Abstract
Hematological malignancies express high levels of CD47 as a mechanism of immune evasion. CD47-SIRPα triggers a cascade of events that inhibit phagocytosis. Preclinical research supports several models of antibody-mediated blockade of CD47-SIRPα resulting in cell death signaling, phagocytosis of cells bearing stress signals, and priming of tumor-specific T cell responses. Four different antibody molecules designed to target the CD47-SIRPα interaction in malignancy are currently being studied in clinical trials: Hu5F9-G4, CC-90002, TTI-621, and ALX-148. Hu5F9-G4, a humanized anti-CD47 blocking antibody is currently being studied in four different Phase I trials. These studies may lay the groundwork for therapeutic bispecific antibodies. Bispecific antibody (CD20-CD47SL) fusion of anti-CD20 (Rituximab) and anti-CD47 also demonstrated a synergistic effect against lymphoma in preclinical models. This review summarizes the large body of preclinical evidence and emerging clinical data supporting the use of antibodies designed to target the CD47-SIRPα interaction in leukemia, lymphoma and multiple myeloma.
Keywords: CD47, immunotherapy, apoptosis, phagocytosis, leukemic stem cell, monoclonal antibody, hematologic malignancy
Introduction
Cluster of Differentiation 47 (CD47) is a heavily glycosylated, ubiquitously expressed cell surface protein in the immunoglobulin superfamily that has characterized roles in important cellular functions like proliferation, adhesion, migration, apoptosis and phagocytosis. Its molecular structure includes an extracellular immunoglobulin variable (IgV)-like domain, a transmembrane spanning domain, and a short, alternatively spliced cytoplasmic tail1. CD47 has been shown to interact in cis with integrins, and in trans with both thrombospondin (TSP-1) and signal regulatory protein alpha (SIRPα)2,3. Research shows that it mediates vascular smooth cell proliferation and migration4, platelet activation and spreading5, and recruitment of granulocytes and T cells to sites of infection6, 7.
Apoptosis or programmed cell death (PCD) is a physiologically important mechanism for maintaining homeostasis. It can be divided into type I, type II and type III PCD; the first two are caspase dependent and type III is caspase-independent8. CD47 also functions as a marker of “self” on host cells within an organism. When expressed, CD47 binds to SIRPα on the surface of circulating immune cells to deliver an inhibitory “don’t eat me” signal9. SIRPα encodes an Ig-superfamily receptor expressed on the surface of macrophages and dendritic cells, whose cytoplasmic region contains immunoreceptor tyrosine-based inhibition motifs (ITIMs) that can trigger a cascade to inhibit phagocytosis. CD47-SIRPα binding results in phosphorylation of ITIMs on SIRPα, which triggers recruitment of Src homology phosphatases, SHP1 and SHP2. These phosphatases can in turn inhibit accumulation of myosin II at the phagocytic synapse, preventing phagocytosis10.
Phagocytosis of target cells by macrophages is ultimately regulated by a balance of activating signals (FcγR, CRT, LRP-1) and inhibitory signals (SIRPα-CD47) (Reviewed in11). This balance is tipped by cancer cells, which co-opt the “self” signal and upregulate CD47 expression to evade immune surveillance and subsequent destruction. Elevated expression of CD47 has been observed in ovarian carcinoma cell lines12, 13, murine myeloid leukemias14, leukemic stem cells14, 15 and several solid tumors16.
Specifically, CD47 expression of human acute lymphoblastic leukemia (ALL) samples was measured as two-fold increased compared to normal bone marrow samples and expression level was predictive of survival and refractoriness to primary treatment in pediatric populations17. Flow cytometry revealed high surface expression of CD47 on 73% of samples collected from the bone marrow of multiple myeloma (MM) patients18. These results corroborate earlier findings by microarray analysis19 and were also mirrored in high CD47 expression of several MM cell lines18. Goto et al (2014) revealed high CD47 expression on six different primary effusion lymphoma (PEL) cell lines compared to peripheral blood mononuclear cells (PBMC)20. Additionally, in acute myeloid leukemia (AML), ALL, and several non-Hodgkin’s lymphoma (NHL) subtypes, increased CD47 expression is correlated with adverse clinical outcomes15, 16, 21.
Hematological malignancies, even at onset, present with widespread bone marrow and peripheral blood involvement and many are still without effective systemic curative therapies22. Several anti-CD47 antibodies have been studied in vitro and in vivo with promising results using cell lines and mouse models of hematological malignancy. From this body of research, three different mechanisms of action of anti-CD47 antibodies have been proposed including: initiation of type III PCD of tumor cells, blockade of tumor cell anti-phagocytic signaling, and stimulation of cytotoxic T cell priming against tumor cells. Thus far it is understood that CD47 blockade on normal cells does not trigger phagocytosis without a pro-phagocytic stress signal, such as calreticulin or phosphatidylserine, which induces phagocytosis by binding to its receptor, low density lipoprotein-receptor related protein (LRP), on phagocytic cells23,24. In addition to interfering with CD47/SIRPα interactions, function-blocking CD47 antibodies may target cancer stem cells25, 26 and enhance tumor sensitivity to radiation therapy while providing protection to normal tissue27, 28. The goal of this review is to provide a systematic and comprehensive overview of the published preclinical data to support the use of anti-CD47 antibody therapies in clinical trials to treat hematological malignancies.
Methods
To perform a comprehensive survey of the available preclinical literature on anti-CD47 antibody treatment in hematological malignancies, we searched literature using key words: “CD47” OR “CD47 antibody” OR “anti-CD47” AND “Lymphoma” OR “Leukemia” OR “hematological malignancy” OR “Hematological malignancies”. A systematic literature search was performed using the databases: PubMed, Embase, SCOPUS, Web of Science, identifying a total of 521 records (Table 1). References from all identified studies were investigated further to eliminate duplicates. Abstracts from 237 records were retrieved and two reviewers (Hua and Russ) independently applied the inclusion and exclusion criteria (Table 2), yielding 38 records. Full articles of potentially relevant studies were reviewed before confirming their inclusion. Any disagreement was resolved by consensus with third author (Anwer). 24 full text articles were independently read by two reviewers, and data was extracted using a standardized form (Figure 1). Additional articles were suggested by independent peer reviewers and two were in turn incorporated into the manuscript after review.
Table 1.
Database Search Count
| Database searched | Date searched | Citation count |
|---|---|---|
| PUBMED | 3/7/2016 | 90 |
| EMBASE | 3/4/2016 | 151 |
| SCOPUS | 3/4/2016 | 164 |
| WEB OF SCIENCE | 3/7/2016 | 116 |
Table 2.
