Abstract
Aim
To evaluate risks of restrictive red-blood cell transfusion strategies (hemoglobin 7–8 g/dL) in patients with and without known cardiovascular disease (CVD).
Background
Recent guidelines recommend restrictive strategies for CVD-patients hospitalized for non-CVD indications, patients without known CVD and patients hospitalized for CVD corrective procedures.
Methods/Materials
Database searches were conducted through December 2017 for randomized-clinical trials that enrolled patients with and without known CVD, hospitalized either for CVD corrective procedures or non-cardiac indications, comparing effects of liberal to restrictive strategies on major adverse coronary events (MACE) and death.
Results
In CVD patients not undergoing cardiac interventions, a liberal strategy decreased (p=0.01) the relative risk (95%CI) (RR) of MACE [0.50 (0.29–0.86)] (I2=0%). Among patients without known CVD, the incidence of MACE was lower (1.7 vs 3.9%), and the effect of a liberal strategy on MACE [0.79, (0.39–1.58)] was smaller and nonsignificant, but not different from CVD patients (p=0.30). Combining all CVD and non-CVD patients, a liberal strategy decreased MACE [0.59, (0.39–0.91); p=0.02]. Conversely, among studies reporting mortality, a liberal strategy decreased mortality in CVD patients (11.7% vs.13.3%), but increased mortality (19.2% vs. 18.0%) in patients without known-CVD [interaction p=0.05; ratio of RR 0.73, (0.53–1.00)]. A liberal strategy also did not benefit patients undergoing cardiac surgery; data was insufficient for percutaneous cardiac procedures.
Conclusions
In patients hospitalized for non-cardiac indications, liberal transfusion strategies are associated with a decrease risk of MACE in both those with and without known CVD. However, this only provides a survival benefit to CVD-patients not admitted for CVD corrective procedures.
Keywords: Blood transfusion, Restrictive transfusion strategy, Liberal transfusion strategy, Cardiovascular disease, Transfusion trigger
INTRODUCTION
Two recently published guidelines from national associations including the AABB, formerly the American Association of Blood Banks (Carson et al. 2016), and the UK National Clinical Guideline Centre (NCGC) (Alexander et al. 2016), suggest that a restrictive red-blood cell (RBC) transfusion threshold of 7–8 g/dL for haemoglobin is safe in most clinical settings. In addition, the current AABB guidelines recommend a transfusion trigger of 8 g/dL for hospitalized patients with pre-existing cardiovascular disease (CVD), including those undergoing cardiac surgery. The AABB makes no recommendation for patients with acute coronary syndrome due to the lack of sufficient data from randomized clinical trials, whereas the NCGC recommends a threshold of 8 g/dL and a target value of 8–10 g/dL for this subset of patients.
Few trials of RBC transfusion triggers have focused on patients with underlying CVD. Most available data on transfusion practices for patients either with or without CVD, stem from decades-old retrospective analyses of large electronic medical record databases (Yang et al. 2005; Alexander et al. 2008; Salisbury et al. 2011; Salisbury et al. 2014; Sherwood et al. 2014; Magruder et al. 2017). These studies compare nadir haemoglobin or haematocrit values among transfused and non-transfused patients with CVD without identifying the specific transfusion thresholds or clinical indicators used to justify RBC transfusion (Alexander et al. 2008; Salisbury et al. 2011). The majority of studies do suggest that there is significant variability in transfusion practices among different centres (Hutton et al. 2005; Sherwood et al. 2014; Magruder et al. 2017). However, no trials have investigated whether the risks of restrictive or liberal strategies differ in patients with CVD compared to those without known CVD or how these risks compare in CVD patients hospitalized for cardiac corrective procedures vs. non-cardiac indications.
Two recent meta-analyses did summarize data evaluating transfusion thresholds in hospitalized patients with pre-existing CVD (Patel et al. 2015; Docherty et al. 2016). One study found a significantly increased incidence of myocardial infarction but not mortality associated with a restrictive transfusion threshold in known-CVD patients hospitalized for non-cardiac indications (Docherty et al. 2016). The other study reported no significant increase in mortality with a restrictive strategy among CVD patients undergoing cardiac corrective procedures (Patel et al. 2015). However, neither review directly addressed whether a restrictive strategy presents different risks in CVD compared to non-CVD patients, or directly compared whether these risks differ in CVD patients admitted for a corrective cardiac procedure (e.g. coronary surgery) vs. non-cardiac indications. These are important questions given that most guidelines still recommend restrictive RBC transfusion triggers for all patients including those with or without known CVD and regardless of whether they are hospitalized for cardiac corrective procedures or not.
A single comprehensive analysis of the literature was performed to address these questions. We investigated whether the effects of restrictive or liberal transfusion policies on the incidence of myocardial infarction and acute coronary syndromes (major acute coronary events, MACE) (Hirji et al. 2017) and mortality in CVD patients admitted for non-cardiac reasons were similar (main effect) or significantly different (qualitative interaction) compared either to patients without known CVD or to CVD patients having cardiac corrective procedures.
