BMP4 shift the cell population toward more ephrinB2+ expression, in all three gel conditions (Fig. 2e,g).
Selection of Flk1 arterial venous EC progenitor populations
From these results, it appears that the hydrogel substrate and media composition have an independent effect on ephrinB2/EphB4 expression: ephrinB2 gel influences EphB4 expression while BMP4 media increases ephrinB2 expression. In order to select two Flk1+ populations with diverging arterial venous phenotypes, we choose the combination of media composition and hydrogel substrate to specifically control the expression of ephrinB2 and EphB4. Based on our new understanding of BMP4/chir99021 and immobilized ephrinB2-Fc/EphB4-Fc signaling, we choose Flk1 populations created from stem cell differentiated on ephrinB2-Fc gel with VEGF, bFGF2, chir99021 media and EphB4-Fc gel with VEGF, bFGF2 and BMP4 media to enrich venous and arterial populations, respectively. These two populations demonstrate arterial venous diverging profiles (Fig. 3a-c) and different molecular ephrinB2/EphB4 profiles (Fig. 3d). Although both sorted populations contain cells that are both ephrinB2+ EphB4+ and cells that express neither (Fig. 3d), EphB4-Fc gel with VEGF, bFGF2, BMP4 media enriched the arterial population (ephrinB2+EphB4-) from 17.65% to 54.04%, and the ephrinB2-Fc gel with VEGF, bFGF2, chir99021 media has an enriched the venous population (ephrinB2-EphB4+) from 1.97% to 16.67%. These two populations also exhibit diverging gene expression profile characteristic of typical arterial and venous ECs. The arterial enriched population has higher expression of arterial marker Nrp1, ephrinB2 and lower expression of venous markers EphB4 and COUP-TFII, compared to the venous enriched population (Fig. 3a,b). These cell populations have similar general EC marker gene expression such as Flk1 and vWF (Fig. 3c), confirming vascular lineage specific differentiation. Moreover, both populations illustrated typical functions of generic ECs including angiogenic tube-like extensions in matrigel tube forming assay (Fig. 3e), cellular uptake of acetylated LDL (Fig. 3f), and expression of VE-Cadherin, a mature endothelial marker (Fig. 3g).
Figure 3. Selected Flk1+ populations demonstrate diverging arterial venous profiles.
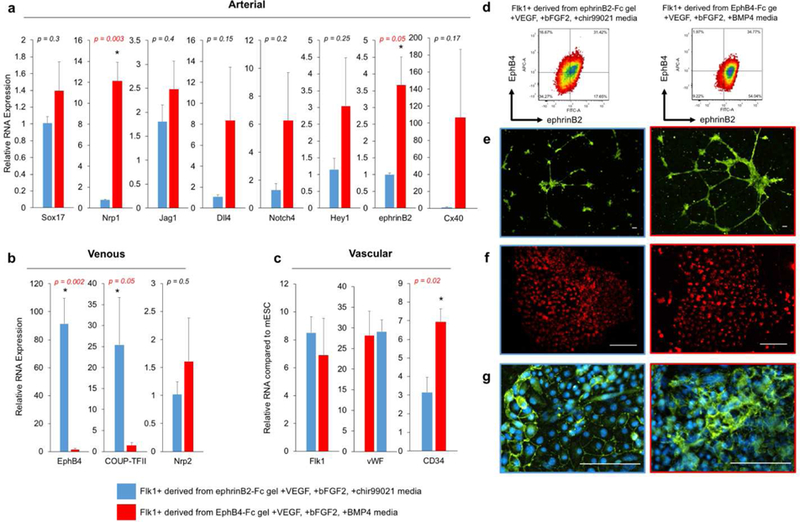
(a,b) Relative gene expression for (a) arterial and (b) venous markers from Flk1+ populations derived from ephrinB2-Fc gel with VEGF, bFGF2, chir99021 (blue) or EphB4-Fc gel with VEGF, bFGF2, BMP4 (red). Values are mean ± standard error of three or more biological replicates. * p<0.05 (unpaired t-test). (c) Relative gene expression of general EC markers for selected Flk1+ populations expressed as folds of increase from undifferentiated mESC. Values are mean ± standard error of three or more biological replicates from independent experiments. * p<0.05 (paired t-test). (d) Representative FACs plots of ephrinB2 (x-axis) and EphB4 (y-axis) of the selected Flk1+ populations. (e) Selected Flk1+ populations in tube formation assay in matrigel using calcein-AM staining (green). (f) Selected Flk1+ populations are positive for LDL uptake using fluorescently labeled acetylated-LDL (red). (g) Immunofluorescent staining of mature EC marker VE-Cadherin (green) and DAPI (blue). All scale bars represent 200 μm.