Inclusion and Exclusion Criteria Table
| Inclusion criteria | Exclusion criteria |
|---|---|
|
| |
| -Studies of CD47/integrin-associated protein/anti-CD47 antibody -Studies of hematological malignancies: [Non Hodgkin Lymphoma/Hodgkin Lymphoma/Myelomas/Leukemias (ALL, AML, CLL, CML)] -Methods include cell lines, animal models or human clinical trial -Published in English language -Date published 1990-2016 |
-No original data -Case report -No mechanism in hematological malignancy -Published in language other than English -Conference proceedings -Poster presentation |
Figure 1.
PRISMA Diagram for CD47 comprehensive review
Results
1. Anticancer effects through Apoptosis
1.1 Leukemia
Early studies on the efficacy of anti-CD47 antibodies proposed that malignant cells were eliminated via type III PCD. Mateo et al (1999) utilized the first anti-CD47 antibody in an in-vitro B cell chronic lymphocytic leukemia (B-CLL) model and showed that the induction of caspase-independent PCD in B-CLL by CD47 ligation using an unspecified anti-CD47 monoclonal antibody was not inhibited by a broad-spectrum caspase inhibitor (zVAD-fmk) or the presence of exogenous rescuing cytokines (IL-4 or IFN-gamma)29. CD47 ligation was shown to induce cell shrinkage, exposure of phosphatidylserine and decrease of mitochondrial membrane potential, all without the disruption of nuclear integrity29.
Utilizing mouse L1210 cells transfected with CD47 cDNA, Uno et al (2007) generated a monoclonal antibody against CD47, denoted as mAb-MABL30. F(ab1)2 of mAb-MABL demonstrated no apoptotic effect on CD34+ hematopoietic stem cells or human endothelial cells in-vitro. Furthermore, the monoclonal antibody had no effect on CD47-negative L1210 cells. They were found to bind to and induce apoptosis in CD47-positive L1210 cells, as well as CCRF-CEM cells and JOK-1 cells. In-vivo, the F(ab1)2 fragment of mAb-MABL also markedly prolonged survival of CCRF-CEM xenograft mice and JOK-1 xenograft mice30. Together, these data suggested that mAb-MABL, and anti CD47 monoclonal antibodies in general, could be developed as anti-leukemia therapy.
Using normal B cells, B lymphocytes from CLL patients, and monoclonal antibody B6H12, Bras et al (2007) demonstrated that CD47 ligation triggers caspase-independent type III PCD via DRP-1 translocation from cytosol to mitochondria without involvement of apoptotic effectors31. In addition, the results in Jurkat cells were DRP-1 independent, suggesting that overexpression of DRP-1 positively modulates cell death response, while DRP-1 down regulation confers resistance to CD47-mediated cell death. The identification of DRP-1 as a downstream death effector of CD47-mediated PCD revealed CD47 as a potential therapeutic target in CLL treatment.
Barbier et al (2009) conducted a study on the type III PCD pathway initiated by ligation of CD47 using either Thrombospondin (TSP-1) or an anti-CD47 antibody (B6H12) and demonstrated that cell death is modulated through F-actin disruption32. In particular, they elucidated a pathway in which ligation of CD47 triggers sequential activation of caspases and serine proteases (serpases), which in turn cause actin rearrangement, allowing for DRP-1 redistribution to the mitochondria and subsequent phosphatidylserine exposure in caspase-independent type III PCD. Additionally, they found that CD47 ligation was more effective than hydrocortisone at inducing cell death in cells with higher Bcl2/Bax ratio that are resistant to type I caspase-dependent PCD. Taken together, Barbier et al (2009) demonstrated that it is possible to initiate PCD in cells lacking a functioning classical apoptotic (type I) mechanism, and suggested that type III PCD can be therapeutically triggered in CLL32.
Sagawa et al (2011) developed a new way to target CD47 by creating a disulfide-linked stabilized dimer of single chain antibody fragment MABL scFv (designated as S-S diabody)33. They showed that S-S diabody induced apoptosis in JOK-1 cells, MOLT-4 cells, and leukemia cells from B-CLL patients in a dose-dependent manner as compared to lack of apoptosis induction in control cells, UCB, HUVEC, and normal peripheral mononuclear blood cells. S-S diabody treatment prolonged median survival of transplanted mice by 9 days compared to control mice (P < 0.0005)33. They also demonstrated the ability to repress this apoptotic induction with knockdown of HIF-1α in MOLT-4 cells, suggesting an apoptotic mechanism via the HIF-1α pathway33.
1.2 Multiple myeloma
Kikuchi et al (2004) developed and characterized a single-chain antibody fragment (scFv) of murine monoclonal antibody, MABL scFv-15, which formed both dimer and monomer to target leukemic cells34. They demonstrated that both dimer and monomer had binding activity for human CD47; however, only treatment with MABL scFv-15 dimer strongly induced apoptosis in human-CD47 introduced mouse leukemic cells in-vitro, and had anti-tumor effect on mouse models of human myeloma. The MABL scFv-15 monomer was non-active and unstable even at 4 °C, thus the antibody was unable to be used in whole form. Building upon this research, Kikuchi et al (2005) developed two stable single-chained Fv fragments: MABL scFv-5 by optimizing the linker, and MABL sc(Fv)2 by covalently linking two scFv fragments together.
They demonstrated that the antibodies strongly induced apoptosis in vitro. Administration of MABL ScFv-5 diabody or MABL sc(Fv)2 significantly reduced serum levels of IgG in a dose dependent manner and significantly prolonged the survival time of xenograft mice in comparison with the untreated control35.
2. Anticancer effect through Phagocytosis
Chao et al (2010b) confirmed earlier findings, showing apoptotic induction in NHL cells after treatment in vitro with immobilized anti-CD47 antibody36. However, when cells were incubated with soluble anti-CD47 mAbs, no induction of apoptosis was observed even after 8 hours of incubation. Though several publications had previously suggested apoptotic induction as the mechanism responsible for the anti-CD47 antibody efficacy, this research suggested that apoptotic induction was perhaps an artifact of fixed antibody conditions in vitro. To discern whether lymphoblasts were undergoing apoptosis before being phagocytosed, Metayer et al (2017) demonstrated that anti-CD47 antibody induced macrophages to phagocytose live lymphoblasts rather than inducing cell death before engulfment. They demonstrated this using JC-1 mitochondrial membrane staining to assess intact membrane potential within lymphoblasts after their engulfment37.
2.1 Leukemia
Contrary to earlier reports, Majeti et al (2009) observed no apoptosis in primary AML leukemic stem cells (LSCs) after treatment with a CD47-blocking antibody15. Working under the hypothesis that high levels of CD47 on human AML LSCs interact with SIRPα to deliver an inhibitory signal for phagocytosis, they demonstrated that this effect can be disrupted with monoclonal antibodies that block the interaction. Using both blocking antibodies (B6H12.2 and BRIC126) and nonblocking (2D3) antibodies designed against CD47 and SIRPα, they demonstrated in vitro that disruption of the CD47-SIRPα interaction enables phagocytosis of AML LSCs by macrophages. Normal hematopoietic progenitor cells, which also express CD47, were not phagocytosed when coated with CD47 antibody15.