MATERIALS AND METHODS
We included all published randomized clinical trials comparing restrictive and liberal transfusion triggers that provided data on patients with and without known CVD admitted for non-cardiac reasons and all published randomized clinical trials comparing the two transfusion strategies in CVD patients having cardiac corrective procedures. We further divided the cardiac corrective procedures into two types; patients having cardiac coronary surgery bypass grafts and/or valve surgery as one type; and those having percutaneous coronary interventions (PCI) as another. Trials were included if they enrolled and reported outcomes of patients with either known CAD (acute coronary syndrome or chronic ischemic heart disease) or other forms of CVD (including cardiac failure, valve disease and peripheral vascular disease). Major acute coronary events (MACE, myocardial infarction or acute coronary syndrome), and mortality rates were the selected end-points of interest.
Literature search and study selection
We searched PubMed, EMBASE, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials from database inception to December 31, 2017 without language restrictions for publications of randomized clinical trials comparing the use of a higher (liberal) and lower (restrictive) RBC transfusion trigger among adult patients with known CVD as well as without known CVD hospitalized for non-cardiac indications and those studies of CVD patients having corrective procedures either heart surgery or PCI. We also scanned the reference lists of analysed studies and references cited in reviews and guidelines identified in the searches.
To retrieve as much information as possible from all previous meta-analyses that included CVD patients, we requested from investigators any data regarding MACE or mortality not reported in the published studies (see S1 Supporting Information).
Statistical analysis
Risk ratios of mortality and MACE were analysed using random-effects models (DerSimonian et al. 1986), with 0.5 added to each cell in the studies with zero cells. Heterogeneity among studies was assessed using the Q statistic and I2 value (Higgins et al. 2002).
For the included trials enrolling both patients with and without known CVD, in order to assess differences in treatment effects between these two patient groups (i.e., interaction), we used two methods to account for the correlation within each study: 1) a “one-step” approach with the study as a blocking factor; and 2) a “two-step” approach by first calculating the difference of log (RR) within each study, and then combining them using a standard random-effect model. These two methods gave almost identical results, so we only presented the latter for better visualization.
All analyses were performed using R version 3.4.3 (R Development Core Team, 2017). Traditional meta-analysis where performed using R packages meta version 4.9-0 (Schwarzer 2015) and metafor version 2.0-0 (Viechtbauer 2010). Two-sided p-values ≤0.05 were considered significant. The Bayesian meta-analysis was performed using R package bmeta version 0.1.2 (cran.r-project.org/web/packages/bmeta). We used RR with normal approximation. Random-effect models with non-informative priors were used.
RESULTS
Study characteristics
Fig. 1 shows a flowchart of study selection and enrolment. Seventeen randomized clinical trials met inclusion criteria. Nine included patients with and without known pre-existing CVD hospitalized for various non-cardiac indications [peripheral vascular surgery (Bush et al. 1997), critical care (Hebert et al. 1999; Walsh et al. 2013), orthopaedics (Carson et al. 2011; Parker 2013; Gregersen et al. 2015), septic shock (Holst et al. 2014), surgical oncology (de Almeida et al. 2015) and acute upper GI bleeding (Jairath et al. 2015)]. Six studies enrolled cardiac surgery patients (Bracey et al. 1999; Murphy et al. 2007; Hajjar et al. 2010; Shehata et al. 2012; Murphy et al. 2015; Mazer et al. 2017), two enrolled PCI patients (Cooper et al. 2011; Carson et al. 2013), Characteristics of the included studies are summarized in Table 1 and S1 Table. Patients were enrolled from July 1994 through March 2017. These seventeen studies enrolled from 45 to 5243 patients each, for a total of 14397 patients. Of these studies; nine provided data for 2048 patients with known CVD and 3337 without known CVD, all admitted for non-cardiac indications; six provided data for 7343 patients with CVD admitted for cardiac surgery; and two provided data for 154 patients with CVD admitted for PCI.
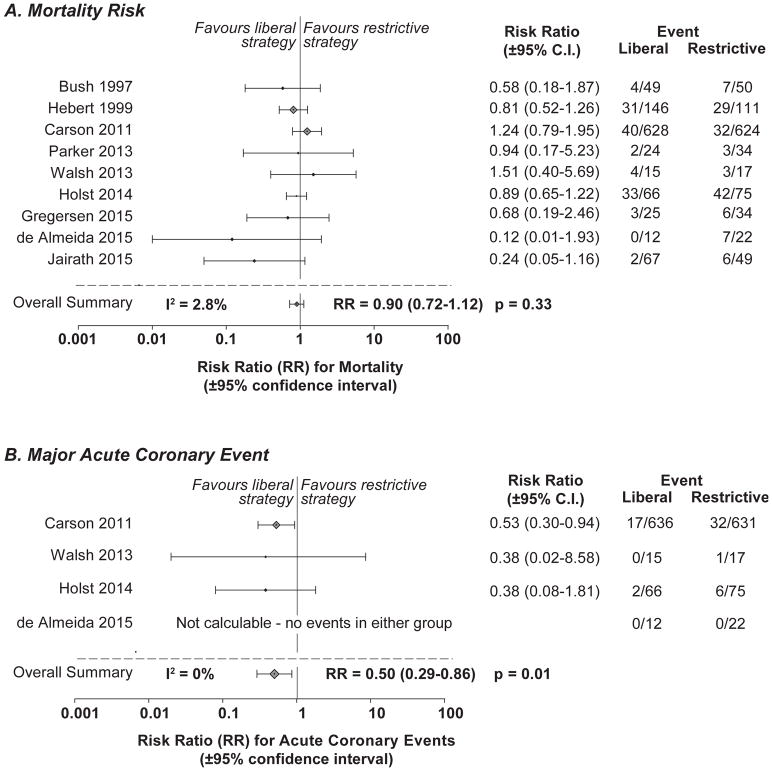
Fig. 1.