To further validate phenotypic differences between these two endothelial progenitor populations, we analyzed genes related to the functional characteristics of arterial vs. venous ECs. Previously in our lab, it has been shown that besides the known arterial venous markers, adult ECs have distinct gene expression profiles related to vascular diseases. Specifically, venous ECs have a higher anti-thrombotic profile. On the other hand, arterial ECs have a higher pro-atherogenic and osteogenic genetic profile44. We observed similar trends within our mESC derived arterial venous enriched EC progenitor populations. Anti-thrombotic markers, were not significantly different between venous enriched EC progenitors and arterial enriched population (Fig. 4a). However, arterial enriched EC progenitors had higher expression of pro-atherogenic genes such as BMP4 and ACE (Fig. 4b), as well as higher osteogenic genes, namely RUNX2, SP7, ALPL, SPP1 and BMP4 (Fig. 4c). To further examine the osteogenic functional differences between the arterial venous progenitor populations, we exposed both sorted populations to osteogenic differentiation media for seven days. The cells were then probed for calcium deposition (Alizarin Red) and alkaline phosphatase (ALP) (Fig. 4d,e). Arterial enriched progenitors stained strongly for both markers compared to venous enriched population (Fig. 4d-e).
Figure 4. Selected Flk1+ populations have distinct functional markers and osteogenic potential.
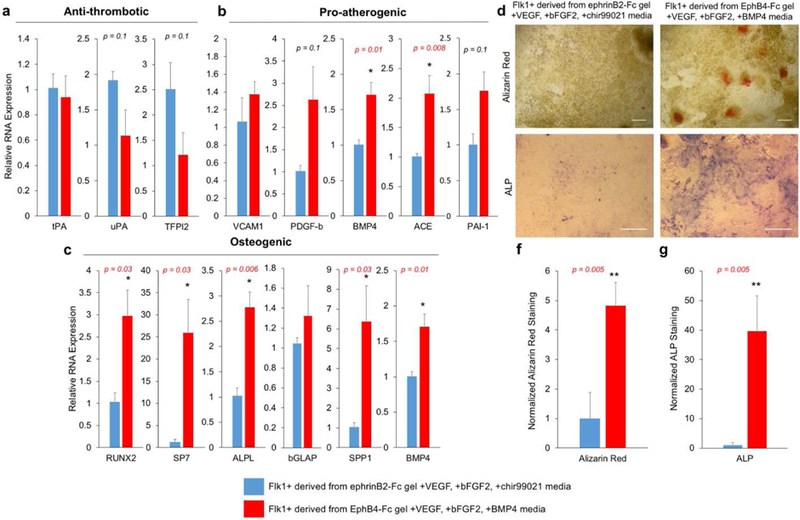
(a-c) Relative gene expression for (a) anti-thrombotic, (b) pro-atherogenic and (c) osteogenic gene markers comparing Flk1+ derived from ephrinB2-Fc gel with VEGF, bFGF2 and chir99021 (blue) and Flk1+ from derived EphB4-Fc gel with VEGF, bFGF2 and BMP4 (red). Values are mean ± standard error of three biological replicates. * p<0.05 (unpaired t-test). (d) Alizarin Red staining of calcium deposition (top) and alkaline phosphatase (ALP) staining (bottom) images after seven days of osteogenic conditioning of Flk1+ selected populations. (e) Image analysis of Alizarin Red staining (left) and ALP (right) comparing Flk1+ selected populations derived from ephrinB2-Fc gel with VEGF, bFGF2 and chir99021 (blue) and EphB4-Fc gel with VEGF, bFGF2 and BMP4 (red). Values are obtained from color thresholding and reported as mean ± standard error of three independent images randomly selected. * p<0.05 (unpaired t-test). Scale bars represent 200 µm.
Arterial and venous progenitor ECs respond differently to flow conditions
To evaluate how these diverging arterial and venous EC progenitor populations are influenced by shear stress, Flk1+ cells were introduced to shear stress at 5 dyne/cm2. After 24 hours, EC progenitors under flow and static conditions were lysed for gene analysis. Arterial enriched EC progenitors under flow increased arterial markers Jag1 and Notch4 (Fig. 5b). However, other arterial markers were downregulated including Sox17, Hey1 and Cx40 (Fig. 5b). No significant changes in venous genes were observed under flow conditions (Fig. 5c). Phase contrast images illustrate EC progenitors under static and flow conditions (Fig. 5d).
Figure 5. Selected Flk1+ populations respond differently to flow condition.