A similar mechanism was elucidated by Jaiswal et al (2009)14. Using a xenograft mouse model, Jaiswal et al (2009) demonstrated that expression of high levels of mouse CD47 on a human leukemia cell line could protect from macrophage phagocytosis, facilitating tumor engraftment and growth. More specifically, low CD47 expressing MOLM-13 clone AML cells showed minimal engraftment in immunodeficient mice while high CD47 expression resulted in high levels of engraftment to bone marrow, spleen and liver. Engraftment of low CD47 expressing cells only reached levels equivalent to those of high expressing cells when macrophages were depleted using clodronate liposomes or macrophage SIRPα was inhibited with a SIRPα blocking antibody14. These experiments further demonstrated that CD47 can deliver a signal to inhibit phagocytosis through its interaction with SIRPα and that targeted antibodies can effectively block the interaction (Figure 1).
Chao et al (2011b) also demonstrated the effectiveness of antibody blockade of the SIRPα-CD47 anti-phagocytic signal17. Two blocking anti-CD47 antibodies (B2H12.2 and BRIC 126) and an anti-SIRPα antibody independently enabled ALL cell phagocytosis when compared to control antibodies in vitro. Treatment of B-ALL and T-ALL cells with a blocking anti-CD47 Ab (B6H12.2) significantly inhibited engraftment into bone marrow and peripheral blood of NSG mice, when compared to treatment with non-blocking control antibodies. Intraperitoneal injection of 100 μg of therapeutic anti-CD47 antibody for 14 days significantly reduced the level of T-ALL and B-ALL in peripheral blood of mice engrafted with human primary ALL cells, but showed varying levels of clearance of leukemic cells from bone marrow. Researchers observed that extremely high levels of leukemic engraftment were in the areas most refractory to therapeutic antibody treatment and hypothesized that insufficient numbers of host macrophages were present in those areas to mediate phagocytosis of ALL cells17.
In secondary transplant models of tumorigenic potential, cells from bone marrow of mice previously treated with anti-CD47 and control antibodies were re-transplanted into sub-lethally irradiated NSG mice. After six weeks, the xenografts from mice previously treated with anti-CD47 blocking antibody had not engrafted compared to controls, suggesting that tumorigenic potential was abrogated long term with anti-CD47 Ab treatment17.
Using mice engineered to express several different SIRPα alleles with differential ability to bind CD47, Theocharides et al demonstrated that macrophage-mediated phagocytosis to clear AML stem cells is dependent on SIRPα signaling38. Specifically, experiments using NS-Idd13 mice that express a SIRPα variant that does not bind to human CD47, resulted in macrophage-mediated elimination of primary human AML cells on engraftment. The same researchers also used a fusion protein, SIRP-Fc, to disrupt the SIRPα-CD47 interaction and found that while phagocytosis of normal hematopoietic cells was not enhanced with SIRP-Fc treatment, phagocytosis of AML cells (AML19) by both mouse and human macrophages was significantly enhanced and leukemic engraftment of AML cells into NS mice was impaired compared to controls38. Curiously, their results also suggested that anti-CD47 antibody B6H12 induces mouse macrophage-mediated phagocytosis of AML cells even in a SIRPα variant that fails to bind human CD47, possibly due to Fc recognition by the macrophage Fc receptor.38 Chao et al further investigated the role of Fc receptor; in their experiments, F(ab’)2 fragments lacking the Fc domain of the B6H12 antibody were able to induce phagocytosis of Raji cells, a Burkitt’s lymphoma cell line. However, other research suggests that antibody induced phagocytosis of cancer cells is dependent on an intact Fc domain. Metayer et al (2017) presented experiments suggesting that the Fc portion of the asnti-CD47 antibody is necessary for the anti-CD47 driven phagocytosis. This was accomplished with experiments comparing the isolated anti-CD47 antibody F(ab’)2 fragment (lacking the Fc portion) to intact antibody and also by blocking antibody-induced uptake of lymphoblasts into macrophages by blocking the Fc portion with anti-Fc, F(ab’)2 fragments37. They propose that anti-CD47 antibody kills cancer cells via antibody-dependent cellular phagocytosis (ADCP). Metayer et al (2017) further suggest that induction of phagocytosis of cancer cells can be triggered with the use of any antibody that targets a highly expressed antigen on cancer cells coupled with a motif that activated phagocytosis Fc receptors, resulting in targeted phagocytosis of cancer cells37.
Wang et al (2015) showed that individual and combination therapy of Ara-C (cytarabine) and anti-CD47 antibody decrease leukemic burden in peripheral blood and bone marrow and significantly prolong survival in a mouse model of AML, xenoplanted with human cell line, THP-139. Individual treatment of Ara-C or anti-CD47 antibody or a combination of the two decreased the leukemic burden and significantly prolonged survival compared to IgG control. This evidence presents combination treatment of cytarabine and anti-CD47 antibody as a novel option for therapeutic targeting of AML39.
Liu et al generated a humanized monoclonal antibody against human CD47, Hu5F9-G4, and demonstrated its induction effects on macrophage-mediated phagocytosis of HL60 AML cells and primary human AML cells40. Use of Hu5F9-G4 antibody in SU048 mice eradicated engrafted AML cells with no recurrence over 22 weeks. Anti-leukemic efficacy of the antibody in this mouse model was optimized at drug serum levels of 50-150 mcg/ml. Additionally, synergy of Hu5F9-G4 with rituximab was demonstrated, eliminating xenografted Raji cells in 3 of 4 mice modeling disseminated NHL. These results support Hu5F9-G4 humanized antibody as a candidate therapeutic and paved the way for its entry into clinical trial40.
2.2 Lymphoma
Researchers have proposed that a potent pro-phagocytic signal must be present in order for target cells to be phagocytosed in response to blockade of the SIRPα-CD47 anti-phagocytic signal. Specifically, Chao et al (2010a) suggested blockade of CD47 on normal cells does not trigger phagocytosis because a pro-phagocytic calreticulin signal is not present24. Cell surface calreticulin is understood to induce phagocytosis by binding to its receptor, low density lipoprotein-receptor related protein (LRP), on phagocytic cells. This occurs in normal physiology to mark cells undergoing DNA damage and serves as a phagocytic activating signal, resulting in engulfment of the damaged cell23, 41. Interestingly, high calreticulin expression correlates with increased CD47 expression in AML, CML and ALL patient samples. Chao et al (2010a) also identified up to 20-fold increase in calreticulin expression on primary human cancer cells from several hematologic malignancies including NHL when compared to normal bone marrow and peripheral blood cells24. They also demonstrated that higher levels of calreticulin mRNA correlate with worse clinical outcomes in mantle cell lymphoma24.