PRISMA flow diagram
Table 1.
Characteristics of included studies
| Study | Year | Cardiac Disease |
Classification | Setting | Enrolled Patients | Years of Enrolment |
Transfusion Triggers | Mortality | Major Acute Coronary Events |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Restrictive | Liberal | Restrictive | Liberal | ||||||||
| CARDIOVASCULAR DISEASE PATIENTS HOSPITALIZED FOR NON-CARDIAC REASONS | |||||||||||
| Bush | 1997 | Chronic | Vascular Surgery | Elective Aortic or infrainguinal arterial reconstruction | 99 randomised - all CVD | August 1995– November 1996 | Hb = 9 g/dL vs 10 g/dL | 7/50 | 4/49 | N/A | N/A |
| Hebert | 1999 | Chronic | Critical Care | Mixed ICU | 838 randomised - 257 CVD / 581 non-CVD | November 1994 – November 1997 | Hb = 7 g/dL vs 10 g/dL | 29/111 | 31/146 | N/A | N/A |
| Carson | 2011 | Chronic | Orthopaedics | Hip Surgery | 2016 randomised - 1268CVD / 748 non-CVD | July 2004 – February 2009 | Hb = 8 g/dL vs 10 g/dL | 32/624 | 40/628 | 32/631 | 17/636 |
| Parker | 2013 | Chronic | Orthopaedics | Hip Surgery | 200 randomised - 87 CVD / 113 non-CVD | August 2002 – May 2012 | Hb = 8 g/dL vs 10 g/dL | 3/34 | 2/24 | N/A | N/A |
| Walsh | 2013 | Chronic | Critical Care | Mixed ICU | 100 randomised - 32 CVD / 68 non-CVD | August 2009 – December 2010 | Hb = 7 g/dL vs 9 g/dL | 3/17 | 4/15 | 1/17 | 0/15 |
| Holst | 2014 | Chronic | Septic Shock | Septic Shock | 1005 randomised - 141 CVD / 857 non-CVD | December 2011 – December 2013 | Hb = 7 g/dL vs 9 g/dL | 42/75 | 33/66 | 6/75 | 2/66 |
| Gregersen | 2015 | Chronic | Orthopaedics | Hip Surgery | 284 randomised - 59 CVD / 225 non-CVD | January 2010 – June 2013 | Hb = 9.7 g/dL vs 11.3 g/dL | 6/34 | 3/25 | N/A | N/A |
| de Almeida | 2015 | Chronic | Surgical Oncology | Surgical Oncology | 198 randomised - 34 CVD / 163 non-CVD | January 2012 – July 2012 | Hb = 7 g/dL vs 9 g/dL | 7/22 | 0/12 | 0/22 | 0/12 |
| Jairath | 2015 | Chronic | Acute Upper GI bleeding | Acute Upper GI bleeding | 936 enrolled - 176 CVD / 760 non-CVD | September 2012 – March 2013 | Hb = 8 g/dL vs 10 g/dL | 6/49 | 2/67 | N/A | N/A |
| CARDIAC SURGERY PATIENTS | |||||||||||
| Bracey | 1999 | Acute | Cardiac Surgery | CABG | 437 randomised | February 1997 – November 1997 | Hb = 8g/dL vs physician orders (9 g/dL by protocol) | 3/215 | 6/222 | 1/212 | 0/216 |
| Murphy | 2007 | Acute | Cardiac Surgery | CABG and/or valve | 321 randomised | September 2005 – October 2006 | Hb = 7g/dL vs 8 g/dL | 5/162 | 3/159 | N/A | N/A |
| Hajjar | 2010 | Acute | Cardiac Surgery | CABG | 512 randomised | February 2009 – February 2010 | HTC = 24% vs 30% | 15/249 | 13/253 | N/A | N/A |
| Shehata | 2012 | Acute | Cardiac Surgery | CABG and/or valve | 50 randomised | January 2007 – June 2010 | Hb = 7–7.5 g/dL vs 9.5–10 g/dL | 4/25 | 1/25 | 1/25 | 0/25 |
| Murphy | 2015 | Acute | Cardiac Surgery or Vascular Surgery | CABG and/or valve or Major Aortic Procedure | 2003 randomised | July 2009 – February 2013 | Hb = 7.5 g/dL vs 9 g/dL | 26/1000 | 19/1003 | 3/987 | 4/981 |
| Mazer | 2017 | Acute | Cardiac Surgery | CABG and/or valve | 5243 randomised – 827 excluded* | January 2014 – March 2017 | Hb < 7.5g/dL vs < 9.5g/dL | 61/1994 | 68/2036 | 117/1994 | 117/2036 |
| PERCUTANEOUS CORONARY INTERVENTION PATIENTS | |||||||||||
| Cooper | 2011 | Acute | PCI | ACS or stable angina | 45 randomised | May 2003 – October 2009 | HTC = 24% vs 30% | 2/24 | 1/21 | 1/24 | 0/21 |
| Carson | 2013 | Acute | PCI | ACS | 110 randomised | March 2010 – May 2012 | Hb = 8 g/dL vs 10 g/dL | 7/54 | 1/55 | 7/54 | 5/55 |
other “non-CABG category” contained non-prespecified groups such as myxoma surgery
Overall mortality and MACE risk in patients with pre-existing CVD admitted for non-CVD indications
Nine trials provided data on the mortality endpoint. Among patients with known CVD hospitalized for non-CVD indications, there were 119 deaths among the 1032 CVD patients reported in the liberal transfusion group and 135 deaths among the 1016 CVD patients in the restrictive transfusion group (Fig. 2A). There was no evidence of significant heterogeneity between studies for the mortality endpoint (I2=2.8%, p=0.41). Overall, a liberal compared to a restrictive transfusion strategy produced a nominally but not statistically significant decreased relative risk of mortality (95%CI) in known CVD patients hospitalized for non-CVD indications [0.90 (0.72–1.12), p=0.33].