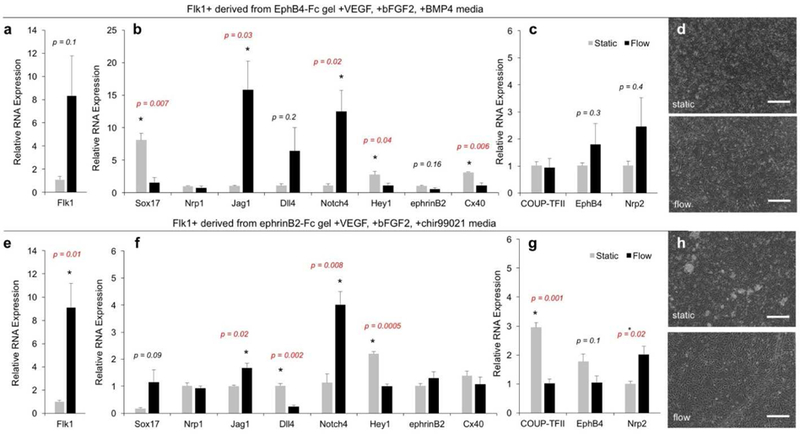
(a-c) Relative gene expression for (a) vascular, (b) arterial and (c) venous markers comparing static culturing (grey) to flow conditioning at 5 dynes/cm2 for 24 hours using Flk1+ cells derived from EphB4-Fc gel with VEGF, bFGF2, BMP4. Values are mean ± standard error of three biological replicates. * p<0.05 (unpaired t-test). (d) Representative phase contrast images of static (top) and flow (bottom), scale bar represents 200 μm. (e-g) Relative gene expression for (e) vascular, (f) arterial and (g) venous markers comparing static and flow conditioning (5 dynes/cm2 for 24 hours) of Flk1+ population derived from ephrinB2-Fc gel with VEGF, bFGF2, chir99021. Values are mean ± standard error of three biological replicates. * p<0.05 (unpaired t-test). (d) Representative phase contrast images of static (top) and flow (bottom) conditions. Scale bar represents 200 µm.
Venous enriched EC progenitors exposed to shear stress also resulted in an increase of general vascular marker, Flk1 (Fig. 5e) and arterial markers Jag1 and Notch4 (Fig. 5f). Again, some arterial markers were also down regulated such as Dll4 and Hey1 (Fig. 5f). Unlike the arterial enriched population, venous enriched progenitors exposed to flow did have changes in their gene expression. Specifically, venous enriched progenitors exposed to flow downregulated venous markers COUPTFII and upregulated Nrp2 (Fig. 5g). Phase contrast images show venous enriched EC progenitors under static and flow conditions (Fig. 5h). Taken together, our data illustrates hemodynamic flow can both upregulate and downregulate arterial markers and is providing no clear direction toward the arterial profile. This suggests that hemodynamic flow alone cannot drive the distinct arterial phenotype.
EphrinB2-Fc gel enhances EphB4 expression via EphB4 receptor
To further our understanding into the ephrinB2-Fc signaling, we generated a time course over of gene expression seven day differentiation. We probed for stem cell marker, Oct-4, mesoderm marker, Brachyury, and vascular marker, Flk1. In both ephrinB2-Fc gel and control gel groups, Oct-4 was reduced over time, Brachyury was dynamically upregulated and Flk1 expression increased over time (Fig. 6a). Dissimilarly, mESCs differentiated on ephrinB2-Fc gel expressed highest Brachyury levels on day two contrary to mESCs differentiated on the control gel where Brachyury expression peaked on day three. This data suggests that ephrinB2-Fc gel leads to an earlier mesoderm commitment. To confirm this observation, we immobilized different ephrinB2-Fc concentrations and observed Brachyury expression on day two and day three of differentiation (Fig. 6b). The results showed that higher concentration of ephrinB2-Fc led to higher Brachyury expression on day two, but lower Brachyury expression on day three, suggesting that higher concentration of ephrinB2-Fc correlates earlier mesoderm differentiation as compared to control which occurs later in day three. Furthermore, vascular differentiation was enhanced on day seven when cultured on ephrinB2-Fc gel compared to the control gel. EphrinB2-Fc gel promoted significantly higher expression of vascular markers including Flk1, CD34 and VE-Cadherin (Fig. 6c). Finally, we observed a linear dose response of EphB4 expression on day seven of differentiation with respect to ephrinB2-Fc gel immobilization concentration (Fig. 6d). This further supports the hypothesis that ephrinB2-Fc gel enhances EphB4 expression.
Figure 6. EphrinB2-Fc gel acts via EphB4 to regulate vascular differentiation.