To demonstrate the interplay between calreticulin and CD47 in cancer, they used a Burkitt’s NHL cell line (Raji cells) with high CD47 and calreticulin expression in phagocytosis assays. 2-fold shRNA-mediated knockdown of CD47 caused more robust phagocytosis by human macrophages when compared to wild type controls, an effect that was abrogated in the presence of a calreticulin blocking peptide24. Additionally, phagocytosis assays performed in the presence of anti-CD47 antibody (B6H12.2) showed similarly robust phagocytosis of tumor cells expressing calreticulin, without phagocytosis of normal cells. Phagocytosis of tumor cells treated with anti-CD47 antibody was again abrogated with the interruption of the calreticulin-LRP interaction24. Furthermore, treatment of cells with a calreticulin blocking peptide caused dose-dependent reduction of anti-CD47 antibody mediated phagocytosis. The authors concluded that calreticulin is necessary for anti-CD47 antibody mediated phagocytosis24.
Utilizing anti-CD47 in combination with rituximab, Chao et al (2010b) demonstrated antibody synergy between rituximab and anti-CD47 mAb, B6H12.2 when used in xenograft mouse models of localized and disseminated NHL36. In NSG and SCID mouse models engrafted with Raji cells, treatment with anti-CD47 blocking antibody regimens reduced lymphoma burden and prolonged survival. Combination of Fc receptor activated, pro-phagocytic signal (rituximab) and simultaneous blockade of the CD47-SIRPα anti-phagocytic signal (B6H12.2) eliminated lymphoma in 60-86% of mice tested36.
In a primary human diffuse large B cell lymphoma (DLBCL) xenotransplant model, 8 out of 9 mice treated with combined anti-CD47 antibody and rituximab were cured of lymphoma, with disease free survival extending greater than 4 months after the end of treatment. Further experiments in SCID mice found that depletion of macrophages, not NK cells or complement, abrogated the therapeutic effect, implicating macrophages as the critical immune cell effectors of antibody treatment36.
Piccione et al developed a bispecific antibody, with a DVD-Ig format, that targets both CD47 and CD20 with a single antibody42. This antibody, CD20-CD47SL, was observed to selectively bind to dual antigen expressing lymphoma cells in the presence of an ‘antigen sink’ of RBCs and recapitulated the synergistic effects of anti-CD47 antibody + rituximab combinations in vitro. CD20-CD47SL eliminated detectable lymphoma and significantly extended survival relative to anti-CD47 antibody or rituximab alone in both localized and disseminated mouse models of NHL42.
Potentially secondary to effects on phagocytosis, CD47 has also been implicated as a regulator of dissemination in an NHL xenotransplant model. Raji cells with intact CD47 expression injected subcutaneously into flank disseminated readily into the liver and other extranodal sites of NSG mice, while knockdown of CD47 significantly reduced liver dissemination. Anti-CD47 antibody treatment of mice transplanted with subcutaneous Raji cells inhibited dissemination to major organs and CNS, while the same antibodies inhibited hematogenous dissemination of primary lymphoma cells as compared to controls43.
Experiments with CD47 knockdown and anti-CD47 antibody in mouse models of primary effusion lymphoma (PEL), an aggressive NHL subtype, recapitulated previous results, showing promotion of macrophage-mediated phagocytosis of PEL cells with disruption of CD47-SIRPα interaction20. When primary PEL cells were IP injected into NRJ mice, concurrent treatment with anti-CD47 antibody inhibited organ invasion and prevented ascites compared with control IgG treated mice. These preclinical results suggest that antibody targeting of CD47 could be an effective therapy in PEL, a disease that lacks CD20 expression, conferring refractoriness to rituximab20. It has also been suggested that anti-CD47 antibody therapies may be considered as an alternative to CNS prophylaxis with toxic chemotherapies in NHL in the future43.
2.3 Multiple Myeloma
Kim et al (2012) showed that macrophages derived from mouse bone marrow displayed increased phagocytosis of MM cell lines and primary human myeloma cells in vitro after incubation with blocking anti-CD47 antibody (B6H12.2) as compared to a non-blocking anti-CD47 antibody (2D3)18. Antibody therapy initiated two weeks after xenotransplantation of RPMI 8226 cells into mice resulted in reduced overall tumor burden compared to controls. Though tumor regression occurred in both anti-CD47 and IgG treated mice, regression was significantly greater in anti-CD47 treated cohort and after stopping treatment, regressed tumors regrew in control treated, but not in previously anti-CD47 Ab treated mice. Researchers reported that sublethal irradiation of mice 2 weeks before antibody treatment abrogated the therapeutic effect, implicating radiation-sensitive cells as necessary effectors of the treatment, as irradiated mice had significant reduction of undifferentiated blasts, monocytes and macrophage progenitors18.
In a mouse model designed to study growth of myeloma in the human bone marrow microenvironment, treatment with anti-CD47 antibodies slowed the growth of patient myeloma cells engrafted into human fetal bone and reduced bone resorption in human bone bearing mice. These results suggest that CD47 is a promising target on myeloma cells and that CD47 antibody therapies have potential to increase quality of life for MM patients by not only reducing myeloma growth, but also reducing bone resorption18.
3. Anticancer effects through Cytotoxic T-cell priming
3.1 Lymphoma
Tseng et al (2013) revealed a new mechanism for anti-CD47 in a model of colorectal carcinoma by using immunocompetent mice44. Researchers found that macrophages phagocytosed cancer cells (DLD1) in the presence of B6H12.2 antibody, but not with a nonblocking antibody, 2D3, demonstrating that phagocytosis is dependent on blockade of CD47-SIRPα interactions and not opsonization effects. Importantly, researchers found that macrophages primed CD8+ cells after phagocytosis of cancer cells and in vivo experimentation showed that those CD8+ T cells primed by macrophages protected mice from subsequent tumor challenge44. These findings suggest that anti-CD47 antibody both enables macrophage phagocytosis to clear cancer cells and initiates a secondary cytotoxic T-cell immune response.
3.2 Leukemia
Research by Liu et al demonstrated that T cells were required for maximal therapeutic effect of CD47 blockade therapy45. Intratumoral treatment with anti-CD47 antibody MIAP301 vs control had greater effects in wild type mice with established A20 tumors than in T cell deficient nude mice with equivalent tumor burden, suggesting that robust anti-tumor response is dependent on the presence of an intact immune system. In these experiments, anti-CD47 antibody intratumoral treatment was combined with intraperitoneal delivery of anti-CD4 or anti-CD8 antibodies. Without the action of CD8+ T cells, effects of anti-CD47 Ab were abrogated, while antibody targeting of CD4+ cells had no effect. These results suggest that anti-CD47 mediated tumor targeting involves both an innate and adaptive immune response that elicits systemic immune memory to prevent relapse45. Researchers found that T cells were primed by dendritic cells45, not macrophages, as was suggested by Tseng et al (2013)44.