Fig. 2. Forest plots of relative risks for mortality and major acute coronary events (MACE, myocardial infarction and acute coronary syndrome) in patients with pre-existing cardiovascular disease hospitalized for non-cardiac indications.
The relative risk (RR) (closed diamonds, liberal versus restrictive) and 95% confidence interval (CI) (horizontal bars) are plotted for each individual trial included in the analysis for mortality (upper panel) and MACE (lower panel).
Four of the nine studies provided data on the MACE endpoint in these known CVD patients admitted for non-CVD indications. MACE occurred in 19 of 729 CVD patients in the liberal transfusion group and 39 of 745 CVD patients in the restrictive transfusion group (Fig. 2B). There was no evidence of heterogeneity across individual studies for the MACE endpoint (I2=0%, p=0.91). A liberal compared to restrictive transfusion strategy was associated with a significantly decreased risk of MACE [0.50, (0.29–0.86), p=0.01] among all patients with known pre-existing CVD hospitalized for non-CVD indications.
Subgroup analyses
Based on significant findings for MACE, we performed a sensitivity analysis to determine the consistency of the risk for both MACE and mortality across studies using lower and higher restrictive transfusion triggers. Fig. 3 shows mortality and MACE endpoints when the restrictive transfusion trigger was less than 8 g/ (~7 g/dL) or 8 g/dL or greater (~ 8 g/dL) in CVD patients. The decreased risk of mortality across the studies comparing a liberal trigger vs. a restrictive trigger of ~ 7 g/dL [0.87 (0.68–1.12), I2=0%], and across studies comparing a liberal trigger vs. a restrictive trigger of ~ 8 g/dl [0.83 (0.49–1.42), I2=23%], were each not statistically significant, but were both similar to the overall decrease [0.90 (0.72–1.12)] (Fig. 3A). The decreased risk of MACE in pre-existing CVD patients across the studies comparing a liberal trigger vs. a restrictive transfusion trigger of ~7 g/dL [0.38 (0.09–1.53), I2=0%] and across the studies comparing a liberal vs. a restrictive trigger of ~ 8 g/dl [0.53 (0.30–0.94)] was significant only for the latter (p=0.03), but both were similar to the overall decrease [0.50 (0.29–0.86), I2=0%] (Fig. 3B).
Fig. 3. Sensitivity analysis - Forest plots of relative risks for mortality and MACE for subgroups of patients with pre-existing cardiovascular disease hospitalized for non-cardiac indications.
The relative risk (RR) (closed diamonds, liberal versus restrictive) and 95% confidence interval (CI) (horizontal bars) are plotted for each individual trial included in the analysis for mortality (upper panel) and acute coronary events (lower panel) for two subgroups: the studies employing a restrictive transfusion trigger of < 8 g/dL (left panels), and the studies employing a restrictive transfusion trigger of 8g/dL or greater (right panels).
Comparing MACE and mortality risks in patients with pre-existing CVD to those without known CVD
For these studies, we next compared the overall relative risk of a liberal vs. restrictive transfusion strategy on the primary endpoints of MACE reported in four of the nine trials and death reported in all nine trials among patients with pre-existing CVD compared to those without known CVD. A liberal compared to a restrictive transfusion strategy reduced the relative risk of MACE in patients both with known CVD [2.6% versus 5.2%; 0.50, (0.29–0.86)] and without known CVD (1.5% versus 1.9%; 0.79, (0.39–1.58) in beneficial patterns that were similar (p=0.30 for interaction, Fig. 4B). In fact, the relative decreases in risk of MACE using a liberal rather than a restrictive strategy in patients with vs. without known CVD were remarkably alike (RR 0.50 vs. 0.79 respectively) and had 95% confidence intervals that almost completely overlapped [CI (0.29–0.86) vs. (0.39–1.58) respectively]. As expected, the absolute incidence of MACE among hospitalized patients managed with a restrictive strategy was much greater in those with pre-existing CVD compared to those without known CVD (5.2% vs. 1.9% absolute incidence of MACE, respectively). However, a similar relative decrease in MACE was found employing a liberal strategy, despite these unsurprising differences in the absolute incidence of MACE. When examined over all patients from these four studies, whether they had known or unknown pre-existing CVD, a liberal compared to a restrictive transfusion strategy was associated with a significant decrease in MACE [0.59 (0.39–0.91), p=0.02].