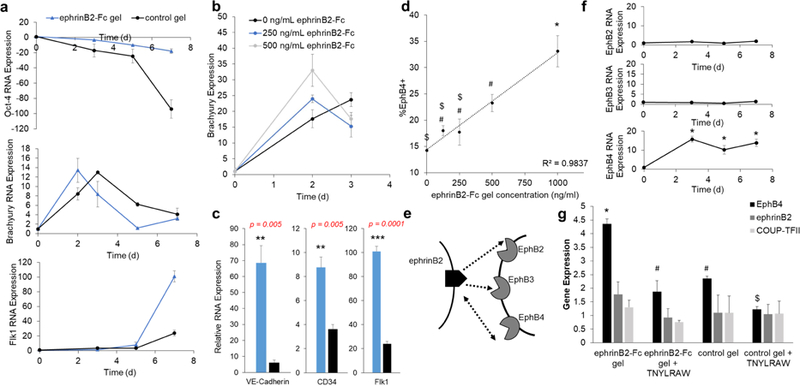
(a) Gene expression time course of Oct-4 (top), Brachyury (middle) and Flk1 (bottom) for mESC differentiated on ephrinB2-Fc gel (250 ng/mL, blue) and control gel (black) with VEGF, bFGF2 and chir99021 media. Values are mean ± standard error of three biological replicates. (b) Brachyury RNA expression profiles of mESCs differentiated on 0 ng/mL (black), 250 ng/mL (blue) and 500 ng/mL(grey) immobilized ephrinB2-Fc gel with VEGF, bFGF2 and chir99021 media. Values are mean ± standard error of three biological replicates. (c) Seven-day differentiation results in expression of VE-Cadherin (left), CD34 (middle) and Flk1 (left) for ephrinB2-Fc gel (250 ng/mL, blue) and control gel (black). Values are mean ± standard error of three biological replicates. * p<0.05; ** p<0.005; *** p<0.0005 (unpaired t-test). (d) Dose dependent expression of percent EphB4+ expression within Flk1+ population after seven-day differentiated with VEGF, bFGF2 and chir99021 media. Values are mean ± standard error of three biological replicates. *, #, $ p<0.05 (ANOVA). (e) Schematic illustrating potential interactions between ephrinB2 and EphB receptors. (f) Gene expression time course of Eph receptors: EphB2 (top), EphB3 (middle), EphB4 (bottom) gene expression relative to mESC over seven-day differentiation cultured on control gel with VEGF, bFGF2 and chir99021 media. Values are mean ± standard error of three biological replicates. * p<0.05 (unpaired t-test). (g) RNA expression on day three of mESC differentiation cultured on ephrinB2-Fc gel (250 ng/mL) or control gel with or without TNYLRAW blocking peptide (50μM) for EphB4, ephrinB2 and COUP-TFII genes. Values are mean ± standard error of three biological replicates. *, #, $ p < 0.05 (ANOVA).
EphrinB2 can interact with three different Eph receptors: EphB2, EphB3 and EphB4 while receptor EphB4 only receives signals from ligand ephrinB24 (Fig. 6e). To investigate which receptor ephrinB2-Fc hydrogel is activating to elicit upregulation of EphB4, we observed time course of EphB2, EphB3 and EphB4 over seven day of differentiation. While there was very little change in EphB2 and EphB3 expression overtime, EphB4 increased early and remained upregulated (Fig. 6f). Therefore, we hypothesized that ephrinB2-Fc is acting through EphB4 receptor. Using our new knowledge into ephrinB2 signaling and mesoderm transition, we predict this interaction is taking place early during the differentiation process. To validate this hypothesis, we performed a receptor blocking experiment by adding soluble EphB4 blocking peptide, TNYLRAW (50μM), to differentiating mESCs on ephrinB2-Fc gel or control gel. Results showed at day three of the differentiation ephrinB2-Fc gel enhanced overall mESC EphB4 RNA expression, however, in the presence of EphB4 blocking peptide this upregulation was no longer observed (Fig. 6g). Previously, we found ephrinB2-Fc gel did not alter gene expression of ephrinB2 or COUP-TFII and therefore these genes were probed as a negative control. As expected, we did measure any change across treatment groups (Fig. 6g). From this data, we believe the ephrinB2-Fc gel is acting through the EphB4 receptor to upregulate EphB4 gene expression.
Compared to gene profiles of adult ECs and other common phenotypic modulating approaches
Finally, we compared our arterial venous enriched EC progenitor populations to in vivo arterial and venous EC gene profiles as well as other common approaches for deriving arterial ECs such as introducing shear stress and high VEGF concentrations (Fig. 7). In vivo, arterial ECs have higher expression of arterial markers, pro-atherogenic and osteogenic genes, while venous ECs have higher expression venous markers and anti-thrombotic genes. Our arterial and venous enriched EC progenitor populations also demonstrated diverging genetic profile similar to those in vivo features. Importantly, media alone (BMP4 or chir99021) nor ephrinB2-Fc/EphB4 gels does not give this diverging functional profiles (Fig. 7). Also, it is evident that both flow shear stress (Supplemental Fig. S6) and high VEGF treatment (Supplemental Fig. S7) does not lead to phenotypic profiles that resemble the in vivo characteristics (Fig. 7), suggesting that the definitive arterial or venous ECs cannot be achieved by adding shear stress or VEGF alone.