Discussion
There is a large body of preclinical evidence and emerging clinical data supporting the use of anti-CD47 antibodies in several hematological malignancies both as a monotherapy and as a combination strategy (Table 3). In hematological cancers, where tumor cells express high levels of CD47 as a mechanism of immune evasion, antibodies designed to block the SIRPα-CD47 interaction offer a compelling therapeutic strategy to prevent escape and promote innate and adaptive immune system clearance of cancer cells. The antibody-mediated blockade of CD47-SIRPα results in phagocytosis of cells bearing stress signals and primes tumor-specific T cell responses (Figure 2B).
Table 3.
Anti-CD47 antibody constructs developed for use in hematologic malignancy with their mechanism of action
| antibody construct | antibody name | animal model | cell lines | publication | murine or human ab | mechanism of action | hematological malignancies |
|---|---|---|---|---|---|---|---|
| monoclonal antibody | B6H12 | none | B lymphocytes isolated from CLL patients | Barbier et al 2009 | human | apoptosis | CLL |
| monoclonal antibody | B6H12 | none | B lymphocytes isolated from CLL patients, Jurkat cells | Bras et al 2007 | human | apoptosis | CLL |
| monoclonal antibody | B6H12 | OT-I transgenic mice, OT-II transgenic mice, OT-II/Foxp3-GFP+ double-transgenic mice | human colon cancer cell DLD1 | Tseng et al 2013 | human | phagocytosis | Hodgkin Lymphoma/colorectal adenocarcinoma |
| monoclonal antibody | B6H12.2 | NSG or SCID mice transplanted with Raji cells | Burkitts lymphoma cell line Raji, DLBCL cell line SUDHL4, and NHL-17 | Chao et al 2010 Cell | human | phagocytosis | B cell NHL, localized and disseminated lymphoma |
| monoclonal antibody | B6H12.2 | sublethally irradiated NOD mice injected intravenously with AML LSCs | Raji cells, AML LSCs, MOLM13 cells (CD47 deficient) | Chao et al 2010 Sci Trans | human | phagocytosis | NHL |
| monoclonal antibody | B6H12.2 | NSG mice engrafted with primary human ALL cells or CCRF-CEM cells | human ALL cells, CCRF-CEM human T-ALL cell line | Chao et al 2011 Cancer Res | human | phagocytosis | ALL |
| monoclonal antibody | B6H12.2 | localized lymphoma model: NSG mice injected subQ with Raji cells | DLBCL cells (primary cells), Raji cells-NHL cell line | Chao et al 2011 Blood | human | phagocytosis | Burkitt lymphoma and DLBCL |
| monoclonal antibody | B6H12.2 | NRJ mice injected peritoneally with primary PEL cells | PEL cells isolated from patient | Goto et al 2014 | human | phagocytosis | primary effusion lymphoma (NHL) |
| monoclonal antibody | B6H12.2 | xenografts and human bone-bearing RAG2-/gamma c-mice | RPMI 8226 human myeloma cell line NCI H929 human myeloma cell line | Kim et al 2012 | murine | phagocytosis | multiple myeloma |
| monoclonal antibody | B6H12.2 | NOG mice | human AML cells | Majeti et al 2009 | human | phagocytosis | AML |
| monoclonal antibody | BRIC126 | none | Raji- Burkitts lymphoma cell line, SUDHL4- DLBCL cell line, NHL-17-DLBCL cell line | Chao et al 2010 Cell | human | phagocytosis | NHL |
| monoclonal antibody | BRIC126 | NSG mice engrafted with primary human ALL cells or CCRF-CEM cells | human ALL cells, CCRF-CEM human T-ALL cell line | Chao et al 2011 Cancer Res | human | phagocytosis | ALL |
| monoclonal antibody | BRIC126 (blocking) | NOG mice | human AML cells | Majeti et al 2009 | murine | phagocytosis | AML |
| monoclonal antibody | SIRPα antibody | NSG mice engrafted with primary human ALL cells or CCRF-CEM cells | human ALL cells, CCRF-CEM human T-ALL cell line | Chao et al 2011 Cancer Res | murine | phagocytosis | ALL |
| monoclonal antibody | SIRPα antibody | NOG, Rag2 −/−, and gamma c −/− mice transplanted with HL-60, Kasumi-I, MOLM-13, and Tet-CD47 MOLM-13 cells | AML cell line MOLM-13, HL-60, Jurkat, U937, K562, and Kasumi-1 cells | Jaiswal et al 2009 | murine | phagocytosis | AML |
| monoclonal antibody | 5F9 | xenograft mice injected via tail vein with human primary AML cells, nonhuman primates treated with ab for toxicokinetic profiling | Primary AML cells, SU048, SU028, SU266 | Liu et al 2015 Plos One | murine | phagocytosis | AML |
| monoclonal antibody | 5F9-G4 | xenograft mice injected with human primary AML cells, nonhuman primates treated with ab for toxicokinetic profiling | Primary AML cells, SU048, SU028, SU266 | Liu et al 2015 Plos One | human | phagocytosis | AML/also works on NHL when synergized with rituximab |
| monoclonal antibody | MIAP301 (ebioscience/biolegend) *blocks mouse SIRPα binding) | syngeneic wild type mice that were subQ inoculaed with A20 B-CLL cells | murine colon adenocarcinoma cell line MC38; murine B cell lymphoma cell line A20 | Liu et al 2015 Nat Med | murine | T cell-mediated elimination of immunogenic tumors | B-CLL, colon adenocarcinoma |