Fig. 4. Combined mortality and MACE rates for enrolled patients with and without known pre-existing cardiovascular disease hospitalized for non-cardiac indications.
The combined percentage rate for mortality (left panel) and MACE (right panel) in patients with (closed circles) and without (open circles) cardiovascular disease are plotted here for a liberal and restrictive strategy (x axis) from the eight trials providing mortality data and four trials providing MACE data on enrolled patients with and without cardiovascular disease.
Conversely, among the nine studies reporting on the mortality endpoint separately for patients with and without known pre-existing CVD and hospitalized for non-cardiac indications, the overall effect of a liberal strategy on mortality was significantly different and opposite depending on whether patients had known CVD or not (p=0.05, qualitative interaction) (Fig. 4A, S1 Fig). In patients randomized to a liberal strategy, mortality rates were lower among those with known pre-existing CVD (11.7% vs.13.3%, respectively), but higher in those without known CVD (19.2% vs. 18.0%, respectively). The ratio (95%CI) of the relative risks of death with a liberal strategy in patients with compared to those without known CVD was significant [0.73 (0.53–1.00), p=0.05]. This divergent effect of a liberal strategy on mortality in patient with and without known CVD is at odds with its directionally similar effects on MACE.
MACE and mortality risks comparing patients with CVD hospitalized for non-cardiac indications versus cardiac corrective procedures including either cardiac surgery or PCI
The effect of a liberal vs. restrictive transfusion strategy on mortality was not statistically significantly different (p=0.26, interaction) in patients with CVD hospitalized for non-cardiac indications compared to those hospitalized for cardiac corrective procedures including either PCI or cardiac surgery (Fig. 5B). The relative risk of death was not statistically significantly decreased with a liberal strategy in patients with known CVD hospitalized for non-cardiac reasons [0.90 (0.73–1.11), p=0.32] or for those having either PCI [0.26 (0.06–1.22), p=0.09] or cardiac surgery [0.96 (0.74–1.24), p=0.73]. Overall though, among the 17 studies providing data for patients with CVD hospitalized for non-cardiac indications and for cardiac corrective procedures, a liberal strategy was associated with a nominal decrease in death that was not statistically significant [0.91 (0.77–1.07), p=0.25].
Fig. 5. Combined mortality and MACE rates for cardiovascular disease patients hospitalized for non-cardiac indications, or percutaneous cardiac corrective procedures or cardiac surgery.
The combined overall relative risk for mortality (left panels) and MACE (right panels) in patients with cardiovascular disease are plotted here for a liberal vs. restrictive strategy for cardiovascular disease patients hospitalized for cardiac surgery (top panels), or percutaneous cardiac corrective procedures (middle panels) or non-cardiac indications (bottom panels).
There was a quantitative interaction for the effect of a liberal transfusion strategy on MACE comparing these three different CVD groups (p=0.08 for interaction) that appeared ordered and prevented averaging over them (Fig. 5). Specifically, a liberal strategy decreased the relative risk of MACE significantly in patients with CVD hospitalized for non-cardiac indications [0.50 (0.29–0.86), p=0.01] and nominally in those with CVD having PCI [0.66, (0.24–1.83) p=0.42], but not at all in those with CVD having cardiac surgery [0.98 (0.76–1.24), p=0.84].
Bayesian analysis
The posterior estimate of RR for mortality (liberal vs restrictive transfusion strategy) is 0.85 with 95% Credible Interval (CrI) (0.56, 1.18), with an 83% posterior probability of the liberal strategy being better. The posterior estimate of RR for myocardial infarction (liberal vs restrictive) is 0.47 with 95% CrI (0.02, 9.87), with an 84% posterior probability of the liberal strategy being better.
DISCUSSION
Seventeen randomized clinical trials enrolling 14,397 hospitalized patients provided data for 12,882 patients on the primary endpoints examined here, either for patients with and without known CVD hospitalized for non-cardiac indications or for CVD patients hospitalized for cardiac corrective procedures. Four trials enrolled patients with pre-existing CVD hospitalized for non-cardiac reasons and provided MACE data. Among the 1474 patients included, a liberal transfusion trigger was associated with a 50% decrease in the risk of MACE. This effect was consistent independent of whether a restrictive threshold of ~7 g/dL or ~ 8 g/dL of haemoglobin was studied. This effect was also similar (I2=0%, P=0.91) for these patients regardless of the setting (critical care, orthopaedics, septic shock, and surgical oncology). These four trials also included patients without known underlying CVD and among these 1853 patients, a liberal transfusion strategy similarly decreased the relative risk of MACE. In addition to these four trials of non-cardiac hospital settings, we found six trials including CVD patients hospitalized for corrective cardiac procedures including either PCI (two trials) or cardiac surgery (four trials). When comparing the effect of a liberal transfusion strategy in CVD patients between trials of non-cardiac hospitalizations and those for corrective cardiac procedures, the benefit on MACE was statistically significant for non-cardiac settings, but only nominally so for PCI patients and not at all significant for cardiac corrective surgery patients. Correcting the CVD abnormality with PCI or surgery may decrease or nullify the benefit of a liberal transfusion strategy on MACE during any hospitalization.