Figure 7. Gene expression heat maps comparing different arterial venous ECs differentiation approaches to in vivo arterial venous EC profiles.
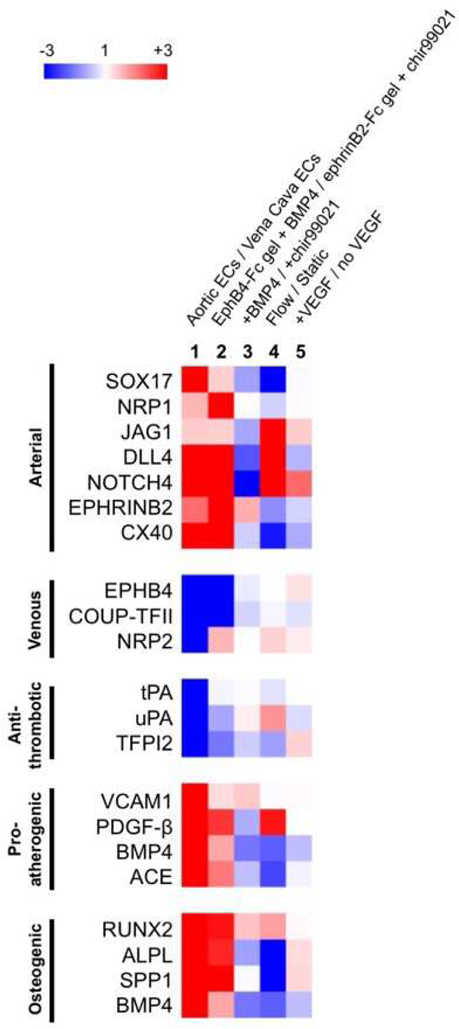
Relative comparison of fold increase (red) or decrease (blue) expression comparing two EC populations. Lane 1: positive control illustrating gene profiles of adult artery ECs (Aorta) compared to adult vein ECs (Vena Cava). Lane 2: Flk1+ derived from EphB4-Fc gel VEGF, bFGF2, BMP4 media compared to Flk1+ derived from ephrinB2-Fc gel VEGF, bFGF2, chir99021 media. Lane 3: Flk1+ derived from VEGF, bFGF2 and BMP4 media compared to Flk1+ derived from VEGF, bFGF2 and chir99021 media. Lane 4: Flk1+ sorted population exposed to 5 dynes/cm2 flow vs. static conditioning. Lane 5: Flk1+ sorted population treated with 30 ng/mL VEGF compared to 0 ng/mL.
Discussion
In this study, we investigated the role of ephrinB2/EphB4 signaling as well as the influence of soluble factors for arterial venous specification. We created a biomaterial approach for differentiating arterial venous EC progenitor populations from pluripotent stem cells. First, we showed that we can promote vascular differentiation by manipulating fibronectin concentration and hydrogel stiffness. Other researchers have also selected fibronectin increases vascular differentiation compared to other ECM adhesion proteins14. Although we observed hydrogel stiffness to enhance vascular differentiation, we believe the real effector was the cell-cell, cell-matrix interaction and fibronectin availability to mESCs. We propose 10% PEGDA gel promoted the most Flk1+ cells because mESCs grew in both 2D and 3D, providing full interactions between cell-cell and cell-fibronectin to drive a more efficient differentiation, whereas 5% or 20% PEGDA gels supported only either 2D monolayer or aggregate as 3D spheroid, respectively.
We also investigated different combinations of soluble factors, specifically BMP4 and small molecule Wnt agonist, chir99021. We demonstrated addition or absence of BMP4 and/or chir99021 influenced arterial venous phenotypic gene expression within the Flk1 population. Particularly, BMP4 or chir99021 can modulate certain arterial venous markers, but not all. We also observed ephrinB2 protein expression was effected by the media composition. We do understand that BMP4 and Wnt pathway have been implicated in mesoderm and vascular differentiation45–52, but the role of BMP4 and Wnt pathways with respect to arterial venous specification has not been studied. Further studies are required to better understand their individual involvement in arterial venous specification. It is clear that using different soluble factors within EC differentiation protocols is changing the phenotype of the resulting EC population.
Additionally, we discovered ephrinB2/EphB4 signaling can influence endothelial phenotypic specification. Specifically, immobilized ephrinB2-Fc was able to enhance overall vascular differentiation and increase expression of EphB4. On the other hand, EphB4-Fc gel increased arterial marker Nrp1 and Jag1 expression.