| monoclonal antibody | 2D3 | NOG mice | human AML cells | Majeti et al 2009 | murine | phagocytosis | AML |
| monoclonal antibody | 2D3 (nonblocking) binds CD47 but does not block its interaction with SIRPα | OT-I transgenic mice, OT-II transgenic mice, OT-II/Foxp3-GFP+ double-transgenic mice | human colon cancer cell DLD1 | Tseng et al 2013 | human | phagocytosis | Hodgkin Lymphoma/colorectal adenocarcinoma |
| monoclonal antibody | MABL | SCID mice with JOK-1 cells | lymphocytic leukemia cell lines MOLT-4; JOK-1. human CLL cells | Sagawa et al 2011 | human | apoptosis | B-CLL |
| monoclonal antibody | F(ab’)2 of mAb-MABL | Male SCID mice intravenously injected with CCRF-CEM or JOK-1 cells; and mice intraperitoneally with JOK-1 cells | acute lymphoblastic leukemia (CCRF-CEM) and B cell chronic lymphocytic leukemia (JOK-1) | Uno et al 2007 | mice | apoptosis | ALL, B-CLL |
| monoclonal antibody | anti-CD47 | THP-1 LSC-engrafted mice | human acute monocytic leukemia cell line THP-1 | Wang et al 2015 | human | phagocytosis | leukemias (targeting LSCs) |
| monoclonal antibody | B6H12/BRIC126 | none | B-CLL human cells | Mateo et al 1999 | Not specified | apoptosis | B-CLL |
| bivalent scFv | MABLscFv-15 | SCID mice xenografted with KPMM2 cells | KPMM2 human myeloma cell line | Kikuchi et al 2004 | murine | apoptosis | Multiple myeloma |
| scFv dimer (diabody) | MABLscFv-5 | SCID mice xenografted with KPMM2 cells | KPMM2 human myeloma cell line | Kikuchi et al 2005 | murine | apoptosis | multiple myeloma |
| sc(Fv)2 monomer (covalently linked) | MABLsc(Fv)2 | SCID mice xenografted with KPMM2 cells | KPMM2 human multiple myeloma cell line | Kikuchi et al 2005 | murine | apoptosis | multiple myeloma |
| bispecific that cotargets CD47 and CD20 (created using Rituximab and B6H12.2) | CD20-CD47 LL (long linker sequence); CD20-CD47 SL (short linker sequence) | NSG mice xenoplanted with luciferase expressing Raji cells, disseminated lymphoma mouse model | YB2/0, Raji, Daudi, Ramos, ST486 and J774 cell lines. B-CLL human cells | Piccione et al 2015 | human | phagocytosis | NHL |
| fusion protein from IgV domain of human SIRPα fused to human IgG-moiety | hSIRPα-FC | NS-mice; NS-Idd13 mice that express a SIRPα variant that does not bind CD47 | human AML cells | Theocarides et al 2012 | human | phagocytosis | AML |
| high affinity SIRPα monomer | CV1 (in combination with rituximab or alemtuzumab | Raji cell lymphoma engrafted mice | Her2/neu+ SK-BR-3 breast cancer cells | Weiskopf et al 2013 | human | phagocytosis | lymphoma |
| high affinity SIRPα monomer | CV1 | NSG mice engrafted with CLBL-1 cells | CLBL-1 canine diffuse large B-cell lymphoma | Weiskopf et al 2016 | human | phagocytosis | canine lymphoma |
| high affinity SIRPα variant | CV1-hIgG4 | NSG mice engrafted with CLBL-1 cells | CLBL-1 canine diffuse large B-cell lymphoma | Weiskopf et al 2016 | mouse | phagocytosis | canine lymphoma |
| high affinity SIRPα variant | Hu5F9-G4 | NSG mice engrafted with CLBL-1 cells | CLBL-1 canine diffuse large B-cell lymphoma | Weiskopf et al 2016 | mouse | phagocytosis | canine lymphoma |
Figure 2.
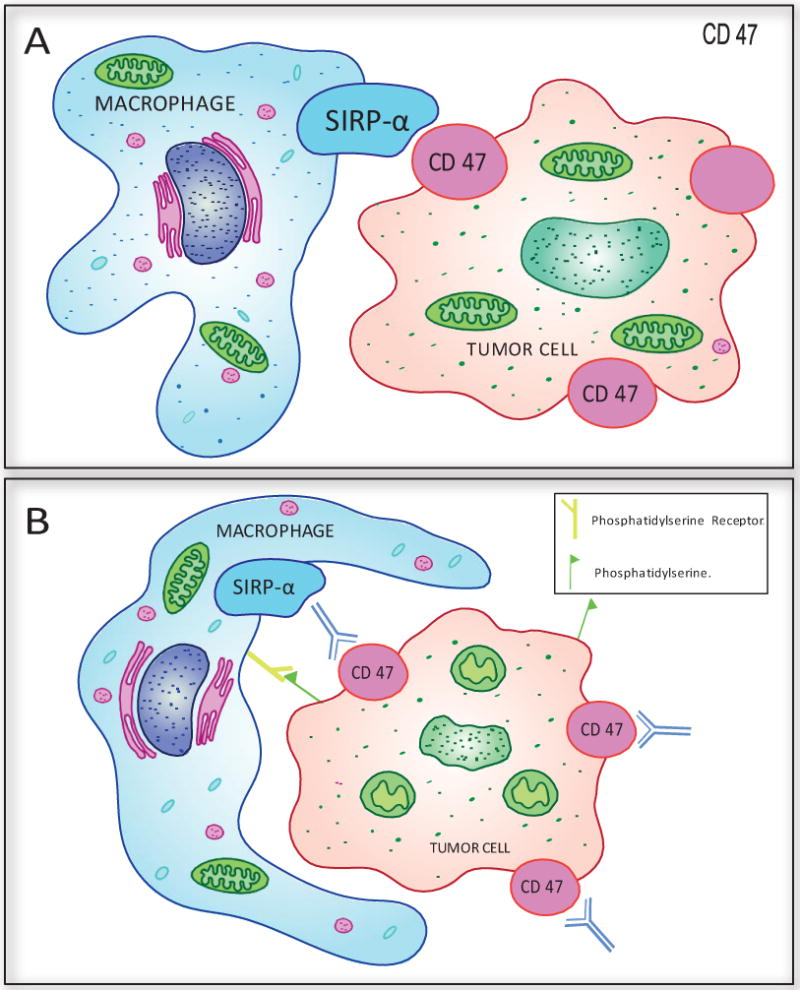
Anti-CD47 mechanisms of cancer cell killing. A. CD47-SIRPα interaction blocks macrophage phagocytosis of cancer cells. B. Treatment of cancer cells treated with anti-CD47 Ab leads to type-III PCD (actin rearrangement, mitochondrial swelling and damage, exposure of phosphatidylserine on plasma membrane) along with induction of phagocytosis by macrophage.
Four different antibody molecules designed to target the CD47-SIRPα interaction in malignancy are currently being studied in clinical trials: Hu5F9-G4, CC-90002, TTI-621, and ALX-148. Nine clinical trials of these drugs are actively recruiting participants (Table 4). Hu5F9-G4, a humanized anti-CD47 blocking antibody46, is currently being studied in four different Phase I trials. Two clinical trials are investigating its safety profile and effective dose as a single therapy in relapsed or refractory AML (NCT 02678338) and in patients with solid tumors (NCT 02216409). Preclinical research using a mouse model of disseminated NHL demonstrated synergy of Hu5F9-G4 when used in combination with rituximab40. One trial is currently studying Hu5F9-G4 with rituximab for relapsed/refractory B-cell NHL (non-Hodgkin lymphoma, large B cell lymphoma, and diffuse indolent lymphoma) (NCT 02953509) and another trial is investigating combination with cetuximab for solid tumors or advanced colorectal cancer (NCT 02953782). A bispecific antibody fusion of anti-CD20 (rituximab) and anti-CD47 (CD20-CD47SL) also demonstrated a synergistic effect against lymphoma in preclinical models42. These studies may lay the groundwork for therapeutic bispecific antibody use in human cancer treatment.
Table 4.