Overall, in nine trials including 1949 patients with pre-existing CVD hospitalized for non-cardiac indications compared to 3337 admitted without known CVD, the effect of a liberal transfusion trigger on mortality was significantly different and opposite (p=0.05, qualitative interaction). A liberal transfusion trigger increased mortality in patients without known CVD, but decreased mortality in those with pre-existing CVD. To explain these unexpected and opposite effects on mortality, it seems likely that some additional and independent adverse survival effect of a liberal strategy was predominately affecting patients without known CVD. This adverse effect was sufficient to reverse the potential beneficial effect on mortality expected from decreasing MACE. These findings are consistent with results from the Transfusion in Critical Care trial (TRICC) for mortality in patients with and without severe cardiovascular disease managed with either a liberal or restrictive strategy (Hebert et al. 2001; Deans et al. 2007). In the TRICC trial, younger and healthier patients who were less likely to have CVD and would not normally be transfused during usual care, had the greatest fold increase in mortality in the liberal versus the restrictive arm. Lastly, comparing patients with CVD hospitalized for non-cardiac indications vs. cardiac interventions, a liberal strategy had a nominally beneficial effect on mortality rates but this difference was not statistically significant.
Two studies in this meta-analysis had a combined weight of over 70% here. One was a large multi-centre transfusion trigger trial, enrolling more than 2000 orthopaedic surgery patients (Carson et al. 2011). One of the most rigorously conducted studies to date using this methodology, this trial found a significant increase after 30-days in the incidence of MACE among orthopaedic patients with CVD and treated with a restrictive strategy (J. Carson, personal communication). The second publication (Mazer et al. 2017) studied more than 5000 cardiac surgery patients and found that higher transfusion triggers were not associated with meaningful differences in the risks of MACE and mortality. Taken together, these data support the notion that for patients with chronic CVD requiring hospitalization for a serious non-cardiac problem, the risk of MACE may be reduced by maintaining haemoglobin levels > 9.5 g/dL. In contrast, if a CVD patient is admitted for correction of a cardiovascular abnormality with surgery, maintaining higher haemoglobin levels does not appear to be beneficial. Determining with any certainty whether there is any benefit to a liberal strategy during less invasive cardiac corrective procedures such as PCI is hampered by insufficient data. There were only two trials enrolling 155 patients providing data on this question. However, one of these trials showed a significant increase in mortality with a restrictive strategy (Carson 2013).
We recognize limitations to the interpretation of these data. We excluded trials not specifying inclusion of patients with CVD and trials in which requests for data on CVD patients were made but not provided or not available. This could have led to unrecognized selection bias. As discussed above, after the enrolment period of studies for our meta-analysis ended, a very large cardiac surgery trial studying transfusion triggers in over 5000 patients was published (Mazer et al. 2017). Due to the influential nature of this trial, we reopened enrolment until December of 2017 with another search. Thus, this analysis is not prospective but retrospective. Other limitations include the following. The specific transfusion thresholds and the definitions of CVD and acute coronary syndrome varied between studies. Even though effect sizes were determined within trials first and combined effects determined, this may have had unrecognized consequences. Some of the outcomes analysed from specific trials were not primary or pre-specified endpoints. Also, some of the subgroups examined were not prospectively stratified in trials and were not part of an original randomization scheme. Therefore, the data analysed here should be considered hypothesis-generating, rather than definitive. However, the importance of these data rests less on the definitive nature of the individual findings than on their consistency.
In patients both with and without known pre-existing CVD and admitted for non-cardiac indications in these studies, the decrease in the relative risk of MACE with a liberal vs. a restrictive strategy was similar across different reasons for hospitalization and regardless of whether a restrictive strategy used a haemoglobin threshold of 7 or 8 g/dL. Moreover, the overall point estimates we examined were always on the side of increased mortality and MACE rates for a restrictive transfusion trigger regardless of whether the patient was admitted for non-cardiac reasons, PCI or cardiac surgery, although the benefit was minimal or non-existent for mortality and MACE in patients hospitalized for cardiac surgery. From 17 trials, we obtained 26 individual estimates in this analysis for risk of death and MACE with a restrictive versus liberal transfusion strategy (see Fig. 5). Of these 26 estimates, 21 favoured a liberal strategy, and five a restrictive strategy. No study to date has found a restrictive transfusion strategy to be significantly beneficial in CVD patients and at least two studies (Carson et al. 2011 and 2013) and one other meta-analysis (Docherty et al. 2016) found that a liberal strategy was significantly beneficial in CVD patients not undergoing cardiac surgery.