Mechanistically, our data suggests that the ephrinB2-Fc gel is acting through the EphB4 receptor and this signaling effects the timing of mesodermal transition during differentiation. Specifically, mESCs cultured on ephrinB2-Fc gel expressed mesoderm marker Brachyury earlier than the control gel. These results further support previously published work that found without EphB4 expression, mESCs had a delay in Brachyury and Flk1 expression during vascular differentiation in vitro53. In cardiac differentiation, EphB4 signal has also been studied as a regulator of early vascular lineage commitment54. Besides its effect on the mesoderm and vascular differentiation, ephrinB2-Fc gel also has a robust effect on EphB4 expression in Flk1+ cells. It is known that ephrinB2 and EphB4 are expressed in the early arterial and venous progenitors and their interaction is critical for proper vascular network formation2. This raises an interesting question on whether ephrinB2 expressed in arterial ECs can further enhance the venous EC phenotype by upregulating its EphB4 expression in vivo. Further studies are warranted to validate this hypothesis.
Overall, we identified two novel treatment conditions that provide diverging arterial venous EC populations. Specifically, EphB4-Fc hydrogel with soluble VEGF, bFGF2 and BMP4 and ephrinB2-Fc hydrogel with soluble VEGF, bFGF2 and chir99021 were able to enrich arterial and venous Flk1 progenitor ECs, respectively (Fig. 8). Not only do these two populations have distinctive arterial venous gene expression, they also display anti-thrombotic, pro-atherogenic and osteogenic profiles similar to those found in artery and vein ECs in vivo. In particular, arterial enriched EC progenitors expressed higher levels of pro-atherogenic and osteogenic genes, while venous EC progenitors expressed higher levels of anti-thrombotic genes. Interestingly, researchers studying osteogenic differentiation have found benefits of over expressing ephrinB2 in stem cells to increase osteogenic differentiation40. Additionally, recent studies in out lab have identified COUP-TFII as the critical regulator of the EC phenotypic profile related to anti-thrombotic, pro-atherogenic and osteogenic functions44. In this work, our arterial venous enriched progenitor populations have significantly different COUP-TFII expression, which may be responsible for the differential phenotypic profile observed in this study.
Figure 8. Proposed signaling to create diverging Flk1+ populations.
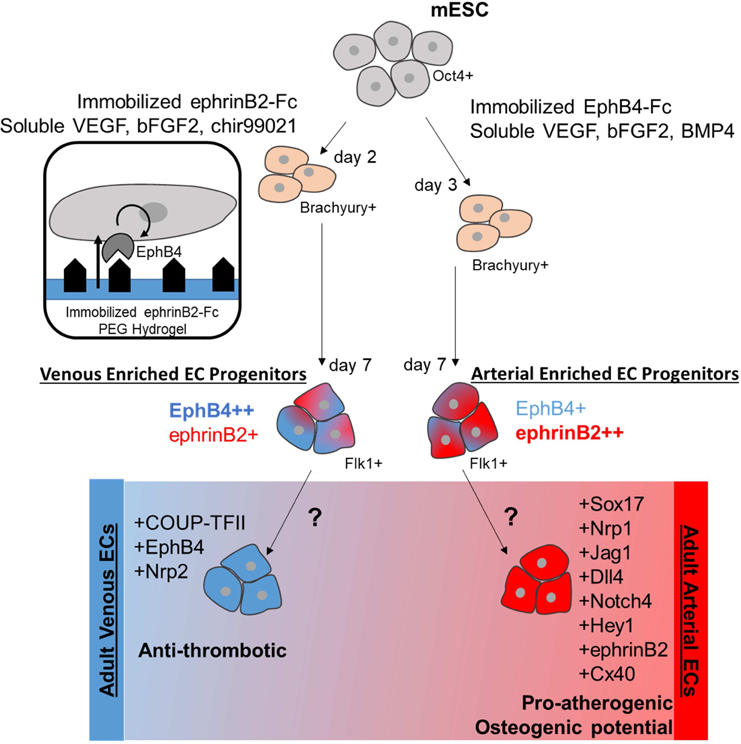
EphrinB2-Fc immobilized signal with media containing VEGF, bFGF2 and chir99021 media results in Flk1+ population enriched venous phenotype, specifically ephrinB2-Fc interacting with EphB4 receptor to upregulate EphB4 expression EphB4-Fc immobilized signal combine with VEGF, bFGF2 and BMP4 signals produces an arterial enriched Flk1+ population. Further signaling is required to differentiate committed and fully distinct adult arterial and venous ECs.
Previous studies have suggested that dynamic flow culture conditioning can modulate the arterial phenotype, specifically we anticipated shear stress induction of notch activation markers, including Jag1, Dll4 and Notch4 15, 31. As expected, our ephrinB2 enriched EC progenitor populations were able to increase Notch related markers, including Jag1 and Notch4. Nevertheless, some other arterial markers were downregulated and there was limited response from venous markers. In particular, arterial enriched EC progenitor population saw no change in venous markers in response to flow while venous enriched EC progenitor population showed certain venous markers were downregulated. Some previous studies have reported no change in venous markers when shear stress is applied15 while others have observed downregulation of venous markers in response to flow31. We speculate these discrepancies may be a result of different phenotypes of EC populations been studied, suggesting the importance of phenotypic evaluation for stem cell derived ECs. Aside from regulating several Notch related factors, we found flow alone cannot derive an EC population that resemble the in vivo phenotypic profile. This data suggests that flow, in combination of some other developmental signals, are necessary to drive the full arterial specification.