Anti CD47 antibodies currently in clinical trials for hematological malignancies.
| Open Clinical Trial | Disease | Phase | Status | Identifier | Antibody |
|---|---|---|---|---|---|
| CAMELLIA: Anti-CD47 Antibody Therapy in Relapsed/Refractory AML |
AML | I | Recruiting | NCT02678338 | Hu5F9-G4 |
| Study of CC-90002 in Subjects With AML and High-risk MDS | Leukemia, Myeloid, Acute Myelody splastic Syndromes |
I | Recruiting | NCT02641002 | B6H12 |
| Trial of TTI-621 for Patients With Hematologic Malignancies | Hematologic Malignancies | I | Recruiting | NCT02663518 | SIRPα-Fc |
| Trial of Hu5F9-G4 With cetuximab in Patients With Solid Tumors and Advanced Colorectal Cancer | Colorectal Neoplasms Solid Tumors | I/II | Recruiting | NCT02953782 | Hu5F9-G4/with cetuximab |
| Phase 1, Dose Finding Study of CC-90002 in Subjects With Advanced Solid and Hematologic Cancers | Hematologic Neoplasms | I | Recruiting | NCT02367196 | B6H12 |
| Phase 1 Trial of Hu5F9-G4, a CD47-targeting Antibody | Solid Tumor | I | Recruiting | NCT02216409 | Hu5F9-G4 |
| Trial of Hu5F9-G4With rituximab in Relapsed/Refractory B-cell NHL | Lymphoma, Non-Hodgkin Lymphoma, Large B-Cell, Diffuse Indolent Lymphoma |
I/II | Recruiting | NCT02953509 | Hu5F9-G4/with rituximab |
| Trial of Intratumoral Injections of TTI-621 in Subjects With Relapsed and Refractory Solid Tumors and Mycosis Fungoides | Solid Tumors Mycosis Fungoides Melanoma Merkel-cell Carcinoma SCC Breast Carcinoma HPV Related Malignant Neoplasm Soft Tissue Sarcoma |
I | Recruiting | NCT02890368 | SIRPα-Fc |
| Study of ALX148 in Patients With Advanced Solid Tumors and Lymphoma | Metastatic Cancer Solid Tumor Advanced Cancer NHL |
I | Recruiting | NCT03013218 | SIRPα-hIgG4 (high affinity) |
In mouse models of leukemia, lymphoma, and myeloma, treatment with anti-CD47 blocking antibody B6H12 has demonstrated induction of stress signaling, macrophage phagocytosis, priming of T cells, and has shown synergy in combination with rituximab15, 17, 18, 32, 36, 44, 47 (Table 3). CC-90002, a proprietary, humanized anti-CD47 monoclonal antibody developed by Celgene, is currently under investigation in two Phase I clinical trials, one treating patients with AML and high risk MDS (NCT02641002) and another treating patients with advanced or refractory solid and hematological cancers (NCT 02367196).
Anti-SIRPα antibodies have also been shown to block the SIRPα-CD47 interaction, successfully inhibiting the anti-phagocytic signal and inducing phagocytosis of tumor cells in several models of hematological cancer14, 38, 48. TTI-621 is a recombinant fusion protein of the CD47 binding domain of SIRPα linked to the Fc region of human IgG1 (SIRPαFc) that promotes phagocytosis of tumors by blocking CD47 and engaging the FcR on macrophages49. This decoy receptor is being studied in patients with relapsed or refractory hematologic malignancies (Hodgkin lymphoma, DLBCL, follicular, and mantle cell), AML, and MDS (NCT 02663518). Early results suggest that the drug is reasonably well-tolerated, with initial reports of dose dependent thrombocytopenia and minimal binding to erythrocytes as evidenced by stable hemoglobin levels in patients treated49. NCT 02890368 is a dose escalation and dose expansion trial testing intratumoral injections of TTI-621 in patients with mycosis fungoides or relapsed or refractory tumors that are percutaneously accessible.
Weiskopf et al (2013 and 2016) engineered SIRPα variants with high affinity for CD47 that did not induce phagocytosis or reduce tumor growth when used alone, yet when combined with approved antibody therapies: trastuzumab, cetuximab, and rituximab, showed synergistic effects50, 51. ALX-148, is a fusion protein composed of two of these high affinity SIRPα variant CD47 binding domains linked to an inactive Fc region of human IgG, that can simultaneously block the CD47-SIRPα interaction, while providing an FcR docking site for macrophages50. Phase I NCT 03013218 is a dose escalation study currently underway examining ALX-148 treatment in patients with metastatic solid tumors or relapsed/refractory non-Hodgkin lymphoma.
Given the nearly ubiquitous expression of CD47 at low levels in tissues, there has been some concern for off-target effects with anti-CD47 treatment. However, research has shown that without the expression of a stress signal or pro-phagocytic signal, such as calreticulin or phosphatidylserine, normal cells are minimally affected24. On the other hand, some studies have revealed high levels of surface calreticulin on circulating neutrophils52. Similarly, research has suggested that treatment with anti-CD47 blocking antibody leads to depletion of neutrophils15. Normal cells can upregulate calreticulin after stress, including radiation exposure and treatment with anthracycline based chemotherapy41, 53. These concerns regarding combination therapy bring to light the importance of nuanced timing of multifaceted treatment regimens, as the overlap of chemotherapy or radiation with anti-CD47 antibody therapy may amplify off target effects. Likewise, synergy or even interference may be time dependent, as has been suggested by several researchers in the field24, 40. To further limit toxicity, design adjustments to capitalize upon antibody affinity differences may reduce off target effects of bispecific agents, as suggested by Weiskopf et al (2017) whereby a tumor-specific component could be engineered with a greater affinity for its target than the CD47 blocking component54.
In following with the cancer stem cell (CSC) model, a cancer therapy must effectively eliminate all cancer stem cells (CSCs) to be considered curative. Antibodies targeting the CD47-SIRPα interaction may be well positioned to fulfill this role, given the preclinical data that supports their antigen specificity, minimal toxicity, and demonstrated ability to target CSCs. Higher expression of CD47 has been demonstrated in AML leukemic stem cells (LSCs) and they can be targeted in vivo with an anti-CD47 antibody that enables phagocytosis of macrophages and inhibits engraftment15. Anti-CD47 antibody treatment has also been shown to act synergistically with cytarabine (Ara-C) chemotherapy in a model of AML. While Ara-C effectively eliminated TSP-1 cancer cells in the proliferative phase, anti-CD47 antibodies were putatively able to target quiescent LSCs that were not susceptible to Ara-C treatment, but highly expressed CD4739.
PRACTICE POINTS
A large body of preclinical evidence and emerging clinical data supports the use of anti-CD47 antibodies in several hematological malignancies both as a monotherapy and as a combination strategy.
Anti-cancer effects of CD47-SIRPα are exerted via phagocytosis and cytotoxic T cell priming.
RESEARCH AGENDA
Improved standard protocol for treating hematological malignancies with additional Phase I testing using antibody molecules designed to target the CD47-SIRPα interaction.