To determine probabilities for the significant adverse effect of a restrictive strategy on MACE in patients with pre-existing CVD hospitalized for non-cardiac indications, we performed a Bayesian analysis. The probability that a restrictive strategy is worse than a liberal one was 84% for MACE and 83% for mortality. These findings are based on nine studies enrolling thousands of patients with and without known CVD. Likewise, a recent meta-analysis of transfusion trigger trials examining older hospitalized patients (>65 years old) likely to be at increased risk for CVD reported a significant increase in 30 and 90-day mortality with a restrictive transfusion strategy (Simon et al. 2017). Nevertheless, an appropriately designed prospective, large multicentre transfusion trial of patients with known pre-existing CVD or at high risk for CVD and admitted for non-cardiac procedures would be needed to provide definitive evidence and determine best transfusion practices. Risks in such a study could be minimized by enrolling only patients cared for by physicians who currently approve of and/or use a restrictive RBC transfusion strategy in CVD patients, or by including only centres with policies mandating restrictive strategies in CVD patients.
The present study supports transfusion guidelines that recommend restrictive rather than liberal transfusion strategies for CVD patients undergoing cardiac corrective procedures. There are insufficient data to make such a recommendation for less invasive cardiac procedures such as PCI. In contrast, for hospitalized patients admitted for non-cardiac reasons with pre-existing CVD or at high risk for CVD, clinicians should be reluctant to adopt restrictive transfusion strategies until prospective studies provide additional information.
Supplementary Material
Acknowledgments
The contributions of authors are as follows: Irene Cortés-Puch and Charles Natanson (performed the research, designed the research study, wrote the paper, analysed the paper); Junfeng Sun (analysed the paper); Judith Welsh (Software, Data Curation); Brandon W. Wiley, Harvey G. Klein, Robert L. Danner and Peter Q. Eichacker (analysed the paper, wrote the paper). The authors would like to thank Julie Maltagliati and Kelly Byrne for help editing and submitting the manuscript.
Funding
This research was supported by the Intramural Research Program of the NIH, Clinical Center.
Footnotes
Competing interests: The authors have no competing interests.
References
- Alexander J, Cifu AS. Transfusion of red blood cells. Journal of the American Medical Association. 2016;316:2038–2039. doi: 10.1001/jama.2016.12870. [DOI] [PubMed] [Google Scholar]
- Alexander KP, Chen AY, Wang TY, Rao SV, Newby LK, LaPointe NM, Ohman EM, Roe MT, Boden WE, Harrington RA, Peterson ED. Transfusion practice and outcomes in non-ST-segment elevation acute coronary syndromes. American Heart Journal. 2008;155:1047–1053. doi: 10.1016/j.ahj.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Angus CW, Klivington D, Wyman J, Kovacs JA. Nucleic acid vaccination against Toxoplasma gondii in mice. Journal of Eukaryotic Microbiology. 1996;43:117S. doi: 10.1111/j.1550-7408.1996.tb05034.x. [DOI] [PubMed] [Google Scholar]
- Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, Radovancevic B, McAllister HA, Jr, Cooley DA. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070–1077. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. American Journal of Surgery. 1997;174:143–148. doi: 10.1016/s0002-9610(97)00073-1. [DOI] [PubMed] [Google Scholar]
- Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV, Noveck H, Passano E, Hardison RM, Smitherman T, Vagaonescu T, Wimmer NJ, Williams DO. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. American Heart Journal. 2013;165:964–971. e961. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. Journal of the American Medical Association. 2016;316(19):2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. New England Journal of Medicine. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, Panza JA. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study) American Journal of Cardiology. 2011;108:1108–1111. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- de Almeida JP, Vincent JL, Galas FR, de Almeida EP, Fukushima JT, Osawa EA, Bergamin F, Park CL, Nakamura RE, Fonseca SM, Cutait G, Alves JI, Bazan M, Vieira S, Sandrini AC, Palomba H, Ribeiro U, Jr, Crippa A, Dalloglio M, del Diz MP, Kalil Filho R, Auler JO, Jr, Rhodes A, Hajjar LA. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology. 2015;122(1):29–38. doi: 10.1097/ALN.0000000000000511. [DOI] [PubMed] [Google Scholar]
- Deans KJ, Minneci PC, Suffredini AF, Danner RL, Hoffman WD, Ciu X, Klein HG, Schechter AN, Banks SM, Eichacker PQ, Natanson C. Randomization in clinical trials of titrated therapies: unintended consequences of using fixed treatment protocols. Critical Care Medicine. 2007;35:1509–1516. doi: 10.1097/01.CCM.0000266584.40715.A6. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst L, Parker M, Gregersen M, De Almeida JP, Walsh TS, Stanworth SJ. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis. British Medical Journal (online) 2016 Mar 29;352:i1351. doi: 10.1136/bmj.i1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthopaedica. 2015;86:363–372. doi: 10.3109/17453674.2015.1006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leao WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO., Jr Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. Journal of the American Medical Association. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. New England Journal of Medicine. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Critical Care Medicine. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hirji SA, Stevens SR, Shaw LK, Campbell EC, Granger CB, Patel MR, Sketch MH, Jr, Wang TY, Ohman EM, Peterson ED, Brennan JM. Predicting risk of cardiac events among ST-segment elevation myocardial infarction patietns with conservatively managed non-infarct-related artery coronary artery disease: An analysis of the Duke Databank for Cardiovascular Disease. American Heart Journal. 2017;194:116–124. doi: 10.1016/j.ahj.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JR, Nielsen JS, Oldner A, Pettila V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Muller RG, Moller MH, Steensen M, Tjader I, Kilsand K, Odeberg-Wernerman S, Sjobo B, Bundgaard H, Thyo MA, Lodahl D, Maerkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A TRISS Trial Group & Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. New England Journal of Medicine. 2014;371(15):1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- Hutton B, Fergusson D, Tinmouth A, McIntyre L, Kmetic A, Hebert PC. Transfusion rates vary significantly amongst Canadian medical centres. Canadian Journal of Anaesthesia. 2005;52:581–590. doi: 10.1007/BF03015766. [DOI] [PubMed] [Google Scholar]
- Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, Stanley AJ, Everett SM, Bailey AA, Dallal H, Greenaway J, Le Jeune I, Darwent M, Church N, Reckless I, Hodge R, Dyer C, Meredith S, Llewelyn C, Palmer KR, Logan RF, Travis SP, Walsh TS, Murphy MF. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137–144. doi: 10.1016/S0140-6736(14)61999-1. [DOI] [PubMed] [Google Scholar]
- Magruder JT, Blasco-Colmenares E, Crawford T, Alejo D, Conte JV, Salenger R, Fonner CE, Kwon CC, Bobbitt J, Brown JM, Nelson MG, Horvath KA, Whitman GR. Variation in red blood cell transfusion practices during cardiac operations among centers in Maryland: results from a state quality-improvement collaborative. Annals of Thoracic Surgery. 2017;103:152–160. doi: 10.1016/j.athoracsur.2016.05.109. [DOI] [PubMed] [Google Scholar]
- Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, Khanykin B, Gregory AJ, de Médici E, McGuinness S, Royse A, Carrier FM, Young PJ, Villar JC, Grocott HP, Seeberger MD, Fremes S, Lellouche F, Syed S, Byrne K, Bagshaw SM, Hwang NC, Mehta C, Painter TW, Royse C, Verma S, Hare GMT, Cohen A, Thorpe KE, Jüni P, Shehata N TRICS Investigators and Perioperative Anesthesia Clinial Trials Group. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. New England Journal of Medicine. 2017;377:2133–2144. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, Reeves BC TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. New England Journal of Medicine. 2015;372:997–1008. doi: 10.1056/NEJMoa1403612. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rizvi SIA, Battaglia F, Culliford L, Rogres C, Cohen A, Angelini GD. A pilot randomized controlled trial of the effect of transfusion threshold reduction on transfusion rates and morbidity after cardiac surgery. Transfusion Alternatives in Transfusion Medicine. 2007;9(Suppl 1):41–42. [Google Scholar]
- Parker MJ. Randomised trial of blood transfusion versus a restrictive transfusion policy after hip fracture surgery. Injury. 2013;44:1916–1918. doi: 10.1016/j.injury.2013.04.033. [DOI] [PubMed] [Google Scholar]
- Patel NN, Avlonitis VS, Jones HE, Reeves BC, Sterne JA, Murphy GJ. Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. The Lancet Haematology. 2015;2:e543–553. doi: 10.1016/S2352-3026(15)00198-2. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. URL http://www.R-project.org/ [Google Scholar]
- Salisbury AC, Amin AP, Reid KJ, Wang TY, Masoudi FA, Chan PS, Alexander KP, Bach RG, Spertus JA, Kosiborod M. Hospital-acquired anemia and in-hospital mortality in patients with acute myocardial infarction. American Heart Journal. 2011;162:300–309. e303. doi: 10.1016/j.ahj.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Salisbury AC, Reid KJ, Marso SP, Amin AP, Alexander KP, Wang TY, Spertus JA, Kosiborod M. Blood transfusion during acute myocardial infarction association with mortality and variability across hospitals. Journal of the American College of Cardiology. 2014;64:811–819. doi: 10.1016/j.jacc.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Schwarzer G. meta: General Package for Meta-Analysis. R package version 4.1-0. 2015 http://CRAN.R-project.org/package=meta.
- Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, Mazer CD. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion. 2012;52:91–99. doi: 10.1111/j.1537-2995.2011.03236.x. [DOI] [PubMed] [Google Scholar]
- Sherwood MW, Wang Y, Curtis JP, Peterson ED, Rao SV. Patterns and outcomes of red blood cell transfusion in patients undergoing percutaneous coronary intervention. Journal of the American Medical Association. 2014;311:836–843. doi: 10.1001/jama.2014.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GI, Craswell A, Thom O, Fung YL. Outcomes of restrictive versus liberal transfusion strategies in older adults from nine randomised controlled trials: a systematic review and meta-anaysis. The Lancet. Haematology. 2017 Oct;4(10):e465–e474. doi: 10.1016/S2352-3026(17)30141-2. Epub 2017 Sep 11. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishan A, Forbes JF, Ramsay P, Pearse R, Wallis C, Cairns C, Cole S, Wyncoll D RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Critical Care Medicine. 2013;41:2354–2363. doi: 10.1097/CCM.0b013e318291cce4. [DOI] [PubMed] [Google Scholar]
- Yang X, Alexander KP, Chen AY, Roe MT, Brindis RG, Rao SV, Gibler WB, Ohman EM, Peterson ED CRUSADE Investigators. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. Journal of the American College of Cardiology. 2005;46:1490–1495. doi: 10.1016/j.jacc.2005.06.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.