Over time, the EC progenitor populations do exhibit plasticity in their phenotypic gene expression. In particular, post sort culturing of Flk1 populations results in phenotypic markers drifting over time (Supplemental Fig. S8), with loss of some arterial markers after two weeks. This is expected as blood flow starts after the early arterial venous markers are established, but this is missing in the experimental setup. This is consistent with previous studies demonstrating EC phenotypical plasticity and the timely initiation of blood flow is critical to maintain the arterial phenotype55. These data support the notion that arterial venous fate decision is pre-determined before the onset of flow, but environmental cues such as blood flow play indispensable roles in maintaining the cell fate decision later on56–61. Overall, our data demonstrates the role of ephrin-B2/EphB4 signal, BMP4/Wnt pathway and biomechanical flow on arterial venous differentiation. We believe proper arterial venous specification may require precise combination of these signals and other signals at the right timing.
Taken together, these data suggest that our current understanding of the arterial venous differentiation process is very limited, and further fine-tuning of the developmental signals is necessary in order to derive functional arterial venous ECs in the future.
Conclusion
Our combinatory biomaterial approach demonstrates a new method to differentiate phenotypic specific EC populations from pluripotent stem cells. We propose that both ephrinB2/EphB4 bidirectional signaling and Wnt/BMP4 pathways play roles in the specification of arterial venous endothelial phenotypes. Our developed arterial venous enriched ECs progenitor populations possess phenotypic and functional gene profiles similar to adult artery and vein ECs. Furthermore, these populations provided more distinct phenotypic and functional distinction compared to other common methods, including introduction of shear stress or modulating VEGF concentration. Our data suggest ephrinB2 signaling occurs via the EphB4 receptor and plays a role in the timing of the mesodermal transition. Moving forward, bioactive biomaterials are a promising approach for mimicking immobilized signals such as Sonic Hedgehog and Notch that occur during development to control stem cell fate. Improvements in differentiating phenotypic specific ECs has great potential to aid our understanding of vascular development, arterial venous disease susceptibility and give rise to new function-specific ECs for tissue engineering applications.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported mainly by grants from American Heart Association Scientist Development Grant (12SDG12050083 to G.D.), National Institute of Health (R21HL102773, R01HL118245 to G.D.) and National Science Foundation (CBET-1263455, CBET-1350240 to G.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
- The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
- 1.Aird WC Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100, 174–190 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Wang HU, Chen ZF & Anderson DJ Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Shin D et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol 230, 139–150 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Gale NW et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol 230, 151–160 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Chong DC, Koo Y, Xu K, Fu S & Cleaver O Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn 240, 2153–2165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo FA et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arteriosclerosis, thrombosis, and vascular biology 27, 1562–1571 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Muto A et al. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. The Journal of experimental medicine 208, 561–575 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita J et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92–96 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Ferreira LS et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res 101, 286–294 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Levenberg S, Ferreira LS, Chen-Konak L, Kraehenbuehl TP & Langer R Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nature Protocols 5, 1115–1126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James D et al. Lentiviral Transduction and Clonal Selection of hESCs with Endothelial-Specific Transgenic Reporters. Current protocols in stem cell biology Chapter 1, Unit1F.12 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Hu S, Ghosh Z, Han Z & Wu JC Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev 20, 1701–1710 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang P-M & Wong PC Differentiation of an embryonic stem cell to hemogenic endothelium by defined factors: essential role of bone morphogenetic protein 4. Development 138, 2833–2843 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blancas AA, Shih AJ, Lauer NE & McCloskey KE Endothelial cells from embryonic stem cells in a chemically defined medium. Stem Cells Dev 20, 2153–2161 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivarapatna A et al. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials 53, 621–633 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa M et al. Derivation of endothelial cells from human embryonic stem cells in fully defined medium enables identification of lysophosphatidic acid and platelet activating factor as regulators of eNOS localization. Stem cell research 10, 103–117 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Glaser DE et al. Functional Characterization of Embryonic Stem Cell-Derived Endothelial Cells. Journal of vascular research 48, 415–428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchand M et al. Concurrent generation of functional smooth muscle and endothelial cells via a vascular progenitor. Stem Cells Transl Med 3, 91–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurugi-Kobayashi T et al. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arteriosclerosis, thrombosis, and vascular biology 26, 1977–1984 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Narazaki G et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation 118, 498–506 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Kane NM et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol 30, 1389–1397 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Pick M, Azzola L, Mossman A, Stanley EG & Elefanty AG Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells 25, 2206–2214 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Sumi T, Tsuneyoshi N, Nakatsuji N & Suemori H Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Rufaihah AJ et al. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. American journal of translational research 5, 21–35 (2013). [PMC free article] [PubMed] [Google Scholar]