Evaluation of testing in combination with immune-inhibitor and immune-modulators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Atlantis Russ MD, PhD, Anh B. Hua BS, William R. Montfort PhD, Bushra Rahman MS, MD, Irbaz Bin Riaz MS, MD, Muhammad Umar Khalid MD, Jennifer S. Carew PhD, Steffan T. Nawrocki PhD, Daniel Persky MD, and Faiz Anwer MD, FACP report nothing to declare as conflict regarding this publication.
References
- 1.Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108:3419–25. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- 2.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–62. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 3.Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633–43. [PubMed] [Google Scholar]
- 4.Lymn JS, Patel MK, Clunn GF, Rao SJ, Gallagher KL, Hughes AD. Thrombospondin-1 differentially induces chemotaxis and DNA synthesis of human venous smooth muscle cells at the receptor-binding level. J Cell Sci. 2002;115:4353–60. doi: 10.1242/jcs.00119. [DOI] [PubMed] [Google Scholar]
- 5.Chung J, Gao AG, Frazier WA. Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J Biol Chem. 1997;272:14740–6. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–8. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 7.Ticchioni M, Deckert M, Mary F, Bernard G, Brown EJ, Bernard A. Integrin-associated protein (CD47) is a comitogenic molecule on CD3-activated human T cells. J Immunol. 1997;158:677–84. [PubMed] [Google Scholar]
- 8.Elmore S. Apoptosis: a review of programmed cell death. Toxicol pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 10.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–99. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leuk Lymphoma. 2004;45:1319–27. doi: 10.1080/1042819042000201989. [DOI] [PubMed] [Google Scholar]
- 12.Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52:5416–20. [PubMed] [Google Scholar]
- 13.Poels LG, Peters D, van Megen Y, Vooijs GP, Verheyen RN, Willemen A, et al. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986;76:781–91. [PubMed] [Google Scholar]
- 14.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–85. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell. 2009;138:286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–84. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–45. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 19.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–57. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 20.Goto H, Kojima Y, Matsuda K, Kariya R, Taura M, Kuwahara K, et al. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer. 2014;50:1836–46. doi: 10.1016/j.ejca.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. New Engl J Med. 2008;359:613–26. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 23.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget. 2016;7:10133–52. doi: 10.18632/oncotarget.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur S, Roberts DD. Divergent modulation of normal and neoplastic stem cells by thrombospondin-1 and CD47 signaling. Int J Biochem Cell Biol. 2016;81:184–94. doi: 10.1016/j.biocel.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep. 2013;3:1038. doi: 10.1038/srep01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, et al. CD47 Receptor Globally Regulates Metabolic Pathways That Control Resistance to Ionizing Radiation. J Biol Chem. 2015;290:24858–74. doi: 10.1074/jbc.M115.665752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mateo V, Lagneaux L, Bron D, Biron G, Armant M, Delespesse G, et al. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5:1277–84. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- 30.Uno S, Kinoshita Y, Azuma Y, Tsunenari T, Yoshimura Y, Iida S, et al. Antitumor activity of a monoclonal antibody against CD47 in xenograft models of human leukemia. Oncol Rep. 2007;17:1189–94. [PubMed] [Google Scholar]
- 31.Bras M, Yuste VJ, Roué G, Barbier S, Sancho P, Virely C, et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–88. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbier S, Chatre L, Bras M, Sancho P, Roue G, Virely C, et al. Caspase-independent type III programmed cell death in chronic lymphocytic leukemia: the key role of the F-actin cytoskeleton. Haematologica. 2009;94:507–17. doi: 10.3324/haematol.13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagawa M, Shimizu T, Fukushima N, Kinoshita Y, Ohizumi I, Uno S, et al. A new disulfide-linked dimer of a single-chain antibody fragment against human CD47 induces apoptosis in lymphoid malignant cells via the hypoxia inducible factor-1alpha pathway. Cancer Sci. 2011;102:1208–15. doi: 10.1111/j.1349-7006.2011.01925.x. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi Y, Uno S, Yoshimura Y, Otabe K, Iida S, Oheda M, et al. A bivalent single-chain Fv fragment against CD47 induces apoptosis for leukemic cells. Biochem Biophys Res Commun. 2004;315:912–8. doi: 10.1016/j.bbrc.2004.01.128. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi Y, Uno S, Kinoshita Y, Yoshimura Y, Iida S, Wakahara Y, et al. Apoptosis inducing bivalent single-chain antibody fragments against CD47 showed antitumor potency for multiple myeloma. Leuk Res. 2005;29:445–50. doi: 10.1016/j.leukres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metayer LE, Vilalta A, Burke GAA, Brown GC. Anti-CD47 antibodies induce phagocytosis of live, malignant B cells by macrophages via the Fc domain, resulting in cell death by phagoptosis. Oncotarget. 2017;8:60892–903. doi: 10.18632/oncotarget.18492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theocharides AP, Jin L, Cheng PY, Prasolava TK, Malko AV, Ho JM, et al. Disruption of SIRPα signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J ExpMed. 2012;209:1883–99. doi: 10.1084/jem.20120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Yin C, Feng L, Wang C, Sheng G. Ara-C and anti-CD47 antibody combination therapy eliminates acute monocytic leukemia THP-1 cells in vivo and in vitro. Genet Mol Res. 2015;14:5630–41. doi: 10.4238/2015.May.25.15. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 42.Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, et al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7:946–56. doi: 10.1080/19420862.2015.1062192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118:4890–901. doi: 10.1182/blood-2011-02-338020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103–8. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XJ, Pu Y, Cron K, Deng LF, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209. doi: 10.1038/nm.3931. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Wang LJ, Zhao FF, Tseng S, Narayanan C, Shura L, et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao MP, Alizadeh AA, Tang CZ, Myklebust JH, Varghese B, Jan M, et al. CD47 is a prognostic factor and an antibody target that synergizes with rituximab to eradicate non-Hodgkin lymphoma. Cancer Res. 2010;70 [Google Scholar]
- 48.Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao FF, et al. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res. 2011;71:1374–84. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansell S, Chen RW, Flinn IW, Maris MB, O’Connor OA, Johnson LD, et al. A Phase 1 Study of TTI-621, a Novel Immune Checkpoint Inhibitor Targeting CD47, in Patients with Relapsed or Refractory Hematologic Malignancies. Blood. 2016;128:1812. [Google Scholar]
- 50.Weiskopf K, Ring AM, Ho CCM, Volkmer JP, Levin AM, Volkmer AK, et al. Engineered SIRP alpha Variants as Immunotherapeutic Adjuvants to Anticancer Antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs. 2015;7:303–10. doi: 10.1080/19420862.2015.1011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghiran I, Klickstein LB, Nicholson-Weller A. Calreticulin is at the surface of circulating neutrophils and uses CD59 as an adaptor molecule. J Biol Chem. 2003;278:21024–31. doi: 10.1074/jbc.M302306200. [DOI] [PubMed] [Google Scholar]
- 53.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 54.Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer. 2017;76:100–9. doi: 10.1016/j.ejca.2017.02.013. [DOI] [PubMed] [Google Scholar]