- 25.Kitajima K et al. GSK3beta inhibition activates the CDX/HOX pathway and promotes hemogenic endothelial progenitor differentiation from human pluripotent stem cells. Exp Hematol 44, 68–74 e61–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao X et al. Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res 15, 122–129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto K et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol 288, H1915–1924 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Yamamizu K et al. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. The Journal of Cell Biology 189, 325–338 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita JK Differentiation of arterial, venous, and lymphatic endothelial cells from vascular progenitors. Trends Cardiovasc Med 17, 59–63 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Lanner F, Sohl M & Farnebo F Functional arterial and venous fate is determined by graded VEGF signaling and notch status during embryonic stem cell differentiation. Arterioscler Thromb Vasc Biol 27, 487–493 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Obi S et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol 106, 203–211 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Masumura T, Yamamoto K, Shimizu N, Obi S & Ando J Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arteriosclerosis, thrombosis, and vascular biology 29, 2125–2131 (2009). [DOI] [PubMed] [Google Scholar]
- 33.You LR et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98–104 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Oike Y et al. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood 100, 1326–1333 (2002). [PubMed] [Google Scholar]
- 35.Lisabeth EM, Falivelli G & Pasquale EB Eph receptor signaling and ephrins. Cold Spring Harbor Perspectives in Biology 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasquale EB Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Ashton RS et al. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nature neuroscience 15, 1399–1406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conway A et al. Multivalent ligands control stem cell behaviour in vitro and in vivo. Nature Nanotechnology (2013). [DOI] [PMC free article] [PubMed]
- 39.Zhao C, Irie N, Takada Y, Shimoda K & Miyamoto T. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell metabolism (2006). [DOI] [PubMed]
- 40.Tierney EG et al. High levels of ephrinB2 over-expression increases the osteogenic differentiation of human mesenchymal stem cells and promotes enhanced cell mediated mineralisation in a polyethyleneimine-ephrinB2 gene-activated matrix. J Control Release 165, 173–182 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Hayashi S. i., Asahara T, Masuda H, Isner JM & Losordo DW Functional ephrin-B2 expression for promotive interaction between arterial and venous vessels in postnatal neovascularization. Circulation 111, 2210–2218 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Lawson ND & Weinstein BM Arteries and veins: making a difference with zebrafish. Nat Rev Genet 3, 674–682 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Germain S & Eichmann A VEGF and ephrin-B2: a bloody duo. Nat Med 16, 752–754 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Cui X et al. Venous Endothelial Marker COUP-TFII Regulates the Distinct Pathologic Potentials of Adult Arteries and Veins. Scientific reports 5, 16193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldman O et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells 27, 1750–1759 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Park S-W et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood 116, 5762–5772 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Bai H et al. BMP4 regulates vascular progenitor development in human embryonic stem cells through a Smad-dependent pathway. Journal of cellular biochemistry 109, 363–374 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang D-H et al. Wnt5a is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving both Wnt/beta-catenin and protein kinase Calpha. Circulation Research 104, 372–379 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Corada M et al. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Developmental Cell 18, 938–949 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Umeda K & Nakayama N Collaboration between WNT and BMP signaling promotes hemoangiogenic cell development from human fibroblast-derived iPS cells. Stem cell research 4, 223–231 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Lian X et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America 109, E1848–1857 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lian X et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports 3, 804–816 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z et al. Ephrin receptor, EphB4, regulates ES cell differentiation of primitive mammalian hemangioblasts, blood, cardiomyocytes, and blood vessels. Blood 103, 100–109 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Chen K et al. EphB4 forward-signaling regulates cardiac progenitor development in mouse ES cells. J Cell Biochem 116, 467–475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.le Noble F et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 131, 361–375 (2004). [DOI] [PubMed] [Google Scholar]
- 56.dela Paz NG & D’Amore PA Arterial versus venous endothelial cells. Cell Tissue Res 335, 5–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swift MR & Weinstein BM Arterial-venous specification during development. Circulation Research 104, 576–588 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Kume T Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol 25, 637–646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Bras A, Vijayaraj P & Oettgen P Molecular mechanisms of endothelial differentiation. Vasc Med 15, 321–331 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Jones EA The initiation of blood flow and flow induced events in early vascular development. Semin Cell Dev Biol 22, 1028–1035 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Atkins GB, Jain MK & Hamik A Endothelial differentiation: molecular mechanisms of specification and heterogeneity. Arteriosclerosis, thrombosis, and vascular biology 31, 1476–1484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


